Abstract
Background
Mulibrey nanism (MUL) is a rare condition with profound growth delay. Congestive heart failure is a major determinant of prognosis. The aim was to delineate pericardial constriction and myocardial functional abnormalities in a pediatric MUL sample.
Methods
A total of 23 MUL patients and 23 individually sex- and age-matched healthy control subjects were prospectively assessed in a cross-sectional study with echocardiography.
Results
Clinical signs of heart failure were present in 7 MUL patients, with severe congestive heart failure in 2. Significant diastolic dysfunction, mainly related to constriction, was found in MUL patients without pericardiectomy (N = 18)—septal bounce, pronounced hepatic vein atrial reversal and right heart inflow–outflow variations, and decreased inferior vena cava collapse during respiration. The appearance of the pericardium was not different from that of control subjects. Longitudinal diastolic myocardial velocities were similar to those in control subjects, suggesting an absence of significant myocardial restriction. Right ventricular free wall longitudinal systolic strain and bilateral longitudinal myocardial systolic velocities were decreased in MUL patients, indicating mild biventricular systolic dysfunction. Myocardial motion abnormalities and persistent congestive heart failure were common (in 3 of 6) in MUL patients with a history of pericardiectomy. Cardiac dimensions were similar between MUL patients and control subjects when adjusting for body size, except for smaller biventricular volumes.
Conclusions
MUL disease presents with significant constriction-related diastolic dysfunction and mild bilateral systolic dysfunction. Constriction–restriction assessments during follow-up could be of benefit in decision-making regarding pericardiectomy in MUL disease. Myocardial abnormalities were prevalent among MUL patients who had undergone pericardiectomy and are consistent with progression of myocardial disease in a significant proportion of patients.
Résumé
Contexte
Le nanisme Mulibrey (MUL) est une maladie rare qui donne lieu à un retard de croissance marqué. L’insuffisance cardiaque congestive est un déterminant majeur du pronostic. L’objectif de cette étude était de caractériser la constriction péricardique et les anomalies fonctionnelles myocardiques dans un échantillon de cas de MUL pédiatrique.
Méthodologie
Au total, 23 patients atteints de MUL et 23 sujets témoins en bonne santé ont été appariés individuellement selon le sexe et l’âge et soumis à une évaluation prospective dans le cadre d’une étude transversale avec échocardiographie.
Résultats
Sept patients atteints de MUL présentaient des signes cliniques d’insuffisance cardiaque, et deux, une insuffisance cardiaque congestive sévère. Une dysfonction diastolique significative, principalement liée à la constriction, a été observée chez les patients atteints de MUL n’ayant pas subi de péricardiectomie (N = 18) – rebond septal, inversion auriculaire marquée du flux de la veine hépatique, variations prononcées du flux entrant et sortant du cœur droit, diminution du collapsus de la veine cave inférieure pendant la respiration. L’apparence du péricarde n’était pas différente de celle notée chez les sujets témoins. Les vélocités myocardiques longitudinales pendant la diastole étaient similaires à celles relevées chez les sujets témoins, ce qui suggère l’absence de restriction myocardique significative. La déformation longitudinale de la paroi libre du ventricule droit et les vélocités myocardiques longitudinales bilatérales étaient diminuées pendant la systole chez les patients atteints de MUL, ce qui indique une dysfonction systolique biventriculaire légère. Les anomalies de la cinétique myocardique et la persistance de l’insuffisance cardiaque congestive étaient fréquentes (dans trois cas sur six) chez les patients atteints de MUL ayant des antécédents de péricardiectomie. Les dimensions cardiaques chez les patients atteints de MUL étaient similaires à celles observées chez les sujets témoins après les ajustements en fonction de la taille corporelle, à l’exception des volumes biventriculaires, qui étaient plus petits.
Conclusions
Le MUL entraîne une dysfonction diastolique significative liée à la constriction et une légère dysfonction systolique bilatérale. Les évaluations axées sur la constriction et la restriction effectuées au cours du suivi pourraient être utiles pour la prise de décisions concernant le recours à la péricardiectomie dans les cas de MUL. Les anomalies myocardiques étaient fréquentes chez les patients atteints de MUL qui avaient subi une péricardiectomie et concordent avec la progression de la myocardiopathie dans une proportion significative de cas.
Mulibrey (muscle–liver–brain–eye) nanism (short stature; MUL, OMIM #253250) is an autosomal recessive disease caused by mutations in the TRIM37 gene.1 This multiorgan peroxisomal disease is particularly prevalent in the Finnish population. MUL patients suffer from severe growth disturbance and variable cardiovascular manifestations of congestive heart failure, which is a major predictor of prognosis.2, 3, 4 Significant pericardial thickening and fibrosis, mild myocardial fibrosis, and left ventricular hypertrophy have been described.5 Long-term clinical benefit of pericardiectomy has been reported in one third of patients, some of whose condition deteriorated after the procedure, presumably due to progression of cardiomyopathy-related heart failure. Classic constriction–restriction evaluations related to respiratory changes observed during spontaneous breathing during noninvasive echocardiography6 have not been assessed previously in MUL disease. Furthermore, case reports describe significant right heart failure among MUL patients,7 but no previous study has to date systematically assessed the right heart in MUL.
