Abstract
Background
Oral squamous cell carcinoma is characterized by high degree of local invasion with metastasis as well as characteristic angiogenic features. Angiogenesis is a critical step in the growth and metastasis of tumors. Cluster of differentiation 44 (CD44) is a cell surface glycoprotein which is widely expressed in both physiological and pathological conditions.
Aim
The study was designed to assess the expression of CD44 (NKI-P1) in oral squamous cell carcinoma associated endothelial cells and to correlate this expression with matrix metalloprpteinase-9 (MMP-9) and transforming growth factor-beta (TGF- β) expression immunohistochemically.
Materials and methods
One hundred fourteen archival oral squamous cell carcinoma cases were used in this study. Immunohistochemistry was performed for CD44 (NKI-P1), Ki-67, cluster of differentiation 31(CD31), podoplanin (D2-40), MMP-9 and TGF- β. Microvessel density was also determined morphologically. Results: CD44 was expressed in (CD31+/ Podoplanin –) blood vascular endothelial cells in a strong cytoplasmic fashion. In addition, the extracellular matrix proteins (MMP-9 and TGF-β were expressed in oral squamous cell carcinoma stroma which was enriched with CD44 + blood vessels. The mean numbers of microvessel density in connective tissue beneath normal epithelium and different grades of oral squamous cell carcinoma stroma were 5.8, 22.1, 22.4 and 23.5, respectively with the P-value < 0.05, where a significant statistical difference between microvessel density in the connective tissue beneath normal epithelia and stroma of all grades of oral squamous cell carcinoma was found. Conclusion: CD44 (NKI-P1) is considered a potential marker of oral squamous cell carcinoma angiogenesis and it can be used as a valuable biomarker of tumor invasion and as a therapeutic target for anti-angiogenic therapies.
Keywords: Angiogenesis, CD44, Extracellular matrix, Oral squamous cell carcinoma
1. Introduction
Oral squamous cell carcinoma (OSCC) has a confrontational malignancy characteristic features originating from squamous epithelium of the oral mucosa and is considered the most common type of head and neck cancers (Siegel et al., 2017). One of the characteristics of OSCC is the high degree of local invasiveness as well as high metastatic and recurrence rates (Bettendorf et al., 2004).
Despite the improved therapeutic modalities, the survival of patients with oral cancer has remained consistent (Neville and Day, 2002). The high rate of relapse in OSCC indicates insufficiency of current prognostic factors in predicting the metastatic potential and tumor outcomes (Cortesina and Martone, 2006). Moreover, it has been widely accepted that growth and metastasis are angiogenesis-dependent where the new blood vessels nourish the tumor by supplying oxygen and nutrients and are crucial for its growth. Therefore, blocking angiogenesis could be a strategy for tumor growth arrest (Folkman, 2000).
Cluster of differentiation 44 (CD44) is a transmembrane 80–90 kDa cell surface adhesion molecule glycoprotein which is widely expressed in physiological and pathological conditions with different splicing variants (Senbanjo and Chellaiah, 2017). It is a cell surface receptor for hyaluronic acid (HA) and the binding of both occurs through the amino-terminal HA-conserved binding region of CD44 (Isacke and Yarwood, 2002). Some of the cells such as epithelial cells, leukocytes, tumor cells and vascular endothelial cells, show the cell surface adhesion receptor characteristics (Isacke and Yarwood, 2002).
Interaction of CD44 with ligands at the extracellular domain is essential for cell signaling regulation (Basakran, 2015). In this context, CD44 acted as a protein responsible for cellular attachment to the extracellular matrix, angiogenesis, migration and invasion depending on its splicing variant (Desai et al., 2007). In different types of cancers, CD44 is also a known marker of cancer stem cells and carcinoma cells produce many variants of CD44 (Hiraga et al., 2013).
Several angiogenic markers that play a role in tumor angiogenesis have been extensively investigated such as fibroblast growth factor (FGF), transforming growth factor-α (TGF- α), TGF-β, vascular endothelial growth factor (VEGF), angiopoietins, tumor necrosis factor (TNF), interleukin-8 (IL-8), hepatocyte growth factor (HGF) and angiogenin (Ferrara, 2011). However, little is known about the expression pattern and functional characteristics of CD44 in OSCC associated endothelial cells. It has been clarified that TGF- β and matrix metalloproteinase-9 (MMP-9) play a role in tumor angiogenesis and progression (Qin and Ivan, 2000). Moreover, previous reports demonstrated an interaction between CD44 and both TGF- β and MMP-9 in different neoplasms and that interaction is required to enhance tumor growth and invasion (Olofsson and Porsch, 2014, Ludwig et al., 2019). Therefore, this study was aimed to correlate the immunohistochemical expression of CD44 in OSCC associated vascular endothelial cells with that of TGF- β and MMP-9 to verify the relationship of CD44 with tumor angiogenesis and progression.
