Abstract
We report the case of an 83-year-old man with paroxysmal atrial fibrillation who underwent successful percutaneous left atrial appendage closure with the LAmbre device, being in sinus rhythm at implantation. Suddenly, the patient experienced cardiac tamponade and died within a few minutes. Autopsy revealed a slight protrusion of the LAmbre device into the atrial appendage wall, and pulmonary artery laceration.
This is the first published report of pulmonary artery perforation by the LAmbre device. This case highlights the need for a detailed imaging study before this procedure is performed, to assess left atrial appendage movement/contraction in patients in sinus rhythm.
Résumé
Nous présentons le cas d’un homme de 83 ans atteint de fibrillation auriculaire paroxystique qui était en rythme sinusal lors de l’implantation réussie du dispositif de fermeture de l’appendice auriculaire LAmbre par voie percutanée. Le patient a soudainement subi une tamponnade cardiaque et est mort en quelques minutes. L’autopsie a révélé que le dispositif LAmbre formait une légère saillie dans la paroi de l’appendice auriculaire et qu’il avait occasionné une lacération de l’artère pulmonaire.
C’est la première fois qu’un cas de perforation de l’artère pulmonaire par le dispositif LAmbre est publié. Ce cas montre l’importance de faire un examen d’imagerie complet pour évaluer le mouvement et la contraction de l’appendice auriculaire gauche chez les patients en rythme sinusal avant de réaliser l’intervention.
Atrial fibrillation (AF) is the most frequent arrythmia, and its association with an increased risk of ischemic stroke is widely documented. Percutaneous left atrial appendage (LAA) closure devices have been developed as an alternative to oral anticoagulants, and their effectiveness in reducing ischemic stroke risk, and their safety, has been documented in various studies.1 The Watchman LAAC device (Boston Scientific Corporation, Natick, MA) and the Amplatzer Amulet (Abbott, Kansas city).1,2 were the first LAA closure devices used in clinical practice, followed in 2012 by the LAmbre device (Lifetech Scientific, Shenzhen, China). Two 12-month follow-up studies of the LAmbre device both observed an incidence of major complications (pericardial hemorrhage, major bleeding, or stroke) of 3.3%,3 similar to the rates observed with the Watchman and Amulet devices.
We describe a case of pulmonary artery (PA) laceration and perforation produced by the LAmbre device in a patient in sinus rhythm with vigorous LAA contraction.
Case
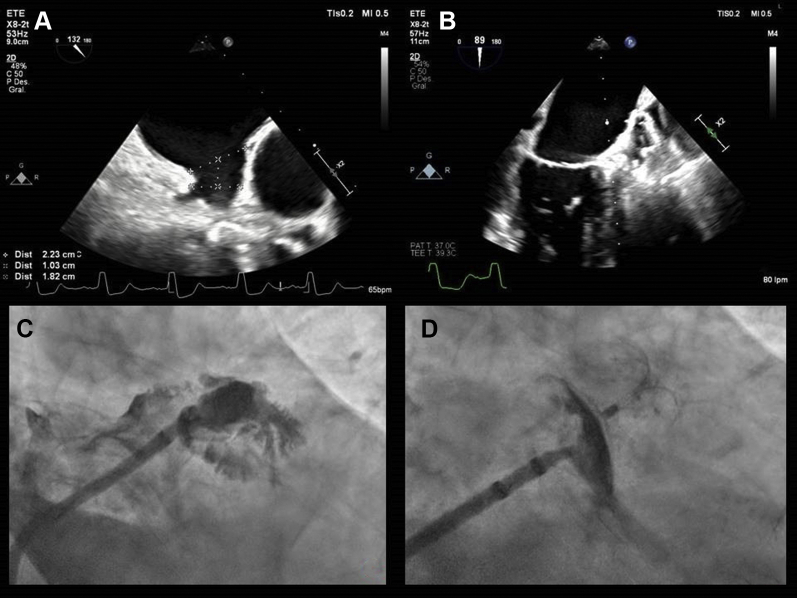
An 83-year-old man with paroxysmal AF was admitted to the hospital after presenting with ischemic stroke. He was assigned a CHA2DS2-VASc (Congestive Heart Failure, Hypertension, Age ≥ 75 years, Diabetes, Stroke/Transient Ischemic Attack, Vascular Disease, Age [65-74 years], Sex Category) score of 6 points. Various therapeutic options were suggested, and finally percutaneous LAA closure was selected. A transesophageal echocardiography study showed an LAA ostium diameter of 22.3 × 23 mm, an LAA diameter of 18.2 × 19.5 mm at a distance of 10 mm from the ostium, and a depth of 30 mm (Fig. 1A). The angiography revealed a “cauliflower”-type morphology, an LAA ostium diameter of 20 × 22 mm, and a depth of 30 mm (Fig. 1C).
Figure 1.
