Abstract
Purpose
Single agent PD-1 inhibitors have yielded durable responses in a minority of gastroesophageal cancers. Radiation therapy has been recognized to promote antitumor immune responses and may synergize with anti-PD-1 agents. We sought to evaluate if combining palliative radiation therapy with pembrolizumab can augment antitumor immune responses in gastroesophageal cancer.
Methods and Materials
Patients had metastatic gastroesophageal cancer with indication for palliative radiation therapy with ≥2 disease sites outside of the radiation field assessable for abscopal response and biopsies for laboratory correlative analyses. Palliative radiation was delivered to a dose of 30 Gy over 10 fractions. Pembrolizumab, 200 mg, was administered concurrently intravenously every 3 weeks until disease progression, unacceptable toxicity, or study withdrawal, for up to 2 years. Endpoints included PD-L1 expression in pre- and posttreatment biopsies and abscopal objective response rate per Response Evaluation Criteria in Solid Tumors.
Results
Of 14 enrolled patients, the objective response rate was 28.6% (95% confidence interval, 8.4%-58.1%), and the median duration of response was not reached (95% confidence interval, 6.9-NR months). Overall, 2 patients had treatment-related grade 3 to 4 adverse events with no grade 5 events. One patient discontinued therapy due to grade 4 colitis. We did not observe an association between radiation and abscopal changes in PD-L1 expression via assessment of an analogous PD-L1 Combined Positive Score, Tumor Proportion Score, Mononuclear Immune Cell Density Score, or proportion of PD-L1-expressing immune cells between pre- and posttreatment tumor biopsies.
Conclusions
Combining palliative radiation therapy and pembrolizumab provided promising durable responses in this patient population but we were unable to definitively distinguish abscopal biologic changes. Biomarker analyses beyond PD-L1 expression are needed to better understand putative mechanisms and identify patients who will benefit from this approach.
Introduction
The PD-1 inhibitor pembrolizumab has demonstrated clinical activity in metastatic gastroesophageal cancers (both squamous cell and adenocarcinomas), but single agent response is predominantly enriched in certain gastroesophageal cancer (GEC) molecular subsets such as those with high microsatellite instability (MSI-H) and Epstein-Barr virus (EBV) association.1, 2, 3 Conversely, the more predominant GEC molecular subtypes classified by The Cancer Genome Atlas of tumors with chromosomal instability and genomically stable tumors have reported response rates of only 5% and 12%, respectively.3 Tumor PD-L1 expression as enumerated by combined positive scoring (CPS) of both PD-L1 expressing tumor cells and immune cells garnered regulatory approval as a biomarker for single agent pembrolizumab prescribing in GEC, but more recent studies point toward higher baseline expression cutoffs as opposed to the dichotomous absence or presence of PD-L1 to be more associated with clinical benefit.4,5 More recently, the US Food and Drug Administration approved adding nivolumab to first-line chemotherapy for metastatic GECs with adenocarcinoma histology alone, and the addition of pembrolizumab to first-line chemotherapy for esophageal squamous cell or adenocarcinomas regardless of PD-L1 biomarker assessment.6,7 However, the benefit for incorporation of anti-PD-1 therapy still appears better delineated if PD-L1 CPS assessment exceeds certain thresholds of expression such as ≥5 in the CheckMate 649 trial adding nivolumab or ≥10 in the KEYNOTE-590 trial adding pembrolizumab.8,9 As such, there still remains a fair proportion of patients with lower PD-L1 CPS expression for which the combination of chemotherapy and anti-PD-1 therapy does not yet yield improved clinical outcomes.
The abscopal effect is a well annotated clinical phenomenon in cancer radiation therapy (RT), in which metastatic lesions outside the field of RT regress and is attributed to immune-mediated mechanisms.10,11 In preclinical models, combination RT and PD-1/PD-L1 axis blockade exhibits synergistic antitumor activity and upregulates tumor PD-L1 and infiltrating T-cell PD-1 expression to restrain immune responses.12, 13, 14 This background lends credence to ongoing efforts to combine RT and immune checkpoint inhibition in attempts to augment higher likelihood of response among a greater proportion of GEC patients. We present the study results for a phase 2 trial combining pembrolizumab and palliative RT in metastatic GEC patients.
Methods and Materials
Study design, treatment, participants
This was a single center, nonrandomized, phase 2 trial for patients 18 years or older with metastatic gastric, gastroesophageal junction, or esophageal either squamous cell or adenocarcinoma. Patients had indication for palliative radiation for symptoms related to their primary tumor or metastatic site including pain, dysphagia, or bleeding, with exception of patients needing radiation to a symptomatic CNS metastasis. There were no limits on prior lines of therapy and patients may be treatment-naïve with the exception that patients with adenocarcinoma histology and known HER2 overexpression were required to have progressed or be intolerant of prior trastuzumab-containing therapy. No prior anti-PD-1 or anti-PD-L1 therapy was allowed. To measure the abscopal effect, patients were required to have a radiographically measurable lesion outside of the field of radiation, and a disease site amenable to image guided or endoscopic pre- and posttreatment biopsies. Palliative radiation was standardized for a dose of 30 Gy in 10 fractions, with the first fraction of radiation coinciding with cycle 1, day 1 of pembrolizumab. Both 3-dimensional conformal radiation therapy and intensity modulated radiation therapy were allowed, with full details of the radiation treatment planning available in the Trial Protocol Supplementary File. Pembrolizumab was administered intravenously at 200 mg every 3 weeks, and continued until disease progression, unacceptable toxicity, investigators’ decision to withdraw treatment, or completion of 35 administrations corresponding to 2 years of therapy. The study received local institutional review board approval and all patients provided written informed consent before the onset of any research interventions.
