Abstract
Background:
Inconsistent sleep patterns may promote excess weight gain by increasing food cravings and loss-of-control (LOC)-eating; however, these relationships have not been elucidated in youth.
Objective:
We tested whether sleep duration and timing were associated with food cravings and LOC-eating.
Method:
For 14 days, youths wore actigraphy monitors to assess sleep and reported severity of food cravings and LOC-eating using ecological momentary assessment. Generalized linear mixed models tested the associations between weekly and nightly shifts in facets of sleep (i.e., duration, onset, midpoint, and waketime) and next day food cravings and LOC-eating. Models were re-run adjusting for relevant covariates (e.g., age, sex, adiposity).
Results:
Among 48 youths (12.88±2.69 years, 68.8% female, 33.3% with overweight/obesity), neither weekly nor nightly facets of sleep were significantly associated with food cravings (ps=.08–.93). Youths with shorter weekly sleep duration (est. ß=−0.31, p=.004), earlier weekly midpoints (est. ß=−0.47, p=.01) and later weekly waketimes (est. ß=0.49, p=.01) reported greater LOC-eating severity; findings persisted in adjusted models.
Conclusions:
In youth, weekly, but not nightly, shifts in multiple facets of sleep were associated with LOC-eating severity; associations were not significant for food cravings. Sleep should be assessed as a potentially modifiable target in pediatric LOC-eating and obesity prevention programs.
Keywords: food cravings, loss-of-control eating, youth, ecological momentary assessment, sleep, actigraphy
Introduction
Loss-of-control (LOC)-eating, and the factors that promote LOC-eating, may be modifiable targets for the prevention of obesity. LOC-eating, a subjective sense of feeling out of control while eating, is prospectively predictive of excessive gain in BMI, the development of overweight and obesity, and worsening of components of metabolic syndrome in youth.1–3 Food cravings [i.e., intense desires to eat specific food(s) or type of food]4 are positively linked to the severity of LOC-eating5 as well as greater energy intake, weight gain, and obesity in adult and pediatric samples.6 One preliminary study suggests food cravings are modifiable among youth;7 therefore, the identification of factors that promote food cravings among youth across a wide weight range could inform the enhancement of obesity prevention programs.
One modifiable factor that may promote or exacerbate food cravings and subsequent LOC-eating is sleep. Sleep patterns are comprised of several facets that have been linked to obesity risk in youth. Specifically, fewer hours of nightly sleep (sleep duration),8,9 greater within-person sleep duration variability (the within-subject standard deviation of average sleep duration across a multi-day period),10,11 and later sleep onset and wake timing (falling asleep and waking up at a time that is later than one’s average fall asleep or wake time)12–14 have been associated with poorer dietary habits, aberrant eating behaviors, and increased obesity risk in youth.15 While the mechanisms linking sleep to pediatric obesity are largely unknown, acute changes in sleep duration and timing may indirectly contribute to obesity through a positive association with LOC-eating and with the desire to eat. However, data exploring the link between these facets of sleep and food cravings and LOC-eating are sparse.
Findings from the few studies examining the association between sleep and food cravings, or food craving-related constructs (e.g., desire for food and food appeal) in youth are inconsistent. For example, Kracht, et al. 16 cross-sectionally analyzed multiple facets of sleep and frequency of food cravings and found that sleep efficiency (i.e., the proportion of time one is asleep while lying in bed), but not sleep duration or variability, was related to cravings for foods high in fat and carbohydrates. Yet, preliminary experimental studies among adolescents have linked earlier bedtimes to lower desires for sweet foods17 and shorter sleep duration to increased desires for sweets.18 Although there is no consensus regarding which facets of sleep are associated with food cravings, preliminary research suggests a link between sleep duration/timing and food cravings.
The only two studies among children and adolescents examining the link between sleep duration and LOC-eating did not find a significant association. Kelly, et al. 19 studied adolescent girls with overweight/obesity at high-risk for type 2 diabetes and found that self-reported sleep duration was not linked to LOC-eating. Using ecological momentary assessment (EMA), Goldschmidt, et al. 20 failed to detect between-subject associations among characteristics of prior night’s sleep (e.g., sleep duration, sleep efficiency, wake after sleep onset, number of nighttime awakenings) and odds of reporting LOC-eating the following day among 40 youth with overweight and obesity. However, given the paucity of studies testing the associations of sleep with LOC-eating among youth across the weight strata, examination of the link between sleep and LOC-eating in more heterogeneous samples of youth is needed. Such data would elucidate whether insufficient and inconsistent sleep are relevant for the development of pediatric obesity.
Inconsistent and statistically nonsignificant findings across previous studies testing the associations among sleep, food cravings and LOC-eating may be due, in part, to multiple methodological and statistical limitations. First, studies that rely solely on retrospective self-reports of sleep duration and timing17,19 are limited due to potential bias and inaccuracy in measurement of sleep.15 Second, there are distinct yet interrelated facets of sleep linked to pediatric obesity (e.g., timing of sleep onset, midpoint, and waketime) that may impact food cravings and LOC-eating that have not been consistently examined in previous studies. This is potentially problematic because going to bed later than usual but arising at one’s typical waketime constitutes a shift or deviation from one’s average sleep onset and duration. Yet, previous studies16–20 have examined the effects of independent facets of sleep on food cravings/LOC-eating without accounting for how individual facets of sleep duration and timing relate to one-another ‒ thereby insufficiently considering this interdependence conceptually and statistically.