We hypothesized that MUL patients have abnormalities in diastolic function, due to both pericardial constriction and myocardial restriction that may be delineated during spontaneous breathing. We further hypothesized that heart failure is, in addition, related to myocardial changes affecting both right and left ventricular systolic function. Due to significantly impaired body growth in MUL, the assessment of cardiac structure in comparison with that of healthy children has to account for not only age and sex, but also differences in body size. Our aim was then to compare MUL patients with age- and sex-matched healthy control subjects, adjusting for anthropometric parameters in the analyses, with the goal of studying the following: (i) differences in right and left heart morphology and dimensions; (ii) differences in right and left heart diastolic and systolic function; (iii) pericardial constriction, by evaluating respiratory variations in biventricular inflows and outflows, as well as signs of interventricular dependence during spontaneous breathing; and (iv) how cardiac findings relate to clinical history of pericardiectomy and signs of heart failure.
Study Subjects and Methods
This is a prospective case–control cross-sectional study. The study included 23 pediatric MUL patients aged 1-16 years, alive in Finland in the period 2015-2017. The studies were performed by one investigator (T.S.) during outpatient visits at the Children’s Hospital, Helsinki University Hospital, Helsinki, Finland. The Finnish major mutation (c.493-2A > G) was seen in all patients, with one being compound heterozygous combined with a Fin-minor (c.2212delG) mutation. Of all pediatric MUL patients aged 1-16 years in Finland, alive in the period 20152017, the participation rate was 23 of 27. None of the contacted MUL patients declined participation, and none was excluded. A healthy control, individually matched for age and sex for each MUL patient, was recruited among hospital personnel’s children and patients referred for assessment of innocent murmurs, syncope or palpitations in sinus rhythm during evaluation, and with no abnormalities found on echocardiography. Exclusion criteria for control subjects were current disease of any kind, a hereditary cardiovascular disorder in a parent or sibling, or regular use of medication, significant arrhythmia, hypertension, or respiratory disease during echocardiography. The study conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Helsinki University Hospital ethics board for women, children, and psychiatry (269/13/03/03/2015/227). Informed consent was obtained at recruitment.
Patient records were reviewed for data on previous and current diagnoses, disease history, medications, and interventions, including invasive hemodynamic catheterization findings, pericardiectomy, and other operations. Current signs of respiratory or heart failure symptoms, medications, and other treatments were recorded during the study visit. Both MUL and control subject clinical status was prospectively assessed with a standard data-collection form. Presence and characteristics of heart murmurs, right internal jugular vein height from estimated right atrial level (mamilla) in sitting position (estimated central venous pressure in mm Hg calculated as height divided by 1.36), presence of pulsus paradoxus, resting heart and respiratory rates, size of liver and presence of ascites or other fluid collections, 12-lead standard electrocardiogram, as well as detailed anthropometric measures of weight, height, and thorax circumference dimensions were recorded. Heart failure was graded using the modified Ross classification for heart failure in children.8 Subject height was assessed by an electronic stadiometer (Seca gmbh & co. kg, Hamburg, Germany) and measured to the nearest 0.1 cm. Weight was assessed using an electronic scale (Seca 770, Seca gmbh & co. kg) to the nearest 0.1 kg. All other anthropometric and jugular vein height measures were assessed with a tape measure, to the nearest 0.1 cm. Z-scores for child height, weight, body mass index, and head circumference were derived using the recent Finnish reference data.9 Lean body mass (LBM) was calculated as described elsewhere.10 Blood pressure was measured according to National High Blood Pressure Education Program 4th report guidelines, using 3 repeated oscillometric measurements (Dinamap ProCare 200; GE Healthcare, Finland) taken with subject at rest in sitting position after 15 minutes of rest, and the mean of the 2 lowest readings was used in analyses. Systolic and diastolic blood pressure z-scores were generated using the 4th report reference.11 Pro-brain natriuretic peptide (BNP) and alanine aminotransferase levels were measured from a fasting blood venous blood sample obtained during morning hours from all MUL subjects.
Cardiac and pericardial assessments with echocardiography
A complete 2-dimensional echocardiogram was obtained, including B-mode, M-Mode and Doppler, tissue Doppler, and cardiac deformation (strain) assessments from standard views and in accordance with American Society of Echocardiography recommendations12 using a Vivid 7 (GE Medical Systems, Horten, Norway) equipped with M4S and 5S transducers as appropriate for patient size, and including simultaneous electrocardiogram and respirometer recordings. Clips including at least 3-5 respiratory cycles were obtained at rest to assess respiratory variations in flow and to study interventricular dependence. Measurements (see Supplemental Appendix S1 for details) were performed offline, with the operator blinded for subject background and clinical characteristics using EchoPac (version 113; GE Healthcare, Finland).