The primary endpoint was a biomarker-driven input of assessing immune cell populations in pre- and posttreatment tumor biopsy specimens outside the field of radiation. Secondary endpoints included objective response rate, duration of response, progression-free survival (PFS), and overall survival (OS). Tumor objective response was assessed per Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1, by investigator assessment every 6 weeks through the first 18 weeks then every 9 weeks thereafter. Only tumor lesions outside of the field of radiation were included as measurable target lesions for strict assessment of abscopal RECIST 1.1 responses, but the irradiated lesion was included in nontarget assessment to still enable determination of RECIST 1.1 progression if it were to exhibit unequivocal worsening. Adverse events (AEs) were collected as per NCI CTCAE version 4.0.
Biomarker analysis
Pretreatment biopsy was performed during screening, and posttreatment biopsy around 21 days after completion of radiation therapy (corresponding to approximately cycle 2, day 15 of pembrolizumab). Both biopsies were outside of the field of radiation therapy to assess abscopal biomarker correlates. PD-L1 expression in tumor and immune cell populations within pre- and posttreatment tumor biopsies were conducted using multispectral immunofluorescence via the Opal-TSA based assay (Akoya Biosciences). This method distinguished PD-L1-expressing or nonexpressing pan-cytokeratin staining tumor cells (CK) and macrophages (CD68). In one multicolor panel, staining antibodies were applied against CD68 (KP1, Biocare), PD-L1 (SP142, Spring), and CK (AE1/AE3, Agilent Dako). Images were acquired by Vectra 3.0 automated quantitative pathology imaging system (Akoya Biosciences), and the cell annotation was performed using inForm Cell Analysis software, which uses machine-learning based cell phenotyping. In this study, only immune cells proximate to the cancer cells were counted to score only cells directly associated with response against the tumor. To achieve this selection, a k-nearest neighbor algorithm was used to identify the immune cells which can reach the cancer cells within 100 microns (k = 1).
PD-L1 staining was performed using SP142 antibody and tumor cells were counted for PD-L1 expression only if membranous staining was present while immune cells were counted if either membranous or cytoplasmic staining was present. Fibroblasts and endothelial cells that may falsely contribute PD-L1 signal were subtracted based on their elongated cell shape and lower nuclear to cytoplasmic ratio compared with tumor cells and immune cells. As such the remaining PD-L1 staining of CD68 negative cells were representative of mononuclear immune cells. Viable carcinoma cells were confirmed by assessment of corresponding hematoxylin and eosin stain slide sections by the pathologist (M.D.) as to exclude benign cells that were CK-staining. Analogous CPS, Tumor Proportion Scores (TPS), and Mononuclear Immune Cell Density Scores (MIDS) were calculated per published algorithms.15 The proportion of PD-L1 expressing immune cells was calculated as per the following formula:
Statistical analysis
For a target accrual of 14 patients, there was 96% power to detect at least one responder if the true response rate is ≥21%, which is in the range of 22% from the phase 1 trial of pembrolizumab for gastric cancer that was the available historical reference at study conception.16 Failure to observe any abscopal response in the 14 patients would have resulted in lack of enthusiasm for the combination of pembrolizumab and RT in this population. PFS and OS were determined using the Kaplan-Meier method. Descriptive statistics were used to characterize differences in PD-L1 biomarker scoring between pre- and posttreatment biopsies. Additional details of statistical analyses are provided in the Trial Protocol Supplementary File.
Results
Between December 13, 2016, and September 19, 2018, 14 patients were treated. The median age was 67 years (range, 40-91), 12 patients (86%) were male, 5 (36%) had no prior therapy, and 3 (21%) had ESCC (Table 1). As of data cut-off of December 31, 2019, the median duration of follow-up was 23.2 months (95% CI, 1.9-29.9), and 1 patient continued to receive pembrolizumab (Fig. E1). Most common treatment-related AEs were fatigue and pruritus that were grade 1 to 2 in severity (Table 2). Grade 3 to 4 AEs included 1 patient with grade 3 diarrhea and an additional 1 patient with grade 4 enterocolitis that required discontinuation of protocol therapy. No grade 5 treatment-related AEs were observed.
Table 1.
