Structured summary
Background:
Ozanimod, a high selective sphingosine 1 phosphate (S1P) receptor (S1PR) 1/5 modulator was approved by the Food and Drug Administration for the treatment of adult patients with moderately to severely active ulcerative colitis. Additional S1PR modulators are being tested in clinical development programs for both ulcerative colitis and Crohn’s disease.
Aim:
To provide an overview of advances in understanding S1PRs biology and summarize preclinical and clinical investigations of S1P receptor modulators in chronic inflammatory disease with special emphasis on inflammatory bowel diseases (IBD).
Methods:
We performed a narrative review using PubMed and ClinicalTrials.gov.
Results:
Through S1PRs, S1P regulates multiple cellular processes, including proliferation, migration, survival, and vascular barrier integrity. The S1PRs function of regulating lymphocyte trafficking is well known, but new functions of S1PRs expand our knowledge of S1PRs biology. Several S1PR modulators are in clinical development for both ulcerative colitis and Crohn’s disease and have shown promise in phase II and III studies with Ozanimod now being approved for ulcerative colitis.
Conclusions:
S1P receptor modulators constitute a novel, promising, safe, and convenient strategy for the treatment of IBD.
Keywords: Inflammatory bowel diseases, S1P / S1PR axis, S1PRs modulators, preclinical studies, clinical trials
Sphingosine 1 phosphate (S1P) is a bioactive lipid mediator involved in the regulation of multiple cellular processes, including proliferation, migration, survival, and vascular barrier integrity. S1P signals through its receptors known as S1P receptors (S1PRs) belonging to the G protein-coupled receptor family. Several S1PRs modulators have been under clinical investigation for the treatment of chronic inflammatory and autoimmune diseases. This review will provide a brief summary of current and emerging evidence of the role of the S1P/S1PR axis in chronic inflammation with a special emphasis on inflammatory bowel disease (IBD) and discusses the therapeutic potential of S1P modulators.
Sphingosine 1-phosphate
Sphingosine 1-phosphate (2S-amino-1-(dihydrogen phosphate)-4E-octadecene-1,3R-diol, S1P) is a multifunctional, bioactive lipid molecule derived from the metabolism of membrane sphingolipids 1. Sphingolipid metabolites include ceramide, sphingosine, and S1P. As shown in Figure 1, intracellular S1P synthesis occurs through sphingosine phosphorylation, catalyzed by two sphingosine kinase isoenzymes, sphingosine kinases (SPHK)1 and SPHK2, which maintain a metabolic balance of sphingosine and control the levels of S1P 2. Once generated, S1P is excreted by an adenosine triphosphate-binding cassette transporter into the extracellular space, where it binds to its receptors and exerts biological functions 3. Concentrations of S1P are high in blood and lymph fluid, but they are low in interstitial fluids, thus creating an S1P gradient between the blood and tissues 4. Increased levels of S1P have been observed at sites of inflammation, inducing the recruitment of immune cells and soluble mediators and the exacerbation of inflammation 5. In the blood, S1P is derived mainly from hematopoietic cells, particularly red blood cells, whereas lymphatic endothelial cells are the major source of S1P in lymphatic fluid 6. S1P degrading enzymes, such as S1P lyase (SPL) and S1P phosphatase (S1PP), play an important role in maintaining low tissue levels of S1P and together with SPHK and S1P transporters, create the S1P gradient7,8. SPL inactivates S1P irreversibly by cleaving its acyl chain, whereas S1PP removes phosphate from S1P to produce sphingosine (Figure 1). Importantly, the S1P concentration gradient directs immune cell trafficking, such as that of lymphocytes, out of the lymph node into the circulation, and plays a role in many inflammatory conditions 9.
Figure 1. S1P/S1PRs signaling pathways.
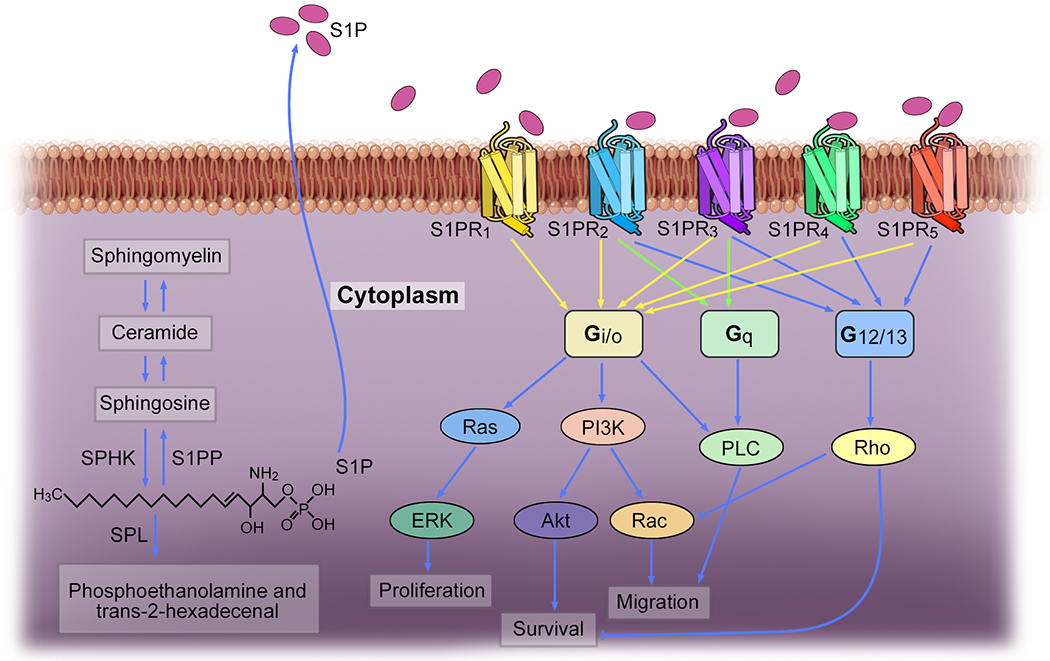
Intracellular S1P synthesis is catalyzed by SPHK1 and SPHK2. The degradation of S1P is catalyzed by S1PP and SPL. S1P signals primarily through five G protein-coupled receptor subtypes named S1PR1-5.
Advances in the understanding of S1PRs biology in cellular processes
S1P signals primarily through five specific G protein-coupled receptor subtypes, named S1PR1-5, with S1PR1 being the most extensively studied (Figure 1) 10. The expression patterns of S1PR1-5 vary significantly among tissues, which may be related to specific roles in various organ systems, including the vascular, immune, nervous system, and others 11. S1PR1, S1PR2, and S1PR3 are ubiquitously expressed, whereas S1PR4 and S1PR5 have a narrower expression pattern restricted to distinct cell types 5. Although the S1PRs function of regulating vascular barrier function and lymphocyte trafficking is well known, recent research on S1PRs biology in several human diseases, such as chronic inflammatory pathologies, autoimmunity, and cancer, together with new functions of S1PRs, such as a pro-fibrotic function in some organs, expanded our knowledge of S1PRs in cellular process and increased the interest to use them as therapeutic targets 11. Table 1 provides an overview of the S1PR functions in health and disease.
Table 1.
Main functions of S1PRs
| Name | Main functions |
|---|---|
| S1PR1 | 1) Cell growth and proliferation 2) Cell migration 3) Cell differentiation 4) Vascular integrity 5) Angiogenesis 6) Lymphocyte egress 7) Fibrosis 8) Chemotaxis |
| S1PR2 | 1) Cell growth and proliferation 2) Cell migration 3) Endothelial barrier function 4) Fibrosis |
| S1PR3 | 1) Dendritic cell maturation 2) Macrophage chemotaxis and killing 3) Neutrophil and eosinophil recruitment 4) Leukocyte rolling on endothelial cells 5) Fibrosis 6) Cytokine production |
| S1PR4 | 1) Cell migration 2) Cytokine secretion 3) Cell differentiation |
| S1PR5 | 1) NK cells egress 2) Monocytes egress 3) Cell proliferation 4) endothelial barrier integrity |
S1PR1
S1PR1 is expressed in most mammalian cell types and is functional in many biological processes, including cell migration, proliferation, cell differentiation, vascular barrier function, and others 12. Upon the activation by S1P, S1PR1 exclusively couples with the Gi/o alpha subunit of heterotrimeric G proteins, and then signals mainly via phospholipase C, phosphoinositide 3-kinase/Akt or Rac, Ras guanosine triphosphatase/extracellular-signal-regulated kinase and adenylyl cyclase 13. An elevated concentration of S1P is usually coupled with an up-regulation of S1PR1 expression within the inflamed tissue of many diseases, such as chronic inflammatory pathologies, autoimmunity, and cancer 12. In addition to inflammation, S1PR1 has been linked to fibrosis 14–17. The role of S1PR1 in angiogenesis has also been studied, but with controversial results. It has been reported that S1PR1-deficient mice show a denser vascular network, while S1PR1-overexpressing mice have a sparser network, suggesting S1PR1 signaling acts as a vascular-intrinsic stabilization mechanism, protecting developing blood vessels against aberrant angiogenic responses 18. Conversly, it has been suggested that S1PR1 is involved in the development of angiogenesis 19. Downregulation of S1PR1 or functional inhibition of S1PR1 by fingolimod leads to suppression of tumor angiogenesis 20,21. These findings suggest S1PR1 has an important role in inflammation, but further investigation of its exact role in angiogenesis and endothelial barrier function is needed.
S1PR2
S1PR2 often exerts cellular functions that are opposed to those of S1PR1, such as preventing recruitment rather than attracting cells in response to S1P 22. However, the function and mechanisms of S1PR2 are complex and vary in different cells types with contrasting results 23. For example, S1PR2 can both activate and inhibit Akt 24, an important signaling pathway for many cellular processes such as survival, migration, and proliferation. Furthermore, its inhibitory role in fibroblasts and endothelial cells is dependent on Rho-dependent activation of the phosphoinositide phosphatase and tensin homolog deleted on chromosome 10 (PTEN), whereas in macrophages S1PR2-mediated inhibition of Akt and migration were independent of PTEN 24. Recent research suggests the involvement of S1PR2 in organ fibrosis 25–27 . Consistent with other S1PRs, S1PR2 also exerts influence on endothelial cells. S1PR2 regulated lymphatic endothelial cells layer structure, permeability, and expression of the junction molecules through the extracellular-signal-regulated kinase pathway, and facilitated T cell transcellular migration through vascular cell adhesion molecule-1 expression and recruitment of T cells to lymphatic endothelial cells migration sites 28. So, while S1PR2 is involved in several cellular processes, its exact role in specific physiological and pathological courses requires further research.
