Abstract
Aims
Reactive astrocytes in Alzheimer's disease (AD) have traditionally been demonstrated by increased glial fibrillary acidic protein (GFAP) immunoreactivity; however, astrocyte reaction is a complex and heterogeneous phenomenon involving multiple astrocyte functions beyond cytoskeletal remodelling. To better understand astrocyte reaction in AD, we conducted a systematic review of astrocyte immunohistochemical studies in post‐mortem AD brains followed by bioinformatics analyses on the extracted reactive astrocyte markers.
Methods
NCBI PubMed, APA PsycInfo and WoS‐SCIE databases were interrogated for original English research articles with the search terms ‘Alzheimer's disease’ AND ‘astrocytes.’ Bioinformatics analyses included protein–protein interaction network analysis, pathway enrichment, and transcription factor enrichment, as well as comparison with public human ‐omics datasets.
Results
A total of 306 articles meeting eligibility criteria rendered 196 proteins, most of which were reported to be upregulated in AD vs control brains. Besides cytoskeletal remodelling (e.g., GFAP), bioinformatics analyses revealed a wide range of functional alterations including neuroinflammation (e.g., IL6, MAPK1/3/8 and TNF), oxidative stress and antioxidant defence (e.g., MT1A/2A, NFE2L2, NOS1/2/3, PRDX6 and SOD1/2), lipid metabolism (e.g., APOE, CLU and LRP1), proteostasis (e.g., cathepsins, CRYAB and HSPB1/2/6/8), extracellular matrix organisation (e.g., CD44, MMP1/3 and SERPINA3), and neurotransmission (e.g., CHRNA7, GABA, GLUL, GRM5, MAOB and SLC1A2), among others. CTCF and ESR1 emerged as potential transcription factors driving these changes. Comparison with published ‐omics datasets validated our results, demonstrating a significant overlap with reported transcriptomic and proteomic changes in AD brains and/or CSF.
Conclusions
Our systematic review of the neuropathological literature reveals the complexity of AD reactive astrogliosis. We have shared these findings as an online resource available at www.astrocyteatlas.org.
Keywords: Alzheimer's disease, astrocyte, bioinformatics, immunohistochemistry, neuropathology, reactive astrogliosis, systematic review
We aimed to define the functional changes characteristic of reactive astrocytes in Alzheimer's disease (AD) via a systematic review of immunohistochemical studies in post‐mortem AD brains followed by bioinformatics analyses (e.g., protein–protein interaction network, pathway and transcription factor enrichment); 306 eligible articles rendered 196 proteins, which underscore a complex phenotypic change involving inflammation, oxidative stress, lipid metabolism, proteostasis, extracellular matrix remodelling, neuromodulation and blood–brain barrier integrity, among other alterations. These markers were catalogued in a new online resource: www.astrocyteatlas.org.
Key points
This is a systematic review of human post‐mortem immunohistochemical studies followed by bioinformatics analyses to define the functional changes of reactive astrocytes in Alzheimer's disease (AD).
A total of 306 eligible articles rendered 196 markers of AD reactive astrocytes (ADRA), implicating inflammation, oxidative stress, lipid metabolism, proteostasis, extracellular matrix remodelling, neuromodulation and blood–brain barrier integrity, among other alterations.
This ADRA protein set is catalogued in a new online resource available at www.astrocyteatlas.org.
INTRODUCTION
Astrocytes are known to undergo profound morphological and functional changes in central nervous system diseases, collectively termed astrocyte reaction or reactive astrogliosis [1]. This astrocyte reaction has traditionally been depicted by an increased immunoreactivity for the cytoskeletal intermediate filament glial fibrillary acidic protein (GFAP). However, in recent years, transcriptomic studies of acutely isolated astrocytes or single nuclei from mouse models and post‐mortem human brains have revealed that astrocyte reaction is heterogeneous, context‐dependent (e.g., different in acute injuries vs chronic neurodegenerative diseases) [1, 2] and complex beyond cytoskeletal rearrangement (reviewed in previous studies [3, 4, 5, 6]). Unfortunately, transcriptomic and proteomic approaches in the Alzheimer's disease (AD) brain are limited by lack of spatial information, which is relevant as there are layer‐specific subtypes of astrocytes [7] and reactive (GFAP+) astrocytes tend to localise near both dense‐core neuritic amyloid‐β (Aβ) plaques and neurofibrillary tangles (NFTs) [8, 9, 10]. While ongoing efforts to develop spatial ‐omics methods will eventually overcome this constraint [11, 12], immunohistochemistry remains the gold‐standard technique to capture spatial expression patterns of astrocytes in post‐mortem tissue sections.
Here, we conducted a systematic review of post‐mortem human brain neuropathological immunohistochemical studies describing potential markers of AD reactive astrocytes (ADRA). We hypothesised that compiling the neuropathological literature could provide a catalogue of dysregulated proteins in ADRA around plaques and tangles, shed light on the complexity of their associated functional changes, and inform the development of fluid (CSF and plasma/serum) and positron emission tomography (PET) imaging biomarkers to detect ADRA in patients.
MATERIAL AND METHODS
Systematic review
We followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines [13] as explained in detail in the Supporting Information [14, 15].
Bioinformatics analyses
Additional details regarding the bioinformatics analyses can be found in the Supporting Information.
Pathway enrichment analysis
We first classified the astrocyte proteins into one of 18 functional categories based on published evidence. The interconnectivity between these assigned functions was demonstrated via chord and Circos plots. In addition, to validate these functional pathways, we performed pathway enrichment analysis (PEA) on the ADRA markers against the Gene Ontology (GO) and Reactome databases available from the Molecular Signatures Database (MSigDB) [16, 17, 18, 19].
Protein‐protein interaction network analysis
To examine direct (physical) and indirect (functional) interactions between the ADRA protein set, a protein–protein interaction (PPI) network was constructed via the application programming interface (API) to the STRING biological database of known and predicted PPIs (version 11.0, Homo sapiens) [20].
Transcription factor enrichment analysis
We identified transcription factors potentially regulating the expression of the ADRA markers with two analogous tools: TFEA.ChIP [21] and Enrichr [22]. While sequence‐based tools using position weight matrices to predict transcription factor binding sites proximal to genes of interest have suboptimal specificity, TFEA.ChIP and Enrichr compute enrichment analysis based on databases of publicly available chromatin immunoprecipitation sequencing (ChIP‐seq) experiments [23, 24, 25, 26].
Comparison with transcriptomic and proteomic studies
To corroborate the results of our systematic review of AD neuropathological post‐mortem studies, we compared the ADRA protein set with recent transcriptomic and proteomic studies on human control and AD brains [27, 28, 29]. First, enrichment analysis against differentially expressed genes or proteins (between control and AD individuals) was conducted with Fisher's exact test. Next, we created heatmaps to illustrate the expression levels of the overlapping transcripts and proteins.
RESULTS
Summary of systematic review results
Figure 1 depicts the PRISMA flowchart with the number of articles obtained after each step (i.e., identification, screening, eligibility and inclusion). A total of 306 articles were ultimately included in the systematic review and reviewed by either LV or AS‐P to extract relevant data. Across these 306 articles, a total of 196 proteins were identified, which we refer to as the ADRA protein set. Table 1 summarises these 196 markers by functional category; a more detailed description of the neuropathological studies included in this systematic review is provided in Table S1.
FIGURE 1.
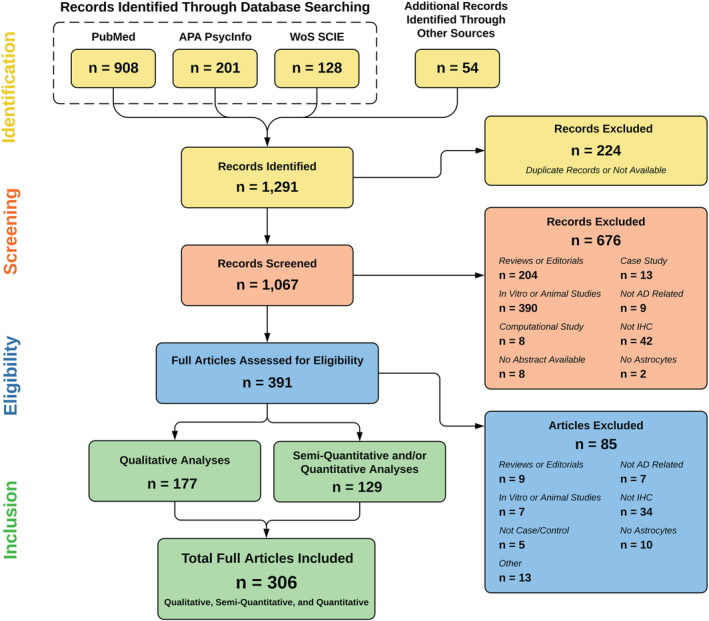
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flowchart of the systematic review. The PubMed, APA PsycInfo and Web of Science‐Science Citation Index Expanded (WoS‐SCIE) databases were queried with the search terms ‘Alzheimer's disease’ AND ‘astrocytes,’ yielding 1237 records, plus 54 additional records identified by scanning reference lists. The titles and abstracts of the 1067 unique records were screened. Of these, 391 were assessed for eligibility based on prespecified inclusion and exclusion criteria. Finally, a total of 306 original articles were thoroughly reviewed to extract relevant information, including markers of Alzheimer's disease reactive astrocytes (ADRA)
TABLE 1.
Functional categorisation of 196 ADRA proteins resulting from the systematic review
| Functional category | Protein markers | Count |
|---|---|---|
| Aβ metabolism | APP, BACE1, CAV3, IDE, MME, PSEN1, PSEN2, SHC1, YY1 | 9 |
| Blood–brain barrier | CLDN11, CLDN2, CLDN5, EDN1, FGA, IGHA1, IGHG1, IGHM | 8 |
| Calcium homeostasis | CALB1, CALB2, KCNIP3, S100A6, S100B | 5 |
| Cytoskeleton | ANK1, FLNA, GFAP, NES, VIM | 5 |
| Extracellular matrix | A2M, CD44, HSPG2, ITIH1, LOX, MMP1, MMP3, NRG1, PLAU, SERPINA3, SERPINE1, TGM1, TGM2 | 13 |
| Inflammation | AEBP1, CASP1, CCL2, CCL4, CEBPD, CHI3L1, CXCL10, CXCL12, GMFB, HPGDS, ICAM1, IL18, IL1B, IL1RL1, IL33, IL6, NFATC2, NFATC4, PLA2G4A, PTGES, PTGS2, RELA, S1PR1, S1PR3, TNF, TSPO | 26 |
| Insulin signalling | AMY2A, IGF1, IGF1R, IGF2R, IGFBP3 | 5 |
| Intracellular trafficking | CLTA, CLTB, CPE, HOOK2, KIF21B, SCG3 | 6 |
| Kinase/phosphatase | CSNK2A1, FYN, GSK3B, MAPK1, MAPK14, MAPK3, MAPK8, PPP1CA, PPP2CA, PPP3CA, PRKDC | 11 |
| Lipid metabolism | APOA1, APOC1, APOD, APOE, CETP, CLU, CYP46A1, LDLR, LRP1 | 9 |
| Miscellaneous | ALDH1L1, ESR1, ESR2, FKBP1A, MAPT, PADI2 | 6 |
| Neurotransmission | ADORA2A, CHAT, CHRM1, CHRNA7, DRD3, DRD5, GAD1, GLUL, GRM5, HTR2A, IDO1, MAOB, SLC1A2, SLC1A3, SLC6A11, SRR | 16 |
| Oxidative stress | AGER, AKR7A2, EPHX1, H2AX, HAMP, HMOX1, MPO, MT1A, MT2A, MT3, NFE2L2, NOS1, NOS2, NOS3, PRDX6, SLC40A1, SOD1, SOD2, TXN | 19 |
| Phagocytosis | C3, CR1, MFGE8, SCARB1 | 4 |
| Proliferation/apoptosis | APC, BCL2, CASP3, CCNC, CDK1, CDK7, CDK8, CDKN2A, DCX, E2F1, EEF1A2, ETS2, FAS, FASLG, FOXO3, GLB1, MYC, MYCN, PTEN, RB1, RBL2, TP53 | 22 |
| Proteostasis | BECN1, CAPN10, CD68, CRYAB, CTSB, CTSD, CTSH, CTSL, HEXA, HSPB1, HSPB2, HSPB6, HSPB8, PARK7, PREP, PRKN, SYVN1 | 17 |
| Trophic factors | EGR1, FGF1, FGF2, FGFR1, GAP43, HGF, HGFAC, PEA15, TGFB2, TGFB3, TGFBR2 | 11 |
| Water/K+ homeostasis | AQP1, AQP4, KCNJ11, KCNN4 | 4 |
Note: The 196 AD reactive astrocyte (ADRA) proteins were classified into one of 18 functional categories based on published evidence. The constituent proteins of each functional category are shown here.
Abbreviation: ADRA, Alzheimer's disease reactive astrocyte.
Functional characterisation of markers of astrocyte reaction
As expected, an increase in GFAP immunoreactivity was the most frequently described hallmark of astrocyte reaction, even without considering studies that only used GFAP to colocalise their marker of interest with reactive astrocytes. However, the ADRA protein set revealed many other functional changes beyond cytoskeletal rearrangement, which we review below.
Cytoskeletal remodelling
Of note, while GFAP isoform 1 (full length) is the main isoform in the brain, less abundant splice forms [30, 31] and caspase‐3‐cleaved GFAP fragments [32] have also been reported to increase in ADRA. Additionally, the cytoskeletal remodelling that reactive astrocytes undergo in AD involves the upregulation of other intermediate filaments proteins such as vimentin (VIM) [31, 33] and nestin (NES) [31, 34], as well as actin‐interacting proteins such as the 280‐kDa actin‐binding protein filamin‐A (FLNA) [35] and ankyrin‐1 (ANK1) [36].
Inflammation
Multiple studies report increased immunoreactivity for a wide variety of inflammatory cytokines, including the inflammasome‐activating enzyme caspase‐1 (CASP1) [37]; interleukins such as interleukin‐1 beta (IL1B) [37, 38], interleukin‐6 (IL6) [37, 39, 40], interleukin‐18 (IL18) [41], interleukin‐33 (IL33) [42] and its receptor interleukin‐1 receptor‐like 1 (IL1RL1) [42], as well as tumour necrosis factor (TNF) [43]; chemokines such as C–C motif chemokine ligand 2 (CCL2) [40], C–C motif chemokine ligand 4 (CCL4) [44], C–X–C motif chemokine ligand 10 (CXCL10) [45] and stromal cell‐derived factor 1 (CXCL12) [46]; cell adhesion molecules such as intercellular adhesion molecule 1 (ICAM1) [47]; eicosanoid metabolism enzymes such as prostaglandin G/H synthase 2 (PTGS2, also known as cyclooxygenase‐2) [48], cytosolic phospholipase A2 (PLA2G4A) [49] and haematopoietic prostaglandin D synthase (HPGDS) [50] (while the microsomal prostaglandin E synthase (PTGES) was reported to be decreased in ADRA [51]); and the immunomodulatory receptor sphingosine 1‐phosphate receptor 3 (S1PR3) [52] but not sphingosine 1‐phosphate receptor 1 (S1PR1) [53]. In addition, some studies have shown increased immunoreactivity for transcription factors known to mediate immune and inflammatory responses such as nuclear factor of activated T‐cells, cytoplasmic 2 (NFATC2) [54]; nuclear factor of activated T‐cells, cytoplasmic 4 (NFATC4) [54]; transcription factor p65 (RELA, also known as nuclear factor NF‐kappa‐B p65 subunit) [55, 56]; adipocyte enhancer‐binding protein 1 (AEBP1) [57]; CCAAT/enhancer‐binding protein delta (CEBPD) [58]; and glia maturation factor (e.g., GMFB) [59, 60]. Lastly, reactive astrocytes also express chitinase‐3‐like protein 1 (CHI3L1, also known as YKL‐40) [61, 62, 63, 64] and the 18 kDa translocator protein (TSPO) [65, 66]; although their function remains to be fully elucidated, these two proteins are commonly interpreted as evidence of inflammation when measured in CSF and detected via PET imaging, respectively.
Oxidative stress
Numerous studies implicate ADRA in oxidative stress, based on an increased immunoreactivity for advanced glycation end‐products (AGEs) [67, 68, 69] and the AGE‐specific receptor (AGER, also known as RAGE) [69, 70]; DNA [71, 72, 73] and protein [74] oxidative damage markers; and pro‐oxidant enzymes such as myeloperoxidase (MPO) [75] and the three nitric oxide synthase isoforms: brain (NOS1) [76], inducible (NOS2) [43, 77] and endothelial (NOS3) [78]. In addition, ADRA exhibit decreased immunoreactivity for nuclear factor erythroid 2‐related factor 2 (NFE2L2) [79]—the main transcription factor orchestrating the antioxidant response—and for solute carrier family 40 member 1 (SLC40A1, also known as ferroportin‐1) and hepcidin (HAMP) [80], both of which regulate iron homeostasis. Conversely, there is also strong evidence for a role of ADRA in antioxidant defence, including increased immunoreactivity for antioxidant enzymes such as the metallothioneins ‐1 (e.g., MT1A), ‐2 (MT2A) and, to a lesser extent, ‐3 (MT3, also known as growth inhibitory factor or GIF) [73, 81, 82, 83, 84, 85], which are also important for zinc and copper homeostasis; the superoxide dismutases [Cu‐Zn] (SOD1) and [Mn], mitochondrial (SOD2) [86, 87]; heme oxygenase 1 (HMOX1) [88]; thioredoxin (TXN) [89]; peroxiredoxin‐6 (PRDX6) [90]; epoxide hydrolase 1 (EPHX1) [91]; and aflatoxin B1 aldehyde reductase member 2 (AKR7A2) [92].
