Abstract
Background: Evidence suggests that the total bilirubin has a protective effect on coronary heart disease (CHD), but the dose-response relationship remains controversial, and there is no meta-analysis to assess the relationship.
Methods: As of October 1, 2021, relevant literature was selected from four databases (PubMed, Web of Science, Cochrane Library, and Embase) by using a retrieval strategy. The dose-response curve between the total bilirubin and CHD was fitted by a restricted cubic spline. Stata 12.0 was used for statistical analysis.
Results: A total of 170,209 (6,342 cases) participants from 7 prospective studies were analyzed in our meta-analysis. We calculated the pooled relative risks (RRs) and 95% CIs for the association between serum bilirubin level and risk of CHD using random-effects models. Compared with the first quantile, the bilirubin level in the third quantile had a protective effect on the risk of CHD (RR, 0.90; 95% CI, 0.82–0.99). The restricted cubic spline functions depicted a U-type curve relationship between bilirubin (3.42–49 μmol/L) and CHD (P linear < 0.001). When the bilirubin level was in the range of 3.42–13μmol/L, the protective effect of bilirubin on CHD was enhanced with increasing bilirubin levels. When the bilirubin level exceeded 13μmol/L, the protective effect of bilirubin weakened, and a dangerous effect gradually appeared with further increases in bilirubin levels.
Conclusions: Compared with a low bilirubin level, a high bilirubin level has a protective effect on the risk of CHD, and there was a U-shaped dose-response relationship between them.
Keywords: bilirubin, coronary heart disease, nonlinear, dose response, meta-analysis
Introduction
Coronary heart disease (CHD) accounts for one-third to one-half of cardiovascular diseases and is the most common heart disease (1). According to WHO estimates, the number of deaths from cardiovascular disease has increased by more than 2 million since 2000 to nearly 9 million in 2019 (2). The increase in cardiovascular disease deaths was due to an increase in the prevalence of CHD (3). Bilirubin is formed from biliverdin through heme catabolism and biliverdin reductase activity (4). Traditionally, bilirubin is considered toxic and can cause irreversible damage to the nervous system (5). In the clinic, bilirubin concentration is used as a marker for the diagnosis of some liver diseases (6). However, there is evidence that bilirubin has antioxidant activity, and its antioxidant effect is stronger than that of bilirubin α-tocopherol (7). It plays an important role in the occurrence and development of oxidative stress diseases. Recent studies have shown that bilirubin has a certain clinical value in the diagnosis and prognosis of stroke (8), diabetes (9), peripheral arterial disease (10), Parkinson's disease (11), and heart failure (12), so it may be used as a new marker for cardiovascular and cerebrovascular diseases in clinical practice.
At present, the relationship between bilirubin and the risk of CHD has attracted increasing attention from scholars. However, the conclusions of these studies are not consistent. Some studies reported that bilirubin was not associated with the occurrence of CHD (13–15); some studies believe that there is a negative linear relationship between them, and with the increase of bilirubin level, the risk of CHD decreases accordingly (16); interestingly, other studies believe that there is a nonlinear relationship between them, such as L-type (17) and U-type (18–20). Stender et al. (21) conducted a meta-analysis of the relationship between hereditary elevated bilirubin levels and the risk of CHD and found no evidence of the association between bilirubin and CHD. Subsequently, a prospective meta-analysis of the relationship between bilirubin and cardiovascular disease reported consistent results (22). Their study only considered whether bilirubin was related to CHD but did not evaluate the relationship between bilirubin exposure level and CHD.
Considering that bilirubin may have certain potential value in the occurrence and development of CHD and no consistent conclusion has been reached on the relationship between the two, it is necessary to explore bilirubin and CHD. Based on published epidemiological studies, we calculated the relative risk of CHD by dose-response meta-analysis, and a dose-response curve was drawn by restrictive cubic spline function to obtain more accurate and intuitive evidence of the dose-response relationship between them.
Methods
This systematic review was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (23) (Supplementary Table 1). Since it is an observational study, the principle of Population, Intervention, Comparator, and Outcomes (PICO) is not suitable, and we adopt the concept of population exposure comparator outcome (PECO) as pillars of the question (24):
P: People of any age,
E: Third quantile of bilirubin level,
C: First quantile of bilirubin level,
O: CHD.
Search Strategy
An extensive search strategy was carried out in four databases (PubMed, Web of Science, Cochrane Library, and Embase) for studies published before October 1, 2021. The following search keywords were used: “bilirubin and CHD,” “bilirubin and cardiovascular diseases,” and “bilirubin and ischemia heart disease.” Relevant MeSH terms were searched in combination to identify relevant studies. Refer to Table 1 for the detailed method used to search PubMed. The strategies for the other databases were similar but adapted where necessary. We also searched references to reviews with related or similar themes for additional research (21, 22, 25–27).
Table 1.
Strategies for the database search.
