Abstract
Forty-eight clinical Acinetobacter isolates with different epidemic behavior were investigated for the presence of integrons and plasmids and for antibiotic susceptibility. Integrons were demonstrated in 50% of the strains by an integrase gene PCR. Epidemic strains of Acinetobacter baumannii were found to contain significantly more integrons than nonepidemic strains. Also, the presence of integrons was significantly correlated with simultaneous resistance to several antibiotics. Plasmids were detected in 42% of the strains. However, there was no significant correlation between the numbers of plasmids and integrons in Acinetobacter species strains, no significant difference in the number of plasmids between epidemic and nonepidemic A. baumannii strains, and no significant correlation between the presence of plasmids and antibiotic resistance. Hence, it is likely that integrons play an important role in antibiotic resistance and thereby in the epidemic behavior of A. baumannii. Because the integrase gene PCR identified almost three-quarters of the epidemic A. baumannii isolates (17 of 23), this seems to be a rapid and simple technique for the routine screening and identification of clinical A. baumannii isolates with epidemic potential.
Acinetobacter baumannii is an important opportunistic pathogen responsible for severe nosocomial infections, especially in intensive-care-unit (ICU) patients (3). The majority of infections are of epidemic origin, and treatment has become difficult because many strains are resistant to a wide range of antibiotics, including broad-spectrum β-lactams, aminoglycosides, and fluoroquinolones (13, 20, 24, 27). Studies of antibiotic resistance mechanisms in A. baumannii have demonstrated the presence of specific genes located on transferable plasmids and transposons (1, 22, 26). Natural transformation has been described in Acinetobacter calcoaceticus, but its role in the genetic spread of antibiotic resistance within clinical A. baumannii isolates has yet to be defined (15).
In recent years, a novel mechanism of resistance gene dissemination among bacteria has been described (25). This mechanism is based on the location of these genes on integrons. Integrons are conserved, transposon-like DNA elements which have the ability to capture and mobilize gene cassettes. Insertion and excision of these cassettes occur via a site-specific recombinase that belongs to the integrase family. A distinguishing feature of an integron is the presence of three components within the conserved 5′ region: (i) an integrase gene (intI) encoding the IntI integrase, (ii) a gene (attI) encoding the cassette integration site, and (iii) one or more promoters responsible for the expression of gene cassettes if present. Based on the sequence of their intI genes, four classes of integrons have been described, three of which (classes 1 to 3) contain antibiotic resistance gene cassettes. At present, approximately 60 different gene cassettes have been identified, most of which encode resistance to antibiotics (6, 8, 17, 25). Class 1 integrons are predominantly associated with a sulI gene as part of a 3′-conserved segment (25). Integrons of class 2 include transposon Tn7 and relatives (9, 16). In class 3 only one integron has been described (2, 21). The majority of integrons belong to class 1 and have been found predominantly in clinical isolates of gram-negative bacteria, including Acinetobacter species (14, 18, 19, 23; M. E. Jones, E. Peters, A. M. Weersink, A. Fluit, and J. Verhoef, Letter, Lancet 349:1742–1743, 1997). A. baumannii strains may vary considerably in their epidemiological potential, and those strains that have been known to spread widely and rapidly among hospitalized patients have been designated epidemic A. baumannii strains. Antibiotic resistance has been shown to be one of the factors which can influence the nosocomial dissemination of A. baumannii (5). In this study the presence of integrons and plasmids was investigated in a collection of unrelated epidemic and sporadic Acinetobacter isolates from different parts of the world. In addition, the association of integrons and plasmids with antibiotic resistance and epidemic behavior was determined.
MATERIALS AND METHODS
Strains.
The Acinetobacter strains used in the present study comprise two sets of isolates. The first set consisted of 25 isolates recovered from patients from 25 independent hospital outbreaks in 11 countries (Table 1). Acinetobacter strains from The Netherlands were obtained from recognized nosocomial outbreaks, and strains from other countries were obtained from reported outbreaks. These epidemic strains were each isolated from at least three different patients. The second set consisted of 25 Acinetobacter species strains that were isolated only once from patients in each outbreak hospital; these strains were defined as nonepidemic, or sporadic.
TABLE 1.