Baseline characteristics
| Characteristic | All patients (N = 14) |
|---|---|
| Age (y) | |
| Median (min, max) | 67 (40, 91) |
| Sex, n (%) | |
| Female | 2 (14%) |
| Male | 12 (86%) |
| Race, n (%) | |
| White | 7 (50%) |
| Asian | 5 (36%) |
| Black or African-American | 1 (7%) |
| Hispanic | 1 (7%) |
| Histologic subtypes, n (%) | |
| Squamous cell carcinoma | 3 (21%) |
| Adenocarcinoma | 11 (79%) |
| HER2 status,* n (%) | |
| Negative | 11 (100%) |
| Positive | 0 (0%) |
| ECOG Performance Status, n (%) | |
| 0 | 3 (21%) |
| 1 | 11 (79%) |
| Prior lines of therapy, n (%) | |
| 0 | 5 (36%) |
| 1 | 7 (50%) |
| ≥ 2 | 2 (14%) |
Among adenocarcinoma histologies only.
Abbreviation: EGOG = Eastern Cooperative Oncology Group.
Table 2.
Treatment-related adverse events
| Adverse events | No. (%) (N = 14) |
|---|---|
| Grade 1-2 (≥10% incidence) | |
| Fatigue | 4 (29) |
| Pruritus | 4 (29) |
| Pneumonitis | 2 (14) |
| Skin rash | 2 (14) |
| Constipation | 2 (14) |
| Xerostomia | 2 (14) |
| Thrombocytopenia | 2 (14) |
| Grade 3-4* | |
| Fatigue | 0 (0) |
| Pruritus | 0 (0) |
| Pneumonitis | 0 (0) |
| Skin rash | 0 (0) |
| Constipation | 0 (0) |
| Xerostomia | 0 (0) |
| Thrombocytopenia | 0 (0) |
| Diarrhea | 1 (7) |
| Enterocolitis† | 1 (7) |
No grade 5 treatment-related events.
Led to treatment discontinuation.
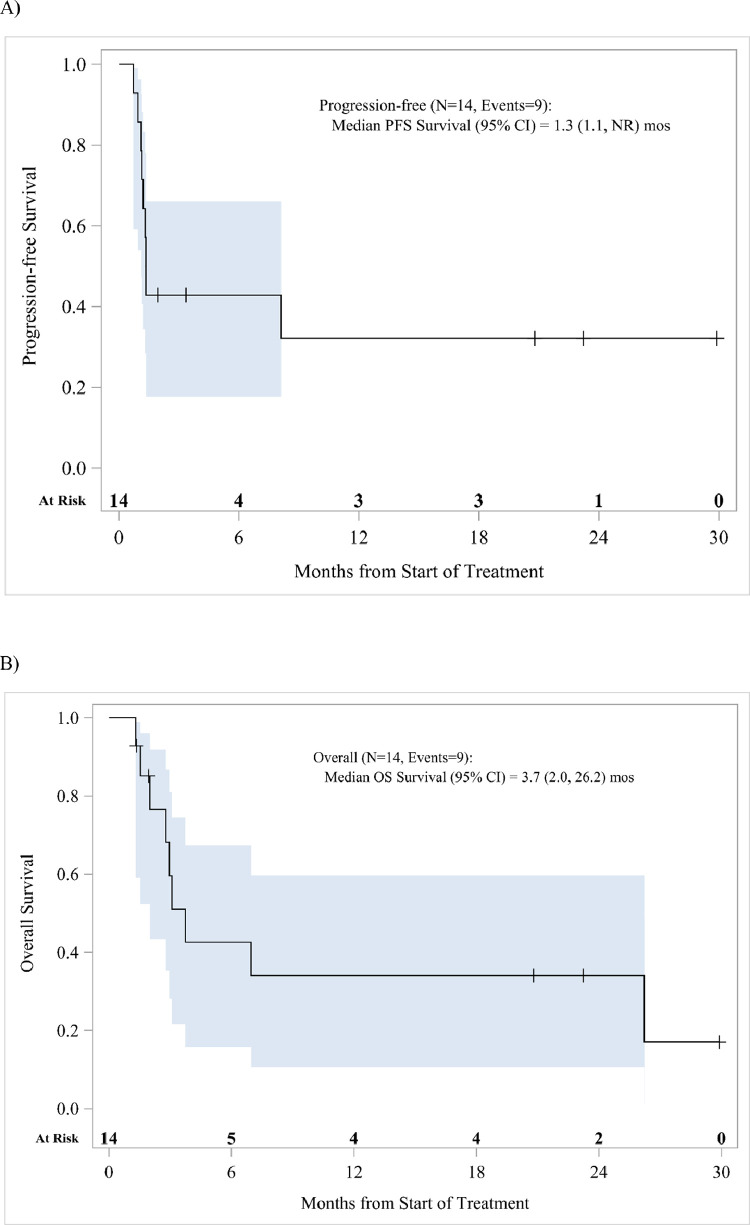
Confirmed partial responses via RECIST 1.1 assessment of only abscopal sites of disease were observed in 4 patients with the median duration of response not being reached (95% CI, 6.9-NR mo; Table 3). The tumor site indicated for palliative radiation therapy for each respective patient and the corresponding metastatic disease areas used for out of the RT field tumor biopsies and RECIST 1.1 assessment are listed in Table 4. Ongoing response lasting >12 months was exhibited in 3 of the 4 patients at data cutoff. All of the patients with confirmed response were of adenocarcinoma histology, with 3 of the 4 responders enrolled into the protocol as their first-line therapy. Median PFS and OS for all patients was 1.3 (95% CI, 1.1-NR mo) and 3.7 months (95% CI, 2.0-26.2 mo), respectively (Fig. 1). MSI status was determined via standard of care mismatch repair protein IHC, microsatellite PCR, or broad panel NGS testing, with no patients having tumors that were MSI-H. Post hoc analysis of tumor EBV status was performed among the 3 patients with ongoing response with none exhibiting positivity for EBV viral RNA by EBER in situ hybridization.
