Abstract
Purpose:
Older cancer patients are susceptible to long-term effects of chemotherapy, including cancer-related cognitive decline and impairments to quality of life. Taxane-based chemotherapies are associated with physical declines among older women and may negatively impact cognitive performance. We sought to examine whether changes in objective and subjective measures of cognitive performance and well-being differ among older breast cancer survivors as a function of taxane-based chemotherapy treatment regimens.
Methods:
Individual-level data was pooled and harmonized from two large prospective studies of older (greater than 60 years) breast cancer survivors. Assessments were conducted prior to systemic therapy and up to 36-months after. Cognitive performance was assessed with objective (working memory, processing speed and executive functions) and subjective tests and physical, emotional and functional well-being was also assessed.
Results:
One hundred and sixty-seven (M age = 67.3 years) women, with 116 receiving chemotherapy with taxanes and 51 without taxanes contributed data. Declines in subjective cognition for both groups were significant between pre-treatment and 12-month follow-up. Significant improvements were seen on a measure of objective cognition (working memory) from 12 to 36-months. Measures of well-being improved from prior to systemic therapy to 12-months. Longitudinal changes across all measures did not vary as a function of receipt of taxane-based treatment.
Conclusion:
Older women who received treatment with taxanes did not have greater declines in cognitive performance or well-being than women receiving other chemotherapy regimens. Despite older cancer survivors being at greater risk for negative outcomes, treatment with taxane-based chemotherapies does not appear to exacerbate these health consequences.
Keywords: Breast cancer, cognition, chemotherapy, taxanes, longitudinal changes
A majority of the observed declines in breast cancer mortality rates over the past decades have been attributed to advances in adjuvant chemotherapy.[1] Among the millions of women now living after breast cancer, approximately three-quarters of them are older than age 65.[2] The number of older survivors will increase over the coming decades due to population dynamics.[3] However, survivors face lifelong risks of adverse health effects from initial therapy, especially chemotherapy, and these effects can have lasting impact on quality of life.[3, 4] Older survivors may be especially vulnerable to long-term chemotherapy-related adverse effects, since they are also facing normative aging-related processes.[5] Cancer-related cognitive decline (CRCD) is an important long-term chemotherapy-related adverse effect.[6–10] Older age is one risk factor for CRCD after chemotherapy either due to vulnerability to direct neurotoxicity and/or unmasking of underlying age-related neurodegenerative processes.[6, 11, 12]
Adjuvant rates of chemotherapy use are expected to decrease in the future with the growth of evidence on use of gene-expression profile tests to guide treatment among women with hormone receptor positive early stage breast cancers.[13] However, chemotherapy will likely continue to be indicated for women newly diagnosed with breast cancers with high recurrence risk factors, advanced stage disease and/or hormonal receptor negative. Taxane-based regimens have rapidly diffused into practice and are a key component of regimens for women with indications for chemotherapy.[14] However, this class of drugs is associated with greater physical functional declines in older women with breast cancer [15, 16]. Preclinical studies indicate treatment with taxanes induce cognitive impairment and the negative impact of these treatments on synaptic plasticity and inflammatory processes may also compromise cognitive performance in humans.[17] However, this idea has been difficult to study for several reasons. The inclusion of taxanes for cancer treatment among older adults was not standard of care and as a result, no research has examined the impact of taxanes on cognitive performance among older adults. There are limited numbers of older women in chemotherapy trials and fewer still that include sufficient numbers of older women and/or data on quality of life and cognitive endpoints for specific regimens and agents.[13] Data on regimen-specific effects of chemotherapy would be useful to oncologists and their older patients who require chemotherapy.
To fill this gap, we pooled and harmonized individual-level data from two large prospective cohorts of older breast cancer survivors (TLC and CogAge).[6, 11, 15, 18] The overall goal of this study was to test whether older women receiving adjuvant taxane-based treatment regimens had greater short-term (pre-treatment to 12-months) or longer-term (12- to 36-months) longitudinal declines in subjective and objective measures of neurocognitive performance and quality of life (QOL) than those receiving non-taxane regimens. The results are intended to guide future research and to inform discussions about breast cancer treatment choices among older women who will undergo adjuvant chemotherapy.
METHODS
Participants
Participants were selected from the Thinking and Living with Cancer Study (TLC) [6, 11] and the CogAge [15, 18] studies. These studies are among the only cohort studies designed to specifically examine longitudinal outcomes among older women with localized breast cancer. Both included pre- and post-systemic therapy assessments of self-reported cognition and the same or comparable neuropsychological tests and domains of cognition. The studies also each measured longitudinal quality of life dimensions.
TLC was Institutional Review Board approved (ClinicalTrials.gov Identifier: NCT03451383) and was conducted at five US sites. TLC enrolled breast cancer survivors were 60 years of age or older, newly diagnosed with primary nonmetastatic breast cancer, and able to complete assessments in English.[11] Women were evaluated at enrollment (post-surgery, pre-systemic therapy) and annually for up to 36-months. We included women enrolled from August 2010 to Dec 2016; enrollment and follow-up is ongoing. CogAge was conducted in three French comprehensive cancer centers.[15, 18] Eligible women were ages 65 and older newly diagnosed with breast cancer AJCCv6 stages 1 and 2 enrolled between January 2009 to August 2012. Pre-treatment assessments occurred after surgery, but before the start of adjuvant therapy. Women were further assessed at 6-, 12-, 18- and 30-months following the pre-treatment assessment.
Both studies excluded women with a history of prior cancer and cancer systemic therapy, metastatic breast cancer, neurological disease, major psychiatric disorders or addictions, a Mini-Mental State Examination (MMSE) score <25 or reading level below 3rd grade or <5 years of formal education.
Measures
The primary predictor variable was treatment regimen based on medical records and dichotomized as containing taxanes (yes vs. no) for adjuvant treatment. The large number of combinations of drugs precluded analyses for each combination.