S1PR3
Initially, S1PR3 has been studied mainly in immune cells and its multifactorial role in the immune system is well documented, including effects on dendritic cell maturation, macrophage chemotaxis and killing, neutrophil and eosinophil recruitment, leukocyte rolling on endothelial cells, and other effects 22. Recent research indicates that S1PR3 is also involved in pro-fibrotic pathways 29,30 .In rheumatoid arthritis, S1PR3 is associated with the development of autoimmune arthritis and the pathogenic function of synoviocytes via increased interleukin (IL)-6 production 31.
S1PR4
Expression of S1PR4 is primarily restricted to hematopoietic and lymphoid tissue 32, but there is a limited investigation about its role in other cellular functions. In T cells, the S1P/S1PR4 axis does not affect migration, but suppresses T cell proliferation, inhibits the generation of trophic and effector cytokines, such as IL-2 and IL-4, interferon (IFN)-γ, and enhances the production of IL-10 33. On the contrary, T cells from S1PR4-deficient mice show no difference in proliferation and cytokine secretion compared to that from wild-type mice 34. Instead, dendritic cell migration and cytokine secretion are profoundly affected by S1PR4 deficiency, leading to reduced differentiation towards T-helper (Th)17 cells 34. In a recent study, the S1PR4-mediated suppression of the 5-lipoxygenase enzyme contributes to the S1P-induced anti-inflammatory effects in vitro and in vivo 35. S1PR4 deficiency also impacts anaphylaxis, resulting in exacerbation of IgE-mediated systemic anaphylaxis, and S1PR4 has a role in the negative regulation of innate mast cell degranulation in response to co-stimulation with IgE/Ag and IL-33 36. In human platelets, S1PR4 mediates the inhibitory role of S1P on collagen-induced activation 37. High S1PR4 expression is associated with shorter disease-free and disease-specific survival in estrogen receptor-negative breast cancer 38. Interestingly, in dextran sodium sulfate (DSS)-induced colitis, S1PR4 deficiency increases mucosal IgA levels under inflammatory conditions and alleviates DSS-induced colitis 34, indicating that S1PR4 functional antagonism may have a potential role in the treatment of IBD, via the effects on dendritic cell migration, cytokine secretion, and Th17-cell differentiation.
S1PR5
S1PR5 expression was previously thought to be restricted to oligodendrocytes, but recent research indicates S1PR5 can be expressed and is important also for natural killer cells and monocytes. The activation of S1PR5 modulates oligodendrocyte progenitor migration, oligodendrocyte myelination survival, and process retraction 39,40. S1PR5-deficient mice exhibit decreased numbers of natural killer cells in the peripheral circulation and increased numbers in the lymph nodes and bone marrow 41. Monocytes require S1PR5 to egress from the bone marrow and enter the systemic circulation 42. In human brain endothelial cells, S1PR5 contributes to optimal barrier formation and maintains barrier integrity 43. In a mouse model of systemic sclerosis, S1PR5 modulates early-stage processes of fibrogenesis, potentially promoting the pathogenesis of the disease 44. In a recent study, S1PR5 signaling promotes chromosome segregation and mitotic progression, indicating potential therapeutic targets to inhibit the proliferation of cancer cells 45. In all, accumulating evidence suggests an important role of S1PR5 in cell migration, proliferation, and barrier integrity.
The S1P/S1PRs axis in IBD
The pathogenesis of IBD is incompletely understood but believed to involve an interplay between genetic and environmental factors which may lead to the impairment of intestinal barrier function. This subsequently promotes the translocation of luminal microbes into the bowel wall, where they and their products are recognized by dendritic cells and macrophages 46. These innate immune cells become activated and produce cytokines and chemokines resulting in the recruitment of additional immune cells and initiation of adaptive immune responses 47,48. Excessive or uncontrolled innate and adaptive immune pro-inflammatory responses lead to the development of chronic intestinal inflammation and further impairment of barrier function, culminating in gut tissue damage 49.
IBD is characterized by altered immune cell circuits and trafficking 49. Increased infiltration of T lymphocytes, a crucial component of the intestinal immune system, correlated with endoscopic activity in patients with IBD 50. Mucosal lymphocytes originate from the bone marrow and enter secondary lymphoid tissues such as peripheral lymph nodes, spleen, and gut-associated lymphoid tissue, where they are primed via antigen-presenting cells 51. Primed T cells are then released from lymph nodes back into the peripheral circulation, entering inflamed tissue by extravasation 5. In the context of IBD, altered immune cell trafficking leads to the accumulation of T lymphocytes in the gut and drives inflammation, together with local immune cells such as macrophages, dendritic cells, and innate lymphoid cells 52. Anti-integrins were the first group of medications to selectively block immune cell trafficking to the gut. Vedolizumab, an α4β7 integrin blocker is currently the only anti-integrin approved for both ulcerative colitis and Crohn’s disease in clinical practice 53. However, since the response is not universal and due to their invasive route of administration (i.e. intravenous or subcutaneous), there is a need for new drugs, specifically small molecules that are given orally. The fact that the S1P/ S1PR1 axis acts as a key regulator for lymphocyte migration from lymph nodes has sparked great interest in the role of the S1P/S1PRs axis as a therapeutic target for IBD 2.
Enzymes that control tissue S1P levels in human and experimental IBD are dysregulated, favoring synthesis over degradation 2,10. Upon deletion of SPHK1, which is responsible for S1P generation, lower S1P concentrations and reduced severity of DSS-induced colitis have been observed 54. However, SPHK2 deletion was shown to aggravate DSS-induced colitis and colitis-associated cancer 55. The underlying mechanism remains unclear. In conditional gut-specific SPL-deficient mice, which show an 8-fold increase in S1P levels in tissues of the lower gastrointestinal tract including the colon, IBD symptoms of diarrhea, blood in stool, and weight loss are more severe than wild type mice 56. However, inhibition of SPL by 4-deoxypyridoxine hydrochloride and 2-acetyl-4 (tetrahydroxybutyl) imidazole markedly increases local intestinal S1P levels, induces peripheral lymphopenia, downregulated pro-inflammatory cytokines, and attenuates Crohn’s disease-like TNF∆ARE chronic murine ileitis 57. These seemingly contradictory observations might be caused by the diverse function of S1P in several biological processes. This suggests that SPHKs and SPL are involved in IBD, but more selective strategies are needed for a therapeutic application. The development of S1P receptor-selective modulators has expanded our understanding of how to selectively target the diverse S1P function and this will be discussed in the next section. In addition, cell types involved in IBD pathogenesis, including T cells, B cells, dendritic cells, and endothelial cells, have a higher S1PR1 expression in response to chronic but not acute inflammatory signals 58. Higher S1PR1 expression levels are observed in the colonic mucosa of patients with ulcerative colitis and is associated with increased vascular density in the inflamed mucosa 59. S1PR1-deficient mice exhibit increased colonic vascular permeability under basal conditions and increased bleeding in experimental colitis 59.
S1PRs modulators and their roles in preclinical studies or clinical trials in IBD
The fact that S1P signaling modulates lymphocyte egress from the thymus or secondary lymphoid organs into the circulatory system makes S1P signaling a possible target for inflammatory and autoimmune diseases 60. Treatment with an S1PR1 modulator, which induces downregulation and attenuates signaling through this receptor, blocks lymphocyte trafficking without reducing global immune function (Figure 2) 60. This selective mechanism proved to be therapeutically effective in the treatment of multiple sclerosis 61. S1P receptor modulators are used in two major therapeutic ways. One is as pro-drugs, such as in the case of fingolimod, which can be converted to an active metabolite by SPHKs; the other is to use them as directly acting drugs, such as ponesimod, siponimod and ozanimod 62. In vitro S1P receptor modulators act as potent selective S1P receptor agonists, binding with highly selective affinity at their target S1P receptor (Table 2), whereas in vivo they are functional antagonists, resulting in marked and long-lasting internalization of the targeted S1P receptors, especially S1PR1. Preclinical studies employing various mouse models of colitis suggest that S1PR modulators may also be effective in the treatment of IBD 63,64. The promising phase II & III results of the S1PR1 modulator Ozanimod led to its approval by Food and Drug Administration for ulcerative colitis and several other S1PR modulators are undergoing clinical trials of both ulcerative colitis and Crohn’s disease (Table 3) 23. Here we will discuss the S1PR modulators that have been proven to be effective in preclinical studies or clinical trials in IBD.
Figure 2. S1PR modulators regulate sequestration of T lymphocytes.
