Abstract
Background
Evidence from clinical trials suggests a differential effect of sex on the effectiveness and safety of direct oral anticoagulants (DOACs) for stroke prophylaxis in atrial fibrillation (AF).
Methods
This population-based cohort study examined the independent effect of sex on hemorrhage and ischemic stroke in 23,884 patients (55% females; age ≥ 66 years) with AF starting apixaban or rivaroxaban treatment in Ontario, Canada. Patients were followed for 90 days after their DOAC prescription. Using female sex as the exposure of interest, differences in baseline characteristics were balanced between sexes using inverse probability weights based on propensity scores. Applying weighted modified Poisson regression, risk ratios (RRs) were estimated for major hemorrhage, ischemic stroke/systemic embolism/transient ischemic attack (hereafter stroke), myocardial infarction, and all-cause mortality, with males as a reference.
Results
Females were older, had higher predicted stroke risk (based on CHADS2 score), and had fewer comorbidities than did males. Males had a higher prevalence of coronary artery disease, diabetes, and cancer, and similar predicted bleeding risk (based on HAS-BLED score). After weighting, baseline characteristics were well balanced. The 90-day risks for hemorrhage (RR 0.96; 95% confidence interval [CI] 0.80-1.15; P = 0.69) and stroke (RR 1.01; 95% CI 0.86-1.19; P = 0.94) were similar between sexes, which remained true when assessing each DOAC separately by dosing regimen. Compared to males, females had a lower risk for myocardial infarction (RR 0.66; 95% CI 0.52-0.84; P = 0.0008), and for all-cause mortality (RR 0.76; 95% CI 0.67-0.87; P < 0.0001).
Conclusions
Our findings do not suggest an association of sex with the 90-day risk of hemorrhage or ischemic stroke in older AF patients prescribed apixaban or rivaroxaban.
Résumé
Contexte
Les données probantes issues des essais cliniques donnent à penser que l’efficacité et l’innocuité des anticoagulants oraux directs (AOD) utilisés pour la prophylaxie des accidents vasculaires cérébraux (AVC) dans un contexte de fibrillation auriculaire (FA) varient selon le sexe du patient.
Méthodologie
Cette étude de cohorte populationnelle a examiné l’effet indépendant du sexe sur l’hémorragie et l’AVC ischémique chez 23 884 patients (55 % de femmes; âge ≥ 66 ans) atteints de FA ayant amorcé un traitement par l’apixaban ou le rivaroxaban en Ontario (Canada). Les patients ont été suivis pendant 90 jours après avoir reçu une ordonnance d’AOD. Le sexe féminin constituant l’exposition d’intérêt, les différences quant aux caractéristiques initiales ont été réparties de façon équilibrée entre les sexes au moyen d’une pondération par probabilité inverse reposant sur le score de propension. La régression de Poisson modifiée avec pondération a servi à estimer les rapports de risques (RR) d’hémorragie majeure, d’AVC ischémique/d’embolie systémique/d’accident ischémique transitoire (ci-après AVC), d’infarctus du myocarde et de mortalité toutes causes confondues, les hommes formant la population de référence.
Résultats
Les femmes étaient plus âgées, présentaient un risque prévu d’AVC plus élevé (d’après le score CHADS2) et présentaient moins de maladies concomitantes que les hommes. Les hommes présentaient une prévalence plus élevée de coronaropathie, de diabète et de cancer, et un risque prévu d’hémorragie similaire (compte tenu du score HAS-BLED). Après la pondération, la répartition des caractéristiques initiales des patients était bien équilibrée. Le risque d’hémorragie (RR : 0,96; intervalle de confiance [IC] à 95 % : 0,80-1,15; P = 0,69) et d’AVC (RR : 1,01; IC à 95 % : 0,86-1,19; P = 0,94) sur 90 jours était similaire pour les deux sexes, et il en était de même lorsque chaque AOD a été évalué séparément en fonction du schéma posologique. Par rapport aux hommes, les femmes présentaient un risque plus faible d’infarctus du myocarde (RR : 0,66; IC à 95 % : 0,52-0,84; P = 0,0008) et de mortalité toutes causes confondues (RR : 0,76; IC à 95 % : 0,67-0,87; P < 0,0001).
Conclusions
Nos résultats ne laissent présumer aucune association entre le sexe et le risque d’hémorragie ou d’AVC ischémique sur une période de 90 jours chez les patients âgés atteints de FA à qui l’apixaban ou le rivaroxaban sont prescrits.
Non-valvular atrial fibrillation (AF) is a common cardiac arrhythmia among elderly patients that is associated with increased risks for stroke and death, affecting approximately 200,000 Canadians.1, 2, 3 Oral anticoagulation (OAC) therapy is effective in preventing ischemic strokes and other embolic events in patients with AF,4,5 and it comprises the vitamin K antagonist warfarin, and more recently, direct-acting oral anticoagulants (DOACs), including dabigatran, rivaroxaban, apixaban, and edoxaban. DOACs cause rapid anticoagulation by directly targeting clotting factors, with no need for routine monitoring and reduced potential for food effects or drug interactions, and serve as clinically favored alternatives to warfarin, with similar or superior efficacy.6,7 In Canada, DOAC prescriptions in AF patients increased from 22.1% of annual anticoagulant prescriptions in 2011 to 87.3% in 2017, with apixaban and rivaroxaban accounting for 60% and 23% of prescriptions in the final year.8
Sex differences in thromboembolic events among AF patients are well documented. In non-anticoagulated AF patients, female sex has been independently associated with increased stroke risk,9, 10, 11, 12 and risk may remain elevated despite anticoagulant therapy, as shown for warfarin.11, 12, 13 Given the recency of the approval of DOACs, studies evaluating sex-specific differences in their effectiveness and safety in AF patients are sparse and limited to secondary analyses of clinical trials data. Although evidence remains conflicting (Supplemental Table S1),14, 15, 16, 17, 18 women may have higher stroke and/or lower bleeding risks than men. Moreover, these trials were not designed to investigate the independent effect of sex on DOAC-related outcomes, comprising more male and on average younger patients than are seen in routine practice, yet AF-related stroke risk tends to be greatest among elderly women (age > 75 years).10,11 Accordingly, data for patients in routine practice remain largely unexplored.