Epidemiological data and results of integron and plasmid analysis of 50 clinical Acinetobacter species strains used in this study
| Strain no.a | Location of hospitalb | Yr of isolation | Body site or tract | Ward type | No. of patients involved | Presence of plasmids | Integron PCR result | Integrase gene PCR resultc |
|---|---|---|---|---|---|---|---|---|
| 1O | Amsterdam (NL) | 1994 | Respiratory | Surgical | 36 | − | + | 1 |
| 2O | Groningen (NL) | 1987 | Urine | Surgical | 10 | − | + | 1 |
| 3O | Amsterdam (NL) | 1995 | Respiratory | ICU | 5 | + | − | 2 |
| 4O | Amsterdam (NL) | 1997 | Respiratory | ICU | 10 | + | − | − |
| 5O | Rotterdam (NL) | 1997 | Skin/mucosa | Burns unit | 6 | + | − | − |
| 6O | Rotterdam (NL) | 1997 | Digestive | ICU | 6 | − | + | 1 |
| 7O | Dordrecht (NL) | 1995 | Skin/mucosa | ICU | 5 | + | + | 1 |
| 8O | Utrecht (NL) | 1997 | Digestive | ICU | 6 | − | + | 1 |
| 9O | Nottingham (U.K.) | 1992 | Respiratory | ICU | 37 | − | + | 1 |
| 10O | Brussels (B) | 1990 | Blood | Surgical | 9 | − | − | 1 |
| 11O | New York (U.S.) | 1991 | Digestive | ICU | 59 | − | − | − |
| 12O | Trieste (I) | 1996 | Respiratory | ICU | >3 | + | + | 1 |
| 13O | Madrid (S) | 1992 | Respiratory | ICU | >100 | − | + | 1 |
| 14O | Ghent (B) | 1991 | Unknown | ICU | >8 | − | + | 1 |
| 15O | Leeds (U.K.) | 1993 | Respiratory | ICU | 5 | − | − | − |
| 16O | Paris (F) | 1991 | Unknown | ICU | 31 | − | + | 1 |
| 17Od | Berlin (G) | 1990 | Respiratory | ICU | Unknown | + | − | − |
| 18O | Freiburg (G) | 1994 | Unknown | ICU | 5 | + | + | 1 |
| 19Od | Berlin (G) | 1992 | Respiratory | ICU | 13 | + | − | − |
| 20O | Hamburg (G) | 1996 | Respiratory | ICU | 3 | − | − | − |
| 21O | St. Etienne (F) | 1993 | Respiratory | Surgical | 15 | − | + | 1 |
| 22O | Vienna (A) | 1996 | Respiratory | ICU | 13 | − | + | 1 |
| 23O | Barcelona (S) | 1986 | Respiratory | ICU | 25 | − | + | 1 |
| 24O | Prague (CR) | 1994 | Skin/mucosa | Burns unit | Unknown | − | + | 1 |
| 25O | Toronto (Can) | 1993 | Respiratory | ICU | 121 | + | − | − |
| 1Ne | Amsterdam (NL) | 1994 | Respiratory | ICU | 1 | − | − | − |
| 2N | Groningen (NL) | Unknown | Unknown | Unknown | 1 | Not tested | + | 1 |
| 3Nf | Amsterdam (NL) | 1997 | Skin/mucosa | Outpatient | 1 | − | − | − |
| 4N | Amsterdam (NL) | 1997 | Skin/mucosa | Surgical | 1 | − | + | 1 |
| 5N | Rotterdam (NL) | 1997 | Respiratory | Pediatric | 1 | − | − | − |
| 6N | Rotterdam (NL) | 1997 | Blood | ICU | 1 | − | − | − |
| 7N | Dordrecht (NL) | 1997 | Blood | ICU | 1 | + | − | − |
| 8Ng | Utrecht (NL) | 1997 | Digestive | ICU | 1 | − | − | − |
| 9N | Nottingham (U.K.) | 1994 | Respiratory | ICU | 1 | − | − | − |
| 10N | Brussels (B) | 1990 | Respiratory | Urology | 1 | + | + | 1 |
| 11N | New York (U.S.) | Unknown | Respiratory | Internal | 1 | + | − | − |
| 12N | Trieste (I) | 1993 | Urine | Urology | 1 | − | + | 1 |
| 13N | Madrid (S) | 1992 | Respiratory | ICU | 1 | + | + | 1 |
| 14Ne | Ghent (B) | 1993 | Unknown | ICU | 1 | − | − | − |
| 15N | Leeds (U.K.) | 1993 | Respiratory | ICU | 1 | + | − | − |
| 16Nf | Paris (F) | Unknown | Unknown | Unknown | 1 | + | − | − |
| 17N | Kiel (G) | Unknown | Respiratory | Unknown | 1 | − | − | − |
| 18Nf | Freiburg (G) | 1994 | Unknown | Pediatric | 1 | + | − | − |
| 19N | Berlin (G) | Unknown | Urine | Unknown | 1 | − | + | 1 |
| 20N | Hamburg (G) | 1997 | Respiratory | ICU | 1 | − | − | − |
| 21Nf | St. Etienne (F) | 1992 | Pus | Geriatric | 1 | + | − | − |
| 22N | Vienna (A) | 1996 | Blood | Oncology | 1 | + | − | − |
| 23N | Barcelona (S) | 1996 | Urine | Surgical | 1 | − | + | 1 |
| 24N | Prague (CR) | 1992 | Skin/mucosa | Burns unit | 1 | + | − | − |
| 25Nf | Toronto (Can) | 1993 | Skinh | Unknown | 1 | + | − | − |
Strains with the same number originated from the same location, except for isolates 17O and 17N. O, outbreak strains; N, nonoutbreak strains. Strains were identified as A. baumannii except where otherwise specified.