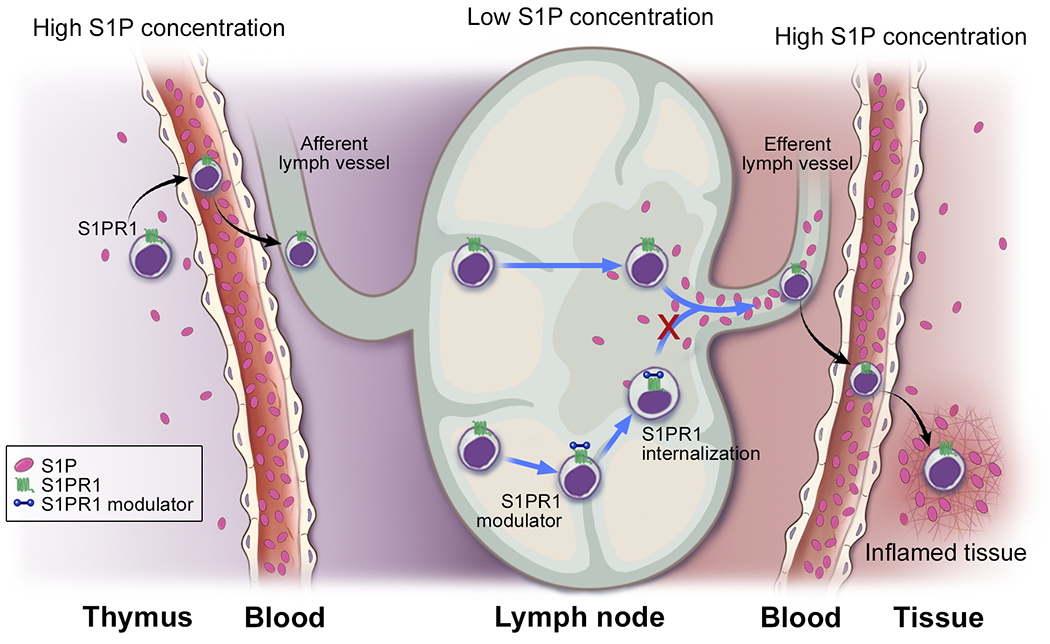
Treatment with an S1PR1 modulator, which induces downregulation and attenuates signaling through this receptor, blocks lymphocyte trafficking.
Table 2.
Characterization and preclinical investigations of S1PR modulators
| Name | Synonym | Formula | Affinity to S1PRs | Animal colitis models investigated | REF |
|---|---|---|---|---|---|
| Ozanimod | RPC-1063 | C23H24N4O3 | S1PR1 and 5 | TNBS-induced colitis in Sprague Dawley rats, Colitis in SCID mice by adoptive transfer of CD4+CD45RBhigh T cells | 65,68 |
| Etrasimod | APD-334 | C26H26F3NO3 | S1PR1, 4 and 5 | Colitis in SCID mice by adoptive transfer of CD4+CD45RBhigh T cells | 80,81 |
| Amiselimod | MT-1303 | C19H30F3NO3 | S1PR1, 4 and 5 | Colitis in SCID mice by adoptive transfer of CD4+CD45RBhigh T cells | 83–86,92 |
| KRP-203 | KRP 203 | C24H26ClNO3S | S1PR1 | Colitis in IL-10−/− mice | 96–100 |
| Fingolimod | FTY720 | C19H34ClNO2 | S1PR1,3,4 and 5 | Colitis in IL-10−/− mice, oxazolone-induced colitis, TNBS-induced colitis, DSS-induced colitis | 102,104–106,122–124 |
| Siponimod | BAF312 | C29H35F3N2O3 | S1PR1 and 5 | None | 107,108 |
| Ponesimod | ACT-128800 | C23H25ClN2O4S | S1PR1 | None | 116 |
| Cenerimod | ACT-334441 | C25H31N3O5 | Mainly S1PR1 and 5 | None | 115,125 |
Table 3.
Clinical trials of S1PR modulators
| Name | Clinical trials | FDA Approvals | REF (ClinicalTrials.gov number) | ||
|---|---|---|---|---|---|
| UC | CD | Non-IBD | |||
| Ozanimod | Phase III, completed | Phase III, recruiting | Phase III in MS, completed | MS, 2020; UC, 2021 | NCT02294058, NCT01628393, NCT02047734, NCT02435992, NCT03467958, NCT03440385, NCT03440372, NCT03464097, and others. |
| Etrasimod | Phase III, recruiting; Phase III, active | Phase II and III, recruiting | Phase II in Eosinophilic Esophagitis, recruiting; Phase II in Atopic Dermatitis, active | None | NCT04706793, NCT04176588, NCT03945188, NCT04173273, NCT04682639, NCT04162769, and others |
| Amiselimod | None | Phase II, completed | Phase II in Plaque Psoriasis, completed; Phase II in SLE, completed | None | NCT02378688, NCT02389790, NCT01987843, NCT02307643 |
| KRP-203 | Phase II, Terminated | None | Phase II in Subacute Cutaneous Lupus Erythematosus, completed | None | NCT01375179, NCT01294774 |
| Fingolimod | None | None | Phase III in MS, completed | RRMS, 2010 | NCT00289978 |
| Siponimod | None | None | Phase III in SPMS, completed; Phase III in MS, recruiting; Phase II in Active Dermatomyositis. | None | NCT01665144, NCT03623243, NCT02029274 |
| Ponesimod | None | None | Phase III in MS, completed; Phase II in Psoriasis, completed | None | NCT02425644, NCT01208090 |
| Cenerimod | None | None | Phase II in SLE, completed | None | NCT02472795 |
CD, Crohn’s disease; FDA, Food and Drug Administration; MS, multiple sclerosis; RRMS, relapsing–remitting multiple sclerosis; SLE, systemic lupus erythematosus; UC, ulcerative colitis
Ozanimod (RPC-1063)
Ozanimod (RPC-1063), (S)-5-(3-(1-((2-hydroxyethyl) amino)-2, 3-dihydro-1H-inden-4-yl)-1, 2, 4-oxadiazol-5-yl)-2-isopropoxybenzonitrile hydrochloride 65, is being developed for the treatment of multiple sclerosis, ulcerative colitis and Crohn’s disease 66. It was approved in the USA for use in the treatment of relapsing forms of adult multiple sclerosis, including relapsing–remitting disease and active secondary progressive disease 66. Meanwhile, in the European union, ozanimod received a positive Committee for Medicinal Products for Human Use (CHMP) opinion recommending approval for the treatment of adult patients with relapsing–remitting multiple sclerosis with active disease defined by clinical or imaging features 67. In 2021, ozanimod was approved by Food and Drug Administration for the treatment of adult patients with moderately to severely active ulcerative colitis.
Ozanimod is a potent agonist of the S1PR1 and S1PR5 receptors with 27-fold selectivity for S1PR1 over S1PR5 receptors and > 10,000-fold selectivity for S1PR1 over S1PR2, 3, 4 receptors 68. Stimulation with ozanimod induces a sustained internalization and degradation of S1PR1 receptors. The downregulation of S1PR1 receptors on lymphocytes prevents their egress from peripheral lymphoid organs, thus reducing circulating lymphocytes and their trafficking to sites of inflammation 68. Therapeutic administration of ozanimod in the 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis in Sprague Dawley rats reduces weight loss in a dose-dependent manner, and inhibits clinical and histological disease scores, with a reduction of circulating lymphocytes 68. Similar results are observed in a T cell adoptive transfer colitis model, indicating ozanimod is a potential candidate for the treatment of IBD 68.
Results from the first-in-human study with ozanimod in 88 healthy volunteers using a range of single and multiple doses (7 and 28 days) and a dose-escalation regimen demonstrated that ozanimod was generally well tolerated up to a maximum single dose of 3 mg and multiple doses of 2 mg/d, with no severe adverse events and no dose-limiting toxicities, only with a dose-dependent negative cardiac chronotropic effect following the first dose69. The treatment of ozanimod induced a robust dose-dependent reduction in total peripheral lymphocytes, with a selective effect on lymphocyte subtypes. Ozanimod caused marked decreases in cells expressing C-C motif chemokine receptor 7 and variable decreases in subsets lacking C-C motif chemokine receptor 7. After the promising results from a randomized, placebo-controlled, phase II trial of ozanimod in relapsing multiple sclerosis (RADIANCE) were released 70,71, the efficacy and safety of ozanimod for adults with relapsing multiple sclerosis was further evaluated in the randomized, double-blind, double-dummy, and multinational phase III SUNBEAM and RADIANCE 72,73. SUNBEAM was a minimum 12-month phase III trial done at 1346 participants who were randomly assigned to ozanimod 1.0 mg (n=447), ozanimod 0.5 mg (n=451), or IFNβ-1a (n=448) 73. The results indicated, in relapsing multiple sclerosis patients treated for at least 12 months, that ozanimod was well tolerated and demonstrated a significantly lower relapse rate than IFNβ-1a 73. RADIANCE was a 24-month phase III trial done at 1320 participants who were randomly assigned to ozanimod 1.0 mg (n=433), ozanimod 0.5 mg (n=439), or IFNβ-1a (n=441) 72.
The efficacy and safety of ozanimod have also been evaluated in ulcerative colitis and Crohn’s disease patients. In a double-blind, placebo-controlled phase II trial of ozanimod of 197 adults with moderate-to-severe ulcerative colitis, patients were randomly assigned to ozanimod at a dose of 0.5 mg or 1 mg or placebo daily for up to 32 weeks 74. At weeks 8 and 32, more patients receiving ozanimod 1 mg achieved clinical remission compared to placebo 74. Clinical response was achieved by 54% and 57% of ozanimod 0.5 mg and 1 mg recipients at week 8 and 35% and 51% at week 32 74. At week 8, absolute lymphocyte counts declined in the group that received ozanimod 74. The most common adverse events overall were anemia and headache 74. In 2020, the results from the TOUCHSTONE open-label extension were released, including efficacy data with up to 4 years of follow-up and safety through the end of openlabel extension 75. The findings indicated durable efficacy by clinical, endoscopic, histologic, and biomarker measures with ozanimod, with no new safety risk, identified 75. In 2020, a single-arm, phase II, prospective observer-blinded endpoint study of ozanimod induction therapy for patients with moderate to severe Crohn’s disease was published 76. All patients began treatment with a 7-day dose escalation (4 days on ozanimod 0.25 mg daily followed by 3 days at 0.5 mg daily), and then received ozanimod 1.0 mg oral capsule daily for a further 11 weeks, for a total of 12-week induction period, followed by a 100-week extension 76. Of the 69 patients, at week 12, 16 patients experienced endoscopic response 76. A reduction from baseline in Crohn’s disease Activity Index (CDAI) score also was observed, with clinical remission (CDAI <150 points) in 27 patients and clinical response (CDAI decrease from baseline ≥100) in 39 patients 76. In addition, ozanimod treatment reduced circulating lymphocytes counts, including total T cells, Th cells, cytotoxic T cells cells, and B cells, but not natural killer cells and monocytes 77. The T cell subsets were not completely inhibited by ozanimod, with CD8+ TEMRA cells largely unaffected 77. These findings suggest ozanimod treatment does not significantly reduce immune surveillance, indicating a low risk of infection or malignancy 77. In 2020, a phase III, randomized, double-blind, placebo-controlled study on ozanimod as induction therapy was reported in moderate-to-severe ulcerative colitis 78,79. 645 patients received ozanimod 1.0 mg (n=429) or placebo (n=216) once-daily for 10 weeks 78,79. Statistically significant differences between ozanimod and placebo-treated patients were reported in clinical remission at week 10 and clinical response, including Mayo rectal bleeding score, endoscopic improvement and mucosal healing 78,79. Then 457 patients who had a clinical response to ozanimod 1.0 mg were given ozanimod 1.0 mg or placebo during the maintenance of the study, and at week 52, more patients receiving ozanimod 1 mg than placebo achieved clinical remission 78,79.