Apixaban and rivaroxaban elicit their pharmacologic effects in a concentration-dependent manner, and factors that significantly alter drug concentration may influence anticoagulant response.19,20 Reduced doses are recommended in AF patients who have clinical characteristics that may enhance blood concentration, such as low body weight, old age, and impaired renal function.21,22 We reported female sex as an independent predictor of elevated apixaban concentration among AF patients after controlling for age, weight, and serum creatinine level,23 corroborating previous findings that women, on average, have a 15% higher apixaban drug exposure than men.20 Elderly female patients on apixaban may be more susceptible to bleeding or better protected from stroke, compared to their male counterparts, whereas rivaroxaban concentration does not appear to be influenced by sex.24
Taken together, the rising popularity of DOACs, the on-average higher drug exposure in women, and evidence for differential sex-dependent effects on their safety and effectiveness being limited to clinical trials data highlight the need to assess the association of sex with DOAC-related outcomes in AF routine care. Using propensity score weights, this study determined whether females, compared to males, prescribed either apixaban or rivaroxaban, differ in their 90-day risk of major bleeding or stroke in a large population-based cohort of elderly AF patients.
Methods
Study design
We conducted a retrospective cohort study using provincial healthcare databases to assess sex-related differences in clinical outcomes related to the use of rivaroxaban and apixaban in AF patients. These databases capture health information of Ontario, Canada residents and include patient utilization of hospital and physician services and prescription drugs in those age ≥ 65 years. Datasets were linked using unique identifiers and analyzed at ICES Western. The use of data herein was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require research ethics board review. This study followed the Reporting of Studies Conducted Using Observational Routinely Collected Data (RECORD) guidelines (Supplemental Table S2).
Study cohort
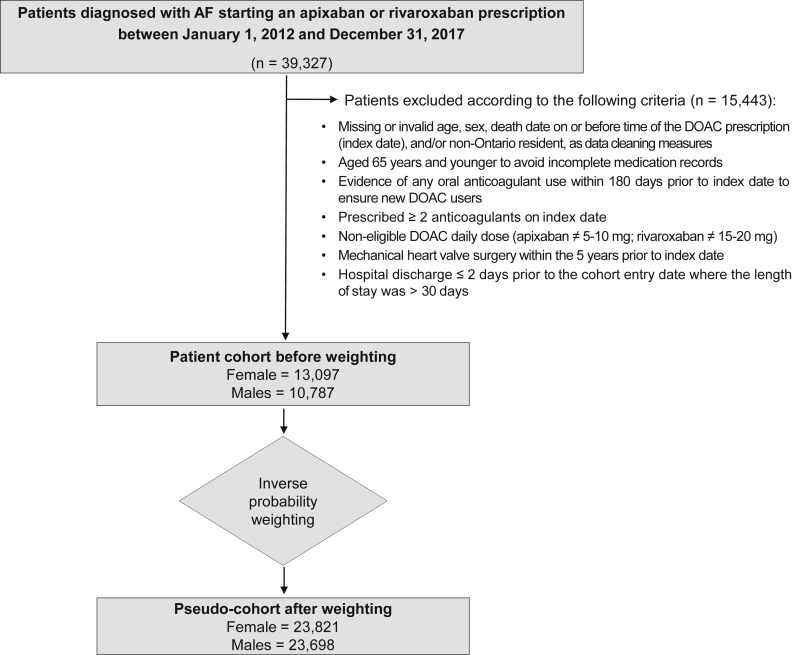
Our study cohort comprised adults aged ≥ 66 years in Ontario, Canada. We considered all patients with a prescription of rivaroxaban or apixaban for at least 7 days between January 1, 2012 and December 31, 2017 and within 30 days of an AF diagnosis, referred to as newly treated AF. The AF diagnosis was defined based on the following codes of the International Statistical Classification of Diseases and Related Health Problems (ICD-10): I4800, paroxysmal atrial fibrillation; I4801, persistent atrial fibrillation; I4802, chronic atrial fibrillation; I4890, atrial fibrillation, unspecified. We looked back as far as 2002 to ensure that patients did not have a diagnosis of AF prior to their cohort inclusion diagnosis date. Exclusion criteria for patients were defined a priori and are listed in Figure 1.
Figure 1.
Patient flow diagram. AF, atrial fibrillation; DOAC, direct oral anticoagulant.
Data sources and covariates
Baseline covariates, including demographics, comorbidities, prescription drugs, hospital services, and laboratory data, were defined a priori based on previous literature suggesting an effect on outcomes studied, and they were collected along with outcomes data using records from 9 linked databases (Supplemental Appendix S1). Laboratory measurements of serum creatinine level and estimated glomerular filtration rate (eGFR) were available for ∼45% of patients.