Country names are abbreviated in parentheses as follows: A, Austria; B, Belgium; Can, Canada; CR, Czech Republic; F, France; G, Germany; I, Italy; NL, The Netherlands; S, Spain; U.K., United Kingdom; U.S., United States.
1, intI1 detected; 2, intI2 detected; −, no integrase gene detected.
Acinetobacter DNA group 13.
Nontypeable.
Acinetobacter DNA group 3.
A. calcoaceticus (DNA group 1).
Strain isolated from a hand wash of one of the nursing staff.
Identification of strains.
Presumptive identification of the isolates was performed by the analytical profile index procedure (API 20NE system; bioMérieux, Marcy l'Etoile, France). Species identification was confirmed by amplified fragment length polymorphism (AFLP), as described previously (11). All strains belonged to the A. calcoaceticus-A. baumannii complex (A. calcoaceticus [n = 1], A. baumannii [n = 40], Acinetobacter genospecies 3 [n = 5], Acinetobacter genospecies 13 [n = 3]), except for two (1N and 14N) which could not be identified at the species level and were therefore excluded from the final results (Table 1). Of all 25 epidemic strains, 23 were identified as A. baumannii.
Genomic DNA isolation.
DNA was prepared from fresh overnight cultures grown on Luria-Bertani (LB) agar plates (Difco Laboratories, Detroit, Mich.) as described previously (4). Extracted DNA was resolved in 100 μl of TE buffer (10 mM Tris, 1 mM EDTA [pH 8.0]) supplemented with 10 μg of RNase (Sigma, St. Louis, Mo.). Purified DNA was aliquoted and stored at −20°C.
PCR amplification.
PCR amplifications were carried out in 20-μl volumes containing 5 μl of template DNA, 0.2 mM (each) deoxynucleoside triphosphate (dNTP), 2 μl of 10× PCR buffer, 1 U of Taq polymerase (Perkin-Elmer [PE] Applied Biosystems, Foster City, Calif.), 1.5 mM MgCl2, and 1.25 μM each primer. PCR amplification was performed with the GeneAmp PCR System 9700 thermal cycler (PE Applied Biosystems). Amplification products were resolved by electrophoresis at 120 V for 2 h on 2% agarose gels with 0.5× Tris-borate-EDTA buffer containing ethidium bromide and were visualized under UV light. All PCR amplifications were performed in duplicate.
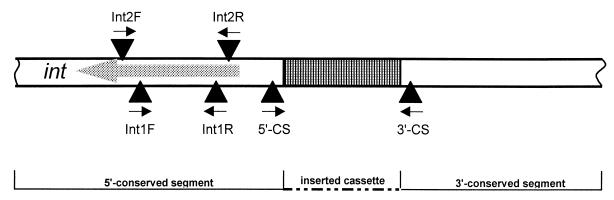
PCR amplification for the detection of class 1 integron cassettes (integron PCR) was performed with primers 5′CS and 3′CS, as described previously (12). For PCR detection of the IntI1 and IntI2 integrase genes (integrase gene PCR), oligonucleotide primers based on the intI1 and intI2 genes were designed (Table 2). Primers IntIF and IntIR were used to amplify a 160-bp fragment of the intI1 gene. The combination of primers Int2F and Int2R amplified a fragment of 288 bp, specific for the intI2 gene. The positions of the primers relative to the integron are indicated in Fig. 1. PCR amplification of integrase gene type 1 and type 2 was performed simultaneously for 35 cycles: 30 s of denaturation at 94°C, 30 s of annealing at 55°C, and 30 s of extension at 72°C.