Table 3.
Objective tumor response
| Best overall response | No. (%) N = 14 |
|---|---|
| Objective responses (CR + PR) | 4 (28.6) 95% CI 8.4-58.1 |
| CR | 0 (0) |
| PR | 4 (28.6) |
| SD | 1 (7.1) |
| Unconfirmed PR | 1 (7.1) |
| Progressive disease | 7 (50.0) |
| No assessment* | 1 (7.1) |
| Duration of response, median (range), mo | Not reached (8.4-35.2 +) |
Patient experienced symptomatic disease progression before posttreatment tumor assessment.
Abbreviations: CI = confidence interval; CR = complete response; PR = partial response; SD = stable disease.
Table 4.
Histology, primary tumor characteristics, palliative radiation therapy indications, and abscopal sites of biopsy and tumor response assessment
| Primary tumor location | Histology | Indication for palliative RT | Out of RT field sites of biopsy | Out of RT field RECIST 1.1 target lesions | |
|---|---|---|---|---|---|
| 1 | GEJ | Adenocarcinoma: poorly differentiated | Dysphagia from primary tumor | Liver metastasis | Liver metastases |
| 2 | Gastric antrum | Adenocarcinoma: poorly differentiated | Bleeding from primary tumor | Liver metastasis | Liver metastases |
| 3 | Gastric cardia and fundus | Adenocarcinoma: moderate to poorly differentiated | Bleeding from primary tumor | FDG avid axillary lymph node; biopsy deferred as not visualized on ultrasound | Mediastinal lymph node |
| 4 | GEJ | Adenocarcinoma: poorly differentiated | Pain from right pelvic bone metastasis | Left arm subcutaneous metastasis | Liver metastasis and left scapular soft tissue metastasis |
| 5 | Gastric body | Adenocarcinoma: signet ring cell | Bleeding from primary tumor | Liver metastasis | Liver metastases and portacaval lymph node |
| 6 | Midesophagus | Squamous cell carcinoma: moderate to poorly differentiated | Pain from right pleural metastasis | Left lung lower lobe metastasis | Lung metastases and gastrohepatic lymph node |
| 7 | GEJ | Adenocarcinoma: signet ring cell | Dysphagia from primary tumor | Left adrenal metastasis | Subcarinal lymph node |
| 8 | Midesophagus | Squamous cell carcinoma: poorly differentiated | Dysphagia from primary tumor | Right gluteal metastasis | Mesenteric and peritoneal metastases and paraspinal muscle metastasis |
| 9 | Gastric antrum | Adenocarcinoma: moderately differentiated | Gastric outlet obstruction from primary tumor | Liver metastasis | Liver metastases |
| 10 | Gastric antrum | Adenocarcinoma: signet ring cell | Pain from left thigh metastasis | Left inguinal lymph node metastasis | Pericardiac soft tissue metastasis |
| 11 | Gastric antrum | Adenocarcinoma: poorly differentiated | Pain from left shoulder metastasis | Right breast metastasis |
Right gluteal metastasis |
| 12 | Midesophagus | Squamous cell carcinoma: poorly differentiated | Dysphagia from primary tumor | Right clavicular soft tissue metastasis | Left supraclavicular lymph node, thoracic spine, and mesenteric lymph node metastases |
| 13 | Gastric body | Adenocarcinoma: poorly differentiated | Pain from retroperitoneal lymph node metastasis | Gastric primary tumor | Gastrohepatic lymph node |
| 14 | GEJ | Adenocarcinoma: poorly differentiated | Dysphagia from primary tumor | Liver metastasis | Liver metastases and porta hepatis lymph node |
Abbreviations: FDG = fluorodeoxyglucose; GEJ = gastroesophageal junction; RECIST = Response Evaluation Criteria in Solid Tumors; RT = radiation therapy.
Figure 1.
(A) Progression free and (B) overall survival for the entire trial population. Blue lines represent 95% confidence intervals.
Multicolor immunofluorescence was performed to quantitate analogous PD-L1 CPS, TPS, and MIDS expression in pre- and posttreatment biopsies (Table 5). One patient did not undergo any biopsies due to site of metastasis not being visualized under image guidance and 2 patients did not undergo the second biopsy for patient safety. Despite sampling of the same disease site for each patient where the pretreatment biopsies had yielded viable tumor, 4 patients had posttreatment biopsies without any viable cancer cells but only residual stromal tissue (Table 5, patients 1, 7, 11, and 13). Among the 7 remaining patients, PD-L1 CPS, TPS, and MIDS expression was not significantly different between pre- and posttreatment samples, with none of these 7 patients deriving a response to therapy. For patients with a confirmed PR, 3 of 4 had available paired biopsies, but no viable cancer cells on the posttreatment biopsies to enable determination of temporal changes in CPS, TPS, or MIDS. Among the 3 confirmed PR patients with an assessable pretreatment biopsy, the baseline PD-L1 CPS values were 1, 43, and 11 (Table 5). There was one additional patient whose posttreatment biopsy yielded no viable carcinoma cells, but this patient exhibited progressive disease as best response (Table 5, patient 11). As such, we explored any changes in the proportion of PD-L1-expressing assessable immune cells in paired biopsies and observed no significant differences or trends before and after therapy (Table 5).
