Abstract
Intermittent hypoxemia is a risk factor for numerous diseases. However, the reverse pathway remains unclear. Therefore, we investigated whether pre-existing hypertension, diabetes, or cardiovascular diseases (CVD) are associated with the worsening of intermittent hypoxemia. Among the included 2535 Sleep Heart Health Study participants, hypertension (n=1164), diabetes (n=170), and CVD (n=265) were frequently present at baseline. All participants had undergone two polysomnographic recordings approximately 5.2 years apart. Covariate-adjusted linear regression analyses were utilized to investigate the difference in the severity of intermittent hypoxemia at baseline between each comorbidity group and the group of participants free from all comorbidities (n=1264). Similarly, we investigated whether the pre-existing comorbidities are associated with the progression of intermittent hypoxemia. Significantly higher oxygen desaturation index (ODI, β=1.77 [95%CI: 0.41–3.13], p=0.011), desaturation severity (DesSev, β=0.07 [95%CI: 0.00–0.14], p=0.048), and desaturation duration (DesDur, β=1.50 [95%CI: 0.31–2.69], p=0.013) was observed in participants with pre-existing CVD at baseline. Furthermore, the increase in ODI (β=3.59 [95%CI: 1.78–5.39], p<0.001), DesSev (β=0.08 [95%CI: 0.02–0.14], p=0.015), and DesDur (β=2.60 [95%CI: 1.22–3.98], p<0.001) during the follow-up were higher among participants with diabetes. Similarly, the increase in ODI (β=2.73 [95%CI: 1.15–4.32], p=0.001) and DesDur (β=1.85 [95%CI: 0.62–3.08], p=0.003) were higher among participants with CVD. These results suggest that patients with pre-existing diabetes or CVD are at increased risk for an expedited worsening of intermittent hypoxemia. As the intermittent hypoxemia is an essential feature of sleep apnea, these patients could benefit from the screening and follow-up monitoring of sleep apnea.
Keywords: cardiovascular disease, diabetes, desaturation, hypoxemia, progression, sleep apnea
Introduction
Interest towards manifestation of sleep apnea (SA) has increased in recent years as the significant medical, social, and economic consequences of SA has become clearer (Jennum & Kjellberg, 2011). Recently it has been estimated that globally, nearly one billion adults are affected by SA (Benjafield et al., 2019). SA is associated with various daytime symptoms, such as excessive daytime sleepiness and reduced vigilance, lowering the quality of life (Al Lawati et al., 2009). Moreover, the prevalence of SA is high in patients with diabetes, hypertension and numerous cardiovascular diseases (CVD) (Floras, 2018).
Currently, SA is diagnosed based on polysomnography (PSG) from which the apnea-hypopnea index (AHI) is determined (Kapur et al., 2017). The AHI simply describes the average number of respiratory events per hour of sleep, thus ignoring the durations and severities of individual respiratory events and related intermittent hypoxemias (IH). However, IH severity has been more strongly associated with adverse CVD outcomes than the AHI (Azarbarzin et al., 2019, 2020; Stone et al., 2016). To overcome these issues, we have previously introduced new oxygen desaturation parameters (Kulkas et al., 2013) which take into account the duration and depth information of the desaturations, rather than just their counts. We have recently shown that these new parameters are associated more strongly with impaired psychomotor vigilance task performance (Kainulainen et al., 2020) and objective daytime sleepiness (Kainulainen et al., 2019) than conventional parameters used in sleep apnea diagnostics (i.e. AHI and oxygen desaturation index (ODI)). In addition, IH is a key component in the pathophysiology of SA and in the development of SA-related comorbidities (Aurora & Punjabi, 2013; Dempsey et al., 2010; Dewan et al., 2015; Floras, 2018; Kasai et al., 2012; Rajan & Greenberg, 2015). Therefore, the usage of more detailed analysis of the oxygen saturation signal could be beneficial and bring new information on the severity estimation of SA among patients with co-existing morbidities.
In general, the severity of SA has been associated with the incidence of CVD events (Jose et al., 2005; Peker et al., 2002) and diabetes (Anothaisintawee et al., 2016; Huang et al., 2018; Liu & Wu, 2017). In addition, SA has been widely accepted to affect the development of hypertension (Konecny et al., 2014; Marin et al., 2012; Peppard et al., 2000), although conflicting findings have been reported (Cano-Pumarega et al., 2011; O’Connor et al., 2009). As SA is a progressive disease, especially in its early stages (Berger et al., 2009), the reverse pathway has also been proposed meaning that pre-existing comorbidities could expedite the worsening of SA and IH (Aurora & Punjabi, 2013; Floras, 2018; Kasai et al., 2012; Rajan & Greenberg, 2015). Yet significantly less literature is available on this regard. We hypothesize that co-existing diseases could further expedite the worsening of IH in various ways, e.g. by increasing the number or the severity of desaturation events. Thus we investigated whether the pre-existing hypertension, diabetes, or CVD are associated with the severity of IH, determined based on ODI and our novel desaturation parameters (Kulkas et al., 2013), and whether these pre-existing comorbidities are associated with the progression of IH over a mean follow-up period of 5.2 years.
Methods
Dataset and study population
Data from the Sleep Heart Health Study (SHHS) (Quan et al., 1997; (The National Sleep Research Resource, 2021); Zhang et al., 2018), a multi-center community-based cohort study of participants enrolled between 1995 and 2006 in the United States was used in this study. The SHHS was designed to determine the associations between sleep-disordered breathing and related health consequences, such as cardiovascular diseases. SHHS data was available through the National Sleep Research Resource (Quan et al., 1997; The National Sleep Research Resource, 2021; Zhang et al., 2018) and it includes baseline and follow-up PSG recordings for 2647 participants. In addition, the dataset includes information on the pre-existing medical conditions at the baseline measurement and the incidence of new medical conditions during the follow-up. The SHHS study protocol was approved by the institutional review board of each participating site and all participants provided written consent. More detailed study details and design have been reported previously (Quan et al., 1997; Zhang et al., 2018).
Participants with incomplete background information (n=112) were excluded from the further analyses (Figure 1). In the present study, CVD was defined to consists of myocardial infarction, heart failure, coronary angioplasty, coronary artery bypass graft, and stroke. The existence of these CVD conditions was defined based on medical history interview. Hypertension was defined if systolic blood pressure was ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or hypertension medication was in use. The existence of diabetes was defined based on self-reported diabetes status and usage of oral hypoglycemic agents or insulin.
Figure 1.