Pericardial constriction vs cardiac restriction was assessed by the following means: (i) ventricular septal left shift during early inspiration; (ii) ratio of hepatic vein diastolic reversal velocity to forward velocity during early expiration; (iii) inferior vena cava collapse during peak inspiration; (iv) absence of respiratory variation in superior vena cava flow velocities; and (v) septal and mitral lateral e’ PWD-TDI velocity. In adults, the presence of ventricular septal shift in combination with either septal e' ≥ 9 cm/s or hepatic vein expiratory diastolic reversal ratio ≥ 0.80 (hepatic vein diastolic reversal velocity / diastolic forward flow velocity) has been shown to be accurate for the diagnosis of constrictive pericarditis vs restrictive cardiomyopathy.6 The inferior vena cava assessment was included to evaluate the severity of constriction-related elevations in right atrial pressure, and the superior vena cava flow was added to differentiate false-positive findings related to obstructive lung disease or other conditions associated with exaggerated respiratory efforts.13
Data analysis
Categorical data are presented as numbers and proportions, and continuous data are reported as mean ± standard deviation, or median (range), as appropriate. Continuous variables were assessed for normal distribution using the Shapiro-Wilks test. Group-wise comparisons were performed using the independent Student t test for normally distributed variables, the Mann-Whitney U test for non-normally distributed variables, and Fisher’s exact test for binominal variables. Group differences on cardiac dimensions were compared using analysis of covariance, adjusting for anthropometry as indicated. Associations between different parameters were assessed with scatter plots and statistical testing with Pearson correlation and Spearman rank-order correlation, as appropriate. Sample size calculations and power analyses were not applicable, as the MUL condition is rare, and the aim was to include the complete Finnish pediatric MUL cohort. Data analyses were performed with SPSS version 24 (IBM, Finland).
Results
Background and clinical characteristics
Anthropometrics and clinical findings regarding study subjects at the study visit are presented in Table 1. As expected, MUL patients were significantly smaller on several anthropometric measures, compared with control subjects. In 18 MUL patients, the liver margin was palpable and enlarged, and the jugular vein was visible above the clavicle in the upright position. There were no signs of ascites or pulsus paradoxus. No abnormalities in heart sounds were noted, including absence of diastolic precordial knock. Heart rate was higher and systolic blood pressure lower in MUL patients, compared with control subjects. However, no difference was observed in blood pressure z-scores between groups, accounting for body size. The modified Ross score for heart failure in children was elevated (> 0) in 16 MUL patients. Among these, the score was low (1-2) in 9 patients, moderate (3-4) in 5, and high (6 and 13) in 2. For the 2 patients with high modified Ross scores, both had a history of premature birth, respiratory distress syndrome/bronchopulmonary dysplasia during infancy, severe growth restriction, and failure to thrive, with one patient requiring long-term intermittent continuous positive airway pressure (CPAP) support. Plasma pro-hormone B-type natriuretic peptide (pro-BNP) levels were variably elevated (> 300 ng/l) in 9 of 23 MUL patients and in 4 of 18 MUL patients older than 5 years. Alanine aminotransferase level was mildly elevated (> 40 U/l) in 6 MUL patients.
Table 1.
Characteristics, anthropometrics, and clinical findings of study subjects at study visit
| Characteristic | MUL patients (n = 23) |
Control subjects (n = 23) |
P | ||
|---|---|---|---|---|---|
| Mean or median | SD or range | Mean or median | SD or range | ||
| Male sex, n | 11 | 11 | |||
| Age, y | 10.3 | 1.9–15.2 | 10.0 | 1.2–15.8 | NS |
| Weight, kg | 14.8 | 6.6–47.0 | 33.0 | 13.0–68.7 | < 0.001 |
| Weight-for-age, Z | –5.17 | 2.78 | 0.20 | 0.96 | < 0.001 |
| Weight-for-height, Z | –2.11 | 1.70 | 0.08 | 0.94 | < 0.001 |
| Weight-for-height, % | –13.3 | 8.83 | 0.92 | 12.7 | < 0.001 |
| Height, cm | 110.5 | 69.5–157 | 146.4 | 93–175.5 | < 0.001 |
| Height-for-age, Z | –4.47 | –10.4–1.10 | 0.20 | –3.34–1.85 | < 0.001 |
| Lean body mass∗ | 14.7 | 6.8–36.1 | 29.5 | 11.3 – 46.2 | < 0.001 |
| Waist-to-hip ratio | 0.93 | 0.73–1.23 | 0.85 | 0.71–1.22 | 0.007 |
| Body surface area, m2 | 0.66 | 0.36–1.43 | 1.15 | 0.58–1.78 | < 0.001 |
| Thorax circumference, mm | 508 | 395–790 | 655 | 507–800 | < 0.001 |
| Laboratory data | |||||
| Alanine aminotransferase, U/l | 35 | 19 | NA | ||
| Pro-BNP, ng/l | 385 | 682 | |||
| Clinical findings | |||||
| Estimated jugular venous pressure, mm Hg | 7 | 3 | Veins not observable | ||
| Liver (cm from right costal margin) | 2 | 0–5 | Liver not palpable | ||
| Modified Ross score | 1 | 0–13 | 0 | ||
| Modified Ross class | 1 | 1–3 | 1 | ||
| Systolic BP, mm Hg | 95 | 13 | 104 | 10 | 0.009 |
| Systolic BP, Z | –0.14 | 1.32 | 0.27 | 0.67 | 0.210 |
| Diastolic BP, mm Hg | 58 | 7 | 60 | 7 | 0.405 |
| Diastolic BP, Z | –0.16 | 1.22 | 0.13 | 0.52 | 0.310 |
| Heart rate, bpm | 91 | 20 | 76 | 14 | 0.005 |
Data are presented as mean and standard deviation (SD), median and range, or count and percentage, unless otherwise specified.
BP, blood pressure. bpm, beats per minute; NA, not assessed; NS nonsignificant. pro-BNP, pro-hormone brain natriuretic peptide.
N = 18 for lean body mass for mulibrey nanism (MUL) patients and individually matched control subjects.