Our primary outcome measure was objective scores on neuropsychological tests; secondary outcomes were self-reported cognition and quality of life domains using measures that were common between the TLC and CogAge samples. Objective cognitive performance was assessed using forward and backward Digit Span [19] (number of correct trials) and primarily measures working memory. Trail making tests (TMT) A and B (time to complete) [20] measure mainly information processing speed (TMT A) and executive functions (TMT B). A shorter time indicates better performance, but for ease of interpretation we reverse scored these so that higher scores indicated better performance. The objective cognitive measures were rescaled to z-scores to permit comparisons. The CogAge z-scores for the Trail making tests were based on age- and education-specific French normative data.[21] TLC results were based on age- and education-specific US normative data.[22–24]
Measures of self-reported cognitive performance included the total score (score range: 0–132) from the Fact-Cog [25, 26] (version 3) and the perceived cognitive impairment (PCI; score range = 0–72) subscale. Since there are slight differences between the number of items in English and French versions of the questionnaire, a modified total score was created. Namely, the four-item “Comments from Others” subscale in the English questionnaire was not included in the French questionnaire because they had been added to the English questionnaire prior to the development of the French instrument.
Three measures of quality of life from the FACT-G scales were evaluated,[27] including emotional well-being (EWB), physical well-being (PWB) and functional well-being (FWB).
In addition to the primary outcomes, each study provided data on demographic (e.g., age, education), clinical (e.g., stage at diagnosis, chemotherapy regimens) and instrument activities of daily living (ADL)[28].
Statistical Analysis
We used t-tests and chi-square tests to compare characteristics of the two cohorts and survivors treated with taxanes vs. no taxanes. Longitudinal changes in subjective and objective cognitive performance were estimating using piecewise multilevel models.[29]. This analytic approach allows for sporadic missing data and differences in follow-up periods, which is particularly relevant here given variation in the follow-up schedule for participants from each study. When combined, the studies provide data at 6-month intervals for up to 3 years following surgery. The longitudinal follow-up period was divided into short-term (pretreatment to 12-month) and a longer-term (12- to 36-month) segments. At baseline, 167 survivors (nTLC = 110, nCogAge = 57) contributed data at baseline and 138 survivors provided data at the one-year follow-up assessment (nTLC = 82, nCogAge = 56). Across the longer-term follow-up period 86 survivors (nTLC = 38, nCogAge = 48) contributed data at their last assessment point.
Separate models were used for each outcome (objective neurocognitive performance, self-reported cognitive problems and quality of life [EWB, PWB, SWB]). Age, education (high school versus more than high school), instrumental activities of daily living (IADL) score and study (TLC versus CogAge) and treatment with hormone therapies served as covariates. Stage was not included in the models since it was strongly colinear with treatment regimen. Time in study was estimated separately for the short- and longer-term segments. Finally, the impact of taxanes was assessed as a main effect in relation to functioning at the 12-month assessment, as well as interacted with longitudinal changes over the short- and longer-term time segments.
We also examined the percent of survivors that reported meaningful change in self-reported cognition (stayed the same vs. declined) between the pre-treatment and 12-month assessment (the timepoint that both the TLC and CogAge studies shared). Clinically meaningful change was defined as a 0.4 SD unit decline, corresponding to an 6.3-point decrement in FACT-Cog total score and a 0.42 SD unit decline, corresponding to an 4.3-point decrement for the PCI subscale [30].
The sample sizes examined here allow for at least 80% statistical power to detect 0.5 SD differences in longitudinal changes between the two chemotherapy groups. All analyses were conducted using SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Sample Characteristics
Patient characteristics included in each study were similar (see supplemental Table 1), except that the French cohort was older and less likely to have a high school education than the US cohort. The combined sample was almost 70 years of age, over half had greater than a high school education, more than half were diagnosed with Stage 1 or 2 breast cancer and the majority were treated with hormone therapies (Table 1). The chemotherapy without taxane group had worse Trail making A scores at pre-treatment than those with taxanes.
Table 1.
Baseline, Pre-Systemic Therapy Characteristics of Older Women with Breast Cancer Treated with Chemotherapy by Taxane Regimen in a Pooled Cohort Sample
| Chemotherapy with taxanes (N=116) | Chemotherapy without taxanes (N=51) | p-value | |
|---|---|---|---|
| Mean (SD) or n (%) | |||
| Demographics | |||
| Age, years | 67.0 years (4.8) | 68.0 years (4.6) | 0.23 |
| More than high school education | 68 (58.6%) | 30 (58.0%) | 0.94 |
| Clinical | |||
| Stage at diagnosis | 0.07 | ||
| Stage 1 | 39 (33.3%) | 26 (51.0%) | |
| Stage 2 | 51 (43.9%) | 19 (37.3%) | |
| Stage 3 | 26 (22.8%) | 6 (11.8%) | |
| Chemotherapy Regimens | |||
| Taxane alone | 38 (32.8%) | 0 (0%) | |
| Taxane + anthracycline | 78 (67.2%) | 0 (0%) | |
| Anthracycline-based alone* | 0 (0%) | 34 (65.7%) | |
| Non-anthracycline-based alone* | 0 (0%) | 17 (34.3%) | |
| Hormonal therapy regimens | 0.43 | ||
| Aromatase inhibitor | 75 (64.7%) | 24 (47.1%) | |
| Tamoxifen | 2 (1.7%) | 0 (0%) | |
| No hormonal therapy | 39 (33.6%) | 27 (52.9%) | |
| Instrumental ADL score* | 0.50 (0.95) | 0.37 (0.92) | 0.41 |
| Cognition | |||
| FACT-Cog Total | 112.7 (15.44) | 115.9 (16.36) | 0.28 |
| FACT-Cog Perceived Cognitive Impairment | 61.44 (10.14) | 63.13 (10.51) | 0.37 |
| Trail Making A z-score** | 0.11 (1.15) | −0.35 (1.85) | 0.05 |
| Trail Making B z-score** | −0.30 (2.55) | −0.82 (2.80) | 0.24 |
| Digits Span z-score | −0.18 (0.89) | −0.03 (0.95) | 0.35 |
| Quality of Life/Function | |||
| FACT-G Physical Well-Being | 21.09 (4.86) | 22.30 (3.28) | 0.15 |
| FACT-G Social Well-Being | 18.84 (3.33) | 18.05 (2.84) | 0.19 |
| FACT-G Emotional Well-Being | 14.14 (4.46) | 14.75 (4.75) | 0.47 |
Note: Ranges for FACT self-report measures: FACT-Cog total (range 0–132), FACT-Cog PCI range (0–72), FACT Physical Well-Being (range 0–24), FACT Social Well-Being (range 0–20), FACT Emotional Well-Being (range 0–16)
total number with any impairment
reversed scores: higher=better
Longitudinal Changes in Objective Cognitive Performance
The results for the cognitive outcomes are shown in Table 2. As a guide to the table, time in study was divided into short-term (pre-treatment to 12-months) and longer-term (12-months to 36-months) time segments based on expected decrements in the post-treatment period, and potential recovery or decline over time. We compared the impact on taxanes on performance at the 12-month assessment (taxane vs. non taxane regimen), as well as changes across the two-time segments, as well as whether taxane impacted longitudinal changes. If either interaction was statistically significant, this would indicated that taxane treatment was related to changes during that time period.