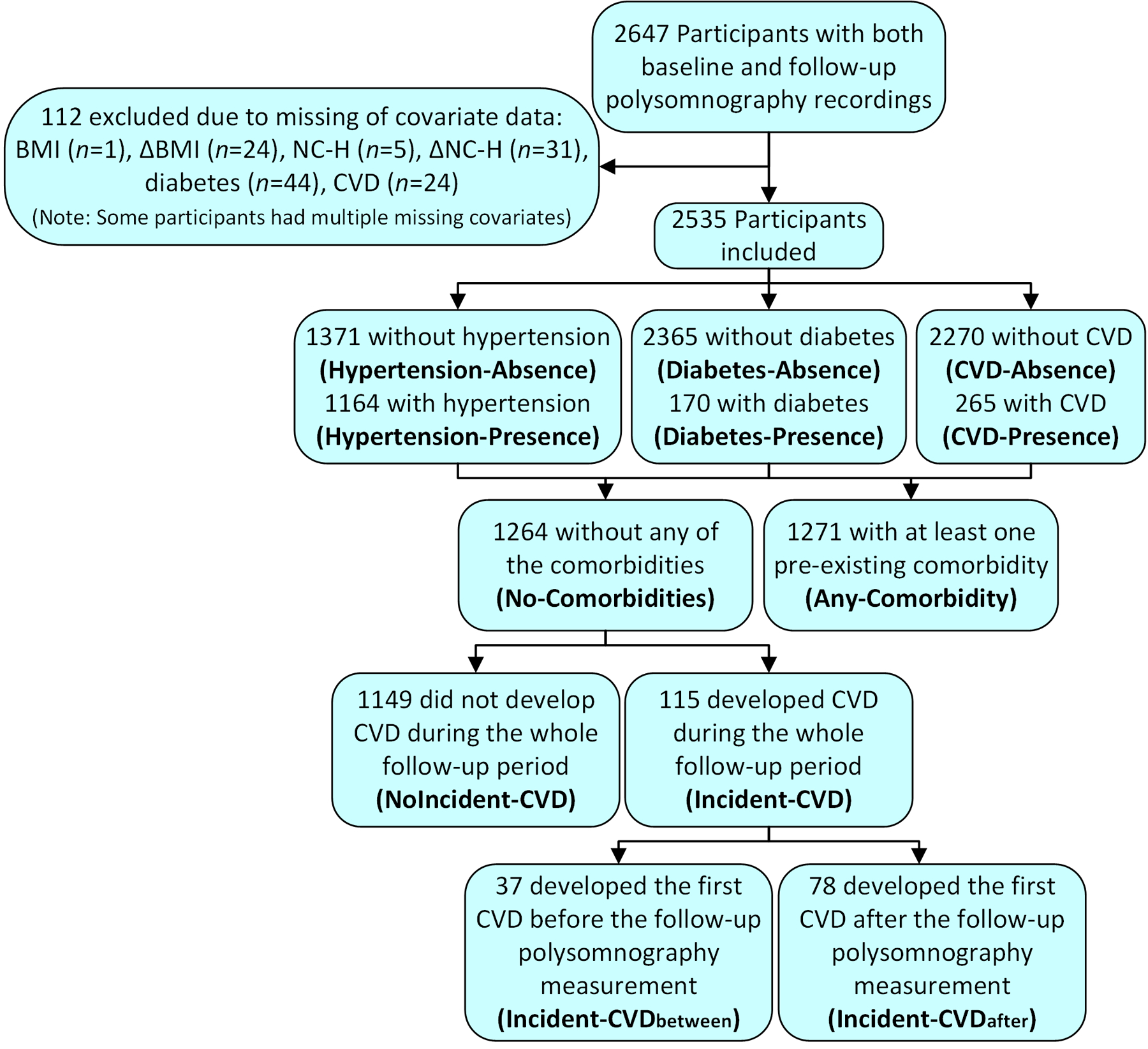
Flow chart of the study population and sizes of each presence and absence comorbidity groups.
Polysomnography and SpO2-derived parameters
PSG recordings were performed at participants homes with Compumedics P-series portable monitor (Abbotsford, Victoria, Australia). Oxygen saturation (SpO2) was measured using finger-pulse oximetry (Nonin XPOD Model 3011, Minneapolis, US). Because some of the originally scored desaturation event annotations had disappeared due to known data corruption (The National Sleep Research Resource, 2021), SpO2 signals were rescored automatically with Noxturnal software (version 5.1.19824, Nox Medical, Reykjavík, Iceland). Agreement between the automatic and manual scorings using the same methods in the same dataset has been previously presented extensively and been shown to be excellent (Karhu et al., 2021). No desaturation scoring criteria exist in the American Academy of Sleep Medicine (AASM) guidelines besides a minimum transient drop of 3% or 4% from the baseline (Berry et al., 2017). Therefore, scoring criteria of 1) minimum transient drop of 3%, 2) minimum event duration of 3 seconds, 3) maximum plateau duration of 45 seconds, and 4) lowest acceptable SpO2 value of 50%, were applied. It was observed that automatically scored desaturation events started systematically one data point too early and this issue was corrected in further parameter calculations. Afterwards desaturation events that fulfilled the 4% transient drop criterion were chosen for the final analysis due to the suspicion based on previous findings (Myllymaa et al., 2015) that without associating desaturation events to the respiratory events, the 3% criterion could be too sensitive. In addition to ODI, desaturation parameters describing the severity of the desaturation events were calculated and used to represent the severity of IH and indicate the severity of SA. These parameters consisted of desaturation severity (DesSev), desaturation duration (DesDur), average desaturation duration (avg. DesDur), and average desaturation area (avg. DesArea) (online supplement, Figure S1) (Kulkas et al., 2013). Furthermore, changes in the parameter values between the two PSGs (5.2 years apart on average) were calculated, and these changes were used to assess the progression of IH.
Statistical analyses
Demographic information and desaturation parameter values were calculated for all the groups of participants with (presence groups) and without (absence groups) pre-existing comorbidities (Figure 1) at the baseline and after the follow-up PSG. Each presence group was compared to the corresponding absence group, and all the groups were compared to the group of participants without any pre-existing comorbidities (No-Comorbidities) at the baseline. Statistical comparison between the groups was performed with Mann-Whitney U test. Baseline desaturation parameter values between each presence group and No-Comorbidities group were further investigated with linear regression analyses adjusted with body mass index (BMI), age, sex, and neck circumference-height ratio (NC-H). Thus, regression coefficients (β-values) correspond to the increase in parameter value associated with the existence of the comorbidity.
Linear regression was also utilized to investigate the association between pre-existing comorbidities at the baseline and the progression of IH. Change in the desaturation parameter values between the two PSGs (i.e., PSG2-PSG1) was used as a continuous dependent variable. The existence of comorbidity at the baseline was the primary independent variable and the No-Comorbidities group was used as a reference group. Therefore, regression coefficients correspond to the expedited increase in parameter value between the PSG recordings that is associated with the existence of the comorbidity at baseline. The model was adjusted with the baseline values of ODI, avg. DesArea, and avg. DesDur, sex, age, baseline BMI, baseline NC-H, change in BMI, change in NC-H, and time between the PSG recordings. The adjusting covariates were selected based on their known clinical relevance to the severity and progression of SA. For example, older age, higher BMI, and male sex are known risk factors for SA (Young et al., 2004). In addition, an increase in weight over time is the most significant factor affecting the worsening of SA (Berger et al., 2009). Multicollinearity between the independent variables was tested with variance inflation factor and no values larger than 5 were observed indicating no serious correlation between the included variables. However, all five desaturation parameters could not be included in the model simultaneously without serious multicollinearity.
In addition, we investigated whether the baseline desaturation parameters and the changes in these parameters between the PSG recordings differed between the participants who developed CVD after the baseline examination and those who remained CVD-free during the whole follow-up period. To eliminate the potential effect of pre-existing comorbidities on the incidence of CVD and the progression of IH, only the participants in the No-Comorbidities group were included. The No-Comorbidities group was divided into Incident-CVD and NoIncident-CVD groups based on whether the participants had incident CVD during the follow-up period or whether they remained CVD-free during the whole follow-up period (Figure 1). The Incident-CVD group was further divided into Incident-CVDbetween and Incident-CVDafter groups based on whether the CVD occurred between the two PSGs or after the follow-up PSG, respectively. Incident-CVD, Incident-CVDbetween, and Incident-CVDafter groups were compared to the NoIncident-CVD group, and Incident-CVDbetween group to the Incident-CVDafter group.
Potential selection bias was addressed by comparing the baseline characteristics between participants who underwent only the baseline PSG and participants who participated in both PSGs.
To reduce the likelihood of obtaining significant results by chance and since we investigated five different desaturation parameters, Bonferroni-corrected p-value of <0.01 was used as the limit of statistical significance, while findings with p<0.05 were interpreted as nominal evidence. All parameter calculations and statistical analyses were conducted with MATLAB® (version 2018b, MathWorks, Natick, MA, USA).