| #1 | “Bilirubin”[Mesh] |
|---|---|
| #2 | (((((((((((((((Bilirubin IX alpha[Text Word]) OR (Bilirubin, (4E)-Isomer[Text Word])) OR (Bilirubin, (4E,15E)-Isomer[Text Word])) OR (Hematoidin[Text Word])) OR (Bilirubin, Disodium Salt[Text Word])) OR (Disodium Salt Bilirubin[Text Word])) OR (Bilirubin, Monosodium Salt[Text Word])) OR (Monosodium Salt Bilirubin[Text Word])) OR (delta-Bilirubin[Text Word])) OR (delta Bilirubin[Text Word])) OR (Bilirubin, (15E)-Isomer[Text Word])) OR (Bilirubin, Calcium Salt[Text Word])) OR (Calcium Salt Bilirubin[Text Word])) OR (Salt Bilirubin, Calcium[Text Word])) OR (Calcium Bilirubinate[Text Word])) OR (Bilirubinate, Calcium[Text Word]) |
| #3 | #1 OR #2 |
| #4 | (((“Cardiovascular Diseases”[Mesh]) OR “Coronary Disease”[Mesh])) OR “Myocardial Ischemia”[Mesh] |
| #5 | ((((((((((((((((((((Cardiovascular Disease[Text Word]) OR (Disease, Cardiovascular[Text Word])) OR (Diseases, Cardiovascular[Text Word])) OR (Coronary Diseases[Text Word])) OR (Disease, Coronary[Text Word])) OR (Diseases, Coronary[Text Word])) OR (Coronary Heart Disease[Text Word])) OR (Coronary Heart Diseases[Text Word])) OR (Disease, Coronary Heart[Text Word])) OR (Diseases, Coronary Heart[Text Word])) OR (Heart Disease, Coronary[Text Word])) OR (Heart Diseases, Coronar[Text Word])) OR (Ischemia, Myocardial[Text Word])) OR (Ischemias, Myocardial[Text Word])) OR (Myocardial Ischemias[Text Word])) OR (Ischemic Heart Disease[Text Word])) OR (Heart Disease, Ischemic[Text Word])) OR (Disease, Ischemic Heart[Text Word])) OR (Diseases, Ischemic Heart[Text Word])) OR (Heart Diseases, Ischemic[Text Word])) OR (Ischemic Heart Diseases[Text Word]) |
| #6 | #4 OR #5 |
| #7 | #3 OR #6 |
Inclusion and Exclusion Criteria
According to the inclusion criteria, the possibly relevant articles that had been collected were independently assessed by two authors (CXL and YMS). The third author (XMW) resolved any disagreements by discussion or consultation.
Articles were included if they fulfilled all of the following criteria: (1) the article reported the risk estimate and 95% CI of the relationship between bilirubin and CHD; (2) the design of the study was prospective; (3) Provided risk estimates at different bilirubin exposure levels, or provided corresponding data to calculate them; (4) Provided sample sizes at different bilirubin exposure levels, or provided corresponding data to calculate them; (5) the sample source of the article was human; (6) if multiple articles were published from the same/similar population, we included data from the study with the largest sample size and/or the latest or highest-quality result. Reviews, case reports, lab experiments, duplicate publications, and articles without full text or available data were excluded.
Risk of Bias and Methodological Quality
The risk of bias and methodological quality of the included studies were independently assessed by two reviewers (CXL and WYW) using the Newcastle Ottawa scale (NOS), with an asterisk assigned to each article. A study can get up to one star for each numbered item in the selection and outcome categories and up to two stars for comparability. One asterisk equals one score, and the quality of the studies was graded as poor (fewer than four scores), fair (four to six scores), and good (seven or more scores) (28, 29).
Data Extraction
Data extraction was managed in Microsoft Excel. The following information was independently extracted from the included articles by two authors (CXL and WYW): author, year of publication, country, study design, sample source, follow-up period, sample size (case/total), effect value, adjusted risk factors, baseline characteristics [age, body mass index (BMI), smokers, alcohol], diseases (hypertension, diabetes, hyperlipidemia, and liver disease), drug, and biochemical indicators of the subjects. For studies that presented several estimates adjusted for different numbers of potential confounders, the estimate that adjusted for the maximum number of potential confounders was selected for analysis.
In addition, we also extracted the following information for the dose-response meta-analysis: (1) the risk estimates and their corresponding 95% CIs in each group; (2) the median or mean of total bilirubin concentration in each group; and (3) the number of cases and total number in each group.
Statistical Analysis
In our meta-analysis, pooled risk ratios (RRs) and 95% CIs were used to analyze the association between serum bilirubin and the risk of CHD. The result of the pooled estimate was generated using the adjusted odds ratio (OR), relative risk (RR), hazard ratio (HR), and a baseline measure of total bilirubin exposure. In the original research, the effect value (OR/RR/HR) was reported with different distributions of bilirubin levels (such as per tertiles, quartiles, quintiles in bilirubin levels). To enable a consistent approach to the meta-analysis and enhance the interpretation of findings, ORs were converted into RRs by the formula, and HRs were regarded as approximate to RRs (30, 31). At the same time, as reported in previous studies, the results were transformed into RR between the upper tertiles and lower tertiles (32). The chi-square-based Q-test was used to assess the heterogeneity among the individual studies (33). Heterogeneity was quantified based on I2, which ranged from 0 to 100% (I2 = 0–25%, no heterogeneity; I2 = 25–50%, low heterogeneity; I2 = 50–75%, moderate heterogeneity; I2 = 75–100%, high heterogeneity). Considering the underlying clinical and methodological heterogeneity (e.g., baseline characteristics of the patients, adjustment for confounders, and follow-up duration), a random-effects model was used as the primary approach to combine results across studies (34). The possible sources of heterogeneity were explored by subgroup analysis. Egger tests were used to evaluate the possibility of publication bias (35). The sensitivity analysis was performed by excluding one study at a time to evaluate whether the results could have been affected by a single study (36). A two-sided value of P < 0.05 in the statistical process was considered statistically significant (37).
We used the two-stage generalized least squares method to estimate the relative risk of the original research report and established a restrictive cubic spline curve with five nodes (5, 25, 50, 75, and 95) to fit the dose-response relationship between bilirubin and CHD (38–40). To ensure unit consistency in all included studies, we converted mg/dL to μmol/L by multiplying by 17.1. If the article reported exposure category by a range, the midpoint was calculated by averaging the lower and upper bounds; if the lowest boundary was open-ended, the midpoint was set at half of the upper boundary; and when the upper boundary for the highest group was not provided, the midpoint was set at 1.5 times the lower boundary.
The dose-response meta-analysis was conducted using the Stata software package (Version 12.0; Stata Crop, College Station, TX, USA).