Table 2.
Factors Associated with Longitudinal Scores for Objective Cognitive Performance1
| Trail making A | Trail making B | Digit Span | |
|---|---|---|---|
| Covariates | β (95% CI) | β (95% CI) | β (95% CI) |
| Age, per 1 year increase | .00 (−.04 – .03) | .00 (−.08 – .07) | .01 (−.01 – .03) |
| CogAge vs. TLC | .64 (.18 – 1.18)** | 1.29 (.39 – 2.19)** | −.31 (−.60 - −.01)* |
| High School vs <=HS Education | .32 (−.10 – .75) | .30 (−.52 – 1.12) | .74 (.48 – 1.01)*** |
| IADL, per increase in limitations | −.15 (−.32 – .02) | −.42 (−.75 – −.09)* | −.15 (−.25 – −.04)** |
| Hormone Therapy (Yes versus No) | .04 (−.30 – .39) | .08 (−.60 – .75) | −.05 (−.26 – .17) |
| Impact of Taxanes | |||
| Taxane vs. non taxane regimen | −.21 (−.63 – .23) | .34 (−.49 – 1.16) | .04 (−.23 – .32) |
| Time effect, pre-treatment to 12-months | .04 (−.16 – .25) | .05 (−.34 – .44) | .04 (−.09 – .17) |
| Taxane group × pre-treatment to 12-month | .31 (−.08 – .70) | .78 (.04 – 1.52)* | −.05 (−.30 – .21) |
| Time effect, 12- to 36-months | .06 (−.08 – .20) | .03 (−.25 – .30) | .16 (.06 – .25)** |
| Taxane group × 12- to 36-months | −.01 (−.29 – .27) | −.51 (−1.04 – .02) | −.11 (−.29 – .07) |
p < .05,
p < .01,
p < .001
Results for piecewise multilevel models modeling change in objective cognitive performance. Sample reference group is TLC; Education reference group is high school completion; treatment group reference is chemotherapy with taxane.
For the measures of objective cognition, performance was associated with CogAge versus TLC study and as expected, women with lower (vs. higher) education and greater (vs. fewer) limits in activities of daily living had significantly lower cognitive performance. None of the effects for time were significant for Trail Making A. For Trail Making B, the significant interaction with taxane group showed improvements in performance between pre-treatment and 12-months for the non-taxane group (see Figure 1). Finally, there were statistically significant improvements in Digit Span performance between the 12- and 36-month assessment points, but these did not vary by taxane group.
Figure 1:
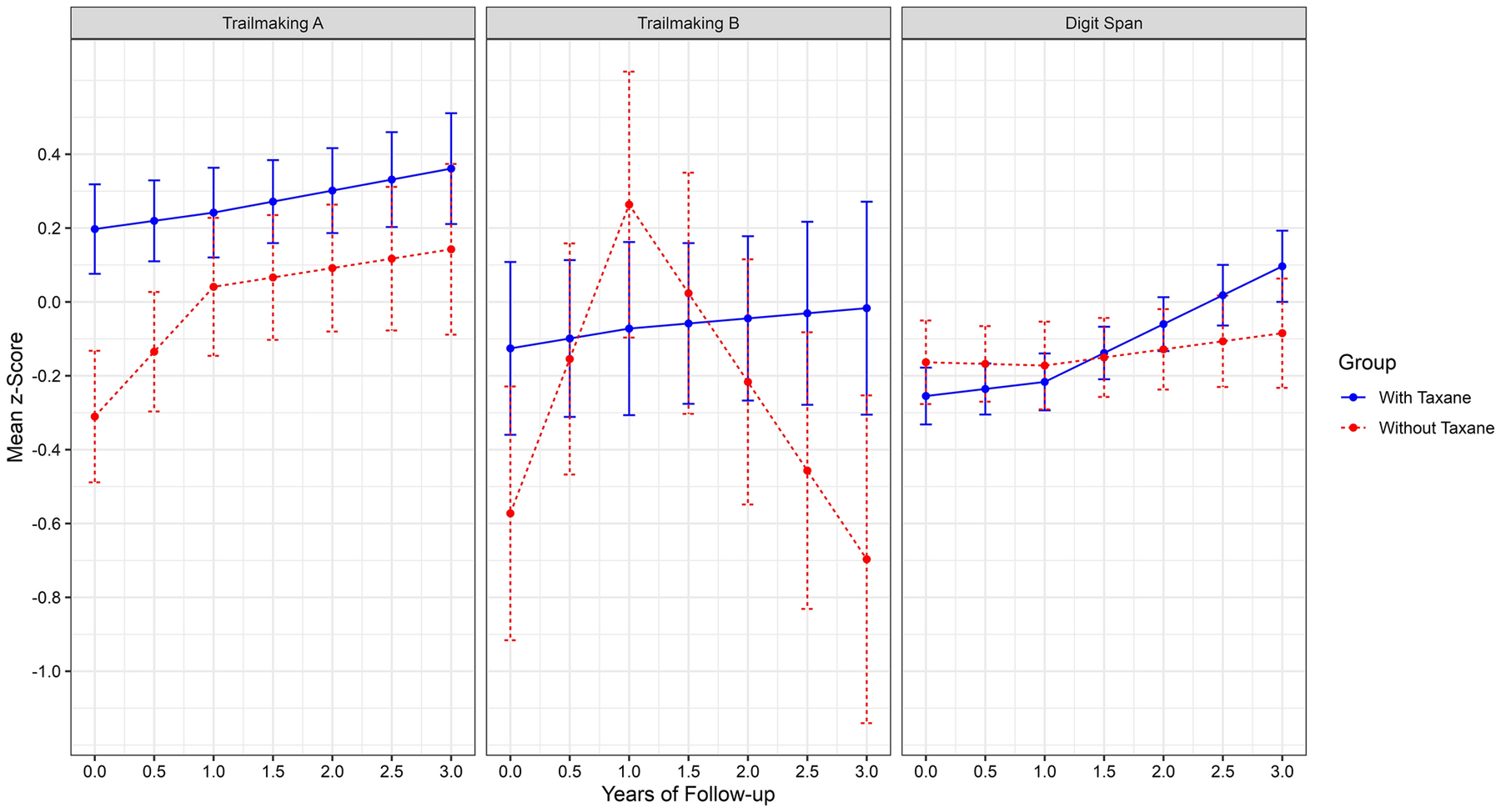
Longitudinal Changes in Objective Cognitive Scores of Processing Speed (Trailmaking A), Executive Functioning (Trailmaking B) and Working Memory (Digit Span). Note: Trail Making A and Trail Making B are reverse scored (higher=better)
Longitudinal Changes in Subjective Cognitive Performance
There were statistically significant declines in self-reported cognitive function (i.e., more problems) from pre-treatment to the 12-month assessment, but no significant changes after that. There were no difference in this pattern of longitudinal change across the follow-up periods based on use of taxanes vs. no taxanes (Table 3 and Figure 2). There were also no differences in the proportion of survivors having clinically meaningful decrement in self-reported cognition based on taxane use (eFigure 1; Fact-Cog Total, p = .614; Fact-Cog PCI, p = .832).
Table 3.
