Abstract
Clinical trials have demonstrated cardiovascular benefits of sodium-glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide 1 receptor agonists (GLP1RA). However, their impact on all-cause and cause-specific hospitalization in real-world practice remains unclear. We identified patients with diabetes who initiated SGLT2i (n=2492), GLP1RA (n=1982), or dipeptidyl peptidase-4 inhibitors (DPP4i, n=2492) between 2015 and 2018 in Geisinger Health System. We examined all-cause hospitalization (net benefit indicator) and cardiovascular disease (CVD) hospitalization (CV benefit indicator), as well as non-CVD hospitalization (harm indicator), using Cox proportional hazards regression. During a median follow-up of 16 months, SGLT2i and GLP1RA were associated with lower risk of all-cause hospitalization (HR [95% CI] 0.85 [0.75–0.95] for SGLT2i; 0.89 [0.78, 0.98] for GLP1RA) as well as CVD hospitalization (0.61 [0.47–0.79] for SGLT2i; 0.77 [0.60, 0.99] for GLP1RA) compared with DPP4i. The risks of all-cause and CVD hospitalization were similar between SGLT2i and GLP1RA. SGLT2i was associated with substantially lower risk of myocardial infarction and heart failure (HF) hospitalization compared with DPP4i and lower risk of HF hospitalization compared with GLP1RA. The risk of non-CVD hospitalization did not differ by the treatment groups. These results from real-world comparison further encourage SGLT2i and GLP1RA use in routine diabetes care, particularly among patients at high risk of cardiovascular events.
Keywords: SGLT2i, GLP1RA, hospitalization, CVD benefits
Introduction
Type 2 diabetes mellitus (T2DM) is a major public health concern around the world.1 Multiple clinical trials have shown cardiovascular protective effects of sodium-glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide 1 receptor agonists (GLP1RA).2–6 Hospitalization increases risk of death and other adverse outcomes and increases medical cost for patients with T2DM.7,8 Although SGLT2i lowers the risk of hospitalization for heart failure (HF),5,6 the impact of SGLT2i and GLP1RA on all-cause hospitalization and cause-specific hospitalization is unclear. Potential adverse events of SGLT2i, such as diabetic ketoacidosis (DKA), lower extremity amputation, and Fournier gangrene, may result in increased risk of hospitalization.9 Better understanding of all-cause hospitalization as well as cause-specific hospitalization by head-to-head comparison of commonly used anti-diabetic medications will be informative to provide optimal therapies for patients with T2DM. In the current study, we examined the risk of all-cause hospitalization and cause-specific hospitalization by three newer classes of antidiabetic medications: SGLT2i, GLP1RA, and dipeptidyl peptidase-4 inhibitors (DPP4i).
Methods
We used electronic health record (EHR) data from the Geisinger Health System that serves 45 counties in central and northeastern Pennsylvania. The EHR data provide information on patient demographics, inpatient and outpatient encounters, outpatient prescriptions and laboratory results. Patients with diabetes who initiated SGLT2i, GLP1RA, or DPP4i between 2015 and 2018, had at least 1 year of prior engagement with Geisinger system, were free of end-stage kidney disease, and had at least 1 serum creatinine and hemoglobin A1c (HbA1c) measurement 1 year prior to medication initiation (T0) were included. Diabetes was diagnosed using international classification of diseases (ICD) diagnosis codes (250, E10, E11, or E13), prescription of anti-diabetic medication (excluding conditions such as polycystic ovarian syndrome and gestational diabetes), HbA1c ≥ 7% and at least two fasting glucose >7 mmol/L within one year.10 This study was approved by the Geisinger Medical Center Institutional Review Board and the Johns Hopkins University Institutional Review Board.
Prescriptions of SGLT2i, GLP1RA, or DPP4i were identified from outpatient prescription records (Supplemental Table 1). Patients were characterized by their initial prescription per an intention-to-treat approach. Patients who initiated more than one study medication were excluded. We did pair-wise comparisons among the three treatment groups.
The primary endpoint was all-cause hospitalization, an indicator of net benefit. Patients were followed from the first prescription date (T0) until first hospitalization after T0, death, last encounter with Geisinger, or January 30, 2019, whichever came first. Secondary endpoints included cardiovascular disease (CVD) hospitalization and non-CVD hospitalization. CVD hospitalization, a CV benefit indicator, was defined as hospitalization due to myocardial infarction (MI), HF, or stroke; we also evaluated the risk of each individual component. Since severe adverse events of antidiabetic medications may lead to hospitalization, we treated non-CVD hospitalization as a potential harm indicator. Lastly, we explored the number of rare severe adverse events of SGLT2i including DKA, lower extremity amputation, and Fournier Gangrene during hospitalization. Cause of hospitalization was ascertained based on inpatient ICD codes (Supplemental Table 2).
