Introduction
Repetitive loss of conduction following a period of excitation has been described in patients with manifest accessory pathways (AP). We describe this phenomenon in a concealed AP.
Case report
A 45-year-old woman with recurrent palpitations and structurally normal heart had supraventricular tachycardia with 2 different RP intervals. On 1 occasion, it was responsive to adenosine.
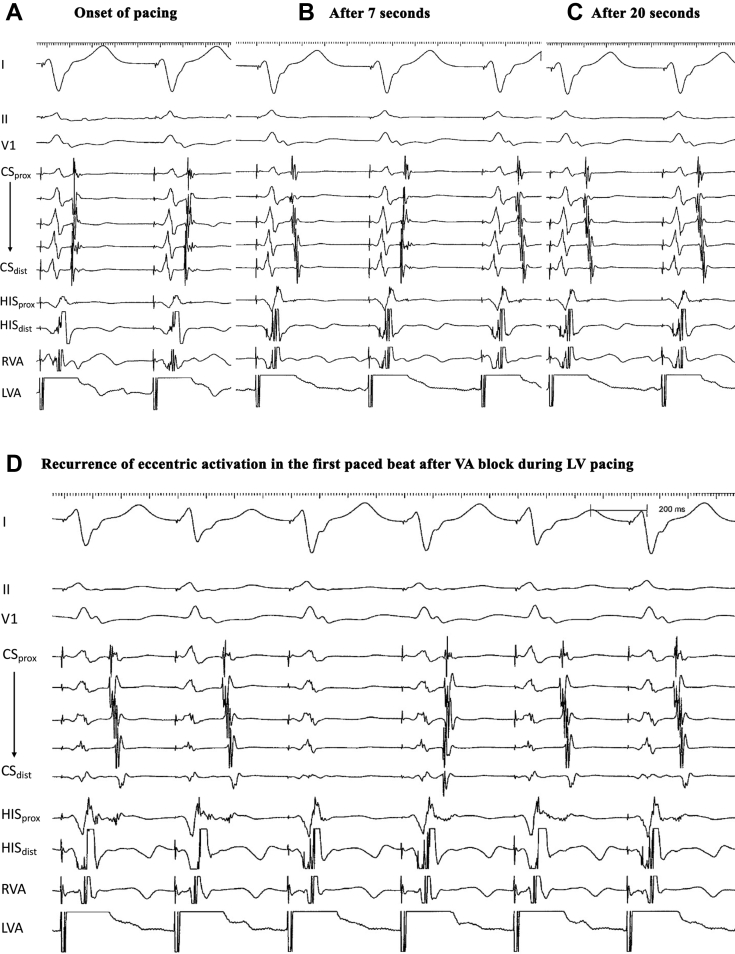
Low doses of intravenous midazolam (usually 2–3 mg) and fentanyl (20–30 mcg) were administered for sedation during the electrophysiology study. Quadripolar catheters were positioned in the high right atrium, right ventricular apex, and His bundle, and a decapolar catheter in the coronary sinus (CS). Conduction intervals were normal (AH 87 ms, HV 37 ms). Pacing from the right ventricular apex revealed variable, eccentric atrial activation sequences. During right atrial and distal CS pacing, anterograde conduction was decremental without preexcitation. A narrow complex tachycardia, initiated by a premature atrial contraction, with a cycle length (CL) of 349 ms, VA interval of 103 ms, and eccentric VA activation, was entrained from right ventricular overdrive pacing with a VAHV sequence, excluding the differential diagnosis of a left atrial tachycardia. Via transseptal access, targeted radiofrequency ablations at 12-, 2-, and 3-o’clock positions on the mitral valve annulus were successful in eliminating conduction over 3 separate concealed AP during right ventricular pacing. Subsequent pacing from CS 9,10 initiated yet another narrow complex tachycardia (CL 580 ms, VA 120 ms), with distal CS again the earliest site of atrial activation. A quadripolar catheter was positioned at the high lateral left ventricle via the same transseptal puncture for pacing (Figure 1). During left ventricular (LV) pacing, 4 observations were encountered. First, a distinct, repetitive pattern of atrial activation was seen during fixed CL LV pacing (Figure 2). A transient eccentric retrograde atrial activation pattern (earliest at CS 1,2) at the onset of pacing was followed by alternating eccentric and concentric activation patterns, before transitioning to a stable concentric activation pattern. Reinitiating LV pacing at the same CL after a period of sinus rhythm would elicit the same cycle of eccentric activation, alternating eccentric and concentric activation, and finally sustained concentric atrial activation pattern. Second, a time-dependent effect of pacing cessation on the duration of eccentric activation was observed. Consistent eccentric activation lasted for 10 seconds after discontinuing pacing for 7 seconds, while eccentric activation was sustained for at least 40 seconds after discontinuing pacing for 43 seconds. Third, intermittent VA block during LV pacing while retrograde conduction was concentric would result in an eccentric retrograde activation pattern in the subsequent paced beat, before returning to a sustained concentric activation pattern (Figure 2). Finally, within a second of starting ablation at the lateral mitral annulus at the 4-o’clock position, closely located to the distal CS, retrograde activation transitioned from an eccentric pattern to an alternating eccentric and concentric activation pattern, before switching to a sustained concentric activation pattern. The administration of isoproterenol increased the rate of AV node conduction but did not have an effect on pathway fatigue.