Results
Out of the included 2535 participants, 1164 (45.9%) had hypertension (Hypertension-Presence), 170 (6.7%) had diabetes (Diabetes-Presence), and 265 (10.5%) had CVD (CVD-Presence) at the baseline (Figure 1). A total of 1264 (49.9%) participants were free from all these diseases (No-Comorbidities), whereas 1271 (50.1%) had at least one of these comorbidities at the baseline (Any-Comorbidity). Participants belonging to the Hypertension-Presence, Diabetes-Presence, or CVD-Presence groups were generally older and had higher BMI and NC-H compared to the corresponding absence groups and No-Comorbidities group (Table 1). A similar observation was made between No-Comorbidities and Any-Comorbidity groups.
Table 1.
Demographic and anthropometric data at the baseline, after the follow-up, and changes in parameter values between the two PSG recordings.
| No- | Any- | Hypertension- | Diabetes- | CVD- | ||||
|---|---|---|---|---|---|---|---|---|
| Comorbidities | Comorbidity | Absence | Presence | Absence | Presence | Absence | Presence | |
| 1264 (42.9) | 1271 (49.4) | 1371 (44.7) | 1164 (47.9) | 2365 (45.5) | 170 (55.3) | 2270 (43.6) | 265 (67.9) | |
| Time between PSGs (y) | 5.3 (0.3) | 5.2 (0.3)*# | 5.3 (0.3) | 5.2 (0.3)*# | 5.2 (0.3)# | 5.2 (0.3)*# | 5.2 (0.3)# | 5.2 (0.2)*# |
| Total follow-up time (y) | 12.1 (1.5) | 11.5 (1.8)*# | 12.0 (1.5) | 11.5 (1.8)*# | 11.8 (1.6)# | 11.2 (2.0)*# | 11.9 (1.6)# | 11.1 (1.8)*# |
| Age (y) | ||||||||
| Baseline (1st PSG) | 58.9 (10.0) | 66.1 (9.6)*# | 59.4 (10.1) | 66.1 (9.7)*# | 62.1 (10.4)# | 67.2 (9.6)*# | 61.8 (10.4)# | 68.6 (8.4)*# |
| Follow-up (2nd PSG) | 64.2 (10.0) | 71.2 (9.5)*# | 64.7 (10.0) | 71.3 (9.6)*# | 67.4 (10.3)# | 72.4 (9.5)*# | 67.0 (10.3)# | 73.8 (8.3)*# |
| Change | 5.3 (0.5) | 5.2 (0.6)*# | 5.3 (0.5) | 5.2 (0.6)*# | 5.2 (0.5) | 5.2 (0.5) | 5.2 (0.5) | 5.2 (0.5)# |
| BMI (kg/m2) | ||||||||
| Baseline (1st PSG) | 27.5 (4.5) | 28.9 (5.1)*# | 27.5 (4.5) | 28.9 (5.2)*# | 28.0 (4.8)# | 30.7 (5.3)*# | 28.2 (4.9)# | 28.2 (4.2)# |
| Follow-up (2nd PSG) | 27.8 (4.7) | 28.7 (5.3)*# | 27.9 (4.7) | 28.8 (5.3)*# | 28.2 (4.9) | 30.1 (5.9)*# | 28.3 (5.1)# | 28.1 (4.5) |
| Change | 0.4 (2.0) | −0.2 (2.3)*# | 0.4 (2.1) | −0.2 (2.3)*# | 0.2 (2.2)# | −0.7 (2.6)*# | 0.1 (2.2)# | −0.1 (2.3)# |
| NC-H (%) | ||||||||
| Baseline (1st PSG) | 22.0 (1.9) | 22.9 (2.0)*# | 22.1 (1.9) | 22.9 (2.0)*# | 22.3 (2.0)# | 23.6 (2.0)*# | 22.3 (2.0)# | 23.0 (1.9)*# |
| Follow-up (2nd PSG) | 22.1 (2.1) | 22.9 (2.0)*# | 22.2 (2.1) | 22.9 (2.0)*# | 22.5 (2.1)# | 23.5 (1.9)*# | 22.4 (2.1)# | 23.2 (2.0)*# |
| Change | 0.1 (1.4) | 0.1 (1.4) | 0.1 (1.4) | 0.1 (1.4) | 0.1 (1.4) | −0.1 (1.5) | 0.1 (1.4) | 0.2 (1.3) |
Parameter values are presented as means and standard deviations.
denotes statistical significance between No-Comorbidities group and the group under the hashtag.
denotes statistically significant difference compared to the corresponding absence group.
Statistical significance of differences (p<0.05) was determined with Mann-Whitney U test. PSG = polysomnography, BMI = body mass index, NC-H = neck circumference-height ratio, CVD = cardiovascular disease (consists of myocardial infarction, heart failure, coronary angioplasty, coronary artery bypass graft, and stroke).
ODI, DesSev, and DesDur were significantly higher at the baseline in Hypertension-Presence, Diabetes-Presence, and CVD-Presence groups compared to the No-Comorbidities group (Table 2). After adjusting the linear regression analyses with BMI, age, sex, and NC-H, ODI (p=0.011), DesSev (p=0.048), and DesDur (p=0.013) remained significantly higher in CVD-Presence group reaching the level of nominal association (Table 3). Furthermore, lower avg. DesDur (p=0.004) and avg. DesArea (p=0.023) were observed in Diabetes-Presence group, reaching the Bonferroni-corrected threshold and the limit of nominal evidence, respectively.
Table 2.
Desaturation parameter values at the baseline, after the follow-up, and changes in parameter values between the two PSG recordings.