Given the experience with non-selective S1PR modulators, such as fingolimod, special emphasis was given to exploring the safety profile of ozanimod. The most commonly observed adverse events overall were anemia, nasopharyngitis, alanine aminotransferase increase, and headache79. The overall safety signals and adverse events are described in Table 4. The safety concerns led to a thorough recommendation for a pre-treatment assessment of Ozanimod that can be found in Table 5. Currently, ozanimod is undergoing phase III evaluation in moderate to severe Crohn’s disease in various countries ((NCT03467958, NCT03440385, NCT03440372, NCT03464097).).
Table 4.
Ozanimod safety signals and adverse events
| System | Specific adverse reaction | Patients at risk | Timing of reaction | Preventive measures | Follow-up measures | Ref. |
|---|---|---|---|---|---|---|
| CVS | Bradycardia/transient AV conduction delays/AV blocks | Preexisting ischemic heart disease, heart failure, bradyarrythmias and conduction blocks | During induction | Contraindicated in high risk populations, including ischemic heart disease, heart failure, bradyarrythmias and conduction blocks | Assess when clinically indicated | 126,127 |
| Orthostatic hypotension | During induction, usually transient | 126,127 | ||||
| Hypertension | During maintenance, usually persistent | Avoidance of tyramine-containing foods and contraindicated with MAO inhibitors | Monitor blood-pressure during treatment and manage appropriately if clinically significant | 127 | ||
| Respiratory | Decline in pulmonary function | ~3 months after treatment initiation | Spirometric evaluation of respiratory function during therapy only when clinically indicated | 128 | ||
| Hematologic | Lymphopenia, rarely severe (<0.2 × 109/L) | During induction | Continue treatment unless severe lymphopenia develops. Resolves after treatment discontinuation | 69,128 | ||
| Ophthalmic | Macular edema | History of uveitis or diabetes mellitus | Two weeks to 1 year from first dose | Patients at risk should undergo an ophthalmic evaluation of the fundus, including the macula, prior to treatment initiation | Patients at risk should undergo regular ophthalmic follow-up examinations and in any case of new change in vision | 128 |
| GI | Liver injury | History of significant liver disease | Any time throughout course of treatment, mostly transient | Measure baseline liver function test | Assess if clinically indicated. Discontinue treatment if significant liver injury is confirmed | 70 |
| Immune defense | Oral Herpes and Herpes zoster | Any time throughout course of treatment | Discontinue treatment in case of serious infection | 70,128 | ||
| Nasopharyngitis and upper respiratory tract infection | Any time throughout course of treatment | Discontinue treatment in case of serious infection | 70,128 | |||
| Urinary tract infection | Any time throughout course of treatment | Assess if clinically indicated | 70,128 |
CVS, cardiovascular system; AV, atrioventricular; MAO, monoamine oxidase; GI, gastrointestinal
Table 5.
Recommended Ozanimod pre-treatment assessment
| Baseline Assessment | Test | Advice |
|---|---|---|
| Cardiac Evaluation& | ECG to exclude baseline bradyarrythmias or conduction blocks, blood pressure, check drug history for medications that may slow heart rate or AV conduction | Cardiac contraindications: MMI, unstable angina, class III or IV heart failure, type 2 second degree or type 3 degree AV block, sick sinus syndrome, significant QTc prolongation |
| Complete Blood Count | Including lymphocyte count | Patients with counts <0.2 × 109/L were excluded from trial programs, a mean 50% reduction in total lymphocyte count is expected |
| Liver Function Tests | Transaminases and bilirubin level | 5% develop transaminitis > 3x ULN |
| Ophthalmic Assessment | Fundoscopy | Patients with a history of diabetes, uveitis or macular edema |
| Virology and TB | Hep B, VZV serology and TB IGRA | Consider VZV vaccination if VZV IgG negative (live vaccines require administration 3 months prior to initiation). Herpes zoster is the most common opportunistic infection |
| Other contraindications | Patient history | TIA or stroke < 6 months, severe untreated sleep apnea |
| Review current or prior medications | Patient history | Anti-neoplastic, immunosuppressive, or immune-modulating therapies may lead to unintended additive immunosuppression. Monoaminooxidase inhibitors are contraindicated |
| Dose titration at start | Titrate once daily dose to maintenance dose at one week: 0.25mg days 1–4, 0.5mg days 5–7, then 1mg QD | |
Request cardiologist consultation in patients with a preexisting cardiac condition.AV, atrioventricular; ECG, electrocardiogram; VZV, varicella-zoster virus; MI, myocardial infarction; QTc, QT Interval; ULN, upper limit of norm; TB, tuberculosis; IGRA, interferon-gamma release assays; Hep B, hepatitis B virus; TIA, transient ischemic attack; QD, every day.
Etrasimod (APD-334)
Etrasimod is a synthetic, next-generation, oral S1P receptor modulator in clinical development for the treatment of immune-mediated inflammatory disorders 80. Etrasimod is a full agonist of human S1PR1 and a partial agonist of S1PR4 and S1PR5 in a β-arrestin recruitment assay, whereas neither agonist nor antagonist activity was observed on either the human recombinant S1PR2 or S1PR3 receptors 80,81. In the adoptive transfer of CD4+CD45RBhigh T cells mouse colitis model, treatment with etrasimod results in significant reductions in mucosal thickness, and lower histopathology scores with fewer inflammatory infiltrates 81. In the colons of CD4+CD45RBhigh severe combined immunodeficient (SCID) mice, treatment with etrasimod significantly reduces the expression of T cell and monocyte markers, such as CD4 (T cells, natural killer and dendritic cells), CD3γ (T lymphocytes), CD11b (monocytes, natural killer and dendritic cells), and Ly6G (neutrophils, and granulocytes), suggesting that etrasimod treatment decreases infiltration and expansion of these cell populations 81. Consistently, T-cell and/or monocyte-derived pro-inflammatory cytokines Tumor necrosis factor-α, IL-1β, IL-6, and IL-17A are also significantly lower in CD4+CD45RBhigh SCID mice treated with etrasimod, whereas the anti-inflammatory cytokine IL-10 is induced by etrasimod treatment 81.
The result of etrasimod in phase II, proof of concept, double-blind, parallel-group, randomized trial of patients with moderately to severely active ulcerative colitis was published in 2019 62. Adult outpatients with modified Mayo clinic scores (stool frequency, rectal bleeding, and endoscopy findings) of 4-9, endoscopic subscores of 2 or more, and rectal bleeding subscores of 1 or more were randomly assigned to groups given once-daily etrasimod 1 mg (n=52), etrasimod 2 mg (n=50), or placebo (n=54) for 12 weeks at 87 centers in 17 countries 62. At week 12, etrasimod 2 mg led to a significantly greater mean improvement in modified Mayo clinic scores from baseline than placebo, and endoscopic improvement occurred in 41.8% of patients receiving etrasimod 2 mg vs 17.8% receiving placebo 62. These results indicated the efficacy of etrasimod in the treatment of moderate to severe ulcerative colitis. Current safety data show a favorable safety profile but are limited by a relatively small number of participants 82. Currently, there are several ongoing phase III studies in severely active ulcerative colitis (NCT04706793, NCT03950232, NCT03996369, NCT04176588, and NCT03945188) and moderate to severe Crohn’s disease (NCT04173273).
Amiselimod (MT-1303)
Amiselimod (MT-1303), 2-amino-2-{2-[4-(heptyloxy)-3-(trifluoromethyl) phenyl] ethyl} propan-1, 3-diol hydrochloride, a structural analogue of fingolimod, was designed as a prodrug lacking S1PR3 agonism for the treatment of autoimmune disease. Amiselimod is converted to its active metabolite, (S)-amiselimod phosphate (amiselimod-P), by SPHKs in vivo 83,84. Amiselimod-P shows high selectivity for S1PR1 and S1PR5, minimal agonist activity for S1PR4, and no distinct agonist activity for S1PR2 or S1PR3 83. The conversion of amiselimod into its active metabolite is slower than fingolimod 85. In both preclinical and phase I studies, amiselimod shows a favorable cardiac safety profile with approximately 5-fold weaker G protein-gated inwardly rectifying potassium (GIRK) activation than fingolimod-phosphate (fingolimod-P) 83,86. In a phase I study, no clinically significant bradycardia was observed after administration of amiselimod up to 0.75mg 83.
A randomized, double-blinded, placebo-controlled phase II trial in relapsing multiple sclerosis reported 0.2 mg and 0.4 mg amiselimod significantly reduced the total number of gadolinium-enhanced T1-weighted lesions with no serious adverse events in any group and no clinically significant heart rate reduction at any dose 87. These findings were confirmed in a phase II extension study of oral amiselimod in relapsing multiple sclerosis, showing that amiselimod was well tolerated and dose-dependently effective in controlling disease activity for up to 2 years of treatment 88. The effects of amiselimod were evaluated in murine systemic lupus erythematosus models and preclinical results suggested that it inhibits the progression of lupus nephritis by reducing the infiltration of autoreactive T cells into the kidneys 89. Two clinical phases II trials in systemic lupus erythematosus (NCT02307643) and psoriasis (NCT01987843) have been completed without released reports 90,91 .
