Abstract
Background
The safety of drug use by nursing-home residents can be impaired by polypharmacy, potentially inappropriate medications (PIM), and neuroleptics, as well as by a lack of adequate interprofessional coordination in the nursing home. The goal of the HIOPP-3-iTBX Trial was to improve drug safety in nursing-home residents, including a reduction of PIM and/or neuroleptic use, by means of a complex interprofessional intervention.
Methods
This cluster-randomized, controlled trial was performed in nursing homes in Germany. Residents over age 65 were included in the trial. The intervention was carried out over six months and consisted of four elements: a drug review by trained pharmacists, educational sessions for general practitioners and nurses, a drug safety toolbox, and change management seminars for members of the three participating professions. The nursing homes in the control group continued to provide usual care. The primary endpoint was the prescription of at least one PIM and/or at least two neuroleptic drugs simultaneously. The secondary endpoints were the incidence of falls and hospitalizations, quality of life, and health-care costs. This trial is registered in the German Clinical Trials Registry (DRKS00013588).
Results
44 nursing homes with 862 residents were randomized, 23 of them (with 452 residents) to the intervention group and 21 (with 410 residents) to the control group. 41% of all nursing-home residents initially took at least one PIM and/or at least two neuroleptic drugs simultaneously. Follow-up data (including, among other things, the current drug regimen) were obtained for 773 residents. The intention-to-treat analysis continued to show no difference between the intervention group and the control group with respect to the primary endpoint.
Conclusion
This trial of an intervention to improve drug safety in nursing homes led neither to reduced prescribing of PIM and/or neuroleptic drugs, nor to any improvement in the overall health status of the nursing-home residents.
The majority of nursing-home residents are exposed to polypharmacy (concurrent use of five or more different medications) (1). Polypharmacy is associated with an increased risk for adverse drug events (ADEs), more falls, higher hospitalization rates, and higher risk of mortality (2). In addition, nursing-home residents are far more likely to use potentially inadequate medications (PIM) compared to elderly people living at home (3). In conjunction with a fragile physical disposition (for example, frailty), PIM are associated with a particularly high risk for ADEs (4, 5). Psychopharmacological drugs (including antidepressants, neuroleptic drugs, and benzodiazepines) also merit specific attention. Approximately 50% of nursing-home residents receive neuroleptic drugs (6) that have been shown to be associated with cardiovascular events and hip fractures, as well as well with higher all-cause mortality (7, 8).
Drug management poses a challenge not only with regard to polypharmacy and the prescription of PIM/neuroleptic drugs, but also in terms of the collaboration between the professionals involved (9). To date, there has been little instruction on interprofessional drug management; recommendations, such as the use of a “drug therapy safety card” by nursing staff, are profession-specific (10). Structured pathways of collaboration and information sharing are not mandatory for general practitioners, physicians in other specialties, pharmacists, or nurses, nor are medication reviews required (11).
Efforts have been made meanwhile to improve drug therapy safety for nursing-home residents, and to demonstrate their effects in randomized controlled trials (RCTs). Three reviews carried out in the years 2010–2012 of the handful of RCTs conducted up to that time found evidence that while effective collaboration or medication reviews can reduce inadequate prescribing, this produced no health benefits (12– 14). According to Alldred et al., the available evidence is based on studies of insufficient methodological quality and, therefore, does not permit reliable conclusions to be drawn (14). More recently, additional cluster-randomized studies (cRCTs) from the European area have been published (15– 17), demonstrating optimization of medication in nursing-home residents, although two studies found no improvement in the clinical endpoints (15, 16). The COSMOS study (17), in contrast, showed an improvement in quality of life as well as activities of daily living functions among participants.
The HIOPP-3-iTBX study (HIOPP, primary care initiative to optimize patient safety in polypharmacy; iTBX, interprofessional toolbox) is the first cRCT on medication optimization in nursing homes in Germany (18). Preliminary studies (19– 21) used a variety of interventional elements (medication reviews, further training for nursing staff, physicians, and pharmacists, as well as a toolbox). These were further developed as part of the project and supplemented with moderated change management seminars for the three professional groups involved.
The aim of this study was to increase drug therapy safety by reducing the proportion of nursing-home residents using ≥ one PIM and/or ≥ two neuroleptic drugs through complex, multiprofessional interventions, including medication reviews.
Methods
Study design
Between May 2018 and July 2019, a cluster-randomized controlled intervention study was conducted in nursing homes. In a first step, four centers (the Institutes for General Practice in Düsseldorf, Hannover, Rostock, and Tübingen) recruited nursing homes with care agreements according to § 72 of Book XI of the German Social Code (SGB). Each home formed a cluster, meaning that the residents were either fully in the invention arm or fully in the control arm. This was to prevent contamination occurring between intervention and routine measures.
Study participants
Firstly, all nursing homes in the Hannover, Düsseldorf, and Tübingen regions, as well as in the federal state of Mecklenburg-Western Pomerania, were contacted. All treating general practitioners and pharmacists in the interested nursing homes were approached. Only if a multiprofessional team could be formed from these professionals were the residents of the respective nursing home approached. The inclusion criterion for participants was age ≥ 65 years. Exclusion criteria included: no consent from the resident or their legal guardian, short-term care, and a life expectancy of less than 6 months. Detailed information on the recruitment process can be found in Kirsch et al. (22).
Complex intervention
The multiprofessional HIOPP-3-iTBX intervention comprised elements I–IV shown in the Box.