Lipid metabolism
The lipid metabolism markers found in our systematic review can be classified as (1) apolipoproteins, which bind and transport cholesterol and phospholipids packed as lipoproteins; (2) lipoprotein receptors, which internalise these via endocytosis; and (3) enzymes that metabolise lipids. As expected, the most studied apolipoprotein is apolipoprotein E (APOE), followed by clusterin (CLU, also known as APOJ). Most studies investigating APOE report increased expression in ADRA [93, 94, 95, 96, 97, 98], but some authors have described reduced expression restricted to the vicinity of amyloid plaques [99, 100] or unchanged [101] expression compared with normal brain astrocytes. In general, all other apolipoproteins including CLU [102, 103] and the apolipoproteins A‐I (APOA1) [98], C‐I (APOC1) [104] and D (APOD) [105] have been shown to increase in ADRA. Among the lipoprotein receptors, low‐density lipoprotein (LDL) receptor‐related protein 1 (LRP1) is the most frequently studied and is increased in ADRA according to the majority of studies [39, 95, 106, 107] (but unchanged in the basal ganglia [101]). In contrast, the LDL receptor (LDLR) is unchanged in ADRA vs normal brain astrocytes [95]. Finally, two cholesterol enzymes, cholesteryl ester transfer protein (CETP) [108] and cholesterol 24‐hydroxylase (CYP46A1) [109, 110], have been shown to be increased in ADRA, while two immunohistochemical studies have shown accumulation of ceramide in ADRA [52, 111].
Extracellular matrix
Reactive astrocytes in AD also play an essential role in reorganisation of the extracellular matrix, as judged by their increased immunoreactivity for secreted proteases such as the matrix metalloproteinases interstitial collagenase (MMP1) [112] and stromelysin‐1 (MMP3) [113] as well as urokinase‐type plasminogen activator (PLAU) [107]; protease inhibitors such as alpha‐1‐antichymotrypsin (SERPINA3, also known as ACT) [114, 115, 116], the inter‐alpha‐trypsin inhibitors (e.g., ITIH1) [117] and plasminogen activator inhibitor 1 (SERPINE1) [107]; protein‐lysine 6‐oxidase (LOX), which oxidises ECM proteins [118, 119]; the protein‐glutamine gamma‐glutamyltransferases K (TGM1) and 2 (TGM2), which crosslink ECM proteins [120, 121] (but are unchanged in ADRA based on [122]); and cell surface and extracellular matrix adhesion receptors and ligands such as CD44 antigen (CD44) [64, 123, 124], the heparan sulphate proteoglycans (e.g., HSPG2) [125], neuregulin 1 (NRG1) [126, 127] and the ganglioside GM1 [128].
Proteostasis
Evidence for an activation of protein degradation systems in ADRA is indicated by increased immunoreactivity for lysosomal enzymes such as the cathepsins B (CTSB) [129], D (CTSD) [94, 129, 130], H (CTSH) [130] and L (CTSL) [130], beta‐hexosaminidase subunit alpha (HEXA) [130] and lysosomal membrane proteins including macrosialin (CD68) [131] and Beclin‐1 (BECN1) [132]; small chaperones such as alpha‐crystallin B chain (CRYAB) [133, 134, 135], the heat shock proteins beta‐1 (HSPB1, also known as HSP27) [134, 135, 136], beta‐2 (HSPB2) [134, 135], beta‐6 (HSPB6) [134, 135], beta‐8 (HSPB8) [135] and Parkinson disease protein 7 (PARK7, also known as DJ‐1) [137]; the E3 ubiquitin‐protein ligases Parkin (PRKN) [138] and synoviolin (SYVN1, also known as HRD1) [139]; and proteases such as calpain‐10 (CAPN10) [140] and prolyl endopeptidase (PREP) [141].
Neurotransmission
The fine processes from protoplasmic astrocytes are structural components of excitatory synapses and modulate glutamatergic transmission by taking up glutamate via the membrane‐bound excitatory amino acid transporters 1 (SLC1A3, better known as EAAT1 or GLAST‐1) and 2 (SLC1A2, better known as EAAT2 or GLT‐1) and converting glutamate into glutamine via glutamine synthetase (GLUL). GLUL immunoreactivity has been reported to be increased [142, 143], decreased [144, 145] and unchanged [146] in ADRA, whereas SLC1A2 levels have more consistently been documented as reduced [147, 148, 149], and SLC1A3 levels appear to be stable [147, 148]. In addition, metabotropic glutamate transporter 5 (GRM5) [56, 150, 151] and serine racemase (SRR, which converts L‐serine into D‐serine, a gliotransmitter that modulates neuronal NMDA receptors) [152] are increased in ADRA. Besides glutamatergic neurotransmission, other neurotransmitter systems have also been associated with ADRA based on increased immunoreactivity for specific markers, including (1) GABAergic: γ‐aminobutyric acid (GABA) [153, 154], the 67‐kDa glutamate decarboxylase 1 (GAD1, also known as GAD‐67) [153] and sodium‐ and chloride‐dependent GABA transporter 3 (SLC6A11) [153]; (2) cholinergic: neuronal acetylcholine receptor subunit alpha‐7 (CHRNA7) [155, 156, 157, 158, 159], choline O‐acetyltransferase (CHAT) [158] and muscarinic receptors (e.g., CHRM1) [160]; (3) catecholaminergic: amine oxidase (flavin‐containing) B (MAOB) [154] (but see Pugliese et al. [34] as well) and D(1B) dopamine receptor (DRD5) (but not D (3) dopamine receptor [DRD3], which is unchanged) [161]; (4) serotoninergic: 5‐hydroxytryptamine receptor 2A (HTR2A) [162]; (5) kynurenergic: quinolinic acid and indoleamine 2,3‐dioxygenase (IDO1) [163]; and (6) purinergic: adenosine receptor A2a (ADORA2A) [164].
Trophic factors
Although homeostatic astrocytes have traditionally been considered as ancillary cells providing trophic support to neurons for neuronal development and survival, remarkably, multiple trophic growth factors have been reported to increase in ADRA by immunohistochemistry. These trophic factors include hepatocyte growth factor (HGF) [165, 166] and its activator (HGFAC) [167]; fibroblast growth factors 1 (FGF1, also known as acidic FGF) [168, 169] and 2 (FGF2, also known as basic FGF) [170, 171, 172, 173] and FGF receptor 1 (FGFR1) [174]; transforming growth factors beta‐2 (TGFB2) [175, 176, 177] and beta‐3 (TGFB3) [177]; and neuromodulin (GAP43) [178]. By contrast, TGF‐beta receptor type‐2 (TGFBR2) has been reported to be reduced in ADRA [177], whereas both early growth response protein 1 (EGR1) [179] and astrocytic phosphoprotein PEA‐15 (PEA15) [180] are unchanged.
Proliferation and apoptosis
Several studies have suggested that ADRA are actively proliferating based on an increased immunoreactivity for proliferative markers including the proto‐oncogenes apoptosis regulator Bcl‐2 (BCL2) [181, 182], Myc (MYC) [183], N‐myc (MYCN) [183] and protein C‐ets‐2 (ETS2) [184], as well as the cell cycle proteins cyclin C (CCNC) [185] and the cyclin‐dependent kinases ‐1 (CDK1) [186] (although CDK1 is unchanged according to [187]), ‐7 (CDK7) [185] and ‐8 (CDK8) [185]. Conversely, multiple studies argue against ADRA proliferation based on increased immunoreactivity for tumour suppressors such as cellular tumour antigen p53 (TP53) [188] (although unchanged based on [72]), the hyperphosphorylated form of retinoblastoma‐associated protein (RB1, also known as pRb) [189], retinoblastoma‐like protein 2 (RBL2, also known as p130) [190], adenomatous polyposis coli protein (APC) [191], transcription factor E2F1 (E2F1) [189], Forkhead box protein O3 (FOXO3) [143], phosphatidylinositol 3,4,5‐trisphosphate 3‐phosphatase and dual‐specificity protein phosphatase PTEN (PTEN) [192] and cyclin‐dependent kinase inhibitor 2A (CDKN2A, also known as p16INK4a) [112, 193], together with decreased immunoreactivity for the microtubule‐associated neuronal migration protein doublecortin (DCX) [194]. Lastly, ADRA apoptosis has been suggested due to an increased immunoreactivity for activated caspase‐3 (CASP3) [32, 195] (but unchanged according to Simpson et al. [72]), caspase‐cleaved actin (ACTB, where the caspase‐cleaved form is known as fragment of actin, or fractin) [72] and caspase‐cleaved GFAP [32], as well as the death receptor TNF receptor superfamily member 6 (FAS) [188, 196, 197] and its ligand, TNF ligand superfamily member 6 (FASLG) [197]. Similarly, increased DNA fragmentation has been shown using dUTP nick‐end labelling by many authors [131, 182, 197, 198] but not others [199].
Kinase/phosphatase activity
ADRA have been shown to upregulate multiple kinases and phosphatases. Among these kinases, besides the cyclin‐dependent kinases described above, there is also evidence of increased immunoreactivity for the mitogen‐activated protein kinases 1 (MAPK1, also called extracellular signal‐regulated kinase 2, or ERK2) [200] (but unchanged based on Webster et al. [201]), 3 (MAKP3, also called ERK1) [200, 201], 8 (MAPK8, also called SAPK1c or JNK1) [200] and 14 (MAPK14, also called p38) [200]; glycogen synthase kinase‐3 beta (GSK3B) [200]; casein kinase II (e.g., CSNK2A1, also known as CK II) [202]; and tyrosine‐protein kinase Fyn (FYN) [203], whereas DNA‐dependent protein kinase (PRKDC) is unchanged [72]. Regarding phosphatases, besides PTEN listed as a tumour suppressor above, there is evidence of increased immunoreactivity for the serine/threonine‐protein phosphatases 2A (PPP2CA) [204] and 2B (PPP3CA, also known as calcineurin) [56, 204, 205, 206], whereas serine/threonine‐protein phosphatase PP1‐alpha (PPP1CA) remains unchanged [204].
Insulin signalling
ADRA appear to mobilise their energy metabolism, as indicated by an increased immunoreactivity for the limiting enzyme in glycogenolysis, pancreatic alpha‐amylase (AMY2A, also called amylase alpha 2A) [207]. In addition, ADRA exhibit increased immunoreactivity for insulin‐like growth factor I (IGF1) [208, 209] and its receptor (IGF1R) [210], as well as for cation‐independent mannose‐6‐phosphate receptor (IGFR2, which is the receptor for insulin‐like growth factor II) [211] and for insulin‐like growth factor‐binding protein 3 (IGFBP3) [205].
Intracellular trafficking
ADRA feature active intracellular trafficking, as assessed by an increased immunoreactivity for clathrin light chains A (CLTA) and B (CLTB) [212], which are critical for clathrin‐mediated endocytosis; protein Hook homolog 2 (HOOK2), which is a microtubule‐binding protein that participates in endosomal transport [213]; kinesin‐like protein KIF21B (KIF21B) [214], a motor protein that transports cargo along microtubules; carboxypeptidase E (CPE) [215], which functions as a sorting receptor for processing of pro‐peptides and secretion of the resulting peptides via the regulated secretory pathway; and secretogranin‐3 (SCG3) [215], which is also involved in the sorting of peptides within secretory granules in the regulated secretory pathway.
Blood–brain barrier integrity
Astrocytes are a key structural element of the blood–brain barrier, with their vascular endfeet wrapping capillaries as well as small arteries and veins. ADRA have increased levels of the vasoconstrictor endothelin‐1 (EDN1) [216, 217] and of the tight junction proteins claudin‐2 (CLDN2) and claudin‐11 (CLDN11), but not claudin‐5 (CLDN5) [218]. Moreover, ADRA surrounding leaky capillaries take up plasma proteins such as fibrinogen (e.g., FGA) [219] and immunoglobulins A (e.g., IGHA1), G (e.g., IGHG1) and M (e.g., IGHM) [220].
Calcium homeostasis
Increased immunoreactivity for cytosolic calcium (Ca2+)‐binding proteins, which buffer any excess of Ca2+, provides indirect evidence of Ca2+ dyshomeostasis in ADRA. Indeed, protein S100‐A6 (S100A6, also known as calcyclin) [221], calsenilin (KCNIP3) [222], calbindin (CALB1) [223] and calretinin (CALB2) [223] have all been shown to increase in ADRA by immunohistochemistry. The Ca2 +‐binding protein S100‐B (S100B) has been reported to be increased by some studies [38, 224, 225, 226, 227] but not others [148, 149].
Water/K+ homeostasis
Astrocytes regulate brain water and potassium (K+) homeostasis through specific membrane channels. ADRA show increased immunoreactivity for aquaporin‐1 (AQP1) [228, 229], whereas aquaporin‐4 (AQP4) levels have been reported to be unchanged [228, 230, 231], decreased [232] and elevated [233] by different authors. Moreover, ADRA exhibit increased immunoreactivity for K+ channel subunits, including intermediate conductance calcium‐activated potassium channel protein 4 (KCNN4, also known as KCa3.1) [234] and ATP‐sensitive inward rectifier potassium channel 11 (KCNJ11, also known as Kir6.2) [235].
Phagocytosis
ADRA have increased immunoreactivity for the opsonin complement C3 (C3) [152, 236] and for scavenger receptor class B member 1 (SCARB1) [237]. One study reported that the C3b/C4b receptor complement receptor type 1 (CR1)—which is a genome‐wide association study AD risk locus—is expressed by astrocytes but unchanged in AD vs control brains [238]. Another study reported reduced immunoreactivity for lactadherin (MGFE8) in ADRA, which has been involved in Aβ phagocytosis by astrocytes [239].
Aβ and tau
Numerous studies have reported Aβ immunoreactivity in ADRA, especially in subpial cortical astrocytes in close proximity with extracellular diffuse Aβ deposits [69, 101, 158, 240, 241, 242, 243, 244, 245, 246, 247, 248, 249, 250, 251, 252], supporting effective Aβ phagocytosis by astrocytes. Of note, Aβ within ADRA has been described as granules or dot‐like staining and is best observed with antibodies against its mid‐segment and C‐terminus (Aβ40 or Aβ42) due to prominent N‐terminal truncation [241, 242, 243, 244, 247, 250, 251, 252]. Aβ oligomeric species have also been shown in ADRA using a conformation‐specific antibody [251]. Remarkably, amyloid‐beta precursor protein (APP) [223, 253, 254, 255], beta‐secretase 1 (BACE1) [256, 257], the BACE1‐cleaved soluble APP ectodomain fragment (i.e., sAPPβ) [258] and the presenilins ‐1 (PSEN1) [35, 255, 259, 260, 261] and ‐2 (PSEN2) [255, 259] have all been reported to increase in ADRA as well, raising the possibility that ADRA contribute to the production and secretion of Aβ. Indeed, APP and PSEN1/2‐positive ADRA exhibit enhanced immunoreactivity for caveolin‐3 (CAV3), which has been implicated in APP cleavage by BACE1 [255], and ADRA also have increased immunoreactivity for the transcriptional repressor protein YY1 (YY1, also known as Yin Yang 1), which can activate BACE1 transcription [262]. In addition, ADRA are immunoreactive for the adaptor protein SHC‐transforming protein 1 (SHC1, also known as ShcA), which is known to interact with APP C‐terminal fragments and may mediate its intracellular signalling [263, 264]. Conversely, the Aβ‐degrading enzymes neprilysin (MME) and insulin‐degrading enzyme (IDE) have also been reported to increase in ADRA [265].
Similar to Aβ, multiple studies have reported the existence of the microtubule‐associated protein tau (MAPT, hereafter referred to as tau) in ADRA [200, 266, 267, 268, 269, 270, 271] with two morphologies: thorn‐shaped astrocytes and granular fuzzy astrocytes, collectively termed ageing‐related tau astrogliopathy (ARTAG). ARTAG tau species are misfolded based on immunoreactivity for the conformation‐specific mouse monoclonal antibodies Alz50 [200, 267, 268, 270] and MC1 [200] and hyperphosphorylated based on positive staining with antibodies against pSer202, pSer202/205 (AT8), pSer214, pSer396, pSer396/404 (PHF1), pSer422, pThr181 and pThr231 [200, 267, 268, 269, 271]. One study also reported nitration of tau in ADRA at Tyr18 [270].
Miscellaneous
Lastly, ADRA have been reported to display increased immunoreactivity for the oestrogen receptors alpha (ESR1) [272] and beta (ESR2) [273], peptidyl‐prolyl cis‐trans isomerase FKBP1A (FKBP1A, also known as 12‐kDa FK506‐binding protein or FKBP12) [274] and protein‐arginine deiminase type‐2 (PADI2) [275], which catalyses the citrullination of GFAP and VIM. On the other hand, cytosolic 10‐formyltetrahydrofolate dehydrogenase (ALDH1L1, better known as aldehyde dehydrogenase 1 family member L1) was described as unchanged in ADRA [146]. Finally, one immunohistochemical study found deficient DNA methylation and hydroxymethylation in ADRA [276], whereas other authors observed no change [277].
Bioinformatics analyses
Pathway enrichment analysis
To further evaluate the functional changes associated with astrocyte reaction in AD, we applied PEA on the 196 ADRA proteins against the curated GO and Reactome pathway databases available from MSigDB [16, 17, 18, 19]. The most salient enriched functional pathways included inflammatory cytokines and innate immune response (e.g., MAPK, toll‐like receptor and interleukin signalling, as well as inflammasomes), response to nitrosative and oxidative stress, lipoprotein metabolism, extracellular matrix organisation, protein degradation, signalling by nuclear receptors (including oestrogen receptor and ERBB4‐mediated signalling) and trophic factors (e.g., FGF) (Figure 2, Table S2).
FIGURE 2.