Covariates included were demographic characteristics, the calendar year of medication initiation, insurance status, smoking and drinking history, blood pressure, lab measurements, comorbidities, concurrent use of medications, and number of outpatient encounters and number of hospitalizations in the prior year (see supplemental methods for detailed definition). Approximately 35% of patients were missing at least one covariate, with urine albumin-creatinine ratio (ACR, 25%) and body mass index, (BMI, 16%) being the most commonly missed. We used multiple imputation by chained equation to impute 40 datasets.11 Aforementioned patient characteristics, exposure, and outcomes were included in the imputation model.
Within each imputed dataset, we estimated the generalized propensity score of receiving each of the three treatments using multinomial logistic regression.12 Aforementioned patient characteristics were included in the model. Inverse probability of treatment weighting (IPTW) based on the propensity scores was applied to minimize differences in baseline characteristics. Estimated weights were truncated at 99% to prevent outliers from strongly affecting the analyses. Balance in covariates was evaluated using the standardized mean difference (SMD) before and after IPTW. A SMD below 0.1 indicates good balance.13 We further adjusted for covariates that did not achieve good balance in analyses. We randomly selected one imputed dataset to present characteristics of the study population.
Baseline characteristics of the study population were reported as number (percentage), mean ± standard deviation (SD), or median (interquartile interval, IQI), as appropriate. Before IPTW, we used the log rank test to compare unadjusted Kaplan-Meier curves between treatments. After multiple imputation and IPTW, we combined Kaplan-Meier estimators and hazard ratios (HRs) estimated from Cox proportional hazard regression in each imputed dataset using Rubin’s rules.14 We tested the proportional hazard assumption by checking Schoenfeld’s partial residuals.
We assessed whether the associations differed by prespecified patient characteristics, including baseline age (<60 vs ≥60 years), sex, baseline eGFR level (<60 vs ≥60 mL/min/1.73m2), ACR (<30, 30–300, or >300 mg/g), and history of CVD (CHD, stroke, or HF). We further stratified the analysis by history of CHD, stroke, and HF, separately in sensitivity analysis. To evaluate for potential residual confounding, we assessed risk of hospitalization due to severe bleeding as a negative control. We selected severe bleeding as an outcome thought to be unaffected by anti-diabetic medication. A two-sided p value <0.05 was considered statistically significant. Statistical analyses were performed with SAS 9.4 (SAS Institute, Cary, NC) and R (www.R-project.org/).15
Results
A total of 9236 patients with diabetes from Geisinger met the inclusion criteria: 2492 SGLT2i, 1982 GLP1RA, and 4762 DPP4i initiators between 2015 and 2018 (Supplemental Figure 1). Before IPTW, SGLT2i users had, on average, a lower risk profile than DPP4i or GLP1RA users (Supplemental Table 3). After IPTW, the weighted mean ± SD age was 58 ±12 years in the SGLT2i group, 59 ±14 years in the DPP4i group, and 58 ±13 years in the GLP1RA group (Table 1). Good covariate balance was achieved for all covariates except the proportion of patients with eGFR<30 ml/min/1.73 m2 in pair-wise comparison between SGLT2i vs. DPP4i (SMD 0.1) and SGLT2i vs. GLP1RA (SMD 0.1) (Supplemental Table 4). We further adjusted for eGFR categories in outcome analyses.
Table 1.
Patient characteristics by antidiabetic medication initiation after inverse probability of treatment weighting.
| Variable | SGLT2i (n=2492) |