Lastly, measures of sleep duration and timing are often averaged over a one- or two-week period, but it is possible that daily shifts in sleep acutely impact eating drives and behaviors. Indeed, an experimental study using a randomized and balanced within-subject design found that, among young adult men, just one night of total sleep deprivation was associated with greater portion size intake the next day compared to one night of adequate sleep.21 However, to date, the effects of acute shifts in sleep on next day eating drives and behaviors have not been examined in youth using a within-subject design. Thus, methodologically and conceptually rigorous studies that address these limitations are necessary to clarify previous discrepant findings.
Given the possibility that variations in sleep patterns may promote greater food cravings, which may in turn increase LOC-eating severity, further exploration of these relationships is warranted. We therefore utilized concurrent actigraphy (an objective measure of sleep) and EMA (repeated measurement of current behaviors and experience in real time) to examine associations between weekly and nightly facets of sleep duration and timing and intensity of food cravings and LOC-eating in the natural environment during a 14-day period. We hypothesized that after adjusting for all facets of sleep 1) youth with, on average, fewer hours of weekly sleep and later sleep onset, midpoint, and waketimes would report greater food cravings and LOC-eating severity, and 2) greater shifts in previous night’s sleep duration, onset, midpoint, and waketime would be associated with greater next day food cravings and LOC-eating severity.
Method
Participants
A secondary analysis of data collected as part of the Children’s Growth and Behavior Study (Clinical Trials Identifier: NCT02390765) was conducted. Findings from this study related to sleep and eating in the absence of hunger, as assessed by a self-report questionnaire, and energy intake, as assessed by a buffet test meal, have been previously reported.22,23 However the impact of sleep on LOC-eating severity reported in one’s natural environment, has not yet been examined. Youth in good general health were recruited to participate in a study designed to understand the relationships between eating behaviors and body weight in children. Youth were eligible if they were 8–17 years old, cognitively capable of completing study procedures, and had a BMI ≥ 5th percentile for age and sex according to the Centers for Disease Control and Prevention 2000 US standards24. Exclusion criteria included: history of brain injuries, major medical or psychiatric illness, current or past pregnancy, current and regular use of illicit drugs, greater than 5% body weight reduction during the three months prior to recruitment, or regular use of medications known to impact eating behavior, weight, autonomic functioning, or endocrine functioning. In-person study visits were completed at the outpatient Pediatric Clinic at the National Institutes of Health Hatfield Clinical Research Center. The study procedures were approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board. Signed informed consent and assent were obtained from parents/guardians and children, respectively. All data were collected prior to the onset of the COVID-19 pandemic.
Measures
Ecological Momentary Assessment
Participants were given the option to complete a two-week EMA protocol while concurrently wearing an actigraphy watch, which was an adjunctive study to the primary protocol. All participants who opted to complete the EMA protocol were provided with a smartphone, regardless of whether they had their own, and trained on how to complete the EMA surveys. The EMA surveys assessed multiple cognitions and behaviors, including food cravings and LOC-eating, and were collected using the Real Time Assessment in the Natural Environment system.25 Consistent with prior studies in youth,5,26 the EMA protocol contained three types of surveys: signal-, interval-, and event-contingent. Randomly, around stratified daily intervals (i.e., at approximately 11:10am, 1:50pm, 3:30pm, 5:40pm, and 8:20pm), participants received a notification instructing them to complete a survey (signal-contingent). On weekends, participants received five signal-prompted surveys between the hours of 11am and 9pm. On weekdays, participants only received three signal-prompted surveys between the hours of 3pm and 9pm, to minimize interference with school. Participants were also asked to complete a survey before bed and, on weekdays, immediately after the end of school (interval-contingent), and following every time they ate or drank a meal or snack (event-contingent). The day after their in-person visit, participants practiced completing the EMA surveys. If compliance on this day was poor (completed ≤ 80% of signal- and interval-contingent surveys) youth were contacted by study personnel to solve barriers to compliance and completed another practice day. When compliance on the practice day was satisfactory, participants began the 14-day protocol. Participants received additional compensation for completion of the EMA protocol.
Food Cravings.
Food cravings were assessed in all interval and signal contingent surveys, regardless of whether an eating episode occurred. We utilized items from the Intense Desire to Eat subscale of the Food Cravings Questionnaire-state version,27 because we were interested in the intensity of one’s craving, rather than hunger or one’s expected outcomes from eating food, which are assessed by all other subscales of the measure. A Likert type scale from 1= ‘not at all’ to 5= ‘extremely’ was used to measure the following items: “How strong is your desire to eat one or more specific foods?”; “How much do you crave one or more specific foods?”; “How strongly do you want to eat one or more specific foods?”; and “How much does your desire or craving to eat have power over you?” All items were averaged to obtain a composite score of food cravings. Internal consistency was excellent (Cronbach’s alpha = .96).
LOC-Eating Severity.
When an eating episode was reported, participants rated the degree to which they experienced LOC-eating during that meal/snack, using items adapted from the Eating Disorder Examination.28 Questions in the EMA surveys were similar to previous studies5,26 and included the following items: “How much did you lose control during this eating episode?”; “Did you feel that you could not keep yourself from eating?”; “Did you feel that you could not stop eating once you started?”; “During the eating episode you just finished, how much did you feel a sense of loss of control?”; “How upset or distressed are you about how much you just ate?”; and “How much did you feel driven to eat?”. Participants indicated their responses on a Likert scale ranging from 1= ‘not at all’ to 5= ‘extremely’. All items were averaged to obtain a composite score of LOC-eating severity at each eating episode. Internal consistency was good (Cronbach’s alpha = .87).
