Abstract
Objectives:
Improvements in acute stroke care have led to an increase in ischemic stroke survivors, who are at risk for development of post-ischemic stroke epilepsy (PISE). The impact of therapies such as thrombectomy and thrombolysis on risk of hospital revisits for PISE is unclear. We utilized administrative data to investigate the association between stroke treatment and PISE-related visits.
Methods:
Using claims data from California, New York, and Florida, we performed a retrospective analysis of adult survivors of acute ischemic strokes. Patients with history of epilepsy, trauma, infections, or tumors were excluded. Included patients were followed for a primary outcome of revisits for seizures or epilepsy. Cox proportional hazards regression was used to identify covariates associated with PISE.
Results:
In 595,545 included patients (median age 74 [IQR 21], 52% female), the 6-year cumulative rate of PISE-related revisit was 2.20% (95% CI 2.16–2.24). In multivariable models adjusting for demographics, comorbidities, and indicators of stroke severity, IV-tPA (HR 1.42, 95% CI 1.31–1.54, p<0.001) but not MT (HR 1.62, 95% CI 0.90–1.50, p=0.2) was associated with PISE-related revisit. Patients who underwent decompressive craniectomy experienced a 2-fold increase in odds for returning with PISE (HR 2.35, 95% CI 1.69–3.26, p<0.001). In-hospital seizures (HR 4.06, 95% CI 3.76–4.39, p<0.001) also elevated risk for PISE.
Significance:
We demonstrate that ischemic stroke survivors who received IV-tPA, underwent decompressive craniectomy, or experienced acute seizures were at increased risk PISE-related revisit. Close attention should be paid to these patients with increased potential for long-term development of and re-hospitalization for PISE.
Keywords: IV-tPA, Mechanical thrombectomy, Decompressive craniectomy, HCUP, Ischemic stroke, Epilepsy, Seizures
INTRODUCTION
Improvements in acute stroke care, namely the use of intravenous alteplase (IV-tPA) and mechanical thrombectomy (MT), have led to a considerable increase in the proportion of patients who survive a first-time ischemic stroke.1 Despite demonstrated benefits of acute stroke interventions, their impact on long term complications is not yet clear. One such complication is post-ischemic stroke epilepsy (PISE). PISE is estimated to occur in 2–6% of stroke survivors,2–4 and is associated with poor-quality of life and high mortality in young patients.5–7 It is possible that acute stroke interventions decrease the likelihood of PISE by minimizing ischemic brain injury.8 But neurotoxic properties of IV-tPA and the potential for reperfusion injury following MT may instead place patients at increased risk for PISE.9–11 Further, MT increases the number of stroke patients surviving with partial infarcts. These partial infarcts may present a nidus for epileptogenesis, consequently increasing the risk for PISE after endovascular intervention.
Previous analyses of PISE have investigated the impact of acute stroke therapies but have reported conflicting findings. Single-center studies found no association between acute stroke interventions and development of post-stroke seizures,12,13 but these studies were likely underpowered to detect differences in the rate of PISE development. Another study of more than 1,000 patients did describe an increased risk for epilepsy in patients receiving either IV-tPA or MT,14 but this study failed to follow patients for more than 2 years. Additionally, atrial fibrillation (AF) is a known risk factor for cortical strokes, and has been shown to correlate with PISE,15,16 while other studies support that PISE is more common in stroke patients who undergo neurosurgical interventions, such as decompressive craniectomy.17
Ischemic stroke survivors also inherit a significant risk for hospital readmission. It is estimated that the likelihood of hospital readmission is nearly 40% by 360 days18 and correlates with a significant increase in 5-year mortality.19 Given the notable proportion of stroke survivors at risk for PISE, hospital revisit for PISE is likely a considerable source of morbidity and mortality faced by survivors. No existing studies have used long-term follow-up in a state-wide population to study the effect of acute stroke interventions on the risk for hospital revisit after development of PISE. We therefore deployed a longitudinal study of administrative claims data from three large and diverse US states to determine the association between acute stroke interventions, demographic and clinical characteristics, and the risk of hospital revisit for PISE.
METHODS
Study Design
We performed a retrospective, longitudinal analysis of prospectively collected administrative claims data from the State Inpatient Databases and State Emergency Department Databases as part of the Healthcare Cost and Utilization Project. All data were de-identified, standardized, and checked for quality. Data from the State Inpatient Databases were available from California between 2005 and 2011, New York between 2005 and 2014, and Florida between 2005 and 2014. The State Emergency Department Database was available from California for years 2005–2011. These states were chosen because of their large, diverse populations and availability of desired data elements. Given that de-identified subject data from publicly available databases were used, this study was exempt from review from our institutional review board.
Subjects
We identified hospital admissions for ischemic stroke using a previously validated algorithm and International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes.20 Acute ischemic stroke was defined by ICD-9-CM codes 433.X1 or 434.X1 in any diagnosis code position without a primary discharge code for rehabilitation (V57) or any diagnosis of intracerebral hemorrhage (431), subarachnoid hemorrhage (430), or trauma (800–804, 850–854). We included adult subjects (age ≥ 18) who were admitted in California from 2005–2010 and in New York and Florida from 2005–2013 to allow for at least one year of follow-up in 2011 and 2014, respectively. We excluded patients who were not residents of California, New York or Florida to increase the likelihood that hospital revisits would be captured in the state-level data. Patients who did not survive to discharge and patients who were discharged to hospice were excluded from the analysis. A unique patient identifier included in the HCUP databases allows for linkage of subsequent admissions. Patients who lacked this identifier were not included. Due to the possibility of confounding, we only included patients without a pre-existing diagnosis of epilepsy or prior seizures or history of known risk factors for the development of epilepsy, including brain trauma, infection, and tumors. Pre-existing epilepsy was identified using the placement ICD-9-CM codes 345.X in any diagnosis code position when indicated as present prior to admission.
Outcome
The primary outcome, PISE, was defined as any hospital readmission or emergency room visit for seizures at least 30 days after the index stroke admission. This definition was based on the fact that a single late, unprovoked seizure after ischemic stroke constitutes epilepsy.21 Visits for seizures were identified using ICD-9-CM codes 780.39 or 345.X in the primary diagnosis position, which has been previously validated.22 Patients were followed from the time of their index ischemic stroke through the end of the available follow-up period. We tracked all subsequent hospital admissions and emergency room visits (in California only) in included subjects. In the case that patients had multiple admissions or visits for seizures, the first readmission or revisit was considered the event of interest.