Exposure and outcomes
Patients were grouped as females and males for analyses with sex as the exposure variable. Primary outcomes were hospital encounters (hospitalization or emergency department [ED] visit) with (i) major hemorrhage or (ii) stroke, systemic embolism (SE), or transient ischemic attack (TIA). Secondary outcomes were ischemic stroke or SE (excluding TIA), myocardial infarction (MI), and all-cause mortality. Diagnostic codes used to ascertain outcomes are listed in Supplemental Table S3. Outcomes data were collected over a 90-day period after a new rivaroxaban or apixaban prescription. This prescription date served as the index date (cohort entry date), which was the start of follow-up. Patients were followed until a hospital admission or ED visit with the study outcome, death, or completion of follow-up. We chose 90 days in order to focus on acute events, and to avoid medication crossovers that may occur with DOAC use over longer periods. In routine care, about one quarter of AF patients are no longer adherent to their medication one year after starting a DOAC.8 Moreover, our initial data showed that the highest event rate occurred during the first 90 days, compared to that for one year, confirming a previous report.25
Statistical analysis
To adjust for confounding variables in baseline characteristics between female (exposed) and male (unexposed/reference) cohorts, we used the inverse probability of treatment weights based on propensity scores.26, 27, 28 Weights were defined as the inverse of the predicted probability of receiving the “treatment” they received, with “treatment” herein being unmodifiable sex. Specifically, propensity scores were calculated using multivariable logistic regression including 60 baseline characteristics (Supplemental Table S4) to estimate the probability of being a female AF patient starting treatment. Propensity score-based weights were then applied to all subjects to create synthetic weighted pseudo-cohorts of males and females that were balanced in their baseline characteristics. These weights have been shown to produce unbiased estimates of the average treatment effect, similar to the result that would be obtained from a randomized controlled trial. The use of a robust sandwich variance estimator accounts for the correlation induced in the data by the weighting. Standardized differences, rather than P values, were used to evaluate this balance before and after weighting, calculated as the difference between group means relative to the pooled standard deviation; differences of ≥ 0.10 were considered to be meaningful. Given that they are dependent on sample size, P values may not adequately reflect a meaningful difference.29
Analyses were carried out on weighted cohorts. Weighted risk ratios (RRs) with 95% confidence intervals (CIs) comparing females to males for primary outcomes were estimated using modified Poisson regression.28 Secondary analyses were carried out to ascertain initial findings. Poisson regression was repeated, adjusting for dosing regimens to account for the potential effect of a residual imbalance in prescribed dosage between cohorts. Cox proportional hazards regression (sensitivity analysis) was used to compare 90-day outcomes between sexes, censoring for death. The cumulative outcome probability was plotted using the complement of the Kaplan-Meier product limit estimator. Prespecified subgroup analyses were performed, assessing each DOAC separately by dosing regimen, for example, comparing females vs males on apixaban 2.5 mg twice daily. Statistical significance was determined using 2-tailed P values (P < 0.05).
Results
Baseline characteristics
We identified 39,327 AF patients starting DOAC treatment, with 23,884 patients (55% females) who met the inclusion criteria (Fig. 1), with baseline characteristics as listed in Table 1 and Supplemental Table S5. Before weighting, females were older and had fewer comorbidities, indicated by a lower mean Charlson comorbidity index (0.63 for women; 0.83 for men). Specifically, 73% of females, compared with 62% of males, were ≥ 75 years old. Apart from hypertension (females, 79.1%; males, 75.1%), females had a lower prevalence, compared with males, of coronary artery disease (37.1% vs 48.3%), diabetes (30.0% vs 37.6%), cancer (36.8% vs 45.2%), and alcoholism (0.5% vs 1.7%). Although baseline stroke risk estimated by CHADS2 score (Congestive Heart Failure, Hypertension, Age ≥ 75 years, Diabetes, and Prior Stroke or Transient Ischemic Attack or Thromboembolism) was higher in females than males (mean ± SD: females, 2.81 ± 1.36; males, 2.63 ± 1.43), the baseline bleeding risks as determined by HAS-BLED score (Hypertension, Abnormal Renal/Liver Function, Stroke History, Bleeding History or Predisposition, Labile INR, Elderly (> 65 Years), Drugs predisposing to bleeding and Alcohol use; labile INR excluded) were similar (females, 2.45 ± 0.96; males, 2.38 ± 0.97). Apixaban was more commonly prescribed than rivaroxaban (females, 60.8%; males, 58.1%). Although the standard dosing regimens for both apixaban and rivaroxaban were prescribed more frequently for males, a higher fraction of females were prescribed the reduced apixaban dosing regimen. Regarding co-medications, cytochrome P450 (CYP) 3A4/P-glycoprotein inhibitor or inducer prescriptions were balanced between the sexes; statin prescription was more common for males, and acetaminophen and proton pump inhibitor use were higher for females. Both sexes had similar numbers of primary care visits, ED visits, and hospitalizations. However, males had a higher prevalence of coronary angiography and revascularization. Females had, on average, lower hemoglobin level, serum creatinine level, and eGFR. After weighting, baseline characteristics were well balanced between pseudo-cohorts (standardized difference < 10%), except for the apixaban dosing regimen and the serum creatinine level, with the latter intentionally not included in our propensity score model.
Table 1.