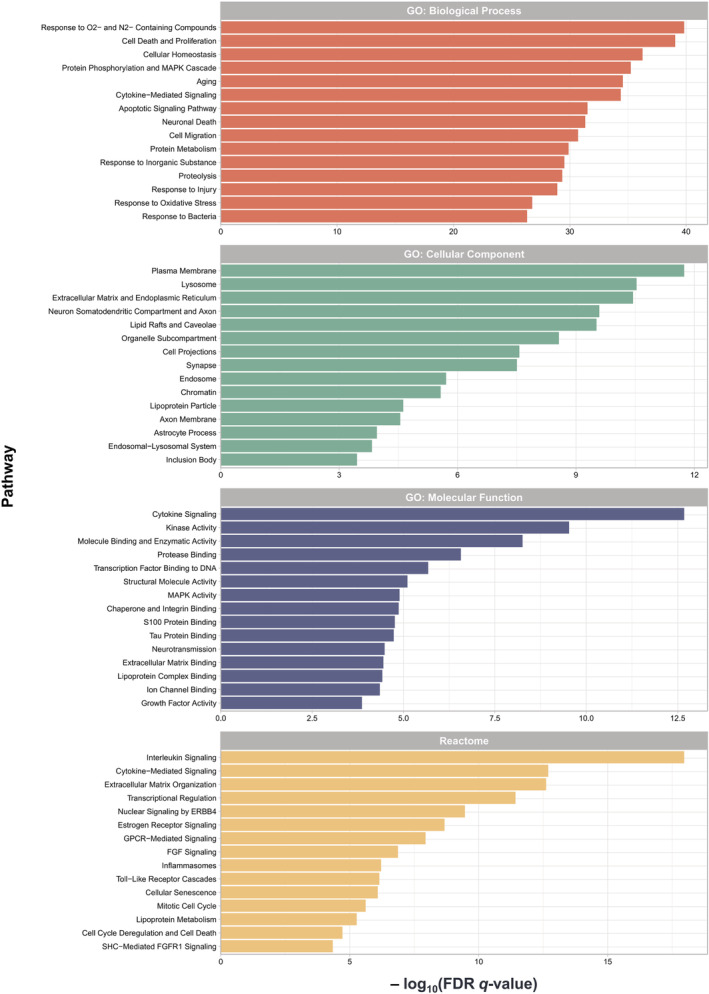
Pathway enrichment analysis (PEA) highlights neuroinflammation, oxidative stress, lipid metabolism and extracellular matrix in Alzheimer's disease reactive astrocytes (ADRA). PEA was performed on the 196 ADRA proteins against the following curated pathway databases: Gene Ontology (GO): Biological Process (BP); GO: Cellular Component (CC); GO: Molecular Function (MF); and Reactome. Bar graphs illustrate the statistical significance of enrichment (i.e., −log10[FDR q value]) for the top 15 pathways in each database
Protein‐protein interaction network analysis
PPI network analysis on the ADRA protein set (n = 196) using the STRING database [20] rendered a highly connected functional network with 193 nodes, 2331 edges (expected 836), an average node degree of 24.2, an average local clustering coefficient of 0.563 and a PPI enrichment p value of < 1.0e‐16 (Figure 3). Based on centrality scores, IL6, TP53, CASP3, TNF, MAPK3, MAPK8, MAPK1, MYC, PTGS2, IGF1, APP, IL1B, CCL2, FGF2 and ESR1 were the top 15 hub proteins. The remarkable interconnectivity between the individual markers of the ADRA protein set was visualised with a network plot (Figure 3A), a Circos plot (Figure S1) and a network heatmap (Figure S2). Similarly, a chord plot (Figure 3B) illustrated the high interconnectivity of these ADRA proteins at the functional pathway level, with inflammation as the most prominent functional alteration in AD reactive astrogliosis.
FIGURE 3.
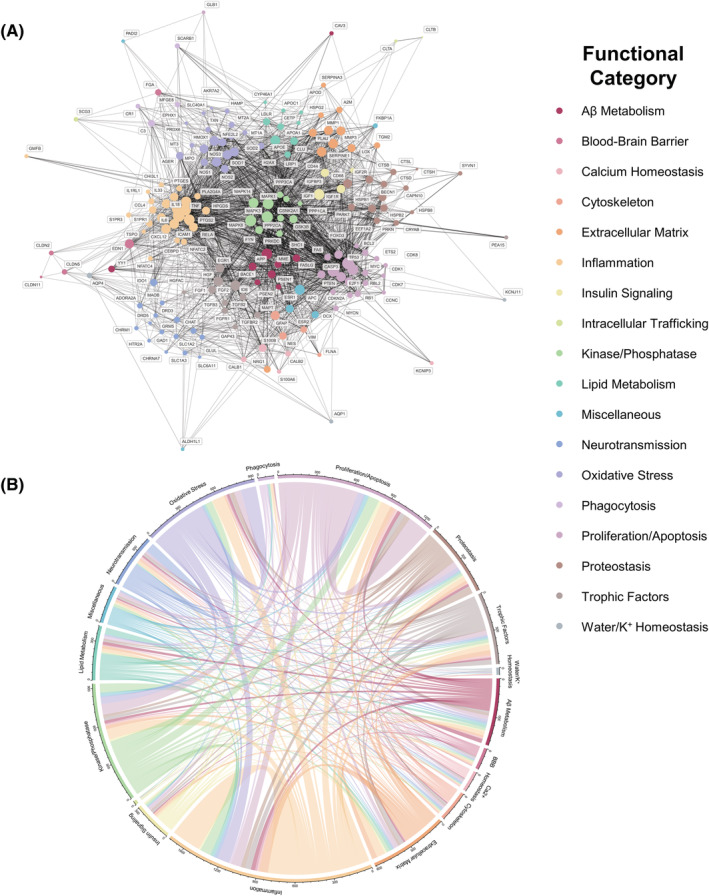
Protein–protein interaction (PPI) network analysis reveals complex functional changes in Alzheimer's disease reactive astrocytes (ADRA). (A) STRING PPI functional network analysis on the 196 ADRA proteins resulting from our systematic review demonstrates a highly connected network with IL6, TNF and MAPK 1, 3, and 8 as top hub proteins. (B) Chord diagram based on expert annotation of the 196 ADRA markers in one of 18 functional categories shows the high interconnectivity of the functional alterations of ADRA
Transcription factor enrichment analysis
To explore the main transcription factors driving the expression changes associated with ADRA, we performed transcription factor enrichment analysis (TFEA) against publicly available datasets of ChIP‐seq studies in a wide variety of cell lines [21, 22, 278] (see Methods). TFEA using two separate bioinformatics tools—namely, TFEA.ChIP and Enrichr—uncovered transcriptional repressor CTCF (CTCF, also known as CCCTC‐binding factor) and ESR1 as novel transcription factors potentially implicated in astrocyte reaction (Figure 4). The antioxidant defence orchestrator NFE2L2 reached statistical significance in Enrichr but not in TFEA.ChIP, whereas RELA (the catalytic subunit of NF‐kappa‐B) and signal transducer and activator of transcription 3 (STAT3) were mostly not significantly enriched (Table S3).
FIGURE 4.
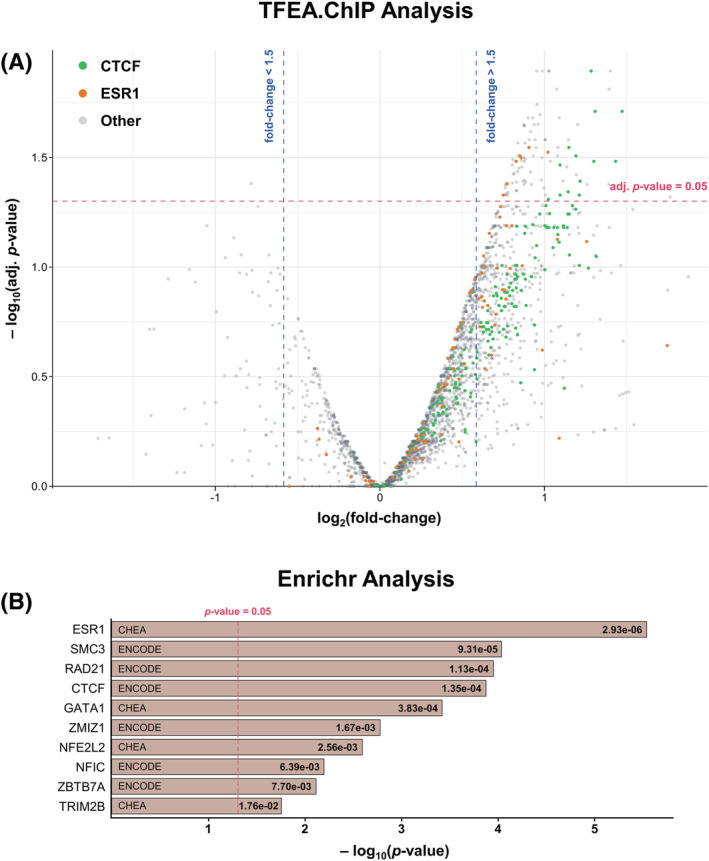
Transcription factor enrichment analysis (TFEA) reveals novel potential drivers of astrocyte reaction. (A) Volcano plot represents the effect size (i.e., log[fold‐change] on the x‐axis) against the statistical significance (i.e., −log[p value] on the y‐axis) of the TFEA.ChIP enrichment analysis for each of the ChIP‐seq experiments. The horizontal red line corresponds to p value = 0.05, whereas the vertical blue lines represent fold‐changes of +1.5 and −1.5. (B) Bar graph represents the statistical significance (nominal p values; adjusted p values are available in Table S3) of the Enrichr TFEA results. The vertical red line corresponds to p value = 0.05. Both methods showed CTCF and ESR1 as novel transcription factors potentially implicated in astrocyte reaction
Comparison with transcriptomic and proteomic studies
Lastly, we aimed to compare the ADRA markers with publicly available human ‐omics datasets, including a microarray study of laser‐capture microdissected GFAP+ astrocytes in the temporal neocortex [27], the astrocyte subset of a single‐nuclei RNA‐sequencing (RNA‐seq) study of the entorhinal cortex [28], the AMP‐AD Consortium bulk brain proteomic dataset (total n = 419) [29] and Cohort 1 of the AMP‐AD CSF proteomic study (total n = 297) [29] (see Supporting Information). We found a substantial representation of ADRA proteins in each of the four datasets examined. Enrichment analysis of the 196 ADRA markers against all genes or proteins differentially expressed between control and AD dementia in Simpson et al. [27], Grubman et al. [28] and Johnson et al. (bulk brain) [29] revealed that this overlap was consistently significant, with p values of 1.55e‐2, 3.45e‐12 and 2.25e‐13, respectively (calculated by Fisher's exact test). Cross‐validation with the two astrocyte‐specific transcriptomic studies listed above indicated that the expression of the genes encoding ADRA proteins correlates with disease progression as measured by Braak NFT stage [27] (Figures 5A and S3) and/or with AD vs control diagnosis [28] (Figures 5B and S4). Notably, cross‐validation with the AMP‐AD CSF proteomics dataset showed that the levels of many ADRA proteins in CSF correlate with the Aβ42/p‐tau ratio, which is a surrogate biomarker of the severity of brain AD neuropathological changes [29] (Figure 5C). Similarly, cross‐validation with the AMP‐AD bulk tissue proteomics dataset revealed that a considerable proportion of ADRA proteins change in parallel to the diagnostic group (control vs asymptomatic AD vs AD dementia) and/or Braak NFT stage [29] (Figures 5D and S5).
FIGURE 5.
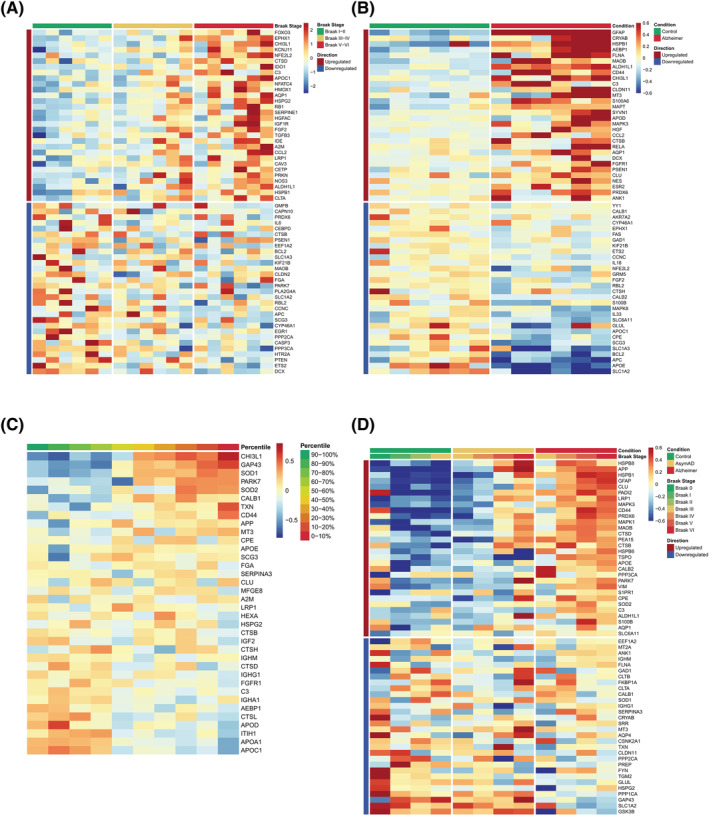
Comparison of Alzheimer's disease reactive astrocytes (ADRA) protein set with publicly available human ‐omics datasets. (A) Heatmap shows the z scores of gene expression of the top 30 upregulated and downregulated ADRA markers across all 18 subjects (n = 6 Braak I/II, n = 6 Braak III/IV and n = 6 Braak V/VI) included in a microarray study of laser‐capture microdissected GFAP+ astrocytes from the temporal neocortex (Simpson et al. [27]). (B) Heatmap shows the z scores of gene expression of the top 30 upregulated and downregulated ADRA markers across all 12 subjects (n = 6 control and n = 6 AD) included in a single nuclei RNA‐sequencing (RNA‐seq) study from the entorhinal cortex (Grubman et al. [28]). (C) Heatmap represents the z scores of protein expression of all available ADRA markers averaged by deciles of cerebrospinal fluid (CSF) Aβ42/p‐tau ratio across the n = 147 control and n = 150 AD subjects from Cohort 1 of the Accelerating Medicines Partnership‐Alzheimer's Disease (AMP‐AD) Consortium CSF proteomic study (Johnson et al. [29]). (D) Heatmap illustrates the z scores of protein expression of the top 30 upregulated and downregulated ADRA markers averaged by Braak neurofibrillary tangle (NFT) stage within each diagnostic group (n = 91 control, n = 98 asymptomatic AD and n = 230 AD dementia subjects) described in the AMP‐AD Consortium bulk brain proteomic dataset (Johnson et al. [29])
DISCUSSION
We demonstrate the feasibility and validity of a systematic review of post‐mortem human neuropathological studies to characterise the protein expression changes associated with astrocyte reaction in the AD brain. Some limitations of this approach should be acknowledged in advance. Systematic reviews are inherently affected by a risk of publication bias; in this case, increased immunoreactivity indicating protein upregulation is typically more obvious to the examiner (and likely more readily reported) than decreased immunoreactivity associated with protein downregulation; therefore, loss of normal astrocyte functions might be underreported. Moreover, the possibility of missing studies because they are published in non‐indexed journals, rarely cited and therefore less likely to be captured from reference lists, inappropriately indexed in databases or simply not encompassed by our search strategy should be considered. Other limitations are inherent to post‐mortem neuropathological studies: antibody specificity was not always tested; the effects of ante‐mortem agonal period and post‐mortem interval on marker immunoreactivity were usually not investigated; most studies compared healthy control brains with advanced AD brains, hence, the described changes in immunoreactivity may only reflect end‐stage status and may differ at earlier stages of disease; and qualitative and semi‐quantitative reports could be affected by examiner subjectivity. Nevertheless, some of these biases are offset by the significant overlap demonstrated between the ADRA protein set and various human ‐omics datasets, which have different inherent methodological biases. Therefore, our thorough bioinformatics analyses on the ADRA protein set provide important clues about the physiological changes central to astrocyte reaction in AD.
PEA on the 196 ADRA proteins revealed that astrocyte reaction is a complex process involving multiple astrocyte functions beyond cytoskeletal remodelling (viz., beyond the classic upregulation of GFAP). These include neuroinflammation (e.g., the cytokines IL1B, IL6 and TNF and the stress kinases MAPK 1, 3 and 8), oxidative stress (e.g., the metallothioneins MT1A, MT2A and MT3; the nitric oxide synthases NOS1, NOS2 and NOS3; and the superoxidase dismutases SOD1 and SOD2), lipid metabolism (e.g., APOE, CLU and LRP1), extracellular matrix (e.g., CD44, SERPINA3, the matrix metalloproteinases MMP1 and MMP3 and the tissue transglutaminases TGM1 and TGM2) and proteostasis (e.g., CRYAB, HSPB1 and the cathepsin protease family).
PPI network analysis of the ADRA protein set revealed an extraordinary interconnectivity across many of these functions, suggesting that one or more parallel pathogenic cascades occur within the reactive astrocyte (i.e., domino effect). This analysis identified IL6 as the protein with the highest connectivity by eigenvalue centrality score. Importantly, mouse models genetically engineered to overexpress IL6 in astrocytes (i.e., Gfap‐Il6 transgenic mice) exhibit a neurodegenerative phenotype with loss of cortical synapses and cerebellar atrophy [279], indicating that IL6 secretion by reactive astrocytes could be neurotoxic. Although our TFEA on the 196 ADRA genes did not detect STAT3, IL6 signalling is known to activate the JAK/STAT pathway, which is thought to be key for astrocyte reaction in transgenic mouse models of β‐amyloidosis [280, 281]. IL6 signalling could be blocked by repurposing IL6 inhibitors (e.g., siltuximab), IL6 receptor inhibitors (e.g., sarilumab and tocilizumab) and JAK inhibitors (e.g., bariticinib, tofacitinib and ruxolitinib). Interestingly, a recent machine learning study to identify candidates for drug repurposing in AD concluded that Food and Drug Administration‐approved JAK inhibitors could be beneficial in AD [282]. Taken together, these observations strongly support the design of clinical trials aimed at inhibiting the IL6/JAK/STAT signalling axis to attenuate astrocyte reaction in AD.