Covariates
Demographic characteristics (age, sex, race/ethnicity) and baseline comorbidities (atrial fibrillation [AF], hypertension [HTN], hyperlipidemia [HLD], smoking, congestive heart failure [CHF], myocardial infarction [MI], chronic kidney disease [CKD], prior malignancy, diabetes [DM], smoking) were identified using validated ICD-9-CM codes and used as covariates in multivariable models. Drug and alcohol use were identified using the AHRQ Elixhuaser comorbidity index ICD-9-CM codes and evaluated as covariates.23 In the State Inpatient Databases, race/ethnicity is categorized into White, Black, Hispanic, Asian or Pacific Islander, Native American, and Other, with ethnicity taking precedence over race. Given that we identified very few Native American patients, Native Americans were grouped into the “Other” category. Acute seizures that occurred during the index hospital admission were identified using ICD-9-CM code 780.39, which includes both electrographic and clinical seizures.24 Procedure codes were used to gather information on treatments administered during the index hospitalization. This included the use of IV-tPA, mechanical thrombectomy, decompressive craniectomy, placement of an external ventricular drain, and electroencephalogram (EEG). Additional markers of stroke severity, including tracheostomy, percutaneous endoscopic gastrostomy (PEG) placement, and length of stay, as well as insurance payer were included. Insurance payer lists the expected primary payer for the hospital admission and includes Medicare, Medicaid, private, self-pay, or Other. Dual eligible Medicare/Medicaid patients are included in the “Medicare” category.
Statistical Analysis
R Version 4.0.0 was used for all analyses. We report descriptive statistics using means (standard deviation [SD]) and medians (interquartile range [IQR]) for normally and non-normally distributed continuous variables, respectively. Discrete variables are reported using counts (percentage [%]). Missing data was not imputed. Unadjusted comparisons involving categorical and continuous variables were performed using Chi-square and unpaired t tests, respectively. We used right-censored survival analysis to determine the incidence of PISE. Patients were censored after the first admission or ED visit for epilepsy or seizures, at the time of an in-hospital death during follow-up, or at the end of the follow-up period. We used the log-rank test to check for differences in the probability of PISE revisits between groups. Unadjusted and adjusted Cox proportional hazards regression was used to determine the association between covariates and the development of epilepsy. Visual inspection of Kaplan-Meier curves was used to confirm the proportional hazards assumption. Multivariable models were built by selecting covariates with p<0.1 in univariable analyses and forcing universal confounders age and sex into the model.
In a sensitivity analysis, we compared the rate of PISE-related revisits between patients who received acute stroke interventions to age and sex matched patients who received neither IV-tPA nor MT. Patients were matched in a 1:1 ratio.
Data Availability
The study was completed using publicly available data provided by the Healthcare Cost and Utilization Project, as described above. Information about acquiring the data can be found at https://hcup-us.ahrq.gov/.
RESULTS
Study Population
We identified 595,545 survivors of index ischemic stroke without prior history of epilepsy or risk factors for seizure development (Figure 1). The median age of included patients was 74 years (interquartile range [IQR] 62–83), and 52% were female (Table 1). Patients were followed for a median time of 4.66 years (IQR 2.50–6.83 years).
Figure 1.
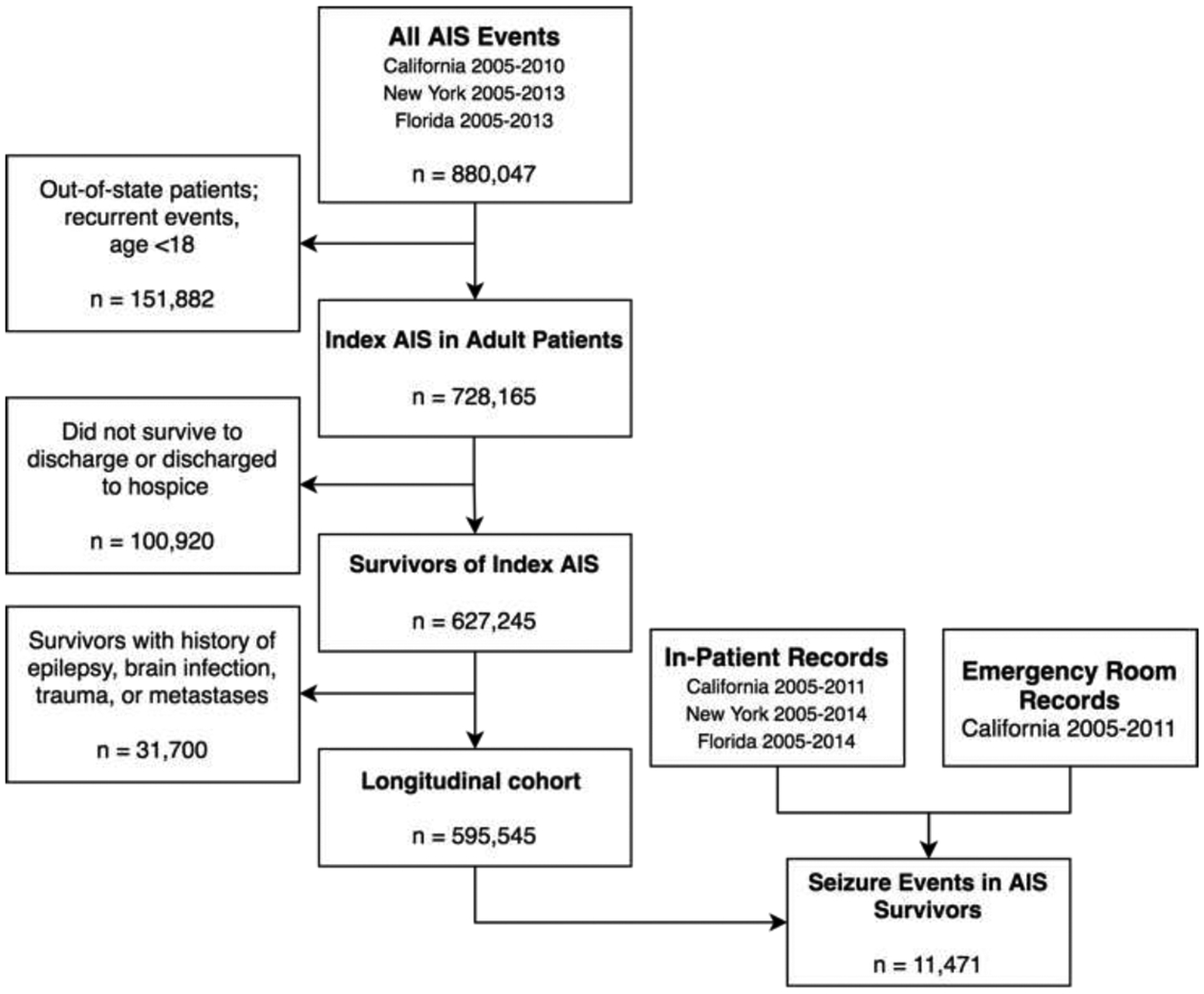
Summary of the application of study inclusion/exclusion criteria across the included datasets. The study cohort was identified from the State Inpatient Datasets from California (2005–2010), New York (2005–2013), and Florida (2005–2013). First-time stroke survivors without pre-existing risk factors for the development of epilepsy (n=595,545) were followed longitudinally for the development of PISE. Abbreviations: AIS, acute ischemic stroke; ICH, intracerebral hemorrhage; SAH, subarachnoid hemorrhage.
Table 1.