TABLE 2.
Oligonucleotides for PCR analysis of integrons
| Primer | Nucleotide sequence (5′ to 3′) |
|---|---|
| 5′-CSa | GGC ATC CAA GCA GCA AG |
| 3′-CSa | AAG CAG ACT TGA CCT GA |
| Int1F | CAG TGG ACA TAA GCC TGT TC |
| Int1R | CCC GAG GCA TAG ACT GTA |
| Int2F | TTG CGA GTA TCC ATA ACC TG |
| Int2R | TTA CCT GCA CTG GAT TAA GC |
Described by Lévesque et al. (12).
FIG. 1.
Scheme for PCR detection of class 1 and class 2 integron structures. The grey arrow shows the direction of transcription. Primers 5′CS and 3′CS are specific to the 5′- and 3′-conserved segments of class 1 integrons, respectively, and were used to amplify the variable regions of class 1 integrons (integron PCR). Primers Int1F and Int1R were used to detect IntI1 integrase (integrase gene 1 PCR). Primers Int2F and Int2R were used to detect IntI2 integrase (integrase gene 2 PCR).
DNA sequencing of PCR products.
Template PCR products were purified with the Qiaquick PCR Purification kit (Qiagen, Chatsworth, Calif.). Purified PCR products were sequenced with dye terminators on an ABI 377 automatic sequencer (Applied Biosystems). DNA sequences were compared to the National Center for Biotechnology Information (NCBI) database.
Isolation of plasmids.
From each strain, plasmid DNA was prepared in duplicate, as described by Hartstein et al. (10), with minor modifications. Briefly, isolates were grown on LB agar plates at 37°C for 24 h. Cells from half of the plate were suspended in 1.5 ml of a solution containing 2.5 M NaCl–10 mM EDTA (pH 8.0), 250 μl of 0.5% alkyltrimethylammonium bromide (ATAB), 250 μl of 1% Triton X-100, and 200 μl of lysozyme (10 mg/ml). After incubation in a water bath at 56°C for 15 min, protein was extracted with phenol-chloroform-isoamyl alcohol (25:24:1), and plasmid DNA was precipitated with ice-cold isopropanol. The precipitate was collected by centrifugation and dissolved in 80 μl of TAE buffer (0.04 M Tris-acetate, 0.001 M EDTA [pH 8.2]). After addition of 1 μl of RNase (500 μg/ml), the solutions were incubated at 37°C for 30 min. Ten microliters of the samples and 5 μl of running dye were loaded onto the gel and run for 18 h at 25 V. Gels were stained with ethidium bromide and photographed under UV illumination.
Antibiotic susceptibility.
MICs of selected antimicrobial agents were determined with the Vitek System (bioMérieux, Vitek, Inc., Hazelwood, Mo.). Thirteen antibiotics were tested: ampicillin, ampicillin-clavulanate, piperacillin-tazobactam, cefuroxime, cefotaxime, ceftazidime, imipenem, meropenem, gentamicin, tobramycin, amikacin, ciprofloxacin, and trimethoprim-sulfamethoxazole. MIC data were interpreted according to the guidelines of the NCCLS.
RESULTS
Epidemiological data for the different strains and hospital outbreaks are shown in Table 1. Almost three-quarters of the outbreaks (18 of 25) occurred in ICUs and affected 3 to more than 100 patients.
Detection of class 1 integrons by integron PCR.
For the detection of complete class 1 integrons, PCR amplification was performed with primers for the 5′- and 3′-conserved segments. This PCR also permitted the determination of the size of any inserted gene cassette. Integrons with various insert sizes were found in 44% (22 of 48) of the Acinetobacter species strains. The range of inserted gene cassette sizes detected varied from 800 to 3,000 bp. Sixty-five percent (15 of 23) of epidemic A. baumannii isolates were integron positive. Strikingly, in nonepidemic Acinetobacter species isolates, the frequency of integron carriage was only 30% (7 of 23).
Detection of class 1 and class 2 integrons by integrase gene PCR.
PCR detection of the intI1 and intI2 genes demonstrated the presence of integrons in two more strains, compared to the integron PCR. Overall, the integrase gene PCR resulted in a frequency of integron-positive isolates of 50% (24 of 48). All the integrons were found in isolates of A. baumannii (24 of 40). Class 1 integrons were detected in 58% (23 of 40) of the A. baumannii isolates, whereas only one A. baumannii strain (3O) contained a class 2 integron (Fig. 2). The correlation between the presence of integrons, as determined by integrase gene PCR, and the epidemic character of the Acinetobacter strains was statistically significant (P < 0.05).