Patient characteristics, including diagnoses, medications, and interventions, are outlined in Supplemental Table S1. Five MUL patients had a history of pericardiectomy, and one was pericardiectomized 2 years after study evaluation. Elevated (mean: 12-20 mm Hg) and equalized right and left atrial pressures with absence of pulmonary hypertension (mean pressure: < 25 mm Hg) were confirmed on diagnostic catheterization in all 6 MUL patients prior to pericardiectomy. Other diagnoses included one case of atrial septal defect secundum closed with a device, one case of moderate-size atrial septal defect secundum without intervention showing interatrial right-to-left shunting and desaturation on exercise, and 2 cases of Wolff-Parkinson-White syndrome.
Cardiac dimensions and appearance of pericardium
Cardiac dimensions among MUL patients and control subjects are outlined in Supplemental Table S2, with mean difference and 95 confidence intervals reported, adjusted for body surface area. Most differences in dimensions were attenuated to nonsignificant levels when adjusting for body surface area. Right and left ventricular length and area were smaller in MUL patients, compared with those of control subjects, but no other statistically significant differences were found, including for right ventricular anterior wall thickness and left ventricular mass. Left atrium antero-posterior length was increased in MUL patients, but no difference in left atrial area or volume was found. The appearance of the pericardium was normal in MUL patients and not different from that in control subjects.
Cardiac systolic and diastolic function
Cardiac systolic and diastolic function parameters for the right and left heart are outlined in Supplemental Table S3. For the right heart, the end-expiratory hepatic vein S/D-wave velocity ratio was decreased due to increased D-wave velocity, and A-wave velocity and duration were increased in MUL patients, compared with control subjects. A-wave duration was also increased in MUL patients, when adjusting for heart rate. Biphasic hepatic vein S-wave was common among MUL patients and tended to be pronounced in MUL patients with more severe right heart failure. Tricuspid E:A ratio was decreased due to an increased A-wave velocity. There were no significant differences in tricuspid lateral annular E-prime and A-prime velocities, but S-prime velocities were decreased in MUL patients, compared with control subjects. Although tricuspid annular plane systolic excursion was significantly reduced, no difference was found when indexing this measure for right ventricular length. Also, no difference in right ventricular fractional area change was found between groups. Right-ventricle free wall global longitudinal strain was, however, significantly reduced, but no differences in strain rates were found between groups.
For the left heart, end-expiratory pulmonary venous waves were similar between groups, with absence of A-waves in both MUL and control subjects. The mitral valve E:A ratio was decreased due to an increased A-wave velocity. A-wave duration and mitral valve propagation velocity were also increased in MUL patients, compared with that in control subjects. This difference was also significant when adjusting for heart rate. E:E’ ratios were increased, and S-primes were reduced, for both mitral valve lateral as well as septal areas. Although mitral annular plane systolic excursion was decreased among MUL patients, there was no difference when indexing mitral annular plane systolic excursion for ventricular length. No statistically significant differences in left ventricular fractional shortening, ejection fraction, global longitudinal strain rate, or global circumferential strain rate were found between the MUL patients and control subjects. There were also no differences in right or left ventricular global strain rates (results not presented). No relationships between systolic and diastolic ventricular abnormalities were found. None of the MUL patients showed signs of annulus reversus with septal E' higher than MV E' velocities.
Signs of constriction during spontaneous breathing
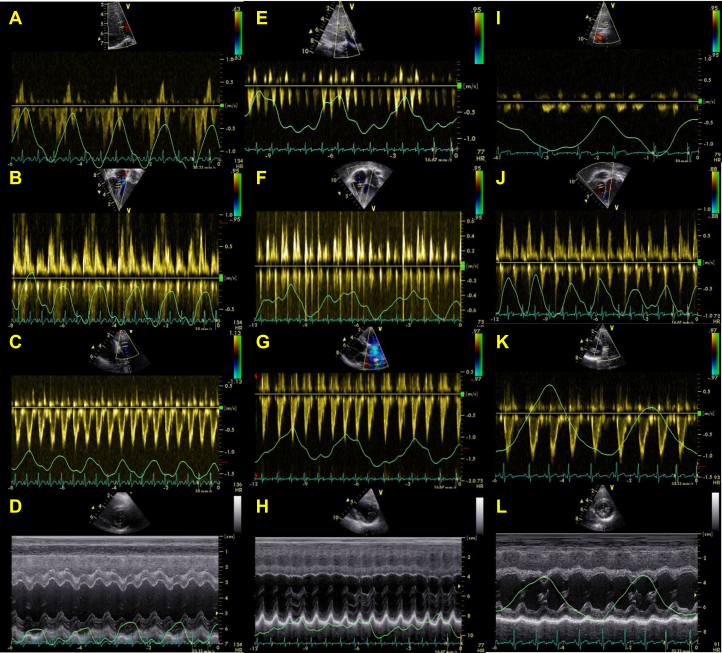
Respiratory variations inflows and outflows, interventricular dependence (septal bounce), and systemic veins are outlined in Table 2. Sample images from an MUL patient without pericardiectomy, an MUL patient with pericardiectomy, and a healthy control subject are provided in Figure 1. Sample images from an MUL patient pre-pericardiectomy, with right-to-left interatrial shunting and absent respiratory variations, are provided in Supplemental Figure S1. No pericardial thickening, echolucency, or effusion was observed during the examination. Changes during peak early inspiration in relation to end-expiration values were calculated. The change in inferior vena cava diameter was less in MUL patients compared with that in control subjects. The hepatic vein A-wave reversal and the A/D ratio were increased during early expirium, and A-wave duration was decreased during peak inspiration in MUL patients, compared with control subjects. Tricuspid inflow E-wave and right ventricle outflow velocities increased more in MUL patients during peak inspiration, compared with control subjects. For the left heart, pulmonary venous S- and D-waves decreased, and mitral valve inflow E and A waves decreased during peak inspiration. The left ventricle eccentricity index decreased (septal shift) during peak inspiration among MUL patients, compared with that of control subjects, and this was not related to heart rate. Constriction parameters (Table 3) were not related to heart rate.