Select baseline characteristics of study cohorts
| Characteristics | Unweighted cohort |
Weighted cohort |
||||
|---|---|---|---|---|---|---|
| Male (n = 10,787) | Female (n = 13,097) | Std. Diff∗ | Male (n = 23,698) | Female (n = 23,821) | Std. Diff∗ | |
| Age at index date, y | 77.6 ± 7.4 | 80.0 ± 7.6 | 0.31 | 78.8 ± 11.3 | 78.9 ± 10.3 | 0.01 |
| Age ≥ 75 | 6688 (62.0) | 9591 (73.2) | 0.24 | 16,057 (67.8) | 16,242 (68.2) | 0.01 |
| DOAC type, mg daily | ||||||
| Apixaban | ||||||
| 10 | 4213 (39.1) | 4191 (32.0) | 0.15 | 9023 (38.1) | 7890 (33.1) | 0.10 |
| 5 | 2055 (19.1) | 3778 (28.8) | 0.23 | 5057 (21.3) | 6318 (26.5) | 0.12 |
| Rivaroxaban | ||||||
| 20 | 3314 (30.7) | 3458 (26.4) | 0.10 | 6838 (28.9) | 6667 (28.0) | 0.02 |
| 15 | 1205 (11.2) | 1670 (12.8) | 0.05 | 2781 (11.7) | 2945 (12.4) | 0.02 |
| Medical history† | ||||||
| CHADS2 score‡ | 2.63 ± 1.43 | 2.81 ± 1.36 | 0.13 | 2.72 ± 2.04 | 2.73 ± 1.91 | 0.01 |
| HAS-BLED score§ | 2.38 ± 0.97 | 2.45 ± 0.96 | 0.07 | 2.42 ± 1.43 | 2.42 ± 1.31 | 0.00 |
| Congestive heart failure | 3107 (28.8) | 3669 (28.0) | 0.02 | 6713 (28.3) | 6785 (28.5) | 0.00 |
| Hypertension | 8096 (75.1) | 10,355 (79.1) | 0.10 | 18,249 (77.0) | 18,381 (77.2) | 0.00 |
| Diabetes | 4054 (37.6) | 3932 (30) | 0.16 | 8021 (33.8) | 7996 (33.6) | 0.00 |
| Prior ischemic stroke/systemic embolism | 500 (4.6) | 577 (4.4) | 0.01 | 1057 (4.5) | 1059 (4.4) | 0.00 |
| Prior TIA | 126 (1.2) | 152 (1.2) | 0 | 268 (1.1) | 275 (1.2) | 0.01 |
| Chronic kidney disease | 1117 (10.4) | 1095 (8.4) | 0.07 | 2194 (9.3) | 2197 (9.2) | 0.00 |
| Chronic liver disease | 458 (4.2) | 420 (3.2) | 0.05 | 888 (3.7) | 878 (3.7) | 0.00 |
| Prior hemorrhage | 610 (5.7) | 666 (5.1) | 0.03 | 1289 (5.4) | 1289 (5.4) | 0.00 |
| Anemia | 2155 (20.0) | 2489 (19.0) | 0.03 | 4,365 (19.6) | 4630 (19.4) | 0.01 |
| Alcoholism | 188 (1.7) | 59 (0.5) | 0.12 | 249 (1.0) | 212 (0.9) | 0.01 |
| CAD (incl. angina) | 5209 (48.3) | 4853 (37.1) | 0.23 | 9988 (42.1) | 9964 (41.8) | 0.01 |
| Prior myocardial infarction | 841 (7.8) | 728 (5.6) | 0.09 | 1578 (6.7) | 1567 (6.6) | 0.00 |
| Prior deep vein thrombosis | 36 (0.3) | 57 (0.4) | 0.02 | 88 (0.4) | 93 (0.4) | 0.00 |
| Prior pulmonary embolism | 50 (0.5) | 91 (0.7) | 0.03 | 137 (0.6) | 141 (0.6) | 0.00 |
| Cancer | 4874 (45.2) | 4818 (36.8) | 0.17 | 9746 (41.1) | 9721 (40.8) | 0.01 |
| Charlson comorbidity index‖ | 0.83 ± 1.44 | 0.63 ± 1.25 | 0.15 | 0.73 ± 1.98 | 0.73 ± 1.86 | 0.00 |
| Medication history¶ | ||||||
| Anti-arrhythmic drugs | 380 (3.5) | 446 (3.4) | 0.01 | 791 (3.3) | 820 (3.4) | 0.01 |
| Clopidogrel | 1145 (10.6) | 1038 (7.9) | 0.09 | 2157 (9.1) | 2161 (9.1) | 0.00 |
| Other oral anti-platelets | 133 (1.2) | 135 (1.0) | 0.02 | 11,494 (48.5) | 11,472 (48.2) | 0.01 |
| Acetylsalicylic acid | 332 (3.1) | 346 (2.6) | 0.03 | 671 (2.8) | 668 (2.8) | 0.00 |
| NSAIDs | 1080 (10.0) | 1505 (11.5) | 0.05 | 2559 (10.8) | 2596 (10.9) | 0.00 |
| Corticosteroids | 2826 (26.2) | 3537 (27.0) | 0.02 | 6362 (26.8) | 6388 (26.8) | 0.00 |
| Statins | 5772 (53.5) | 5817 (44.4) | 0.18 | 11,494 (48.5) | 11,472 (48.2) | 0.01 |
| Proton pump inhibitors | 3025 (28.0) | 4341 (33.1) | 0.11 | 7228 (30.5) | 7354 (30.9) | 0.01 |
| CYP3A4/P-gp inducers∗∗ | 79 (0.7) | 70 (0.5) | 0.03 | 151 (0.6) | 150 (0.6) | 0.00 |
| CYP3A4/P-gp inhibitors†† | 1053 (9.8) | 1513 (11.6) | 0.06 | 2482 (10.5) | 2532 (10.6) | 0.00 |
Data are presented as n (%) or mean ± standard deviation (SD). Boldface indicates a standardized difference ≥ 10%, which is considered meaningful.
CAD, coronary artery disease; CHADS2, Congestive Heart Failure, Hypertension, Age ≥ 75, Diabetes, and Prior Stroke/Transient Ischemic Attack; CYP3A4, cytochrome P450 3A4; DOAC, direct oral anticoagulant; HAS-BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly (> 65 Years), Drugs and Alcohol; incl., including; INR, international normalized ratio; NSAIDs, nonsteroidal anti-inflammatory drugs; P-gp, P-glycoprotein; Std. Diff, standardized difference; TIA, transient ischemic attack.
A standardized difference ≥ 10% is considered meaningful.
Medical history 5 years prior to index date.
CHADS2 score estimates ischemic stroke risk in non-anticoagulated patients. Score awards 1 point each for congestive heart failure, hypertension, diabetes, and age ≥ 75 years, and 2 points for stroke/TIA/ thromboembolism.
HAS-BLED score estimates major bleeding risk for patients on anticoagulation. Score awards 1 point each for hypertension, abnormal renal function, abnormal liver function, history of stroke, bleeding or anemia, labile INR, age > 65 years, medication usage predisposing to bleeding, and alcohol use; labile INR was excluded as it is not monitored in these patients.
3-year look-back period for Charlson comorbidity index.
Co-medication use 120 days prior to index date.
Carbamazepine/ phenobarbital/ phenytoin/ rifampin.
Amiodarone/ clarithromycin/ cyclosporine/ diltiazem/ ketoconazole/ ritonavir/ verapamil.