Baseline characteristics of included subjects separated by patients who did vs. did not return to the hospital with PISE.
| Variable | Without PISE N=584,047 |
With PISE N=11,471 |
p |
|---|---|---|---|
| Age (years), mean [SD) | 71 [14] | 67 [15] | <0.001 |
| Female, n (%) | 305,385 (52) | 5,932 (52) | 0.2 |
| Race, n (%) | <0.001 | ||
| White | 368,696 (64) | 6,206 (55) | |
| Black | 87,914 (15) | 2,661 (24) | |
| Hispanic | 71,463 (13) | 1,579 (14) | |
| Asian | 24,354 (4) | 375 (3) | |
| Other | 20,106 (4) | 373 (3) | |
| Insurance Payer, n (%) | <0.001 | ||
| Medicare | 394,937 (68) | 7,060 (62) | |
| Medicaid | 49,623 (9) | 1,530 (13) | |
| Private Insurance | 100,879 (17) | 2,108 (18) | |
| Comorbidities, n (%) | |||
| AF | 99,331 (17) | 2,039 (18) | 0.03 |
| HTN | 300,829 (52) | 5,719 (50) | <0.001 |
| HLD | 219,696 (38) | 4,033 (35) | <0.001 |
| DM | 157,279 (27) | 3,287 (29) | <0.001 |
| CKD | 58,769 (10) | 1,233 (11) | 0.02 |
| Smoking | 12,564 (2) | 507 (4) | <0.001 |
| Drug use | 22,495 (4) | 683 (6) | <0.001 |
| Alcohol use | 99,331 (17) | 2,039 (18) | 0.03 |
| Hospital Course, n (%) | |||
| IV-tPA | 23,296 (4) | 661 (6) | <0.001 |
| MT | 1,878 (0.3) | 63 (0.5) | <0.001 |
| Craniectomy | 700 (0.1) | 77 (0.7) | <0.001 |
| EEG | 7,840 (1) | 268 (2) | <0.001 |
| Tracheostomy | 6,741 (1) | 220 (2) | <0.001 |
| PEG | 30,054 (5) | 1,009 (9) | <0.001 |
| LOS (days), mean [SD] | 7 [9] | 9 [13] | <0.001 |
During the follow-up period, 11,471 ischemic stroke survivors (1.93%) were readmitted to the hospital or presented to the emergency room with seizures, which we classified as PISE. The median time to seizure visit was 11.47 months (IQR 5.88–21.06 months). Among patients included in the study, the 6-year cumulative risk for revisit for PISE was 2.20% (95% confidence interval [CI] 2.16–2.24). PISE was more common in patients who were younger (mean age 67 vs. 71 years, p<0.001) and patients who had more pre-existing comorbidities, including AF (18 vs. 17%, p=0.03), HTN (52 vs. 50%, p<0.001), and DM (29 vs. 27%, p<0.001). The 6-year cumulative rate of PISE-related revisit in California, where ED data were available, was 2.74% (95% CI 2.66–2.82), compared to 1.96% (95% CI 1.89–2.02) in Florida New York and 1.97% (95% CI 1.91–2.04) in New York, where only seizures that led to hospital admissions were captured (Figure 2).
Figure 2.
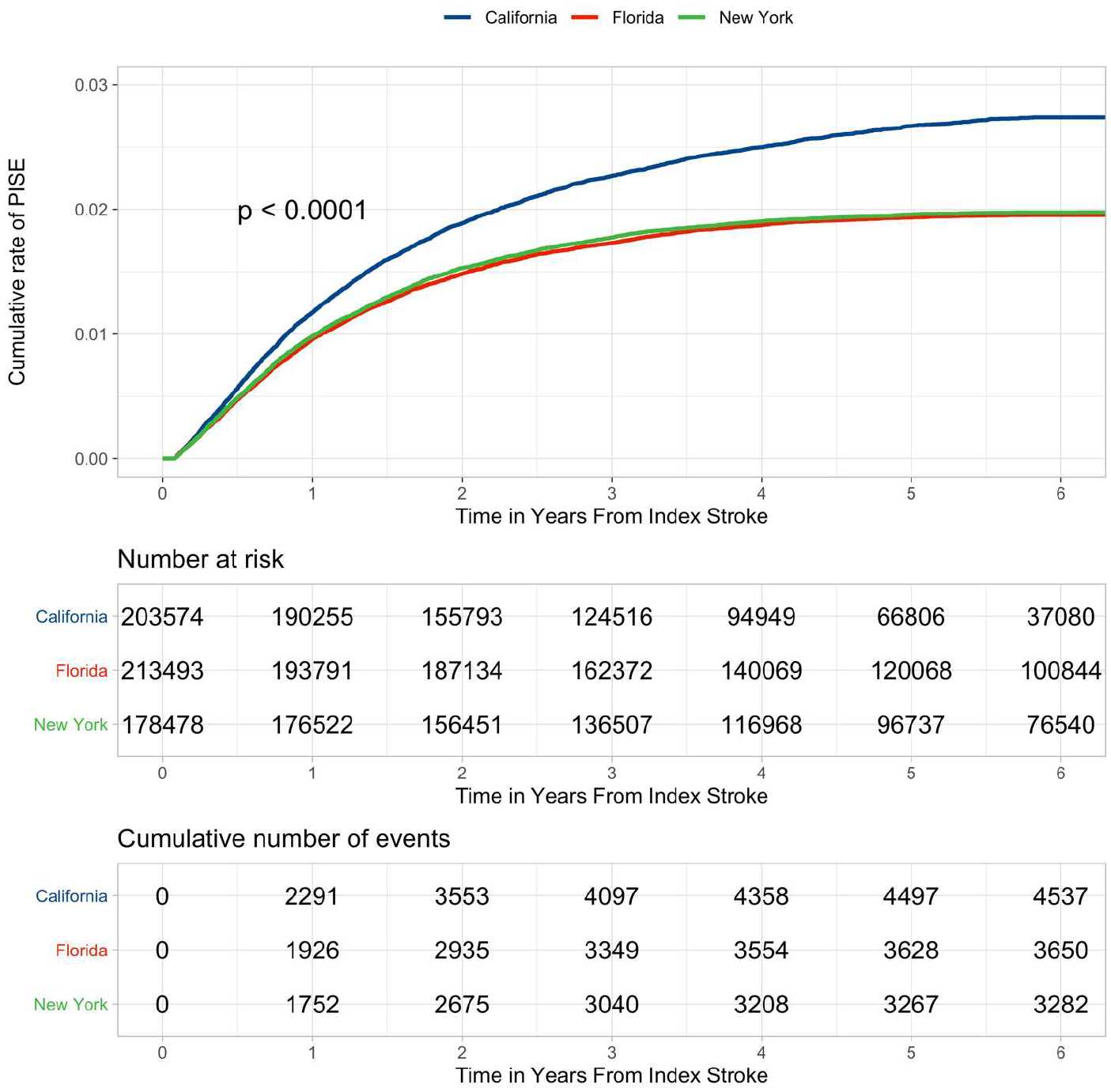
Kaplan-Meier curves demonstrating the cumulative risk for revisits for PISE separated by state – California, Florida, and New York. Shown are the results of the longitudinal analysis of ischemic stroke survivors who were followed for post-stroke seizure events in each included state. More revisits for PISE were captured in California, where emergency department data were available. P-value for the log-rank test indicates a significant difference in cumulative rate of PISE between groups. Abbreviations: PISE, post-ischemic stroke epilepsy.