Table 2.
Change in cardiac inflows and outflows, interventricular dependence, and inferior vena cava diameter change during peak inspiration, compared with end-expiration
| MUL patients (n = 23) |
Control subjects (n = 23) |
P | |||
|---|---|---|---|---|---|
| Mean or median | SD or range | Mean or median | SD or range | ||
| Right heart | |||||
| IVC max diameter, cm | 0.80 | 0.50–1.80 | 1.19 | 0.60–1.86 | 0.063 |
| IVC min diameter, cm | 0.50 | 0.20–1.16 | 0.46 | 0.16–1.12 | 0.466 |
| IVC diameter | –39 | 21 | –59 | 11 | < 0.001 |
| HV S-wave | 50 | –5 – 219 | 69 | 26–189 | 0.162 |
| HV S/D-ratio | –26 | –47–57 | –21 | –64–34 | 0.432 |
| HV A-wave velocity | 37 | –39–269 | 67 | 0–113 | 0.442 |
| A-wave duration | 5 | –36–128 | 36 | –7–165 | 0.011 |
| HV D-wave velocity early expirium, cm/s | 39 | 15 | 42 | 13 | 0.489 |
| HV A-wave velocity early expirium, cm/s | 46 | 15 | 31 | 9 | < 0.001 |
| HV A/D -ratio early expirium | 1.32 | 0.67 | 0.79 | 0.27 | < 0.001 |
| TV E-wave | 58 | 18–156 | 36 | 8–81 | 0.004 |
| TV A-wave | 32 | –26–120 | 8 | –16–66 | 0.166 |
| TV E/A-ratio | 26 | 37 | 25 | 29 | 0.768 |
| Pulmonary artery peak velocity | 16 | 12 | 0 | 4 | < 0.001 |
| Left heart | |||||
| PV S-wave | –19 | 28 | 4 | 31 | 0.010 |
| PV D-wave | –20 | 26 | 7 | 17 | < 0.001 |
| MV E-wave | –15 | 9 | 3 | 7 | < 0.001 |
| MV A-wave | –4 | 26 | 21 | 37 | 0.010 |
| MV E/A ratio | –13 | –36–61 | –14 | –43–72 | 0.796 |
| Ascending aorta peak velocity | –7 | 16 | –1 | 3 | 0.033 |
| LV eccentricity index in diastole | |||||
| End expiratory | 0.99 | 0.05 | 1.00 | 0.02 | 0.125 |
| Peak inspiratory | 0.81 | 0.12 | 0.95 | 0.07 | < 0.001 |
| Absolute change | –0.18 | 0.10 | –0.06 | 0.06 | < 0.001 |
| LV eccentricity index in systole | |||||
| End expiratory | 1.00 | 0.05 | 0.98 | 0.05 | 0.248 |
| Peak inspiratory | 0.98 | 0.05 | 0.99 | 0.03 | 0.257 |
| Absolute change | –0.02 | 0.05 | 0.009 | 0.05 | 0.045 |
Values are %, unless otherwise indicated. Septal bounce is reported as absolute change in LV eccentricity index from end expiration to peak inspiration.
A, late-diastolic; D, diastolic; E, early diastolic; HV, hepatic vein; IVC, inferior vena cava; LV, left ventricle; MUL, mulibrey nanism; MV, mitral valve; PV, pulmonary vein; S, systolic; TV, tricuspid valve.
Figure 1.
Sample images of respiratory changes in (A, E, I) hepatic vein flow, (B, F, J) right ventricular inflow, and (C, G, K) outflow, (D, H, L) septal curvature (M-mode) in a (A-D) 3-year-old mulibrey nanism (MUL) patient without pericardiectomy; (E-H) a 10-year-old MUL patient with pericardiectomy performed 2 years earlier; and (I-L) a 3-year-old healthy control. Note (A) early expiratory flow reversals during atrial contraction in hepatic veins, (B) significant increase in right ventricular inflow, (C) minor increase in right ventricular outflow, as well as (D) septal shift during inspiration in MUL patient without pericardiectomy. Note (F) moderate increase in right ventricular inflow, (E) minor increase in right ventricular outflow during inspiration, but (E, H) absence of other respiratory changes in MUL patient with pericardiectomy. Note (J) minor inspiratory increase in right ventricular inflow, but (I, K, L) absence of other respiratory changes in healthy control subject.
Table 3.