In the intestine, the effects of amiselimod were evaluated in chronic colitis of immune-deficient SCID mice by adoptive transfer of CD4+CD45RBhigh T cells. The results demonstrated that amiselimod regulates lymphocyte trafficking, limits infiltration of colitogenic Th1 and Th17 cells into the colon, and inhibits the development of chronic colitis 92. The investigation of the effects of amiselimod in moderate-to-severe, active Crohn’s disease in a 14-week, randomized, placebo-controlled, phase II trial 93 with an open-label extension of ≥22 weeks 94 has been completed. However, amiselimod 0.4 mg/day for 12 weeks did not show a significant effect on clinical or biochemical disease activity in refractory Crohn’s disease 95. The effects of amiselimod in ulcerative colitis patients may be further evaluated 89.
KRP-203
KRP-203, 2-amino2-propanediol hydrochloride, an S1P receptor agonist with some similarities in molecular structure to fingolimod, has been developed for immunomodulation in autoimmune diseases and organ transplantation 5,96,97. KRP-203, which is supposed to be selective for S1PR1, prolonges graft survival and attenuates chronic rejection in rat allograft models, indicating that KRP-203 might regulate both T cell trafficking and B cell response 98. KRP-203 administration substantially reduced atherosclerotic lesion formation and induced lymphopenia in LDL-R−/− mice, with reduced numbers of total (CD4+, CD8+) and activated (CD69+/CD8+, CD69+/CD4+) T cells in peripheral lymphoid organs, and lower levels of cytokine and chemokine (tumor necrosis factor-α, regulated and normal T cell expressed and secreted) levels in plasma and aortas 99. In IL-10 gene-deficient mice, KRP-203 significantly inhibits ongoing colitis in part through decreasing the infiltration of lymphocytes at inflammatory sites and by blocking T-helper 1 cytokine production in the colonic mucosa 100.
In 2011, a clinical trial evaluated the efficacy and safety of KRP-203 in moderately active refractory ulcerative colitis patients, with approximately 72 randomized subjects 101. The trial has been terminated, but results have not been published yet 101.
Other S1PRs modulators as potential future inflammatory therapies
Fingolimod (FTY720), Siponimod (BAF312), Ponesimod (ACT-128800), and Cenerimod (ACT-334441) have been investigated in preclinical studies or clinical trials of several diseases but have yet to be tested in IBD.
Fingolimod was characterized as a non-selective agonist of all of the S1P receptors (S1PR), except for S1PR2, and in addition, as a selective S1PR1 functional antagonist of S1PR1 102. Fingolimod exhibits clear efficacy in reducing the frequency of relapses and disability progression on long-term follow-up of patients with multiple sclerosis compared to placebo, in phase III trials (FREEDOMS, FREEDOMS II, and TRANSFORMS) and was the first approved oral treatment for relapsing-remitting multiple sclerosis, licensed by the Food and Drug Administration in 2010 and by the European Medicines Agency in 2011 103. Treatment with fingolimod decreases the clinical and histopathologic severity of oxazolone-induced colitis104, TNBS colitis mouse model105, and DSS colitis model106. Despite the clinical efficacy of fingolimod in the treatment of multiple sclerosis and the promising preclinical results of fingolimod in the treatment of other diseases, the occurrence of adverse events raised concerns about the safety profile of fingolimod 102. Infections, bradyarrhythmias and atrioventricular blocks, increased blood pressure, respiratory adverse effects, liver injury, and basal cell carcinoma were observed, perhaps related to the nonselective mechanism of action of fingolimod 102, suggesting that more selective S1PR1 modulators may be needed.
Siponimod (BAF312), targeting S1PR1 and S1PR5, is the second S1P receptor modulator to enter clinical trials for multiple sclerosis 107. It was designed using fingolimod as the lead structure, and optimizing it for potency at S1PR1, with limiting effects on S1PR3 (for risk of side effects), and pharmacokinetics (allowing for once a day oral dosing, but also for rapid recovery of peripheral lymphocyte counts following discontinuation) 108. According to the data of a phase I study, siponimod induces profound but rapidly reversible inhibition of lymphocyte trafficking 109. However, although siponimod lacks affinity with S1PR3, which was associated with bradycardia in mice and it induces rapid but transient bradycardia in humans 109. In a phase II study and its extension study of siponimod in patients with relapsing-remitting multiple sclerosis, promising results are observed with siponimod on MRI lesion activity in model-based analyses and its tolerability in relapsing-remitting multiple sclerosis 110,111. In a subsequent phase III study in secondary progressive multiple sclerosis, siponimod reduced the risk of disability progression with a safety profile similar to that of other S1P modulators 112.
Ponesimod, is a reversible, orally active, selective S1PR1 modulator with at least 10-fold more activation potency on the S1PR1 than on any other S1P receptor subtype113. A double-blind, placebo-controlled, dose-finding phase II study evaluated the efficacy and safety for the treatment of patients with relapsing–remitting multiple sclerosis showed that ponesimod was generally well tolerated and significantly reduced the number of new lesions, showing a beneficial effect on clinical endpoints 114. In the meantime, a double-blind, randomised, placebo-controlled, parallel-group, multicentre phase II study of oral ponesimod to assess efficacy, safety, and tolerability in patients with moderate to severe chronic plaque psoriasis indicated more patients in the ponesimod groups than the placebo group experienced improvements at week 16 115. However, ponesimod was associated with dyspnea, elevated liver enzymes, and dizziness 116.
Cenerimod, is a selective S1PR1 modulator with a 16-fold higher activation potency on the S1PR1 compared to the natural ligand S1P, whereas displaying no detectable agonist activity on the S1PR2, at least 2000-fold less active at the S1PR3 than S1P, 30- to 35-fold lower potency at the S1PR4 than S1P, but two-fold more potent on the S1PR5 receptor than S1P 115. A phase I study in healthy participants showed that cenerimod is well tolerated with no significant safety concerns 117. A double-blind, randomized, placebo-controlled, proof-of-concept study showed that cenerimod has the potential to treat patients with systemic lupus erythematosus with an acceptable safety profile 118. Cenerimod caused a statistically significant dose-dependent reduction in total lymphocyte count from baseline to end of treatment 118. Compared with placebo, cenerimod 4 mg showed a clinical and biological improvement on change from baseline in systemic lupus erythematosus disease activity index-2000 score, and on biomarker anti-dsDNA antibodies 118.
Summary and future perspective
Most IBD patients show either a primary non-response or lose responsiveness during the long-term maintenance treatment with current therapies, including anti-Tumor necrosis factor, anti-integrin, anti-IL-23 monoclonal antibodies and JAK inhibitors. This justifies the need for alternate, efficient, and well-tolerated new medical therapies 10. S1P receptor modulators have been investigated in several preclinical and clinical trials related to chronic inflammatory disorders, such as relapsing-remitting multiple sclerosis, systemic lupus erythematosus, psoriasis, and IBD 119 showing efficacy in several of those diseases. Solid information on the biology of the different S1P receptors, their cellular function, and their clinical relevance is emerging, mostly confirming the role of S1PR1 in inflammation. One advantage of the S1P receptor modulators is that all of them can be conveniently administered by the oral route 120. Furthermore, the second generation of S1P receptor modulators, such as siponimod, amiselimod, and ozanimod, are more selective and might offer better safety and tolerability with fewer cardiac side-effects 119. The promising phase II and III data of ozanimod in the treatment of ulcerative colitis and Crohn’s disease patients and of etrasimod in ulcerative colitis suggest S1P receptor modulators targeting S1P signaling may constitute a novel, promising, safe, and convenient strategy for the alleviation of the symptoms of IBD 62,74,121. What remains to be clarified in this therapeutic area? The distinct and exact mechanisms of different modes of action of the highly selective S1P receptor modulators, which will help to find the best combination therapeutic strategies for optimal clinical efficacy and safety with limited side effects. This is particularly true as there appears to be a partial disconnect between peripheral lymphocyte numbers and inflammation at the level of the mucosa, suggesting direct anti-inflammatory efficacy independent of a reduction of lymphocytes in the peripheral circulation. The exact profile of lymphocyte subpopulations in the mucosa influenced by S1P modulation is still unclear, and defining it would help better understand the immunomodulatory function of these novel drugs. Finally, long-term safety data collected post-approval of this novel class of medications will shed light on safety and tolerability, critical factors for therapeutic success. S1P modulators are here to stay, and future work will determine their place in the treatment algorithms for Crohn’s disease and ulcerative colitis.
Acknowledgments
F.R. is the Guarantor of the article. J.W.: Study concept and design, literature search, manuscript writing, and illustrations; I.G.: manuscript writing, and illustrations; B.Y.: literature search, and illustrations; S.L., J.L., M.E.: literature search; C.F.: critical revision of the manuscript; F.R.: study concept and design, supervision, and critical revision of the manuscript. All authors approved the final version of the manuscript for publication.
Statement of Interests
This work was supported by a grant from the Leona M. and Harry B. Helmsley Charitable Trust (3081), and grants from The National Institute of Diabetes and Digestive and Kidney Diseases, part of the National Institutes of Health (K08-110415, and R01-123233) for F.R.