GLP1RA (n=1982) |
DPP4i (n=4762) |
|---|---|---|---|
| Age (years) | 58±12 | 58±13 | 59±14 |
| Women | 1167 (49%) | 957 (49%) | 2320 (49%) |
| White | 2174 (91%) | 1775 (91%) | 4314 (91%) |
| Body mass index (kg/m2) | 36±8 | 367±8 | 36±9 |
| Systolic blood pressure (mmHg) | 130±15 | 129±15 | 129±15 |
| HbA1c (%) | 8±2 | 8±2 | 8±2 |
| eGFR (ml/min/1.73m2) | 85±23 | 84±25 | 84±25 |
| eGFR categories (ml/min/1.73m2) | |||
| ≥90 | 1150 (48%) | 923 (48%) | 2205 (47%) |
| 60–89 | 840 (35%) | 667 (34%) | 1621 (34%) |
| 45–59 | 262 (11%) | 205 (11%) | 507 (11%) |
| 30–44 | 122 (5%) | 112 (6%) | 306 (7%) |
| <30 | 15 (1%) | 37 (2%) | 93 (2%) |
| Urine albumin creatinine ratio (mg/g) | 12 [6, 40] | 12 [5, 42] | 12 [5, 46] |
| ACR categories (mg/g) | |||
| <30 | 1674 (70%) | 1347 (70%) | 3227 (68%) |
| 30–300 | 559 (23%) | 462 (24%) | 1191 (25%) |
| >300 | 155 (7%) | 135 (6%) | 315 (7%) |
| Atrial fibrillation | 182 (8%) | 149 (8%) | 382 (8%) |
| Stroke | 169 (7%) | 155 (8%) | 382 (8%) |
| Carotid atherosclerosis | 96 (4%) | 86 (4%) | 227 (5%) |
| Peripheral artery disease | 81 (3%) | 71 (4%) | 159 (3%) |
| Coronary heart disease | 519 (22%) | 441 (23%) | 1059 (22%) |
| Heart failure | 175 (7%) | 148 (8%) | 397 (8%) |
| Acute kidney injury | 77 (3%) | 73 (4%) | 192 (4%) |
| Charlson comorbidity index | 5±3 | 5±3 | 5±3 |
| Hospitalization in the prior year | |||
| 0 | 2106 (88%) | 1723 (89%) | 4136 (87%) |
| 1 | 201 (8%) | 156 (8%) | 414 (9%) |
| >1 | 83 (4%) | 65 (3%) | 182 (4%) |
| Outpatient visits in the prior year, median [IQI] | 5 [3,9] | 5 [3,9] | 5 [3,9] |
| Concurrent use of medication | |||
| Insulin | 519 (22%) | 392 (20%) | 906 (19%) |
| Sulfonylurea | 825 (35%) | 705 (36%) | 1676 (35%) |
| Metformin | 1655 (69%) | 1325 (68%) | 3204 (68%) |
| Thiazolidinediones | 75 (3%) | 54 (3%) | 138 (3%) |
| Statins | 1407 (59%) | 1148 (59%) | 2794 (59%) |
| ACEI | 1021 (43%) | 803 (41%) | 1980 (42%) |
| ARB | 377 (16%) | 307 (16%) | 751 (16%) |
| Beta blocker | 734 (31%) | 614 (32%) | 1500 (32%) |
| Diuretics | 812 (34%) | 663 (34%) | 1613 (34%) |
| Antiplatelet medications | 972 (41%) | 773 (40%) | 1929 (41%) |
| Medication initiation year | |||
| 2015 | 461 (19%) | 387 (20%) | 933 (20%) |
| 2016 | 506 (21%) | 442 (23%) | 1032 (22%) |
| 2017 | 674 (28%) | 519 (27%) | 1314 (28%) |
| 2018 | 748 (32%) | 596 (31%) | 1453 (30%) |
| Insured | 2072 (87%) | 1685 (87%) | 4115 (87%) |
| Smoker | |||
| Never | 1039 (43%) | 845 (43%) | 2124 (45%) |
| Past | 946 (40%) | 772 (40%) | 1833 (39%) |
| Current | 405 (17%) | 327 (17%) | 776 (16%) |
| Alcohol drinker | 994 (42%) | 800 (41%) | 1921 (41%) |
ACEI=angiotensin-converting-enzyme inhibitors; ACR=urine albumin-creatinine ratio; ARB=angiotensin receptor blocker; DPP4i=dipeptidyl peptidase-4 inhibitors; eGFR=estimated glomerular filtration rate; GLP1RA=glucagon-like peptide 1 receptor agonists; HbA1c=hemoglobin A1c; SGLT2i=sodium-glucose cotransporter 2 inhibitors.
Variables were presented as mean ± standard deviation, median [interquartile range], or n (%).
During a median (IQI) follow-up of 16 (7, 28) months, 329 (13%) patients in the SGLT2i group, 369 (19%) in the GLP1RA group, and 1073 (23%) in the DPP4i group were hospitalized. Before IPTW, the Kaplan-Meier curve suggested substantially lower risk of all-cause hospitalization in the SGLT2i group compared with the DPP4i as well as the GLP1RA group (Supplemental Figure 2.a). After IPTW, both SGLT2i and GLP1RA were associated with lower risk of all-cause hospitalization compared with DPP4i (HR 0.85, 95% CI: 0.75–0.95 for SGLT2i; HR 0.89, 95% CI: 0.78–0.98 for GLP1RA, Figure 1.a and Table 2). No significant difference was observed in risk of all-cause hospitalization between SGLT2i vs. GLP1RA.
Figure 1.