Results
Literature Search
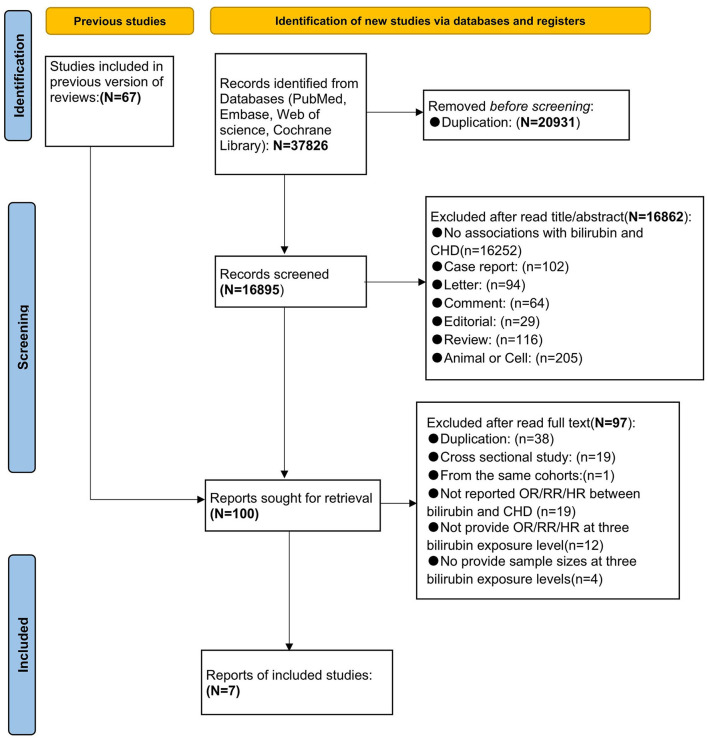
A total of 37,826 potential studies were retrieved. A total of 20,931 articles were excluded according to automation tools. The remaining literature was imported into EndNote X9 (Clarivate Analytics) for further screening. According to the analysis of titles and abstracts, 16,862 articles were excluded and 33 potential articles were included. In addition, 67 articles were included from the reference list of previous reviews. After reading the full text and detailed screening, 93 articles were excluded for various reasons. Finally, 7 prospective studies (16, 18, 20, 21, 41–43) were included in the meta-analysis. The PRISMA flowchart of literature retrieval and selection is presented in Figure 1.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart of literature retrieval and selection.
Characteristics of Studies and Risk of Bias
Table 2 shows the details of the included studies in our meta-analysis. All the effect values in the included studies were adjusted for the maximum number of potential confounders. All of the studies were based on community populations. The publication year was from 1996 to 2018. The follow-up period ranged from 5 to 21.9 years. The included studies involved three regions, including Europe (20, 21, 42), Asia (16, 18) and America (41, 43).
Table 2.
Characteristics of articles included in the meta-analysis.
| ID | Author | year | Country | sample source | Follow up period | Subgroup | Case/Total | Effect value | Adjusted Basic indicators |
Adjusted Biochemical |
Adjusted Disease | Adjust-Relevant treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Breimer | 1996 | British | Population base | 11.5 years | measured before 16 o'clock | 470/4916 | RR:0.99(0.73–1.34) | age, sex, BMI, smoking, alcohol, SBP, social class, physical activity, | TC, HDL, FEV1, blood glucose, serum albumin | diabetes, preexisting IHD | antihypertensive |
| measured after 16 o'clock | 267/2769 | RR:1.20(0.80–1.80) | age, sex, BMI, smoking, alcohol, SBP, social class, physical activity, | TC, HDL, FEV1, blood glucose, serum albumin | diabetes, preexisting IHD | antihypertensive | ||||||
| 2 | Luc | 2001 | American | Population base | 21.9 ± 4.3 years | men | 196/1983 | HR:0.75(0.49–1.14) | sex, age, smoking, alcohol | TC | hypertension, diabetes | / |
| women | 61/2293 | HR:0.53(0.18–1.52) | Sex, age, smoking, alcohol | TC | hypertension, diabetes | / | ||||||
| 3 | Stender | 2013 | Denmark | Population base | 7.5 years | / | 1583/43708 | HR:0.93(0.82–1.06) | age, sex, smoking, alcohol, physical activity, | HDL-c, LDL-c, TG, CRP | hypertension, diabetes | use of lipid-lowering drugs |
| 4 | Jorgens | 2014 | British | 6 years | / | 355/9742 | HR:0.73(0.545–0.979) | age, sex | / | overweight and obese | sibutramine/ placebo, | |
| 5 | Lai | 2018 | China | Population base | 5 years | / | 1204/12097 | HR:1.01(0.84–1.2) | age, sex, BMI, smoking, alcohol, education levels, physical activity, | ALT, AST, ALP | hypertension, diabetes, hyperlipidemia, history of CHD, | / |
| 6 | Suh | 2018 | Korea | Population base | 8.1 years | / | 444/8844 | HR:0.769(0.593–0.996) | age, sex, BMI, SB, | LDL, HSCRP, HbA1c, | / | / |
| 7 | Marconi | 2018 | American | Population base | 5.7 years | / | 1762/83857 | HR:0.81(0.7–0.93) | age, sex, smoking, alcohol, SBP, race-ethnicity | / | diabetes, HIV, hepatitis C, liver fibrosis measured by FIB-4 | / |
IHD, ischemic heart disease; MI, myocardial infarction; CHD, coronary heart disease; RR, relative risk; HR, hazard risk; SBP, systolic blood pressure; TC, total cholesterol; HDL-c, high-density lipoprotein cholesterol; FEV1,forced expiratory volume in 1; LDL-c, low-density lipoprotein cholesterol; TG, triglycerides; CPR, C-reaction protein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; BMI, body mass index; HIV, human immunodeficiency virus; FIB-4,fibrinogen; HbA1c, hemoglobin A1c.