| No- | Any- | Hypertension- | Diabetes- | CVD- | ||||
|---|---|---|---|---|---|---|---|---|
| Comorbidities | Comorbidity | Absence | Presence | Absence | Presence | Absence | Presence | |
| ODI (1/h) | ||||||||
| Baseline (1st PSG) | 3.2 (1.4, 8.1) | 5.9 (2.6, 12.2)*# | 3.3 (1.5, 8.3) | 5.9 (2.6, 12.2)*# | 4.2 (1.7, 10.0)# | 7.3 (3.1, 12.7)*# | 4.1 (1.7, 9.7)# | 7.2 (2.9, 14.5)*# |
| Follow-up (2nd PSG) | 8.1 (4.1, 15.8) | 12.3 (6.4, 22.7)*# | 8.6 (4.4, 16.6) | 12.2 (6.3, 22.8)*# | 9.8 (4.9, 18.6)# | 16.9 (8.4, 28.6)*# | 9.6 (4.8, 18.6)# | 14.9 (8.9, 26.5)*# |
| Change | 4.1 (1.3, 8.7) | 5.1 (1.1, 12.6)*# | 4.4 (1.3, 8.9) | 5.0 (1.1, 12.5)*# | 4.5 (1.2, 9.9) | 6.9 (2.0, 19.2)*# | 4.5 (1.2, 9.9) | 7.0 (1.4, 14.8)*# |
| DesSev (%) | ||||||||
| Baseline (1st PSG) | 0.08 (0.03, 0.22) | 0.16 (0.06, 0.34)*# | 0.08 (0.03, 0.23) | 0.16 (0.06, 0.34)*# | 0.11 (0.04, 0.29)# | 0.19 (0.08, 0.32)*# | 0.10 (0.04, 0.27)# | 0.21 (0.08, 0.41)*# |
| Follow-up (2nd PSG) | 0.19 (0.09, 0.41) | 0.31 (0.15, 0.64)*# | 0.20 (0.09, 0.43) | 0.31 (0.15, 0.64)*# | 0.24 (0.11, 0.50)# | 0.37 (0.19, 0.84)*# | 0.24 (0.11, 0.49)# | 0.38 (0.23, 0.72)*# |
| Change | 0.09 (0.02, 0.22) | 0.12 (0.02, 0.30)*# | 0.09 (0.02, 0.22) | 0.12 (0.02, 0.30)*# | 0.10 (0.02, 0.25) | 0.15 (0.05, 0.50)*# | 0.10 (0.02, 0.24) | 0.16 (0.03, 0.35)*# |
| DesDur (%) | ||||||||
| Baseline (1st PSG) | 2.6 (1.0, 7.0) | 5.2 (2.1, 10.4)*# | 2.8 (1.1, 7.4) | 5.2 (2.1, 10.4)*# | 3.5 (1.4, 8.6)# | 6.1 (2.6, 10.4)*# | 3.5 (1.4, 8.3)# | 6.3 (2.7, 11.9)*# |
| Follow-up (2nd PSG) | 6.9 (3.5, 13.7) | 10.7 (5.6, 19.4)*# | 7.2 (3.6, 14.1) | 10.6 (5.6, 19.4)*# | 8.5 (4.0, 16.1)# | 12.8 (6.7, 24.9)*# | 8.3 (3.9, 15.7)# | 12.6 (7.7, 22.4)*# |
| Change | 3.5 (1.0, 7.4) | 4.3 (1.0, 10.0)*# | 3.6 (1.1, 7.6) | 4.3 (0.9, 10.0)*# | 3.9 (1.0, 8.4) | 5.4 (2.2, 15.2)*# | 3.8 (1.0, 8.2) | 5.8 (1.4, 11.5)*# |
| avg. DesArea (s%) | ||||||||
| Baseline (1st PSG) | 88.2 (70.6, 109.6) | 95.4 (78.5, 116.6)*# | 88.8 (71.7, 110.1) | 94.9 (78.4, 117.2)*# | 91.8 (74.8, 113.6)# | 92.8 (76.8, 112.8) | 91.1 (73.8, 113.0)# | 98.6 (82.8, 116.3)*# |
| Follow-up (2nd PSG) | 85.6 (70.2, 103.5) | 90.0 (76.5, 109.3)*# | 85.6 (70.7, 103.8) | 90.6 (76.5, 109.3)*# | 88.2 (73.1, 107.2)# | 86.6 (73.9, 105.7) | 87.8 (72.3, 106.4)# | 90.2 (79.5, 111.1)*# |
| Change | −2.1 (−19.0, 15.5) | −3.7 (−22.4, 13.3) | −2.4 (−19.2, 15.2) | −2.9 (−22.6, 13.8) | −2.6 (−20.5, 14.6) | −1.6 (−22.3, 12.2) | −2.3 (−20.2, 14.7) | −5.2 (−23.8, 11.0) |
| avg. DesDur (s) | ||||||||
| Baseline (1st PSG) | 28.6 (23.4, 34.1) | 29.3 (24.8, 34.4)*# | 28.8 (23.5, 34.1) | 29.1 (24.7, 34.4)*# | 29.0 (24.1, 34.4)# | 28.5 (24.2, 32.2) | 28.8 (24.0, 34.2) | 30.3 (26.2, 34.5)*# |
| Follow-up (2nd PSG) | 29.1 (24.8, 33.7) | 29.5 (25.5, 33.8) | 29.1 (24.9, 33.7) | 29.5 (25.6, 33.8) | 29.4 (25.3, 33.8) | 28.1 (24.4, 32.6)* | 29.3 (25.1, 33.7) | 29.6 (26.0, 34.6)# |
| Change | 0.6 (−4.6, 5.7) | 0.0 (−4.7, 4.4)*# | 0.5 (−4.7, 5.5) | 0.1 (−4.7, 4.4) | 0.3 (−4.7, 5.2) | 0.3 (−4.4, 3.4) | 0.4 (−4.7, 5.2) | −0.6 (−4.9, 4.1) |
Parameter values are presented as medians and interquartile ranges.
denotes statistical significance between No-Comorbidities group and the group under the hashtag.
denotes statistically significant difference compared to the corresponding absence group.
Statistical significance of differences (p<0.05) was determined with Mann-Whitney U test. PSG = polysomnography, ODI = oxygen desaturation index, DesSev = desaturation severity, DesDur = desaturation duration, avg. DesArea = average desaturation area of individual desaturation events, avg. DesDur = average desaturation duration of individual desaturation events.
Table 3.
Regression coefficients (β-values) with 95% confidence intervals from adjusted linear regression analyses utilized to investigate the difference in desaturation parameter values at the baseline between participants without pre-existing comorbidities and participants with hypertension, diabetes, or CVD. β-values correspond to the increase in parameter value associated with the existence of the comorbidity.
| ODI | DesSev | DesDur | avg. DesArea | avg. DesDur | |
|---|---|---|---|---|---|
| Any-Comorbidity | 0.71 (−0.13, 1.55) | 0.01 (−0.03, 0.05) | 0.46 (−0.26, 1.18) | 0.94 (−1.95, 3.83) | −0.37 (−1.06, 0.32) |
| Hypertension | 0.83 (−0.03, 1.69) | 0.01 (−0.03, 0.05) | 0.54 (−0.19, 1.27) | 0.97 (−2.00, 3.94) | −0.40 (−1.11, 0.31) |
| Diabetes | 0.46 (−1.15, 2.07) | −0.02 (−0.09, 0.05) | 0.01 (−1.34, 1.36) | −6.40 (−11.92, −0.88)* | −2.07 (−3.48, −0.66)** |
| CVD | 1.77 (0.41, 3.13)* | 0.07 (0.00, 0.14)* | 1.50 (0.31, 2.69)* | 0.82 (−4.03, 5.67) | −0.83 (−2.02, 0.36) |
Regression analyses were adjusted for sex, age, body mass index, and neck circumference-height ratio. Statistically significant differences in the parameter values at the baseline between patients with comorbidity and comorbidity-free patients is marked with an asterisk (p<0.05, *; Bonferroni-corrected threshold p<0.01, **). Participants free from hypertension, diabetes, and CVD (i.e. No-comorbidity group) were used as a reference group in the case of all comorbidities. ODI = oxygen desaturation index, DesSev = desaturation severity, DesDur = desaturation duration, avg. DesArea = average desaturation area of individual desaturation events, avg. DesDur = average desaturation duration of individual desaturation events, CVD = cardiovascular disease (consists of myocardial infarction, heart failure, coronary angioplasty, coronary artery bypass graft, and stroke).
ODI, DesSev, and DesDur values increased in all absence and presence groups between the PSG recordings (Table 2). However, covariate-adjusted linear regression analyses revealed that the pre-existing diabetes was associated with an expedited increase in ODI (p<0.001) and DesDur (p<0.001) at the Bonferroni-corrected threshold and DesSev (p=0.015) at the limit of nominal evidence. Furthermore, an expedited increase in ODI (p=0.001) and DesDur (p=0.003) was observed among participants with pre-existing CVD reaching the Bonferroni-corrected threshold. However, pre-existing comorbidities were not associated with the progression of avg. DesArea or avg. DesDur (except of diabetes on avg. DesDur, p=0.015, Table 4). Overall, pre-existing diabetes had the largest β-values, thus indicating the strongest association of the investigated comorbidities on the worsening of IH (Table 4).
Table 4.