Acute Stroke Treatment
In our study population, 22,818 patients (3.8%) received IV-tPA alone, 802 (0.1%) underwent mechanical thrombectomy (MT) alone, and 1,139 (0.2%) received both IV-tPA and MT. The 6-year cumulative rate of PISE after receiving IV-tPA was 3.20% (95% CI 2.94–3.45), compared to 4.11% (95% CI 2.52–5.67) after MT, 4.27% (95% CI 2.71–5.81) after both IV-tPA and MT, and 2.16% (95% CI 2.12–2.20) after neither treatment (Table 2, Figure 3). In unadjusted regression analyses, we found that patients who received IV-tPA (hazard ratio [HR] 1.50, 95% confidence interval [CI] 1.39–1.63, p<0.001) or who underwent MT (HR 1.86, 95% CI 1.86–2.38, p<0.001) were at greater risk for developing PISE (Figure 4) compared to patients who received neither treatment.
Table 2.
Cumulative rates of revisits for PISE among patients treated with acute stroke therapies.
| All Patients | IV-tPA | MT | Both IV-tPA and MT | Neither | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 595,545 | N = 22,818 | N = 802 | N = 1,139 | N = 570,786 | |||||||||||
| Risk | At risk | Events | Rate (%) | At risk | Events | Rate (%) | At risk | Events | Rate (%) | At risk | Events | Rate (%) | At risk | Events | Rate (%) |
| 1-Year Risk | 560,568 | 5,969 | 1.03 | 21,566 | 342 | 1.55 | 737 | 14 | 1.84 | 1,054 | 18 | 1.66 | 537,211 | 5,595 | 1.02 |
| 6-Year Cumulative Risk | 214,464 | 5,500 | 2.20 | 6,165 | 283 | 3.20 | 87 | 13 | 4.11 | 168 | 18 | 4.27 | 208,044 | 5,186 | 2.16 |
Figure 3.
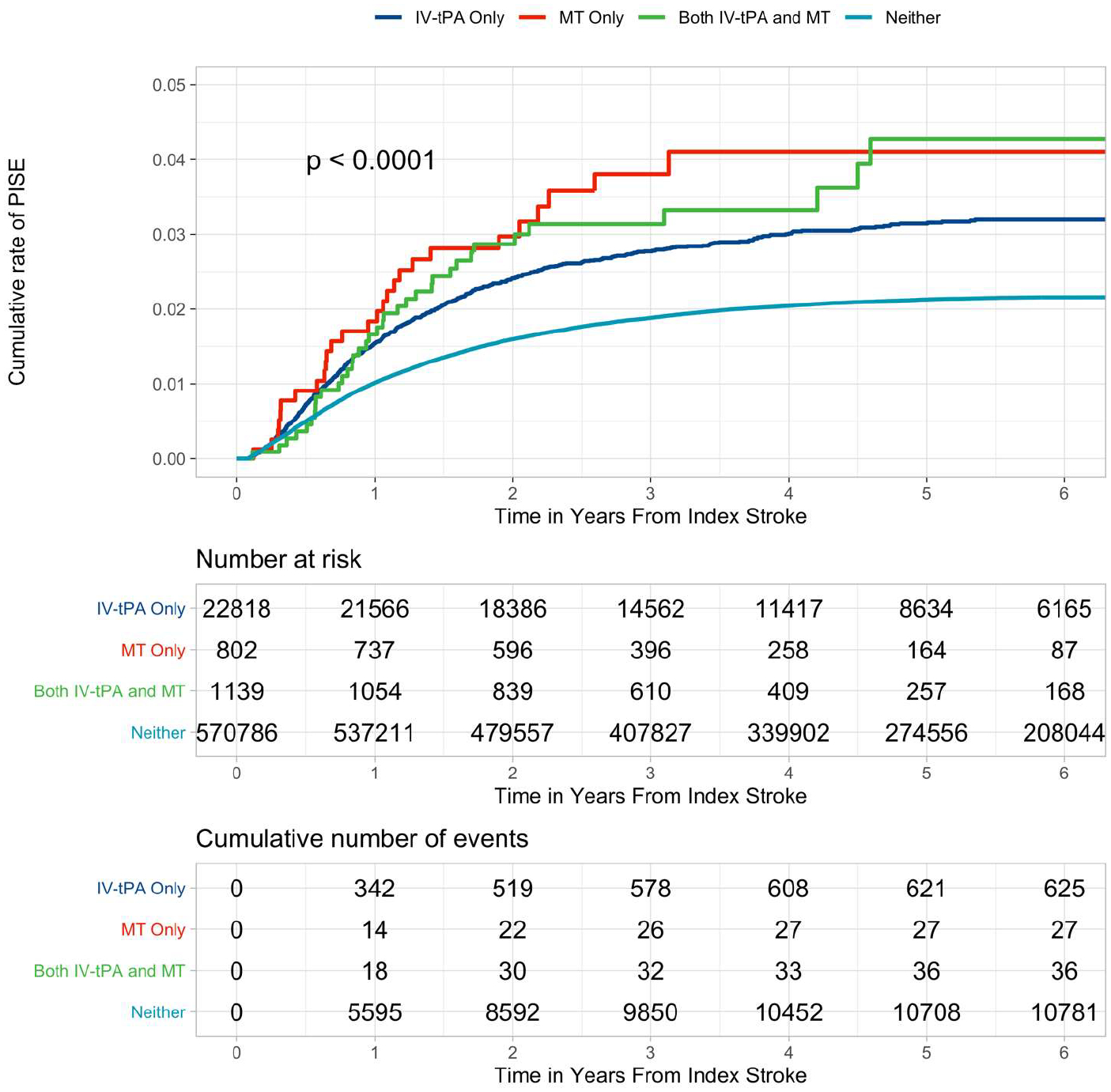
Kaplan-Meier curves demonstrating the cumulative risk for PISE-related revisits separated by treatment exposure at index stroke – IV-tPA, MT, both treatments, and neither treatment. Shown are the results of the longitudinal analysis of ischemic stroke survivors who were followed for post-stroke seizure events. Patients who received IV-tPA, underwent MT, or received both treatments were at increased risk for hospital revisit for PISE. P-value for the log-rank test indicates a significant difference in cumulative rate of PISE between groups. Abbreviations: IV-tPA, intravenous alteplase; MT, mechanical thrombectomy; PISE, post-ischemic stroke epilepsy.
Figure 4.