2. Materials and methods
2.1. Selection of cases
The present study was conducted in oral pathology department, Faculty of Dentistry, Tanta University. The period of the study was five years, from January 2015 to January 2020. A total of 114 archival OSCC cases were collected. These cases were histologically diagnosed as OSCC with the following grades well differentiated (n = 54), moderately differentiated (n = 42) and poorly differentiated (n = 18). Normal oral epithelium is included from 20 gingivectomy cases (as a control group) after obtaining their written consent.
The research proposal and the experimental protocol of the surgical materials was reviewed and analyzed after having the approval of the Ethical Board, Faculty of Dentistry, Tanta University.
2.2. Inclusion criteria
All OSCC cases of primary origin in all intraoral sites were included in this study.
2.3. Exclusion criteria
Cases with distant OSCC metastasis as well as regional lymph node OSCC metastasis were excluded.
2.4. Conventional hematoxylin and eosin staining
The samples were fixed using formalin (10%) and were processed and embedded in paraffin. Hematoxylin, eosin and immunohistochemical staining was performed using the serial sections cut at 5 μm from paraffin blocks.
2.5. Antibodies
Mouse monoclonal antibodies against human CD44 (clone NKI-P1), Ki-67 (MIB 1), podoplanin (D2-40, IgG1), CD31 (JC70A, IgG1) were purchased from Dako (Glostrup, Denmark). Mouse monoclonal antibodies against human MMP −9 (clone 56-2A4) was purchased from Daiichi fine chemical co. ltd. Rabbit polyclonal antibody raised against a peptide mapping at the C-terminus of TGF-ß1 [(V): sc-146] of human origin were obtained from Santa Cruz Biotechnology, Inc.
2.6. Immunohistochemistry
Immunohistochemistry was done using the ChemMate EnvisionTM system (Dako). For CD44, Ki-67, podoplanin and CD31, sections were autoclaved in citrate buffer (pH 6.0) for 10 min at 121 °C. Then, sections were treated with 0.3% hydrogen peroxide in methanol for 30 min at room temperature to block endogenous peroxidase activity and incubated with 5% milk protein in 0.01 M phosphate-buffered saline (PBS, pH 7.4) containing 0.05% Triton X-100 (T-PBS) for 1 h at room temperature to block non-specific protein binding sites. They were then incubated overnight at 4˚C with the primary antibodies diluted at 1:100 (CD44, Ki-76, podoplanin, CD31 and MMP – 9) and at 1: 50 for (TGF-ß1) in PBS. Once the overnight incubation was completed, the sections were incubated with the Envision reagents for 1 h at room temperature. Reaction products were visualized with 0.02% 3, 30-diaminobenzidine in 0.05 M Tris-HCl buffer (pH 7.6) containing 0.005% hydrogen peroxide. Finally, the sections were counterstained with hematoxylin. For control studies, the primary antibodies were replaced with preimmune IgGs.
2.7. Counting of microvessel density (MVD)
The entire sections were scanned at low magnification in order to identify the most highly vascularized areas (hot spots), and three hotspot areas were photographed at high magnification using a 20 × objective lens. Blood vessels that were positive for CD44 were counted manually in a unit field of 0.54 mm2 and considered MVD. Then, the average was computed and the statistical significance of differences was determined using Anova test (Maturana-Ramírez et al., 2015).
2.8. Statistical evaluation
The data obtained in the present study were collected, tabulated and analyzed statistically using the “SPSS 21” (Statistical Package for Scientific Studies) (SPSS Inc., Chicago, Illinois, USA). The probability value (p -value with 0.05) was used in the assessment of the significance. The data was analyzed with segregation of the data based on the group. The mean and standard deviation with the test performed was ANOVA with Post Hoc test to study and analyze the difference between the groups and In between the group (Intra and Inter).