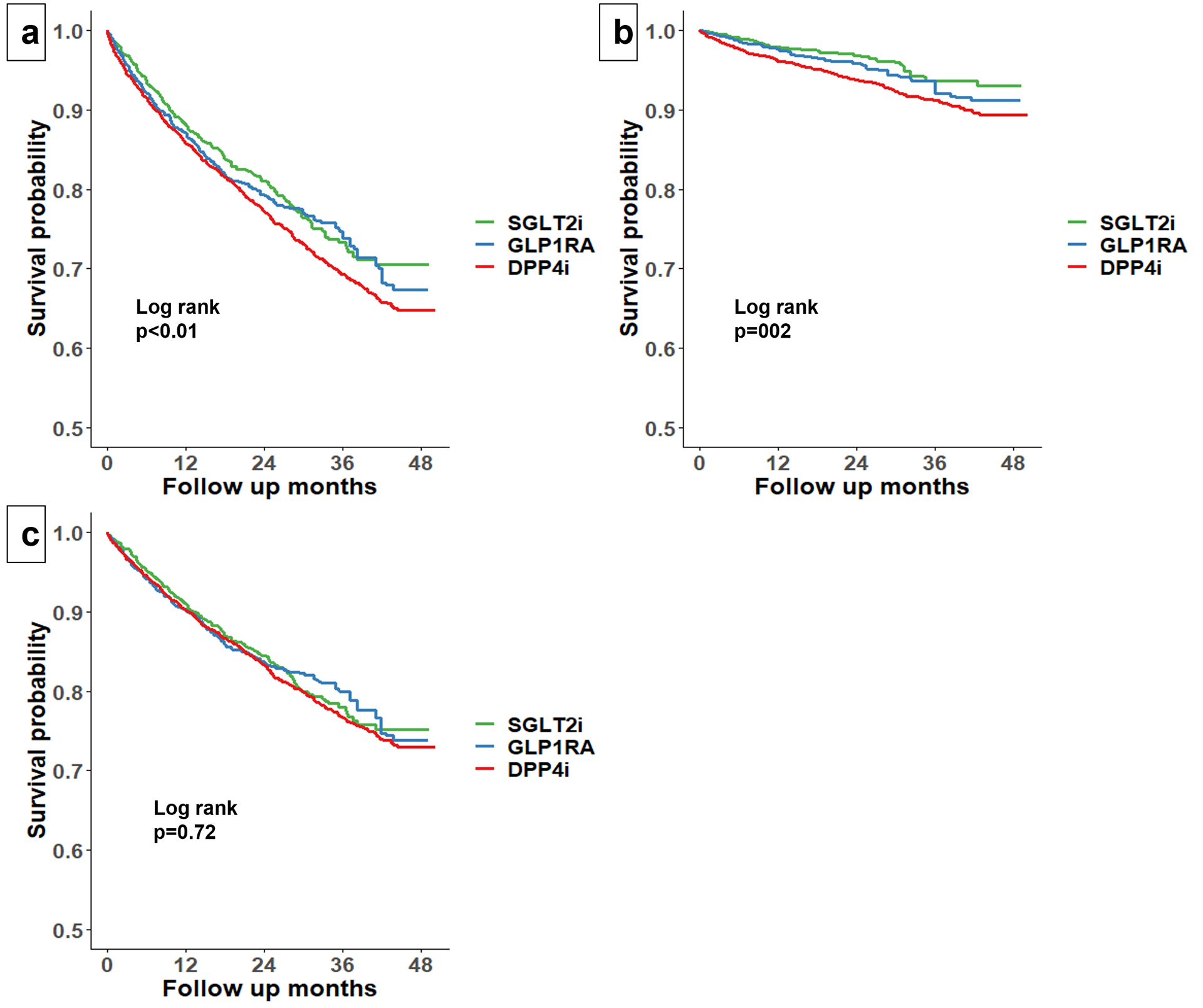
Adjusted Kaplan Meier Curve of all-cause hospitalization (a), CVD hospitalization (b), and non-CVD hospitalization (c) by treatment groups after inverse probability of treatment weighting.
CVD=cardiovascular disease; DPP4i= dipeptidyl peptidase-4 inhibitors; GLP1RA=glucagon-like peptide 1 receptor agonists; SGLT2i=sodium glucose-cotransporter 2 inhibitors.
Table 2.
IPTW-Hazard ratio (95% confidence interval) for hospitalization comparing SGLT2i (n=2492), GLP1RA (n=1982), and DPP4i (n=4762).
| Variable | SGLT2i vs. DPP4i (ref.) | GLP1RA vs. DPP4i (ref.) | SGLT2i vs. GLP1RA (ref.) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Unweighted no. of events (%) | IPTW-HR (95% CI) | Unweighted no. of events (%) | IPTW-HR (95% CI) | Unweighted no. of events (%) | IPTW-HR (95% CI) | ||||
| SGLT2i | DPP4i | GLP1RA | DPP4i | SGLT2i | GLP1RA | ||||
| All cause hospitalization | 329 (13%) | 1073 (23%) | 0.85 (0.75, 0.95)* | 369 (19%) | 1073 (23%) | 0.89 (0.78, 0.98)* | 329 (13%) | 369 (19%) | 0.92 (0.80, 1.07) |
| CVD hospitalization | 56 (2%) | 309 (7%) | 0.61 (0.47, 0.79)* | 89 (5%) | 309 (7%) | 0.77 (0.60, 0.99)* | 56 (2%) | 89 (5%) | 0.79 (0.58, 1.08) |
| MI hospitalization | 10 (0.4%) | 61 (1%) | 0.48 (0.25, 0.90)* | 19 (1%) | 61 (1%) | 0.79 (0.45,1.39) | 10 (0.4%) | 19 (1%) | 0.65 (0.30, 1.44) |
| HF hospitalization | 36 (1.4%) | 229 (5%) | 0.59 (0.41, 0.76)* | 73 (4%) | 229 (5%) | 0.84 (0.63, 1.11) | 36 (1.4%) | 73 (4%) | 0.66 (0.45, 0.95)* |
| Stroke hospitalization | 53 (2%) | 13 (0.5%) | 0.98 (0.56, 1.69) | 12 (0.6%) | 13 (0.5%) | 0.74 (0.39, 1.42) | 53 (2%) | 12 (0.6%) | 1.37 (0.63, 2.95) |
| Non-CVD hospitalization | 273 (11%) | 764 (16%) | 0.94 (0.83, 1.07) | 280 (14%) | 764 (16%) | 0.77 (0.60, 1.01) | 273 (11%) | 280 (14%) | 0.99 (0.84, 1.16) |
CVD=cardiovascular disease; DPP4i=dipeptidyl peptidase-4 inhibitors; HF=heart failure; GLP1RA= glucagon-like peptide 1 receptor agonists; IPTW=inverse probability of treatment weighting; MI=myocardial infarction; SGLT2i=sodium glucose-cotransporter 2 inhibitors.