FIG. 2.
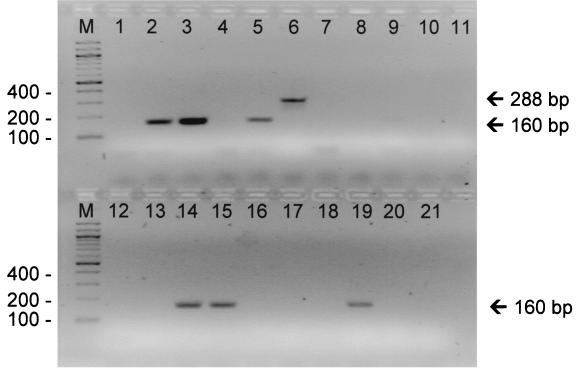
Gel image of representative PCR amplification products from different clinical strains of Acinetobacter species after amplification with intI1- and intI2-specific primers. Lane M, molecular weight marker; lane 1, PCR-negative buffer control; lanes 2 to 11, strains 1O, 2O, 3N, 4O, 3O, 5O, 5N, 6N, 7N, and 8N, respectively; lanes 12 to 21, strains 9N, 11O, 6O, 7O, 14N, 15O, 15N, 16O, 2O, and 20N, respectively. Strain designations are as presented in Table 1.
Sequence analysis.
To confirm that primers Int1 and Int2 correctly identified the intI1 and intI2 genes, amplification products of strain 1O and 3O were sequenced. The sequences of these products were 100% identical to previously published sequences of the intI1 and intI2 genes.
Detection of plasmids.
Plasmids were found in 42% (20 of 48) of the Acinetobacter strains. The distribution of plasmids in epidemic isolates was 36% (9 of 25) versus 48% (11 of 23) for nonepidemic isolates; this difference was not significant. Interestingly, only six isolates of A. baumannii contained both an integron and a plasmid.
Antibiotic susceptibility and integron carriage.
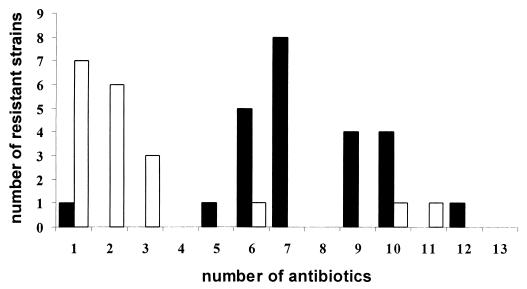
Susceptibility to 13 different antibiotics was related to the presence or absence of an integron within the Acinetobacter strains. Table 3 shows the antibiotic susceptibilities of integron-positive and integron-negative isolates to each of the antibiotics tested, expressed in terms of the MIC at which 50% of the isolates tested were inhibited (MIC50). Integron carriage was significantly associated with an increase in antibiotic resistance. In addition, integron-positive A. baumannii strains showed resistance to a significantly higher number of different antibiotics compared to integron-negative isolates (Fig. 3). Eighty percent (20 of 25) of the epidemic strains were resistant to five or more of the antibiotics tested. Strikingly, all integron-positive strains except one (23 of 24) showed resistance to five or more of the antibiotics tested. Integron detection identified 74% (17 of 23) of the epidemic A. baumannii isolates.
TABLE 3.
Antibiotic susceptibilities of integron-positive A. baumannii and integron-negative Acinetobacter species isolates
| Antibacterial agent | Susceptibility of isolates
|
Pb | |||
|---|---|---|---|---|---|
| Integron positive (n = 24)
|
Integron negative (n = 24)
|
||||
| MIC50 (mg/liter) | % Sa | MIC50 (mg/liter) | % S | ||
| Amikacin | 32 | 38 | 1 | 77 | <0.01 |
| Gentamicin | 32 | 4 | 0.25 | 69 | <0.001 |
| Tobramycin | 32 | 25 | 0.25 | 77 | <0.001 |
| Ciprofloxacin | 8 | 17 | 0.25 | 88 | <0.001 |
| Trimethoprim-sulfamethoxazole | 160 | 21 | 5 | 73 | <0.001 |
| Ampicillin | 64 | 0 | 16 | 27 | |
| Amoxicillin-clavulanic acid | 64 | 8 | 4 | 69 | <0.001 |
| Piperacillin-tazobactam | 64 | 42 | 4 | 73 | <0.05 |
| Cefuroxime | 64 | 4 | 64 | 4 | NS |
| Cefotaxime | 32 | 8 | 16 | 38 | <0.05 |
| Ceftazidime | 16 | 38 | 4 | 81 | <0.01 |
| Imipenem | 2 | 83 | 2 | 92 | NS |
| Meropenem | 1 | 96 | 1 | 96 | NS |
Percentage of strains that are susceptible according to NCCLS breakpoints.