Table 3 shows the baseline characteristics and biochemical indicators of the subjects in each study. The total sample size is 170,209 (age: 27.5–70 years). The percentage of men ranged from 45 to 100%. The percentages of smoking and alcohol were 67 and 70%, respectively. The percentages of hypertension patients, diabetic patients, hyperlipidemia patients, and liver disease were 60, 70, 80, and 22.95%, respectively. The biochemical indicators included AST, ALT, TC, TG, LDL, and HDL. The biochemical indicators of the subjects in all the included studies are shown in detail in Table 3.
Table 3.
Characteristics of subjects included in the study.
| Author | Sample size |
Male (%) |
Age (year) |
BMI (kg/m2) |
Smoker (%) |
Alcohol (%) |
Hypertension (%) |
Diabetes (%) |
Hyperlipidemia (%) | Liver disease (%) | Drug (%) |
bilirubin level (μmol/L) |
TC (mmol/L) |
TG (mmol/L) |
HDL (mmol/L) |
LDL (mmol/L) |
AST (U/l) |
ALT (U/l) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breimer | 7685 | 100% | / | / | / | / | / | / | / | / | / | 8.47 | / | / | / | / | / | / |
| Luc | 4276 | 46% | 37.42 | / | / | / | 19% | 2% | / | 0% | / | 12.60 | 5.09 | / | / | / | / | / |
| Stender | 43708 | 59% | 54.65 | 25.35 | 22% | 42% | 54% | 3% | 7% | / | / | 10.05 | 5.59 | 1.51 | 1.64 | 3.22 | / | / |
| Jorgens | 9742 | 57% | 62.91 | 34.46 | 42% | / | / | / | / | 5% | 66% | 11.02 | / | / | 46.23 | 107.15 | 23.25 | 26.03 |
| Lai | 12097 | 45% | 62.7 | 24.26 | 19% | 23% | 48% | 16% | 49% | / | / | 13.75 | / | / | / | / | 23.15 | 20.37 |
| Suh | 8844 | 47% | 52.3 | 24.56 | 25% | / | 31% | / | 18% | / | / | 12.83 | 5.01 | 1.79 | 1.12 | 3.08 | 28.19 | 26.82 |
| Marconi | 83857 | 97% | 48.56 | / | 52% | 27% | 25% | 14% | / | 46% | 17% | 10.56 | / | / | / | / | / | / |
TC, total cholesterol; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; TG, triglycerides; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; BMI, body mass index.
Age, BMI, bilirubin, TG, TC, HDL, LDL, AST, and ALT are expressed as means; smoker, alcohol, hypertension, diabetes, hyperlipidemia, liver disease, and drug are expressed as percentages.
Table 4 shows the quality scores of the included studies based on the NOS assessment tool. All 7 articles were assessed as good.
Table 4.
Newcastle-Ottawa Scale (NOS) scores of the studies included in the meta-analysis.
| Newcastle-Ottawa Quality Assessment Scale | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | outcome | Quality scores a | ||||||
| Is the case definition adequate/ Representativeness of the exposed cohort | Representativeness of the cases/ Selection of the non-exposed cohort | Selection of Controls/ Ascertainment of exposure to implants | Definition of Controls/ Demonstration that outcome of interest was not present at start of study | Comparability of cases and controls on the basis of the design or analysis/ Comparability of cohorts on the basis of the design or analysis | Ascertainment of exposure/ Assessment of outcome | Same method of ascertainment for cases and controls/ Was follow up long enough for outcomes to occur | Non-Response Rate/ Adequacy of follow up of cohorts | ||
| Breimer | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | ⋆ | 9 |
| Luc | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | ⋆ | 9 |
| Stender | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | ⋆ | 9 |
| Jorgens | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | ⋆ | 9 |
| Lai | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | ⋆ | 9 |
| Suh | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | ⋆ | 9 |
| Marconi | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | ⋆ | 9 |
a, One asterisk equals one score.
Correlation Between Total Bilirubin and CHD
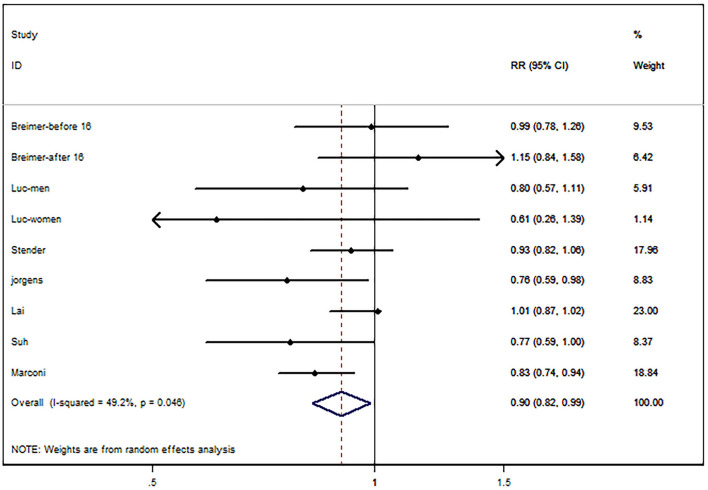
We extracted 9 sets of statistical data from 7 articles, and 1 study reported the relationship between bilirubin and CHD by sex (43); in another study, the relationship between bilirubin and CHD was reported by two different populations, which were divided according to the time of bilirubin measurement (before and after 16 o'clock) (20). There was low heterogeneity in 7 studies (I2 = 49.2%, P = 0.046), and a random effect model was used for analysis. Compared with the first quantile, the bilirubin level in the third quantile had a protective effect on the risk of CHD (RR, 0.90; 95% CI, 0.82–0.99; P = 0.027; Figure 2.
Figure 2.
Forest map of bilirubin and risk of coronary heart disease (CHD).