3. Results
3.1. CD44 expression beneath normal epithelia and in oral squamous cell carcinoma associated blood vessels
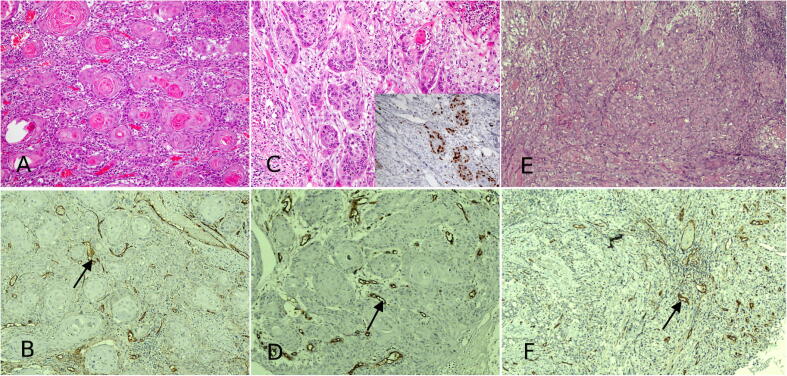
In normal epithelia, there was less CD44 expression in the underlying connective tissue stroma associated blood vessels with low MVD (Fig. 1 A and B). The mean numbers of MVD in connective tissue beneath normal epithelium and different grades of OSCC stroma were 5.8, 22.1, 22.4 and 23.5 respectively (Table 1). The difference was statistically significant (P-value < 0.05) only between MVD in the connective tissue beneath normal epithelia and stroma of all grades of OSCC. It was observed with probability value more than 0.05 between the MVD in stroma of all grades of OSCC. Table 2 showing the statistical significance (P-value 0.001) within and between the groups. In well-differentiated OSCC, where there are numerous islands of malignant epithelial cells with central keratin formation (Fig. 2A), OSCC stroma was enriched with CD44 + blood vessels (Fig. 2B).
Fig. 1.

A photomicrograph of H&E stained tissue sections reveal normal oral epithelia (A) with underlying connective tissue containing less number of CD44 + blood vessels (B). Hematoxylin and eosin (HE) (a) and immunoperoxidase stains for CD44 (b) hematoxylin counterstain. (a, b) × 100.
Table 1.
Blood vessel density in the connective tissue beneath normal epithelia and in different grades of OSCC stroma. ANOVA * p-value < 0.05.
| mean BVD number | |
|---|---|
| Normal epithelia (control) (n = 20) | 5.8 |
| Well differentiated OSCC stroma (n = 54) | 22.1 |
| Moderately differentiated OSCC stroma (n = 42) | 22.4 |
| Poorly differentiated OSCC stroma (n = 18) | 23.5 |
Table 2.
ANOVA analysis with pairwise comparison of inter and intra groups. ANOVA/ post-hoc * p-value < 0.001.
| ANOVA analysis | ||
|---|---|---|
| Significance | ||
| MMP-9 | Between Groups | <0.001* |
| Within Groups | ||
| Total | ||
| TGF- beta | Between Groups | <0.001* |
| Within Groups | ||
| Total | ||
| CD44 | Between Groups | <0.001* |
| Within Groups | ||
| Total | ||
Fig. 2.
A photomicrograph of H&E stained tissue sections exhibit a case of OSCC (well-differentiated) showing islands of malignant epithelial cells with keratin pearl formation (A); CD44 + blood vessels are shown in the stroma of OSCC (B). Invasive malignant epithelial cells in a case of moderately differentiated OSCC (C) most of these malignant cells exhibit Ki-67 immunostaining (inset). Another case of OSCC reveals invasive scattered malignant epithelial cells in poorly differentiated OSCC (E) The stroma of OSCC shows numerous blood vessels with CD44 positivity with intense cytoplasmic expression for CD44 in vascular endothelial cells (F) (arrows). Hematoxylin and eosin (HE) (a, c and e) and immunoperoxidase stains for CD 44 (b, d and f)); (a -f) × 200.
Moderately differentiated OSCC (Fig. 2C and D) where there are islands of malignant epithelial cells invading the connective tissue was observed. Most of the malignant cells were Ki-67 positive. (Fig. 2C, inset). Expression of CD444 was evident in OSCC associated endothelial cells (F 2D). In OSCC, poorly differentiated (Fig. 2E) where there are scattered malignant epithelial cells invading the connective tissue with loss of cellular cohesiveness as well as increased mitotic figures, there were numerous blood vessels that were positive for CD44 (Fig. 2F) in a strong cytoplasmic fashion.
3.2. CD44 positive endothelial cells are of vascular but not of lymphatic origin
In OSCC stroma, numerous endothelial cells showed strong cytoplasmic expression for CD44 (Fig. 3A). These CD44 positive cells were positive for CD31 (Fig. 3B) but not for podoplanin (Fig. 2C). These findings indicate that CD 44 positive endothelial cells are of vascular but not of lymphatic origin.
Fig. 3.