Intriguingly, CTCF and ESR1 emerged as potential novel transcription factors involved in astrocyte reaction according to two separate TFEA tools. While these methods use curated databases of ChIP‐seq experiments and outperform methods based on ascertaining binding motifs within the DNA sequence, most ChIP‐seq experiments correspond to tumour cell lines of various organs; hence, any extrapolation to astrocytes in the human brain should be taken with caution. However, CTCF was also enriched in the ChIP‐seq experiments conducted on primary astrocytes and astrocytoma cell lines in our TFEA.ChIP analysis (Table S3). CTCF regulates 3‐D genome architecture and facilitates enhancer–promoter interactions across multiple cell types in the brain [283]; mutations in this transcription factor cause a neurodevelopmental disorder with intellectual disability, possibly by deregulating the expression of multiple genes [284]. Conditional deletion of CTCF from excitatory glutamatergic neurons in mice impairs synaptic plasticity, learning and memory and causes both neurodegeneration and reactive gliosis [285, 286, 287]. Further, upon TGF‐beta stimulation of astrocytes in vitro, CTCF enhances APP expression [288]; of note, APP ranked 11th in our PPI network by centrality score and is significantly upregulated in AD vs controls in both CSF and bulk brain proteomic studies [29]. The oestrogen receptors ESR1 and ESR2 are present in the plasma membrane and are trafficked to the nucleus, where they act as transcription factors regulating gene expression. Consistent with the results of our PEA and TFEA, ESR1 ranked 15th in our PPI network by centrality score. Further, ESR1 and ESR2 have been reported as upregulated in reactive astrocytes in AD [272, 273], in male primates after transection of the fimbria fornix [289] and in male rats after kainic acid‐induced status epilepticus [290]. Hence, together with our findings, the available literature suggests that CTCF and ESR1 warrant further investigation as regulators of AD astrocyte reaction.
Finally, a significant subset of the ADRA protein set was also altered in AD vs control individuals in several reference transcriptomics and proteomics studies. For example, in their microarray study of laser‐capture microdissected GFAP‐immunoreactive astrocytes, Simpson et al. highlighted actin cytoskeleton, proliferation/apoptosis and proteostasis, as well as stress and immune responses as the main dysregulated astrocyte functions, and implicated the MAPK signalling pathway [27]. The pro‐inflammatory astrocyte phenotype has also been shown in aged wild‐type mice and APP/PS1 AD transgenic mice [2, 291, 292]. These similarities are remarkable given the large technical and biological discrepancies frequently observed between these methods [293]. Moreover, these findings have two important implications. First, astrocyte‐specific transcriptomic studies and bulk brain or CSF proteomics studies could be validated by cyclic multiplex immunohistochemistry methods in post‐mortem brain sections [294]. Second, our ADRA protein atlas may inform ongoing efforts to discover serum/plasma, CSF and PET imaging biomarkers of reactive astrocytes, which may assist the early diagnosis and prognostication of AD. For example, many of the overlapping proteins between the ADRA protein set and a public CSF proteomic dataset strongly correlated with the levels of AD CSF biomarkers, which are used for the clinical diagnosis of AD and as a proxy for the severity of AD neuropathological changes (Aβ plaques and NFTs) in the brain.
In summary, our systematic review of the neuropathological literature reveals the complexity of AD‐associated astrocyte reaction, which has been increasingly recognised [1, 3, 4, 5, 6]. Besides biomarker discovery, these findings could inform future astrocyte‐centric single‐cell and single‐nuclei RNA‐seq studies as well as spatial transcriptomic and proteomic investigations. To this end, we have shared these results as a web‐based resource available at www.astrocyteatlas.org.
CONFLICT OF INTEREST
The authors declare no conflicts of interest related to the content of this article.
AUTHOR CONTRIBUTIONS
Lucía Viejo performed the data curation, investigation and writing—original draft; Ayush Noori performed the formal analysis, software, visualisation and writing—original draft; Emily Merrill provided resources; Sudeshna Das performed the funding acquisition, supervision and writing—review and editing; Bradley T. Hyman performed the funding acquisition, supervision and writing—review and editing; Alberto Serrano‐Pozo performed the conceptualisation, data curation, funding acquisition, investigation, methodology, supervision and writing—original draft.
ETHICS STATEMENT
This research did not involve interaction with living human subjects or animal models. Data in this article were extracted from published neuropathological studies, which only included deidentified post‐mortem brain specimens.
CODE AVAILABILITY
The source code for all bioinformatics analyses is available on GitHub at https://github.com/serrano-pozo-lab/astrocyte-review (DOI: 10.5281/zenodo.5140749).
Supporting information
Figure S1. Circos plot depicting interconnectivity within the Alzheimer's disease reactive astrocyte (ADRA) protein set. The interconnectivity between the 196 constituents of the ADRA protein set and across functional categories is demonstrated via a Circos plot, where each node is a protein marker and each colour represents a functional group.
Figure S2. Heatmap of protein‐protein interaction (PPI) network. Heatmap of the PPI network of the 196 ADRA proteins grouped by expert‐annotated functional category. Note that markers belonging to the same functional group are highly connected, but substantial interactions are also observed between markers from different functional categories (e.g., oxidative stress and inflammation, proliferation/apoptosis and kinase/phosphatase).
Figure S3. Full heatmap of the overlap between the ADRA protein set and a microarray dataset of laser‐capture microdissected GFAP+ astrocytes. Heatmap shows the z‐scores of gene expression of all available ADRA markers across the 18 subjects (n = 6 Braak I/II, n = 6 Braak III/IV, and n = 6 Braak V/VI) included in a microarray study of laser‐capture microdissected GFAP+ astrocytes from the temporal neocortex (Simpson et al., 2011).
Figure S4. Full heatmap of the overlap between the ADRA protein set and a human astrocyte single‐nuclei RNA‐seq dataset. Heatmap shows the z‐scores of gene expression of all available ADRA markers across all 12 subjects (n = 6 control and n = 6 AD) included in a single‐nuclei RNA‐seq study from the entorhinal cortex (Grubman et al., 2019).
Figure S5. Full heatmap of the overlap between the ADRA protein set and a human bulk brain proteomic dataset. Heatmap illustrates the z‐scores of protein expression of all available ADRA markers averaged by Braak NFT stage within each diagnostic group (n = 91 control, n = 98 asymptomatic AD, and n = 230 AD dementia subjects) described in the AMP‐AD Consortium bulk brain proteomic dataset (Johnson et al., 2020).
Table S1. Studies and markers included in the systematic review. Detailed information collected from studies included in the systematic review. Studies are grouped by the functional categories of their respective markers. Colour code corresponds to Figures 3 and S1.
Table S2. Detailed pathway enrichment analysis results. Output of the pathway enrichment analysis against the curated pathway databases (i.e., Gene Ontology (GO): Biological Process (BP), GO: Cellular Component (CC), GO: Molecular Function (MF), and Reactome). Pathways are grouped by hierarchical clustering based on the Jaccard similarity coefficient and expert annotated.
Table S3. Detailed transcription factor enrichment analysis results. Output of the TFEA.ChIP and Enrichr transcription factor enrichment analysis.
ACKNOWLEDGEMENTS
This work was supported by the Alzheimer's Association (AACF‐17‐524184 to AS‐P), the National Institute on Aging (K08AG064039 to AS‐P and P30AG062421 to BTH and SD), the JPB Foundation (to BTH), a MassLife Sciences MassCATS award (to BTH and SD), and the Instituto de Salud Carlos III of the Spanish Ministry of Science and Innovation (FI17/00079 and MV19/00033 to LV).
Viejo L, Noori A, Merrill E, Das S, Hyman BT, Serrano‐Pozo A. Systematic review of human post‐mortem immunohistochemical studies and bioinformatics analyses unveil the complexity of astrocyte reaction in Alzheimer's disease. Neuropathol Appl Neurobiol. 2022;48(1):e12753. 10.1111/nan.12753
Lucía Viejo and Ayush Noori contributed equally to this work.
Funding information Alzheimer's Association; JPB Foundation; MassLife Sciences MassCATS; National Institute on Aging; Instituto de Salud Carlos III (Spanish Ministry of Science and Innovation)
Contributor Information
Lucía Viejo, Email: lucia.viejo@estudiante.uam.es.
Ayush Noori, Email: anoori1@mgh.harvard.edu.
Sudeshna Das, Email: sdas5@mgh.harvard.edu.
Bradley T. Hyman, Email: bhyman@mgh.harvard.edu
Alberto Serrano‐Pozo, Email: aserrano1@mgh.harvard.edu.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. The source code for all bioinformatics analyses is available on GitHub at https://github.com/serrano-pozo-lab/astrocyte-review (DOI: 10.5281/zenodo.5140749).
REFERENCES
- 1. Escartin C, Galea E, Lakatos A, et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci. 2021;15:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Das S, Li Z, Noori A, Hyman BT, Serrano‐Pozo A. Meta‐analysis of mouse transcriptomic studies supports a context‐dependent astrocyte reaction in acute CNS injury versus neurodegeneration. J Neuroinflammation. 2020;17(1):227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Perez‐Nievas BG, Serrano‐Pozo A. Deciphering the astrocyte reaction in Alzheimer's disease. Front Aging Neurosci. 2018;10:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garwood CJ, Ratcliffe LE, Simpson JE, Heath PR, Ince PG, Wharton SB. Review: astrocytes in Alzheimer's disease and other age‐associated dementias: a supporting player with a central role. Neuropathol Appl Neurobiol. 2017;43(4):281‐298. [DOI] [PubMed] [Google Scholar]
- 5. Ferrer I. Diversity of astroglial responses across human neurodegenerative disorders and brain aging. Brain Pathol Zurich Switz. 2017;27(5):645‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Verkerke M, Hol EM, Middeldorp J. Physiological and pathological ageing of astrocytes in the human brain. Neurochem Res. 2021. Feb 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bayraktar OA, Bartels T, Holmqvist S, et al. Astrocyte layers in the mammalian cerebral cortex revealed by a single‐cell in situ transcriptomic map. Nat Neurosci. 2020;23(4):500‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Serrano‐Pozo A, Mielke ML, Gómez‐Isla T, et al. Reactive glia not only associates with plaques but also parallels tangles in Alzheimer's disease. Am J Pathol. 2011;179(3):1373‐1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Serrano‐Pozo A, Muzikansky A, Gómez‐Isla T, et al. Differential relationships of reactive astrocytes and microglia to fibrillar amyloid deposits in Alzheimer disease. J Neuropathol Exp Neurol. 2013;72(6):462‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Serrano‐Pozo A, Betensky RA, Frosch MP, Hyman BT. Plaque‐associated local toxicity increases over the clinical course of Alzheimer disease. Am J Pathol. 2016;186(2):375‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prokop S, Miller KR, Labra SR, et al. Impact of TREM2 risk variants on brain region‐specific immune activation and plaque microenvironment in Alzheimer's disease patient brain samples. Acta Neuropathol (Berl). 2019;138(4):613‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen W‐T, Lu A, Craessaerts K, et al. Spatial transcriptomics and in situ sequencing to study Alzheimer's disease. Cell. 2020;182(4):976‐991.e19. [DOI] [PubMed] [Google Scholar]
- 13. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. The UniProt Consortium . UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 2021. Jan 8;49(D1):D480‐D409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tweedie S, Braschi B, Gray K, et al. Genenames.org: the HGNC and VGNC resources in 2021. Nucleic Acids Res. 2021;49(D1):D939‐D946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ashburner M, Ball CA, Blake JA, et al. Gene Ontology: tool for the unification of biology. Nat Genet. 2000;25(1):25‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jassal B, Matthews L, Viteri G, et al. The Reactome pathway knowledgebase. Nucleic Acids Res. 2020;48(D1):D498‐D503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liberzon A, Subramanian A, Pinchback R, Thorvaldsdóttir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011. Jun 15;27(12):1739‐1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. The Gene Ontology Consortium . The gene ontology resource: 20 years and still GOing strong. Nucleic Acids Res. 2019;47:D330‐D338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein‐protein association networks with increased coverage, supporting functional discovery in genome‐wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607‐D613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Puente‐Santamaria L, Wasserman WW, del Peso L. TFEA.ChIP: a tool kit for transcription factor binding site enrichment analysis capitalizing on ChIP‐seq datasets. Bioinforma Oxf Engl. 2019;35(24):5339‐5340. [DOI] [PubMed] [Google Scholar]
- 22. Kuleshov MV, Jones MR, Rouillard AD, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44(W1):W90‐W97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chèneby J, Gheorghe M, Artufel M, Mathelier A, Ballester B. ReMap 2018: an updated atlas of regulatory regions from an integrative analysis of DNA‐binding ChIP‐seq experiments. Nucleic Acids Res. 2018;46(D1):D267‐D275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fishilevich S, Nudel R, Rappaport N, et al. GeneHancer: genome‐wide integration of enhancers and target genes in GeneCards. Database [Internet]. 2017. Jan 1 [cited 2021 Feb 28];2017(bax028). Available from: 10.1093/database/bax028 [DOI] [PMC free article] [PubMed]
- 25. Dunham I, Kundaje A, Aldred SF, et al. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lachmann A, Xu H, Krishnan J, Berger SI, Mazloom AR, Ma'ayan A. ChEA: transcription factor regulation inferred from integrating genome‐wide ChIP‐X experiments. Bioinforma Oxf Engl. 2010;26(19):2438‐2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simpson JE, Ince PG, Shaw PJ, et al. Microarray analysis of the astrocyte transcriptome in the aging brain: relationship to Alzheimer's pathology and APOE genotype. Neurobiol Aging. 2011;32(10):1795‐1807. [DOI] [PubMed] [Google Scholar]
- 28. Grubman A, Chew G, Ouyang JF, et al. A single‐cell atlas of entorhinal cortex from individuals with Alzheimer's disease reveals cell‐type‐specific gene expression regulation. Nat Neurosci. 2019;22(12):2087‐2097. [DOI] [PubMed] [Google Scholar]
- 29. Johnson ECB, Dammer EB, Duong DM, et al. Large‐scale proteomic analysis of Alzheimer's disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation. Nat Med. 2020;26(5):769‐780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Middeldorp J, van den Berge SA, Aronica E, Speijer D, Hol EM. Specific human astrocyte subtype revealed by affinity purified GFAP antibody; unpurified serum cross‐reacts with neurofilament‐L in Alzheimer. PloS One. 2009;4(11):e7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kamphuis W, Middeldorp J, Kooijman L, et al. Glial fibrillary acidic protein isoform expression in plaque related astrogliosis in Alzheimer's disease. Neurobiol Aging. 2014;35(3):492‐510. [DOI] [PubMed] [Google Scholar]
- 32. Mouser PE, Head E, Ha K‐H, Rohn TT. Caspase‐mediated cleavage of glial fibrillary acidic protein within degenerating astrocytes of the Alzheimer's disease brain. Am J Pathol. 2006;168(3):936‐946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Colombo JA, Quinn B, Puissant V. Disruption of astroglial interlaminar processes in Alzheimer's disease. Brain Res Bull. 2002;58(2):235‐242. [DOI] [PubMed] [Google Scholar]