The association was statistically significant (p<0.05).
During follow-up, 56 (2%) patients in the SGLT2i group, 89 (5%) in the GLP1RA group, and 309 (7%) in the DPP4i group were hospitalized for CVD causes. In unadjusted analyses, SGLT2i was associated with lower risk of CVD hospitalization compared with DPP4i as well as GLP1RA (Supplemental Figure 2.b). After IPTW, both SGLT2i and GLP1RA were associated with lower risk of CVD hospitalization compared with DPP4i (HR 0.61, 95% CI: 0.47–0.79 for SGLT2i; HR: 0.77, 95% CI: 0.60–0.99 for GLP1RA, Figure 1.b). No significant difference was observed in risk of CVD hospitalization between SGLT2i vs. GLP1RA.
For individual component of CVD hospitalization, SGLT2i was associated with lower risk of MI and HF hospitalization compared with DPP4i (HR 0.48, 95% CI: 0.25–0.90 for MI hospitalization; HR 0.59, 95% CI: 0.41–0.76 for HF hospitalization, Table 2). SGLT2i was also associated with lower risk of HF hospitalization compared with GLP1RA (HR 0.66, 95% CI: 0.45–0.95). No significant association was observed between risk of stroke hospitalization and treatment groups.
We did not observe any significant difference in non-CVD hospitalization across the three treatment groups before or after IPTW (Supplemental Figure 2.c, Figure 1.c, and Table 2).
Fifteen (0.6%), 16 (0.8%), and 26 (0.5%) patients developed DKA for SGLT2i, GLP1RA, and DPP4i users, respectively. Eighteen (0.7%) SGLT2i users, 16 (0.8%) GLP1RA users, and 26 (0.5%) DPP4i users had an amputation. Only 1 patient in the SGLT2i group and 1 patient in the GLP1RA group had Fournier gangrene.
The magnitude of reduced risk of all-cause hospitalization associated with SGLT2i vs. DPP4i was greater for patients aged ≥60 years and those with increased albuminuria or CVD than their counterparts (all p for interaction <0.05, Figure 2.a). There was also stronger association between SGLT2i vs. DPP4i and reduced risk CVD hospitalization among patients with CVD than those without CVD (p for interaction <0.05, Figure 2.b). The results were consistent when we further stratified by individual component of CVD (i.e., CHD, stroke, and HF) (Supplemental Table 5). In the comparison of GLP1RA with DPP4i, neither all-cause hospitalization nor CVD hospitalization had significant interaction between treatment and subgroups (Figure 2.c and 2.d).
Figure 2.