BOX. The four elements of the HIOPP-3-iTBX intervention.
| ● Medication review | ● Toolbox |
| – By pharmacist | – Discharge tool, ward round tool |
| – ABDA type 2b | |
| – One-off | – AMTS card, Priscus list, treatment monitoring |
| ● Further training | ● Change management |
| – Pharmacists | – Three sessions per home |
| – Physicians | – Interprofessional |
| – Nursing staff |
ABDA, Federal Association of German Pharmacists (Bundesvereinigung Deutscher Apothekerverbände); AMTS, drug therapy safety (Arzneimitteltherapiesicherheit)
The first of these elements was the mandatory pharmaceutical medication review (23). The pharmacists supplying the nursing homes performed a one-off review for each nursing-home resident in the intervention group. To this end, they used the German ATHINA assessment criteria (drug therapy safety in pharmacies) (24) in the context of a more advanced medication analysis (type 2b). The pharmacists then faxed a form with their recommendations (Med-Check fax) to the responsible general practitioners, who decided on treatment changes.
The second element consisted of training sessions or continuing education. All pharmacists were required to participate in the ATHINA advanced training program organized by the German Chambers of Pharmacists, where they learned the requirements for the medication review, as well as a 1-day study-related training session on the problems of medications in nursing-home residents. The advanced training programs for general practitioners and nursing staff were optional. General practitioners could take part in a 2-h group training session on the topic of “Drug therapy safety in nursing homes” or, alternatively, a personal training session (in person/by telephone). Nursing staff were offered two training sessions on frequent medication-specific adverse effects in nursing homes.
The third intervention element comprised the interprofessional toolbox: a collection of materials to promote collaboration as well as to be used to look up drug-related risks.
Finally, all professionals affiliated to nursing homes in the intervention group attended three change management seminars over the course of the intervention.
Professionals in the control group did not receive any measures. Detailed information on the complex intervention can be found in eMethods Section 1 and the eTable.
eTable. HIOPP-3-iTBX study: a pragmatic complex intervention comprising four elements to increase drug therapy safety in nursing homes.
| Who/what | Content and procedure | Framework | How often | Mandatory |
| 1. Medication review | ||||
| Pharmacists | ATHINA medication review | Duration approximately 1 h per resident | One-off | Yes |
| 2. Training programs/further training | ||||
| Pharmacists | ATHINA training program | 16 Hours | One-off | Yes |
| HIOPP training program | 8 Hours | One-off | Yes | |
| Physicians | Training program Medication management and PIM/neuroleptic drugs in nursing homes | 2-Hour group session; individual training session in the practice or by telephone | One-off | No |
| Nursing staff |
Two further training sessions a) Recognizing adverse drug events b) Managing agitated patients/own role in the prescription of neuroleptic drugs |
Approximately 15 min | Twice | No |
| 3. Toolbox | ||||
| Med-Check fax | Pharmacist sends recommendations to general practitioner; general practitioner transmits any necessary instructions to the nursing home | Joint use by pharmacist and general practitioner with transmission to the nursing staff | One-off | Yes |
| Drug therapy safety (AMTS) card with additional info card for nursing staff | Essential content of geriatric drug therapy safety at a glance | Provision of information for nursing staff, general practitioners, and pharmacists | Continuous | No |
| PRISCUS list | List of potentially inadequate medications for older patients and treatment alternatives | Provision of information for nursing staff, general practitioners, and pharmacists | Continuous | No |
| Treatment monitoring sheet | Structured guide to recognizing and documenting new health complaints | Nursing staff | Continuous | No |
| Hospital discharge tool | Information from the home for the general practitioner after hospital discharge, feedback from general practitioner possible | General practitioners, nursing staff | Upon hospital discharge | No |
| Ward round tool | Sheet for structured recording of decisions taken during a joint round | General practitioners, nursing staff, pharmacists | On ward rounds | No |
| 4. Change management | ||||
|
Interdisciplinary – Pharmacists – Physicians – Nursing staff |
1. Kick-off session – Current situation and analysis of strengths/weaknesses – Jointly agreed changes in medication management: plan of action – Presentation of the toolbox 2. Half-time session – Feedback on implementation and forwarding of medication reviews – Feedback on initial changes: obstacles and opportunities – Fresh motivation 3. Closing session – Experiences: useful/effective versus short-term/ineffective change – Consolidation in routine care – Boundary conditions required for change |
2 Hours 1 Hour 1 Hour |
Three sessions | No |
“AMTS,” German interventional studies on drug therapy safety; ATHINA, drug therapy safety in German pharmacies; HIOPP, German primary care physicians’ initiative to optimize patient safety in polypharmacy; PIM, potentially inadequate medications
Endpoints
The primary endpoint was the proportion of nursing-home residents that were using at least one PIM and/or at least two neuroleptic drugs 6 months following the baseline survey. Secondary endpoints related to the following variables: fall incidence, hospitalizations, quality of life, and health economic outcomes.
Data collection
Study personnel carried out two surveys in nursing homes in both the intervention arm and in the control arm: one shortly before the intervention (T0) and one at the end of the intervention phase at 6 months (T1). In addition to patients’ current medication plans, the following were collected from the nursing records for the preceding 6 months: fall events, diagnoses, number and duration of hospitalizations, and uptake of other health services. Furthermore, using an extensive list of symptoms (20 items), possible adverse drugs events in nursing-home residents were recorded either from the residents themselves or based on third-party accounts. The EQ-5D-3L questionnaire was used to determine health-related quality of life.
Randomization
Nursing homes were randomized to the intervention or to the control arm by an independent person using block randomization with stratification by centers and number of subjects per home and were included parallel in time. Blinding was not possible due to the design of the intervention. Further details on the methodology of the study can be found in the eMethods Section.