Abbreviations:
- CDAI
Crohn’s disease Activity Index
- DSS
dextran sodium sulfate
- IBD
Inflammatory bowel diseases
- IFN
interferon
- IL
interleukin
- PTEN
phosphatase and tensin homolog
- S1P
sphingosine 1 phosphate
- S1PR
sphingosine 1 phosphate reptor
- SCID
severe combined immunodeficient
- SPHK
sphingosine kinases
- SPL
S1P lyase
- S1PP
S1P phosphatase
- Th
T-helper
- TNBS
2,4,6-trinitrobenzene sulfonic acid
Reference
- 1.Dulai PS, Sandborn WJ, Gupta S. Colorectal Cancer and Dysplasia in Inflammatory Bowel Disease: A Review of Disease Epidemiology, Pathophysiology, and Management. Cancer Prev Res (Phila). 2016;9(12):887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sukocheva OA, Furuya H, Ng ML, et al. Sphingosine kinase and sphingosine-1-phosphate receptor signaling pathway in inflammatory gastrointestinal disease and cancers: A novel therapeutic target. Pharmacol Ther. 2020;207:107464. [DOI] [PubMed] [Google Scholar]
- 3.Rivera-Nieves J Strategies that target leukocyte traffic in inflammatory bowel diseases: recent developments. Curr Opin Gastroenterol. 2015;31(6):441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol. 2012;30:69–94. [DOI] [PubMed] [Google Scholar]
- 5.Danese S, Furfaro F, Vetrano S. Targeting S1P in Inflammatory Bowel Disease: New Avenues for Modulating Intestinal Leukocyte Migration. J Crohns Colitis. 2018;12(suppl_2):S678–S686. [DOI] [PubMed] [Google Scholar]
- 6.Nagahashi M, Abe M, Sakimura K, Takabe K, Wakai T. The role of sphingosine-1-phosphate in inflammation and cancer progression. Cancer Sci. 2018;109(12):3671–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar A, Zamora-Pineda J, Degagne E, Saba JD. S1P Lyase Regulation of Thymic Egress and Oncogenic Inflammatory Signaling. Mediators Inflamm. 2017;2017:7685142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pyne S, Lee SC, Long J, Pyne NJ. Role of sphingosine kinases and lipid phosphate phosphatases in regulating spatial sphingosine 1-phosphate signalling in health and disease. Cell Signal. 2009;21(1):14–21. [DOI] [PubMed] [Google Scholar]
- 9.Aoki M, Aoki H, Ramanathan R, Hait NC, Takabe K. Sphingosine-1-Phosphate Signaling in Immune Cells and Inflammation: Roles and Therapeutic Potential. Mediators Inflamm. 2016;2016:8606878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen OH, Li Y, Johansson-Lindbom B, Coskun M. Sphingosine-1-Phosphate Signaling in Inflammatory Bowel Disease. Trends Mol Med. 2017;23(4):362–374. [DOI] [PubMed] [Google Scholar]
- 11.Blaho VA, Hla T. An update on the biology of sphingosine 1-phosphate receptors. J Lipid Res. 2014;55(8):1596–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao L, Zhou Y, Friis T, Beagley K, Xiao Y. S1P-S1PR1 Signaling: the “Sphinx” in Osteoimmunology. Front Immunol. 2019;10:1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obinata H, Hla T. Sphingosine 1-phosphate and inflammation. Int Immunol. 2019;31(9):617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang L, Yue S, Yang L, et al. Sphingosine kinase/sphingosine 1-phosphate (S1P)/S1P receptor axis is involved in liver fibrosis-associated angiogenesis. J Hepatol. 2013;59(1):114–123. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Yue S, Li C, Yang L, You H, Li L. Essential roles of sphingosine 1-phosphate receptor types 1 and 3 in human hepatic stellate cells motility and activation. J Cell Physiol. 2011;226(9):2370–2377. [DOI] [PubMed] [Google Scholar]
- 16.Ohkura SI, Usui S, Takashima SI, et al. Augmented sphingosine 1 phosphate receptor-1 signaling in cardiac fibroblasts induces cardiac hypertrophy and fibrosis through angiotensin II and interleukin-6. PLoS One. 2017;12(8):e0182329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdullah CS, Jin ZQ. Targeted deletion of T-cell S1P receptor 1 ameliorates cardiac fibrosis in streptozotocin-induced diabetic mice. FASEB J. 2018;32(10):5426–5435. [DOI] [PubMed] [Google Scholar]
- 18.Jung B, Obinata H, Galvani S, et al. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev Cell. 2012;23(3):600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yonesu K, Kawase Y, Inoue T, et al. Involvement of sphingosine-1-phosphate and S1P1 in angiogenesis: analyses using a new S1P1 antagonist of non-sphingosine-1-phosphate analog. Biochem Pharmacol. 2009;77(6):1011–1020. [DOI] [PubMed] [Google Scholar]
- 20.Chae SS, Paik JH, Furneaux H, Hla T. Requirement for sphingosine 1-phosphate receptor-1 in tumor angiogenesis demonstrated by in vivo RNA interference. J Clin Invest. 2004;114(8):1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaMontagne K, Littlewood-Evans A, Schnell C, et al. Antagonism of sphingosine-1-phosphate receptors by FTY720 inhibits angiogenesis and tumor vascularization. Cancer Res. 2006;66(1):221–231. [DOI] [PubMed] [Google Scholar]
- 22.Bryan AM, Del Poeta M. Sphingosine-1-phosphate receptors and innate immunity. Cell Microbiol. 2018;20(5):e12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peyrin-Biroulet L, Christopher R, Behan D, Lassen C. Modulation of sphingosine-1-phosphate in inflammatory bowel disease. Autoimmun Rev. 2017;16(5):495–503. [DOI] [PubMed] [Google Scholar]
- 24.Blankenbach KV, Schwalm S, Pfeilschifter J, Meyer Zu Heringdorf D. Sphingosine-1-Phosphate Receptor-2 Antagonists: Therapeutic Potential and Potential Risks. Front Pharmacol. 2016;7:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou L, Yang L, Chang N, et al. Macrophage Sphingosine 1-Phosphate Receptor 2 Blockade Attenuates Liver Inflammation and Fibrogenesis Triggered by NLRP3 Inflammasome. Front Immunol. 2020;11:1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao J, Okamoto Y, Asano Y, et al. Sphingosine-1-phosphate receptor-2 facilitates pulmonary fibrosis through potentiating IL-13 pathway in macrophages. PLoS One. 2018;13(5):e0197604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Q, Rehman J, Chan M, et al. Angiocrine Sphingosine-1-Phosphate Activation of S1PR2-YAP Signaling Axis in Alveolar Type II Cells Is Essential for Lung Repair. Cell Rep. 2020;31(13):107828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong Y, Piao W, Brinkman CC, et al. CD4 T cell sphingosine 1-phosphate receptor (S1PR)1 and S1PR4 and endothelial S1PR2 regulate afferent lymphatic migration. Sci Immunol. 2019;4(33). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gong L, Wu X, Li X, et al. S1PR3 deficiency alleviates radiation-induced pulmonary fibrosis through the regulation of epithelial-mesenchymal transition by targeting miR-495–3p. J Cell Physiol. 2020;235(3):2310–2324. [DOI] [PubMed] [Google Scholar]
- 30.Murakami K, Kohno M, Kadoya M, et al. Knock out of S1P3 receptor signaling attenuates inflammation and fibrosis in bleomycin-induced lung injury mice model. PLoS One. 2014;9(9):e106792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoue T, Kohno M, Nagahara H, et al. Upregulation of sphingosine-1-phosphate receptor 3 on fibroblast-like synoviocytes is associated with the development of collagen-induced arthritis via increased interleukin-6 production. PLoS One. 2019;14(6):e0218090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simmons S, Ishii M. Sphingosine-1-phosphate: a master regulator of lymphocyte egress and immunity. Arch Immunol Ther Exp (Warsz). 2014;62(2):103–115. [DOI] [PubMed] [Google Scholar]
- 33.Wang W, Graeler MH, Goetzl EJ. Type 4 sphingosine 1-phosphate G protein-coupled receptor (S1P4) transduces S1P effects on T cell proliferation and cytokine secretion without signaling migration. FASEB J. 2005;19(12):1731–1733. [DOI] [PubMed] [Google Scholar]
- 34.Schulze T, Golfier S, Tabeling C, et al. Sphingosine-1-phospate receptor 4 (S1P(4)) deficiency profoundly affects dendritic cell function and TH17-cell differentiation in a murine model. FASEB J. 2011;25(11):4024–4036. [DOI] [PubMed] [Google Scholar]
- 35.Fettel J, Kuhn B, Guillen NA, et al. Sphingosine-1-phosphate (S1P) induces potent anti-inflammatory effects in vitro and in vivo by S1P receptor 4-mediated suppression of 5-lipoxygenase activity. FASEB J. 2019;33(2):1711–1726. [DOI] [PubMed] [Google Scholar]
- 36.Kulinski JM, Proia RL, Larson EM, Metcalfe DD, Olivera A. S1P(4) Regulates Passive Systemic Anaphylaxis in Mice but Is Dispensable for Canonical IgE-Mediated Responses in Mast Cells. Int J Mol Sci. 2018;19(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onuma T, Tanabe K, Kito Y, et al. Sphingosine 1-phosphate (S1P) suppresses the collagen-induced activation of human platelets via S1P4 receptor. Thromb Res. 2017;156:91–100. [DOI] [PubMed] [Google Scholar]
- 38.Ohotski J, Long JS, Orange C, et al. Expression of sphingosine 1-phosphate receptor 4 and sphingosine kinase 1 is associated with outcome in oestrogen receptor-negative breast cancer. Br J Cancer. 2012;106(8):1453–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Novgorodov AS, El-Alwani M, Bielawski J, Obeid LM, Gudz TI. Activation of sphingosine-1-phosphate receptor S1P5 inhibits oligodendrocyte progenitor migration. FASEB J. 2007;21(7):1503–1514. [DOI] [PubMed] [Google Scholar]
- 40.Jaillard C, Harrison S, Stankoff B, et al. Edg8/S1P5: an oligodendroglial receptor with dual function on process retraction and cell survival. J Neurosci. 2005;25(6):1459–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walzer T, Chiossone L, Chaix J, et al. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat Immunol. 2007;8(12):1337–1344. [DOI] [PubMed] [Google Scholar]
- 42.Debien E, Mayol K, Biajoux V, et al. S1PR5 is pivotal for the homeostasis of patrolling monocytes. Eur J Immunol. 2013;43(6):1667–1675. [DOI] [PubMed] [Google Scholar]