Statistical significance of the difference between the number of susceptible integron-positive isolates and the number of susceptible integron-negative isolates (by Fisher's exact test). NS, not significant.
FIG. 3.
Comparison of resistance among integron-positive A. baumannii isolates (solid bars) and integron-negative Acinetobacter species isolates (open bars) in terms of the numbers of antibiotics to which isolates were resistant. Five integron-negative strains (two epidemic and three nonepidemic strains) were susceptible to all antibiotics tested.
DISCUSSION
The aim of our study was to investigate the possible role of integrons and plasmids in the epidemic behavior of clinical isolates of A. baumannii. The epidemic A. baumannii isolates as well as the sporadic Acinetobacter species strains included in this study were obtained from hospitals in 11 different countries. Almost half of these strains (48%) carried integrons. Integrons, however, were found significantly more often in epidemic A. baumannii strains than in sporadic isolates. Possibly, these genetic structures play an important role in the epidemic behavior of A. baumannii.
Class 1 integrons were the most common integrons found in this collection of A. baumannii isolates. Only one clinical isolate, strain 3O, was found to contain a class 2 integron structure. Other investigators have also found predominantly class 1 integrons in Acinetobacter species (23). A survey by Gonzalez et al., however, demonstrated predominantly class 2 integrons in A. baumannii isolates from Chilean hospitals (7). Possibly the strains from the latter study were more genetically related.
In the present study, two different PCR assays were used to detect either class 1 integrons by amplification of any inserted gene cassette or class 1 and class 2 integrons by detection of the specific intI1 and intI2 genes. The integron PCR could lead to false-negative results because (i) the number of inserted genes in the cassette could exceed the PCR extension capacity, which is optimized for DNA products of less than 2.5 kb; (ii) a strain can possess an integron without a gene cassette; and (iii) class 2 integrons do not contain the sulI gene at the 5′-conserved segment (18). The integrase gene PCR, however, detects both class 1 and class 2 integrons by amplification of two products of specific small sizes irrespective of the heterogeneity of the inserted gene cassettes. The integrase gene PCR was indeed more sensitive than the integron PCR and detected integrons in two outbreak strains which were negative in the integron PCR.
Integron-positive strains were significantly associated with resistance to multiple antibiotics. This is not surprising, since many antibiotic resistance gene cassettes encoding resistance to a wide range of antibiotics have been reported (6). However, this could not explain resistance to extended-spectrum β-lactams because such integron-encoded resistance genes have never been described. Resistance to extended-spectrum β-lactams could be due to a combination of integrons and resistance genes located on other genetic structures such as plasmids (14). The differences in ciprofloxacin resistance, however, cannot be explained this way, since transferable quinolone resistance encoded by integrons or plasmids has never been described. Most likely, changes in outer membranes of integron-positive Acinetobacter strains, induced by transferable β-lactam resistance, are responsible for these differences. Although plasmids were detected in a substantial number of strains, no significant correlations between plasmid carriage and either antibiotic resistance (data not shown) or epidemic behavior was found. These results indicate that antibiotic resistance in clinical isolates of A. baumannii is associated particularly with the presence of integrons. In the strains analyzed in this study, the integrons are probably not located on plasmids, since only six of the strains investigated harbored both integrons and plasmids.
The analysis of A. baumannii strains with known epidemic behavior demonstrates that early identification of epidemic strains may be possible by detection of integrons or multiple antibiotic resistance. The integrase gene PCR identified almost 75% of the epidemic A. baumannii strains. Multiple antibiotic resistance, defined as resistance to five or more antibiotics, showed good correlation with the presence of integrons and epidemic behavior of the strains. Susceptibility testing, however, has several disadvantages, including its laboriousness, the need for a pure bacterial culture, and subsequent overnight incubation. Also, the chosen cutoff level of five antibiotics is arbitrary and depends on the choice and the number of the antibiotics tested. PCR mapping of integrons, on the other hand, is a rapid and easy technique which can be performed on a single colony.