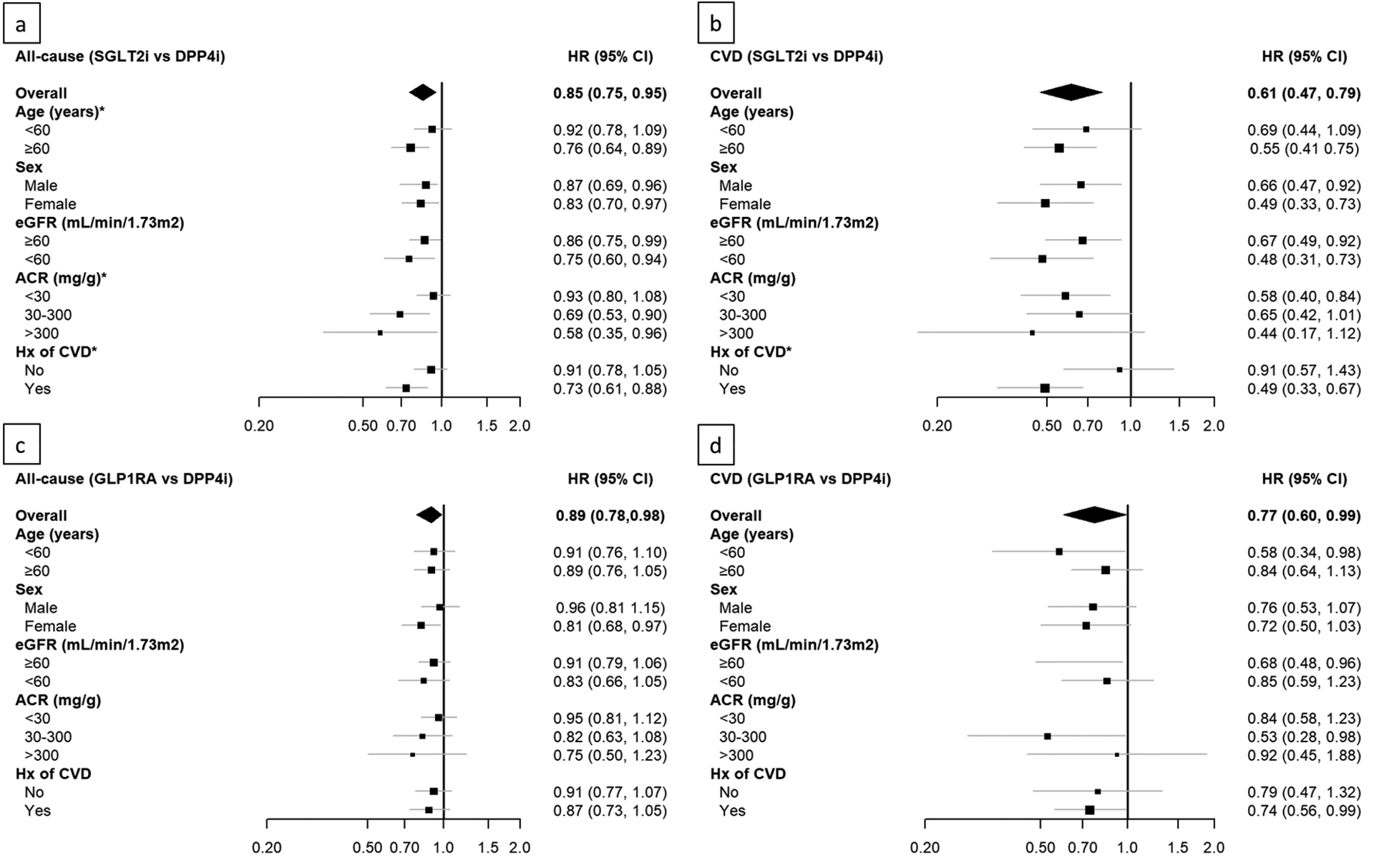
Hazard ratios for risk of all-cause hospitalization between SGLT2i vs. DPP4i (a), CVD hospitalization between SGLT2i vs. DPP4i (b), all-cause hospitalization between GLP1RA vs. DPP4i (c), and CVD hospitalization between GLP1RA vs. DPP4i (d) by age, sex, eGFR, ACR, and history of CVD. *p for interaction<0.05.
ACR= albumin creatinine ratio; CVD=cardiovascular disease; DPP4i= dipeptidyl peptidase-4 inhibitors; eGFR= estimated glomerular filtration rate; GLP1RA=glucagon-like peptide 1 receptor agonists; SGLT2i=sodium glucose-cotransporter 2 inhibitors.
In IPTW analyses, risk of bleeding-related hospitalization was similar across the three treatment groups (Supplemental Table 6).
Discussion
In this real-world comparison of anti-diabetic medications among patients with diabetes, individuals who used SGLT2i or GLP1RA had lower risk of all-cause and CVD hospitalization with similar risk of non-CVD hospitalization, compared with those who used DPP4i. This study provides additional evidence for the effectiveness and safety of SGLT2i and GLP1RA in real-world practice and should motivate additional uptake of these relatively new medications, particularly in patients with high risk of CVD adverse events.
Hospitalization is associated with substantially higher risk of adverse outcomes and greater medical cost among patients with T2DM.7 Previous studies have consistently demonstrated CVD benefits of SGLT2i and GLP1RA compared to other antidiabetic medications.4–6,16–21 Our data further confirmed such benefits in terms of CVD hospitalization. We observed substantial benefits in lowering risk of HF hospitalization of SGLT2i compared with GLP1RA and DPP4i. Potential mechanisms of such benefits of SGLT2i include reduction of adverse histological and molecular remodeling, natriuresis and improvement of blood volume, and reduction in pulmonary artery pressures.22–26 In addition, SGLT2i and GLP1RA were not associated with higher risk of non-CVD hospitalization. Treating non-CVD hospitalization as a potential harm indicator, our results suggest that SGLT2i and GLP1RA have comparable safety profiles compared with DPP4i and provide reassurance that SGLT2i can be safely prescribed without increasing the risk of hospitalization due to severe adverse events compared with DPP4i or GLP1RA.