Factors Associated with Longitudinal Scores for Self-Reported Cognitive Problems1
| Fact-Cog Total | Fact-Cog PCI | |
|---|---|---|
| Covariates | β (SE) | β (SE) |
| Age, per 1 year increase | −.33 (−.87 – .22) | −.26 (−.62 – .09) |
| CogAge vs TLC | −6.40 (−13.5 – .66) | −1.73 (−6.31 – 2.85) |
| High school vs <=HS education | 5.81 (−.76 – 12.38) | 1.46 (−2.76 – 5.67) |
| IADL, per increase in limitations | −3.42 (−5.96 - −.89)** | −2.01 (−3.67 - −.36)* |
| Hormone Therapy (Yes versus No) | 3.37 (−1.81 – 8.55) | 1.33 (−2.05 – 4.71) |
| Impact of Taxanes | ||
| Taxane vs. non taxane regimen | 3.14 (−3.19 – 9.48) | 2.37 (−1.74 – 6.49) |
| Time effect, pre-treatment to 12-months | −4.57 (−7.52 - −1.62)** | −3.65 (−5.55 - −1.75)*** |
| Taxane group × pre-treatment to 12-months | .79 (−5.14 – 6.74) | .54 (−3.25 – 4.32) |
| Time effect, 12- to 36-months | 1.59 (−.51 – 3.69) | .89 (−.47 – 2.24) |
| Taxane group × 12- to 36-months | −2.46 (−6.51 – 1.60) | −.99 (−3.62 – 1.63) |
p < .05,
p < .01,
p < .001
Results for piecewise multilevel models modeling change in self-reported cognitive performance. Sample reference group is TLC; Education reference group is high school completion; treatment group reference is chemotherapy with taxane.
Figure 2:
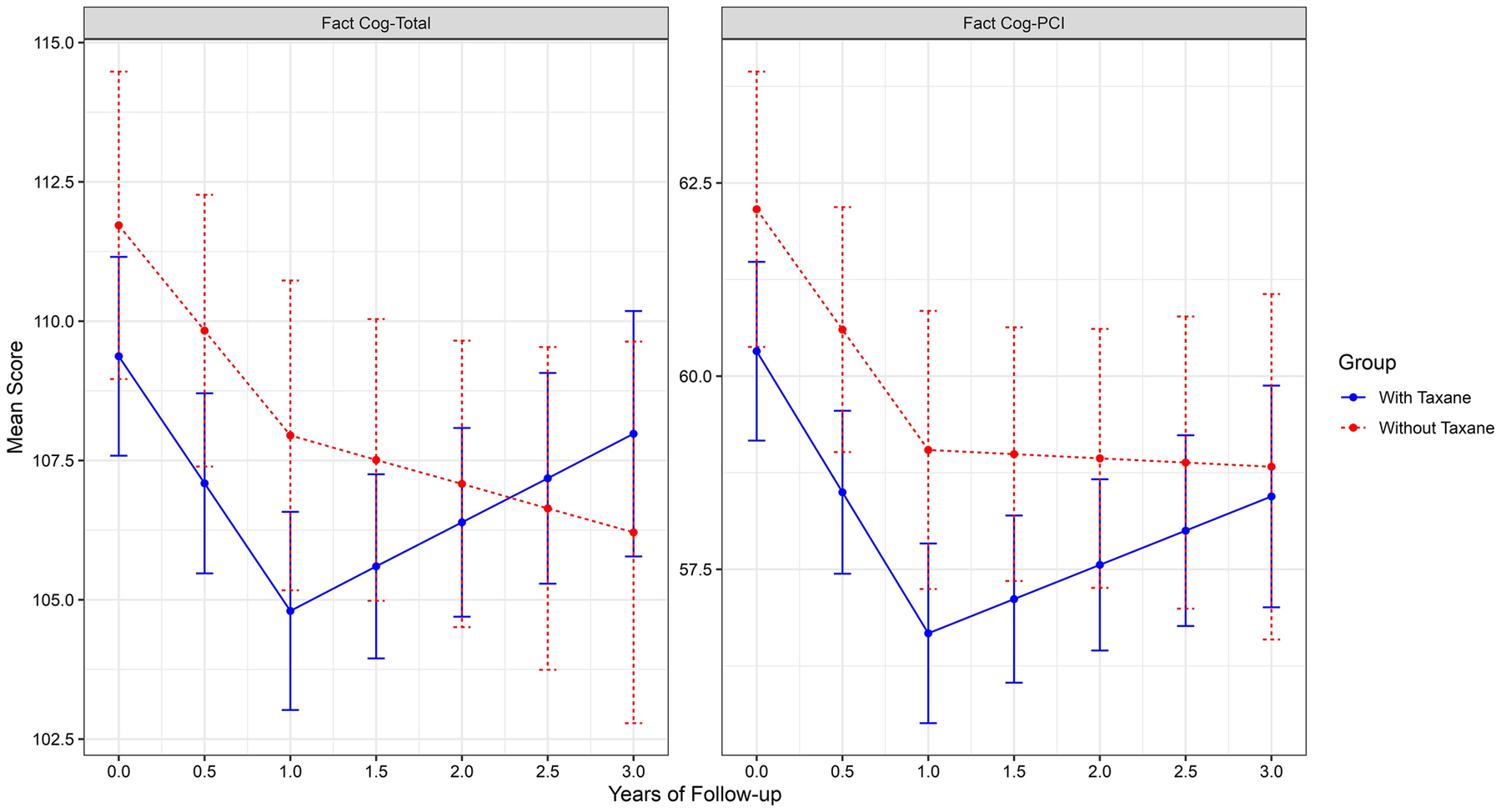
Longitudinal Changes in FACT-Cog Total and FACT-Cog PCI Scores. Note: Higher score=fewer cognitive complaints
Longitudinal Changes in Quality of Life
Adjusted functional and emotional well-being scores increased from pre-treatment to the 12-month assessment (Table 4 and Figure 3) but there were no differences by taxane groups.
Table 4.
Factors Associated with Longitudinal Scores for Well-Being Outcomes1
| Physical Well-being | Functional Well-being | Emotional Well-being | |
|---|---|---|---|
| Covariates | β (95% CI) | β (95% CI) | β (95% CI) |
| Age, per 1 year increase | .01 (−.10 – .11) | .05 (−.09 – .19) | .02 (−.08 – .11) |
| CogAge vs TLC | 3.04 (1.66 – 4.43)*** | .06 (−1.69 – 1.82) | 6.49 (5.25 – 7.74)*** |
| High school vs. <=HS education | 10.2 (−.27 – 2.30) | 1.63 (.00 – 3.26)* | .87 (−.28 – 2.02) |
| IADL, per increase in limitation | −.74 (−1.23 - −.24)** | .89 (−1.52 – .25)** | −.33 (−.78 – .12) |
| Hormone Therapy (Yes versus No) | 1.02 (.00 – 2.05) | 1.55 (.25 – 2.84)* | .61 (−.31 – 1.53) |
| Impact of Taxanes | |||
| Taxane vs. non taxane regimen | .89 (−.66 – 2.23) | −.03 (−1.72 – 1.71) | −.50 (−1.64 – .64) |
| Time effect, pre-treatment to 12-month | .35 (−.53 – 1.24) | 1.02 (.08 – 1.97)* | 1.38 (.83 – 1.93)*** |
| Taxane group × pre-treatment to 12-month | −.62 (−2.36 – 1.12) | −.20 (−2.08 – 1.68) | −.60 (−1.70 – .50) |
| Time effect, 12- to 36-months | .47 (−.16 – 1.10) | .39 (−.28 – 1.08) | .06 (−.34 – .45) |
| Taxane group × 12- to 36 months | −.15 (−1.36 – 1.07) | −.17 (−1.47 – 1.14) | −.02 (−.78 – .74) |
p < .05,
p < .01,
p < .001
Results for piecewise multilevel models modeling change in well-being outcomes. Sample reference group is TLC; Education reference group is high school completion; treatment group reference is chemotherapy with taxane.