Outcomes
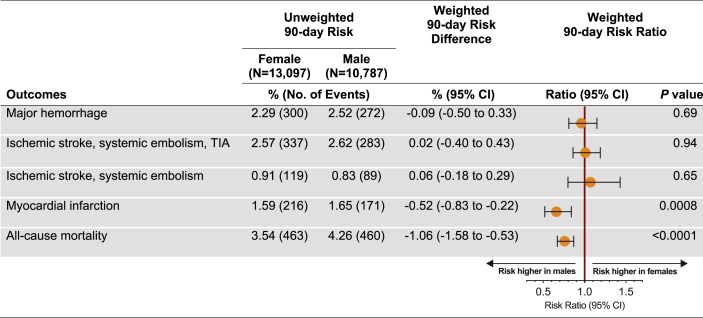
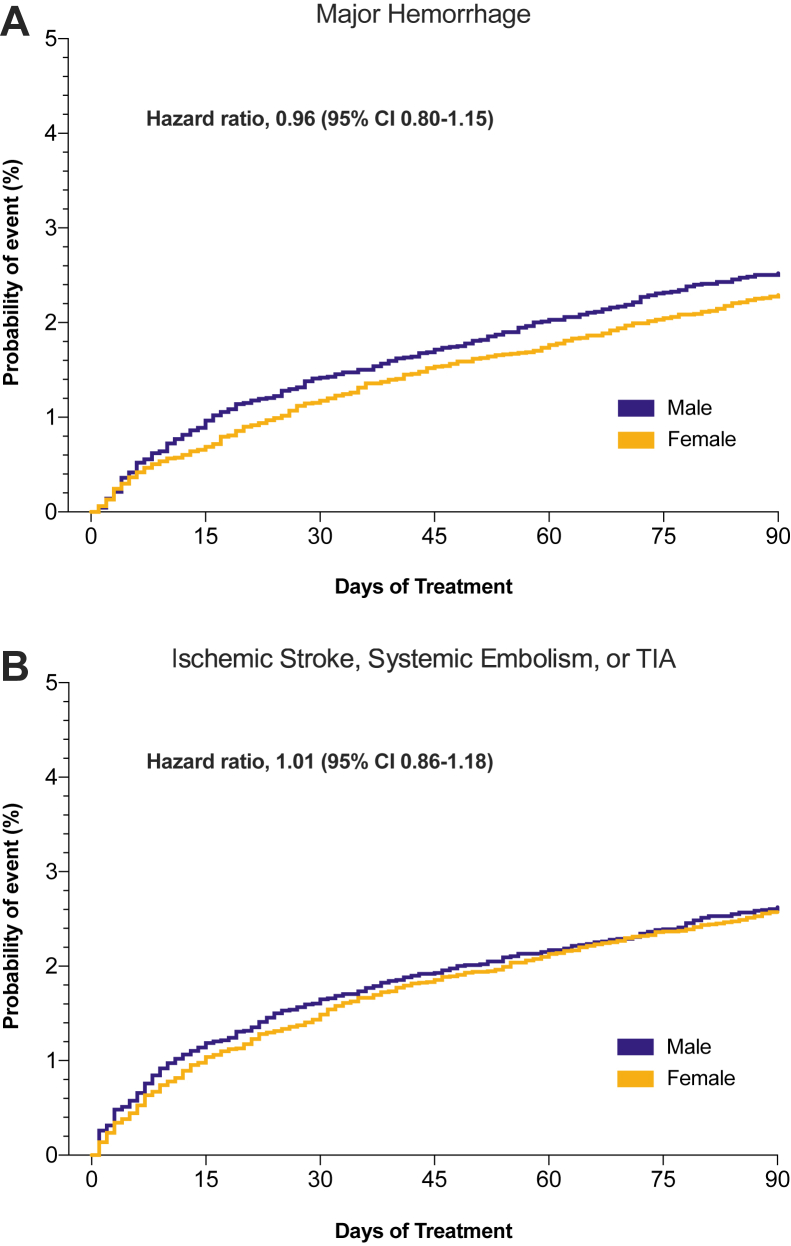
The unweighted numbers of events recorded through hospital encounters over a 90-day follow-up period are listed in Figure 2. After weighting, the risk for major hemorrhage associated with DOAC use in AF patients was similar for the 2 sexes (RR, 0.96; 95% CI, 0.81-1.15; females vs males). With regard to stroke prevention, there was no difference in the risk of ischemic stroke, SE, or TIA between female and male patients (RR, 1.01; 95% CI, 0.86-1.19), a finding that remained unchanged after excluding TIA (RR, 1.07; 95% CI, 0.80-1.43). Similarly, no sex-related differences in these outcomes were observed when repeating the above analyses adjusting for dosing regimen (Supplemental Table S6). Time-to-event analyses further corroborated our findings (Fig. 3), indicating comparable risks for major bleeding (hazard ratio [HR], 0.96; 95% CI, 0.80-1.15) and thromboembolic events (ischemic stroke, SE, or TIA; HR, 1.01; 95% CI, 0.86-1.18). Compared with males, females had a lower risk for MI (RR, 0.66; 95% CI, 0.52-0.84; P = 0.0008) and all-cause mortality (RR, 0.76; 95% CI, 0.67-0.87; P < 0.0001; Fig. 2). In a subgroup analysis, primary outcomes were compared between sexes after stratifying each DOAC according to dosing regimen. Risks for major bleeding and for stroke did not differ between females and males on apixaban and rivaroxaban prescribed in either normal or reduced doses (Fig. 4).
Figure 2.
Risk ratios for the study outcomes. Male sex was used as the reference. CI, confidence interval; TIA, transient ischemic attack.
Figure 3.
Weighted Kaplan-Meier cumulative probability plots for (A) major hemorrhage and (B) thromboembolic events. CI, confidence interval; TIA, transient ischemic attack.
Figure 4.
Risk ratios of primary outcomes stratified by pre-specified subgroups. Male sex was used as the reference. CI, confidence interval; TIA, transient ischemic attack.