- 43.van Doorn R, Lopes Pinheiro MA, Kooij G, et al. Sphingosine 1-phosphate receptor 5 mediates the immune quiescence of the human brain endothelial barrier. J Neuroinflammation. 2012;9:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt KG, Herrero San Juan M, Trautmann S, et al. Sphingosine-1-Phosphate Receptor 5 Modulates Early-Stage Processes during Fibrogenesis in a Mouse Model of Systemic Sclerosis: A Pilot Study. Front Immunol. 2017;8:1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andrieu G, Ledoux A, Branka S, et al. Sphingosine 1-phosphate signaling through its receptor S1P5 promotes chromosome segregation and mitotic progression. Sci Signal. 2017;10(472). [DOI] [PubMed] [Google Scholar]
- 46.Davies JM, Abreu MT. The innate immune system and inflammatory bowel disease. Scand J Gastroenterol. 2015;50(1):24–33. [DOI] [PubMed] [Google Scholar]
- 47.Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13(1):3–10. [DOI] [PubMed] [Google Scholar]
- 48.Huang Y, Chen Z. Inflammatory bowel disease related innate immunity and adaptive immunity. American Journal of Translational Research. 2016;8(6):2490–2497. [PMC free article] [PubMed] [Google Scholar]
- 49.de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13(1):13–27. [DOI] [PubMed] [Google Scholar]
- 50.Smids C, Horjus Talabur Horje CS, Drylewicz J, et al. Intestinal T Cell Profiling in Inflammatory Bowel Disease: Linking T Cell Subsets to Disease Activity and Disease Course. J Crohns Colitis. 2018;12(4):465–475. [DOI] [PubMed] [Google Scholar]
- 51.Habtezion A, Nguyen LP, Hadeiba H, Butcher EC. Leukocyte Trafficking to the Small Intestine and Colon. Gastroenterology. 2016;150(2):340–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neurath MF. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat Immunol. 2019;20(8):970–979. [DOI] [PubMed] [Google Scholar]
- 53.Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66(5):839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Snider AJ, Kawamori T, Bradshaw SG, et al. A role for sphingosine kinase 1 in dextran sulfate sodium-induced colitis. FASEB J. 2009;23(1):143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang J, Nagahashi M, Kim EY, et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell. 2013;23(1):107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suh JH, Saba JD. Sphingosine-1-phosphate in inflammatory bowel disease and colitis-associated colon cancer: the fat’s in the fire. Transl Cancer Res. 2015;4(5):469–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karuppuchamy T, Tyler CJ, Lundborg LR, et al. Sphingosine-1-Phosphate Lyase Inhibition Alters the S1P Gradient and Ameliorates Crohn’s-Like Ileitis by Suppressing Thymocyte Maturation. Inflamm Bowel Dis. 2020;26(2):216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang Y, Chen Z. Inflammatory bowel disease related innate immunity and adaptive immunity. Am J Transl Res. 2016;8(6):2490–2497. [PMC free article] [PubMed] [Google Scholar]
- 59.Montrose DC, Scherl EJ, Bosworth BP, et al. S1P(1) localizes to the colonic vasculature in ulcerative colitis and maintains blood vessel integrity. J Lipid Res. 2013;54(3):843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Degagne E, Saba JD. S1pping fire: Sphingosine-1-phosphate signaling as an emerging target in inflammatory bowel disease and colitis-associated cancer. Clin Exp Gastroenterol. 2014;7:205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427(6972):355–360. [DOI] [PubMed] [Google Scholar]
- 62.Bigaud M, Guerini D, Billich A, Bassilana F, Brinkmann V. Second generation S1P pathway modulators: research strategies and clinical developments. Biochim Biophys Acta. 2014;1841(5):745–758. [DOI] [PubMed] [Google Scholar]
- 63.Kappos L, Antel J, Comi G, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355(11):1124–1140. [DOI] [PubMed] [Google Scholar]
- 64.Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387–401. [DOI] [PubMed] [Google Scholar]
- 65.Rasche L, Paul F. Ozanimod for the treatment of relapsing remitting multiple sclerosis. Expert Opin Pharmacother. 2018;19(18):2073–2086. [DOI] [PubMed] [Google Scholar]
- 66.Lamb YN. Ozanimod: First Approval. Drugs. 2020;80(8):841–848. [DOI] [PubMed] [Google Scholar]
- 67.Agency EM. Zeposia (ozanimod): summary of opinion. 2020. http://wwwemaeuropaeu/ Accessed 20 Apr 2020. 2020.
- 68.Scott FL, Clemons B, Brooks J, et al. Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor-1 (S1P1) and receptor-5 (S1P5) agonist with autoimmune disease-modifying activity. Br J Pharmacol. 2016;173(11):1778–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tran JQ, Hartung JP, Peach RJ, et al. Results From the First-in-Human Study With Ozanimod, a Novel, Selective Sphingosine-1-Phosphate Receptor Modulator. J Clin Pharmacol. 2017;57(8):988–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cohen JA, Comi G, Arnold DL, et al. Efficacy and safety of ozanimod in multiple sclerosis: Dose-blinded extension of a randomized phase II study. Mult Scler. 2019;25(9):1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brian F, William S, Geert D, Stephen H, Allan O. Ozanimod, an Oral S1P Receptor Modulator, Is Effective and Well-Tolerated in the Long-Term Treatment of Moderate to Severe Ulcerative Colitis: P-012. The American Journal of Gastroenterology. 2018;113:S3. [Google Scholar]
- 72.Cohen JA, Comi G, Selmaj KW, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): a multicentre, randomised, 24-month, phase 3 trial. Lancet Neurol. 2019;18(11):1021–1033. [DOI] [PubMed] [Google Scholar]
- 73.Comi G, Kappos L, Selmaj KW, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): a multicentre, randomised, minimum 12-month, phase 3 trial. Lancet Neurol. 2019;18(11):1009–1020. [DOI] [PubMed] [Google Scholar]
- 74.Sandborn WJ, Feagan BG, Wolf DC, et al. Ozanimod Induction and Maintenance Treatment for Ulcerative Colitis. N Engl J Med. 2016;374(18):1754–1762. [DOI] [PubMed] [Google Scholar]
- 75.Sandborn WFBG, Hanauer SB, Vermeire S, Ghosh S, Liu WJ, Petersen A, Charles L, Huang V, Usiskin K, Wolf DC, D’Haens G. LONG-TERM SAFETY AND EFFICACY OF OZANIMOD IN PATIENTS WITH MODERATE-TO-SEVERE ULCERATIVE COLITIS: RESULTS FROM THE TOUCHSTONE OPEN-LABEL EXTENSION P087. UEGW2020 (United European Gastroenterology Week) Abstracts. 2020. [Google Scholar]
- 76.Feagan BG, Sandborn WJ, Danese S, et al. Ozanimod induction therapy for patients with moderate to severe Crohn’s disease: a single-arm, phase 2, prospective observer-blinded endpoint study. Lancet Gastroenterol Hepatol. 2020;5(9):819–828. [DOI] [PubMed] [Google Scholar]
- 77.Harris SSW, Feagan BG, Hanauer SB, Vermeire S, Ghosh S, Liu WJ1, Wolf DC, D’Haens G. P0527 - EFFECTS OF OZANIMOD ON CIRCULATING LYMPHOCYTES IN PATIENTS WITH MODERATELY TO SEVERELY ACTIVE CROHN’S DISEASE: FLOW CYTOMETRY RESULTS FROM THE STEPSTONE PHASE 2 STUDY. UEGW2020 (United European Gastroenterology Week) Abstracts. 2020. [Google Scholar]
- 78.Sandborn WJ, Feagan BG, D’Haens G, et al. Ozanimod as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2021;385(14):1280–1291. [DOI] [PubMed] [Google Scholar]
- 79.Sandborn W LB02 - OZANIMOD AS INDUCTION THERAPY IN MODERATE-TO-SEVERE ULCERATIVE COLITIS: RESULTS FROM THE PHASE 3, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED TRUE NORTH STUDY. UEGW (United European Gastroenterology Week) 2020. 2020. [Google Scholar]
- 80.Buzard DJ, Kim SH, Lopez L, et al. Discovery of APD334: Design of a Clinical Stage Functional Antagonist of the Sphingosine-1-phosphate-1 Receptor. ACS Med Chem Lett. 2014;5(12):1313–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Al-Shamma H, Lehmann-Bruinsma K, Carroll C, et al. The Selective Sphingosine 1-Phosphate Receptor Modulator Etrasimod Regulates Lymphocyte Trafficking and Alleviates Experimental Colitis. J Pharmacol Exp Ther. 2019;369(3):311–317. [DOI] [PubMed] [Google Scholar]
- 82.Vermeire S, Chiorean M, Panes J, et al. Long-term Safety and Efficacy of Etrasimod for Ulcerative Colitis: Results from the Open-label Extension of the OASIS Study. J Crohns Colitis. 2021;15(6):950–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sugahara K, Maeda Y, Shimano K, et al. Amiselimod, a novel sphingosine 1-phosphate receptor-1 modulator, has potent therapeutic efficacy for autoimmune diseases, with low bradycardia risk. Br J Pharmacol. 2017;174(1):15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kifuji T, Inoue S, Furukawa M, et al. Absorption, disposition and metabolic pathway of amiselimod (MT-1303) in healthy volunteers in a mass balance study. Xenobiotica. 2019;49(9):1033–1043. [DOI] [PubMed] [Google Scholar]
- 85.Sugahara K MY, Shimano K, et al. MT-1303, a novel sphingosine 1-phosphate (S1P) receptor modulator, has less potential for bradycardia than fingolimod. Congress of the European Committeefor Treatment and Research in Multiple Sclerosis; Barcelona. 2015. [Google Scholar]
- 86.Harada T, Wilbraham D, de La Borderie G, Inoue S, Bush J, Camm AJ. Cardiac effects of amiselimod compared with fingolimod and placebo: results of a randomised, parallel-group, phase I study in healthy subjects. Br J Clin Pharmacol. 2017;83(5):1011–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kappos L, Arnold DL, Bar-Or A, et al. Safety and efficacy of amiselimod in relapsing multiple sclerosis (MOMENTUM): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2016;15(11):1148–1159. [DOI] [PubMed] [Google Scholar]
- 88.Kappos L, Arnold DL, Bar-Or A, et al. Two-year results from a phase 2 extension study of oral amiselimod in relapsing multiple sclerosis. Mult Scler. 2018;24(12):1605–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sugahara K, Maeda Y, Shimano K, et al. Amiselimod (MT-1303), a Novel Sphingosine 1-Phosphate Receptor-1 Modulator, Potently Inhibits the Progression of Lupus Nephritis in Two Murine SLE Models. J Immunol Res. 2019;2019:5821589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clinicaltrials.gov. NCT02307643: An Exploratory Study of MT-1303 in Subjects With Systemic Lupus Erythematosus (a Multicenter, Open-label Study). https://clinicaltrialsgov/ct2/show/NCT02307643?term=MT-1303&draw=2&rank=7.