Results
Study participants
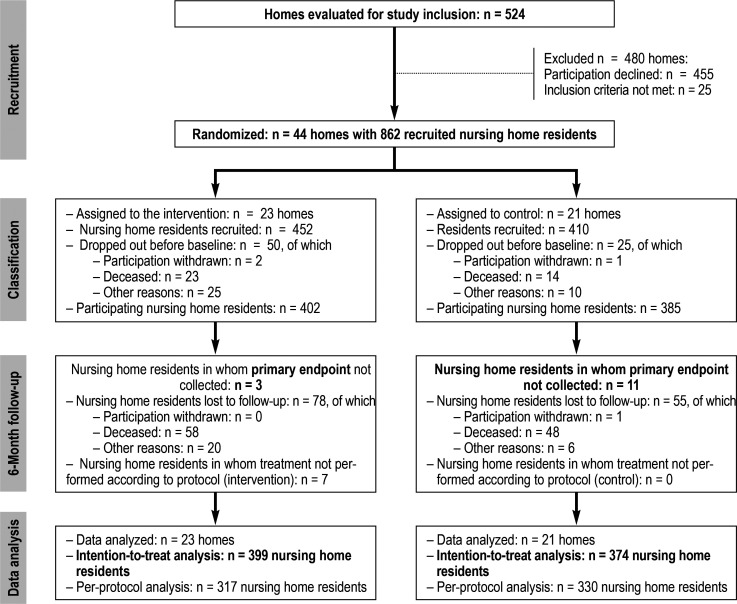
Of the 524 nursing homes contacted in the four regions, 69 agreed to participate (for details see [22]). Ultimately, 44 nursing homes with 862 residents were randomized. A total of 23 nursing homes with 452 participants were assigned to the intervention group (IG) and 21 nursing homes with 410 participants to the control group (CG). In all, 787 residents could be surveyed for the baseline assessment (402 IG, 385 CG). The follow-up assessment could not be performed in a total of 133 residents (78 in the IG and 55 in the CG). Nevertheless, the medication plan was recorded as the primary endpoint in 119 of these subjects that dropped out, meaning that 773 participants (399 in the IG and 374 in the CG) were included in the intention-to-treat analysis (ITT). In addition to the subjects that dropped out, it was also not possible to carry out the intervention according to the protocol in seven residents, meaning that altogether 647 residents (317 in the IG and 330 in the CG) were included in the per-protocol analysis (PP) (figure).
Figure.
CONSORT flow diagram from the HIOPP-3-iTBX study
Patient characteristics
Table 1 shows the baseline survey (787 residents) in detail. Half of residents had been living in their nursing home for at least 29 months. The average age was 84 years and residents were predominantly female. Two thirds of residents were deemed to be suffering from dementia (according to the judgment of nursing staff, medical records, or the general practitioner). Approximately 80% of residents were moderately to severely impaired in terms of the Barthel index for activities of daily living. On average, general practitioners reported seven of 36 predefined chronic disorders for their patients. The most frequent diagnoses included arterial hypertension, dementia, osteoarthritis, heart failure, renal failure, dyslipidemia, depression, and diabetes mellitus. In all, 50% of residents used 11 or more prescription medications (including on-demand medication), thereby exhibiting excessive polypharmacy. A total of 41% of participants concurrently used at least one PIM and/or at least two neuroleptic drugs. Intervention patients did not differ significantly from control patients in terms of characteristics (table 1).
Table 1. Characteristics of nursing home residents (NHR) at baseline (T0).
| Total (N = 787) | Intervention (N = 402) | Control (N = 385) | |
| Ø Age in years (SD) | 84.3 (± 7.7) | 84.7 (± 7.7) | 83.9 (± 8.1) |
| NHR, female, % | 73.8 | 76.4 | 71.2 |
| NHR with legal guardianship, % | 33.1 (19 missing) | 32.6 (9 missing) | 33.6 (10 missing) |
|
NHR with educational status*1, % – Low – Medium – High – Not specified |
45.4 30.5 7.5 16.6 |
46.8 30.8 9.0 13.4 |
43.9 30.1 6.0 20.0 |
|
Nursing home stay in months, – Median (IQA) |
29 (13–53) (6 missing) |
29 (13–52) (2 missing) |
28 (12–54) (4 missing) |
|
NHR with care level, % – None – 1 and 2 – 3 and 4 – 5 – Not specified |
0.4 22.0 64.7 12.6 0.3 |
0 20.0 66.3 13.8 0 |
0.8 24.1 63.1 11.4 0.5 |
| Ø Number of diagnoses (SD) | 7.4 (± 3.5) (34 missing) |
7.7 (± 3.3) (24 missing) |
7.0 (± 3.7) (10 missing) |
|
Barthel Index NHR (U50.0–50), % – None, mild (U50.0–10) – Moderate, moderately severe (U50.20–30) – Severe, very severe (U50.40–50) |
17.5 44.4 38.1 (3 missing) |
18.0 44.9 37.1 (3 missing) |
16.8 43.9 39.3 |
| NHR with signs of dementia*2, % | 64.5 (1 missing) |
65.6 (1 missing) |
63.4 |
| Ø Number of drugs prescribed (SD) | 11.1 (± 4.5) | 11.7 (± 4.7) | 10.5 (± 4.1) |
| NHR receiving ≥ 1 PIM and/or ≥ 2 neuroleptics, % | 41.3 | 42.0 | 40.5 |
*1 Educational status according to CASMIN (25)
*2 Sign of dementia: identified by nursing home or general practitioner
Ø, Average; IQA, interquartile range; PIM, potentially inadequate medications; SD, standard deviation
Primary endpoint
For the follow-up investigation, the most recent medication plans of 773 nursing-home residents (399 in the IG and 374 in the CG) were evaluated. The percentage of residents using at least one PIM and/or at least two neuroleptic drugs did not differ between the two groups (table 2); the percentage in the IG was 40.6% and in the CG 40.4%. In mixed model 1, the homes were accounted for as a random effect; in model 2, the variables age, sex, dementia, number of illnesses, and use of ≥ one PIM and/or ≥ two neuroleptic drugs were additionally included at baseline. Even after adjusting for these variables, no differences were found for the primary endpoint. A sensitivity analysis with the “per protocol” population also yielded no significant differences (table 2).