Evaluation by transesophageal echocardiography (TEE) and angiography shows: (A) left atrial appendage measurements, as shown by TEE at 130°; (B) correct device implantation, as shown by TEE; (C) left atrial appendage, as shown by angiography; and (D) correct device implantation, as shown by angiography.
A LAmbre device of 22/26 mm was implanted successfully, with no residual leak or pericardial hemorrhage observed after the procedure (Fig. 1, B and D). At 3 hours after the procedure, the patient experienced sudden arterial hypotension. Emergency transesophageal echocardiography revealed cardiac tamponade. An emergency pericardiocentesis was performed; nevertheless, the patient died from refractory shock a few minutes later. Necropsy was performed with the consent of the family, confirming that the cause of death was PA dissection, and rupture after penetration of the LAA wall by the LAmbre device (Fig. 2).
Figure 2.
Evaluation by autopsy shows (A) penetration of the left atrial appendage (LAA) by the device (blue arrow) and pulmonary artery rupture (red arrow); (B) LAmbre device in normal position in LAA ostium; (C) After LAA dissection, prominent hooks (red circle) and fissure in the pulmonary artery (green arrow); (D) before LAA dissection, prominence of the device, with hematoma indicating premortem lesion (red circle) and rupture with pulmonary artery hematoma (green arrow); and (E) before LAA dissection, prominence of the device (blue arrow) and dissection and rupture, with hematoma of the pulmonary artery (green arrow).
Discussion
To the best of our knowledge, this is the first published case of PA perforation secondary to LAmbre device implantation, confirmed by autopsy findings. Pericardial hemorrhage has been reported as a complication in all clinical trials of percutaneous atrial appendage closure devices. In 2019, a systematic review described 8 cases of PA rupture, mostly with the Amplatzer device2; none of these patients were in permanent AF, but whether they were in sinus rhythm at the time of implantation was not reported. Only a few cases have been reported, but the mortality incidence was elevated.2 This severe complication may be related to atrial contraction in patients in sinus rhythm during implantation; however, it is not known whether sinus rhythm and vigorous LAA contraction at implantation increase the risk of LAA wall perforation with secondary damage to the PA wall. Correct selection of the type and size of device appears to be even more important in these cases, although this complication has also been detected with the Watchman and Amplatzer devices. Analysis of the recording (Videos 1 and 2
 , view video online) of the present case suggests that a broad movement of the LAA due to atrial contraction produced progressive laceration of the PA that led to its dissection and subsequent perforation.
, view video online) of the present case suggests that a broad movement of the LAA due to atrial contraction produced progressive laceration of the PA that led to its dissection and subsequent perforation.
Conclusion
PA perforation is a rare but very severe complication of LAA closure. Atrial contraction may play an important role in the onset of this complication in patients who are in sinus rhythm at implantation. Special care should be taken in selecting the type and size of device used in these patients, although the comparative advantages and drawbacks of the different devices have yet to be elucidated. However, it appears essential to ensure that the device is small enough to avoid its protrusion into the atrial appendage wall, but large enough to prevent its migration. Further research is also warranted to compare complications of this procedure for patients in sinus rhythm vs persistent AF.
Funding Sources
Support was provided by FIBAO and Department of Cardiology, Virgen de las Nieves University Hospital, Granada, Spain.
Novel Teaching Points.
-
•
Periprocedural complications of LAA occlusion may occur.
-
•
There is a risk of rupture after percutaneous appendage closure in patients in sinus rhythm.
-
•
Selection criteria for the various types of appendage closure devices need to be clarified.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: Necropsy was performed with the consent of the family. The research reported has adhered to the ethical guidelines.
See page 99 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2021.07.021.
Supplementary Material
Fluoroscopic imaging. Correct LAA closure (tested by contrast injection), good device positioning, and wide movement of the device with atrial contraction.
TEE imaging reveals the ample movement of the device with atrial contraction because the patient was in sinus rhythm.
References
- 1.Reddy V.Y., Doshi S.K., Sievert H., et al. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-year follow-up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) Trial. Circulation. 2013;127:720–729. doi: 10.1161/CIRCULATIONAHA.112.114389. [DOI] [PubMed] [Google Scholar]
- 2.Sharma S.P., Murtaza G., Madoukh B., et al. Systematic review of contiguous vessel and valve injury associated with endocardial left atrial appendage occlusion devices. J Atr Fibrillation. 2019;12:2256. doi: 10.4022/jafib.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zwirner J., Bayer R., Hädrich C., et al. Pulmonary artery perforation and coronary air embolism—two fatal outcomes in percutaneous left atrial appendage occlusion. Int J Legal Med. 2017;131:191–197. doi: 10.1007/s00414-016-1486-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fluoroscopic imaging. Correct LAA closure (tested by contrast injection), good device positioning, and wide movement of the device with atrial contraction.
TEE imaging reveals the ample movement of the device with atrial contraction because the patient was in sinus rhythm.