Discussion
In this analysis of administrative healthcare data from 23,884 elderly patients with newly treated AF receiving routine care we assessed the association of sex with risks for bleeding or thromboembolic events of rivaroxaban and apixaban treatment using propensity score weights that produced well-balanced synthetic cohorts of females and males. Compared to males, females had a similar 90-day risk for major bleeding or stroke associated with a DOAC prescription, but they had a lower 90-day risk for MI and all-cause mortality, after differences in baseline risk factors were balanced.
To our knowledge, this is the first population-based study to perform a direct comparison between female and male patients prescribed apixaban or rivaroxaban, to determine the independent effect of sex on therapeutic and adverse outcomes. Previously, 2 population-based studies, one from Canada30 and one from the United States,31 assessed sex differences in dabigatran and rivaroxaban effectiveness and safety, but a different approach was taken, as comparison was made to another OAC treatment in AF patients of the same sex (Supplemental Table S1). Therefore, results from these studies cannot be compared to our findings.
In the current study, careful consideration was given to adjusting for covariates between female and male AF patients that may determine major bleeding or stroke risk in the context of apixaban or rivaroxaban prescription. Using propensity scores, weighted male and female synthetic cohorts were created with a well-balanced distribution of such baseline risk factors, including those considered sex-related, such as stroke risk (higher in females) and MI risk (higher in males).32 We did not include characteristics that represent well-characterized physiological differences between sexes, including hemoglobin level, serum creatinine level, and eGFR.33 Before weighting, the estimated baseline stroke risk (CHADS2 score) was higher in females, compared to males. This difference may be driven primarily by females being older and having a higher prevalence of hypertension. The remaining stroke risk factors were either similarly prevalent (congestive heart failure, history of stroke) or less frequent among females (diabetes). Although these sex-related differences are consistent with previous observations among AF patients,30,34,35 the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study (N = 13,559) found that females had a higher risk than males for AF-related thromboembolism at both younger and older ages that was independent of the presence of other risk factors for stroke.34 In terms of bleeding risk, the difference in HAS-BLED scores was not considered meaningful. After weighting, these known predictors for stroke and bleeding were equally distributed between the 2 cohorts.
Females with AF receiving anticoagulation treatment also were shown to have a higher stroke risk than men, even after adjustment for risk factors, as suggested by 2 prospective real-world studies, including the Global Anticoagulant Registry in the Field-AF (GARFIELD-AF) registry (N = 28,624)12,13 comprising predominantly patients on warfarin. We determined a comparable stroke risk between sexes among rivaroxaban and apixaban AF users in routine care. Previous studies are limited to secondary analyses of randomized controlled trial data, and they have reported conflicting results (Supplemental Table S1). Similar stroke risks for women vs men were reported in a subanalysis of apixaban and warfarin users in the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial and in a meta-analysis of large clinical trials, including those evaluating dabigatran, rivaroxaban, and apixaban.14,17 In contrast, a higher risk of stroke among women taking DOACs was reported in a subanalysis of the Apixaban Versus Acetylsalicylic Acid (ASA) to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment (AVERROES) trial and a more recent meta-analysis of 5 trials also including edoxaban.15,18 However, some of the aforementioned studies did not adjust for differences between the sexes in the baseline risk for stroke (ie, higher CHADS2 scores in women); thus, their findings may not reflect the independent effect of a patient’s sex but rather the result of differences in risk factors for stroke between women and men, revealing another critical aspect of treatment.
Our findings suggest comparable risks for females and males for major bleeding, thus mostly corroborating the findings from clinical trials indicating lower or similar risk in females compared to males.15, 16, 17, 18 This finding was further supported by subgroup analyses according to DOAC type (apixaban, rivaroxaban) and dosing regimen (standard, reduced) that did not show significant differences in major bleeding events between sexes, including for gastrointestinal and intracranial hemorrhage. Although females prescribed apixaban were reported to have about a 15% higher blood exposure, compared to males, after adjusting for dose, body weight, and renal function,20 this study suggests that the effect of sex on apixaban concentration does not translate into increased bleeding risk.
In this study, female patients had a 24% lower risk of all-cause death, compared with the risk for male patients. This finding coincides with results from the ARISTOTLE trial, which reported a 35% lower risk for all-cause mortality in females.14 However, this result is less likely related to sex differences in the effect of DOACs among AF patients than to an overall higher mortality rate in males, compared with that for females, that is seen in the general population of Canada.36 AF has been associated with an increased risk of death in both males and females,2 though the risk is stronger in females. According to a recent meta-analysis of cohort studies (N = 4,371,714), AF is associated with a 12% greater risk of all-cause mortality in females compared to males.37 To more appropriately determine the effect of sex on DOAC-associated mortality in AF patients, future studies require inclusion of data for similarly aged non-AF patients in their analysis, to control for sex differences in mortality rate within the general population. Similarly, the 34% adjusted lower risk of MI among females likely is not due to a sex-specific DOAC effect. Adjusting for baseline characteristics included risk factors for coronary artery disease; thus, differences in risk likely reflect the independent association of female sex with lower MI incidence reported in the general population.32
Dose reductions are recommended when prescribing rivaroxaban in patients with impaired renal function, and with apixaban prescription when patients meet at least 2 of the following criteria: impaired renal function, old age, and low body weight.21,22 Although we were not able to access weight data, female patients in our study were older, had a higher serum creatinine level, and had a lower eGFR, compared to those for men. With increased age, renal function declines, and this effect is even more pronounced among females.38 Accordingly, more females in our study received a reduced dose of rivaroxaban and apixaban, compared to the number of males (41.6% of females vs 30.3% of males), a difference previously reported for dabigatran users.30 Therefore, we also assessed the effect of sex on our primary outcomes, according to dosing regimen, in a subgroup analysis, which did not indicate sex differences in major bleeding or stroke for AF patients on apixaban or rivaroxaban with reduced or normal doses.