Sleep
Wrist-worn actigraphy was used to assess sleep behavior in youth for the 14 days following their in-person visit. Participants were given an ActiGraph GT3X+ activity monitor (ActiGraph, Pensacola, FL), which records 24-hour physical activity and sleep/wake measurements. In young adults, the GT3X+ is a valid and reliable device for detecting sleep/wake diurnal patterns and has good concordance with polysomnography.29 The GT3X+ has been used in previous studies of youth ages 8–18 years.30,31 To be included in analyses, participants were required to have valid data from ≥ 3 weekday nights of sleep and ≥ 1 weekend night of sleep. Sleep data were downloaded from devices using Actilife software version 6.13.3 (Actigraph, Pensacola, FL). The Sadeh sleep detection algorithm, which has been previously validated for children and adolescents,32,33 was applied to data to detect periods of sleep. Data were also visually inspected, and self-reported sleep logs were used to confirm periods when the monitor was removed for contact sports, swimming, or bathing.
Facets of sleep examined included sleep duration, onset, midpoint, and waketime. Sleep duration was defined as the amount of time between sleep onset and wake time. Sleep onset time was defined as the first 5-minute period in which activity counts were all zero and the subsequent activity counts were determined to be sleep by the Sadeh algorithm. Sleep midpoint was defined as halfway between sleep onset and wake time. Waketime was similarly defined as the first 5-minute period in which activity counts were above zero and the subsequent activity was determined by the Sadeh algorithm to be awake time.
Race and Ethnicity
Race and ethnicity were reported by the child’s parents.
Snoring
Presence or absence of participant’s snoring/known sleep apnea was reported by parents during the physical examination.
Socioeconomic status
Socioeconomic status was determined by parents’ reported educational and occupational status via the Hollingshead Two Factor Index of Socioeconomic Status. Possible scores range from 8–66 with higher scores indicating higher family socioeconomic status.34
Anthropometrics
Fasting weight was measured to the nearest 0.1 kg using a calibrated scale. Height (to the nearest 0.1 cm) was measured in triplicate by stadiometer. Body mass index (BMI, kg/m2) was calculated and standard deviation BMI scores (BMIz) were calculated following Center of Disease Control and Prevention growth standards for age and sex24 and used to determine weight status. Given that BMI is known to inconsistently capture differences in adiposity,35 fat mass (kg) was also assessed directly by dual-energy x-ray absorptiometry (GE Healthcare, Madison WI).
Depressive symptoms
Depressive symptoms over the previous two weeks were assessed by the Children’s Depressive Inventory- Second Edition36 a self-report measure that demonstrates excellent reliability and validity among children and adolescents.37
Statistical Analyses
Analyses were conducted in SPSS version 25.38 EMA practice days were not included in analyses. EMA reports and measures of sleep were matched on the date the individual woke up. Such that if an individual went to bed on Sunday and woke up on Monday, characteristics of that night’s sleep were matched with EMA surveys completed on Monday. For descriptive analyses, average food craving and LOC-eating scores were computed by aggregating ratings within persons. To account for variation in the number of EMA surveys reported each day, daily averages in sleep duration/timing were computed. These daily values were then aggregated within-person to obtain a single rating of average sleep duration/timing across the two-weeks.
All weekly sleep variables represent the direction and degree to which a person’s average sleep duration/timing deviated from the sample’s average sleep duration/timing, and nightly sleep variables represent the direction and degree to which participants’ last night’s sleep duration/timing deviated from their average night’s sleep duration/timing. To compute these variables, weekly sleep duration, midpoint and waketime variables were mean-centered by subtracting the entire sample’s average sleep duration/timing (grand means) from each individual’s own average sleep duration/timing (individual means). Nightly shifts in sleep were mean centered by subtracting each participant’s average sleep duration/timing (individual means) from their previous night’s sleep duration/timing (a specific night’s sleep duration/timing).39 Sleep onset time was the only sleep variable to demonstrate a significant linear increase across the study, such that participants fell asleep later over the course of the study. Therefore, sleep onset was detrended by regressing each participant’s sleep onset on day in the study (e.g., day 3 of participation).40 Then, weekly sleep onset was centered on the individual’s intercept (sleep onset on the first day in the study) and daily sleep onset was centered on the individual’s regression slope (predicted sleep onset).
Average sleep midpoint was significantly correlated with average bed and wake times (r = .85 and .87, respectively). However, given the theoretical rational for examining the effect of facets of sleep when adjusting for all other facets, all sleep variables were included a independent variables in the final models. Two generalized linear mixed models (GLMMs) tested the contribution of each facet of sleep while adjusting for all other sleep variables. Outcome variables for the two models were food cravings and LOC-eating severity. In both models, participant ID number was entered as a random effect, as sleep and eating measurements were nested within persons. A standard variance components covariance structure was used for random effects. Distributions of food cravings and LOC-eating severity were positively skewed; therefore, models assumed a Gamma distribution and logit function.
Models were re-run adjusting for covariates that were selected a priori and included weekend vs. weekday sleep (1 = weekday, 0 = weekend day), fat mass (kg), height (cm), snoring41 (1 = presence, 0= absence), sex (1 = Female, 0= Male), socioeconomic status,42 age, and race/ethnicity (1 = non-Hispanic white, 0= other) and depressive symptoms.43,44 All facets of sleep were included in analyses as continuous predictors, but to facilitate visualization of the results, facets of sleep were dichotomized as one standard deviation above and below the sample’s mean.
Results
Sample Characteristics
The opportunity to participate in the EMA protocol was first offered to youth in September 2016, approximately 18 months after protocol initiation. Of the 252 participants in the parent study to date, 169 participants provided usable actigraphy data. Of the 120 youth who elected to complete the optional EMA protocol, 68 concurrently wore an actigraphy watch. Twenty participants were excluded from analyses because they reported one or fewer eating episodes during the EMA protocol, had less than 30% compliance with EMA surveys, or had sleep data from fewer than 3 weekdays and 2 weekends. Participants included in analyses had a higher percentage of females (χ2 = 4.68, p = .03), but did not differ from the parent study sample on percentage of non-Hispanic White individuals (χ2= 0.30, p = .58), age (t = −0.84, p = .41), or BMIz (t = −0.19, p = .85). Data from 48 boys and girls (68.8% female; 12.8 ± 2.7y; 14.6% Non-Hispanic Black or African American; 33.3% with overweight/obesity) were analyzed. See Table 1 for final sample characteristics.