Figure 3:
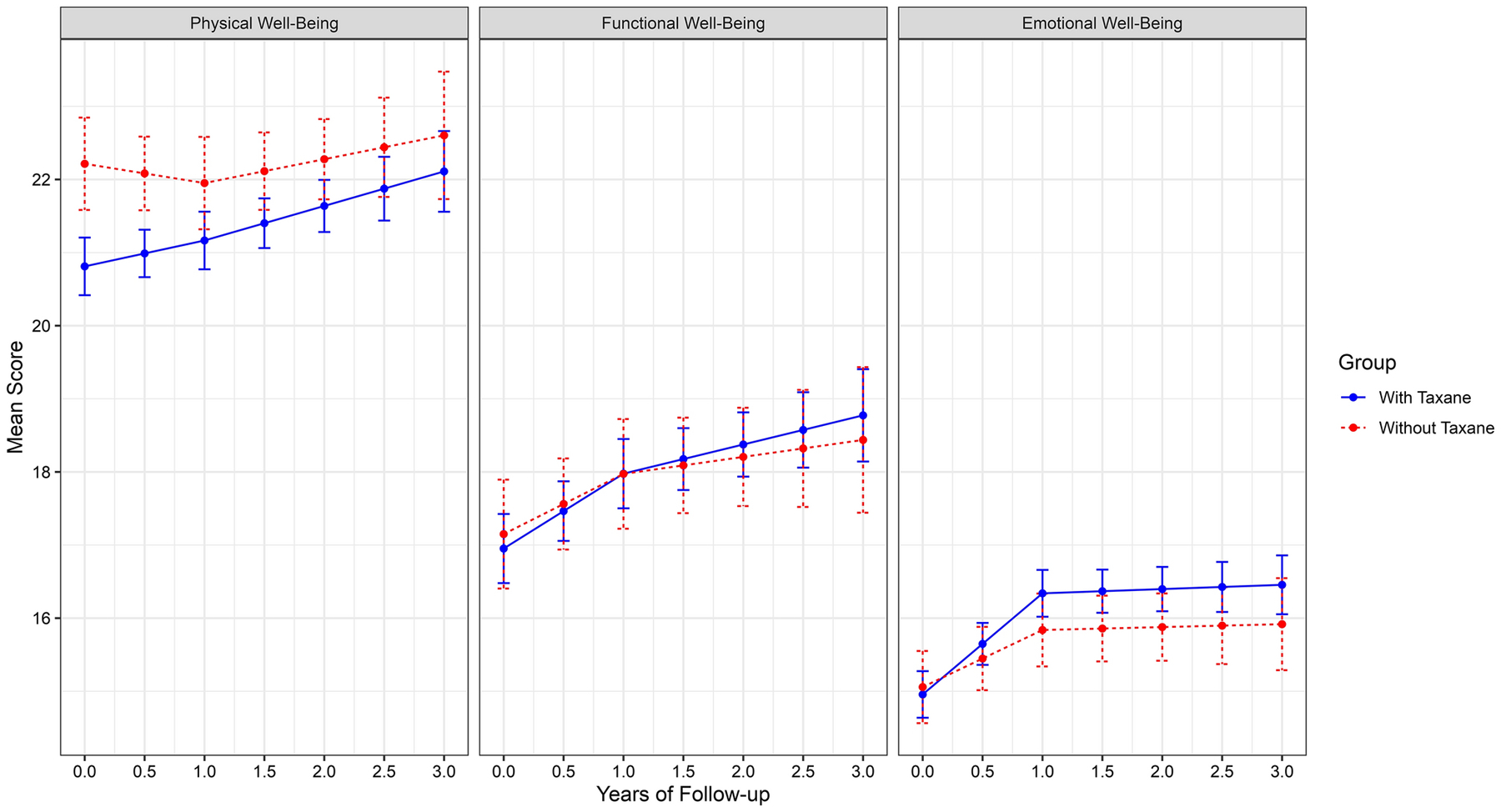
Longitudinal Changes in Well-Being Scores
DISCUSSION
This study pooled data from the largest cohort studies of older women with localized breast cancer to evaluate the effects of taxane-based chemotherapy on cognitive and quality of life outcomes over time. Overall, the results indicated that older women selected to receive treatment with taxanes did not have greater declines in subjective or objective cognitive performance than women receiving other chemotherapy regimens. Use of taxanes also did not also have a negative impact on quality of life.
There have been mixed results in prior studies examining the impact of specific chemotherapy regimens on breast cancer survivors.[31, 32] While we did not find effects of taxane regimes on self-reported cognition, Myers and colleagues [31] reported, in analyses that were uncorrected for any covariates, that women receiving taxanes (n = 214) reported greater cognitive impairment on the FACT-Cog PCI subscale, as compared to those who did not receive taxanes (n = 149). These differences may be attributable to difference in populations of women with breast cancer, study design, availability of pre-treatment data and consideration of important confounding variables. Among the measures of subjective cognitive performance, we observed worsening of self-reported performance from pre-treatment to 12-months, but then no further declines after, suggesting that most survivors returned to pre-treatment levels by 1 year.
Research on objective tests of cognitive performance is more limited, with only one study reporting that younger survivors treated with taxanes performed worse on a test of visuospatial functioning [33], but another failing to observe differential change in cognitive outcomes from pre-treatment to approximately one year post-treatment among taxane recipients with an average age of 50 and all under 70.[34] In animal models, chemotherapy with taxanes was associated with impaired cognitive performance and reduced activity.[17] One potential criticism of the current research is the narrow breadth of objective measures of cognitive performance. Future research should strive to include a wider array of cognitive domains to evaluate the potential for taxane treatment to influence cognition. With that said, we are confident that when the results of the subjective measures of cognitive performance are considered with the objective measures, there appears to be relatively little evidence of a significant impact of taxane treatment on subjective or objective tests of cognitive performance.
We are not aware of any studies that have examined the impact of taxanes on quality of life of older cancer survivors. Our results showed improvements in functional and emotional well-being during the short-term follow-up period, followed by relative stability thereafter. This pattern of improvement is consistent with previous work [35, 36]. We did not find any effects of taxanes on physical, functional or emotional well-being. Interestingly, we observed significant differences between the study samples on physical and emotional well-being, with the CogAge sample exhibiting better scores, even after adjusting for differences in age, years of education, IADL and receipt of hormone therapy.