Regression coefficients (β-values) with 95% confidence intervals from linear regression analyses which were utilized to investigate the associations between pre-existing comorbidities and the progression of desaturation parameters between the two PSG recordings. β-values correspond to the expedited increase in parameter value between the PSG recordings that is associated with the existence of the comorbidity at baseline.
| ΔODI | ΔDesSev | ΔDesDur | Δavg. DesArea | Δavg. DesDur | |
|---|---|---|---|---|---|
| Any-Comorbidity | 0.98 (−0.02, 1.99) | 0.03 (−0.01, 0.06) | 0.74 (−0.05, 1.52) | −0.98 (−3.17, 1.21) | −0.22 (−0.77, 0.32) |
| Hypertension | 0.98 (−0.05, 2.01) | 0.03 (−0.01, 0.06) | 0.74 (−0.06, 1.54) | −1.05 (−3.31, 1.20) | −0.21 (−0.78, 0.35) |
| Diabetes | 3.59 (1.78, 5.39)** | 0.08 (0.02, 0.14)* | 2.60 (1.22, 3.98)** | −3.59 (−8.00, 0.81) | −1.41 (−2.54, −0.27)* |
| CVD | 2.73 (1.15, 4.32)** | 0.06 (0.00, 0.11) | 1.85 (0.62, 3.08)** | −1.19 (−4.97, 2.59) | −0.38 (−1.34, 0.58) |
Regression analyses were adjusted for sex, age, baseline values of body mass index, neck circumference-height ratio, ODI, avg. DesArea, and avg. DesDur, and changes in body mass index and neck circumference-height ratio between the PSG recordings. Statistically significant association between pre-existing comorbidity and the expedited progression of the parameter (Δ) is marked with an asterisk (p<0.05, *; Bonferroni-corrected threshold p<0.01, **). Participants free from hypertension, diabetes, and CVD (i.e. No-Comorbidity group) were used as a reference group in the case of all comorbidities. ODI = oxygen desaturation index, DesSev = desaturation severity, DesDur = desaturation duration, avg. DesArea = average desaturation area of individual desaturation events, avg. DesDur = average desaturation duration of individual desaturation events, CVD = cardiovascular disease (consists of myocardial infarction, heart failure, coronary angioplasty, coronary artery bypass graft, and stroke).
From the No-Comorbidities group, 115 (9.1%) participants had incident CVD during the follow-up period (Incident-CVD) from which 37 (32.2%) had it between the two PSGs (Incident-CVDbetween) and 78 (67.8%) after the follow-up PSG (Incident-CVDafter). A total of 1149 (90.9%) participants remained CVD-free during the whole follow-up period (NoIncident-CVD). All five desaturation parameters were significantly higher in the Incident-CVD group compared to the NoIncident-CVD group at the baseline, although ODI and avg. DesArea did not reach the Bonferroni-corrected threshold (Table 5). Similar findings were observed between Incident-CVDafter and NoIncident-CVD groups. No statistically significant differences between Incident-CVDbetween and Incident-CVDafter groups were observed in any baseline desaturation parameter values or changes in these parameters between the two PSGs.
Table 5.
Desaturation parameter values at the baseline, after the follow-up, and changes in parameter values between the two PSG recordings for participants with and without incident CVD after the baseline measurement.
| NoIncident-CVD | Incident-CVD | Incident-CVDbetween | Incident-CVDafter | |
|---|---|---|---|---|
| n (male%) | 1149 (40.9) | 115 (62.6) | 37 (67.6) | 78 (60.3) |
| Time between PSGs (y) | 5.3 (0.3) | 5.2 (0.4)## | 5.3 (0.4) | 5.2 (0.3)##* |
| Total follow-up time (y) | 12.1 (1.5) | 11.8 (1.6) | 12.1 (1.1) | 11.6 (1.8) |
| ODI (1/h) | ||||
| Baseline (1st PSG) | 3.0 (1.4, 8.0) | 4.6 (2.1, 9.0)# | 3.8 (1.8, 7.2) | 4.6 (2.1, 9.6)# |
| Follow-up (2nd PSG) | 7.9 (3.9, 15.6) | 10.0 (5.8, 18.3)# | 9.5 (6.4, 19.0) | 10.2 (5.5, 17.9)# |
| Change | 4.0 (1.2, 8.6) | 5.1 (2.2, 11.2)# | 4.9 (2.4, 12.2) | 5.1 (1.8, 11.0) |
| DesSev (%) | ||||
| Baseline (1st PSG) | 0.08 (0.03, 0.21) | 0.11 (0.04, 0.24)## | 0.10 (0.03, 0.20) | 0.12 (0.05, 0.26)## |
| Follow-up (2nd PSG) | 0.19 (0.09, 0.40) | 0.24 (0.13, 0.49)## | 0.21 (0.14, 0.46) | 0.26 (0.13, 0.53)# |
| Change | 0.08 (0.02, 0.21) | 0.12 (0.05, 0.29)# | 0.13 (0.06, 0.25) | 0.12 (0.03, 0.30) |
| DesDur (%) | ||||
| Baseline (1st PSG) | 2.6 (1.0, 6.8) | 3.9 (1.7, 7.8)## | 3.5 (1.2, 6.1) | 4.1 (1.9, 8.2)## |
| Follow-up (2nd PSG) | 6.8 (3.3, 13.4) | 8.6 (4.8, 16.0)## | 8.4 (5.0, 14.8) | 9.0 (4.7, 17.2)# |
| Change | 3.4 (1.0, 7.1) | 4.7 (2.2, 9.1)## | 5.2 (2.7, 8.7)# | 4.7 (1.8, 9.3) |
| avg. DesArea (s%) | ||||
| Baseline (1st PSG) | 87.8 (70.2, 108.9) | 95.7 (78.2, 113.0)# | 94.4 (70.4, 111.6) | 97.4 (81.1, 113.5)# |
| Follow-up (2nd PSG) | 84.9 (70.0, 103.3) | 90.1 (77.0, 109.0)# | 91.4 (73.0, 105.9) | 89.7 (77.3, 112.5)# |
| Change | −1.8 (−19.2, 15.6) | −5.5 (−17.9, 14.9) | −5.5 (−16.7, 22.2) | −6.2 (−18.5, 12.9) |
| avg. DesDur (s) | ||||
| Baseline (1st PSG) | 28.3 (23.1, 33.9) | 30.6 (26.0, 36.1)## | 29.0 (24.2, 34.1) | 31.1 (26.5, 36.9)## |
| Follow-up (2nd PSG) | 28.9 (24.6, 33.5) | 30.8 (27.0, 34.9)## | 31.0 (26.9, 34.2) | 30.6 (27.0, 35.9)## |
| Change | 0.6 (−4.6, 5.9) | −0.6 (−4.8, 5.4) | 1.0 (−3.6, 6.5) | −1.3 (−4.9, 5.3) |
Parameter values are presented as medians and interquartile ranges. Incident-CVD group consist of participants who developed CVD after the baseline PSG examination. This group is further divided into Incident-CVDbetween and Incident-CVDafter groups based on whether the first CVD occurred before or after the follow-up PSG, respectively. Mann-Whitney U test was used to investigate statistical significance of differences between incident CVD groups and NoIncident-CVD group (p<0.05, #; p<0.01, ##), and between Incident-CVDbetween and Incident-CVDafter groups (p<0.05, *; p<0.01, **). ODI = oxygen desaturation index, DesSev = desaturation severity, DesDur = desaturation duration, avg. DesArea = average desaturation area of individual desaturation events, avg. DesDur = average desaturation duration of individual desaturation events. CVD = cardiovascular disease (consists of myocardial infarction, heart failure, coronary angioplasty, coronary artery bypass graft, and stroke.
A total of 2729 (1312 men, 1417 women) participants underwent only the baseline PSG recording and had full covariate data available (online supplement, Table S1). These participants were significantly older, had higher NC-H, and had higher proportion of hypertension, diabetes, and CVD (p<0.001 for all) compared to the participants who participated in both PSGs. In addition, ODI (p<0.001), DesSev (p<0.001), DesDur (p<0.001), and avg. DesArea (p=0.001) were significantly higher in participants with only baseline PSG recording.