Table 1.
Participant Characteristics and Descriptive Statistics
| Participant Characteristics | n | % | ||
|---|---|---|---|---|
|
| ||||
| Sex | ||||
| Girls | 33 | 68.8 | ||
| Boys | 15 | 31.2 | ||
| Race | ||||
| White | 24 | 50.0 | ||
| Black | 7 | 14.6 | ||
| Asian | 11 | 22.9 | ||
| Multiracial | 4 | 8.3 | ||
| Unknown | 2 | 4.2 | ||
| Ethnicity | ||||
| Non-Hispanic | 41 | 85.4 | ||
| Hispanic | 6 | 12.5 | ||
| Unknown | 1 | 2.1 | ||
| Presence of Snoring | 13 | 27.1 | ||
| Overweight or Obesity | 16 | 33.3 | ||
| M | SD | Minimum | Maximum | |
| Age (years) | 12.88 | 2.69 | 8.00 | 17.00 |
| Socioeconomic Statusa | 2.23 | 1.06 | 1.00 | 5.00 |
| Depressive Symptoms | 7.29 | 5.66 | 0.00 | 24.00 |
| EMA Ratings | ||||
| Food Cravings | 1.51 | 0.72 | 1.00 | 4.86 |
| LOC-eating Severity | 1.25 | 4.17 | 1.00 | 3.49 |
| Shifts in Facets of Sleep (hours) | ||||
| Sleep Duration Daily | −0.03 | 0.24 | −1.09 | 0.65 |
| Sleep Duration Weekly | 0.09 | 0.90 | −2.01 | 1.83 |
| Sleep Onset Daily | −0.01 | 0.15 | −0.28 | 0.43 |
| Sleep Onset Weekly | 0.23 | 0.81 | −2.86 | 3.61 |
| Midpoint Daily | −0.02 | 0.14 | −0.33 | 0.32 |
| Midpoint Weekly | 0.07 | 1.08 | −1.54 | 3.10 |
| Waketime Daily | 1.01 | 0.71 | 0.18 | 4.25 |
| Waketime Weekly | 0.12 | 1.02 | −1.32 | 3.00 |
BMIz, standardized body mass index; LOC-eating, loss-of-control eating; M, mean; SD, standard deviation.
Three participants did not have reports of socioeconomic status. Average facets of sleep were obtained from the 548 nights of sleep analyzed in the craving analyses. Daily averages and ranges in facets of sleep were obtained by aggregating daily shifts in sleep within persons. Weekly averages and ranges in facets of sleep were obtained by aggregating weekly shifts within days and persons. Negative sleep duration values represent shorter sleep duration (e.g., 6 hours vs. 8 hours). Negative sleep onset, midpoint, and waketime represent later timing (e.g., 3am vs. 12am).
On average, EMA compliance was (M = 73.97%, SD = 17.78). Craving analyses comprised 548 nights of sleep and 2,380 craving ratings. LOC-eating analyses included 518 nights of sleep and 1,073 eating episodes. Generally, food cravings and LOC-eating severity were low, but varied (food cravings: M = 1.51, SD = 0.72; LOC-eating: M = 1.25, SD = 4.17) and weekly/daily shifts in sleep were more variable (range 0.18 – 4.25 hours). Average food cravings, LOC-eating severity, and weekly and nightly shifts in all facets of sleep are reported in Table 1.
Associations of Sleep with Food Cravings
Weekly and nightly measures of sleep were not significantly related to food cravings when accounting for all facets of sleep, (est. ßs = −0.13 – 0.15, ps = .12 – .69) or when adjusting for covariates (est. ßs = −0.15 – 0.10, ps = .08 – .93).
Associations of Sleep with LOC-Eating Severity
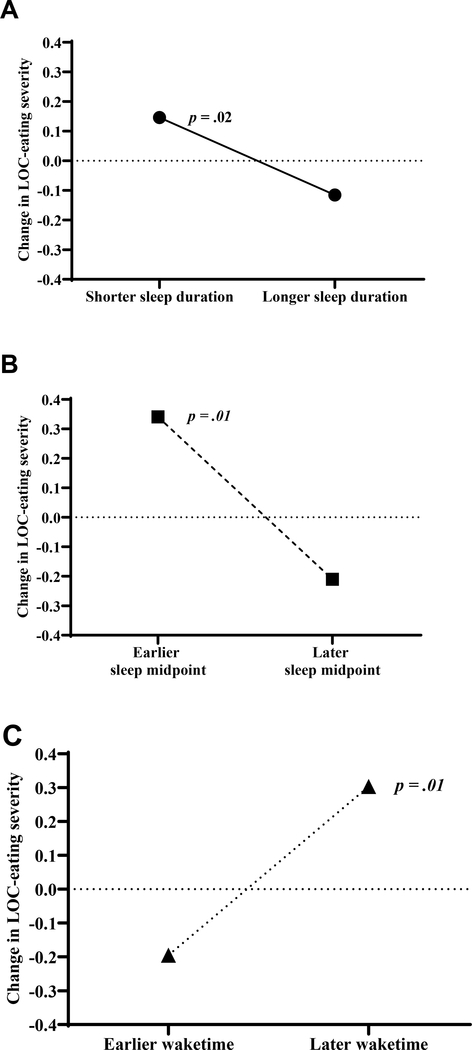
When accounting for all facets of sleep, weekly sleep duration (est. ß = −0.31, p = .004), sleep midpoint (est. ß = −0.47, p = .01), and wake time (est. ß = 0.49, p = .01) were associated with greater LOC-eating severity (Figure 1). Specifically, youth who had shorter average sleep duration, an earlier average sleep midpoint, and, on average, woke up later reported greater LOC-eating severity during the EMA period (Figure 1). Shifts in daily facets of sleep were not significantly related to subsequent LOC-eating severity (est. ßs = −0.01 – 0.02, ps = .11 – .92). Results remained significant after adjusting for all covariates. Results from unadjusted and fully adjusted models are displayed in Table 2.