Subgroup analysis was performed according to sample size (subject <15,000, ≥15,000), which was consistent with the results of the holistic analysis (Table 5). Egger's test (P = 0.202) suggested no publication bias (Figure 3). For sensitivity analysis, the pooled RRs ranged from 0.89 (95% CI:0.81–1) to 0.92 (95% CI:0.83–1.02) (Table 6).
Table 5.
Subgroup analysis of the relationship between total bilirubin and risk of coronary heart disease (CHD).
| Number | RR (95%CI) | I2 | p for I2 | |
|---|---|---|---|---|
| Sample size | ||||
| <15000 | 7 | 0.96(0.91–1.03) | 45.8% | 0.086 |
| ≥15000 | 2 | 0.88(0.78–0.98) | 38.1% | 0.304 |
RR, relative risk; CI, confidence interval.
Figure 3.
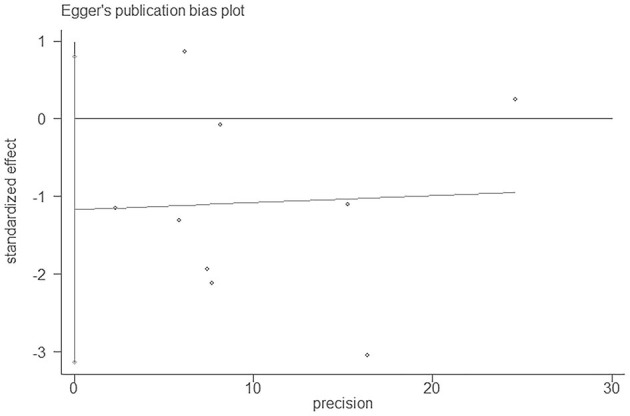
Egger's publication bias plot of bilirubin and CHD.
Table 6.
Sensitivity analysis of bilirubin and CHD.
| Study ommited | RR | 95%CI | |
|---|---|---|---|
| low | up | ||
| Total | 0.9 | 0.82 | 0.99 |
| Bremier-before 16 | 0.89 | 0.81 | 0.99 |
| Bremier-after 16 | 0.89 | 0.81 | 0.97 |
| Luc-men | 0.91 | 0.83 | 1 |
| Luc-women | 0.91 | 0.83 | 0.99 |
| Stend | 0.89 | 0.8 | 1 |
| Jorgens | 0.92 | 0.84 | 1.01 |
| Lai | 0.87 | 0.8 | 0.95 |
| Suh | 0.92 | 0.83 | 1.01 |
| Marco | 0.92 | 0.84 | 1.02 |
RR, relative risk; CI, confidence interval.
Dose-Response Analysis for Bilirubin and CHD
We extracted 9 sets of data from 7 studies for dose-response meta-analysis (16, 18, 20, 21, 41–43) (Supplementary Table 2) The dose-effect relationship between bilirubin (3.42–49 μmol/L) and CHD was nonlinear (p-linear <0.001) (Figure 4). When the bilirubin level was in the range of 3.42–13 μmol/L, the protective effect of bilirubin on CHD was enhanced. When the bilirubin level exceeded 13 μmol/L, the protective effect of bilirubin weakened, and dangerous effects gradually appeared with increasing bilirubin levels.
Figure 4.
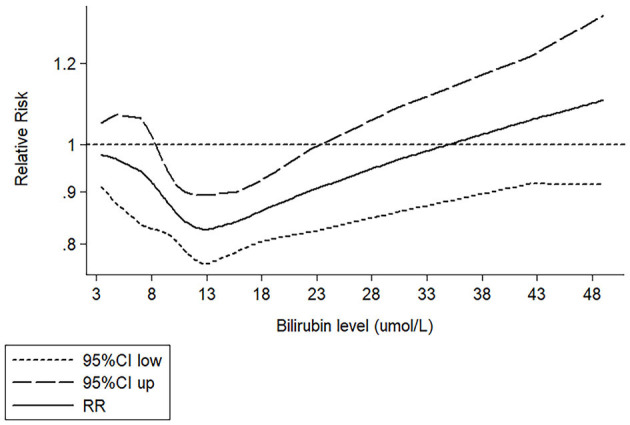
Restrictive cubic spline fitting curve between bilirubin and CHD.
Discussion
This was the first study to assess the relationship between bilirubin and CHD based on a dose-response meta-analysis. Our results showed that compared with the first quantile, the bilirubin level in the third quantile has a protective effect on the risk of CHD; at the same time, dose-response meta-analysis showed that the protective effect was U-type.
Previous studies have reported a nonlinear relationship between bilirubin and CHD, and our study is consistent with these findings. A cross-sectional study in Japan reported a nonlinear relationship between bilirubin and CHD (44). A prospective study of middle-aged people in Britain reported consistent results, and their results showed that the nonlinear relationship may be U-shaped (20). However, these conclusions are limited to male subjects. In another prospective study including Chinese elderly individuals (mean age was 62.7 years; 45.2% men), Lai et al. (18) reported a U-shaped relationship between them. Due to different research methods and subject populations, the conclusions of these studies may have some limitations, but they showed that the protective effect of bilirubin on CHD is not a simple linear relationship. The U-shaped relationship between bilirubin and disease means that when the bilirubin level is approximately 12–16 μmol/L (18, 20, 45, 46), the protective effect of bilirubin on disease will no longer be enhanced. When the bilirubin level exceeds this range, the protective effect will weaken, and a dangerous effect will begin to appear. Other studies have reported an absence of any such association, and the inconsistency of this conclusion can be attributed to the bilirubin exposure level of subjects included in the study not being high enough to detect an effect of bilirubin on CHD (15–17). For example, the results of Kim et al. (15) and Suh et al. (16) showed that there was a negative linear relationship between bilirubin and CHD. In their study, the fourth quartile of bilirubin levels was only 9.5 and 10.77 μmol/L. Therefore, the linear relationship of negative correlation they found may only be part of the U-type relationship. A similar dose-response relationship was also found between bilirubin and other diseases, such as stroke and diabetic retinopathy (DR). Liu et al. (45) reported that the protective relationship between bilirubin and stroke is nonlinear, and the risk of first ischemic stroke was the lowest when the bilirubin level was 17.0 μmol/L. A nonlinear correlation relationship was also reported between bilirubin and diabetic retinopathy (DR). The protective effect of bilirubin level on DR was the strongest in the range of 12–13.8μmol/L (46).