Signs of heart failure, pericardial constriction, right ventricular (RV) and left ventricular (LV) systolic heart failure, and diastolic function among mulibrey nanism (MUL) patients with or without pericardiectomy, and among control subjects
| Sign | MUL patients with pericardiectomy (n = 5) | MUL patients, with no pericardiectomy (n = 18) | Control subjects (n = 23) | P value MUL patients vs control subjects |
|---|---|---|---|---|
| Constriction | ||||
| Septal bounce > –0.05 | 3 | 16 | 4 | < 0.001 |
| HV A/D ratio in early expirium > 0.80 | 2 | 17 | 8 | 0.002 |
| Absent IVC collapse > 50% | 3 | 12 | 3 | 0.001 |
| Normal SepE’ > 9 cm/s | 2 | 16 | 23 | 0.049 |
| No SVC respiratory flow variation | 5 | 18 | 23 | NA |
| RV systolicdysfunction | ||||
| TAPSE/L < 0.20 | 2 | 0 | 0 | 0.489 |
| RVFWLS > –20% | 3 | 6 | 0 | 0.001 |
| TV S’ < 5 cm/s | 2 | 0 | 0 | 0.489 |
| RV-FAC < 30% | 0 | 0 | 0 | NA |
| RV diastolic function | ||||
| Atrial area, cm2/m2 | 13.7 (5.6) | 11.1 (2.3) | 9.7 (1.1) | |
| Hepatic A-wave, cm/s | 30 (13) | 22 (7) | 18 (4) | |
| Tricuspid inflow E:A ratio | 1.4 (0.3) | 1.6 (0.5) | 2.1 (0.6) | |
| LV systolic dysfunction | ||||
| EF Simpson < 50% | 2 | 4 | 0 | 0.022 |
| MV S’ < 6 cm/s | 0 | 2 | 0 | 0.489 |
| MAPSE/L < 0.20 | 2 | 5 | 0 | 0.009 |
| LV-4GLS < –16% | 2 | 3 | 0 | 0.049 |
| LV diastolic function | ||||
| Atrial volume, ml/m2 | 18.6 (7.3) | 21.9 (6.0) | 20.9 (3.9) | |
| Mitral inflow E:A ratio | 2.3 (0.5) | 2.3 (0.5) | 2.9 (1.1) | |
| Septal E:E’ ratio | 11.0 (3.6) | 8.3 (2.3) | 6.9 (1.5) | |
| Signs of heart failure | ||||
| Liver > 2 cm | 2 | 5 | 0 | 0.009 |
| Estimated jugular venous pressure, ≥ 10 mm Hg | 0 | 5 | 0 | 0.049 |
| Modified Ross Score > 1 | 1 | 9 | 0 | 0.001 |
| Plasma pro-BNP, ng/L | 301 (239–3264) | 168 (30–669) | NA |
Septal bounce was defined as > –0.05 absolute change in LV diastolic eccentricity index from end-expiration to peak inspiration. Superior vena cava (SVC) flow variation was assessed as variation in S/D wave velocities between end-expiration and peak inspiration. Absent inferior vena cava (IVC) collapse defined as failure to reach > 50% absolute decrease in IVC diameter from end-expiration to peak inspiration. GLS MUL patients vs control subjects compared with Fisher’s exact test. Mean (standard deviation) is reported for diastolic function parameters; median (range) is reported for plasma pro-hormone B-type natriuretic peptide (pro-BNP); and n is reported for all other variables.
E, early diastolic; E', E prime; EF, ejection fraction; GLS, global longitudinal strain; HV, hepatic vein; MAPSE, mitral annular plane systolic excursion; NA, not assessed. RV-FAC, RV fractional area change; RVFWLS, right ventricle free wall longitudinal strain; S', S prime; TAPSE, tricuspid annular plane systolic excursion.
Relationship between pericardiectomy and heart failure
Signs of constriction, cardiac systolic and diastolic dysfunction, and clinical and laboratory findings of heart failure among MUL patients stratified for history of pericardiectomy are presented in Table 3. No respiratory superior vena cava flow variation among MUL patients or control subjects was observed. Signs of constriction (septal shift/bounce and absence of inferior vena cava collapse during inspirium as well as abnormal hepatic vein A/D ratio during early expirium) were found in a majority of MUL patients without pericardiectomy, but also in 2-3 out of 5 patients with a history of pericardiectomy. Mildly reduced right and left ventricular systolic function was common among MUL patients, occurring in one third. Pro-BNP levels tended to be higher in MUL patients with a history of pericardiectomy, compared with MUL patients of similar age without pericardiectomy (Table 3; Supplemental Fig. S2).
Discussion
Pericardial disease and pericardial constriction without inflammation or effusion is very rare in the pediatric age group, but it is a common problem in MUL patients. This study outlines comprehensive noninvasive measures differentiating constriction- and restriction-related abnormalities in diastolic cardiac function in MUL patients. The study reports significant constriction in MUL patients, and residual myocardial restriction in patients following surgical removal of a fibrotic pericardium. Mild abnormalities in left and right ventricular systolic function were also found. Overall, myocardial right heart disease seems significant in the progression of heart failure among these patients, likely impacting prognosis.