Figure 1. Facets of Weekly Sleep and LOC-Eating Severity.
A: Associations among LOC-eating severity and weekly sleep duration. B: Associations among LOC-eating severity and weekly sleep midpoint. C: Associations among LOC-eating severity and weekly waketime.
In all graphs, LOC-eating, loss-of-control eating. To facilitate visualization, facets of sleep were dichotomized as 1 SD below/above the average for each facet of sleep. Estimated Betas were used to predict LOC-eating severity at 1 SD below the mean, 1 SD above the mean, and at the mean for each facet of sleep. Predicted values were adjusted for weekend/weekday sleep, fat mass, height, snoring, socioeconomic status, age, race/ethnicity, and depressive symptoms. The Y-axis is centered on predicted LOC-eating severity at the average shift in each facet of sleep. Therefore, the difference between LOC-eating severity predicted for the average sleep duration, sleep midpoint, and waketime facets of sleep and 1 SD below/above means of each facet of sleep are presented.
Table 2.
Unadjusted and adjusted GLMMs testing the associations among sleep, food cravings, and LOC-eating
| Food Cravings | LOC-eating Severity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Variable | est. ß | SE | t | p | 95% CI | est. ß | SE | t | p | 95% CI |
|
| ||||||||||
| Unadjusted models | ||||||||||
| Intercept | 0.34 | 0.34 | 1.01 | .32 | −0.32 – 1.00 | 0.19 | 0.21 | 0.90 | .37 | −0.22 – 0.59 |
| Sleep Duration Weekly | 0.05 | 0.10 | 0.52 | .61 | −0.15 – 0.25 | −0.31 | 0.11 | −2.86 | .004 | −0.52 – −0.10 |
| Sleep Duration Daily | 0.01 | 0.01 | 0.77 | .44 | −0.01 – 0.02 | −0.003 | 0.01 | −0.42 | .67 | −0.02 – 0.01 |
| Sleep Onset Weekly | −0.12 | 0.08 | −1.54 | .12 | −0.27 – 0.03 | 0.03 | 0.04 | 0.60 | .55 | −0.06 – 0.11 |
| Sleep Onset Daily | 0.01 | 0.01 | 1.25 | .21 | −0.01 – 0.03 | −0.01 | 0.01 | −1.09 | .27 | −0.03 – 0.01 |
| Midpoint Weekly | 0.15 | 0.17 | 0.85 | .40 | −0.19 – 0.48 | −0.47 | 0.18 | −2.68 | .01 | −0.82 – −0.13 |
| Midpoint Daily | 0.00 | 0.01 | −0.41 | .69 | −0.02 – 0.01 | 0.02 | 0.01 | 1.59 | .11 | −0.004 – 0.04 |
| Waketime Weekly | −0.13 | 0.18 | −0.71 | .48 | −0.49 – 0.23 | 0.49 | 0.18 | 2.75 | .01 | 0.14 – 0.84 |
| Waketime Daily | 0.01 | 0.01 | 1.04 | .30 | −0.01 – 0.04 | 0.001 | 0.01 | 0.10 | .92 | −0.02 – 0.02 |
| Fully adjusted models | ||||||||||
| Intercept | 0.34 | 0.90 | 0.38 | .71 | −1.43 – 2.11 | 0.38 | 0.49 | 0.77 | .44 | −0.58 – 1.33 |
| Weekend/weekday Sleep | 0.06 | 0.02 | 2.48 | .01 | 0.01 – 0.10 | 0.002 | 0.02 | 0.11 | .91 | −0.03 – 0.03 |
| Fat mass | −0.01 | 0.01 | −1.22 | .22 | −0.02 – 0.01 | −0.002 | 0.004 | −0.53 | .59 | −0.01 – 0.01 |
| Height | −0.002 | 0.01 | −0.24 | .81 | −0.02 – 0.01 | −0.01 | 0.004 | −1.28 | .20 | −0.01 – 0.003 |
| Snoring | 0.15 | 0.19 | 0.79 | .43 | −0.22 – 0.52 | −0.09 | 0.10 | −0.89 | .37 | −0.29 – 0.11 |
| Sex | 0.01 | 0.15 | 0.10 | .92 | −0.27 – 0.30 | 0.09 | 0.09 | 1.04 | .30 | −0.08 – 0.26 |
| SES | 0.05 | 0.08 | 0.70 | .49 | −0.09 – 0.20 | 0.01 | 0.04 | 0.30 | .77 | −0.07 – 0.10 |
| Age | 0.01 | 0.04 | 0.12 | .91 | −0.07 – 0.08 | 0.04 | 0.02 | 1.78 | .08 | −0.004 – 0.08 |
| Race/Ethnicity | 0.08 | 0.15 | 0.57 | .57 | −0.20 – 0.37 | 0.15 | 0.08 | 1.76 | .08 | −0.02 – 0.31 |
| Depressive Symptoms | 0.02 | 0.01 | 1.48 | .14 | −0.01 – 0.04 | −0.001 | 0.01 | −0.07 | .94 | −0.02 – 0.01 |
| Sleep Duration Weekly | 0.07 | 0.13 | 0.59 | .56 | −0.17 – 0.32 | −0.26 | 0.11 | −2.40 | .02 | −0.47 – −0.05 |
| Sleep Duration Daily | 0.01 | 0.01 | 0.99 | .32 | −0.01 – 0.03 | −0.003 | 0.01 | −0.40 | .69 | −0.02 – 0.01 |
| Sleep Onset Weekly | −0.15 | 0.08 | −1.76 | .08 | −0.31 – 0.02 | 0.05 | 0.05 | 0.98 | .33 | −0.05 – 0.13 |
| Sleep Onset Daily | 0.02 | 0.01 | 1.76 | .08 | −0.002 – 0.04 | −0.01 | 0.01 | −0.99 | .32 | −0.03 – 0.01 |
| Midpoint Weekly | 0.10 | 0.21 | 0.46 | .65 | −0.31 – 0.50 | −0.49 | 0.18 | −2.78 | .01 | −0.84 – −0.14 |
| Midpoint Daily | 0.00 | 0.01 | −0.09 | .93 | −0.02 – 0.02 | 0.02 | 0.01 | 1.73 | .08 | −0.002 – 0.04 |
| Waketime Weekly | −0.10 | 0.22 | −0.46 | .65 | −0.52 – 0.32 | 0.47 | 0.18 | 2.69 | .01 | 0.13 – 0.82 |
| Waketime Daily | 0.02 | 0.02 | 1.25 | .21 | −0.01 – 0.05 | −0.001 | 0.01 | −0.07 | .95 | −0.02 – 0.02 |
est. ß, coefficient; SE, standard error; CI, confidence interval; LOC-eating, loss-of-control eating, SES; social economic status. Significant p-values are in bold type font
Discussion