Although our study population was large, reflecting routine care-prescribing practices and highlighting the generalizability of our work, the following limitations should be acknowledged. Despite our use of a robust method to balance cohorts using propensity score weights, residual confounding still might have been present. This approach allowed us to maintain a large sample size, as opposed to a propensity score-matching method, which would have excluded about 75% of patients. DOAC exposure was determined based on filling of prescriptions, with no information available on therapy adherence. Due to the limited number of events, TIA was included as a primary outcome with ischemic stroke and SE, despite the potential for misclassification. However, our results were unchanged when the outcome was evaluated with TIA excluded. Similarly, bleeding events included, among other types, gastrointestinal and intracranial hemorrhage; however, exploratory results indicate no increased risks for these specific subtypes in females vs males (Supplemental Table S7). Lastly, considering the chronic nature of AF and the long-term use of these agents, a potential association of sex with these events after 90 days cannot be ruled out, owing to the relatively short duration of follow-up.
Our findings indicate similar 90-day risks for ischemic stroke and hemorrhage leading to an ED visit or hospitalization in older female and male AF patients prescribed apixaban or rivaroxaban. Accordingly, sex-dependent differential effects for these clinical outcomes were not observed in elderly AF patients using current dosing schemes in routine care.
Data Statement
Data access/access to data analysis protocol: The analysis was conducted by members of the ICES Kidney Dialysis & Transplantation (KDT) team at the ICES Western facility (London, Ontario). E.M. was responsible for the data analysis. The protocol can be obtained by emailing U.I.S. at ute.schwarz@lhsc.on.ca.
Acknowledgements
This study was supported by the ICES Western Site. Information was provided by the Canadian Institute for Health Information (CIHI). Analyses, conclusions, opinions, and statements are those of the authors and not those of CIHI. The authors thank IQVIA Solutions Canada Inc. for use of their Drug Information Database.
Funding Sources
This research was supported by the Canadian Institutes of Health Research (CIHR) Personalized Health Catalyst Grant (Application No. 385209; principal investigator, Ute Schwarz). In addition, Richard Kim was supported by the Wolfe Medical Research Chair in Pharmacogenomics at Western University, and Amit Garg was supported by the Dr Adam Linton Chair in Kidney Health Analytics and a CIHR Clinician Investigator Award. ICES is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). Core funding for ICES Western is provided by the Academic Medical Organization of Southwestern Ontario (AMOSO), the Schulich School of Medicine and Dentistry (SSMD), Western University, and the Lawson Health Research Institute (LHRI). The research was conducted by members of the ICES Kidney, Dialysis, and Transplantation team at the ICES Western facility. The opinions, results, and conclusions are those of the authors and are independent from the funding sources. No endorsement by ICES, AMOSO, SSMD, LHRI, CIHR, or the MOHLTC is intended or should be inferred. The study sponsors had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the article; or the decision to submit the article for publication.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The use of data herein was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require research ethics board review. This study followed the Reporting of Studies Conducted Using Observational Routinely Collected Data guidelines (Supplemental Table S2).
See page 63 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2021.09.002.
Supplementary Material
References
- 1.Wolf P.A., Dawber T.R., Thomas H.E., Jr., Kannel W.B. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study. Neurology. 1978;28:973–977. doi: 10.1212/wnl.28.10.973. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin E.J., Wolf P.A., D'Agostino R.B., et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 3.Heart and Stroke Foundation. Atrial fibrillation. Available at: https://www.heartandstroke.ca/heart/conditions/atrial-fibrillation. Accessed 2020.
- 4.Culebras A., Messe S.R., Chaturvedi S., Kase C.S., Gronseth G. Summary of evidence-based guideline update: prevention of stroke in nonvalvular atrial fibrillation: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82:716–724. doi: 10.1212/WNL.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hart R.G., Pearce L.A., Aguilar M.I. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 6.Lip G.Y.H., Banerjee A., Boriani G., et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest. 2018;154:1121–1201. doi: 10.1016/j.chest.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 7.Lip G.Y.H., Keshishian A., Li X., et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients. Stroke. 2018;49:2933–2944. doi: 10.1161/STROKEAHA.118.020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perreault S., de Denus S., White-Guay B., et al. Oral anticoagulant prescription trends, profile use, and determinants of adherence in patients with atrial fibrillation. Pharmacotherapy. 2020;40:40–54. doi: 10.1002/phar.2350. [DOI] [PubMed] [Google Scholar]
- 9.Friberg L., Benson L., Rosenqvist M., Lip G.Y. Assessment of female sex as a risk factor in atrial fibrillation in Sweden: nationwide retrospective cohort study. BMJ. 2012;344:e3522. doi: 10.1136/bmj.e3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikkelsen A.P., Lindhardsen J., Lip G.Y., et al. Female sex as a risk factor for stroke in atrial fibrillation: a nationwide cohort study. J Thromb Haemost. 2012;10:1745–1751. doi: 10.1111/j.1538-7836.2012.04853.x. [DOI] [PubMed] [Google Scholar]
- 11.Avgil Tsadok M., Jackevicius C.A., Rahme E., et al. Sex differences in stroke risk among older patients with recently diagnosed atrial fibrillation. JAMA. 2012;307:1952–1958. doi: 10.1001/jama.2012.3490. [DOI] [PubMed] [Google Scholar]