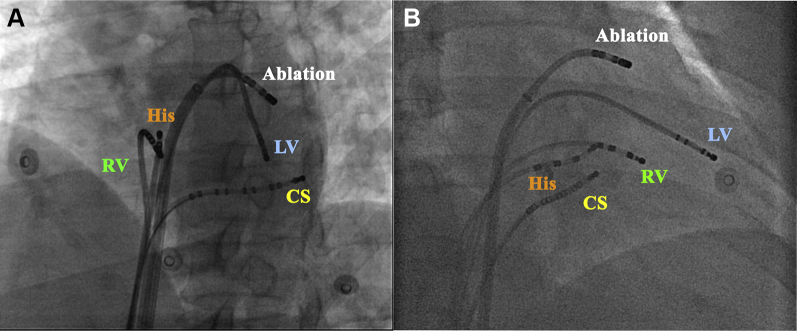
Figure 1.
Angiographic views of a quadripolar catheter positioned in the high lateral left ventricle (LV) for left ventricular pacing. A: Left anterior oblique (LAO) view. B: Right anterior oblique (RAO) view. CS = coronary sinus; RV = right ventricle.
Figure 2.
Repetitive cycle of varying atrial activation patterns with ventricular pacing (A–C), with transient recurrence of eccentric activation in the first paced beat after intermittent VA block during left ventricular pacing (D).
What is the behavior underlying these observations? Should further attempts at ablation of the final AP be made?
Discussion
The observations made in this case are consistent with fatigue phenomenon of the AP. Fatigue phenomenon is characterized by repetitive loss of conduction following a period of repetitive excitation and was first described in diseased AV conduction system.1,2 It is infrequent in AP, with reported cases involving manifest AP.1,3,4 Transient delta wave disappearance after cardiac pacing is akin to fatigue phenomenon in the His-Purkinje system, and could be provoked by either atrial or ventricular pacing.1,3 Our case was unique to previous reports in that the AP was concealed but demonstrated the same characteristics observed with fatigue phenomenon. The reproducible onset of conduction fatigue after ventricular pacing was evidenced by alternating eccentric and concentric activation patterns indicating intermittent AP conduction block. This was followed by complete conduction block over the AP, with retrograde activation now occurring through the AV node, resulting in sustained concentric activation (Figure 2). The discontinuation of pacing allowed time for AP conduction recovery, manifesting as the return of eccentric activation upon resumption of ventricular pacing.
A characteristic of fatigue phenomenon is the time-dependent effect of pacing duration on onset of pathway fatigue.1,3 The time-dependent effects of pacing discontinuation on pathway recovery were demonstrated in this case by the ability to sustain longer periods of retrograde conduction over the AP with longer durations of sinus rhythm. During transient VA block, the AP is able to conduct for 1 beat before reverting to the AV node owing to pathway fatigue. This observation suggests that a fatigued pathway may have highly variable refractory periods.
The rapid transition from eccentric to concentric activation at the onset of ablation is likely a manifestation of the fatigue phenomenon, coinciding with the onset of conduction block over the AP after 7 seconds of pacing. This may potentially be misinterpreted as successful elimination of AP conduction. Recurrence of eccentric activation after a few minutes of rest indicated that the AP was still capable of conduction, necessitating further ablation. Upon successful ablation of the fourth concealed AP demonstrating fatigue phenomenon, supraventricular tachycardia at the same CL but now with a concentric atrial activation pattern was still inducible. This proved to be AV nodal reentrant tachycardia and was successfully treated by slow pathway modification. In the literature, AP demonstrating fatigue phenomenon have not been shown to participate in tachycardia and it is highly probable that the fourth ablated AP was a bystander during AV nodal reentrant tachycardia.