Integrons were also found in seven nonepidemic strains, which may be an indication of the epidemic potential of these strains. Another explanation for this finding could be the definition of the epidemic and nonepidemic phenotypes in terms of the capacity to spread to one or more patients. It is well known that many different circumstances, such as infection control measures, antibiotic policy, and susceptibilities of individual patients, can facilitate or prevent the dissemination of bacteria, including A. baumannii.
In conclusion, integrase gene PCR is a rapid, valuable procedure, which can be easily used in routine clinical microbiology laboratories for the detection of integrons in clinical A. baumannii isolates. It seems to be a rapid and simple tool for the routine screening of A. baumannii isolates in order to identify strains with epidemic potentials. This is important for the immediate introduction of specific infection control measures in the hospital setting in order to limit the nosocomial spread of these strains.
ACKNOWLEDGMENTS
We are indebted to our colleagues who contributed strains of Acinetobacter to this study. We also thank Nabil Atif for technical assistance.
REFERENCES
- 1.Amyes S G B, Young H-K. Mechanisms of antibiotic resistance in Acinetobacter spp.—genetics of resistance. In: Bergogne-Bérézin E, Joly-Guillou M L, Towner K J, editors. Acinetobacter microbiology, epidemiology, infections, management. Boca Raton, Fla: CRC Press; 1996. pp. 185–223. [Google Scholar]
- 2.Arakawa Y, Murakami M, Suzuki K, Ito H, Wacharotayankun R, Ohsuka S, Kato N, Ohta M. A novel integron-like element carrying the metallo-beta-lactamase gene blaIMP. Antimicrob Agents Chemother. 1995;39:1612–1615. doi: 10.1128/aac.39.7.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergogne-Bérézin E, Towner K J. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev. 1996;9:148–165. doi: 10.1128/cmr.9.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boom R, Sol C J A, Salimans M M M, Jansen C L, Wertheim-van Dillen P M E, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dijkshoorn L, Aucken H, Gerner-Smidt P, Janssen P, Kaufmann M E, Garaizar J, Ursing J, Pitt T L. Comparison of outbreak and nonoutbreak Acinetobacter baumannii strains by genotypic and phenotypic methods. J Clin Microbiol. 1996;34:1519–1525. doi: 10.1128/jcm.34.6.1519-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fluit A C, Schmitz F J. Class 1 integrons, gene cassettes, mobility, and epidemiology. Eur J Clin Microbiol Infect Dis. 1999;18:761–770. doi: 10.1007/s100960050398. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez G, Sossa K, Bello H, Dominguez M, Mella S, Zemelman R. Presence of integrons in isolates of different biotypes of Acinetobacter baumannii from Chilean hospitals. FEMS Microbiol Lett. 1998;161:125–128. doi: 10.1111/j.1574-6968.1998.tb12937.x. [DOI] [PubMed] [Google Scholar]
- 8.Hall R M, Brown H J, Brookes D E, Stokes H W. Integrons found in different locations have identical 5′ ends but variable 3′ ends. J Bacteriol. 1994;176:6286–6294. doi: 10.1128/jb.176.20.6286-6294.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall R M, Collis C M. Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination. Mol Microbiol. 1995;15:593–600. doi: 10.1111/j.1365-2958.1995.tb02368.x. [DOI] [PubMed] [Google Scholar]
- 10.Hartstein A I, Morthland V H, Rourke J W, Jr, Freeman J, Garber S, Sykes R, Rashad A L. Plasmid DNA fingerprinting of Acinetobacter calcoaceticus subspecies anitratus from intubated and mechanically ventilated patients. Infect Control Hosp Epidemiol. 1990;11:531–538. doi: 10.1086/646087. [DOI] [PubMed] [Google Scholar]