The current study utilized an objective measurement of sleep and concurrent EMA to examine the associations among weekly and daily shifts in sleep duration/timing and food cravings and LOC-eating severity among a diverse sample of girls and boys of a broad age and weight range. When adjusting for the effects of various facets of sleep, sleep was not associated with food cravings. However, in both unadjusted and adjusted models, youth with shorter average sleep duration, earlier average sleep midpoint, and later average waketime during the two-weeks reported greater LOC-severity. Overall, these results demonstrate that when accounting for the entire sleep pattern, between-person differences in sleep duration and timing may be associated with eating-disordered behaviors.
When using objective measurement of sleep and accounting for the entire sleep pattern and relevant covariates, shifts in weekly and nightly sleep were not associated with food cravings. These findings are inconsistent with previous experimental studies among youth suggesting links between weekly sleep duration and bedtime and food cravings.17,18 The differences in the findings between our study and previous studies could be due to a relatively small sample and analyses that adjusted for all facets of sleep. Our ability to detect an association may have been obscured due to our inclusion of youth of various ages and BMIs. Variability of sleep needs across development45 and weight status could be explored in future studies as potential moderators of the associations among sleep patterns and food cravings. Other characteristics of sleep that were not studied (e.g., sleep efficiency) may also be linked to food cravings. Additionally, acute changes in sleep duration and timing may influence other factors (e.g., hormones, physical activity, affect, executive function) that promote LOC-eating in youth. The mechanisms underlying the relationships between sleep patterns and LOC-eating are likely complex, therefore future studies among larger samples are needed to identify the moderators and mechanisms linking interdependent facets of sleep to LOC-eating.
The present study found shorter weekly sleep duration, later weekly midpoints, and earlier weekly waketimes to be positively associated with LOC-eating, suggesting greater or more frequent shifts, in sleep duration and timing across two weeks may be linked to LOC-eating severity. Sleep midpoint is thought to be a behavioral estimation of one’s biological preference for sleep timing such that individuals with earlier midpoints may have a preference for earlier bedtime and waketime, while those with later midpoints may have a later sleep preference.46 Our results suggest that a discordance in later biological sleep preference (i.e., later sleep midpoint), earlier waketime, and shorter sleep duration appears to be linked to LOC-eating severity. More direct measures of biological sleep preference are needed in future studies to better understand these relationships. These findings are inconsistent with preliminary cross-sectional studies in youth at risk for type 2 diabetes and with overweight and obesity which have failed to detect links between sleep and LOC-eating.19,20 However, extant studies only examined the independent effects of a single facet of sleep on drives to eat and LOC-eating,19,20 and have not disentangled the impact of weekly and nightly shifts in sleep. Thus, differences are likely due to disparate statistical and methodological approaches. Carefully designed experimental studies are needed to elucidate the possible additive and interactive effects of nightly and weekly shifts in sleep duration, timing, and biological preference and their impact on factors that promote LOC-eating.
Study strengths include the use of actigraphy to examine several facets of sleep collected over two weeks. We also utilized EMA, which allowed for the examination of the associations between facets of sleep and both food cravings and LOC-eating severity in the natural environment. Additionally, EMA and sleep data were measured concurrently, which allowed for the examination of weekly and nightly shifts in sleep and food cravings and LOC-eating severity. Examination of youth in generally good health enabled us to study factors associated with LOC-eating severity prior to the development of significant eating issues, thereby elucidating potential risk factors for the onset of obesity and related health comorbidities.