It is encouraging that older women with breast cancer selected to receive adjuvant taxane regimens did as well as those on non-taxane regimens. A strength of the current study was the ability to pool data from two studies of older adult breast cancer survivors. This resulted in sample sizes that were over twice those of previously published work that examined longitudinal changes as a function of taxane treatment among younger women.[33, 34] Our sample was well powered to detect meaningful differences (i.e., 0.5 SD) in longitudinal changes between the two chemotherapy groups. The use of pooled or harmonized approaches to understanding changes in cognitive performance is commonplace in cognitive aging [37, 38] and Alzheimer’s disease research [39, 40], but our study is among the first to pool individual-level data to study CRCD. Although there are challenges to this approach, including the lack of standardization of neuropsychological measures across studies, as well as the lack of breadth of cognitive domains that are assessed,[41] the ability to pool data from diverse samples will likely result in a better understanding of the course of CRCD.
There are several limitations to this study that require consideration when evaluating our results. First, even though we were able to pool results from two studies of older breast cancer survivors, there were differences in the design of the two studies that may have impacted the results that were observed. The objective cognitive assessments in the CogAge study were more frequent during the first 12 months and this could have resulted in differential practice effects across cognitive outcomes. Second, although the results of the current study suggest that there is little impact of taxanes on cognitive performance, clinicians may choose their treatment regimen based on the survivor’s ability to tolerate more aggressive treatment. As such, if those who are treated with taxanes are generally healthier prior to treatment, this may mitigate against seeing an effect of taxanes longitudinally. In our study, the participants averaged less than 70 years and exhibited few limitations to instrumental ADL. We did see that the taxane-treated group had minimally higher cognition and quality of life scores pre-systemic therapy. We also cannot rule out the possibility that women experienced toxicity with taxanes leading to dose reductions or incomplete cycles, attenuating any potential differences and we were unable to control for differences in taxane dose in the current study. Finally, taxanes may impact aspects of cognition that were not captured in the neuropsychological outcomes that were common across the two studies.