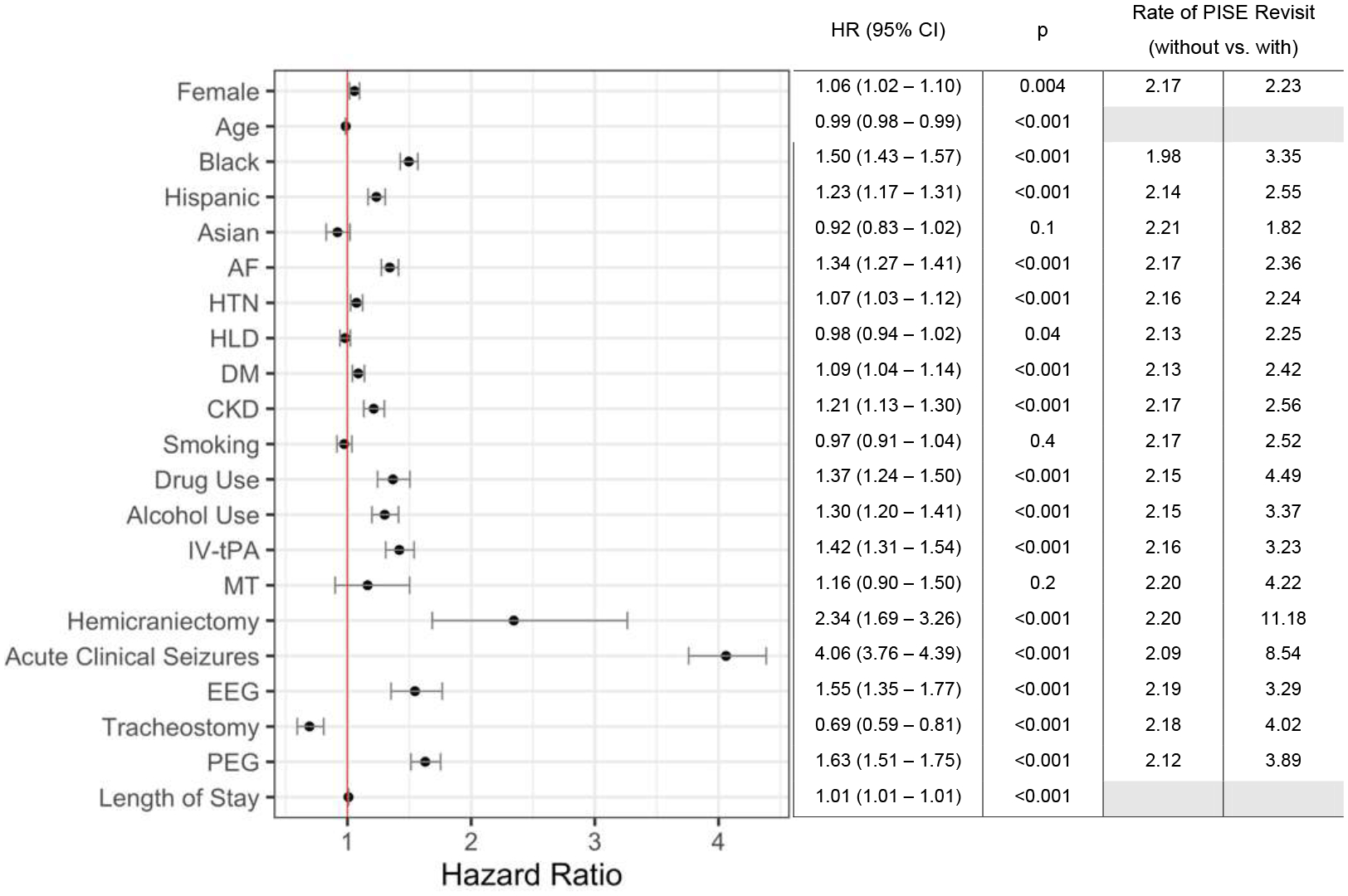
Adjusted Cox proportional hazards regression model evaluating the association between baseline patient characteristics, stroke interventions, and attributes of the index hospitalization with hospital revisits for PISE. The estimated hazard ratio for each covariate is plotted on the X-axis and listed in the adjacent table. Error bars represent 95% confidence intervals. The vertical red line indicates a hazard ratio of 1. EEG refers to patients who underwent EEG but did not experience acute seizures during the index hospitalization. The 6-year cumulative rate of PISE-related revisit in patients with vs. without each binary covariate is listed in the last two columns of the table to clarify the impact of each covariate on the risk for PISE-related revisit. Abbreviations: CI, confidence interval; HR, hazard ratio; PISE, post-ischemic stroke epilepsy.
Stroke Severity
We assessed the impact of surrogate markers of stroke severity on the risk for hospital revisit for PISE. The 6-year cumulative risk for PISE-related revisit was 11.18% (95% CI 7.68–14.54) in patients who underwent decompressive craniectomy compared to 2.20% (95% CI 2.16–2.24) in patients who did not. Craniectomy was associated with the greatest increase in risk for PISE-related revisit (hazard ratio [HR] 5.51, 95% CI 4.01–7.58, p<0.001). We discovered a 1% increase in the risk for PISE-related revisit for each additional day of hospitalization for the index stroke (HR 1.01, 95% CI 1.01–1.01, p<0.001). Further, patients who required a tracheostomy experienced a 2-fold increase in odds of returning to the hospital with PISE (HR 1.89, 95% CI 1.65–2.16, p<0.001).
Multivariable Analysis
In adjusted Cox proportional hazards regression models, we tested the association between acute stroke therapies and revisits for PISE while accounting for baseline demographics, comorbidities, and characteristics of the index hospitalization (Figure 4). IV-tPA was associated with a 42% increase in the odds of PISE-related revisit (HR 1.42, 95% CI 1.31–1.54, p<0.001, Figure 3). We did not find an association between MT and PISE (HR 1.62, 95% CI 0.90–1.50, p=0.2) in the adjusted model. The strongest predictors for PISE-related revisits were decompressive craniectomy (HR 2.35, 95% CI 1.69–3.26, p<0.001) and acute seizures during the index hospital admission (HR 4.06, 95% CI 3.76–4.39, p<0.001). In the absence of acute seizures, the use of EEG during the index hospitalization still predicted revisits for PISE during follow-up (HR 1.55, 95% CI 1.35–1.77, p<0.001). Race, AF, HTN, DM, CKD, and drug and alcohol use were also associated with PISE-related revisits.
We used exploratory model building to determine which covariates explained the lack of association between MT and PISE in adjusted analyses. In a multivariable model that included all covariates listed in Figure 4, but specifically excluded AF and markers of stroke severity (craniectomy, tracheostomy and PEG placement, and length of stay) we did observe a significant correlation between MT and PISE (HR 1.40, 95% CI 1.09–1.81, p=0.009). When AF and severity markers were added to the model, the correlation between MT and PISE did not persist.
Sensitivity Analysis
In a sensitivity analysis, where we compared ischemic stroke patients who received IV-tPA to age- and sex-matched stroke controls who received neither IV-tPA nor MT, the association between IV-tPA and PISE persisted. The 6-year cumulative rate of PISE-related revisit in the control cohort was 2.22% (95% CI 2.01–2.42), while a rate of 3.22% (95% CI 2.96–3.47) was found in the IV-tPA group.
DISCUSSION
In this longitudinal, state-wide analysis, we found that the use of IV-tPA, but not MT, was an independent risk factor for hospital revisits for PISE. There was an increase in risk for revisits for PISE among stroke survivors who received IV-tPA even after adjustment for demographic factors and indicators of stroke severity. MT was associated with PISE in unadjusted models, but the association did not persist in adjusted analyses. Neurosurgical intervention with decompressive craniectomy and acute seizures during the index hospitalization were most strongly predictive of PISE-related revisits. We followed patients for a median of nearly 5 years. While other explanations for the development of epilepsy could arise in this period, there are known latencies between brain injury and development of PISE that warrant longitudinal follow-up.25 Our results support an elevated risk for PISE hospital revisits after ischemic strokes treated with IV-tPA.