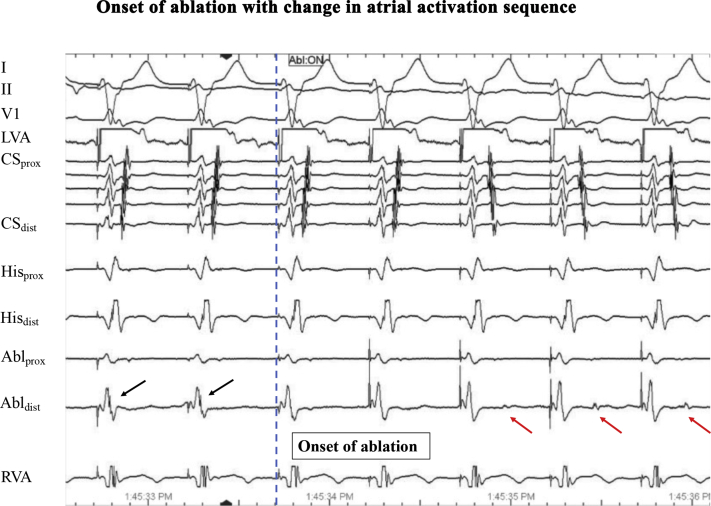
Alternative mechanisms that may account for the observed phenomenon include, firstly, AP injury from previous ablations in proximity to the final ablation location. While successful elimination of different retrograde atrial conduction patterns through ablation at different locations along the mitral annulus suggested the presence of multiple AP, it is possible that this may have been the same left free wall AP with a broad band of insertion on the atrium. Secondly, complete mitral isthmus block during radiofrequency ablation may also account for changes in retrograde atrial activation sequence to a concentric pattern.5,6 However, we think it unlikely in this case, as (1) there was loss of AP potential and atrial electrograms were delayed on the ablation catheter at onset of ablation, suggesting loss of AP conduction; and (2) earliest atrial activation was seen on the His catheter, suggesting retrograde atrial activation through the AV node (Figure 3). Furthermore, mitral isthmus block is also excluded, as pacing was performed more laterally in the left ventricle and septal to any potential line of block where previous ablations were performed. It would therefore have been unlikely for mitral isthmus block to account for the changes in atrial activation sequence encountered.
Figure 3.
Changes in atrial activation sequence at onset of ablation, with atrial electrogram (red arrow) on ablation catheter (Abldistal) now widely separated from the ventricular electrogram. Earliest atrial activation is now seen on the His catheter, suggesting retrograde atrial activation through the AV node, while accessory pathway potentials (black arrows) seen on the ablation catheter prior to onset of ablation are no longer present. Pacing was performed with the high right atrium catheter in the left ventricle.
Conclusion
Pathway fatigue may occur in concealed AP. Repetitive loss of AP conduction during pacing or ablation owing to fatigue phenomenon should be recognized to prevent misinterpretation of successful AP conduction elimination.
Key Teaching Points.
-
•
Contrary to previous reports of fatigue phenomenon in manifest accessory pathways, pathway fatigue may also occur in concealed accessory pathways.
-
•
The time-dependent effects of pacing discontinuation on pathway recovery are suggestive of fatigue phenomenon in the accessory pathway.
-
•
The rapid transition of eccentric activation at onset of ablation to a concentric activation pattern may potentially be misinterpreted as successful elimination of accessory pathway conduction.
-
•
Prolongation in local VA interval at the site of ablation may help distinguish from mitral isthmus block when retrograde atrial activation sequence changes from eccentric to concentric during ablation
Acknowledgments
The contributions of all staff at the National University Heart Centre Singapore cardiac catheterization laboratory are duly acknowledged.
Footnotes
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Conflicts of interest: None declared.
References
- 1.Li H.G., Klein G.J., Yee R., Thakur R.K. Fatigue phenomenon in accessory pathways. J Cardiovasc Electrophysiol. 1994;5:818–823. doi: 10.1111/j.1540-8167.1994.tb01120.x. [DOI] [PubMed] [Google Scholar]
- 2.Narula O.S., Runge M. In: The Conduction System of the Heart. Wellens H.J.J., Lie K.I., Janse M.J., editors. H. E. Stenfert Kroese; Leiden, The Netherlands: 1976. Accommodation of A-V nodal conduction and fatigue phenomenon in the His-Purkinje system; pp. 529–544. [Google Scholar]
- 3.Ohe T., Shimonura K., Shiroeda O. Fatigue phenomenon of the accessory pathway. Int J Cardiol. 1985;8:211–214. doi: 10.1016/0167-5273(85)90290-6. [DOI] [PubMed] [Google Scholar]
- 4.Fujimura O., Smith B.A., Kuo C.S. Effect of verapamil on an accessory pathway manifesting as "fatigue phenomenon" in Wolff-Parkinson-White syndrome. Chest. 1993;104:305–307. doi: 10.1378/chest.104.1.305. [DOI] [PubMed] [Google Scholar]
- 5.Luria D.M., Nemec J., Etheridge S.P., et al. Intra-atrial conduction block along the mitral valve annulus during accessory pathway ablation: evidence for a left atrial "isthmus. J Cardiovasc Electrophysiol. 2001;12:744–749. doi: 10.1046/j.1540-8167.2001.00744.x. [DOI] [PubMed] [Google Scholar]
- 6.Fujiki A., Usuda K., Mizumaki K., et al. Double potentials at successful catheter ablation site of left-sided retrograde accessory pathway: mitral isthmus block or conduction through coronary sinus musculature. Int J Cardiol. 2007;117:90–96. doi: 10.1016/j.ijcard.2006.04.055. [DOI] [PubMed] [Google Scholar]