Table 5.
PD-L1 CPS, TPS, and MIDS expression and proportion of PD-L1 expressing immune cells in available pre- and posttreatment biopsies in all study patients and associated RECIST 1.1. best overall response
| Patient sequence | PD-L1 CPS |
PD-L1 TPS (%) |
PD-L1 MIDS |
Proportion of PD-L1 expressing immune cells (%) |
RECIST 1.1 Best Overall Response | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pretreatment | Posttreatment | Pretreatment | Posttreatment | Pretreatment | Posttreatment | Pretreatment | Posttreatment | ||
| 1 | 1 | NE: no viable carcinoma cells | 0 | NE: no viable carcinoma cells | 1 | NE: no viable carcinoma cells | 1.5 | 4.7 | PR |
| 2 | 10 | 6 | 1 | 1 | 9 | 5 | 3.1 | 2.3 | PD |
| 3 | Biopsy deferred | Biopsy deferred | Biopsy deferred | Biopsy deferred | Biopsy deferred | Biopsy deferred | Biopsy deferred | Biopsy deferred | PR |
| 4 | 9 | 36 | 1 | 2 | 8 | 34 | 1.8 | 15.8 | PD |
| 5 | 6 | Biopsy deferred | 2 | Biopsy deferred | 4 | Biopsy deferred | 1.6 | Biopsy deferred | Not assessed |
| 6 | 13 | 1 | 7 | 0 | 6 | 1 | 4.7 | 2.3 | PD |
| 7 | 43 | NE: no viable carcinoma cells | 1 | NE: no viable `carcinoma cells | 42 | NE: no viable carcinoma cells | 13.7 | 3.7 | PR |
| 8 | 32 | 13 | 0 | 8 | 32 | 5 | 0.9 | 11.0 | Unconfirmed PR |
| 9 | 8 | 1 | 2 | 0 | 6 | 1 | 2.1 | 3.2 | PD |
| 10 | 30 | 4 | 3 | 1 | 27 | 3 | 1.1 | 6.3 | SD |
| 11 | 2 | NE: no viable carcinoma cells | 0 | NE: no viable carcinoma cells | 2 | NE: no viable carcinoma cells | 5.1 | 1.32 | PD |
| 12 | 9 | Biopsy deferred | 0 | Biopsy deferred | 9 | Biopsy deferred | 3.9 | Biopsy deferred | PD |
| 13 | 11 | NE: no viable carcinoma cells | 1 | NE: no viable carcinoma cells | 10 | NE: no viable carcinoma cells | 4.6 | 4.7 | PR |
| 14 | 1 | 15 | 0 | 0 | 1 | 15 | 1.2 | 5.4 | PD |
| Mean PD-L1 values* | 15 | 11 | 2 | 2 | 13 | 9 | 3.6 | 5.5 | |
| P value† | .60 | .87 | .64 | .36 | |||||
Abbreviations: CPS = combined positive scoring; MIDS = Mononuclear Immune Cell Density Scores; NE, not evaluable; PD, progressive disease; PR, partial response; RECIST = Response Evaluation Criteria in Solid Tumors; SD = XXX; TPS = Tumor Proportion Scores.
Among samples with assessable pairs.
Paired t test.
Discussion
This report to our knowledge is the first in metastatic GEC to prospectively administer a PD-1 inhibitor concurrent with palliative RT to query abscopal responses. Durability of responses were encouraging but proportion of such responses were still modest in our study population. However, confirmation of all cases being MSS and EBV negative excluded these molecular subtypes of GEC accounting for the responses observed.3 Median PFS and OS in our study also appeared shorter than larger randomized studies examining single agent pembrolizumab.1,17,18 Arguably, with our study examining patients in need of palliative radiation the vast majority were of lower performance status Eastern Cooperative Oncology Group 1 where a lesser benefit to pembrolizumab and poorer survival has been reported versus patients who are asymptomatic and Eastern Cooperative Oncology Group 0.17 In addition, the requirements for at least 2 abscopal sites outside of the radiation field led to recruitment of patients with significant metastatic disease burden including 6 of 14 patients with liver metastases where recent patient observations of limited immunotherapy efficacy have been supported by preclinical models.19 For our study, we chose a standard fractionation schedule of 30 Gy over 10 fractions given this schedule is still highly relevant to current practice in metastatic GEC.20,21 Research efforts are ongoing to define the optimal radiation dose to use in the clinic to augment immune responses. Preclinical models more recently suggest higher ablative RT doses of >10 to 12 Gy per fraction blunts immune responses through upregulation of the exonuclease TREX1,22 and as such the lower dose per fraction we used may still synergize with anti-PD-1 therapy.