Table 2. Effects of the intervention on the use of PIM and neuroleptic drugs at 6 months following inclusion.
| Study participants | Nursing home residents with ≥ 1 PIM and/or ≥ 2 neuroleptics |
Mixed model 1*1
OR [95% CI] (unadjusted) (p) |
Mixed model 2*2
OR [95% CI] (adjusted) (p) |
|
|
Intervention group
n (%) |
Control group
n (%) |
|||
| ITT (n = 773) | 162 (40.6 %) |
151 (40.4 %) |
1.05 [0.68; 1.62] (p = 0.836) |
0.90 [0.55; 1.46] (p = 0.762) (32 missing) |
| PP (n = 647) | 130 (41.0 %) |
133 (40.3 %) |
1.04 [0.64; 1.69] (p = 0.864) |
0.78 [0.42; 1.42] (p = 0.409) (6 missing) |
*1 The homes are included in the analysis as a cluster or random effect. ICC for ITT = 0.073; ICC for PP = 0.089
*2 The homes are included in the analysis as a cluster or random effect. Adjusting variables include: age, sex, dementia, number of diseases, use of ≥ 1 PIM and/or ≥ 2 neuroleptic drugs at baseline
IICC, intraclass correlation; ITT, intention-to-treat analysis; OR, odds ratio; PIM, potentially inadequate medications; PP, per-protocol analysis; 95% CI, 95% confidence interval
Secondary endpoints
Secondary endpoints were recorded in the follow-up assessment by survey and using the documentation from the nursing home records. Since full follow-up was not possible in 140 nursing-home residents, an evaluation of the per-protocol population was carried out.
Over the 6-month observation period, 39% of residents in the intervention arm and 30% of residents in the control arm experienced at least one fall. The results of the mixed ordinal logistic models clearly show that allocation to the intervention or to the control group had no effect on the number of fall events. Similarly, there were no significant differences in terms of the number of hospitalizations or the proportion of nursing-home residents requiring emergency or rescue services on at least one occasion in the preceding 6 months. Participants in the intervention arm also showed no notably different quality of life (table 3). The overall costs will be presented in a separate publication.
Table 3. Effects of the intervention on fall events, hospitalizations, use of emergency services, and quality of life in nursing home residents.
| Nursing home residents (N) | Intervention group | Control group | Mixed model 1 unadjusted [95% CI] (p) | Mixed model 2adjusted [95% CI] (p) |
| Average number of falls per nursing home resident in the preceding 6 months, (SD) (N = 539) | 0.7 (± 2.1) | 0.5 (± 1.6) | OR = 1.30 [0.78; 2.19] (p = 0.319) |
OR = 0.92 [0.45; 1.88]*1 (p = 0.811) (1 missing) |
| Average number of hospitalizations per nursing home resident in the preceding 6 months, (SD) (N = 645) | 0.4 (± 0.7) | 0.3 (± 0.6) | OR = 1.28 [0.89; 1.85] (p = 0.188) |
OR = 1.39 [0.92; 2.10]*2 (p = 0.115) (9 missing) |
| Rate of residents requiring emergency or rescue services at least once in the preceding 6 months (N = 645) |
24.3 % (N = 77) |
19.2 % (N = 63) |
OR = 1.27 [0.78; 2.099 (p = 0.336) |
OR =1.37 [0.84; 2.27] *3 (p = 0.206) |
| Mean of quality of life questionnaire EQ-5D-3L, (SD) (N = 583)*4 |
0.54 (± 0.30) | 0.53 (± 0.31) | β = 0.0056 [− 0.0503; 0.6167] (p = 0.842) |
β = 0.0007 [− 0.0513; 0.0527]*4 (p = 0.979) (51 missing) |
The homes are included in the analysis as a random effect.
*1 Adjusted variables: age, sex, and number of falls 6 months prior to baseline
*2 Adjusted variables: age, sex, and hospitalizations 6 months prior to baseline survey, number of diseases
*3 Adjusted variables: age, sex, and number of call-outs 6 months prior to baseline
*4 Adjusted variables: age, sex, EQ-5D-3L at baseline
ß, Regression coefficient; OR, odds ratio; SD, standard deviation; 95% CI, 95% confidence interval
Discussion
The complex intervention used in the HIOPP-3-iTBX study to increase drug therapy safety for nursing-home residents consisted of a mandatory medication review and the optional elements of further training, tools to increase awareness of drug therapy safety and to overcome interprofessional/intersectorial boundaries, as well as change management seminars to optimize collaboration. In the intervention nursing homes, these measures resulted neither in less prescribing of PIM and/or neuroleptic drugs nor in a measurable improvement in the health status of residents.
Medication management in nursing homes is an elaborate process: it begins with establishing the indication for a medication and ends with its use by the nursing-home residents, as well as with health checks. In addition to residents and their relatives, this process involves general practitioners, other medical specialists (for example, neurologists, psychiatrists, and urologists), pharmacists, and nurses, and possibly also additional centers, such as service providers who package medications in blister packs for patients on an individual basis (26). Also, nursing-home residents have free choice of physician, leading to a large amount of contact between carers and various (primary care) physicians (9). Furthermore, care culture and boundary conditions, such as nursing staff resources and drug discount contracts, affect the process (9).
The complex HIOPP-3-iTBX intervention was developed and elaborated in preliminary studies taking these factors into account. Nevertheless, this study was unable to demonstrate a relevant effect. A number of limiting factors already became evident during the study. As also observed in the preliminary AMTS I and AMTS-Ampel studies (19, 20), some general practitioners had difficulty complying with the HIOPP-3-iTBX intervention in all its aspects. For example, the participation rate among physicians at the three consecutive change management seminars fell to 46%, whereas the final seminar was still attended by 60% of the nursing staff originally present and 75% of pharmacists. It is possible that this situation was contributed to by the increasing work demands on primary care practices due to a lack of general practitioners, as well as by the prioritization of tasks within practices (27).
In addition, a comparatively low rate of adoption by physicians of recommendations made by pharmacists was observed. For example, written feedback was provided by general practitioners for 239 of the 360 medication reviews carried out by pharmacists in the intervention group. The pharmacists initially proposed that 939 (32.4%) of the medications reviewed required optimization. The general practitioners responded to 590 proposals: they accepted recommendations for 153 medications (25.9%), wanted to potentially respond at a later point regarding 176 medications (29.8%), and made no changes at all for 349 proposals.