- 34. Pugliese M, Rodríguez MJ, Gimeno‐Bayon J, et al. Alzheimer's disease modifies progenitor cell expression of monoamine oxidase B in the subventricular zone. J Neurosci Res. 2010;88(12):2588‐2597. [DOI] [PubMed] [Google Scholar]
- 35. Zhang W, Han SW, McKeel DW, Goate A, Wu JY. Interaction of presenilins with the filamin family of actin‐binding proteins. J Neurosci off J Soc Neurosci. 1998;18(3):914‐922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bosman GJ, Renkawek K, van Workum FP, Bartholomeus IG, de Grip WJ. Involvement of neuronal anion exchange proteins in cell death in Alzheimer's disease. Gerontology. 1997;43(1–2):67‐78. [DOI] [PubMed] [Google Scholar]
- 37. Bouvier DS, Jones EV, Quesseveur G, et al. High resolution dissection of reactive glial nets in Alzheimer's disease. Sci Rep. 2016;6:24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Griffin WS, Stanley LC, Ling C, et al. Brain interleukin 1 and S‐100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S a. 1989;86(19):7611‐7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thal DR, Schober R, Birkenmeier G. The subunits of alpha2‐macroglobulin receptor/low density lipoprotein receptor‐related protein, native and transformed alpha2‐macroglobulin and interleukin 6 in Alzheimer's disease. Brain Res. 1997;777(1–2):223‐227. [DOI] [PubMed] [Google Scholar]
- 40. Sokolova A, Hill MD, Rahimi F, Warden LA, Halliday GM, Shepherd CE. Monocyte chemoattractant protein‐1 plays a dominant role in the chronic inflammation observed in Alzheimer's disease. Brain Pathol Zurich Switz. 2009;19(3):392‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ojala J, Alafuzoff I, Herukka S‐K, van Groen T, Tanila H, Pirttilä T. Expression of interleukin‐18 is increased in the brains of Alzheimer's disease patients. Neurobiol Aging. 2009;30(2):198‐209. [DOI] [PubMed] [Google Scholar]
- 42. Xiong Z, Thangavel R, Kempuraj D, Yang E, Zaheer S, Zaheer A. Alzheimer's disease: evidence for the expression of interleukin‐33 and its receptor ST2 in the brain. J Alzheimers Dis JAD. 2014;40(2):297‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Katsuse O, Iseki E, Kosaka K. Immunohistochemical study of the expression of cytokines and nitric oxide synthases in brains of patients with dementia with Lewy bodies. Neuropathol off J Jpn Soc Neuropathol. 2003;23(1):9‐15. [DOI] [PubMed] [Google Scholar]
- 44. Xia MQ, Qin SX, Wu LJ, Mackay CR, Hyman BT. Immunohistochemical study of the beta‐chemokine receptors CCR3 and CCR5 and their ligands in normal and Alzheimer's disease brains. Am J Pathol. 1998;153(1):31‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xia MQ, Bacskai BJ, Knowles RB, Qin SX, Hyman BT. Expression of the chemokine receptor CXCR3 on neurons and the elevated expression of its ligand IP‐10 in reactive astrocytes: in vitro ERK1/2 activation and role in Alzheimer's disease. J Neuroimmunol. 2000;108(1–2):227‐235. [DOI] [PubMed] [Google Scholar]
- 46. Rostasy K, Egles C, Chauhan A, et al. SDF‐1alpha is expressed in astrocytes and neurons in the AIDS dementia complex: an in vivo and in vitro study. J Neuropathol Exp Neurol. 2003;62(6):617‐626. [DOI] [PubMed] [Google Scholar]
- 47. Akiyama H, Kawamata T, Yamada T, Tooyama I, Ishii T, McGeer PL. Expression of intercellular adhesion molecule (ICAM)‐1 by a subset of astrocytes in Alzheimer disease and some other degenerative neurological disorders. Acta Neuropathol (Berl). 1993;85(6):628‐634. [DOI] [PubMed] [Google Scholar]
- 48. Yermakova AV, O'Banion MK. Downregulation of neuronal cyclooxygenase‐2 expression in end stage Alzheimer's disease. Neurobiol Aging. 2001;22(6):823‐836. [DOI] [PubMed] [Google Scholar]
- 49. Stephenson DT, Lemere CA, Selkoe DJ, Clemens JA. Cytosolic phospholipase A2 (cPLA2) immunoreactivity is elevated in Alzheimer's disease brain. Neurobiol Dis. 1996;3(1):51‐63. [DOI] [PubMed] [Google Scholar]
- 50. Mohri I, Kadoyama K, Kanekiyo T, et al. Hematopoietic prostaglandin D synthase and DP1 receptor are selectively upregulated in microglia and astrocytes within senile plaques from human patients and in a mouse model of Alzheimer disease. J Neuropathol Exp Neurol. 2007;66(6):469‐480. [DOI] [PubMed] [Google Scholar]
- 51. Chaudhry UA, Zhuang H, Crain BJ, Doré S. Elevated microsomal prostaglandin‐E synthase‐1 in Alzheimer's disease. Alzheimers Dement J Alzheimers Assoc. 2008;4(1):6‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. de Wit NM, Snkhchyan H, den Hoedt S, et al. Altered sphingolipid balance in capillary cerebral amyloid angiopathy. J Alzheimers Dis JAD. 2017;60(3):795‐807. [DOI] [PubMed] [Google Scholar]
- 53. Brana C, Frossard MJ, Pescini Gobert R, Martinier N, Boschert U, Seabrook TJ. Immunohistochemical detection of sphingosine‐1‐phosphate receptor 1 and 5 in human multiple sclerosis lesions. Neuropathol Appl Neurobiol. 2014;40(5):564‐578. [DOI] [PubMed] [Google Scholar]
- 54. Abdul HM, Sama MA, Furman JL, et al. Cognitive decline in Alzheimer's disease is associated with selective changes in calcineurin/NFAT signaling. J Neurosci off J Soc Neurosci. 2009;29(41):12957‐12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Terai K, Matsuo A, McGeer PL. Enhancement of immunoreactivity for NF‐kappa B in the hippocampal formation and cerebral cortex of Alzheimer's disease. Brain Res. 1996;735(1):159‐168. [DOI] [PubMed] [Google Scholar]
- 56. Lim D, Iyer A, Ronco V, et al. Amyloid beta deregulates astroglial mGluR5‐mediated calcium signaling via calcineurin and Nf‐kB. Glia. 2013;61(7):1134‐1145. [DOI] [PubMed] [Google Scholar]
- 57. Shijo M, Honda H, Suzuki SO, et al. Association of adipocyte enhancer‐binding protein 1 with Alzheimer's disease pathology in human hippocampi. Brain Pathol Zurich Switz. 2018;28(1):58‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li R, Strohmeyer R, Liang Z, Lue L‐F, Rogers J. CCAAT/enhancer binding protein delta (C/EBPdelta) expression and elevation in Alzheimer's disease. Neurobiol Aging. 2004;25(8):991‐999. [DOI] [PubMed] [Google Scholar]
- 59. Thangavel R, Stolmeier D, Yang X, Anantharam P, Zaheer A. Expression of glia maturation factor in neuropathological lesions of Alzheimer's disease. Neuropathol Appl Neurobiol. 2012;38(6):572‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Stolmeier D, Thangavel R, Anantharam P, Khan MM, Kempuraj D, Zaheer A. Glia maturation factor expression in hippocampus of human Alzheimer's disease. Neurochem Res. 2013;38(8):1580‐1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Craig‐Schapiro R, Perrin RJ, Roe CM, et al. YKL‐40: a novel prognostic fluid biomarker for preclinical Alzheimer's disease. Biol Psychiatry. 2010;68(10):903‐912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Querol‐Vilaseca M, Colom‐Cadena M, Pegueroles J, et al. YKL‐40 (Chitinase 3‐like I) is expressed in a subset of astrocytes in Alzheimer's disease and other tauopathies. J Neuroinflammation. 2017;14(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Llorens F, Thüne K, Tahir W, et al. YKL‐40 in the brain and cerebrospinal fluid of neurodegenerative dementias. Mol Neurodegener. 2017;12(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Moreno‐Rodriguez M, Perez SE, Nadeem M, Malek‐Ahmadi M, Mufson EJ. Frontal cortex chitinase and pentraxin neuroinflammatory alterations during the progression of Alzheimer's disease. J Neuroinflammation. 2020;17(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cosenza‐Nashat M, Zhao M‐L, Suh H‐S, et al. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol Appl Neurobiol. 2009;35(3):306‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gui Y, Marks JD, Das S, Hyman BT, Serrano‐Pozo A. Characterization of the 18 kDa translocator protein (TSPO) expression in post‐mortem normal and Alzheimer's disease brains. Brain Pathol Zurich Switz. 2020;30(1):151‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Takeda A, Yasuda T, Miyata T, et al. Advanced glycation end products co‐localized with astrocytes and microglial cells in Alzheimer's disease brain. Acta Neuropathol (Berl). 1998;95(6):555‐558. [DOI] [PubMed] [Google Scholar]
- 68. Takeda A, Wakai M, Niwa H, et al. Neuronal and glial advanced glycation end product [Nepsilon‐(carboxymethyl)lysine] in Alzheimer's disease brains. Acta Neuropathol (Berl). 2001;101(1):27‐35. [DOI] [PubMed] [Google Scholar]
- 69. Sasaki N, Toki S, Chowei H, et al. Immunohistochemical distribution of the receptor for advanced glycation end products in neurons and astrocytes in Alzheimer's disease. Brain Res. 2001;888(2):256‐262. [DOI] [PubMed] [Google Scholar]
- 70. Wang MY, Ross‐Cisneros FN, Aggarwal D, Liang C‐Y, Sadun AA. Receptor for advanced glycation end products is upregulated in optic neuropathy of Alzheimer's disease. Acta Neuropathol (Berl). 2009;118(3):381‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Myung N‐H, Zhu X, Kruman II, et al. Evidence of DNA damage in Alzheimer disease: phosphorylation of histone H2AX in astrocytes. Age Dordr Neth. 2008. Dec;30(4):209‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Simpson JE, Ince PG, Haynes LJ, et al. Population variation in oxidative stress and astrocyte DNA damage in relation to Alzheimer‐type pathology in the ageing brain. Neuropathol Appl Neurobiol. 2010;36(1):25‐40. [DOI] [PubMed] [Google Scholar]
- 73. Waller R, Murphy M, Garwood CJ, et al. Metallothionein‐I/II expression associates with the astrocyte DNA damage response and not Alzheimer‐type pathology in the aging brain. Glia. 2018;66(11):2316‐2323. [DOI] [PubMed] [Google Scholar]
- 74. Kawaguchi‐Niida M, Shibata N, Morikawa S, et al. Crotonaldehyde accumulates in glial cells of Alzheimer's disease brain. Acta Neuropathol (Berl). 2006;111(5):422‐429. [DOI] [PubMed] [Google Scholar]
- 75. Maki RA, Tyurin VA, Lyon RC, et al. Aberrant expression of myeloperoxidase in astrocytes promotes phospholipid oxidation and memory deficits in a mouse model of Alzheimer disease. J Biol Chem. 2009;284(5):3158‐3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Simic G, Lucassen PJ, Krsnik Z, et al. nNOS expression in reactive astrocytes correlates with increased cell death related DNA damage in the hippocampus and entorhinal cortex in Alzheimer's disease. Exp Neurol. 2000;165(1):12‐26. [DOI] [PubMed] [Google Scholar]
- 77. Wallace MN, Geddes JG, Farquhar DA, Masson MR. Nitric oxide synthase in reactive astrocytes adjacent to beta‐amyloid plaques. Exp Neurol. 1997;144(2):266‐272. [DOI] [PubMed] [Google Scholar]
- 78. de la Monte SM, Bloch KD. Aberrant expression of the constitutive endothelial nitric oxide synthase gene in Alzheimer disease. Mol Chem Neuropathol. 1997;30(1–2):139‐159. [DOI] [PubMed] [Google Scholar]
- 79. Ramsey CP, Glass CA, Montgomery MB, et al. Expression of Nrf2 in neurodegenerative diseases. J Neuropathol Exp Neurol. 2007;66(1):75‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Raha AA, Vaishnav RA, Friedland RP, Bomford A, Raha‐Chowdhury R. The systemic iron‐regulatory proteins hepcidin and ferroportin are reduced in the brain in Alzheimer's disease. Acta Neuropathol Commun. 2013;1:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zambenedetti P, Giordano R, Zatta P. Metallothioneins are highly expressed in astrocytes and microcapillaries in Alzheimer's disease. J Chem Neuroanat. 1998;15(1):21‐26. [DOI] [PubMed] [Google Scholar]
- 82. Adlard PA, West AK, Vickers JC. Increased density of metallothionein I/II‐immunopositive cortical glial cells in the early stages of Alzheimer's disease. Neurobiol Dis. 1998;5(5):349‐356. [DOI] [PubMed] [Google Scholar]
- 83. Maslinska D, Laure‐Kamionowska M, Maslinski KT, Gujski M, Maslinski S. Distribution of tryptase‐containing mast cells and metallothionein reactive astrocytes in human brains with amyloid deposits. Inflamm Res Off J Eur Histamine Res Soc Al. 2007;56(Suppl 1):S17‐S18. [DOI] [PubMed] [Google Scholar]
- 84. Uchida Y, Takio K, Titani K, Ihara Y, Tomonaga M. The growth inhibitory factor that is deficient in the Alzheimer's disease brain is a 68 amino acid metallothionein‐like protein. Neuron. 1991;7(2):337‐347. [DOI] [PubMed] [Google Scholar]
- 85. Arai Y, Uchida Y, Takashima S. Developmental immunohistochemistry of growth inhibitory factor in normal brains and brains of patients with Down syndrome. Pediatr Neurol. 1997;17(2):134‐138. [DOI] [PubMed] [Google Scholar]
- 86. Pappolla MA, Omar RA, Kim KS, Robakis NK. Immunohistochemical evidence of oxidative [corrected] stress in Alzheimer's disease. Am J Pathol. 1992;140(3):621‐628. [PMC free article] [PubMed] [Google Scholar]
- 87. Furuta A, Price DL, Pardo CA, et al. Localization of superoxide dismutases in Alzheimer's disease and Down's syndrome neocortex and hippocampus. Am J Pathol. 1995;146(2):357‐367. [PMC free article] [PubMed] [Google Scholar]
- 88. Schipper HM, Cissé S, Stopa EG. Expression of heme oxygenase‐1 in the senescent and Alzheimer‐diseased brain. Ann Neurol. 1995;37(6):758‐768. [DOI] [PubMed] [Google Scholar]
- 89. Asahina M, Yamada T, Yoshiyama Y, Yodoi J. Expression of adult T cell leukemia‐derived factor in human brain and peripheral nerve tissues. Dement Geriatr Cogn Disord. 1998;9(4):181‐185. [DOI] [PubMed] [Google Scholar]
- 90. Power JHT, Asad S, Chataway TK, et al. Peroxiredoxin 6 in human brain: molecular forms, cellular distribution and association with Alzheimer's disease pathology. Acta Neuropathol (Berl). 2008;115(6):611‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Liu M, Sun A, Shin E‐J, et al. Expression of microsomal epoxide hydrolase is elevated in Alzheimer's hippocampus and induced by exogenous beta‐amyloid and trimethyl‐tin. Eur J Neurosci. 2006;23(8):2027‐2034. [DOI] [PubMed] [Google Scholar]
- 92. Picklo MJ, Olson SJ, Hayes JD, Markesbery WR, Montine TJ. Elevation of AKR7A2 (succinic semialdehyde reductase) in neurodegenerative disease. Brain Res. 2001;916(1–2):229‐238. [DOI] [PubMed] [Google Scholar]
- 93. Namba Y, Tomonaga M, Kawasaki H, Otomo E, Ikeda K. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer's disease and kuru plaque amyloid in Creutzfeldt‐Jakob disease. Brain Res. 1991;541(1):163‐166. [DOI] [PubMed] [Google Scholar]
- 94. Diedrich JF, Minnigan H, Carp RI, et al. Neuropathological changes in scrapie and Alzheimer's disease are associated with increased expression of apolipoprotein E and cathepsin D in astrocytes. J Virol. 1991;65(9):4759‐4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rebeck GW, Reiter JS, Strickland DK, Hyman BT. Apolipoprotein E in sporadic Alzheimer's disease: allelic variation and receptor interactions. Neuron. 1993;11(4):575‐580. [DOI] [PubMed] [Google Scholar]
- 96. Han SH, Einstein G, Weisgraber KH, et al. Apolipoprotein E is localized to the cytoplasm of human cortical neurons: a light and electron microscopic study. J Neuropathol Exp Neurol. 1994;53(5):535‐544. [DOI] [PubMed] [Google Scholar]
- 97. Han SH, Hulette C, Saunders AM, et al. Apolipoprotein E is present in hippocampal neurons without neurofibrillary tangles in Alzheimer's disease and in age‐matched controls. Exp Neurol. 1994;128(1):13‐26. [DOI] [PubMed] [Google Scholar]
- 98. Harr SD, Uint L, Hollister R, Hyman BT, Mendez AJ. Brain expression of apolipoproteins E, J, and A‐I in Alzheimer's disease. J Neurochem. 1996;66(6):2429‐2435. [DOI] [PubMed] [Google Scholar]
- 99. Uchihara T, Duyckaerts C, He Y, et al. ApoE immunoreactivity and microglial cells in Alzheimer's disease brain. Neurosci Lett. 1995;195(1):5‐8. [DOI] [PubMed] [Google Scholar]
- 100. Shao Y, Gearing M, Mirra SS. Astrocyte‐apolipoprotein E associations in senile plaques in Alzheimer disease and vascular lesions: a regional immunohistochemical study. J Neuropathol Exp Neurol. 1997;56(4):376‐381. [DOI] [PubMed] [Google Scholar]
- 101. Utter S, Tamboli IY, Walter J, et al. Cerebral small vessel disease‐induced apolipoprotein E leakage is associated with Alzheimer disease and the accumulation of amyloid beta‐protein in perivascular astrocytes. J Neuropathol Exp Neurol. 2008;67(9):842‐856. [DOI] [PubMed] [Google Scholar]