We were unable to observe consistent upregulation of intratumoral PD-L1 levels in abscopal lesions after radiation or a pharmacodynamic association with response to PD-1 inhibition that has been suggested in preclinical models.12, 13, 14 Retrospective data sets have noted other immune checkpoints such as TIM3, GITR, IDO1, LAG3, and KIR are upregulated in GEC after treatment with radiation, pointing toward the existence of heterogeneity in immunosuppressive mechanisms which may restrain the abscopal response and should be further studied.23 Spatial intrapatient heterogeneity for GEC PD-L1 expression may also confound PD-L1 biomarker analyses, that is, primary and metastatic tumor sites within the same patient may have inherent interlesional variability in PD-L1 expression and should also be factored into future efforts.24,25 In addition, we conducted endoscopic or radiographically guided core biopsies, which is in line with standard of care and patient welfare in terms of limiting the invasiveness of the associated procedures. However, a recent patient case series also points to limited tumor sampling potentially not representing the PD-L1 expression status of whole section surgically resected gastric cancer specimens raising the issue of intralesional heterogeneity.26 Temporal heterogeneity of GEC tumor PD-L1 expression has also been reported in 2 separate case series where discordance of PD-L1 expression in the range of 20% to 50% has been observed between 2 sampling timepoints.24,27 The context for both of these studies were for patients undergoing cytotoxic chemotherapy and the overall trend was for loss of PD-L1 expression posttherapy. Abscopal tumor PD-L1 expression invariably could not be studied with the systemic exposure of cytotoxic drugs achieved in these patient cohorts. Studies are also likely not feasible to assess the temporal variability of PD-L1 expression under no treatment pressure, as that would entail repeated tumor sampling in patients whose metastatic disease is allowed to progress in a typically fatal natural course. Although we ultimately did not observe any significant trends, to our knowledge our study is unique in reporting on PD-L1 expression after exposure to systemic single-agent anti-PD-1 therapy in an exclusively GEC population. Although we had patients undergo a second biopsy after a relatively short duration of initiating protocol therapy (∼36 days), complete tumor cell necrosis had already occurred in a fair proportion that invariably affected the ability to perform tumor biomarker analyses. The one patient with complete tumor cell necrosis but whose overall disease burden did not respond to protocol therapy also speaks to the potential for intrapatient interlesional heterogeneity affecting interpretation of biological correlates from tumor biopsies. Other novel research methodologies should be explored, and liquid biopsy assays such as assessment of circulating immune cell populations may hold promise in interrogating dynamics of the immune response.28,29 In addition, circulating tumor DNA kinetics while initially linked to molecularly targeted strategies may also have potential as pharmacodynamic markers of immunotherapy response.30, 31, 32 Adding further complexity to predictive biomarker assessment is the ascertainment of bacterial diversity in the stool microbiome has also emerged as correlating with clinical efficacy to PD-1 inhibitors.33,34
Three of our 4 responding patients derived a response with receiving palliative RT and pembrolizumab as first-line therapy as opposed to only one patient deriving a response entering the trial as later-line therapy. However, larger prospective GEC trial data sets have reported equivalent proportion of responders to single agent pembrolizumab irrespective of line of therapy, albeit still limited to ∼15% of patients even when tumor PD-L1 expression is detected.1,17,18 Incorporation of PD-1 inhibitors in combination with first-line chemotherapy for metastatic gastroesophageal cancers has become the more pragmatic application in the clinic, although benefit seems allocated to certain PD-L1 CPS cutoffs (≥5 for nivolumab and ≥10 for pembrolizumab).8,9 The need for palliative RT to treat bleeding or dysphagia from the primary tumor before onset of first-line systemic therapy for synchronous metastatic disease is not an uncommon clinical scenario as exemplified by radiation therapy indications for patients enrolled in our trial. For the former cytotoxic chemotherapy may even be contraindicated in exacerbating complications from anemia. Palliative concurrent chemoradiotherapy versus radiation therapy alone in a prospective randomized trial for both esophageal adenocarcinoma and squamous cell carcinoma patients did not demonstrate increased relief of dysphagia or a survival benefit while coming with the cost of greater toxicity.35 Our data would support future larger studies to validate an alternative approach of initially including immune checkpoint inhibition with RT in attempts to induce an abscopal response of the metastatic disease burden. Patients subsequently may not need to compromise starting of systemic therapy if palliative radiation therapy is needed first as anti-PD-1 therapy can be included with the RT and subsequently continued with cytotoxic chemotherapy once RT is concluded. In-field radiation antitumor responses may also be potentiated and has been suggested by other investigators reporting on retrospective data sets of metastatic gastric cancer patients receiving anti-PD-1 therapy preceding RT.36 Although not an objective of our trial, among our durably responding patients the target radiated lesions have also remained in response which exceeds expectations and reported durations of palliative radiation therapy courses.37 Collection of patient-reported outcomes in relation to palliation of symptoms of bleeding, pain, and dysphagia and quality-of-life measurements should be compared in a randomized study to ensure the benefit of adding anti-PD-1 therapy to single modality palliative RT.