The change in bilirubin level plays an important role in the occurrence and development of CHD. On one hand, bilirubin can eliminate reactive oxygen species produced in the process of oxidative stress, inhibit the aggregation of inflammatory cells, improve the bioavailability of Nitric Oxide (NO), and play a protective role in the heart (47, 48). However, an increase in bilirubin exceeding the physiological range may indicate potential liver injury, and ALP, ALT, and GGT released by liver injury are related to an increased risk of CHD (49, 50). On the other hand, we should pay attention to the fact that bilirubin is also cytotoxic. It has been confirmed in animal experiments that when the accumulation of bilirubin in cells reaches a certain threshold, oxidation will change into cytotoxicity (51). Therefore, the U-type relationship between bilirubin and CHD may be a combination of antioxidant, hepatotoxicity, or cytotoxicity.
Our study has some advantages. Firstly, we established a restrictive cubic spline to fit the dose-response relationship between bilirubin and CHD by pooling original research and obtaining a more intuitive curved shape. Secondly, our dose-response meta-analysis included 7 prospective studies, a large sample size (170,209), and a wide range of bilirubin levels (3.42–49μmol/L), providing sufficient statistical validity for the dose-response relationship model. Our study also has some limitations. Firstly, in a longitudinal analysis, biochemical indicators including bilirubin were collected from baseline measurement, which are sensitive to changes during the follow-up. It may fail to (at least partly) show a robust link between bilirubin and CHD. Secondly, bilirubin levels are disturbed by many endogenous and environmental factors, we had no access to individual participants' data and potential confounders could not be ruled out. Under confounding factors, there may be no reliable connection between bilirubin and CHD. Gajdos et al. (52) and Mahaba et al. (53) found a modest link of bilirubin to CHD. However, after adjusting for risk factors, they were no longer statistically significant. Similarly, in a retrospective cohort of patients with HIV, bilirubin was associated with ischemic events. After the addition of related treatment to the Cox model, bilirubin was no longer significantly associated with ischemic events (54). Their results suggested that the correlation between bilirubin and CHD may be related to uncontrolled confounding factors. Considering the influence of potential factors, we prioritize OR/RR/HR adjusted by most factors when collecting and extracting effect values. It is hoped that the combined value can present objective results after controlling the bias factors as much as possible. In future research to explore the relationship between bilirubin and CHD, bilirubin-related factors should also be considered when adjusting the risk factors for cardiovascular disease.
Conclusion
Compared with a low bilirubin level, a high bilirubin level has a protective effect on the risk of CHD, and there was a U-shaped dose-response relationship between them.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
XW and WW designed the whole research. CL, SX, and YS conducted the data collection. CL, WW, and XW analyzed the data. CL and WW wrote the manuscript. All authors discussed the relevant results, read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.761520/full#supplementary-material
References
- 1.Olvera Lopez E, Ballard BD, Jan A. Cardiovascular Disease. Treasure Island, FL: StatPearls; (2021). [PubMed] [Google Scholar]
- 2.World Health Organization . the-top-10-causes-of-death (2019). Available online at: https://www.who.int/zh/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed April 13, 2021).
- 3.Dalen JE, Alpert JS, Goldberg RJ, Weinstein RS. The epidemic of the 20(th) century: coronary heart disease. Am J Med. (2014) 127:807–12. 10.1016/j.amjmed.2014.04.015 [DOI] [PubMed] [Google Scholar]
- 4.Kalakonda A, Jenkins BA, John S. Physiology, Bilirubin. Treasure Island, FL: StatPearls; (2021). [PubMed] [Google Scholar]
- 5.Vodret S, Bortolussi G, Iaconcig A, Martinelli E, Tiribelli C, Muro AF. Attenuation of neuro-inflammation improves survival and neurodegeneration in a mouse model of severe neonatal hyperbilirubinemia. Brain Behav Immun. (2018) 70:166–78. 10.1016/j.bbi.2018.02.011 [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Wang Z, Yu M, Jiang Y. The predictive ability of liver function indexes on 18F-FDG uptake in the liver. Nuklearmedizin. (2017) 56:239–42. 10.3413/Nukmed-0914-17-07 [DOI] [PubMed] [Google Scholar]
- 7.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. (1987) 235:1043–6. 10.1126/science.3029864 [DOI] [PubMed] [Google Scholar]