- 11.Koeleman J G M, Stoof J, Biesmans D J, Savelkoul P H M, Vandenbroucke-Grauls C M J E. Comparison of amplified ribosomal DNA restriction analysis, random amplified polymorphic DNA analysis, and amplified fragment length polymorphism fingerprinting for identification of Acinetobacter genomic species and typing of Acinetobacter baumannii. J Clin Microbiol. 1998;36:2522–2529. doi: 10.1128/jcm.36.9.2522-2529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levesque C, Piche L, Larose C, Roy P H. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob Agents Chemother. 1995;39:185–191. doi: 10.1128/aac.39.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marques M B, Brookings E S, Moser S A, Sonke P B, Waites K B. Comparative in vitro antimicrobial susceptibilities of nosocomial isolates of Acinetobacter baumannii and synergistic activities of nine antimicrobial combinations. Antimicrob Agents Chemother. 1997;41:881–885. doi: 10.1128/aac.41.5.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Freijo P, Fluit A C, Schmitz F J, Grek V S C, Verhoef J, Jones M E. Class I integrons in Gram-negative isolates from different European hospitals and association with decreased susceptibility to multiple antibiotic compounds. J Antimicrob Chemother. 1998;42:689–696. doi: 10.1093/jac/42.6.689. [DOI] [PubMed] [Google Scholar]
- 15.Palmen R, Hellingwerf K J. Uptake and processing of DNA by Acinetobacter calcoaceticus—a review. Gene. 1997;192:179–190. doi: 10.1016/s0378-1119(97)00042-5. [DOI] [PubMed] [Google Scholar]
- 16.Radstrom P, Skold O, Swedberg G, Flensburg J, Roy P H, Sundstrom L. Transposon Tn5090 of plasmid R751, which carries an integron, is related to Tn7, Mu, and the retroelements. J Bacteriol. 1994;176:3257–3268. doi: 10.1128/jb.176.11.3257-3268.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Recchia G D, Stokes H W, Hall R M. Characterisation of specific and secondary recombination sites recognised by the integron DNA integrase. Nucleic Acids Res. 1994;22:2071–2078. doi: 10.1093/nar/22.11.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sallen B, Rajoharison A, Desvarenne S, Mabilat C. Molecular epidemiology of integron-associated antibiotic resistance genes in clinical isolates of Enterobacteriaceae. Microb Drug Resist. 1995;1:195–202. doi: 10.1089/mdr.1995.1.195. [DOI] [PubMed] [Google Scholar]
- 19.Schmitz F J, Martinez-Freijo P, Theis S, Fluit A C, Verhoef J, Heinz H P, Jones M E. Prevalence of class 1 integrons and association with decreased antibiotic susceptibility in German gram-negative blood culture isolates. Clin Microbiol Infect. 1999;5:496–498. doi: 10.1111/j.1469-0691.1999.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 20.Seifert H, Baginski R, Schulze A, Pulverer G. Antimicrobial susceptibility of Acinetobacter species. Antimicrob Agents Chemother. 1993;37:750–753. doi: 10.1128/aac.37.4.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senda K, Arakawa Y, Ichiyama S, Nakashima K, Ito H, Ohsuka S, Shimokata K, Kato N, Ohta M. PCR detection of metallo-beta-lactamase gene (blaIMP) in gram-negative rods resistant to broad-spectrum beta-lactams. J Clin Microbiol. 1996;34:2909–2913. doi: 10.1128/jcm.34.12.2909-2913.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seward R J, Lambert T, Towner K J. Molecular epidemiology of aminoglycoside resistance in Acinetobacter spp. J Med Microbiol. 1998;47:455–462. doi: 10.1099/00222615-47-5-455. [DOI] [PubMed] [Google Scholar]
- 23.Seward R J, Towner K J. Detection of integrons in worldwide nosocomial isolates of Acinetobacter spp. Clin Microbiol Infect. 1999;5:308–318. doi: 10.1111/j.1469-0691.1999.tb00149.x. [DOI] [PubMed] [Google Scholar]
- 24.Shi Z Y, Liu P Y, Lau Y, Lin Y, Hu B S, Shir J M. Antimicrobial susceptibility of clinical isolates of Acinetobacter baumannii. Diagn Microbiol Infect Dis. 1996;24:81–85. doi: 10.1016/0732-8893(96)00017-x. [DOI] [PubMed] [Google Scholar]
- 25.Stokes H W, Hall R M. A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol Microbiol. 1989;3:1669–1683. doi: 10.1111/j.1365-2958.1989.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 26.Vahaboglu H, Ozturk R, Aygun G, Coskunkan F, Yaman A, Kaygusuz A, Leblebicioglu H, Balik I, Aydin K, Otkun M. Widespread detection of PER-1-type extended-spectrum beta-lactamases among nosocomial Acinetobacter and Pseudomonas aeruginosa isolates in Turkey: a nationwide multicenter study. Antimicrob Agents Chemother. 1997;41:2265–2269. doi: 10.1128/aac.41.10.2265. . (Erratum, 42:484, 1998.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vila J, Marcos A, Marco F, Abdalla S, Vergara Y, Reig R, Gomez-Lus R, Jimenez de Anta T. In vitro antimicrobial production of beta-lactamases, aminoglycoside-modifying enzymes, and chloramphenicol acetyltransferase by and susceptibility of clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother. 1993;37:138–141. doi: 10.1128/aac.37.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]