The study shows that cardiac dimensions in MUL patients are largely congruent with body size, similar to findings of previous reports.5,14 Although left atrial anterior-posterior diameter was increased in MUL patients, compared with control subjects, no significant difference between groups in other left atrial dimensions, including major axis and volume, was found. Diastolic biventricular volumes were nevertheless smaller in our MUL patients, as also previously reported.14,15 We found no consistent difference in left ventricular mass or right ventricular anterior wall thickness when adjusted for height or body surface area, but hypertrophy may develop later in life in those with MUL disease.5
Pericardial constriction typically presents with right heart failure, including hepato- and splenomegaly, skin edema, and ascites. In our ambulatory pediatric MUL patients, however, skin edema and ascites were not observed, and hepatomegaly in MUL is apparently related to both congestive heart failure and the disorder itself. Differentiating pericardial constriction from myocardial restriction may be a major diagnostic challenge that is relatively rarely encountered in pediatric pericardial disease but important when assessing infectious perimyocardial disease.16 Major diagnostic signs of constriction include the dissociation between intrathoracic and intracardiac pressures and exaggerated ventricular interdependence during respiration. The diastolic longitudinal myocardial velocities are usually preserved in constriction, but in restriction, the inflow patterns are less altered during respiration, and diastolic myocardial velocities are constantly decreased.6 Our MUL patients presented with a lower mitral early propagation velocity and decreased left and right E:A inflow ratios consistent with diastolic dysfunction. Decreased inferior vena cava collapse during inspiration was also found, consistent with elevated right atrial pressures.17,18 Diagnostic catheterizations were performed only in patients with significant signs of heart failure prior to pericardiectomy and consistently showed elevated and equalized atrial pressures. Longitudinal early diastolic tissue Doppler velocities were similar to those in our healthy control subjects, suggesting a limited role of myocardial restriction in diastolic dysfunction in MUL patients without pericardiectomy. Septal shifting leftward on inspiration (bounce) and marked respiratory variations in hepatic atrial waves and tricuspid early inflows were frequent among MUL patients, and consistent with constriction. Superior vena cava Doppler profiles were in the normal range, and without respiratory variation, making the possibility of a significant impact of pulmonary functional abnormalities on constriction parameters unlikely. This finding is also consistent with results of a recent study showing no signs of airway obstruction in MUL disease.19 Taken together, our findings are consistent with significant pericardial constriction-related diastolic dysfunction in MUL disease.
Although previous autopsy studies report pericardial fibrosis with adhesions between the pericardial layers, visceral pericardium adhering to the epicardium, as well as scattered large calcific plaques occasionally penetrating into the myocardium in MUL patients,3,20 we were unable to show pericardial thickening on echocardiography. Furthermore, a clearly thickened pericardium was described in the surgical notes for only 3 of 6 MUL patients, suggesting a significant variance in pericardial thickness in MUL disease. This finding is similar to that reported by Kivistö et al.15 of normal pericardial thickness ( < 3.5 mm) assessed with magnetic resonance imaging, and to the relatively thin pericardium (2.5 mm) seen on computed tomography, as reported previously in pediatric MUL patients.21 A major challenge in assessment and decision-making in pericardiectomy is the inability to quantify pericardial thickness in pediatric (or adult) MUL patients. Therefore, the noninvasive diagnostic approach we present, assessing respiratory variations as described above, seems a good option during the follow-up and for surgical decision-making for these patients.
This study reports normal left ventricular ejection fraction but mild abnormalities in right and left ventricular longitudinal myocardial motion in a subset of MUL patients when assessed with comprehensive tissue Doppler and speckle tracking strain imaging which is congruent with findings of previous studies.5,14,15 Overall, the role of mild left ventricular systolic dysfunction in MUL seems limited, as systolic function was relatively preserved in our MUL patients with significant clinical heart failure. However, abnormalities in longitudinal myocardial deformation of the right and left ventricle, as well as longitudinal myocardial velocity abnormalities, were commonly found in patients after pericardiectomy. These abnormalities could be related to adhesions of the right ventricular anterolateral wall, but they are more likely consistent with progression of myocardial disease, particularly in MUL patients that do not respond clinically to pericardiectomy, as previously described.5
Our study has a limited sample size due to the rarity of MUL disease, but we included a representative pediatric sample and age range. Cardiac magnetic resonance imaging was not performed. Exercise data were not included due to methodological limitations related to age and body size. In addition, prospective longitudinal data collected pre and post pericardiectomy were not included due to the rarity of interventions.
In conclusion, we report a comprehensive clinical and echocardiographic assessment delineating pericardial constriction and myocardial restriction in pediatric MUL disease known to be variably challenged by noninflammatory pericardial fibrosis and myocardial disease leading to congestive heart failure. Differentiating pericardial constriction and myocardial restriction could potentially be of benefit in the follow-up of MUL patients and particularly in decision-making regarding pericardiectomy.
Funding Sources
T.S. has been supported by grants from the Sigrid Juselius Foundation, The Medical Society of Finland, and Finnish Foundation for Pediatric Research, Perklen foundation, Medicinska understödsföreningen Liv och Hälsa, and the Stockmann Foundation.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The study conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Helsinki University Hospital Ethics Board for Women, Children and Psychiatry (269/13/03/03/2015/227). Informed consent was obtained at recruitment.
See page 35 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2021.08.012.