Study limitations include small average shifts in sleep and low average severity of food cravings and LOC-eating. Further, the craving measure used in the present study has been validated in samples of adults, but not yet in children or adolescents. Therefore, although the measure demonstrated good internal consistency, the low severity reports of food cravings may suggest the need for measures of food cravings that are adapted for and validated in youth. Moreover, it is unknown if continuous severity, rather than a specific cut-off score, is most appropriate for detecting LOC-eating in the natural environment among youth. It is possible that the present study identified “false positives,” such that some reports of LOC-eating may not have met a clinical threshold. There are no empirically determined cutoffs of LOC-eating severity in youth; therefore, we examined LOC-eating severity as a continuous measure to maintain statistical power and consistency with previous EMA studies in children and adolescents.5,26 Future studies are needed to determine the clinical significance of LOC-eating severity and replicate findings from the current study in samples of youth enriched for LOC-eating. Nevertheless, detection of associations among sleep and severity of LOC-eating, even in a sample comprising healthy youths with low scores on this measure, supports sleep as a potentially modifiable risk factor for pediatric obesity and disordered eating behaviors. Lastly, average sleep midpoint was strongly correlated with average sleep onset and wake times in the present study, yet despite associations among facets of sleep, distinct facets of sleep appear to offer unique effects on eating behaviors. Thus, experimental studies should be carefully designed to ensure only the intended facets of sleep are being manipulated when investigating the independent effects of different facets of sleep on eating and weight.
Conclusion
Interactions among sleep duration and sleep timing may be important for the promotion of LOC-eating. We did not observe statistically significant associations among facets of sleep and food cravings. Thus, LOC-eating may be a candidate mechanism linking sleep to excess weight gain. Longitudinal data are needed to provide clarification on how shifts in sleep duration and timing prospectively impact appetite, eating behaviors, and onset of obesity. Future studies that aim to determine the biological and psychological mechanisms that promote risk for developing obesity may further elucidate how sleep can be harnessed to maximize the efficiency of pediatric obesity prevention programs.
Acknowledgments
FUNDING: This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant number ZIA-HD00641; J. Yanovski).
Footnotes
DISCLOSURE: The authors have no conflict to declare. The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the Uniformed Services University of the Health Sciences (USU), or the U.S. Department of Defense.
CLINICAL TRIAL REGISTRATION: NCT02390765
DATA AVAILABILITY STATEMENT:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Tanofsky-Kraff M, Yanovski SZ, Schvey NA, Olsen CH, Gustafson J, Yanovski JA. A prospective study of loss of control eating for body weight gain in children at high risk for adult obesity. Int J Eat Disord. 2009;42(1):26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sonneville KR, Horton NJ, Micali N, et al. Longitudinal associations between binge eating and overeating and adverse outcomes among adolescents and young adults: does loss of control matter? JAMA pediatrics. 2013;167(2):149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanofsky-Kraff M, Shomaker LB, Stern EA, et al. Children’s binge eating and development of metabolic syndrome. Int J Obes (Lond). 2012;36(7):956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill AJ. The psychology of food craving. Proc Nutr Soc. 2007;66(2):277–285. [DOI] [PubMed] [Google Scholar]
- 5.Goldschmidt AB, Smith KE, Crosby RD, et al. Ecological momentary assessment of maladaptive eating in children and adolescents with overweight or obesity. Int J Eat Disord. 2018;51(6):549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boswell RG, Kober H. Food cue reactivity and craving predict eating and weight gain: a meta-analytic review. Obes Rev. 2016;17(2):159–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boutelle KN, Zucker NL, Peterson CB, Rydell SA, Cafri G, Harnack L. Two novel treatments to reduce overeating in overweight children: a randomized controlled trial. J Consult Clin Psychol. 2011;79(6):759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller MA, Kruisbrink M, Wallace J, Ji C, Cappuccio FP. Sleep duration and incidence of obesity in infants, children, and adolescents: a systematic review and meta-analysis of prospective studies. Sleep. 2018;41(4). [DOI] [PubMed] [Google Scholar]
- 9.Fatima Y, Doi S, Mamun A. Sleep quality and obesity in young subjects: a meta-analysis. Obesity reviews. 2016;17(11):1154–1166. [DOI] [PubMed] [Google Scholar]
- 10.He F, Bixler EO, Liao J, et al. Habitual sleep variability, mediated by nutrition intake, is associated with abdominal obesity in adolescents. Sleep Med. 2015;16(12):1489–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He F, Bixler EO, Berg A, et al. Habitual sleep variability, not sleep duration, is associated with caloric intake in adolescents. Sleep medicine. 2015;16(7):856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amigo I, Peña E, Errasti JM, Busto R. Sedentary versus active leisure activities and their relationship with sleeping habits and body mass index in children of 9 and 10 years of age. Journal of health psychology. 2016;21(7):1472–1480. [DOI] [PubMed] [Google Scholar]
- 13.Thivel D, Isacco L, Aucouturier J, et al. Bedtime and sleep timing but not sleep duration are associated with eating habits in primary school children. J Dev Behav Pediatr. 2015;36(3):158–165. [DOI] [PubMed] [Google Scholar]
- 14.Xiu L, Ekstedt M, Hagströmer M, Bruni O, Bergqvist-Norén L, Marcus C. Sleep and adiposity in children from 2 to 6 years of age. Pediatrics. 2020;145(3). [DOI] [PubMed] [Google Scholar]
- 15.Krietsch KN, Chardon ML, Beebe DW, Janicke DM. Sleep and weight-related factors in youth: A systematic review of recent studies. Sleep Medicine Reviews. 2019;46:87–96. [DOI] [PubMed] [Google Scholar]