Our analysis identified an elevated rate of hospital revisits for PISE in patients who were treated with IV-tPA. Mixed results from prior studies have made the relationship between IV-tPA and PISE difficult to clarify. Some studies report no differences in the rate of PISE among patients who did vs. did not receive IV-tPA,12 while others report a significantly increased risk for PISE after IV-tPA that is not explained by stroke severity or baseline comorbidities.14 Even further, one study identified a decreased risk for seizures after IV-tPA treatment.26 Among disparate findings, our results add evidence for a small but statistically significant relationship between ischemic strokes treated with IV-tPA and PISE. We utilized a population-sized sample of demographically and clinically diverse ischemic stroke survivors, who were free of pre-existing risk factors for epilepsy development, to show that treatment with IV-tPA correlates with PISE. In an age- and sex-matched sensitivity analysis, the association persisted. It is possible that this relationship is confounded by clinical predictors that we were unable to assess in our data.
Stroke patients who require treatment with IV-tPA may have larger and more debilitating strokes or may survive with greater volume of partially infarcted tissue than their counterparts. IV-tPA also increases the risk for hemorrhagic conversion, which, even if minor, significantly increases the risk for PISE.27 Our findings nonetheless provide strong evidence that increased vigilance for new-onset seizures is warranted in stroke survivors treated with IV-tPA.
In contrast, our results did not support an association between MT and PISE. Like IV-tPA, the relationship between endovascular therapies and PISE has not been clear. Only one study identified an independent association between MT and post-stroke seizures,14 while other positive studies found that the association depended upon infarct size and occurrence of hemorrhagic transformation.28,29 These findings fit more closely with our results. We found that MT correlated with PISE in univariable analyses, but after adjustment for baseline characteristics, other acute stroke therapies, and indicators of stroke severity, the association did not persist. However, we were unable to assess specific aspects of the intervention that may affect the risk for PISE, such as degree of recanalization success, hemorrhagic transformation, or thrombectomy-related subarachnoid hemorrhage. Additionally, we were limited to data through 2014. Results from trials demonstrating the efficacy of MT for acute stroke treatment were not released until 2015,30 after which MT became both more successful and widely used. Future studies including data from 2015 onwards will be pivotal in identifying or discrediting a potential link between MT and PISE. Patients who underwent MT in our study cohort likely had large stroke syndromes and potentially low rates of successful recanalization. Thus, studies from 2015 onwards are needed to fully understand the association between MT and PISE. It is possible that treatment with MT only correlates with PISE when recanalization is unsuccessful. If true, this may indicate that the risk for seizures is confounded by extensive ischemic injury caused by large vessel occlusions and, as our results support, is not due to an independent epileptogenic effect of MT. Further, hemorrhagic transformation is a known predictor of PISE and occurs in up to 11% of ischemic strokes treated with MT.31 The risk for hemorrhagic transformation after MT may in part explain the association between MT and PISE seen in our univariable analyses. However, we were unable to address this in our dataset given that ICD-9-CM codes cannot be reliably used to identify hemorrhagic transformation.32 Future studies with neuroimaging data would help clarify whether infarct size and hemorrhagic transformation, even if minor, are independent predictors of PISE-related revisits in patients treated with MT. Lastly, reperfusion syndrome, caused by sudden cerebral reperfusion and characterized by early seizures, is one plausible way by which MT predisposes patients to PISE.33,14 But this mechanism again relies on an intermediary, in this case early seizures, to establish a greater risk for PISE. Correspondingly, in our adjusted analyses accounting for the occurrence of in-hospital seizures, MT did not correlate with revisits for PISE.
In our exploratory, multivariable model, we discovered that the association between MT and PISE was lost specifically when pre-existing AF and indicators of stroke severity were included as covariates. AF is a known risk factor for large vessel occlusions, a stroke type that commonly requires intervention with MT.34,35 As well, strokes of cardioembolic etiology are more likely to produce cortically distributed infarcts, which have been shown to increase the risk for PISE.36,37 Thus, AF, as a marker of large vessel occlusions and cortical involvement that may necessitate intervention with MT, may in part explain the increase in risk for PISE after MT. Similarly, increasing stroke severity despite attempted intervention may also explain our findings. It is possible that strokes requiring decompressive craniectomy represent instances of unsuccessful MT. The risk for PISE in patients who underwent MT but still required craniectomies, tracheostomy/PEG placements, or extended hospital stays is likely more closely related to the volume and severity of the stroke than to the MT intervention. We were unable to assess the recanalization rate following MT in this dataset, which may also explain the lack of significance in the multivariable model. Based on our exploratory analysis, we hypothesize that AF and increasing stroke severity, which overlap with unsuccessful MT, are stronger predictors of PISE than undergoing MT without stratification of success rates. Further studies with detailed clinical and imaging data are needed to confirm this hypothesis.
Treatment with decompressive craniectomy imparted a significantly increased risk for returning to the hospital with PISE. The rate of PISE in patients undergoing craniectomy for malignant infarction has been estimated between 45 and 55%.38,39 We identified a rate of hospital revisit of 11.18%, indicating that a strong association between neurosurgical interventions and development of PISE is supported. The risk for PISE associated with craniectomy is likely due to underlying stroke severity, rather than the procedure itself. Additional research is needed to clarify the factors underlying the risk of PISE after craniectomy.40
The greatest risk for PISE-related revisit occurred in patients who experienced in-hospital seizures, aligning with the known risk for post-stroke epilepsy after early seizures and status epilepticus.41,42 Interestingly, we also found that patients who did not experience acute seizures but underwent EEG also faced an elevated risk for epilepsy. It is possible that patients who required EEG without experiencing an event characterized or coded as seizures may have still shown other abnormal EEG patterns, such as periodic epileptiform discharges, a biomarker associated with the development of electrographic seizures. This would support prior evidence provided that periodic discharges that do not meet seizure criteria are a significant independent risk factor for the development of epilepsy later on.43,44 The presence of an early neurophysiological risk factor for PISE indicates that broader use of continuous or extended EEG monitoring after stroke may be necessary. Ongoing research to identify which patients require extended EEG monitoring will be crucial for identifying those at high risk for PISE and eventually preventing its development.43
There are limitations to this study. First, we were unable to account for infarct size, stroke location, or hemorrhagic transformation in our models, which are known risk factors for the development of PISE. Thus, it is possible that the observed associations were confounded by stroke severity, degree of cortical involvement, or hemorrhagic transformation of the index stroke. We demonstrate a robust correlation between IV-tPA use and PISE while understanding that the mechanistic association is not yet clear. Second, the use of ICD-9-CM codes to identify our initial study cohort and outcomes measures may be flawed. It is possible that hospital admissions were incorrectly attributed to ischemic stroke or seizure events. To attempt to mediate this, we used only previously validated ICD-9-CM codes. Also, due to inherent limitations of claims data, we focused only on PISE events that led to hospital revisits and were unable to quantify those who were treated in the outpatient setting. Additionally, we were only able to assess seizure revisits in the ED setting in California and therefore missed seizure events that occurred in the ED in New York or Florida, unless they led to admission. This limitation is reflected in a slightly higher risk observed in California compared to New York and Florida. Also, given that our outcome relies on readmissions, PISE would not have been detected until after discharge in the small proportion of patients (<2%) with an index length of stay longer than 30 days. Lastly, claims data lacks information on prescription medications, rendering us unable to evaluate the effect of prior and post-discharge medications on the risk of PISE. Future studies of stroke survivors that may clarify the mechanism of the correlation between administration of IV-tPA and improving mechanical thrombectomy procedures are warranted. Studies that account for stroke severity, infarct location, hemosiderin deposition, early EEG findings, and medication usage will be crucial.