- 102. Lidström AM, Bogdanovic N, Hesse C, Volkman I, Davidsson P, Blennow K. Clusterin (apolipoprotein J) protein levels are increased in hippocampus and in frontal cortex in Alzheimer's disease. Exp Neurol. 1998;154(2):511‐521. [DOI] [PubMed] [Google Scholar]
- 103. Troakes C, Smyth R, Noor F, et al. Clusterin expression is upregulated following acute head injury and localizes to astrocytes in old head injury. Neuropathol off J Jpn Soc Neuropathol. 2017;37(1):12‐24. [DOI] [PubMed] [Google Scholar]
- 104. Petit‐Turcotte C, Stohl SM, Beffert U, et al. Apolipoprotein C‐I expression in the brain in Alzheimer's disease. Neurobiol Dis. 2001;8(6):953‐963. [DOI] [PubMed] [Google Scholar]
- 105. Kalman J, McConathy W, Araoz C, Kasa P, Lacko AG. Apolipoprotein D in the aging brain and in Alzheimer's dementia. Neurol Res. 2000;22(4):330‐336. [DOI] [PubMed] [Google Scholar]
- 106. Tooyama I, Kawamata T, Akiyama H, Moestrup SK, Gliemann J, McGeer PL. Immunohistochemical study of alpha 2 macroglobulin receptor in Alzheimer and control postmortem human brain. Mol Chem Neuropathol. 1993;18(1–2):153‐160. [DOI] [PubMed] [Google Scholar]
- 107. Rebeck GW, Harr SD, Strickland DK, Hyman BT. Multiple, diverse senile plaque‐associated proteins are ligands of an apolipoprotein E receptor, the alpha 2‐macroglobulin receptor/low‐density‐lipoprotein receptor‐related protein. Ann Neurol. 1995;37(2):211‐217. [DOI] [PubMed] [Google Scholar]
- 108. Yamada T, Kawata M, Arai H, Fukasawa M, Inoue K, Sato T. Astroglial localization of cholesteryl ester transfer protein in normal and Alzheimer's disease brain tissues. Acta Neuropathol (Berl). 1995;90(6):633‐636. [DOI] [PubMed] [Google Scholar]
- 109. Bogdanovic N, Bretillon L, Lund EG, et al. On the turnover of brain cholesterol in patients with Alzheimer's disease. Abnormal induction of the cholesterol‐catabolic enzyme CYP46 in glial cells. Neurosci Lett. 2001;314(1–2):45‐48. [DOI] [PubMed] [Google Scholar]
- 110. Brown J, Theisler C, Silberman S, et al. Differential expression of cholesterol hydroxylases in Alzheimer's disease. J Biol Chem. 2004;279(33):34674‐34681. [DOI] [PubMed] [Google Scholar]
- 111. Satoi H, Tomimoto H, Ohtani R, et al. Astroglial expression of ceramide in Alzheimer's disease brains: a role during neuronal apoptosis. Neuroscience. 2005;130(3):657‐666. [DOI] [PubMed] [Google Scholar]
- 112. Bhat R, Crowe EP, Bitto A, et al. Astrocyte senescence as a component of Alzheimer's disease. PloS One. 2012;7(9):e45069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Yoshiyama Y, Asahina M, Hattori T. Selective distribution of matrix metalloproteinase‐3 (MMP‐3) in Alzheimer's disease brain. Acta Neuropathol (Berl). 2000;99(2):91‐95. [DOI] [PubMed] [Google Scholar]
- 114. Abraham CR, Shirahama T, Potter H. Alpha 1‐antichymotrypsin is associated solely with amyloid deposits containing the beta‐protein. Amyloid and cell localization of alpha 1‐antichymotrypsin. Neurobiol Aging. 1990;11(2):123‐129. [DOI] [PubMed] [Google Scholar]
- 115. Ishiguro K, Shoji M, Yamaguchi H, et al. Differential expression of alpha 1‐antichymotrypsin in the aged human brain. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;64(4):221‐227. [DOI] [PubMed] [Google Scholar]
- 116. Styren SD, Kamboh MI, DeKosky ST. Expression of differential immune factors in temporal cortex and cerebellum: the role of alpha‐1‐antichymotrypsin, apolipoprotein E, and reactive glia in the progression of Alzheimer's disease. J Comp Neurol. 1998;396(4):511‐520. [PubMed] [Google Scholar]
- 117. Yoshida E, Yoshimura M, Ito Y, Mihara H. Demonstration of an active component of inter‐alpha‐trypsin inhibitor in the brains of Alzheimer type dementia. Biochem Biophys Res Commun. 1991;174(2):1015‐1021. [DOI] [PubMed] [Google Scholar]
- 118. Gilad GM, Kagan HM, Gilad VH. Evidence for increased lysyl oxidase, the extracellular matrix‐forming enzyme, in Alzheimer's disease brain. Neurosci Lett. 2005;376(3):210‐214. [DOI] [PubMed] [Google Scholar]
- 119. Wilhelmus MMM, Bol JGJM, van Duinen SG, Drukarch B. Extracellular matrix modulator lysyl oxidase colocalizes with amyloid‐beta pathology in Alzheimer's disease and hereditary cerebral hemorrhage with amyloidosis—Dutch type. Exp Gerontol. 2013;48(2):109‐114. [DOI] [PubMed] [Google Scholar]
- 120. de Jager M, van der Wildt B, Schul E, et al. Tissue transglutaminase colocalizes with extracellular matrix proteins in cerebral amyloid angiopathy. Neurobiol Aging. 2013;34(4):1159‐1169. [DOI] [PubMed] [Google Scholar]
- 121. Wilhelmus MMM, Grunberg SCS, Bol JGJM, et al. Transglutaminases and transglutaminase‐catalyzed cross‐links colocalize with the pathological lesions in Alzheimer's disease brain. Brain Pathol Zurich Switz. 2009;19(4):612‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Yamada T, Yoshiyama Y, Kawaguchi N, et al. Possible roles of transglutaminases in Alzheimer's disease. Dement Geriatr Cogn Disord. 1998;9(2):103‐110. [DOI] [PubMed] [Google Scholar]
- 123. Vogel H, Butcher EC, Picker LJ. H‐CAM expression in the human nervous system: evidence for a role in diverse glial interactions. J Neurocytol. 1992;21(5):363‐373. [DOI] [PubMed] [Google Scholar]
- 124. Akiyama H, Tooyama I, Kawamata T, Ikeda K, McGeer PL. Morphological diversities of CD44 positive astrocytes in the cerebral cortex of normal subjects and patients with Alzheimer's disease. Brain Res. 1993;632(1–2):249‐259. [DOI] [PubMed] [Google Scholar]
- 125. Snow AD, Mar H, Nochlin D, et al. The presence of heparan sulfate proteoglycans in the neuritic plaques and congophilic angiopathy in Alzheimer's disease. Am J Pathol. 1988;133(3):456‐463. [PMC free article] [PubMed] [Google Scholar]
- 126. Chaudhury AR, Gerecke KM, Wyss JM, Morgan DG, Gordon MN, Carroll SL. Neuregulin‐1 and erbB4 immunoreactivity is associated with neuritic plaques in Alzheimer disease brain and in a transgenic model of Alzheimer disease. J Neuropathol Exp Neurol. 2003;62(1):42‐54. [DOI] [PubMed] [Google Scholar]
- 127. Pankonin MS, Sohi J, Kamholz J, Loeb JA. Differential distribution of neuregulin in human brain and spinal fluid. Brain Res. 2009;1258:1‐11. [DOI] [PubMed] [Google Scholar]
- 128. Iwamoto N, Suzuki Y, Makino Y, Haga C, Kosaka K, Iizuka R. Cell membrane changes in brains manifesting senile plaques: an immunohistochemical study of GM1 membranous ganglioside. Brain Res. 1990;522(1):152‐156. [DOI] [PubMed] [Google Scholar]
- 129. Nakamura Y, Takeda M, Suzuki H, et al. Abnormal distribution of cathepsins in the brain of patients with Alzheimer's disease. Neurosci Lett. 1991;130(2):195‐198. [DOI] [PubMed] [Google Scholar]
- 130. Cataldo AM, Paskevich PA, Kominami E, Nixon RA. Lysosomal hydrolases of different classes are abnormally distributed in brains of patients with Alzheimer disease. Proc Natl Acad Sci U S a. 1991;88(24):10998‐11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kobayashi K, Hayashi M, Nakano H, et al. Apoptosis of astrocytes with enhanced lysosomal activity and oligodendrocytes in white matter lesions in Alzheimer's disease. Neuropathol Appl Neurobiol. 2002;28(3):238‐251. [DOI] [PubMed] [Google Scholar]
- 132. Rohn TT, Wirawan E, Brown RJ, Harris JR, Masliah E, Vandenabeele P. Depletion of Beclin‐1 due to proteolytic cleavage by caspases in the Alzheimer's disease brain. Neurobiol Dis. 2011;43(1):68‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Renkawek K, Voorter CE, Bosman GJ, van Workum FP, de Jong WW. Expression of alpha B‐crystallin in Alzheimer's disease. Acta Neuropathol (Berl). 1994;87(2):155‐160. [DOI] [PubMed] [Google Scholar]
- 134. Wilhelmus MMM, Otte‐Höller I, Wesseling P, de Waal RMW, Boelens WC, Verbeek MM. Specific association of small heat shock proteins with the pathological hallmarks of Alzheimer's disease brains. Neuropathol Appl Neurobiol. 2006;32(2):119‐130. [DOI] [PubMed] [Google Scholar]
- 135. Bruinsma IB, de Jager M, Carrano A, et al. Small heat shock proteins induce a cerebral inflammatory reaction. J Neurosci off J Soc Neurosci. 2011;31(33):11992‐12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Renkawek K, Bosman GJ, Gaestel M. Increased expression of heat‐shock protein 27 kDa in Alzheimer disease: a preliminary study. Neuroreport. 1993. ;5(1):14‐16. [DOI] [PubMed] [Google Scholar]
- 137. Rizzu P, Hinkle DA, Zhukareva V, et al. DJ‐1 colocalizes with tau inclusions: a link between parkinsonism and dementia. Ann Neurol. 2004;55(1):113‐118. [DOI] [PubMed] [Google Scholar]
- 138. Witte ME, Bol JGJM, Gerritsen WH, et al. Parkinson's disease‐associated parkin colocalizes with Alzheimer's disease and multiple sclerosis brain lesions. Neurobiol Dis. 2009;36(3):445‐452. [DOI] [PubMed] [Google Scholar]
- 139. Hou H‐L, Shen Y‐X, Zhu H‐Y, et al. Alterations of hHrd1 expression are related to hyperphosphorylated tau in the hippocampus in Alzheimer's disease. J Neurosci Res. 2006;84(8):1862‐1870. [DOI] [PubMed] [Google Scholar]
- 140. Garwood C, Faizullabhoy A, Wharton SB, et al. Calcium dysregulation in relation to Alzheimer‐type pathology in the ageing brain. Neuropathol Appl Neurobiol. 2013;39(7):788‐799. [DOI] [PubMed] [Google Scholar]
- 141. Hannula MJ, Myöhänen TT, Tenorio‐Laranga J, Männistö PT, Garcia‐Horsman JA. Prolyl oligopeptidase colocalizes with α‐synuclein, β‐amyloid, tau protein and astroglia in the post‐mortem brain samples with Parkinson's and Alzheimer's diseases. Neuroscience. 2013;242:140‐150. [DOI] [PubMed] [Google Scholar]
- 142. Tumani H, Shen G, Peter JB, Brück W. Glutamine synthetase in cerebrospinal fluid, serum, and brain: a diagnostic marker for Alzheimer disease? Arch Neurol. 1999;56(10):1241‐1246. [DOI] [PubMed] [Google Scholar]
- 143. Fluteau A, Ince PG, Minett T, et al. The nuclear retention of transcription factor FOXO3a correlates with a DNA damage response and increased glutamine synthetase expression by astrocytes suggesting a neuroprotective role in the ageing brain. Neurosci Lett. 2015;609:11‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Robinson SR. Neuronal expression of glutamine synthetase in Alzheimer's disease indicates a profound impairment of metabolic interactions with astrocytes. Neurochem Int. 2000;36(4–5):471‐482. [DOI] [PubMed] [Google Scholar]
- 145. Robinson SR. Changes in the cellular distribution of glutamine synthetase in Alzheimer's disease. J Neurosci Res. 2001;66(5):972‐980. [DOI] [PubMed] [Google Scholar]
- 146. Serrano‐Pozo A, Gómez‐Isla T, Growdon JH, Frosch MP, Hyman BT. A phenotypic change but not proliferation underlies glial responses in Alzheimer disease. Am J Pathol. 2013;182(6):2332‐2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Li S, Mallory M, Alford M, Tanaka S, Masliah E. Glutamate transporter alterations in Alzheimer disease are possibly associated with abnormal APP expression. J Neuropathol Exp Neurol. 1997;56(8):901‐911. [DOI] [PubMed] [Google Scholar]
- 148. Simpson JE, Ince PG, Lace G, et al. Astrocyte phenotype in relation to Alzheimer‐type pathology in the ageing brain. Neurobiol Aging. 2010;31(4):578‐590. [DOI] [PubMed] [Google Scholar]
- 149. Kobayashi E, Nakano M, Kubota K, et al. Activated forms of astrocytes with higher GLT‐1 expression are associated with cognitive normal subjects with Alzheimer pathology in human brain. Sci Rep. 2018;8(1):1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Casley CS, Lakics V, Lee H, et al. Up‐regulation of astrocyte metabotropic glutamate receptor 5 by amyloid‐β peptide. Brain Res. 2009;1260:65‐75. [DOI] [PubMed] [Google Scholar]
- 151. Iyer AM, van Scheppingen J, Milenkovic I, et al. Metabotropic glutamate receptor 5 in Down's syndrome hippocampus during development: increased expression in astrocytes. Curr Alzheimer Res. 2014;11(7):694‐705. [DOI] [PubMed] [Google Scholar]
- 152. Balu DT, Pantazopoulos H, Huang CCY, et al. Neurotoxic astrocytes express the d‐serine synthesizing enzyme, serine racemase, in Alzheimer's disease. Neurobiol Dis. 2019;130:104511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Wu Z, Guo Z, Gearing M, Chen G. Tonic inhibition in dentate gyrus impairs long‐term potentiation and memory in an Alzheimer's [corrected] disease model. Nat Commun. 2014;5:4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Jo S, Yarishkin O, Hwang YJ, et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer's disease. Nat Med. 2014;20(8):886‐896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Wevers A, Monteggia L, Nowacki S, et al. Expression of nicotinic acetylcholine receptor subunits in the cerebral cortex in Alzheimer's disease: histotopographical correlation with amyloid plaques and hyperphosphorylated‐tau protein. Eur J Neurosci. 1999;11(7):2551‐2565. [DOI] [PubMed] [Google Scholar]
- 156. Teaktong T, Graham A, Court J, et al. Alzheimer's disease is associated with a selective increase in alpha7 nicotinic acetylcholine receptor immunoreactivity in astrocytes. Glia. 2003;41(2):207‐211. [DOI] [PubMed] [Google Scholar]
- 157. Teaktong T, Graham AJ, Court JA, et al. Nicotinic acetylcholine receptor immunohistochemistry in Alzheimer's disease and dementia with Lewy bodies: differential neuronal and astroglial pathology. J Neurol Sci. 2004;225(1–2):39‐49. [DOI] [PubMed] [Google Scholar]
- 158. Nagele RG, D'Andrea MR, Lee H, Venkataraman V, Wang H‐Y. Astrocytes accumulate A beta 42 and give rise to astrocytic amyloid plaques in Alzheimer disease brains. Brain Res. 2003;971(2):197‐209. [DOI] [PubMed] [Google Scholar]
- 159. Yu W‐F, Guan Z‐Z, Bogdanovic N, Nordberg A. High selective expression of alpha7 nicotinic receptors on astrocytes in the brains of patients with sporadic Alzheimer's disease and patients carrying Swedish APP 670/671 mutation: a possible association with neuritic plaques. Exp Neurol. 2005;192(1):215‐225. [DOI] [PubMed] [Google Scholar]
- 160. Messamore E, Bogdanovich N, Schröder H, Winblad B. Astrocytes associated with senile plaques possess muscarinic acetylcholine receptors. Neuroreport. 1994;5(12):1473‐1476. [DOI] [PubMed] [Google Scholar]
- 161. Kumar U, Patel SC. Immunohistochemical localization of dopamine receptor subtypes (D1R‐D5R) in Alzheimer's disease brain. Brain Res. 2007;1131(1):187‐196. [DOI] [PubMed] [Google Scholar]
- 162. Wu C, Singh SK, Dias P, Kumar S, Mann DM. Activated astrocytes display increased 5‐HT2a receptor expression in pathological states. Exp Neurol. 1999;158(2):529‐533. [DOI] [PubMed] [Google Scholar]
- 163. Guillemin GJ, Brew BJ, Noonan CE, Takikawa O, Cullen KM. Indoleamine 2,3 dioxygenase and quinolinic acid immunoreactivity in Alzheimer's disease hippocampus. Neuropathol Appl Neurobiol. 2005;31(4):395‐404. [DOI] [PubMed] [Google Scholar]
- 164. Orr AG, Hsiao EC, Wang MM, et al. Astrocytic adenosine receptor A2A and Gs‐coupled signaling regulate memory. Nat Neurosci. 2015;18(3):423‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Yamada T, Tsubouchi H, Daikuhara Y, et al. Immunohistochemistry with antibodies to hepatocyte growth factor and its receptor protein (c‐MET) in human brain tissues. Brain Res. 1994;637(1–2):308‐312. [DOI] [PubMed] [Google Scholar]
- 166. Fenton H, Finch PW, Rubin JS, et al. Hepatocyte growth factor (HGF/SF) in Alzheimer's disease. Brain Res. 1998;779(1–2):262‐270. [DOI] [PubMed] [Google Scholar]
- 167. Yamada T, Yoshiyama Y, Tsuboi Y, Shimomura T. Astroglial expression of hepatocyte growth factor and hepatocyte growth factor activator in human brain tissues. Brain Res. 1997;762(1–2):251‐255. [DOI] [PubMed] [Google Scholar]
- 168. Tooyama I, Akiyama H, McGeer PL, Hara Y, Yasuhara O, Kimura H. Acidic fibroblast growth factor‐like immunoreactivity in brain of Alzheimer patients. Neurosci Lett. 1991;121(1–2):155‐158. [DOI] [PubMed] [Google Scholar]
- 169. Kimura H, Tooyama I, McGeer PL. Acidic FGF expression in the surroundings of senile plaques. Tohoku J Exp Med. 1994;174(3):279‐293. [DOI] [PubMed] [Google Scholar]
- 170. Gómez‐Pinilla F, Cummings BJ, Cotman CW. Induction of basic fibroblast growth factor in Alzheimer's disease pathology. Neuroreport. 1990;1(3–4):211‐214. [DOI] [PubMed] [Google Scholar]