Conclusions
Our study observed no concerning safety signals with combining PD-1 inhibitors with radiation therapy consistent with studies reported in other tumor types.38,39 A proportion of patients with MSS, EBV-negative tumors demonstrated encouraging durable responses that should be explored in larger trials. Weaknesses of our study include the lack of a comparative control arm of single agent pembrolizumab to clearly delineate if our observed responses may have occurred in the absence of concurrent radiation and our modest trial size limited to a single center. Larger studies should be considered to validate this strategy in metastatic gastroesophageal cancer and collect patient-reported outcomes to ascertain if quality-of-life improvements merits addition of immune checkpoint inhibition to usual symptoms alleviated by palliative radiation. Additional composite biomarkers beyond PD-L1 which use novel testing methodologies and orthogonal approaches such as liquid biopsies should be incorporated.
Acknowledgments
The authors would like to acknowledge the patients and families that participated in this clinical trial and Christopher Ruel for providing additional statistical support.
Footnotes
Sources of Support: This study was funded by a Merck Investigator Studies Program grant and partially supported by National Institutes of Health Grant 5K12CA001727-23. Research reported in this publication included work performed in the Biostatistics Core funded by the National Cancer Institute of the National Institutes of Health under award number P30CA033572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding organization Merck had a partial role in the design and conduct of the study; no role in collection, management, analysis, and interpretation of the data; no role in manuscript preparation, partial role in review, or approval of the manuscript; and partial role in decision to submit the manuscript for publication.
Disclosures: Dr Chao has received research funding (institutional) from Merck and Brooklyn Immunotherapeutics, has served a consultant/advisory role for Amgen, Macrogenics, Ono Pharmaceuticals, Daiichi-Sankyo, and Foundation Medicine, Inc, and serves on the Speakers Bureau for Merck. Dr Klempner has served a consultant/advisory role for Bristol Myers Squibb, Merck, Eli Lilly, Astellas, Pieris, and Foundation Medicine, Inc; reports stock/equity in Turning Point Therapeutics. The remaining authors declare that they have no conflicts of interest.
Research data are stored in an institutional repository and will be shared upon reasonable request to the corresponding author.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.adro.2021.100807.
Appendix. Supplementary materials
References
- 1.Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical keynote-059 trial. JAMA Oncol. 2018;4 doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah MA, Kojima T, Hochhauser D, et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: The phase 2 KEYNOTE-180 study. JAMA Oncol. 2019;5:546–550. doi: 10.1001/jamaoncol.2018.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449–1458. doi: 10.1038/s41591-018-0101-z. [DOI] [PubMed] [Google Scholar]
- 4.Kojima T, Shah MA, Muro K, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38:4138–4148. doi: 10.1200/JCO.20.01888. [DOI] [PubMed] [Google Scholar]
- 5.Wainberg ZA, Fuchs CS, Tabernero J, et al. Efficacy of pembrolizumab monotherapy for advanced gastric/gastroesophageal junction cancer with programmed death ligand 1 Combined Positive Score ≥10. Clin Cancer Res. 2021;27:1923–1931. doi: 10.1158/1078-0432.CCR-20-2980. [DOI] [PubMed] [Google Scholar]
- 6.Food and Drug Administration. FDA approves pembrolizumab for esophageal or GEJ carcinoma. Availale at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-esophageal-or-gej-carcinoma. Accessed March 22, 2021.
- 7.Food and Drug Administration. FDA approves nivolumab in combination with chemotherapy for metastatic gastric cancer and esophageal adenocarcinoma. Available at:https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-nivolumab-combination-chemotherapy-metastatic-gastric-cancer-and-esophageal. Accessed April 16, 2021.
- 8.Moehler M, Shitara K, Garrido M, et al. LBA6_PR Nivolumab (nivo) plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): First results of the CheckMate 649 study. Ann Oncol. 2020;31:S1191. [Google Scholar]
- 9.Kato K, Sun JM, Shah MA, et al. LBA8_PR Pembrolizumab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced esophageal cancer: The phase 3 KEYNOTE-590 study. Ann Oncol. 2020;31:S1192–S1193. [Google Scholar]
- 10.Mole RH. Whole body irradiation: Radiobiology or medicine? Br J Radiol. 1953;26:234–241. doi: 10.1259/0007-1285-26-305-234. [DOI] [PubMed] [Google Scholar]
- 11.Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–5468. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 13.Park SS, Dong H, Liu X, et al. PD-1 restrains radiotherapy-induced abscopal effect. Cancer Immunol Res. 2015;3:610–619. doi: 10.1158/2326-6066.CIR-14-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dovedi SJ, Cheadle EJ, Popple AL, et al. Fractionated radiation therapy stimulates antitumor immunity mediated by both resident and infiltrating polyclonal T-cell populations when combined with PD-1 blockade. Clin Cancer Res. 2017;23:5514–5526. doi: 10.1158/1078-0432.CCR-16-1673. [DOI] [PubMed] [Google Scholar]