Discussion
In this study, we investigated differences in the severity of IH between patients with hypertension, diabetes, or CVD in comparison to the patients free of these diseases at the baseline. Furthermore, it was investigated how these pre-existing comorbidities were associated with the progression of IH during the five-year follow-up. In addition, we examined how the IH severity at the baseline and the change in the IH severity between the PSG recordings differed between participants who developed CVD after the baseline PSG recording and those who remained CVD-free during the whole follow-up period. We showed that in patients with pre-existing CVD, the ODI, DesSev, and DesDur values were elevated at baseline. Moreover, pre-existing diabetes and CVD were associated with an expedited worsening of IH, measured mainly by increased number of desaturations. In general, SA has been accepted to be an independent risk factor for the development of these comorbidities (Anothaisintawee et al., 2016; Floras, 2018; Huang et al., 2018; Jose et al., 2005; Kasai et al., 2012; Liu & Wu, 2017; Peker et al., 2002). Our present findings suggest the possibility of reversible pathway, and thus, bidirectional association between SA and these comorbidities through the effects of IH. Therefore, as IH is an important feature of SA, our present results support the idea that patients with these comorbidities should get more attention in the screening of SA and monitoring of its progression. These actions could enable preventative actions for the incidence of future adverse events.
We showed that patients with hypertension, diabetes, or CVD have more severe IH at the baseline compared to individuals free of comorbidities. However, after adjusting for BMI, age, NC-H, and sex, ODI, DesSev, and DesDur remained significantly higher only in patients with CVD. Findings that the consideration of key covariates, such as obesity and age attenuates the difference in SA severity in terms of AHI between patients with and without comorbidities, such as hypertension (Nieto et al., 2000; Tkacova et al., 2014) and diabetes (Resnick et al., 2003) have been previously reported. This is somewhat expected as SA and related comorbidities share many risk factors, such as obesity and older age (Young et al., 2004). However, parameters describing IH have remained independent predictors for hypertension in other previous studies after considering the relevant covariates (Nieto et al., 2000; Tkacova et al., 2014). Furthermore, weight gain is widely accepted to be the most significant factor affecting the worsening of SA (Berger et al., 2009). Moreover, the prevalence of SA is high in patients with diabetes, hypertension, and CVD (Floras, 2018), however, limited literature is available on how these pre-existing comorbidities affect the progression of SA and IH over time. Our present results implicate that the existence of diabetes or CVD further expedites the worsening of IH independently of age, change in BMI, NC-H, or IH severity. These findings are somewhat in conflict with other previous studies in which pre-existing diabetes or CVD were not linked to the development of SA (Liu & Wu, 2017; Tishler et al., 2003). However, a direct comparison with these previous studies should be done with caution as they were limited on substantially younger patients without existing SA at the baseline (Liu & Wu, 2017; Tishler et al., 2003).
We also investigated how the IH severity and the progression of IH differed between participants who developed CVD after the baseline PSG and those who remained CVD-free during the whole follow-up period. We observed that IH is more severe in Incident-CVD and Incident-CVDafter groups at the baseline, but not in Incident-CVDbetween group, compared to the NoIncident-CVD group. This could be explained by the widely accepted concept that more severe SA increases the risk for the incidence of CVD (Floras, 2018; Kasai et al., 2012). However, as the difference in the absolute values between Incident-CVDafter and NoIncident-CVD groups was rather small, it could be thought that the development of CVD, due to the more severe IH, takes time (over 5 years). However, these findings should be interpreted cautiously as there could be significant differences in follow-up times between the PSG recordings, which would affect the grouping, and in times from the baseline to the incidence of the first CVD event.
Several potential mechanisms explaining how the pre-existing comorbidities could contribute to SA and IH have been suggested. First, diabetes related autonomic dysfunction could increase the loop gain meaning that even small changes in partial carbon dioxide pressure could result in central apnea, and thus, over- and undershooting of carbon dioxide levels, i.e. periodic breathing (Aurora & Punjabi, 2013; Floras, 2018; Rajan & Greenberg, 2015). Second, diabetes could contribute to systemic inflammation and oxidative stress which in turn predisposes to SA and exacerbates existing SA (Aurora & Punjabi, 2013; Rajan & Greenberg, 2015). Third, insulin resistance could reduce ventilatory responses for hypercapnia and hypoxia (Aurora & Punjabi, 2013; Rajan & Greenberg, 2015). Moreover, pre-existing hypertension and heart failure could accumulate the fluid retention and increase the rostral fluid shift from legs to the rostral area when moving from upward position to recumbent position (Floras, 2018; Kasai et al., 2012). This would narrow the upper airways and increase the extraluminal tissue pressure (Floras, 2018). Similarly, increased fluid retention and rostral fluid shift due to the increased activity of sympathetic nervous system and activation of the renin-angiotensin-aldosterone axis caused by heart failure could contribute to SA (Floras, 2018). Heart failure could also increase the loop gain, similarly as diabetes (Dempsey et al., 2010). In addition, heart failure lowers cardiac output which prolongs circulation times to the chemoreceptors in carotid body which increases the delay for the corrective actions against changes in partial carbon dioxide pressure, and thus, could prolong the periodic breathing (Dempsey et al., 2010).
It is important to note that this study has some limitations. As SHHS was designed to investigate the consequences of sleep-disordered breathing, such as cardiovascular diseases, the study population could be enriched with participants with such conditions potentially causing selection bias. In addition, the exclusion of participants with only baseline PSG measurement could cause bias as it was observed that the severity of IH and the proportion of comorbidities was higher in these participants compared with participants who underwent both PSGs (online supplement, Table S1). Furthermore, baseline comorbidity statuses were mostly obtained from the parent cohort studies before the baseline PSG examination, and thus, some newly diagnosed diseases could be missed. Pre-existing diabetes and CVD statuses were also self-reported. In addition, no information was available when patients were diagnosed with these comorbidities for the first time. This could affect the results as the progression of IH could differ in patients with newly diagnosed disease compared to the patients who have suffered with the disease over many decades. Moreover, very limited information was available whether participants received treatment for SA at any point between the two PSG recordings, and thus, no participants were excluded due to this regard.
Another limitation is that we used automatically rescored desaturation events to describe IH which were not manually adjusted due to the massive size of the SHHS dataset. Moreover, as no scoring criteria for desaturations exist in the AASM guidelines (Berry et al., 2017) besides a transient drop of 3% or 4%, the alteration of the used scoring criteria would affect the number and severity of desaturation events. However, the used scorings can be assumed to be valid as correlations and median differences between automatic scorings and subset of manual scorings have been shown to be excellent (Karhu et al., 2021). Furthermore, apneas and hypopneas were not automatically rescored as they were found to be insufficiently accurate without manual editing. Therefore, the current diagnostic standard, AHI, and the severities and durations of apneas and hypopneas could not be investigated. However, as the ODI has been shown to correlate well with the AHI (Tsai et al., 1999) it could be reliably used in the screening of SA (Behar et al., 2019). In addition, IH has been suggested to be a major factor in the development of many SA-related comorbidities (Aurora & Punjabi, 2013; Dempsey et al., 2010; Dewan et al., 2015; Floras, 2018; Kasai et al., 2012; Rajan & Greenberg, 2015), and the parameters describing hypoxemia have been associated more strongly with adverse SA-related events than the AHI (Azarbarzin et al., 2019, 2020; Stone et al., 2016). Therefore, the use of new oxygen saturation-based parameters to investigate the associations between comorbidities and IH can be justified.