We showed that the benefits of SGLT2i in terms of all-cause hospitalization were potentially greater among older patients and patients with greater albuminuria or CVD, populations who are at greater risk of CVD events. The 2021 ADA guidelines emphasize the use of SGLT2i for patients with established atherosclerotic CVD, multiple atherosclerotic CVD risk factors, or diabetic kidney disease with elevated albuminuria.27 However, we observed that patients who initiated SGLT2i were healthier and less likely to have a history of CVD compared with patients who initiated DPP4i or GLP1RA. This risk/treatment paradox is consistent with a previous study,28 and may reflect a concern that adverse events could afflict higher risk patients at higher rates. The comparable safety profiles of SGLT2i and GLP1RA or DPP4i from our analyses may dismiss such concern and encourage the use of SGLT2i and GLP1RA over DPP4i in routine diabetes care, particularly among patients at higher risk of cardiorenal adverse outcomes.
Our study has several strengths. First, our study sample contained patients from routine clinical care, which provided real-world evidence of the comparative safety and effectiveness of SGLT2i, GLP1RA, and DPP4i. Second, we used rigorous statistical methods including multiple imputation and IPTW to minimize potential selection bias introduced by missing data and confounding by indication, respectively.
Our study also has limitations. First, the majority of our study population was white from a single health system, which may limit generalizability of our findings. Second, despite the use of IPTW methods to minimize confounding, there might still exist residual or unmeasured confounding. However, the null results in the analysis with negative control outcome make such case less likely. Third, we had a relatively small sample size, especially for individual causes of hospitalization and subgroup analyses. We also had relatively small number of patients with CKD. Fourth, the follow-up duration was relatively short, which may limit our capacity to detect long-term effect. Fifth, our study only focused on severe adverse events requiring hospitalization and did not include mild adverse events that do not require hospitalization. While mild adverse events are also an important consideration to guide optimal selection of antidiabetic medication, concerns about severe adverse events requiring hospitalization such as DKA may be a bigger barrier to widespread use of SGLT2i.
In conclusion, our real-world data shows that SGLT2i and GLP1RA are associated with lower risk all-cause and CVD hospitalization compared with DPP4i, providing additional support for the widespread use of SGLT2i and GLP1RA in diabetes care to improve clinical outcomes, particularly among patients at higher risk of CVD events.
Supplementary Material
Acknowledgement
Dr. Morgan E. Grams was supported by grant number R01DK115534 (Principal Investigator: M.G.) from the National Institute of Diabetes and Digestive and Kidney Disease. Dr. Jung-Im Shin was supported by grant number K01DK121825 (Principal Investigator: J-I.S.) from the National Institute of Diabetes and Digestive and Kidney Disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Williams R, Karuranga S, Malanda B, Saeedi P, Basit A, Besançon S, Bommer C, Esteghamati A, Ogurtsova K, Zhang P. Global and regional estimates and projections of diabetes-related health expenditure: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract 2020;162:108072. [DOI] [PubMed] [Google Scholar]
- 2.Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, Maggioni AP, Marso SP, Öhman P, Pagidipati NJ, Poulter N, Ramachandran A, Zinman B, Hernandez AF. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2017;377:1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ipp E, Genter P, Childress K. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med 2017;376:890–892. [DOI] [PubMed] [Google Scholar]
- 4.Marso S, D GH, B-F K, K P, M JF, N MA, N SE, P S, P NR, R LS, S WM, S M, Z B, B RM, B JB. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2016;375:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 6.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner–La Rocca H-P, Choi D-J, Chopra V, Chuquiure-Valenzuela E. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021. [Google Scholar]
- 7.Riddle MC, Herman WH. The cost of diabetes cared an elephant in the room. Diabetes Care 2018;41:929–932. [DOI] [PubMed] [Google Scholar]
- 8.Yoshinaga R, Ishikawa S, Ayukawa K, Doi Y. Plasma Glucose Level as a Predictor of In-Hospital Mortality in Patients at an Emergency Room: A Retrospective Cohort Study. Diabetes Care 2019;42:e6–e7. [DOI] [PubMed] [Google Scholar]
- 9.Scheen AJ. An update on the safety of SGLT2 inhibitors. Expert Opin Drug Saf 2019;18:295–311. [DOI] [PubMed] [Google Scholar]
- 10.Williamson T, Green ME, Birtwhistle R, Khan S, Garies S, Wong ST, Natarajan N, Manca D, Drummond N. Validating the 8 CPCSSN case definitions for chronic disease surveillance in a primary care database of electronic health records. Ann Fam Med 2014;12:367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Royston P Multiple imputation of missing values. Stata J 2004;4:227–241. [Google Scholar]
- 12.Feng P, Zhou XH, Zou QM, Fan MY, Li XS. Generalized propensity score for estimating the average treatment effect of multiple treatments. Stat Med 2012;31:681–697. [DOI] [PubMed] [Google Scholar]
- 13.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubin DB. Multiple imputation for nonresponse in surveys. John Wiley & Sons; 2004. [Google Scholar]
- 15.Team RC. R: A language and environment for statistical computing. 2013.