Whereas pharmacists focus on drug therapy safety, general practitioners additionally need to consider other factors such as clinical need in the absence of better alternatives, as well as the expectations of residents and/or their relatives, nursing home staff, and co-treating specialists. However, experience gained in local initiatives shows that direct contact and personal collaboration between physicians and pharmacists can lead to greater acceptance of their mutual expertise (28). Ultimately, it is the vulnerable and changing health status of the nursing-home residents themselves that consistently prompts changes in medication. The extent to which an initially one-off medication review can have a sustained effect under these ever-changing conditions is unclear.
Review articles assess with caution the effects on optimizing home medication due to the variability and quality of outcomes in two respects: on the one hand one sees indications of improved quality of medication, and on the other virtually no effects on the health status of residents. Recently published randomized controlled studies from Europe (15– 17) confirm these previous findings. In contrast to our study, they demonstrate improved drug therapy safety. However, the medication endpoints relate to the reduction in the number of medications, PIM, or PIM and potentially underprescribed medications. Only the COSMOS study (17) specifically deals with psychotropic drugs, with no sustained change being apparent, much like the current PROPER study, in which the reduction in psychotropic drugs in the nursing home was the primary objective (29). Positive effects on health status are demonstrated only in the COSMOS study in terms of quality of life, activities of daily living (ADL) function, and mental health symptoms (agitation). In addition to a medication review, the intervention also included non-pharmacological measures. It is possible that for nursing-home residents in particular, a comprehensive intervention approach of this kind in combination with drug therapy safety management is effective.
Strengths and limitations
With 862 residents recruited from 44 nursing homes, the requisite high number of cases was achieved despite the prevailing challenges. However, it is possible that there may have been an unintentional selection of the primarily interested nursing homes, pharmacists, and general practitioners.
Attention was paid to ensure that the HIOPP-3-iTBX study adhered to the principles of a pragmatic intervention under routine practice conditions, as also reflected in the realistic further training times. Therefore, although it is not possible to demonstrate maximum potential effectiveness, the “real” effectiveness that can be expected in the routine care setting can be shown (30). This has once again highlighted how challenging it is to implement a complex intervention that requires harmonious multiprofessional communication and collaboration under the prevailing conditions of fragmentation and delineation of tasks in medication management (26, 28, 31).
The selected 6-month duration of follow-up can be evaluated critically on the one hand with regard to demonstrating effectiveness. It is possible that at the time of the follow-up survey the complex interventions had not yet been consolidated. However, the generally high mortality rate among nursing-home residents and higher study costs were arguments against a longer follow-up. Comparable nursing home studies have follow-up periods of between 6 weeks and 24 months (14– 17). On the other hand, the primary endpoint chosen by us, “reduction in PIM and neuroleptic drugs,” was possibly not tailor-made for the broad-based intervention. A recommendation that has since been published on the selection of endpoints to demonstrate the effectiveness of medication reviews suggests, moreover, that too little attention has been paid to the expectations of elderly patients in previous medication studies (32).
Supplementary Material
eMethods section 1
More detailed information on the complex intervention
ATHINA medication review
The ATHINA medication review is the product of a further training program for pharmacists offered and coordinated by the chambers of pharmacists in a number of federal states of Germany. The medication review is structured and is aimed at increasing the effectiveness of drug therapy and minimizing drug risks. Pharmacies usually undertake a broader type 2a medication review (medication data and patient medical history). To review the medication of nursing home residents in the HIOPP-3-iTBX study, pharmacists used the type 2b medication review (medication data and clinical data).
The reviews in the nursing homes were based on the residents’ medication plans (medication use, long-term and on-demand medication, times at which medications were taken, and dose) as well as obtaining both personal and clinical data (patient age, sex, long-term diagnoses, list of symptoms, laboratory values [such as sodium, potassium, and glomerular filtration rate]).
Based on these date, the pharmacists assessed the presence of the following potential problems for each medication:
The medication plan was then faxed to general practitioners, together with the Med-Check Fax including the pharmacists’ recommendations on medication. The physicians assessed the pharmacists’ recommendations and, where appropriate, made changes to the drug therapy of the nursing home residents.
ATHINA training program
The ATHINA training program comprises a 2-day seminar that provides a basic understanding of drug therapy safety as well as a structured approach to carrying out a medication review. Attendees work through patient cases taken from routine practice and learn the working methods. The pharmacists, guided by a tutor with an additional clinical pharmaceutical qualification, go on to present at least four patient cases.
HIOPP further training (ATHINA-Plus)
ATHINA-Plus is a standardized further training program for pharmacists supplying nursing homes developed as part of the HIOPP-3-iTBX project. Aspects of geriatric pharmacy (falls, delirium, somnolence, pain; laboratory values, whether drugs can be administered via enteral feeding tube) and potentially inadequate medication, in particular with regard to neuroleptic drug treatment. In addition, tools/instruments to identify drug-related problems, as well as for interdisciplinary communication in the nursing home setting, are presented.
Physician and nurse training
Exchange of experience in the problems of drug therapy safety in nursing homes, recognizing potentially inadequate medications (using corresponding instruments/tools). Other areas of focus: neurolpetic drugs in the nursing home, presenting tools/instruments to identify drug-related problems and promote interdisciplinary communication in the nursing home setting.
The eTable presents additional components of the toolbox and change management.