In conclusion, treatment with IV-tPA was an independent risk factor for hospital revisits for PISE. Patients treated with MT experienced higher rates of PISE although MT was not independently associated with PISE after controlling for AF and severity markers. Decompressive craniectomy and the need for EEG were also independent predictors of PISE-related revisits, as were in-hospital seizures during the index admission. Further investigation is needed to clarify the impact of acute stroke care on PISE while carefully accounting for clinical and neuroimaging stroke characteristics.
Highlights:
Use of IV-tPA was associated with hospital revisit for PISE when adjusting for demographics, comorbidities, and characteristics of the index stroke admission.
There was no independent association between MT and PISE-related revisits in adjusted models.
Decompressive craniectomy was associated with more than a 2-fold increase in the risk for revisit for PISE.
The occurrence of in-hospital seizures correlated most closely with long-term revisits for PISE.
Sources of Support
EJG is supported by the NIH (R01NS117904). KNS is supported by the NIH (U24NS107136, U24NS107215, R01NR018335, R01NS107215, U01NS106513, R03NS112859) and the American Heart Association (18TPA34170180,17CSA33550004). JAK is supported by the NIH (K23NS112596), AAN Clinical Research Training Scholarship and the NIH NeuroNEXT fellowship. The funding entities had no role in the design of the study; collection, management, analysis, or interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The present manuscript was completed within the Department of Neurology at Yale School of Medicine in New Haven, CT. The manuscript complies with all instructions to authors. All authors have met authorship requirements and provided final approval of the manuscript. The manuscript has not previously been published and is not under consideration for publication by another journal. Given that only de-identified subject data from publicly available databases were used, this study was exempt from review from our institutional review board. The STROBE checklist was used for this study.
Declaration of Competing Interest
Ms. Kuohn reports no conflicts of interest. Ms. Herman reports no conflicts of interest. Ms. Soto reports no conflicts of interest. Dr. Brown reports no conflicts of interest. Dr. Gilmore reports no conflicts of interest. Dr. Hirsch reports no conflicts of interest. Dr. Matouk reports no conflicts of interest. Dr. Sheth reports no conflicts of interest. Dr. Kim reports no conflicts of interest.
REFERENCES
- 1.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. The Lancet. 2014;383:245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pitkänen A, Roivainen R, Lukasiuk K. Development of epilepsy after ischaemic stroke. Lancet Neurol. 2016;15:185–197. [DOI] [PubMed] [Google Scholar]
- 3.Roivainen R, Haapaniemi E, Putaala J, Kaste M, Tatlisumak T. Young adult ischaemic stroke related acute symptomatic and late seizures: risk factors. Eur. J. Neurol 2013;20:1247–1255. [DOI] [PubMed] [Google Scholar]
- 4.Burn J, Dennis M, Bamford J, Sandercock P, Wade D, Warlow C. Epileptic seizures after a first stroke: the Oxfordshire Community Stroke Project. BMJ. 1997;315:1582–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C-W, Saposnik G, Fang J, Steven DA, Burneo JG. Influence of seizures on stroke outcomes: a large multicenter study. Neurology. 2014;82:768–776. [DOI] [PubMed] [Google Scholar]
- 6.Kim JS, Choi-Kwon S, Kwon SU, Lee HJ, Park K-A, Seo YS. Factors Affecting the Quality of Life After Ischemic Stroke: Young Versus Old Patients. J. Clin. Neurol 2005;1:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arntz Renate M, Rutten-Jacobs Loes CA, Maaijwee Noortje AM, Schoonderwaldt Hennie C, Dorresteijn Lucille DA, van Dijk Ewoud J, de Leeuw Frank-Erik. Poststroke Epilepsy Is Associated With a High Mortality After a Stroke at Young Age. Stroke. 2015;46:2309–2311. [DOI] [PubMed] [Google Scholar]
- 8.De Reuck J, Van Maele G. Acute ischemic stroke treatment and the occurrence of seizures. Clin. Neurol. Neurosurg 2010;112:328–331. [DOI] [PubMed] [Google Scholar]
- 9.Iyer AM, Zurolo E, Boer K, Baayen JC, Giangaspero F, Arcella A, Di Gennaro GC, Esposito V, Spliet WGM, van Rijen PC, et al. Tissue plasminogen activator and urokinase plasminogen activator in human epileptogenic pathologies. Neuroscience. 2010;167:929–945. [DOI] [PubMed] [Google Scholar]
- 10.Rodan LH, Aviv RI, Sahlas DJ, Murray BJ, Gladstone JP, Gladstone DJ. Seizures during stroke thrombolysis heralding dramatic neurologic recovery. Neurology. 2006;67:2048–2049. [DOI] [PubMed] [Google Scholar]
- 11.Simon Jung, Kaspar Schindler, Oliver Findling, Marie-Luise Mono, Urs Fischer, Jan Gralla, Marwan El-Koussy, Anja Weck, Aekaterini Galimanis, Caspar Brekenfeld, et al. Adverse Effect of Early Epileptic Seizures in Patients Receiving Endovascular Therapy for Acute Stroke. Stroke. 2012;43:1584–1590. [DOI] [PubMed] [Google Scholar]
- 12.Bentes C, Martins H, Peralta AR, Morgado C, Casimiro C, Franco AC, Fonseca AC, Geraldes R, Canhão P, Melo TP e, et al. Epileptic manifestations in stroke patients treated with intravenous alteplase. Eur. J. Neurol 2017;24:755–761. [DOI] [PubMed] [Google Scholar]
- 13.Keller L, Hobohm C, Zeynalova S, Classen J, Baum P. Does treatment with t-PA increase the risk of developing epilepsy after stroke? J. Neurol 2015;262:2364–2372. [DOI] [PubMed] [Google Scholar]
- 14.Naylor J, Thevathasan A, Churilov L, Guo R, Xiong Y, Koome M, Chen Z, Chen Z, Liu X, Kwan P, et al. Association between different acute stroke therapies and development of post stroke seizures. BMC Neurol. 2018;18:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chi N-F, Kuan Y-C, Huang Y-H, Chan L, Hu C-J, Liu H-Y, Chiou H-Y, Chien L-N. Development and validation of risk score to estimate 1-year late poststroke epilepsy risk in ischemic stroke patients. Clin. Epidemiol 2018;10:1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HJ, Park KD, Choi K-G, Lee HW. Clinical predictors of seizure recurrence after the first post-ischemic stroke seizure. BMC Neurol. 2016;16:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pande SD, Lwin MT, Kyaw KM, Khine AA, Thant AA, Win MM, Morris J. Post‐stroke seizure—Do he locations, types and managements of stroke matter? Epilepsia Open. 2018;3:392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin H-J, Chang W-L, Tseng M-C. Readmission after stroke in a hospital-based registry: Risk, etiologies, and risk factors. Neurology. 2011;76:438–443. [DOI] [PubMed] [Google Scholar]