- 12.Camm A.J., Accetta G., Al Mahmeed W., et al. Impact of gender on event rates at 1 year in patients with newly diagnosed non-valvular atrial fibrillation: contemporary perspective from the GARFIELD-AF registry. BMJ Open. 2017;7:e014579. doi: 10.1136/bmjopen-2016-014579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poli D., Antonucci E., Grifoni E., et al. Gender differences in stroke risk of atrial fibrillation patients on oral anticoagulant treatment. Thromb Haemost. 2009;101:938–942. [PubMed] [Google Scholar]
- 14.Vinereanu D., Stevens S.R., Alexander J.H., et al. Clinical outcomes in patients with atrial fibrillation according to sex during anticoagulation with apixaban or warfarin: a secondary analysis of a randomized controlled trial. Eur Heart J. 2015;36:3268–3275. doi: 10.1093/eurheartj/ehv447. [DOI] [PubMed] [Google Scholar]
- 15.Lip G.Y., Eikelboom J., Yusuf S., et al. Modification of outcomes with aspirin or apixaban in relation to female and male sex in patients with atrial fibrillation: a secondary analysis of the AVERROES study. Stroke. 2014;45:2127–2130. doi: 10.1161/STROKEAHA.114.005746. [DOI] [PubMed] [Google Scholar]
- 16.Goodman S.G., Wojdyla D.M., Piccini J.P., et al. Factors associated with major bleeding events: insights from the ROCKET AF trial (rivaroxaban once-daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation) J Am Coll Cardiol. 2014;63:891–900. doi: 10.1016/j.jacc.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pancholy S.B., Sharma P.S., Pancholy D.S., et al. Meta-analysis of gender differences in residual stroke risk and major bleeding in patients with nonvalvular atrial fibrillation treated with oral anticoagulants. Am J Cardiol. 2014;113:485–490. doi: 10.1016/j.amjcard.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 18.Raccah B.H., Perlman A., Zwas D.R., et al. Gender differences in efficacy and safety of direct oral anticoagulants in atrial fibrillation: systematic review and network meta-analysis. Ann Pharmacother. 2018;52:1135–1142. doi: 10.1177/1060028018771264. [DOI] [PubMed] [Google Scholar]
- 19.Solms A., Frede M., Berkowitz S.D., et al. Enhancing the quality of rivaroxaban exposure estimates using prothrombin time in the absence of pharmacokinetic sampling. CPT Pharmacometrics Syst Pharmacol. 2019;8:805–814. doi: 10.1002/psp4.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byon W., Garonzik S., Boyd R.A., Frost C.E. Apixaban: a clinical pharmacokinetic and pharmacodynamic review. Clin Pharmacokinet. 2019;58:1265–1279. doi: 10.1007/s40262-019-00775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bristol-Myers Squibb Canada. Eliquis (apixaban) 2.5 mg and 5 mg product monograph. Available at: https://www.pfizer.ca/eliquis-apixaban. Accessed October 7, 2019.
- 22.Bayer. Xarelto (rivaroxaban) 10 mg, 15 mg and 20 mg product monograph. Available at: https://www.bayer.ca/omr/online/xarelto-pm-en.pdf. Accessed September 18, 2018.
- 23.Gulilat M., Keller D., Linton B., et al. Drug interactions and pharmacogenetic factors contribute to variation in apixaban concentration in atrial fibrillation patients in routine care. J Thromb Thrombolysis. 2020;49:294–303. doi: 10.1007/s11239-019-01962-2. [DOI] [PubMed] [Google Scholar]
- 24.Kubitza D., Becka M., Roth A., Mueck W. The influence of age and gender on the pharmacokinetics and pharmacodynamics of rivaroxaban—an oral, direct Factor Xa inhibitor. J Clin Pharmacol. 2013;53:249–255. doi: 10.1002/jcph.5. [DOI] [PubMed] [Google Scholar]
- 25.Keskar V., McArthur E., Wald R., et al. The association of anticoagulation, ischemic stroke, and hemorrhage in elderly adults with chronic kidney disease and atrial fibrillation. Kidney Int. 2017;91:928–936. doi: 10.1016/j.kint.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 26.Robins J.M., Hernan M.A., Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Xu S., Ross C., Raebel M.A., et al. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health. 2010;13:273–277. doi: 10.1111/j.1524-4733.2009.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 29.Austin P.C. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun StatSimul Comput. 2009;38:1228–1234. [Google Scholar]
- 30.Avgil Tsadok M., Jackevicius C.A., Rahme E., Humphries K.H., Pilote L. Sex differences in dabigatran use, safety, and effectiveness in a population-based cohort of patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2015;8:593–599. doi: 10.1161/CIRCOUTCOMES.114.001398. [DOI] [PubMed] [Google Scholar]
- 31.Shantha G.P.S., Bhave P.D., Girotra S., et al. Sex-specific comparative effectiveness of oral anticoagulants in elderly patients with newly diagnosed atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2017;10:e003418. doi: 10.1161/CIRCOUTCOMES.116.003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albrektsen G., Heuch I., Lochen M.L., et al. Lifelong gender gap in risk of incident myocardial infarction: the Tromso Study. JAMA Intern Med. 2016;176:1673–1679. doi: 10.1001/jamainternmed.2016.5451. [DOI] [PubMed] [Google Scholar]
- 33.Tamargo J., Rosano G., Walther T., et al. Gender differences in the effects of cardiovascular drugs. Eur Heart J Cardiovasc Pharmacother. 2017;3:163–182. doi: 10.1093/ehjcvp/pvw042. [DOI] [PubMed] [Google Scholar]
- 34.Fang M.C., Singer D.E., Chang Y., et al. Gender differences in the risk of ischemic stroke and peripheral embolism in atrial fibrillation: the AnTicoagulation and Risk factors In Atrial fibrillation (ATRIA) study. Circulation. 2005;112:1687–1691. doi: 10.1161/CIRCULATIONAHA.105.553438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomberg-Maitland M., Wenger N.K., Feyzi J., et al. Anticoagulation in women with non-valvular atrial fibrillation in the stroke prevention using an oral thrombin inhibitor (SPORTIF) trials. Eur Heart J. 2006;27:1947–1953. doi: 10.1093/eurheartj/ehl103. [DOI] [PubMed] [Google Scholar]
- 36.Rosella L.C., Calzavara A., Frank J.W., et al. Narrowing mortality gap between men and women over two decades: a registry-based study in Ontario, Canada. BMJ Open. 2016;6:e012564. doi: 10.1136/bmjopen-2016-012564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emdin C.A., Wong C.X., Hsiao A.J., et al. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies. BMJ. 2016;532:h7013. doi: 10.1136/bmj.h7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz J.B. The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clin Pharmacol Ther. 2007;82:87–96. doi: 10.1038/sj.clpt.6100226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.