- 16.Kracht CL, Chaput JP, Martin CK, Champagne CM, Katzmarzyk PT, Staiano AE. Associations of Sleep with Food Cravings, Diet, and Obesity in Adolescence. Nutrients. 2019;11(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asarnow LD, Greer SM, Walker MP, Harvey AG. The impact of sleep improvement on food choices in adolescents with late bedtimes. Journal of Adolescent Health. 2017;60(5):570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon SL, Field J, Miller LE, DiFrancesco M, Beebe DW. Sweet/dessert foods are more appealing to adolescents after sleep restriction. PloS one. 2015;10(2):e0115434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly NR, Shomaker LB, Radin RM, et al. Associations of sleep duration and quality with disinhibited eating behaviors in adolescent girls at-risk for type 2 diabetes. Eat Behav. 2016;22:149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldschmidt AB, Evans EW, Saletin JM, et al. Naturalistic, multimethod exploratory study of sleep duration and quality as predictors of dysregulated eating in youth with overweight and obesity. Appetite. 2020;146:104521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hogenkamp PS, Nilsson E, Nilsson VC, et al. Acute sleep deprivation increases portion size and affects food choice in young men. Psychoneuroendocrinology. 2013;38(9):1668–1674. [DOI] [PubMed] [Google Scholar]
- 22.LeMay-Russell S, Tanofsky-Kraff M, Schvey NA, et al. Associations of Weekday and Weekend Sleep with Children’s Reported Eating in the Absence of Hunger. Nutrients. 2019;11(7):1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mi SJ, Kelly NR, Brychta RJ, et al. Associations of sleep patterns with metabolic syndrome indices, body composition, and energy intake in children and adolescents. Pediatr Obes. 2019;14(6):e12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000. CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002(246):1–190. [PubMed] [Google Scholar]
- 25.Real Time Assessment In the Natural Environment (ReTAINE) [computer program]. Fargo ND: 2019. [Google Scholar]
- 26.Ranzenhofer LM, Engel SG, Crosby RD, et al. Using ecological momentary assessment to examine interpersonal and affective predictors of loss of control eating in adolescent girls. Int J Eat Disord. 2014;47(7):748–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreno S, Rodríguez S, Fernandez MC, Tamez J, Cepeda-Benito A. Clinical validation of the trait and state versions of the Food Craving Questionnaire. Assessment. 2008;15(3):375–387. [DOI] [PubMed] [Google Scholar]
- 28.Cooper Z, Fairburn C. The eating disorder examination: A semi-structured interview for the assessment of the specific psychopathology of eating disorders. Int J Eat Disord. 1987;6(1):1–8. [Google Scholar]
- 29.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2(5):389–396. [DOI] [PubMed] [Google Scholar]
- 30.Cespedes EF, Rifas-Shiman S, Quante M, Redline S, Oken E, Taveras E. Chronotype, Social Jet Lag, and Cardiometabolic Risk Factors in Early Adolescence. JAMA pediatrics. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hjorth MF, Chaput JP, Damsgaard CT, et al. Low physical activity level and short sleep duration are associated with an increased cardio-metabolic risk profile: A longitudinal study in 8–11 year old Danish children. PloS one. 2014;9(8):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sadeh A, Sharkey M, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17(3):201–207. [DOI] [PubMed] [Google Scholar]
- 33.Sadeh A, Raviv A, Gruber R. Sleep patterns and sleep disruptions in school-age children. Dev Psychol. 2000;36(3):291–301. [DOI] [PubMed] [Google Scholar]
- 34.Hollingshead AB. Hollingshead two factor index of social position In: Miller DC, ed. Handbook of research design and social measurement. 5th ed. Newbury Park, CA: Sage Publications; 1991; 1957:351–359. [Google Scholar]
- 35.Daniels SR. The use of BMI in the clinical setting. Pediatrics. 2009;124(Supplement 1):S35–S41. [DOI] [PubMed] [Google Scholar]
- 36.Kovacs M Children’s depression inventory (CDI2) : technical manual. North Tonawanda, NY: Multi-Health Systems, Inc.; 2011. [Google Scholar]
- 37.Smucker MR, Craighead WE, Craighead LW, Green BJ. Normative and reliability data for the Children’s Depressive Inventory. Journal of Abnormal Child Psychology. 1986;14:25–39. [DOI] [PubMed] [Google Scholar]
- 38.IBM SPSS Statistics for Macintosh [computer program]. Version 25.0. Armonk, NY: IBM Corp.; 2017. [Google Scholar]
- 39.Curran PJ, Bauer DJ. The disaggregation of within-person and between-person effects in longitudinal models of change. Annual review of psychology. 2011;62:583–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, Maxwell SE. On disaggregating between-person and within-person effects with longitudinal data using multilevel models. Psychological Methods. 2015;20(1):63–83. [DOI] [PubMed] [Google Scholar]
- 41.Verhulst SL, Schrauwen N, Haentjens D, et al. Sleep-disordered breathing in overweight and obese children and adolescents: prevalence, characteristics and the role of fat distribution. Archives of disease in childhood. 2007;92(3):205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marco CA, Wolfson AR, Sparling M, Azuaje A. Family socioeconomic status and sleep patterns of young adolescents. Behavioral sleep medicine. 2012;10(1):70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubio-Lopez N, Morales-Suarez-Varela M, Pico Y, Livianos-Aldana L, Llopis-Gonzalez A. Nutrient Intake and Depression Symptoms in Spanish Children: The ANIVA Study. Int J Environ Res Public Health. 2016;13(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang SJ, Cha HS. Retrospective cohort study on Korean adolescents’ sleep, depression, school adjustment, and life satisfaction. Nurs Health Sci. 2018;20(4):422–430. [DOI] [PubMed] [Google Scholar]
- 45.Maslowsky J, Ozer EJ. Developmental trends in sleep duration in adolescence and young adulthood: evidence from a national United States sample. J Adolesc Health. 2014;54(6):691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santisteban JA, Brown TG, Gruber R. Association between the Munich Chronotype Questionnaire and Wrist Actigraphy. Sleep Disorders. 2018;2018:5646848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.