- 8.Zhong P, Wu D, Ye X, Wang X, Zhou Y, Zhu X, et al. Association of circulating total bilirubin level with ischemic stroke: a systemic review and meta-analysis of observational evidence. Ann Transl Med. (2019) 7:335. 10.21037/atm.2019.06.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang M, Ni C, Chang B, Jiang Z, Zhu Y, Tang Y, et al. Association between serum total bilirubin levels and the risk of type 2 diabetes mellitus. Diabetes Res Clin Pract. (2019) 152:23–8. 10.1016/j.diabres.2019.04.033 [DOI] [PubMed] [Google Scholar]
- 10.Ozeki M, Morita H, Miyamura M, Fujisaka T, Fujita SI, Ito T, et al. High serum bilirubin is associated with lower prevalence of peripheral arterial disease among cardiac patients. Clin Chim Acta. (2018) 476:60–6. 10.1016/j.cca.2017.11.013 [DOI] [PubMed] [Google Scholar]
- 11.Jin JN, Liu X, Li MJ, Bai XL, Xie AM. Association between serum bilirubin concentration and Parkinson's disease: a meta-analysis. Chin Med J (Engl). (2020) 134:655–61. 10.1097/CM9.0000000000001300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Jia Q, Shi J, Hu Y. Prognostic value of serum bilirubin in patients with heart failure: A protocol for a systematic review and meta-analysis. Medicine (Baltimore). (2021) 100:e26180. 10.1097/MD.0000000000026180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeon C, Lee JY, Lee SJ, Jung KJ, Kimm H, Jee SH. Bilirubin and risk of ischemic heart disease in Korea: a two-sample Mendelian randomization study. Epidemiol Health. (2019) 41:e2019034. 10.4178/epih.e2019034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosma PJ, van der Meer IM, Bakker CT, Hofman A, Paul-Abrahamse M, Witteman JC. UGT1A1*28 allele and coronary heart disease: the rotterdam study. Clin Chem. (2003) 49:1180–1. 10.1373/49.7.1180 [DOI] [PubMed] [Google Scholar]
- 15.Ekblom K, Marklund SL, Jansson JH, Osterman P, Hallmans G, Weinehall L, et al. Plasma bilirubin and UGT1A1*28 are not protective factors against first-time myocardial infarction in a prospective, nested case-referent setting. Circ Cardiovasc Genet. (2010) 3:340–7. 10.1161/CIRCGENETICS.109.861773 [DOI] [PubMed] [Google Scholar]
- 16.Suh S, Cho YR, Park MK, Kim DK, Cho NH, Lee MK. Relationship between serum bilirubin levels and cardiovascular disease. PLoS ONE. (2018) 13:e0193041. 10.1371/journal.pone.0193041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horsfall LJ, Nazareth I, Petersen I. Cardiovascular events as a function of serum bilirubin levels in a large, statin-treated cohort. Circulation. (2012) 126:2556–64. 10.1161/CIRCULATIONAHA.112.114066 [DOI] [PubMed] [Google Scholar]
- 18.Lai X, Fang Q, Yang L, Chen X, Wang H, Ma L, et al. Direct, indirect and total bilirubin and risk of incident coronary heart disease in the Dongfeng-Tongji cohort. Ann Med. (2018) 50:16–25. 10.1080/07853890.2017.1377846 [DOI] [PubMed] [Google Scholar]
- 19.Troughton JA, Woodside JV, Young IS, Arveiler D, Amouyel P, Ferrières J, et al. Bilirubin and coronary heart disease risk in the Prospective Epidemiological Study of Myocardial Infarction (PRIME). Eur J Cardiovasc Prev Rehabil. (2007) 14:79–84. 10.1097/01.hjr.0000230097.81202.9f [DOI] [PubMed] [Google Scholar]
- 20.Breimer LH, Wannamethee G, Ebrahim S, Shaper AG. Serum bilirubin and risk of ischemic heart disease in middle-aged British men. Clin Chem. (1995) 41:1504–8. 10.1093/clinchem/41.10.1504 [DOI] [PubMed] [Google Scholar]
- 21.Stender S, Frikke-Schmidt R, Nordestgaard BG, Grande P, Tybjaerg-Hansen A. Genetically elevated bilirubin and risk of ischaemic heart disease: three Mendelian randomization studies and a meta-analysis. J Intern Med. (2013) 273:59–68. 10.1111/j.1365-2796.2012.02576.x [DOI] [PubMed] [Google Scholar]
- 22.Kunutsor SK, Bakker SJ, Gansevoort RT, Chowdhury R, Dullaart RP. Circulating total bilirubin and risk of incident cardiovascular disease in the general population. Arterioscler Thromb Vasc Biol. (2015) 35:716–24. 10.1161/ATVBAHA.114.304929 [DOI] [PubMed] [Google Scholar]
- 23.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan RL, Whaley P, Thayer KA, Schünemann HJ. Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int. (2018) 121(Pt 1):1027–31. 10.1016/j.envint.2018.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lan Y, Liu H, Liu J, Zhao H, Wang H. Is serum total bilirubin a predictor of prognosis in arteriosclerotic cardiovascular disease? A meta-analysis. Medicine (Baltimore). (2019) 98:e17544. 10.1097/MD.0000000000017544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen H, Zeng C, Wu X, Liu S, Chen X. Prognostic value of total bilirubin in patients with acute myocardial infarction: a meta-analysis. Medicine (Baltimore). (2019) 98:e13920. 10.1097/MD.0000000000013920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao ME, Su MY, Huang Y, Chen W. Physiologically increased total bilirubin is associated with reduced risk of first myocardial infarction: a meta-analysis and dose-response analysis. Nutr Metab Cardiovasc Dis. (2021) 31:1016–26. 10.1016/j.numecd.2021.01.002 [DOI] [PubMed] [Google Scholar]
- 28.GA Wells BS, D O'Connell, J Peterson, V Welch, M Losos, P Tugwell. The Newcastle-Ottawa Scale (Nos) for Assessing The Quality of Nonrandomised Studies in Meta-Analyses. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed November 8, 2021).