Indication cannot be derived from the long-term diagnoses
Negative benefit:risk ratio (according to the PRISCUS list)
Potentially relevant drug interaction
Potentially relevant disease–drug interaction
Duplication of prescriptions
Potentially inadequate in older patients (as per the PRISCUS list)
Possible relevant adverse effects
Inadequate mode of application/administration
Check possible underdosing
Check possible overdosing
Contraindication
Check treatment duration
Other reasons
eMethods section 2
Additional details on methodology
Sample size determination
Sample size determination was based on the assumption that 50% of nursing home residents receive at least one PIM and/or at least two neuroleptic drugs (18). A reduction in the proportion of participants using this medication from 50% to 30% was considered clinically important. At an intracluster correlation (ICC) of ρ = 0.1, a cluster size of n = 5 NHR, an α error of 0.05, and a power of 80%, 632 nursing home residents were required to detect this difference (two-sided χ² test). Taking into account an annual mortality rate of approximately 30% as well as possible withdrawals from participation, a drop-out rate of 20% was assumed, meaning that 760 residents in 32 nursing homes needed to be recruited.
Data quality management
Data were recorded locally at the centers using the web-based data capture system secuTrial® and saved in a central ORACLE database. To this end, on-site training sessions on data capture were held for those involved.
Statistical methods
To evaluate the endpoints, mixed models were used in which residence in the respective home was entered as a random effect. To analyze the primary endpoint, a mixed logistic regression model was used. Here, all subjects were included in the analysis for whom the primary endpoint was recorded, even if they dropped out of the study or did not receive the intervention (intention-to-treat population). For the secondary endpoints “number of falls” and “number of hospitalizations,” mixed ordinal logistic regression models were used, while for the endpoint EQ5D, a mixed linear regression model was used. To analyze the secondary outcomes, as well as for sensitivity analyses, the per-protocol population, which included all subjects in whom the study was carried out according to the protocol, was used. Analyses of the secondary endpoints were exploratory and performed using STATA/SE Version 16.1.
Acknowledgments
Translated from the original German by Christine Rye.
Funding and registration
The HIOPP-3-iTBX study was funded by the Innovation Fund of the Joint Federal Committee (Grant No.: 01VSF16017). The Ethics Committee of the Hannover Medical University approved the Masters Ethics Application (No. 7655). The study was registered in the German Register of Clinical Trials (DRKS00013588).
Members of the HIOPP-3-iTBX study group
Dr. Simone Bernard, Prof. Petra Thürmann, Philipp Klee Institut für Klinische Pharmakologie, Universität Witten/Herdecke; Stefanie Kortekamp, Evangelische Hochschule Rheinland-Westfalen-Lippe; Angela Fuchs, Prof. Achim Mortsiefer, Prof. Stefan Wilm, Institut für Allgemeinmedizin, Heinrich-Heine Universität Düsseldorf; Dr. Anja Wollny, Lisa Sparenberg, Franziska Rebentisch, Prof. Attila Altiner, Institut für Allgemeinmedizin, Universitätsklinik Rostock; Regina Stolz, Dr. Hannah Haumann, Heidi Riescher, Prof. Stefanie Joos, Institut für Allgemeinmedizin und Interprofessionelle Versorgung, Universitätsklinik Tübingen; Birgitt Wiese, Prof. Ulrike Junius-Walker, PD Dr. Olaf Krause, Dr. Ina-Merle Doyle, Claudia Kirsch, Svetlana Usacheva, Trang Le, Silke Mamone, Prof. Nils Schneider, Institut für Allgemeinmedizin, Medizinische Hochschule Hannover; Dr. Antje Freytag, Institut für Allgemeinmedizin, Universitätsklinikum Jena; Dr. Thomas G. Grobe, Jona Frasch, Mike Klora, Sina Weinand, aQua-Institut für angewandte Qualitätsförderung und Forschung im Gesundheitswesen GmbH, Göttingen; Christian Günster, WIdO Institut, Berlin.
Acknowledgments
We would like to thank all participating nursing homes as well as participating nursing-home residents and their carers. We would also like to thank all nursing staff in the homes, as well as all nursing services, general practitioners, medical assistants, and pharmacists for their participation. Our thanks also go to Julia Fabricius, Magdalene Linz, Carina John, Patrick Schäfer, and Christian Gillot from the regional chambers of pharmacists in Lower Saxony, North Rhine-Westphalia, Baden-Württemberg, and Mecklenburg-Western Pomerania for their tremendous support of the project.
Data sharing statement
Based on the informed consent statements provided by the nursing-home residents, the data can be reported in aggregate form only.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Hoffmann F, Boeschen D, Dörks M, Herget-Rosenthal S, Petersen J, Schmiemann G. Renal insufficiency and medication in nursing home residents—a cross-sectional study (IMREN) Dtsch Arztebl Int. 2016;113:92–98. doi: 10.3238/arztebl.2016.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moßhammer D, Haumann H, Mörike K, Joos S. Polypharmacy—an upward trend with unpredictable effects. Dtsch Arztebl Int. 2016;113:627–633. doi: 10.3238/arztebl.2016.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endres HG, Kaufmann-Kolle P, Steeb V, Bauer E, Bottner C, Thürmann P. Association between potentially inappropriate medication (PIM) use and risk of hospitalization in older adults: an observational study based on routine data comparing PIM use with use of PIM alternatives. PLoS One. 2016;11 doi: 10.1371/journal.pone.0146811. e0146811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedna K, Hakkarainen KM, Gyllensten H, Jonsson AK, Petzold M, Hagg S. Potentially inappropriate prescribing and adverse drug reactions in the elderly: a population-based study. Eur J Clin Pharmacol. 2015;71:1525–1533. doi: 10.1007/s00228-015-1950-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cullinan S, O‘Mahony D, O‘Sullivan D, Byrne S. Use of a frailty index to identify potentially inappropriate prescribing and adverse drug reaction risks in older patients. Age Ageing. 2016;45:115–120. doi: 10.1093/ageing/afv166. [DOI] [PubMed] [Google Scholar]