- 16.Neal B, Perkovic V, Mahaffey KW, De Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657. [DOI] [PubMed] [Google Scholar]
- 17.Kosiborod M, Lam CS, Kohsaka S, Kim DJ, Karasik A, Shaw J, Tangri N, Goh SY, Thuresson M, Chen H, Surmont F, Hammar N, Fenici P, Kosiborod M, Cavender MA, Fu AZ, Wilding JP, Khunti K, Norhammar A, Birkeland K, Jørgensen ME, Holl RW, Lam CS, Gulseth HL, Carstensen B, Bollow E, Franch-Nadal J, García Rodríguez LA, Karasik A, Tangri N, Kohsaka S, Kim DJ, Shaw J, Arnold S, Goh SY, Hammar N, Fenici P, Bodegård J, Chen H, Surmont F, Nahrebne K, Blak BT, Wittbrodt ET, Saathoff M, Noguchi Y, Tan D, Williams M, Lee HW, Greenbloom M, Kaidanovich-Beilin O, Yeo KK, Bee YM, Khoo J, Koong A, Lau YH, Gao F, Tan WB, Kadir HA, Ha KH, Lee J, Chodick G, Melzer Cohen C, Whitlock R, Cea Soriano L, Fernándex Cantero O, Riehle E, Ilomaki J, Magliano D. Cardiovascular Events Associated With SGLT-2 Inhibitors Versus Other Glucose-Lowering Drugs: The CVD-REAL 2 Study. J Am Coll Cardiol 2018;71:2628–2639. [DOI] [PubMed] [Google Scholar]
- 18.Tsapas A, Avgerinos I, Karagiannis T, Malandris K, Manolopoulos A, Andreadis P, Liakos A, Matthews DR, Bekiari E. Comparative Effectiveness of Glucose-Lowering Drugs for Type 2 Diabetes: A Systematic Review and Network Meta-analysis. Ann Intern Med 2020;173:278–286. [DOI] [PubMed] [Google Scholar]
- 19.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou F-F, Mann JFE, McMurray JJ V, Lindberg M, Rossing P. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–1446. [DOI] [PubMed] [Google Scholar]
- 20.Ipp E, Genter P, Childress K. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med 2017;376:890–892. [DOI] [PubMed] [Google Scholar]
- 21.Dave CV, Kim SC, Goldfine AB, Glynn RJ, Tong A, Patorno E. Risk of Cardiovascular Outcomes in Patients with Type 2 Diabetes after Addition of SGLT2 Inhibitors Versus Sulfonylureas to Baseline GLP-1RA Therapy. Circulation 2021;143:770–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, Garcia-Ropero A, Mancini D, Pinney S, Macaluso F, Sartori S, Roque M, Sabatel-Perez F. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol 2021;77:243–255. [DOI] [PubMed] [Google Scholar]
- 23.Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, Garcia-Ropero A, Ishikawa K, Watanabe S, Picatoste B, Vargas-Delgado AP, Flores-Umanzor EJ, Sanz J. Empagliflozin ameliorates diastolic dysfunction and left ventricular fibrosis/stiffness in nondiabetic heart failure: a multimodality study. JACC Cardiovasc Imaging 2021;14:393–407. [DOI] [PubMed] [Google Scholar]
- 24.Requena-Ibáñez JA, Santos-Gallego CG, Rodriguez-Cordero A, Vargas-Delgado AP, Mancini D, Sartori S, Atallah-Lajam F, Giannarelli C, Macaluso F, Lala A. Mechanistic insights of empagliflozin in nondiabetic patients with HFrEF: from the EMPA-TROPISM study. Heart Fail 2021;9:578–589. [DOI] [PubMed] [Google Scholar]
- 25.Griffin M, Rao VS, Ivey-Miranda J, Fleming J, Mahoney D, Maulion C, Suda N, Siwakoti K, Ahmad T, Jacoby D. Empagliflozin in heart failure: diuretic and cardiorenal effects. Circulation 2020;142:1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nassif ME, Qintar M, Windsor SL, Jermyn R, Shavelle DM, Tang F, Lamba S, Bhatt K, Brush J, Civitello A. Empagliflozin Effects on Pulmonary Artery Pressure in Patients With Heart Failure: Results From the EMBRACE-HF Trial. Circulation 2021;143:1673–1686. [DOI] [PubMed] [Google Scholar]
- 27.9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetesd―2020. Diabetes Care 2020;43:S98–S110. [DOI] [PubMed] [Google Scholar]
- 28.McCoy RG, Dykhoff HJ, Sangaralingham L, Ross JS, Karaca-Mandic P, Montori VM, Shah ND. Adoption of New Glucose-Lowering Medications in the U.S. - The Case of SGLT2 Inhibitors: Nationwide Cohort Study. Diabetes Technol Ther 2019;21:702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