- 29.Cai X, Zhang Y, Li M, Wu JH, Mai L, Li J, et al. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ. (2020) 370:m2297. 10.1136/bmj.m2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mai L, Wen W, Qiu M, Liu X, Sun L, Zheng H, et al. Association between prediabetes and adverse outcomes in heart failure. Diabetes Obes Metab. (2021) 23:2476–83. 10.1111/dom.14490 [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. (1998) 280:1690–1. 10.1001/jama.280.19.1690 [DOI] [PubMed] [Google Scholar]
- 32.Li W, Huang A, Zhu H, Liu X, Huang X, Huang Y, et al. Gut microbiota-derived trimethylamine N-oxide is associated with poor prognosis in patients with heart failure. Med J Aust. (2020) 213:374–9. 10.5694/mja2.50781 [DOI] [PubMed] [Google Scholar]
- 33.Crippa A, Khudyakov P, Wang M, Orsini N, Spiegelman D. A new measure of between-studies heterogeneity in meta-analysis. Stat Med. (2016) 35:3661–75. 10.1002/sim.6980 [DOI] [PubMed] [Google Scholar]
- 34.Cai X, Liu X, Sun L, He Y, Zheng S, Zhang Y, et al. Prediabetes and the risk of heart failure: a meta-analysis. Diabetes Obes Metab. (2021) 23:1746–53. 10.1111/dom.14388 [DOI] [PubMed] [Google Scholar]
- 35.Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. (2018) 74:785–94. 10.1111/biom.12817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. (2008) 37:1148–57. 10.1093/ije/dyn065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ranganathan P, Pramesh CS, Buyse M. Common pitfalls in statistical analysis: “P” values, statistical significance and confidence intervals. Perspect Clin Res. (2015) 6:116–7. 10.4103/2229-3485.154016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. (1992) 135:1301–9. 10.1093/oxfordjournals.aje.a116237 [DOI] [PubMed] [Google Scholar]
- 39.Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. (2012) 175:66–73. 10.1093/aje/kwr265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrell FE, Jr, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. (1988) 80:1198–202. 10.1093/jnci/80.15.1198 [DOI] [PubMed] [Google Scholar]
- 41.Marconi VC, Duncan MS, So-Armah K, Re VL 3rd, Lim JK, Butt AA, et al. Bilirubin Is Inversely Associated With Cardiovascular Disease Among HIV-Positive and HIV-Negative Individuals in VACS (Veterans Aging Cohort Study). J Am Heart Assoc. (2018) 7:e007792. 10.1161/JAHA.117.007792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jorgensen ME, Torp-Pedersen C, Finer N, Caterson I, James WP, Legler UF, et al. Association between serum bilirubin and cardiovascular disease in an overweight high risk population from the SCOUT trial. Nutr Metab Cardiovasc Dis. (2014) 24:656–62. 10.1016/j.numecd.2013.12.009 [DOI] [PubMed] [Google Scholar]
- 43.Djoussé L, Levy D, Cupples LA, Evans JC, D'Agostino RB, Ellison RC. Total serum bilirubin and risk of cardiovascular disease in the Framingham offspring study. Am J Cardiol. (2001) 87:1196–200; a4, 7. 10.1016/S0002-9149(01)01494-1 [DOI] [PubMed] [Google Scholar]
- 44.Oda E, Kawai R. A possible cross-sectional association of serum total bilirubin with coronary heart disease and stroke in a Japanese health screening population. Heart Vessels. (2012) 27:29–36. 10.1007/s00380-011-0123-7 [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Wang J, Zeng WZ, Lyu QS. Nonlinear relationship between serum total bilirubin levels and initial ischemic stroke in patients with non-valvular atrial fibrillation. J Int Med Res. (2020) 48:300060520962347. 10.1177/0300060520962347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu M, Wang J, He Y. The U-Shaped Association between Bilirubin and Diabetic Retinopathy Risk: A Five-Year Cohort Based on 5323 Male Diabetic Patients. J Diabetes Res. (2018) 2018:4603087. 10.1155/2018/4603087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Z, Vong CT, Gao C, Chen S, Wu X, Wang S, et al. Bilirubin Nanomedicines for the Treatment of Reactive Oxygen Species (ROS)-Mediated Diseases. Mol Pharm. (2020) 17:2260–74. 10.1021/acs.molpharmaceut.0c00337 [DOI] [PubMed] [Google Scholar]
- 48.Li Y, Huang B, Ye T, Wang Y, Xia D, Qian J. Physiological concentrations of bilirubin control inflammatory response by inhibiting NF-kappaB and inflammasome activation. Int Immunopharmacol. (2020) 84:106520. 10.1016/j.intimp.2020.106520 [DOI] [PubMed] [Google Scholar]
- 49.Ndrepepa G, Colleran R, Kastrati A. Gamma-glutamyl transferase and the risk of atherosclerosis and coronary heart disease. Clin Chim Acta. (2018) 476:130–8. 10.1016/j.cca.2017.11.026 [DOI] [PubMed] [Google Scholar]
- 50.Cheraghi M, Ahmadvand H, Maleki A, Babaeenezhad E, Shakiba S, Hassanzadeh F. Oxidative stress status and liver markers in coronary heart disease. Rep Biochem Mol Biol. (2019) 8:49–55. [PMC free article] [PubMed] [Google Scholar]
- 51.Bianco A, Dvorak A, Capkova N, Gironde C, Tiribelli C, Furger C, et al. The extent of intracellular accumulation of bilirubin determines its anti- or pro-oxidant effect. Int J Mol Sci. (2020) 21:8101. 10.3390/ijms21218101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gajdos V, Petit FM, Perret C, Mollet-Boudjemline A, Colin P, Capel L, et al. Further evidence that the UGT1A1*28 allele is not associated with coronary heart disease: The ECTIM Study. Clin Chem. (2006) 52:2313–4. 10.1373/clinchem.2006.078667 [DOI] [PubMed] [Google Scholar]
- 53.Mahabadi AA, Lehmann N, Mohlenkamp S, Kalsch H, Bauer M, Schulz R, et al. Association of bilirubin with coronary artery calcification and cardiovascular events in the general population without known liver disease: the Heinz Nixdorf Recall study. Clin Res Cardiol. (2014) 103:647–53. 10.1007/s00392-014-0697-z [DOI] [PubMed] [Google Scholar]
- 54.Li M, Chan WW, Zucker SD. Association between atazanavir-induced hyperbilirubinemia and cardiovascular disease in patients infected with HIV. J Am Heart Assoc. (2020) 9:e016310. 10.1161/JAHA.120.016310 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.