- 15.Kulangara K, Zhang N, Corigliano E, et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med. 2019;143:330–337. doi: 10.5858/arpa.2018-0043-OA. [DOI] [PubMed] [Google Scholar]
- 16.Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): A multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–726. doi: 10.1016/S1470-2045(16)00175-3. [DOI] [PubMed] [Google Scholar]
- 17.Shitara K, Özgüroğlu M, Bang Y-J, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123–133. doi: 10.1016/S0140-6736(18)31257-1. [DOI] [PubMed] [Google Scholar]
- 18.Shitara K, Van Cutsem E, Bang YJ, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: The KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6:1571–1580. doi: 10.1001/jamaoncol.2020.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu J, Green MD, Li S, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. 2021;27:152–164. doi: 10.1038/s41591-020-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ajani JA, D'Amico TA, Almhanna K, et al. Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14:1286–1312. doi: 10.6004/jnccn.2016.0137. [DOI] [PubMed] [Google Scholar]
- 21.Ajani JA, D'Amico TA, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:855–883. doi: 10.6004/jnccn.2019.0033. [DOI] [PubMed] [Google Scholar]
- 22.Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly RJ, Zaidi AH, Smith MA, et al. The dynamic and transient immune microenvironment in locally advanced esophageal adenocarcinoma post chemoradiation. Ann Surg. 2018;268:992–999. doi: 10.1097/SLA.0000000000002410. [DOI] [PubMed] [Google Scholar]
- 24.Zhou KI, Peterson BF, Serritella A, et al. Spatial and temporal heterogeneity of PD-L1 expression and tumor mutational burden in gastroesophageal adenocarcinoma at baseline diagnosis and after chemotherapy. Clin Cancer Res. 2020;26:6453–6463. doi: 10.1158/1078-0432.CCR-20-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klempner SJ, Upadhyay V, Chao J. A space-time continuum for immunotherapy biomarkers in gastroesophageal cancer? Clin Cancer Res. 2020;26:6401–6403. doi: 10.1158/1078-0432.CCR-20-3389. [DOI] [PubMed] [Google Scholar]
- 26.Ye M, Huang D, Zhang Q, et al. Heterogeneous programmed death-ligand 1 expression in gastric cancer: Comparison of tissue microarrays and whole sections. Cancer Cell Int. 2020;20:186. doi: 10.1186/s12935-020-01273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang JH, Kim H, Roh SY, et al. Discordancy and changes in the pattern of programmed death ligand 1 expression before and after platinum-based chemotherapy in metastatic gastric cancer. Gastric Cancer. 2019;22:147–154. doi: 10.1007/s10120-018-0842-x. [DOI] [PubMed] [Google Scholar]
- 28.Hofman P, Heeke S, Alix-Panabières C, Pantel K. Liquid biopsy in the era of immuno-oncology: Is it ready for prime-time use for cancer patients? Ann Oncol. 2019;30:1448–1459. doi: 10.1093/annonc/mdz196. [DOI] [PubMed] [Google Scholar]
- 29.Griffiths JI, Wallet P, Pflieger LT, et al. Circulating immune cell phenotype dynamics reflect the strength of tumor-immune cell interactions in patients during immunotherapy. Proc Natl Acad Sci U S A. 2020;117:16072–16082. doi: 10.1073/pnas.1918937117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maron SB, Chase LM, Lomnicki S, et al. Circulating tumor DNA sequencing analysis of gastroesophageal adenocarcinoma. Clin Cancer Res. 2019;25:7098–7112. doi: 10.1158/1078-0432.CCR-19-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasi PM. Circulating tumor DNA and plasma microsatellite instability during PD-1 blockade. J Gastrointest Oncol. 2020;11:826–828. doi: 10.21037/jgo-20-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bratman SV, Yang SYC, Iafolla MAJ, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nature Cancer. 2020;1:873–881. doi: 10.1038/s43018-020-0096-5. [DOI] [PubMed] [Google Scholar]
- 33.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sunakawa Y, Matoba R, Inoue E, et al. Genomic pathway of gut microbiome to predict efficacy of nivolumab in advanced gastric cancer: DELIVER trial (JACCRO GC-08) J Clin Oncol. 2021;39(3 Suppl):161. [Google Scholar]
- 35.Penniment MG, De Ieso PB, Harvey JA, et al. Palliative chemoradiotherapy versus radiotherapy alone for dysphagia in advanced oesophageal cancer: A multicentre randomised controlled trial (TROG 03.01) Lancet Gastroenterol Hepatol. 2018;3:114–124. doi: 10.1016/S2468-1253(17)30363-1. [DOI] [PubMed] [Google Scholar]
- 36.Sasaki A, Nakamura Y, Togashi Y, et al. Enhanced tumor response to radiotherapy after PD-1 blockade in metastatic gastric cancer. Gastric Cancer. 2020;23:893–903. doi: 10.1007/s10120-020-01058-4. [DOI] [PubMed] [Google Scholar]
- 37.Tey J, Zheng H, Soon YY, et al. Palliative radiotherapy in symptomatic locally advanced gastric cancer: A phase II trial. Cancer Med. 2019;8:1447–1458. doi: 10.1002/cam4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luke JJ, Lemons JM, Karrison TG, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol. 2018;36:1611–1618. doi: 10.1200/JCO.2017.76.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho AY, Barker CA, Arnold BB, et al. A phase 2 clinical trial assessing the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer. 2020;126:850–860. doi: 10.1002/cncr.32599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.