Our findings indicate that patients with diabetes and CVD are at increased risk for an expedited worsening of IH, and thus, they could benefit from the screening and follow-up monitoring of SA. The detection of SA would enable preventative actions for its further worsening, such as lifestyle counselling or initiation of treatment. These actions could mitigate the development of future SA-related comorbidities and adverse events. Furthermore, the follow-up monitoring could be conducted with a simple pulse oximeter as the investigated parameters only require oxygen saturation signal. Monitoring with a simple pulse oximeter setup would be patient-friendly, cost-effective, and would enable the monitoring over multiple consecutive nights eliminating the significant night-to-night variability in the SA severity (Roeder et al., 2020).
Conclusion
Based on the present findings, intermittent hypoxemia is more severe in patients with pre-existing CVD at the baseline, and that pre-existing diabetes and CVD are associated with an expedited worsening of intermittent hypoxemia. Therefore, as the intermittent hypoxemia is an important pathophysiological feature of sleep apnea increasing the risk for severe health consequences, patients with diabetes or CVD should get more attention related to the screening and follow-up monitoring of sleep apnea.
Supplementary Material
Acknowledgements
The Sleep Heart Health Study (SHHS) was supported by National Heart, Lung, and Blood Institute cooperative agreements U01HL53916 (University of California, Davis), U01HL53931 (New York University), U01HL53934 (University of Minnesota), U01HL53937 and U01HL64360 (Johns Hopkins University), U01HL53938 (University of Arizona), U01HL53940 (University of Washington), U01HL53941 (Boston University), and U01HL63463 (Case Western Reserve University). The National Sleep Research Resource was supported by the National Heart, Lung, and Blood Institute (R24 HL114473, RFP 75N92019R002).
Funding:
This work was funded by the Academy of Finland (decision numbers 313697 and 323536), the Research Committee of the Kuopio University Hospital Catchment Area for the State Research Funding (projects 5041767, 5041768, 5041770, 5041781, 5041787, 5041794, and 5041797), Seinäjoki Central Hospital, the Competitive State Research Financing of Expert Responsibility Area of Tampere University Hospital (grant numbers: VTR3242, VTR3228, and EVO2089), Business Finland (decision number 5133/31/2018), The Research Foundation of the Pulmonary Diseases, Tampere Tuberculosis Foundation, Orion Research Foundation, Instrumentarium Science Foundation, Finnish Anti-Tuberculosis Association, Päivikki and Sakari Sohlberg Foundation, and The Finnish Cultural Foundation - North Regional Fund. This manuscript was also supported by the American Academy of Sleep Medicine Foundation (194-SR-18) and the NHLBI (P01HL094307).
Footnotes
Financial/nonfinancial disclosures: None declared.
Conflict of interest statements: None declared.
Additional information: Table S1 and Figure S1 can be found in the online Supplemental Material.
References:
- Al Lawati NM, Patel SR, & Ayas NT (2009). Epidemiology, Risk Factors, and Consequences of Obstructive Sleep Apnea and Short Sleep Duration. Progress in Cardiovascular Diseases, 51(4), 285–293. 10.1016/j.pcad.2008.08.001 [DOI] [PubMed] [Google Scholar]
- Anothaisintawee T, Reutrakul S, Van Cauter E, & Thakkinstian A (2016). Sleep disturbances compared to traditional risk factors for diabetes development: Systematic review and meta-analysis. Sleep Medicine Reviews, 30, 11–24. 10.1016/j.smrv.2015.10.002 [DOI] [PubMed] [Google Scholar]
- Aurora RN, & Punjabi NM (2013). Obstructive Sleep Apnoea and Type 2 Diabetes Mellitus: A Bidirectional Association. The Lancet Respiratory Medicine, 1(4), 329–338. 10.1016/S2213-2600(13)70039-0 [DOI] [PubMed] [Google Scholar]
- Azarbarzin A, Sands SA, Stone KL, Taranto-montemurro L, Messineo L, Terrill PI, Ancoli-israel S, Ensrud K, Purcell S, White DP, Redline S, & Wellman A (2019). The Hypoxic Burden of Sleep Apnoea Predicts Cardiovascular Disease-Related Mortality: The Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. European Heart Journal, 40(14), 1149–1157. 10.1093/eurheartj/ehy624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarbarzin A, Sands SA, Taranto-Montemurro L, Vena D, Sofer T, Kim SW, Stone KL, White DP, Wellman A, & Redline S (2020). The Sleep Apnea-Specific Hypoxic Burden Predicts Incident Heart Failure. Chest, 158(2), 739–750. 10.1016/j.chest.2020.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar JA, Palmius N, Li Q, Garbuio S, Rizzatti FPG, Bittencourt L, Tufik S, & Clifford GD (2019). Feasibility of Single Channel Oximetry for Mass Screening of Obstructive Sleep Apnea. EClinicalMedicine, 11, 81–88. 10.1016/j.eclinm.2019.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, Nunez CM, Patel SR, Penzel T, Pepin J-L, Peppard PE, Sinha S, Tufik S, Valentine K, & Malhotra A (2019). Estimation of the global prevalence and burden of obstructive sleep apnoea : a literature-based analysis. The Lancet Respiratory Medicine, 7(8), 687–698. 10.1016/S2213-2600(19)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger GB, Berger RL, & Oksenberg AS (2009). Progression of Snoring and Obstructive Sleep Apnoea: The Role of Increasing Weight and Time. European Respiratory Journal, 33(2), 338–345. 10.1183/09031936.00075408 [DOI] [PubMed] [Google Scholar]
- Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Quan SF, Troester MM, & Vaughn BV (2017). The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technocal Specifications - Version 2.4. American Academy of Sleep Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano-Pumarega I, Durán-Cantolla J, Aizpuru F, Miranda-Serrano E, Rubio R, Martínez-Null C, De Miguel J, Egea C, Cancelo L, Álvarez A, Fernández-Bolaños M, & Barbé F (2011). Obstructive sleep apnea and systemic hypertension - Longitudinal study in the general population: The vitoria sleep cohort. American Journal of Respiratory and Critical Care Medicine, 184(11), 1299–1304. 10.1164/rccm.201101-0130OC [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Veasey SC, Morgan BJ, & O’Donnell CP (2010). Pathophysiology of sleep apnea. Physiological Reviews, 90(1), 47–112. 10.1152/physrev.00043.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewan NA, Nieto FJ, & Somers VK (2015). Intermittent hypoxemia and OSA: Implications for comorbidities. Chest, 147(1), 266–274. 10.1378/chest.14-0500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floras JS (2018). Sleep Apnea and Cardiovascular Disease an Enigmatic Risk Factor. Circulation Research, 122(12), 1741–1764. 10.1161/CIRCRESAHA.118.310783 [DOI] [PubMed] [Google Scholar]
- Huang T, Lin BM, Stampfer MJ, Tworoger SS, Hu FB, & Redline S (2018). A population-based study of the bidirectional association between obstructive sleep apnea and type 2 diabetes in three prospective U.S. Cohorts. Diabetes Care, 41(10), 2111–2119. 10.2337/dc18-0675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennum P, & Kjellberg J (2011). Health, social and economical consequences of sleep-disordered breathing: A controlled national study. Thorax, 66(7), 560–566. 10.1136/thx.2010.143958 [DOI] [PubMed] [Google Scholar]