Supplementary Material
References
- 1.Avela K., Lipsanen-Nyman M., Idänheimo N., et al. Gene encoding a new RING-B-box- coiled-coil protein is mutated in mulibrey nanism. Nat Genet. 2000;25:298–301. doi: 10.1038/77053. [DOI] [PubMed] [Google Scholar]
- 2.Perheentupa J., Autio S., Leisti S., et al. Mulibrey nanism, an autosomal recessive syndrome with pericardial constriction. Lancet. 1973;2:351–355. doi: 10.1016/s0140-6736(73)93193-0. [DOI] [PubMed] [Google Scholar]
- 3.Tuuteri L., Perheentupa J., Rapola J. The cardiopathy of mulibrey nanism, a new inherited syndrome. Chest. 1974;65:628–631. doi: 10.1378/chest.65.6.628. [DOI] [PubMed] [Google Scholar]
- 4.Karlberg N., Jalanko H., Perheentupa J., Lipsanen-Nyman M. Mulibrey nanism: clinical features and diagnostic criteria. J Med Genet. 2004;41:92–98. doi: 10.1136/jmg.2003.014118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipsanen-Nyman M., Perheentupa J., Rapola J., Sovijärvi A., Kupari M. Mulibrey heart disease: clinical manifestations, long-term course, and results of pericardiectomy in a series of 49 patients born before 1985. Circulation. 2003;107:2810–2815. doi: 10.1161/01.CIR.0000070949.76608.E2. [DOI] [PubMed] [Google Scholar]
- 6.Welch T.D., Ling L.H., Espinosa R.E., et al. Echocardiographic diagnosis of constrictive pericarditis: Mayo Clinic criteria. CircCardiovasc Imaging. 2014;7:526–534. doi: 10.1161/CIRCIMAGING.113.001613. [DOI] [PubMed] [Google Scholar]
- 7.Kumpf M., Hämäläinen R.H., Hofbeck M., Baden W. Refractory congestive heart failure following delayed pericardectomy in a 12-year-old child with mulibrey nanism due to a novel mutation in TRIM37. Eur J Pediatr. 2013;172:1415–1418. doi: 10.1007/s00431-013-1962-2. [DOI] [PubMed] [Google Scholar]
- 8.Ross R.D. The Ross classification for heart failure in children after 25 years: a review and an age-stratified revision. Pediatr Cardiol. 2012;33:1295–1300. doi: 10.1007/s00246-012-0306-8. [DOI] [PubMed] [Google Scholar]
- 9.Saari A., Sankilampi U., Hannila M.L., et al. New Finnish growth references for children and adolescents aged 0 to 20 years: length/height-for-age, weight-for-length/height, and body mass index-for-age. Ann.Med. 2011;43:235–248. doi: 10.3109/07853890.2010.515603. [DOI] [PubMed] [Google Scholar]
- 10.Foster B.J., Platt R.W., Zemel B.S. Development and validation of a predictive equation for lean body mass in children and adolescents. Ann Hum Biol. 2012;39:171–182. doi: 10.3109/03014460.2012.681800. [DOI] [PubMed] [Google Scholar]
- 11.National High Blood Pressure Education Program Working Group on.High Blood Pressure in Children and Adolescents The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl 4th report):555–576. [PubMed] [Google Scholar]
- 12.Lopez L., Colan S.D., Frommelt P.C., et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr. 2010;23:465–467. doi: 10.1016/j.echo.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Boonyaratavej S., Oh J.K., Tajik A.J., Appleton C.P., Seward J.B. Comparison of mitral inflow and superior vena cava Doppler velocities in chronic obstructive pulmonary disease and constrictive pericarditis. J Am Coll Cardiol. 1998;32:2043–2048. doi: 10.1016/s0735-1097(98)00472-0. [DOI] [PubMed] [Google Scholar]
- 14.Eerola A., Pihkala J.I., Karlberg N., Lipsanen-Nyman M., Jokinen E. Cardiac dysfunction in children with mulibrey nanism. Pediatr Cardiol. 2007;28:155–162. doi: 10.1007/s00246-006-0007-2. [DOI] [PubMed] [Google Scholar]
- 15.Kivistö S., Lipsanen-Nyman M., Kupari M., Hekali P., Lauerma K. Cardiac involvement in mulibrey nanism: characterization with magnetic resonance imaging. J Cardiovasc Magn Reson. 2004;6:645–652. doi: 10.1081/jcmr-120038085. [DOI] [PubMed] [Google Scholar]
- 16.Shakti D., Hehn R., Gauvreau K., Sundel R.P., Newburger J.W. Idiopathic pericarditis and pericardial effusion in children: contemporary epidemiology and management. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwamoto Y., Tamai A., Kohno K., et al. Usefulness of respiratory variation of inferior vena cava diameter for estimation of elevated central venous pressure in children with cardiovascular disease. Circ J. 2011;75:1209–1214. doi: 10.1253/circj.cj-10-0690. [DOI] [PubMed] [Google Scholar]
- 18.Kutty S., Li L., Hasan R., et al. Systemic venous diameters, collapsibility indices, and right atrial measurements in normal pediatric subjects. J Am Soc Echocardiogr. 2014;27:155–162. doi: 10.1016/j.echo.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Sivunen J., Piirilä P., Karlberg S., et al. Restriction of lung volumes but normal function of pulmonary tissue in mulibrey nanism. Pediatr Pulmonol. 2020;55:122–129. doi: 10.1002/ppul.24518. [DOI] [PubMed] [Google Scholar]
- 20.Karlberg N., Karlberg S., Karikoski R., et al. High frequency of tumours in mulibrey nanism. J Pathol. 2009;218:163–171. doi: 10.1002/path.2538. [DOI] [PubMed] [Google Scholar]
- 21.Yasuhara J., Omori S., Maeda J., et al. Successful total pericardiectomy for constrictive pericarditis in the first series of Japanese patients with mulibrey nanism. Can J Cardiol. 2018;34:690. doi: 10.1016/j.cjca.2018.02.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.