- 91.Clinicaltrials.gov. NCT01987843: A Phase IIa, Multicentre, Randomised, Double-Blind, Parallel Group, Placebo-Controlled Study to Evaluate Safety, Tolerability and Clinical Efficacy of MT-1303 in Subjects With Moderate to Severe Chronic Plaque Psoriasis https://clinicaltrialsgov/ct2/show/NCT01987843?term=MT-1303&draw=2&rank=9.
- 92.Shimano K, Maeda Y, Kataoka H, et al. Amiselimod (MT-1303), a novel sphingosine 1-phosphate receptor-1 functional antagonist, inhibits progress of chronic colitis induced by transfer of CD4+CD45RBhigh T cells. PLoS One. 2019;14(12):e0226154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clinicaltrials.gov. NCT02378688: Safety and efficacy of MT-13013 in subjects with moderate to severe active Crohn’s Disease. https://clinicaltrialsgov/ct2/show/NCT02378688.
- 94.Clinicaltrials.gov. NCT02389790: Extension study of MT-1303 in subjects with Crohn’s disease. https://clinicaltrialsgov/ct2/show/NCT02389790.
- 95.D’Haens G, Danese S, Davies M, Watanabe M, Hibi T. DOP48 Amiselimod, a selective S1P receptor modulator in Crohn’s disease patients: a proof-of-concept study. Journal of Crohns & Colitis. 13(Supplement_1):S055–S056. [Google Scholar]
- 96.Yokoyama E, Hashimoto D, Hayase E, et al. Short-term KRP203 and posttransplant cyclophosphamide for graft-versus-host disease prophylaxis. Bone Marrow Transplant. 2020;55(4):787–795. [DOI] [PubMed] [Google Scholar]
- 97.Clinicaltrials.gov. NCT01987843: A Phase IIa, Multicentre, Randomised, Double-Blind, Parallel Group, Placebo-Controlled Study to Evaluate Safety, Tolerability and Clinical Efficacy of MT-1303 in Subjects With Moderate to Severe Chronic Plaque Psoriasis. https://clinicaltrialsgov/ct2/show/NCT01987843?term=MT-1303&draw=2&rank=9.
- 98.Shimizu H, Takahashi M, Kaneko T, et al. KRP-203, a novel synthetic immunosuppressant, prolongs graft survival and attenuates chronic rejection in rat skin and heart allografts. Circulation. 2005;111(2):222–229. [DOI] [PubMed] [Google Scholar]
- 99.Poti F, Gualtieri F, Sacchi S, et al. KRP-203, sphingosine 1-phosphate receptor type 1 agonist, ameliorates atherosclerosis in LDL-R−/− mice. Arterioscler Thromb Vasc Biol. 2013;33(7):1505–1512. [DOI] [PubMed] [Google Scholar]
- 100.Song J, Matsuda C, Kai Y, et al. A novel sphingosine 1-phosphate receptor agonist, 2-amino-2-propanediol hydrochloride (KRP-203), regulates chronic colitis in interleukin-10 gene-deficient mice. J Pharmacol Exp Ther. 2008;324(1):276–283. [DOI] [PubMed] [Google Scholar]
- 101.listed NA. NCT01375179. Efficacy & safety in moderately active refractory ulcerative colitis patients. https://clinicaltrialsgov/ct2/show/NCT01375179?term=NCT01375179&rank=1 2017.
- 102.Stepanovska B, Huwiler A. Targeting the S1P receptor signaling pathways as a promising approach for treatment of autoimmune and inflammatory diseases. Pharmacol Res. 2020;154:104170. [DOI] [PubMed] [Google Scholar]
- 103.Argollo M, Furfaro F, Gilardi D, et al. Modulation of sphingosine-1-phosphate in ulcerative colitis. Expert Opin Biol Ther. 2020;20(4):413–420. [DOI] [PubMed] [Google Scholar]
- 104.Daniel C, Sartory NA, Zahn N, et al. FTY720 ameliorates oxazolone colitis in mice by directly affecting T helper type 2 functions. Mol Immunol. 2007;44(13):3305–3316. [DOI] [PubMed] [Google Scholar]
- 105.Daniel C, Sartory N, Zahn N, Geisslinger G, Radeke HH, Stein JM. FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J Immunol. 2007;178(4):2458–2468. [DOI] [PubMed] [Google Scholar]
- 106.Deguchi Y, Andoh A, Yagi Y, et al. The S1P receptor modulator FTY720 prevents the development of experimental colitis in mice. Oncol Rep. 2006;16(4):699–703. [PubMed] [Google Scholar]
- 107.Dumitrescu L, Constantinescu CS, Tanasescu R. Siponimod for the treatment of secondary progressive multiple sclerosis. Expert Opin Pharmacother. 2019;20(2):143–150. [DOI] [PubMed] [Google Scholar]
- 108.Pan S, Gray NS, Gao W, et al. Discovery of BAF312 (Siponimod), a Potent and Selective S1P Receptor Modulator. ACS Med Chem Lett. 2013;4(3):333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gergely P, Nuesslein-Hildesheim B, Guerini D, et al. The selective sphingosine 1-phosphate receptor modulator BAF312 redirects lymphocyte distribution and has species-specific effects on heart rate. Br J Pharmacol. 2012;167(5):1035–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Selmaj K, Li DK, Hartung HP, et al. Siponimod for patients with relapsing-remitting multiple sclerosis (BOLD): an adaptive, dose-ranging, randomised, phase 2 study. Lancet Neurol. 2013;12(8):756–767. [DOI] [PubMed] [Google Scholar]
- 111.Kappos L, Li DK, Stuve O, et al. Safety and Efficacy of Siponimod (BAF312) in Patients With Relapsing-Remitting Multiple Sclerosis: Dose-Blinded, Randomized Extension of the Phase 2 BOLD Study. JAMA Neurol. 2016;73(9):1089–1098. [DOI] [PubMed] [Google Scholar]
- 112.Kappos L, Bar-Or A, Cree BAC, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391(10127):1263–1273. [DOI] [PubMed] [Google Scholar]
- 113.Piali L, Froidevaux S, Hess P, et al. The selective sphingosine 1-phosphate receptor 1 agonist ponesimod protects against lymphocyte-mediated tissue inflammation. J Pharmacol Exp Ther. 2011;337(2):547–556. [DOI] [PubMed] [Google Scholar]
- 114.Olsson T, Boster A, Fernandez O, et al. Oral ponesimod in relapsing-remitting multiple sclerosis: a randomised phase II trial. J Neurol Neurosurg Psychiatry. 2014;85(11):1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Piali L, Birker-Robaczewska M, Lescop C, et al. Cenerimod, a novel selective S1P1 receptor modulator with unique signaling properties. Pharmacol Res Perspect. 2017;5(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vaclavkova A, Chimenti S, Arenberger P, et al. Oral ponesimod in patients with chronic plaque psoriasis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet. 2014;384(9959):2036–2045. [DOI] [PubMed] [Google Scholar]
- 117.Juif PE, Baldoni D, Reyes M, et al. Pharmacokinetics, Pharmacodynamics, Tolerability, and Food Effect of Cenerimod, a Selective S1P(1) Receptor Modulator in Healthy Subjects. Int J Mol Sci. 2017;18(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hermann V, Batalov A, Smakotina S, Juif PE, Cornelisse P. First use of cenerimod, a selective S1P1 receptor modulator, for the treatment of SLE: a double-blind, randomised, placebo-controlled, proof-of-concept study. Lupus Sci Med. 2019;6(1):e000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Verstockt B, Ferrante M, Vermeire S, Van Assche G. New treatment options for inflammatory bowel diseases. J Gastroenterol. 2018;53(5):585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Derfuss T, Mehling M, Papadopoulou A, Bar-Or A, Cohen JA, Kappos L. Advances in oral immunomodulating therapies in relapsing multiple sclerosis. Lancet Neurol. 2020;19(4):336–347. [DOI] [PubMed] [Google Scholar]
- 121.Feagan BG, D’Haens G, Usiskin K, Liu J, Paul D, Pai RK. P661 Early histological improvement demonstrated with oral ozanimod in patients with moderately to severely active Crohn’s disease in the STEPSTONE trial. Journal of Crohn s and Colitis. (Supplement_1):S450–S450. [Google Scholar]
- 122.Chiba K Discovery of fingolimod based on the chemical modification of a natural product from the fungus, Isaria sinclairii. J Antibiot (Tokyo). 2020;73(10):666–678. [DOI] [PubMed] [Google Scholar]
- 123.Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol. 2010;33(2):91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mizushima T, Ito T, Kishi D, et al. Therapeutic effects of a new lymphocyte homing reagent FTY720 in interleukin-10 gene-deficient mice with colitis. Inflamm Bowel Dis. 2004;10(3):182–192. [DOI] [PubMed] [Google Scholar]
- 125.Kano M, Kobayashi T, Date M, et al. Attenuation of murine sclerodermatous models by the selective S1P1 receptor modulator cenerimod. Sci Rep. 2019;9(1):658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tran JQ, Hartung JP, Olson AD, et al. Cardiac Safety of Ozanimod, a Novel Sphingosine-1-Phosphate Receptor Modulator: Results of a Thorough QT/QTc Study. Clin Pharmacol Drug Dev. 2018;7(3):263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.FDA drug approval, accessed October 20, 2021: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209899s000lbl.pdf. 2021.
- 128.Selmaj KW, Cohen JA, Comi G, et al. Ozanimod in relapsing multiple sclerosis: Pooled safety results from the clinical development program. Mult Scler Relat Disord. 2021;51:102844. [DOI] [PubMed] [Google Scholar]