- Jose M, Santiago J, & Alvar GN (2005). Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet, 365(9464), 1046–1053. 10.1016/S0140-6736(05)74229-X [DOI] [PubMed] [Google Scholar]
- Kainulainen S, Duce B, Korkalainen H, Oksenberg A, Leino A, Arnardottir ES, Kulkas A, Myllymaa S, Töyräs J, & Leppänen T (2020). Severe Desaturations Increase Psychomotor vigilance Task-based Median Reaction Time and Number of Lapses in Obstructive Sleep Apnoea Patients. European Respiratory Journal, 55(4), art. no. 1901849. 10.1183/13993003.01849-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kainulainen S, Töyräs J, Oksenberg A, Korkalainen H, Sefa S, Kulkas A, & Leppänen T (2019). Severity of Desaturations Reflects OSA-Related Daytime Sleepiness Better Than AHI. Journal of Clinical Sleep Medicine, 15(8), 1135–1142. 10.5664/jcsm.7806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, & Harrod C (2017). Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. Journal of Clinical Sleep Medicine, 13(3), 479–504. 10.5664/jcsm.6506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karhu T, Myllymaa S, Nikkonen S, Mazzotti DR, Töyräs J, & Leppänen T (2021). Longer and Deeper Desaturations Are Associated With the Worsening of Mild Sleep Apnea: The Sleep Heart Health Study. Frontiers in Neuroscience, 15, art.no. 657126. 10.3389/fnins.2021.657126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai T, Floras JS, & Bradley TD (2012). Sleep apnea and cardiovascular disease: A bidirectional relationship. Circulation, 126(12), 1495–1510. 10.1161/CIRCULATIONAHA.111.070813 [DOI] [PubMed] [Google Scholar]
- Konecny T, Kara T, & Somers VK (2014). Obstructive sleep apnea and hypertension: An update. Hypertension, 63(2), 203–209. 10.1161/HYPERTENSIONAHA.113.00613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkas A, Tiihonen P, Eskola K, Julkunen P, Mervaala E, & Töyräs J (2013). Novel Parameters for Evaluating Severity of Sleep Disordered Breathing and for Supporting Diagnosis of Sleep Apnea-Hypopnea Syndrome. Journal of Medical Engineering and Technology, 37(2), 135–143. 10.3109/03091902.2012.754509 [DOI] [PubMed] [Google Scholar]
- Liu C-L, & Wu C (2017). Assessing Whether the Association Between Sleep Apnea and Diabetes is Bidirectional. Canadian Journal of Diabetes, 41(2), 197–203. 10.1016/j.jcjd.2016.09.009 [DOI] [PubMed] [Google Scholar]
- Marin JM, Agusti A, Villar I, Forner M, Nieto D, Carrizo SJ, Barbe F, Vicente E, Wei Y, Nieto J, & Jelic S (2012). Association Between Treated and Untreated Obstructivd Sleep Apnea and Risk of Hypertension. Journal of the American Medical Association, 307(20), 2169–2176. 10.1001/jama.2012.3418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllymaa S, Myllymaa K, Kupari S, Kulkas A, Leppänen T, Tiihonen P, Mervaala E, Seppä J, Tuomilehto H, & Töyräs J (2015). Effect of different oxygen desaturation threshold levels on hypopnea scoring and classification of severity of sleep apnea. Sleep and Breathing, 19(3), 947–954. 10.1007/s11325-015-1118-x [DOI] [PubMed] [Google Scholar]
- Nieto FJ, Young TB, Lind BK, Redline S, Agostino RBD, Newman AB, Lebowitz MD, & Pickering TG (2000). Association of Sleep-Disordered Brathing, Sleep Apnea, and Hypertension in a Large Community-Based Study for the Sleep Heart Health Study. JAMA, 284(14), 1829–1837. 10.1046/j.0022-202X.2001.01432.x [DOI] [PubMed] [Google Scholar]
- O’Connor GT, Caffo B, Newman AB, Quan SF, Rapoport DM, Redline S, Resnick HE, Samet J, & Shahar E (2009). Prospective study of sleep-disordered breathing and hypertension: The sleep heart health study. American Journal of Respiratory and Critical Care Medicine, 179(12), 1159–1164. 10.1164/rccm.200712-1809OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peker Y, Hedner J, Norum J, Kraiczi H, & Carlson J (2002). Increased Incidence of Cardiovascular Disease in Middle-Aged Men with Obstructive Sleep Apnea: A 7-year Follow-up. American Journal of Respiratory and Critical Care Medicine, 166(2), 159–165. 10.1164/rccm.2105124 [DOI] [PubMed] [Google Scholar]
- Peppard PE, Young T, Palta M, & Skatrud J (2000). Prospective Study of the Association Between Sleep-Disordered Breathing and Hypertension. The New England Journal of Medicine, 342(19), 1378–1384. [DOI] [PubMed] [Google Scholar]
- Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O’Connor GT, Rapoport D, Redline S, Robbins J, Samet JM, & Wahl PW (1997). The Sleep Heart Health Study: Design, Rationale, and Methods. Sleep, 20(12), 1077–1085. 10.1093/sleep/20.12.1077 [DOI] [PubMed] [Google Scholar]
- Rajan P, & Greenberg H (2015). Obstructive Sleep Apnea as a Risk Factor for Type 2 Diabetes Mellitus. Nature and Science of Sleep, 7, 113–125. 10.2147/NSS.S90835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick HE, Redline S, Shahar E, Gilpin A, Newman A, Walter R, Ewy GA, Howard BV, & Punjabi NM (2003). Diabetes and Sleep Disturbances: Findings from the Sleep Heart Health Study. Diabetes Care, 26(3), 702–709. 10.2337/diacare.26.3.702 [DOI] [PubMed] [Google Scholar]
- Roeder M, Bradicich M, Schwarz EI, Thiel S, Gaisl T, Held U, & Kohler M (2020). Night-to-night variability of respiratory events in obstructive sleep apnoea: A systematic review and meta-analysis. Thorax, 75(12), 1095–1102. 10.1136/thoraxjnl-2020-214544 [DOI] [PubMed] [Google Scholar]
- Stone KL, Blackwell TL, Ancoli-Israel S, Barrett-Connor E, Bauer DC, Cauley JA, Ensrud KE, Hoffman AR, Mehra R, Stefanick ML, Varosy PD, Yaffe K, & Redline S (2016). Sleep Disordered Breathing and Risk of Stroke in Older Community-Dwelling Men. Sleep, 39(3), 531–540. 10.5665/sleep.5520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The National Sleep Research Resource. (2021). https://sleepdata.org/datasets/shhs/pages/05-polysomnography-introduction.md
- Tishler PV, Larkin EK, Schluchter MD, & Redline S (2003). Incidence of Sleep-Disordered Breathing in an Urban Adult Population: The Relative Importance of Risk Factors in the Development of Sleep-Disordered Breathing. Journal of the American Medical Association, 289(17), 2230–2237. 10.1001/jama.289.17.2230 [DOI] [PubMed] [Google Scholar]
- Tkacova R, McNicholas WT, Javorsky M, Fietze I, Sliwinski P, Parati G, Grote L, & Hedner J (2014). Nocturnal Intermittent Hypoxia Predicts Prevalent Hypertension in the European Sleep Apnoea Database Cohort Study. European Respiratory Journal, 44(4), 931–941. 10.1183/09031936.00225113 [DOI] [PubMed] [Google Scholar]
- Tsai WH, Flemons WW, Whitelaw WA, & Remmers JE (1999). A Comparison of Apnea-Hypopnea Indices Derived from Different Definitions of Hypopnea. American Journal of Respiratory and Critical Care Medicine, 159(1), 43–48. 10.1164/ajrccm.159.1.9709017 [DOI] [PubMed] [Google Scholar]
- Young T, Skatrud J, & Peppard PE (2004). Risk Factors for Obstructive Sleep Apnea in Adults. Journal of the American Medical Association, 291(16), 2013–2016. 10.1001/jama.291.16.2013 [DOI] [PubMed] [Google Scholar]
- Zhang G, Cui L, Mueller R, Tao S, Kim M, Rueschman M, Mariani S, Mobley D, & Redline S (2018). The National Sleep Research Resource: Towards a Sleep Data Commons. Journal of the American Medical Informatics Association, 25(10), 1351–1358. 10.1093/jamia/ocy064 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