- 171. Stopa EG, Gonzalez AM, Chorsky R, et al. Basic fibroblast growth factor in Alzheimer's disease. Biochem Biophys Res Commun. 1990;171(2):690‐696. [DOI] [PubMed] [Google Scholar]
- 172. Cummings BJ, Su JH, Cotman CW. Neuritic involvement within bFGF immunopositive plaques of Alzheimer's disease. Exp Neurol. 1993;124(2):315‐325. [DOI] [PubMed] [Google Scholar]
- 173. Pike CJ, Cummings BJ, Monzavi R, Cotman CW. Beta‐amyloid‐induced changes in cultured astrocytes parallel reactive astrocytosis associated with senile plaques in Alzheimer's disease. Neuroscience. 1994;63(2):517‐531. [DOI] [PubMed] [Google Scholar]
- 174. Takami K, Matsuo A, Terai K, Walker DG, McGeer EG, McGeer PL. Fibroblast growth factor receptor‐1 expression in the cortex and hippocampus in Alzheimer's disease. Brain Res. 1998;802(1–2):89‐97. [DOI] [PubMed] [Google Scholar]
- 175. Flanders KC, Lippa CF, Smith TW, Pollen DA, Sporn MB. Altered expression of transforming growth factor‐beta in Alzheimer's disease. Neurology. 1995;45(8):1561‐1569. [DOI] [PubMed] [Google Scholar]
- 176. Peress NS, Perillo E. Differential expression of TGF‐beta 1, 2 and 3 isotypes in Alzheimer's disease: a comparative immunohistochemical study with cerebral infarction, aged human and mouse control brains. J Neuropathol Exp Neurol. 1995;54(6):802‐811. [DOI] [PubMed] [Google Scholar]
- 177. Tashiro H, Dohura K, Iwaki T. Differential expression of transforming growth factor‐beta isoforms in human prion diseases. Neuropathol Appl Neurobiol. 1998;24(4):284‐292. [DOI] [PubMed] [Google Scholar]
- 178. de la Monte SM, Ng SC, Hsu DW. Aberrant GAP‐43 gene expression in Alzheimer's disease. Am J Pathol. 1995;147(4):934‐946. [PMC free article] [PubMed] [Google Scholar]
- 179. Rensink AAM, Gellekink H, Otte‐Höller I, et al. Expression of the cytokine leukemia inhibitory factor and pro‐apoptotic insulin‐like growth factor binding protein‐3 in Alzheimer's disease. Acta Neuropathol (Berl). 2002;104(5):525‐533. [DOI] [PubMed] [Google Scholar]
- 180. Thomason LAM, Smithson LJ, Hazrati L‐N, McLaurin J, Kawaja MD. Reactive astrocytes associated with plaques in TgCRND8 mouse brain and in human Alzheimer brain express phosphoprotein enriched in astrocytes (PEA‐15). FEBS Lett. 2013;587(15):2448‐2454. [DOI] [PubMed] [Google Scholar]
- 181. O'Barr S, Schultz J, Rogers J. Expression of the protooncogene bcl‐2 in Alzheimer's disease brain. Neurobiol Aging. 1996;17(1):131‐136. [DOI] [PubMed] [Google Scholar]
- 182. Kobayashi K, Hayashi M, Nakano H, Shimazaki M, Sugimori K, Koshino Y. Correlation between astrocyte apoptosis and Alzheimer changes in gray matter lesions in Alzheimer's disease. J Alzheimers Dis JAD. 2004;6(6):623‐632. discussion 673‐681 [DOI] [PubMed] [Google Scholar]
- 183. Ferrer I, Blanco R. N‐myc and c‐myc expression in Alzheimer disease, Huntington disease and Parkinson disease. Brain Res Mol Brain Res. 2000;77(2):270‐276. [DOI] [PubMed] [Google Scholar]
- 184. Helguera P, Pelsman A, Pigino G, Wolvetang E, Head E, Busciglio J. ets‐2 promotes the activation of a mitochondrial death pathway in Down's syndrome neurons. J Neurosci off J Soc Neurosci. 2005;25(9):2295‐2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Ueberham U, Hessel A, Arendt T. Cyclin C expression is involved in the pathogenesis of Alzheimer's disease. Neurobiol Aging. 2003;24(3):427‐435. [DOI] [PubMed] [Google Scholar]
- 186. Tsujioka Y, Takahashi M, Tsuboi Y, Yamamoto T, Yamada T. Localization and expression of cdc2 and cdk4 in Alzheimer brain tissue. Dement Geriatr Cogn Disord. 1999;10(3):192‐198. [DOI] [PubMed] [Google Scholar]
- 187. Vincent I, Jicha G, Rosado M, Dickson DW. Aberrant expression of mitotic cdc2/cyclin B1 kinase in degenerating neurons of Alzheimer's disease brain. J Neurosci off J Soc Neurosci. 1997;17(10):3588‐3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. de la Monte SM, Sohn YK, Wands JR. Correlates of p53‐ and Fas (CD95)‐mediated apoptosis in Alzheimer's disease. J Neurol Sci. 1997;152(1):73‐83. [DOI] [PubMed] [Google Scholar]
- 189. Jordan‐Sciutto KL, Malaiyandi LM, Bowser R. Altered distribution of cell cycle transcriptional regulators during Alzheimer disease. J Neuropathol Exp Neurol. 2002;61(4):358‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Previll LA, Crosby ME, Castellani RJ, et al. Increased expression of p130 in Alzheimer disease. Neurochem Res. 2007;32(4–5):639‐644. [DOI] [PubMed] [Google Scholar]
- 191. Leroy K, Duyckaerts C, Bovekamp L, Müller O, Anderton BH, Brion JP. Increase of adenomatous polyposis coli immunoreactivity is a marker of reactive astrocytes in Alzheimer's disease and in other pathological conditions. Acta Neuropathol (Berl). 2001;102(1):1‐10. [DOI] [PubMed] [Google Scholar]
- 192. Sonoda Y, Mukai H, Matsuo K, et al. Accumulation of tumor‐suppressor PTEN in Alzheimer neurofibrillary tangles. Neurosci Lett. 2010;471(1):20‐24. [DOI] [PubMed] [Google Scholar]
- 193. Turnquist C, Horikawa I, Foran E, et al. p53 isoforms regulate astrocyte‐mediated neuroprotection and neurodegeneration. Cell Death Differ. 2016;23(9):1515‐1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Verwer RWH, Sluiter AA, Balesar RA, et al. Mature astrocytes in the adult human neocortex express the early neuronal marker doublecortin. Brain J Neurol. 2007;130(Pt 12):3321‐3335. [DOI] [PubMed] [Google Scholar]
- 195. Su JH, Zhao M, Anderson AJ, Srinivasan A, Cotman CW. Activated caspase‐3 expression in Alzheimer's and aged control brain: correlation with Alzheimer pathology. Brain Res. 2001;898(2):350‐357. [DOI] [PubMed] [Google Scholar]
- 196. Nishimura T, Akiyama H, Yonehara S, et al. Fas antigen expression in brains of patients with Alzheimer‐type dementia. Brain Res. 1995. Oct 16;695(2):137‐145. [DOI] [PubMed] [Google Scholar]
- 197. Ferrer I, Puig B, Krupinsk J, Carmona M, Blanco R. Fas and Fas ligand expression in Alzheimer's disease. Acta Neuropathol (Berl). 2001;102(2):121‐131. [DOI] [PubMed] [Google Scholar]
- 198. Smale G, Nichols NR, Brady DR, Finch CE, Horton WE. Evidence for apoptotic cell death in Alzheimer's disease. Exp Neurol. 1995;133(2):225‐230. [DOI] [PubMed] [Google Scholar]
- 199. Li WP, Chan WY, Lai HW, Yew DT. Terminal dUTP nick end labeling (TUNEL) positive cells in the different regions of the brain in normal aging and Alzheimer patients. J Mol Neurosci MN. 1997;8(2):75‐82. [DOI] [PubMed] [Google Scholar]
- 200. López‐González I, Carmona M, Blanco R, et al. Characterization of thorn‐shaped astrocytes in white matter of temporal lobe in Alzheimer's disease brains. Brain Pathol Zurich Switz. 2013;23(2):144‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Webster B, Hansen L, Adame A, et al. Astroglial activation of extracellular‐regulated kinase in early stages of Alzheimer disease. J Neuropathol Exp Neurol. 2006;65(2):142‐151. [DOI] [PubMed] [Google Scholar]
- 202. Rosenberger AFN, Morrema THJ, Gerritsen WH, et al. Increased occurrence of protein kinase CK2 in astrocytes in Alzheimer's disease pathology. J Neuroinflammation. 2016;13:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Lee C, Low CYB, Francis PT, et al. An isoform‐specific role of FynT tyrosine kinase in Alzheimer's disease. J Neurochem. 2016;136(3):637‐650. [DOI] [PubMed] [Google Scholar]
- 204. Pei JJ, Grundke‐Iqbal I, Iqbal K, Bogdanovic N, Winblad B, Cowburn RF. Elevated protein levels of protein phosphatases PP‐2A and PP‐2B in astrocytes of Alzheimer's disease temporal cortex. J Neural Transm Vienna Austria 1996. 1997;104(11–12):1329‐1338. [DOI] [PubMed] [Google Scholar]
- 205. Watanabe K, Uemura K, Asada M, et al. The participation of insulin‐like growth factor‐binding protein 3 released by astrocytes in the pathology of Alzheimer's disease. Mol Brain. 2015;8(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Pleiss MM, Sompol P, Kraner SD, et al. Calcineurin proteolysis in astrocytes: implications for impaired synaptic function. Biochim Biophys Acta. 2016;1862(9):1521‐1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Byman E, Schultz N, Netherlands Brain Bank , Fex M, Wennström M. Brain alpha‐amylase: a novel energy regulator important in Alzheimer disease? Brain Pathol Zurich Switz. 2018;28(6):920‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Connor B, Beilharz EJ, Williams C, et al. Insulin‐like growth factor‐I (IGF‐I) immunoreactivity in the Alzheimer's disease temporal cortex and hippocampus. Brain Res Mol Brain Res. 1997;49(1–2):283‐290. [DOI] [PubMed] [Google Scholar]
- 209. Jafferali S, Dumont Y, Sotty F, Robitaille Y, Quirion R, Kar S. Insulin‐like growth factor‐I and its receptor in the frontal cortex, hippocampus, and cerebellum of normal human and alzheimer disease brains. Synap N Y N. 2000;38(4):450‐459. [DOI] [PubMed] [Google Scholar]
- 210. Moloney AM, Griffin RJ, Timmons S, O'Connor R, Ravid R, O'Neill C. Defects in IGF‐1 receptor, insulin receptor and IRS‐1/2 in Alzheimer's disease indicate possible resistance to IGF‐1 and insulin signalling. Neurobiol Aging. 2010;31(2):224‐243. [DOI] [PubMed] [Google Scholar]
- 211. Kar S, Poirier J, Guevara J, et al. Cellular distribution of insulin‐like growth factor‐II/mannose‐6‐phosphate receptor in normal human brain and its alteration in Alzheimer's disease pathology. Neurobiol Aging. 2006;27(2):199‐210. [DOI] [PubMed] [Google Scholar]
- 212. Nakamura Y, Takeda M, Yoshimi K, et al. Involvement of clathrin light chains in the pathology of Alzheimer's disease. Acta Neuropathol (Berl). 1994;87(1):23‐31. [DOI] [PubMed] [Google Scholar]
- 213. Herrmann L, Wiegmann C, Arsalan‐Werner A, et al. Hook proteins: association with Alzheimer pathology and regulatory role of hook3 in amyloid beta generation. PloS One. 2015;10(3):e0119423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Kreft KL, van Meurs M, Wierenga‐Wolf AF, et al. Abundant kif21b is associated with accelerated progression in neurodegenerative diseases. Acta Neuropathol Commun. 2014;2:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Plá V, Paco S, Ghezali G, et al. Secretory sorting receptors carboxypeptidase E and secretogranin III in amyloid β‐associated neural degeneration in Alzheimer's disease. Brain Pathol Zurich Switz. 2013;23(3):274‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Jiang MH, Höög A, Ma KC, Nie XJ, Olsson Y, Zhang WW. Endothelin‐1‐like immunoreactivity is expressed in human reactive astrocytes. Neuroreport. 1993;4(7):935‐937. [DOI] [PubMed] [Google Scholar]
- 217. Zhang WW, Badonic T, Höög A, et al. Astrocytes in Alzheimer's disease express immunoreactivity to the vaso‐constrictor endothelin‐1. J Neurol Sci. 1994;122(1):90‐96. [DOI] [PubMed] [Google Scholar]
- 218. Romanitan MO, Popescu BO, Spulber S, et al. Altered expression of claudin family proteins in Alzheimer's disease and vascular dementia brains. J Cell Mol Med. 2010;14(5):1088‐1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Ryu JK, McLarnon JG. A leaky blood‐brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer's disease brain. J Cell Mol Med. 2009;13(9A):2911‐2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. D’Andrea MR. Evidence linking neuronal cell death to autoimmunity in Alzheimer's disease. Brain Res. 2003;982(1):19‐30. [DOI] [PubMed] [Google Scholar]
- 221. Boom A, Pochet R, Authelet M, et al. Astrocytic calcium/zinc binding protein S100A6 over expression in Alzheimer's disease and in PS1/APP transgenic mice models. Biochim Biophys Acta. 2004;1742(1–3):161‐168. [DOI] [PubMed] [Google Scholar]
- 222. Jin J‐K, Choi J‐K, Wasco W, et al. Expression of calsenilin in neurons and astrocytes in the Alzheimer's disease brain. Neuroreport. 2005;16(5):451‐455. [DOI] [PubMed] [Google Scholar]
- 223. Alvarez M‐I, Rivas L, Lacruz C, Toledano A. Astroglial cell subtypes in the cerebella of normal adults, elderly adults, and patients with Alzheimer's disease: a histological and immunohistochemical comparison. Glia. 2015;63(2):287‐312. [DOI] [PubMed] [Google Scholar]
- 224. Marshak DR, Pesce SA, Stanley LC, Griffin WS. Increased S100 beta neurotrophic activity in Alzheimer's disease temporal lobe. Neurobiol Aging. 1992;13(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 225. Murphy GM, Eng LF, Ellis WG, Perry G, Meissner LC, Tinklenberg JR. Antigenic profile of plaques and neurofibrillary tangles in the amygdala in Down's syndrome: a comparison with Alzheimer's disease. Brain Res. 1990;537(1–2):102‐108. [DOI] [PubMed] [Google Scholar]
- 226. Sheng JG, Mrak RE, Rovnaghi CR, Kozlowska E, van Eldik LJ, Griffin WS. Human brain S100 beta and S100 beta mRNA expression increases with age: pathogenic implications for Alzheimer's disease. Neurobiol Aging. 1996;17(3):359‐363. [DOI] [PubMed] [Google Scholar]
- 227. van Eldik LJ, Griffin WS. S100 beta expression in Alzheimer's disease: relation to neuropathology in brain regions. Biochim Biophys Acta. 1994;1223(3):398‐403. [DOI] [PubMed] [Google Scholar]
- 228. Pérez E, Barrachina M, Rodríguez A, et al. Aquaporin expression in the cerebral cortex is increased at early stages of Alzheimer disease. Brain Res. 2007;1128(1):164‐174. [DOI] [PubMed] [Google Scholar]
- 229. Misawa T, Arima K, Mizusawa H, Satoh J. Close association of water channel AQP1 with amyloid‐beta deposition in Alzheimer disease brains. Acta Neuropathol (Berl). 2008;116(3):247‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Rodríguez A, Pérez‐Gracia E, Espinosa JC, Pumarola M, Torres JM, Ferrer I. Increased expression of water channel aquaporin 1 and aquaporin 4 in Creutzfeldt‐Jakob disease and in bovine spongiform encephalopathy‐infected bovine‐PrP transgenic mice. Acta Neuropathol (Berl). 2006;112(5):573‐585. [DOI] [PubMed] [Google Scholar]
- 231. Wilcock DM, Vitek MP, Colton CA. Vascular amyloid alters astrocytic water and potassium channels in mouse models and humans with Alzheimer's disease. Neuroscience. 2009;159(3):1055‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Zeppenfeld DM, Simon M, Haswell JD, et al. Association of perivascular localization of aquaporin‐4 with cognition and Alzheimer disease in aging brains. JAMA Neurol. 2017;74(1):91‐99. [DOI] [PubMed] [Google Scholar]
- 233. Boespflug EL, Simon MJ, Leonard E, et al. Targeted assessment of enlargement of the perivascular space in Alzheimer's disease and vascular dementia subtypes implicates astroglial involvement specific to Alzheimer's disease. J Alzheimers Dis JAD. 2018;66(4):1587‐1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Yi M, Yu P, Lu Q, Geller HM, Yu Z, Chen H. KCa3.1 constitutes a pharmacological target for astrogliosis associated with Alzheimer's disease. Mol Cell Neurosci. 2016;76:21‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Griffith CM, Xie M‐X, Qiu W‐Y, et al. Aberrant expression of the pore‐forming KATP channel subunit Kir6.2 in hippocampal reactive astrocytes in the 3xTg‐AD mouse model and human Alzheimer's disease. Neuroscience. 2016;336:81‐101. [DOI] [PubMed] [Google Scholar]
- 236. Grimaldi A, Pediconi N, Oieni F, et al. Neuroinflammatory processes, A1 astrocyte activation and protein aggregation in the retina of Alzheimer's disease patients. Possible Biomarkers for Early Diagnosis Front Neurosci. 2019;13:925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Husemann J, Silverstein SC. Expression of scavenger receptor class B, type I, by astrocytes and vascular smooth muscle cells in normal adult mouse and human brain and in Alzheimer's disease brain. Am J Pathol. 2001;158(3):825‐832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Fonseca MI, Chu S, Pierce AL, et al. Analysis of the putative role of CR1 in Alzheimer's disease: genetic association. Expression and Function PloS One. 2016;11(2):e0149792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Boddaert J, Kinugawa K, Lambert J‐C, et al. Evidence of a role for lactadherin in Alzheimer's disease. Am J Pathol. 2007;170(3):921‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Takashima S, Kuruta H, Mito T, Nishizawa M, Kunishita T, Tabira T. Developmental and aging changes in the expression patterns of beta‐amyloid in the brains of normal and Down syndrome cases. Brain Dev. 1990;12(4):367‐371. [DOI] [PubMed] [Google Scholar]