- 6.Richter T, Mann E, Meyer G, Haastert B, Kopke S. Prevalence of psychotropic medication use among German and Austrian nursing home residents: a comparison of 3 cohorts. J Am Med Dir Assoc. 2012;13 doi: 10.1016/j.jamda.2011.03.007. e7-e13. [DOI] [PubMed] [Google Scholar]
- 7.Liperoti R, Sganga F, Landi F, et al. Antipsychotic drug interactions and mortality among nursing home residents with cognitive impairment. J Clin Psychiatry. 2017;78 doi: 10.4088/JCP.15m10303. e76-e82. [DOI] [PubMed] [Google Scholar]
- 8.Bakken MS, Schjott J, Engeland A, Engesaeter LB, Ruths S. Antipsychotic drugs and risk of hip fracture in people aged 60 and older in Norway. J Am Geriatr Soc. 2016;64:1203–1209. doi: 10.1111/jgs.14162. [DOI] [PubMed] [Google Scholar]
- 9.Fleischmann N, Geister C, Hoell A, Hummers-Pradier E, Mueller CA. Interprofessional collaboration in nursing homes (interprof): a grounded theory study of nurse experiences of general practitioner visits. Appl Nurs Res. 2017;35:118–125. doi: 10.1016/j.apnr.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 10.ABDA - Bundesvereinigung Deutscher Apothekerverbände e. V. Leitlinien und Arbeitshilfen - Heimversorgung. www.abda.de/fuer-apotheker/qualitaetssicherung/leitlinien/leitlinien-und-arbeitshilfen/ (last accessed on 23 July 2020) [Google Scholar]
- 11.Thürmann PA. Medication safety—models of interprofessional collaboration. Dtsch Arztebl Int. 2016;113:739–740. doi: 10.3238/arztebl.2016.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loganathan M, Singh S, Franklin BD, Bottle A, Majeed A. Interventions to optimise prescribing in care homes: systematic review. Age Ageing. 2011;40:150–162. doi: 10.1093/ageing/afq161. [DOI] [PubMed] [Google Scholar]
- 13.Forsetlund L, Eike MC, Gjerberg E, Vist GE. Effect of interventions to reduce potentially inappropriate use of drugs in nursing homes: a systematic review of randomised controlled trials. BMC Geriatr. 2011;11 doi: 10.1186/1471-2318-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alldred DP, Kennedy MC, Hughes C, Chen TF, Miller P. Interventions to optimise prescribing for older people in care homes. Cochrane Database Syst Rev. 2016;2 doi: 10.1002/14651858.CD009095.pub3. CD009095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strauven G, Anrys P, Vandael E, et al. Cluster-controlled trial of an intervention to improve prescribing in nursing homes study. J Am Med Dir Assoc. 2019;20:1404–1411. doi: 10.1016/j.jamda.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Wouters H, Scheper J, Koning H, et al. Discontinuing inappropriate medication use in nursing home residents: a cluster randomized controlled trial. Ann Intern Med. 2017;167:609–617. doi: 10.7326/M16-2729. [DOI] [PubMed] [Google Scholar]
- 17.Husebo BS, Ballard C, Aarsland D, et al. The effect of a multicomponent intervention on quality of life in residents of nursing homes: a randomized controlled trial (COSMOS) J Am Med Dir Assoc. 2019;20:330–339. doi: 10.1016/j.jamda.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Krause O, Wiese B, Doyle I, et al. Multidisciplinary intervention to improve medication safety in nursing home residents: protocol of a cluster randomised controlled trial (HIOPP-3-iTBX study) BMC Geriatrics. 2019;19 doi: 10.1186/s12877-019-1027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaehde U, Thürmann P. Arzneimitteltherapiesicherheit in Alten- und Pflegeheimen. Z Evid Fortbild Qual Gesundhwes. 2012;106:712–716. doi: 10.1016/j.zefq.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 20.Thürmann P. Abschlussbericht zum Projekt: Arzneimitteltherapiesicherheit bei Patienten in Einrichtungen der Langzeitpflege (AMTS-AMPEL) www.bundesgesundheitsministerium.de/fileadmin/Dateien/5_Publikationen/Gesundheit/Berichte/AMPELAbschlussbericht-gesamt-15-12-16.pdf (last accessed on 16 August 2021) [Google Scholar]
- 21.Schleef T, Junius-Walker U, Krause O. Sicheres Medikamentenmanagement im Pflegeheim: Die interdisziplinäre Visite im Praxistest. Z Allg Med. 2019;95:59–65. [Google Scholar]
- 22.Kirsch C, Doyle I, Krause O, et al. „Lessons learned“ - Herausforderungen im Rekrutierungsprozess in der cluster-randomisierten Pflegeheimstudie „HIOPP-3 iTBX“. Z Evid Fortbild Qual Gesundhwes. 2020;156-157:24–32. doi: 10.1016/j.zefq.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 23.ABDA - Bundesvereinigung Deutscher Apothekerverbände e. V. Grundsatzpapier zur Medikationsanalyse und zum Medikationsmanagement. www.abda.de/themen/arzneimitteltherapiesicherheit/foerderinitiative-pharmazeutische-betreuung/medikationsmanagement/ (last accessed on 23 July 2020) [Google Scholar]
- 24.Seidling HM, Send AFJ, Bittmann J, et al. Medication review in German community pharmacies—post-hoc analysis of documented drug-related problems and subsequent interventions in the ATHINA-project. Res Social Adm Pharm. 2017;13:1127–1134. doi: 10.1016/j.sapharm.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 25.König W, Lüttinger P, Müller W. A comparative analysis of the development and structure of educational systems. CASMIN Working Paper No 12. 1988 Universität Mannheim. [Google Scholar]
- 26.Laag S. Meyer H, Kortekamp S, editors. Drunter und drüber - die ärztliche Versorgung in Pflegeheimen neu denken Medikationsmanagement in stationären Pflegeeinrichtungen: Teamarbeit der Solisten. Cuvillier Verlag, Göttingen. 2014;2014:38–176. [Google Scholar]
- 27.Koch K, Miksch A, Schürmann C, Joos S, Sawicki PT. The German health care system in international comparison: the primary care physicians´ perspective. Dtsch Arztebl Int. 2011;108:255–261. doi: 10.3238/arztebl.2011.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weissenborn M, Schulz M, Kraft M, Haefeli WE, Seidling HM. Potential benchmarks for successful interdisciplinary collaboration projects in Germany: a systematic review. Gesundheitswesen. 2019;81:1057–1068. doi: 10.1055/a-0592-7184. [DOI] [PubMed] [Google Scholar]
- 29.Smeets CHW, Smalbrugge M, Koopmans R, et al. Can the PROPER intervention reduce psychotropic drug prescription in nursing home residents with dementia? Results of a cluster-randomized controlled trial. Int Psychogeriatr. 2021;33:577–586. doi: 10.1017/S1041610220000629. [DOI] [PubMed] [Google Scholar]