The overall goal of this study was to evaluate whether older women receiving taxane-based treatment regimens had greater longitudinal declines on multiple measures of cognitive performance and well-being as compared to women treated without taxanes. To do so, we pooled data from two large prospective studies of older breast cancer survivors. Our results demonstrated little impact of taxane-based treatments on longitudinal changes in performance. The results suggest that despite taxane-based chemotherapies being associated with more physical declines among older cancer survivors, we found little evidence of these treatments leading to greater declines in cognitive performance or well-being, at least among the measures that were considered here.
Supplementary Material
Acknowledgements
Funding:
This research was supported by the National Cancer Institute at the National Institutes of Health grants R01CA129769 and R35CA197289 to JM. This work was supported in part by the National Institute of Aging at the National Institutes of Health grant R01 AG068193 to JM. As a member of the Cancer and Aging Research Group (CARG), JM’s work on this project was also supported in part by grant R33AG059206 (MPI: W Dale, S Mohile, H Klepin) from the National Institute of Aging at the National Institutes of Health. This study was also supported in part by the National Cancer Institute at the National Institutes of Health grant P30CA51008 to Georgetown-Lombardi Comprehensive Cancer Center for support of the Biostatistics and Bioinformatics Resource and the Non-Therapeutic Shared Resource. The work of AJS was supported in part by the National Institute of Aging at the National Institutes of Health grants P30AG10133, R01AG19771 and R01LM01136. TAA was supported in part by National Cancer Institute at the National Institutes of Health grants R01CA172119, U54 CA137788, and P30CA008748. JR was supported in part by National Cancer Institute at the National Institutes of Health grant R01CA172119. BCM was supported in part by National Cancer Institute at the National Institutes of Health grant R01CA244673. KVD was supported in part by the National Cancer Institute at the National Institutes of Health grant K08CA241337. The work of JEC was supported in part by the American Cancer Society Research Scholars grant 128660-RSG-15-187-01-PCSM and the National Cancer Institute at the National Institutes of Health grant R01CA237535. HJC was supported in part by the National Institute of Aging at the National Institutes of Health grant P30AG028716 for the Duke Pepper Center. SKP was supported in part by the American Cancer Society Research Scholars grant RSG-17-023-01-CPPB. The Cog-Age study was supported by a national grant (Inca Programme Hospitalier de Recherche Clinique, Grant APN 2008 n°06-08) and Sanofi.
Footnotes
Publisher's Disclaimer: This AM is a PDF file of the manuscript accepted for publication after peer review, when applicable, but does not reflect post-acceptance improvements, or any corrections. Use of this AM is subject to the publisher’s embargo period and AM terms of use. Under no circumstances may this AM be shared or distributed under a Creative Commons or other form of open access license, nor may it be reformatted or enhanced, whether by the Author or third parties. See here for Springer Nature’s terms of use for AM versions of subscription articles: https://www.springernature.com/gp/open-research/policies/accepted-manuscript-terms
Conflicts of interest/Competing interests: The authors have no conflicts of interest to report
Ethics approval: Ethical approval was obtained from relevant organizations.
Consent to participate: All participants provided informed consent.
Consent for publication: All authors consent for publication
Availability of data and material:
Data is made available upon request
References
- [1].Plevritis SK, Munoz D, Kurian AW, Stout NK, Alagoz O, Near AM, et al. Association of Screening and Treatment With Breast Cancer Mortality by Molecular Subtype in US Women, 2000–2012. JAMA. 2018;319:154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bluethmann S, Mariotto A, Rowland J. Anticipating the “Silver Tsunami”: Prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25:1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25:1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rowland JH, Bellizzi KM. Cancer survivorship issues: life after treatment and implications for an aging population. J Clin Oncol. 2014;32:2662–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mandelblatt JS, Zhai W, Ahn J, Small BJ, Ahles TA, Carroll JE, et al. Symptom burden among older breast cancer survivors: The Thinking and Living With Cancer (TLC) study. Cancer. 2020;126:1183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mandelblatt JS, Small BJ, Luta G, Hurria A, Jim H, McDonald BC, et al. Cancer-Related Cognitive Outcomes Among Older Breast Cancer Survivors in the Thinking and Living With Cancer Study. J Clin Oncol. 2018;36:Jco1800140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol. 2012;30:3675–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Janelsins MC, Heckler CE, Peppone LJ, Kamen C, Mustian KM, Mohile SG, et al. Cognitive Complaints in Survivors of Breast Cancer After Chemotherapy Compared With Age-Matched Controls: An Analysis From a Nationwide, Multicenter, Prospective Longitudinal Study. J Clin Oncol. 2017;35:506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lange M, Rigal O, Clarisse B, Giffard B, Sevin E, Barillet M, et al. Cognitive dysfunctions in elderly cancer patients: A new challenge for oncologists. Cancer TreatRev. 2014;40:810–7. [DOI] [PubMed] [Google Scholar]
- [10].Ahles TA, Li Y, McDonald BC, Schwartz GN, Kaufman PA, Tsongalis GJ, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: the impact of APOE and smoking. Psychooncology. 2014;23:1382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mandelblatt JS, Stern RA, Luta G, McGuckin M, Clapp JD, Hurria A, et al. Cognitive impairment in older patients with breast cancer before systemic therapy: is there an interaction between cancer and comorbidity? J Clin Oncol. 2014;32:1909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. JClinOncol. 2010;28:4434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wagner LI, Gray RJ, Sparano JA, Whelan TJ, Garcia SF, Yanez B, et al. Patient-Reported Cognitive Impairment Among Women With Early Breast Cancer Randomly Assigned to Endocrine Therapy Alone Versus Chemoendocrine Therapy: Results From TAILORx. J Clin Oncol. 2020;38:1875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Denduluri N, Somerfield MR, Eisen A, Holloway JN, Hurria A, King TA, et al. Selection of Optimal Adjuvant Chemotherapy Regimens for Human Epidermal Growth Factor Receptor 2 (HER2) -Negative and Adjuvant Targeted Therapy for HER2-Positive Breast Cancers: An American Society of Clinical Oncology Guideline Adaptation of the Cancer Care Ontario Clinical Practice Guideline. J Clin Oncol. 2016;34:2416–27. [DOI] [PubMed] [Google Scholar]