- 241. Akiyama H, Schwab C, Kondo H, et al. Granules in glial cells of patients with Alzheimer's disease are immunopositive for C‐terminal sequences of beta‐amyloid protein. Neurosci Lett. 1996;206(2–3):169‐172. [DOI] [PubMed] [Google Scholar]
- 242. Funato H, Yoshimura M, Yamazaki T, et al. Astrocytes containing amyloid beta‐protein (Abeta)‐positive granules are associated with Abeta40‐positive diffuse plaques in the aged human brain. Am J Pathol. 1998;152(4):983‐992. [PMC free article] [PubMed] [Google Scholar]
- 243. Yamaguchi H, Sugihara S, Ogawa A, Saido TC, Ihara Y. Diffuse plaques associated with astroglial amyloid beta protein, possibly showing a disappearing stage of senile plaques. Acta Neuropathol (Berl). 1998;95(3):217‐222. [DOI] [PubMed] [Google Scholar]
- 244. Akiyama H, Mori H, Saido T, Kondo H, Ikeda K, McGeer PL. Occurrence of the diffuse amyloid beta‐protein (Abeta) deposits with numerous Abeta‐containing glial cells in the cerebral cortex of patients with Alzheimer's disease. Glia. 1999;25(4):324‐331. [DOI] [PubMed] [Google Scholar]
- 245. Kurt MA, Davies DC, Kidd M. beta‐Amyloid immunoreactivity in astrocytes in Alzheimer's disease brain biopsies: an electron microscope study. Exp Neurol. 1999;158(1):221‐228. [DOI] [PubMed] [Google Scholar]
- 246. Thal DR, Härtig W, Schober R. Diffuse plaques in the molecular layer show intracellular A beta(8‐17)‐immunoreactive deposits in subpial astrocytes. Clin Neuropathol. 1999;18(5):226‐231. [PubMed] [Google Scholar]
- 247. Thal DR, Schultz C, Dehghani F, Yamaguchi H, Braak H, Braak E. Amyloid beta‐protein (Abeta)‐containing astrocytes are located preferentially near N‐terminal‐truncated Abeta deposits in the human entorhinal cortex. Acta Neuropathol (Berl). 2000;100(6):608‐617. [DOI] [PubMed] [Google Scholar]
- 248. Gyure KA, Durham R, Stewart WF, Smialek JE, Troncoso JC. Intraneuronal abeta‐amyloid precedes development of amyloid plaques in Down syndrome. Arch Pathol Lab Med. 2001;125(4):489‐492. [DOI] [PubMed] [Google Scholar]
- 249. Busciglio J, Pelsman A, Wong C, et al. Altered metabolism of the amyloid beta precursor protein is associated with mitochondrial dysfunction in Down's syndrome. Neuron. 2002;33(5):677‐688. [DOI] [PubMed] [Google Scholar]
- 250. Oide T, Kinoshita T, Arima K. Regression stage senile plaques in the natural course of Alzheimer's disease. Neuropathol Appl Neurobiol. 2006;32(5):539‐556. [DOI] [PubMed] [Google Scholar]
- 251. Lasagna‐Reeves CA, Kayed R. Astrocytes contain amyloid‐β annular protofibrils in Alzheimer's disease brains. FEBS Lett. 2011;585(19):3052‐3057. [DOI] [PubMed] [Google Scholar]
- 252. Perez‐Garmendia R, Hernandez‐Zimbron LF, Morales MA, et al. Identification of N‐terminally truncated pyroglutamate amyloid‐β in cholesterol‐enriched diet‐fed rabbit and AD brain. J Alzheimers Dis JAD. 2014;39(2):441‐455. [DOI] [PubMed] [Google Scholar]
- 253. Tate‐Ostroff B, Majocha RE, Marotta CA. Identification of cellular and extracellular sites of amyloid precursor protein extracytoplasmic domain in normal and Alzheimer disease brains. Proc Natl Acad Sci U S a. 1989;86(2):745‐479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Yamaguchi H, Yamazaki T, Ishiguro K, Shoji M, Nakazato Y, Hirai S. Ultrastructural localization of Alzheimer amyloid beta/A4 protein precursor in the cytoplasm of neurons and senile plaque‐associated astrocytes. Acta Neuropathol (Berl). 1992;85(1):15‐22. [DOI] [PubMed] [Google Scholar]
- 255. Nishiyama K, Trapp BD, Ikezu T, et al. Caveolin‐3 upregulation activates beta‐secretase‐mediated cleavage of the amyloid precursor protein in Alzheimer's disease. J Neurosci Off J Soc Neurosci. 1999;19(15):6538‐6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Hartlage‐Rübsamen M, Zeitschel U, Apelt J, et al. Astrocytic expression of the Alzheimer's disease beta‐secretase (BACE1) is stimulus‐dependent. Glia. 2003;41(2):169‐179. [DOI] [PubMed] [Google Scholar]
- 257. Leuba G, Wernli G, Vernay A, Kraftsik R, Mohajeri MH, Saini KD. Neuronal and nonneuronal quantitative BACE immunocytochemical expression in the entorhinohippocampal and frontal regions in Alzheimer's disease. Dement Geriatr Cogn Disord. 2005;19(4):171‐183. [DOI] [PubMed] [Google Scholar]
- 258. Sennvik K, Bogdanovic N, Volkmann I, Fastbom J, Benedikz E. Beta‐secretase‐cleaved amyloid precursor protein in Alzheimer brain: a morphologic study. J Cell Mol Med. 2004;8(1):127‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Huynh DP, Vinters HV, Ho DH, Ho VV, Pulst SM. Neuronal expression and intracellular localization of presenilins in normal and Alzheimer disease brains. J Neuropathol Exp Neurol. 1997;56(9):1009‐1017. [DOI] [PubMed] [Google Scholar]
- 260. Weggen S, Diehlmann A, Buslei R, Beyreuther K, Bayer TA. Prominent expression of presenilin‐1 in senile plaques and reactive astrocytes in Alzheimer's disease brain. Neuroreport. 1998;9(14):3279‐3283. [PubMed] [Google Scholar]
- 261. Diehlmann A, Ida N, Weggen S, et al. Analysis of presenilin 1 and presenilin 2 expression and processing by newly developed monoclonal antibodies. J Neurosci Res. 1999;56(4):405‐419. [DOI] [PubMed] [Google Scholar]
- 262. Nowak K, Lange‐Dohna C, Zeitschel U, et al. The transcription factor Yin Yang 1 is an activator of BACE1 expression. J Neurochem. 2006;96(6):1696‐1707. [DOI] [PubMed] [Google Scholar]
- 263. Russo C, Dolcini V, Salis S, et al. Signal transduction through tyrosine‐phosphorylated carboxy‐terminal fragments of APP via an enhanced interaction with Shc/Grb2 adaptor proteins in reactive astrocytes of Alzheimer's disease brain. Ann N Y Acad Sci. 2002;973:323‐333. [DOI] [PubMed] [Google Scholar]
- 264. Russo C, Dolcini V, Salis S, et al. Signal transduction through tyrosine‐phosphorylated C‐terminal fragments of amyloid precursor protein via an enhanced interaction with Shc/Grb2 adaptor proteins in reactive astrocytes of Alzheimer's disease brain. J Biol Chem. 2002;277(38):35282‐35288. [DOI] [PubMed] [Google Scholar]
- 265. Dorfman VB, Pasquini L, Riudavets M, et al. Differential cerebral deposition of IDE and NEP in sporadic and familial Alzheimer's disease. Neurobiol Aging. 2010;31(10):1743‐1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Arima K, Izumiyama Y, Nakamura M, et al. Argyrophilic tau‐positive twisted and non‐twisted tubules in astrocytic processes in brains of Alzheimer‐type dementia: an electron microscopical study. Acta Neuropathol (Berl). 1998;95(1):28‐39. [DOI] [PubMed] [Google Scholar]
- 267. Ikeda K, Akiyama H, Arai T, Nishimura T. Glial tau pathology in neurodegenerative diseases: their nature and comparison with neuronal tangles. Neurobiol Aging. 1998;19(1 Suppl):S85‐S91. [DOI] [PubMed] [Google Scholar]
- 268. Schultz C, Ghebremedhin E, del Tredici K, Rüb U, Braak H. High prevalence of thorn‐shaped astrocytes in the aged human medial temporal lobe. Neurobiol Aging. 2004;25(3):397‐405. [DOI] [PubMed] [Google Scholar]
- 269. Munoz DG, Woulfe J, Kertesz A. Argyrophilic thorny astrocyte clusters in association with Alzheimer's disease pathology in possible primary progressive aphasia. Acta Neuropathol (Berl). 2007;114(4):347‐357. [DOI] [PubMed] [Google Scholar]
- 270. Reyes JF, Reynolds MR, Horowitz PM, et al. A possible link between astrocyte activation and tau nitration in Alzheimer's disease. Neurobiol Dis. 2008;31(2):198‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Kovacs GG, Robinson JL, Xie SX, et al. Evaluating the patterns of aging‐related tau astrogliopathy unravels novel insights into brain aging and neurodegenerative diseases. J Neuropathol Exp Neurol. 2017;76(4):270‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Lu Y‐P, Zeng M, Hu X‐Y, et al. Estrogen receptor alpha‐immunoreactive astrocytes are increased in the hippocampus in Alzheimer's disease. Exp Neurol. 2003;183(2):482‐428. [DOI] [PubMed] [Google Scholar]
- 273. Savaskan E, Olivieri G, Meier F, Ravid R, Müller‐Spahn F. Hippocampal estrogen beta‐receptor immunoreactivity is increased in Alzheimer's disease. Brain Res. 2001;908(2):113‐119. [DOI] [PubMed] [Google Scholar]
- 274. Sugata H, Matsuo K, Nakagawa T, et al. A peptidyl‐prolyl isomerase, FKBP12, accumulates in Alzheimer neurofibrillary tangles. Neurosci Lett. 2009;459(2):96‐99. [DOI] [PubMed] [Google Scholar]
- 275. Acharya NK, Nagele EP, Han M, et al. Neuronal PAD4 expression and protein citrullination: possible role in production of autoantibodies associated with neurodegenerative disease. J Autoimmun. 2012;38(4):369‐380. [DOI] [PubMed] [Google Scholar]
- 276. Phipps AJ, Vickers JC, Taberlay PC, Woodhouse A. Neurofilament‐labeled pyramidal neurons and astrocytes are deficient in DNA methylation marks in Alzheimer's disease. Neurobiol Aging. 2016;45:30‐42. [DOI] [PubMed] [Google Scholar]
- 277. Coppieters N, Dieriks BV, Lill C, Faull RLM, Curtis MA, Dragunow M. Global changes in DNA methylation and hydroxymethylation in Alzheimer's disease human brain. Neurobiol Aging. 2014;35(6):1334‐1344. [DOI] [PubMed] [Google Scholar]
- 278. Chen EY, Tan CM, Kou Y, et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics. 2013;14:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Gyengesi E, Rangel A, Ullah F, et al. Chronic microglial activation in the GFAP‐IL6 mouse contributes to age‐dependent cerebellar volume loss and impairment in motor function. Front Neurosci. 2019;13:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Ben Haim L, Ceyzériat K, Carrillo‐de Sauvage MA, et al. The JAK/STAT3 pathway is a common inducer of astrocyte reactivity in Alzheimer's and Huntington's diseases. J Neurosci off J Soc Neurosci. 2015;35(6):2817‐2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Ceyzériat K, Ben Haim L, Denizot A, et al. Modulation of astrocyte reactivity improves functional deficits in mouse models of Alzheimer's disease. Acta Neuropathol Commun. 2018;6(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Rodriguez S, Hug C, Todorov P, et al. Machine learning identifies candidates for drug repurposing in Alzheimer's disease. Nat Commun. 2021;12(1):1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Ong C‐T, Corces VG. CTCF: an architectural protein bridging genome topology and function. Nat Rev Genet. 2014;15(4):234‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Konrad EDH, Nardini N, Caliebe A, et al. CTCF variants in 39 individuals with a variable neurodevelopmental disorder broaden the mutational and clinical spectrum. Genet Med off J am Coll Med Genet. 2019;21(12):2723‐2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Sams DS, Nardone S, Getselter D, et al. Neuronal CTCF is necessary for basal and experience‐dependent gene regulation, memory formation, and genomic structure of BDNF and arc. Cell Rep. 2016;17(9):2418‐2430. [DOI] [PubMed] [Google Scholar]
- 286. McGill BE, Barve RA, Maloney SE, et al. Abnormal microglia and enhanced inflammation‐related gene transcription in mice with conditional deletion of Ctcf in Camk2a‐Cre‐expressing neurons. J Neurosci off J Soc Neurosci. 2018;38(1):200‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Kim S, Yu N‐K, Shim K‐W, et al. Remote memory and cortical synaptic plasticity require neuronal CCCTC‐binding factor (CTCF). J Neurosci off J Soc Neurosci. 2018. 30;38(22):5042‐5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Burton T, Liang B, Dibrov A, Amara F. Transforming growth factor‐beta‐induced transcription of the Alzheimer beta‐amyloid precursor protein gene involves interaction between the CTCF‐complex and Smads. Biochem Biophys Res Commun. 2002;295(3):713‐723. [DOI] [PubMed] [Google Scholar]
- 289. Blurton‐Jones M, Tuszynski MH. Reactive astrocytes express estrogen receptors in the injured primate brain. J Comp Neurol. 2001;433(1):115‐123. [DOI] [PubMed] [Google Scholar]
- 290. Sakuma S, Tokuhara D, Hattori H, Matsuoka O, Yamano T. Expression of estrogen receptor alpha and beta in reactive astrocytes at the male rat hippocampus after status epilepticus. Neuropathol off J Jpn Soc Neuropathol. 2009;29(1):55‐62. [DOI] [PubMed] [Google Scholar]
- 291. Orre M, Kamphuis W, Osborn LM, et al. Isolation of glia from Alzheimer's mice reveals inflammation and dysfunction. Neurobiol Aging. 2014;35(12):2746‐2760. [DOI] [PubMed] [Google Scholar]
- 292. Orre M, Kamphuis W, Osborn LM, et al. Acute isolation and transcriptome characterization of cortical astrocytes and microglia from young and aged mice. Neurobiol Aging. 2014;35(1):1‐14. [DOI] [PubMed] [Google Scholar]
- 293. Andrés‐Benito P, Gelpi E, Povedano M, et al. Combined transcriptomics and proteomics in frontal cortex area 8 in frontotemporal lobar degeneration linked to C9ORF72 expansion. J Alzheimers Dis JAD. 2019;68(3):1287‐1307. [DOI] [PubMed] [Google Scholar]
- 294. Du Z, Lin J‐R, Rashid R, et al. Qualifying antibodies for image‐based immune profiling and multiplexed tissue imaging. Nat Protoc. 2019;14(10):2900‐2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Circos plot depicting interconnectivity within the Alzheimer's disease reactive astrocyte (ADRA) protein set. The interconnectivity between the 196 constituents of the ADRA protein set and across functional categories is demonstrated via a Circos plot, where each node is a protein marker and each colour represents a functional group.
Figure S2. Heatmap of protein‐protein interaction (PPI) network. Heatmap of the PPI network of the 196 ADRA proteins grouped by expert‐annotated functional category. Note that markers belonging to the same functional group are highly connected, but substantial interactions are also observed between markers from different functional categories (e.g., oxidative stress and inflammation, proliferation/apoptosis and kinase/phosphatase).
Figure S3. Full heatmap of the overlap between the ADRA protein set and a microarray dataset of laser‐capture microdissected GFAP+ astrocytes. Heatmap shows the z‐scores of gene expression of all available ADRA markers across the 18 subjects (n = 6 Braak I/II, n = 6 Braak III/IV, and n = 6 Braak V/VI) included in a microarray study of laser‐capture microdissected GFAP+ astrocytes from the temporal neocortex (Simpson et al., 2011).
Figure S4. Full heatmap of the overlap between the ADRA protein set and a human astrocyte single‐nuclei RNA‐seq dataset. Heatmap shows the z‐scores of gene expression of all available ADRA markers across all 12 subjects (n = 6 control and n = 6 AD) included in a single‐nuclei RNA‐seq study from the entorhinal cortex (Grubman et al., 2019).
Figure S5. Full heatmap of the overlap between the ADRA protein set and a human bulk brain proteomic dataset. Heatmap illustrates the z‐scores of protein expression of all available ADRA markers averaged by Braak NFT stage within each diagnostic group (n = 91 control, n = 98 asymptomatic AD, and n = 230 AD dementia subjects) described in the AMP‐AD Consortium bulk brain proteomic dataset (Johnson et al., 2020).
Table S1. Studies and markers included in the systematic review. Detailed information collected from studies included in the systematic review. Studies are grouped by the functional categories of their respective markers. Colour code corresponds to Figures 3 and S1.
Table S2. Detailed pathway enrichment analysis results. Output of the pathway enrichment analysis against the curated pathway databases (i.e., Gene Ontology (GO): Biological Process (BP), GO: Cellular Component (CC), GO: Molecular Function (MF), and Reactome). Pathways are grouped by hierarchical clustering based on the Jaccard similarity coefficient and expert annotated.
Table S3. Detailed transcription factor enrichment analysis results. Output of the TFEA.ChIP and Enrichr transcription factor enrichment analysis.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The source code for all bioinformatics analyses is available on GitHub at https://github.com/serrano-pozo-lab/astrocyte-review (DOI: 10.5281/zenodo.5140749).