- 19.Bjerkreim AT, Khanevski AN, Thomassen L, Selvik HA, Waje-Andreassen U, Naess H, Logallo N. Five-year readmission and mortality differ by ischemic stroke subtype. J. Neurol. Sci 2019;403:31–37. [DOI] [PubMed] [Google Scholar]
- 20.Tirschwell David L, Longstreth WT Validating Administrative Data in Stroke Research. Stroke. 2002;33:2465–2470. [DOI] [PubMed] [Google Scholar]
- 21.Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, Engel J, Forsgren L, French JA, Glynn M, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–482. [DOI] [PubMed] [Google Scholar]
- 22.Kee VR, Gilchrist B, Granner MA, Sarrazin NR, Carnahan RM. A systematic review of validated methods for identifying seizures, convulsions, or epilepsy using administrative and claims data. Pharmacoepidemiol. Drug Saf 2012;21 Suppl 1:183–193. [DOI] [PubMed] [Google Scholar]
- 23.Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying Increased Risk of Readmission and In-hospital Mortality Using Hospital Administrative Data: The AHRQ Elixhauser Comorbidity Index. Med. Care 2017;55:698–705. [DOI] [PubMed] [Google Scholar]
- 24.Barkley GL. Coding of Seizures and Epilepsy. :11. [Google Scholar]
- 25.Lowenstein DH. Epilepsy after head injury: an overview. Epilepsia. 2009;50 Suppl 2:4–9. [DOI] [PubMed] [Google Scholar]
- 26.Nesselroth D, Gilad R, Namneh M, Avishay S, Eilam A. Estimation of seizures prevalence in ischemic strokes after thrombolytic therapy. Seizure. 2018;62:91–94. [DOI] [PubMed] [Google Scholar]
- 27.Brondani R, de Almeida AG, Cherubini PA, Secchi TL, de Oliveira MA, Martins SCO, Bianchin MM. Risk Factors for Epilepsy After Thrombolysis for Ischemic Stroke: A Cohort Study. Front. Neurol [Internet]. 2020. [cited 2020 Nov 22];10. Available from: https://www.frontiersin.org/articles/10.3389/fneur.2019.01256/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anadani M, Lekoubou A, Almallouhi E, Alawieh A, Chatterjee A, Vargas J, Spiotta AM. Incidence, predictors, and outcome of early seizures after mechanical thrombectomy. J. Neurol. Sci 2019;396:235–239. [DOI] [PubMed] [Google Scholar]
- 29.Thevathasan A, Naylor J, Churilov L, Mitchell PJ, Dowling RJ, Yan B, Kwan P. Association between hemorrhagic transformation after endovascular therapy and poststroke seizures. Epilepsia. 2018;59:403–409. [DOI] [PubMed] [Google Scholar]
- 30.Fransen PS, Beumer D, Berkhemer OA, van den Berg LA, Lingsma H, van der Lugt A, van Zwam WH, van Oostenbrugge RJ, Roos YB, Majoie CB, et al. MR CLEAN, a multicenter randomized clinical trial of endovascular treatment for acute ischemic stroke in the Netherlands: study protocol for a randomized controlled trial. Trials [Internet]. 2014. [cited 2020 Aug 12];15. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4162915/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The Penumbra Pivotal Stroke Trial. Stroke. 2009;40:2761–2768. [DOI] [PubMed] [Google Scholar]
- 32.Mullen MT, Moomaw CJ, Alwell K, Khoury JC, Kissela BM, Woo D, Flaherty ML, Khatri P, Adeoye O, Ferioli S, et al. ICD9 Codes Cannot Reliably Identify Hemorrhagic Transformation of Ischemic Stroke. Circ. Cardiovasc. Qual. Outcomes 2013;6:505–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silverman IE, Restrepo L, Mathews GC. Poststroke Seizures. Arch. Neurol 2002;59:195–201. [DOI] [PubMed] [Google Scholar]
- 34.Inoue M, Noda R, Yamaguchi S, Tamai Y, Miyahara M, Yanagisawa S, Okamoto K, Hara T, Takeuchi S, Miki K, et al. Specific Factors to Predict Large-Vessel Occlusion in Acute Stroke Patients. J. Stroke Cerebrovasc. Dis 2018;27:886–891. [DOI] [PubMed] [Google Scholar]
- 35.Palaniswami M, Yan B. Mechanical Thrombectomy Is Now the Gold Standard for Acute Ischemic Stroke: Implications for Routine Clinical Practice. Interv. Neurol 2015;4:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ringelstein EB, Koschorke S, Holling A, Thron A, Lambertz H, Minale C. Computed tomographic patterns of proven embolic brain infarctions. Ann. Neurol 1989;26:759–765. [DOI] [PubMed] [Google Scholar]
- 37.Graham Neil SN, Crichton Siobhan, Koutroumanidis Michael, Wolfe Charles DA, Rudd Anthony G Incidence and Associations of Poststroke Epilepsy. Stroke. 2013;44:605–611. [DOI] [PubMed] [Google Scholar]
- 38.Brondani R, Garcia de Almeida A, Abrahim Cherubini P, Mandelli Mota S, de Alencastro LC, Antunes ACM, Bianchin Muxfeldt M. High Risk of Seizures and Epilepsy after Decompressive Hemicraniectomy for Malignant Middle Cerebral Artery Stroke. Cerebrovasc. Dis. Extra 2017;7:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Creutzfeldt CJ, Tirschwell DL, Kim LJ, Schubert GB, Longstreth WT, Becker KJ. Seizures after decompressive hemicraniectomy for ischaemic stroke. J. Neurol. Neurosurg. Psychiatry 2014;85:721–725. [DOI] [PubMed] [Google Scholar]
- 40.Stretz C, Sheikh Z, Maciel CB, Hirsch LJ, Gilmore EJ. Seizures, periodic and rhythmic patterns in primary intraventricular hemorrhage. Ann. Clin. Transl. Neurol 2018;5:1104–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rumbach L, Sablot D, Berger E, Tatu L, Vuillier F, Moulin T. Status epilepticus in stroke. Neurology. 2000;54:350. [DOI] [PubMed] [Google Scholar]
- 42.Labovitz DL, Allen Hauser W, Sacco RL. Prevalence and predictors of early seizure and status epilepticus after first stroke. Neurology. 2001;57:200. [DOI] [PubMed] [Google Scholar]
- 43.Bentes C, Martins H, Peralta AR, Morgado C, Casimiro C, Franco AC, Fonseca AC, Geraldes R, Canhão P, Pinho e Melo T, et al. Early EEG predicts poststroke epilepsy. Epilepsia Open. 2018;3:203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Punia V, Bena J, Krishnan B, Newey C, Hantus S. New onset epilepsy among patients with periodic discharges on continuous electroencephalographic monitoring. Epilepsia. 2018;59:1612–1620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study was completed using publicly available data provided by the Healthcare Cost and Utilization Project, as described above. Information about acquiring the data can be found at https://hcup-us.ahrq.gov/.