- [15].Lange M, Heutte N, Rigal O, Noal S, Kurtz JE, Levy C, et al. Decline in Cognitive Function in Older Adults With Early-Stage Breast Cancer After Adjuvant Treatment. Oncologist. 2016;21:1337–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hershman DL, Till C, Wright JD, Awad D, Ramsey SD, Barlow WE, et al. Comorbidities and Risk of Chemotherapy-Induced Peripheral Neuropathy Among Participants 65 Years or Older in Southwest Oncology Group Clinical Trials. J Clin Oncol. 2016;34:3014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].da Costa R, Passos GF, Quintão NLM, Fernandes ES, Maia J, Campos MM, et al. Taxane-induced neurotoxicity: Pathophysiology and therapeutic perspectives. Br J Pharmacol. 2020;177:3127–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lange M, Giffard B, Noal S, Rigal O, Kurtz JE, Heutte N, et al. Baseline cognitive functions among elderly patients with localised breast cancer. Eur J Cancer. 2014;50:2181–9. [DOI] [PubMed] [Google Scholar]
- [19].Wechsler D Wechsler Adult Intelligence Scale-III (WAIS-III). San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- [20].Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. Tucson, AZ: Neuropsychology Press; 1993. [Google Scholar]
- [21].Godefroy O Réflexion pour l’évaluation des Fonction sEXécutives. [Executive Functions and neurological and psychiatric diseases: assessment in clinical practice]. Marseille, France: France:Solal; 2008. [Google Scholar]
- [22].Wechsler D WAIS-III Administration and Scoring Manual. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- [23].Tombaugh T, McIntyre N. The mini-mental state examination: A comprehensive review. J Am Geriatr Soc. 1992;40:922–35. [DOI] [PubMed] [Google Scholar]
- [24].Stern RA, White T. Neuropsychological assessment battery (NAB). Lutz, FL: Psychological Assessment Resources; 2003. [Google Scholar]
- [25].Wagner LI, Sweet J, Butt Z, Lai J-s, Cella D. Measuring patient self-reported cognitive function: development of the functional assessment of cancer therapy-cognitive function instrument. J Support Oncol. 2009;7:W32–W9. [Google Scholar]
- [26].Joly F, Lange M, Rigal O, Correia H, Giffard B, Beaumont JL, et al. French version of the Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog) version 3. Support Care Cancer. 2012;20:3297–305. [DOI] [PubMed] [Google Scholar]
- [27].Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997;15:974–86. [DOI] [PubMed] [Google Scholar]
- [28].Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of Illness in the Aged. The Index of Adl: A Standardized Measure of Biological and Psychosocial Function. JAMA. 1963;185:914–9. [DOI] [PubMed] [Google Scholar]
- [29].Hoffman L Longitudinal analysis: Modeling within-person fluctuation and change. New York, NY: Routledge; 2015. [Google Scholar]
- [30].Bell ML, Dhillon HM, Bray VJ, Vardy JL. Important differences and meaningful changes for the Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog). Journal of Patient-Reported Outcomes. 2018;2:48. [Google Scholar]
- [31].Myers JS, Wick JA, Klemp J. Potential factors associated with perceived cognitive impairment in breast cancer survivors. Support Care Cancer. 2015;23:3219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Liou KT, Ahles TA, Garland SN, Li QS, Bao T, Li Y, et al. The Relationship Between Insomnia and Cognitive Impairment in Breast Cancer Survivors. JNCI Cancer Spectr. 2019;3:pkz041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jansen CE, Cooper BA, Dodd MJ, Miaskowski CA. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Support Care Cancer. 2011;19:1647–56. [DOI] [PubMed] [Google Scholar]
- [34].Cerulla N, Arcusa À, Navarro JB, Garolera M, Enero C, Chico G, et al. Role of taxanes in chemotherapy-related cognitive impairment: A prospective longitudinal study. Breast Cancer Res Treat. 2017;164:179–87. [DOI] [PubMed] [Google Scholar]
- [35].Kobayashi LC, Cohen HJ, Zhai W, Zhou X, Small BJ, Luta G, et al. Cognitive function prior to systemic therapy and subsequent well-being in older breast cancer survivors: Longitudinal findings from the Thinking and Living with Cancer Study. Psychooncology. 2020;29:1051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hurria A, Soto-Perez-de-Celis E, Allred JB, Cohen HJ, Arsenyan A, Ballman K, et al. Functional Decline and Resilience in Older Women Receiving Adjuvant Chemotherapy for Breast Cancer. J Am Geriatr Soc. 2019;67:920–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].McArdle JJ, Grimm KJ, Hamagami F, Bowles RP, Meredith W. Modeling Life-Span Growth Curves of Cognition Using Longitudinal Data With Multiple Samples and Changing Scales of Measurement. Psychological Methods. 2009;14:126–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Duggan EC, Piccinin AM, Clouston S, Koval AV, Robitaille A, Zammit AR, et al. A Multi-study Coordinated Meta-analysis of Pulmonary Function and Cognition in Aging. J Gerontol A Biol Sci Med Sci. 2019;74:1793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gross AL, Sherva R, Mukherjee S, Newhouse S, Kauwe JS, Munsie LM, et al. Calibrating longitudinal cognition in Alzheimer’s disease across diverse test batteries and datasets. Neuroepidemiology. 2014;43:194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rabin LA, Smart CM, Crane PK, Amariglio RE, Berman LM, Boada M, et al. Subjective Cognitive Decline in Older Adults: An Overview of Self-Report Measures Used Across 19 International Research Studies. Journal of Alzheimer’s disease : JAD. 2015;48 Suppl 1:S63–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wefel JS, Kesler SR, Noll KR, Schagen SB. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA: a cancer journal for clinicians. 2015;65:123–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is made available upon request