- 30.Donner-Banzhoff N. Pragmatic trials in a routine care setting. Z Evid Fortbild Qual Gesundhwes. 2009;103:404–409. doi: 10.1016/j.zefq.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 31.Tsakitzidis G, Anthierens S, Timmermans O, Truijen S, Meulemans H, Van Royen P. Do not confuse multidisciplinary task management in nursing homes with interprofessional care! Prim Health Care Res Dev. 2017;18:591–602. doi: 10.1017/S146342361700024X. [DOI] [PubMed] [Google Scholar]
- 32.Beuscart JB, Knol W, Cullinan S, et al. International core outcome set for clinical trials of medication review in multi-morbid older patients with polypharmacy. BMC Med. 2018;16 doi: 10.1186/s12916-018-1007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods section 1
More detailed information on the complex intervention
ATHINA medication review
The ATHINA medication review is the product of a further training program for pharmacists offered and coordinated by the chambers of pharmacists in a number of federal states of Germany. The medication review is structured and is aimed at increasing the effectiveness of drug therapy and minimizing drug risks. Pharmacies usually undertake a broader type 2a medication review (medication data and patient medical history). To review the medication of nursing home residents in the HIOPP-3-iTBX study, pharmacists used the type 2b medication review (medication data and clinical data).
The reviews in the nursing homes were based on the residents’ medication plans (medication use, long-term and on-demand medication, times at which medications were taken, and dose) as well as obtaining both personal and clinical data (patient age, sex, long-term diagnoses, list of symptoms, laboratory values [such as sodium, potassium, and glomerular filtration rate]).
Based on these date, the pharmacists assessed the presence of the following potential problems for each medication:
The medication plan was then faxed to general practitioners, together with the Med-Check Fax including the pharmacists’ recommendations on medication. The physicians assessed the pharmacists’ recommendations and, where appropriate, made changes to the drug therapy of the nursing home residents.
ATHINA training program
The ATHINA training program comprises a 2-day seminar that provides a basic understanding of drug therapy safety as well as a structured approach to carrying out a medication review. Attendees work through patient cases taken from routine practice and learn the working methods. The pharmacists, guided by a tutor with an additional clinical pharmaceutical qualification, go on to present at least four patient cases.
HIOPP further training (ATHINA-Plus)
ATHINA-Plus is a standardized further training program for pharmacists supplying nursing homes developed as part of the HIOPP-3-iTBX project. Aspects of geriatric pharmacy (falls, delirium, somnolence, pain; laboratory values, whether drugs can be administered via enteral feeding tube) and potentially inadequate medication, in particular with regard to neuroleptic drug treatment. In addition, tools/instruments to identify drug-related problems, as well as for interdisciplinary communication in the nursing home setting, are presented.
Physician and nurse training
Exchange of experience in the problems of drug therapy safety in nursing homes, recognizing potentially inadequate medications (using corresponding instruments/tools). Other areas of focus: neurolpetic drugs in the nursing home, presenting tools/instruments to identify drug-related problems and promote interdisciplinary communication in the nursing home setting.
The eTable presents additional components of the toolbox and change management.
Indication cannot be derived from the long-term diagnoses
Negative benefit:risk ratio (according to the PRISCUS list)
Potentially relevant drug interaction
Potentially relevant disease–drug interaction
Duplication of prescriptions
Potentially inadequate in older patients (as per the PRISCUS list)
Possible relevant adverse effects
Inadequate mode of application/administration
Check possible underdosing
Check possible overdosing
Contraindication
Check treatment duration
Other reasons
eMethods section 2
Additional details on methodology
Sample size determination
Sample size determination was based on the assumption that 50% of nursing home residents receive at least one PIM and/or at least two neuroleptic drugs (18). A reduction in the proportion of participants using this medication from 50% to 30% was considered clinically important. At an intracluster correlation (ICC) of ρ = 0.1, a cluster size of n = 5 NHR, an α error of 0.05, and a power of 80%, 632 nursing home residents were required to detect this difference (two-sided χ² test). Taking into account an annual mortality rate of approximately 30% as well as possible withdrawals from participation, a drop-out rate of 20% was assumed, meaning that 760 residents in 32 nursing homes needed to be recruited.
Data quality management
Data were recorded locally at the centers using the web-based data capture system secuTrial® and saved in a central ORACLE database. To this end, on-site training sessions on data capture were held for those involved.
Statistical methods
To evaluate the endpoints, mixed models were used in which residence in the respective home was entered as a random effect. To analyze the primary endpoint, a mixed logistic regression model was used. Here, all subjects were included in the analysis for whom the primary endpoint was recorded, even if they dropped out of the study or did not receive the intervention (intention-to-treat population). For the secondary endpoints “number of falls” and “number of hospitalizations,” mixed ordinal logistic regression models were used, while for the endpoint EQ5D, a mixed linear regression model was used. To analyze the secondary outcomes, as well as for sensitivity analyses, the per-protocol population, which included all subjects in whom the study was carried out according to the protocol, was used. Analyses of the secondary endpoints were exploratory and performed using STATA/SE Version 16.1.