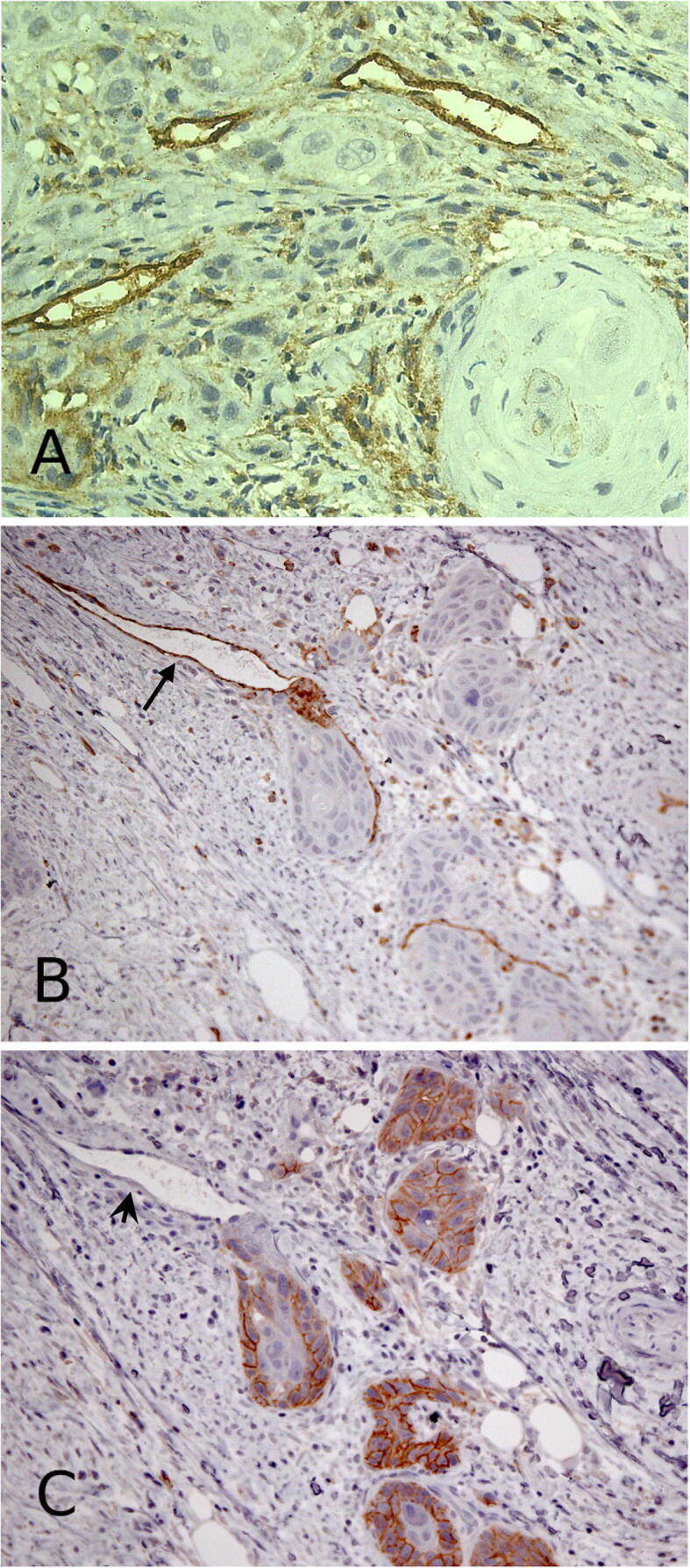
A photomicrograph shows a stroma of OSCC demonstrating numerous endothelial cells with strong positivity for CD44 (A). These vessels exhibit CD31 positivity (B) (arrow) but no immunoreactivity for D2-40 is seen (C) (arrow head). CD31+/ Podoplanin – blood vessels were also strongly positive for CD44. Immunoperoxidase stains for CD44 (a), CD31 (b), and D2-40 (c) (a) × 400 (b, c) × 200.
3.3. CD44 correlation with OSCC extracellular matrix proteins
In this study, we investigated the expression of two extracellular matrix (ECM) proteins MMP-9 and TGF-β to assess the correlation between CD44 with these proteins in the process of tumor angiogenesis. The extracellular matrix proteins were expressed in OSCC stroma that was enriched with CD44 + blood vessels (Fig. 4A), MMP-9 (Fig. 4B) and TGF-β (Fig. 4C). We found that CD44 was highly expressed in OSCC associated blood vessels due to exposure to these ECM proteins. The association of CD44 with MMP-9 and TGF-β may increase CD44 expression on OSCC associated blood vessels, which enables endothelial cell union with ECM components and facilitates tumor neovascularization and induction of new vessel formation in OSCC stroma.
Fig. 4.
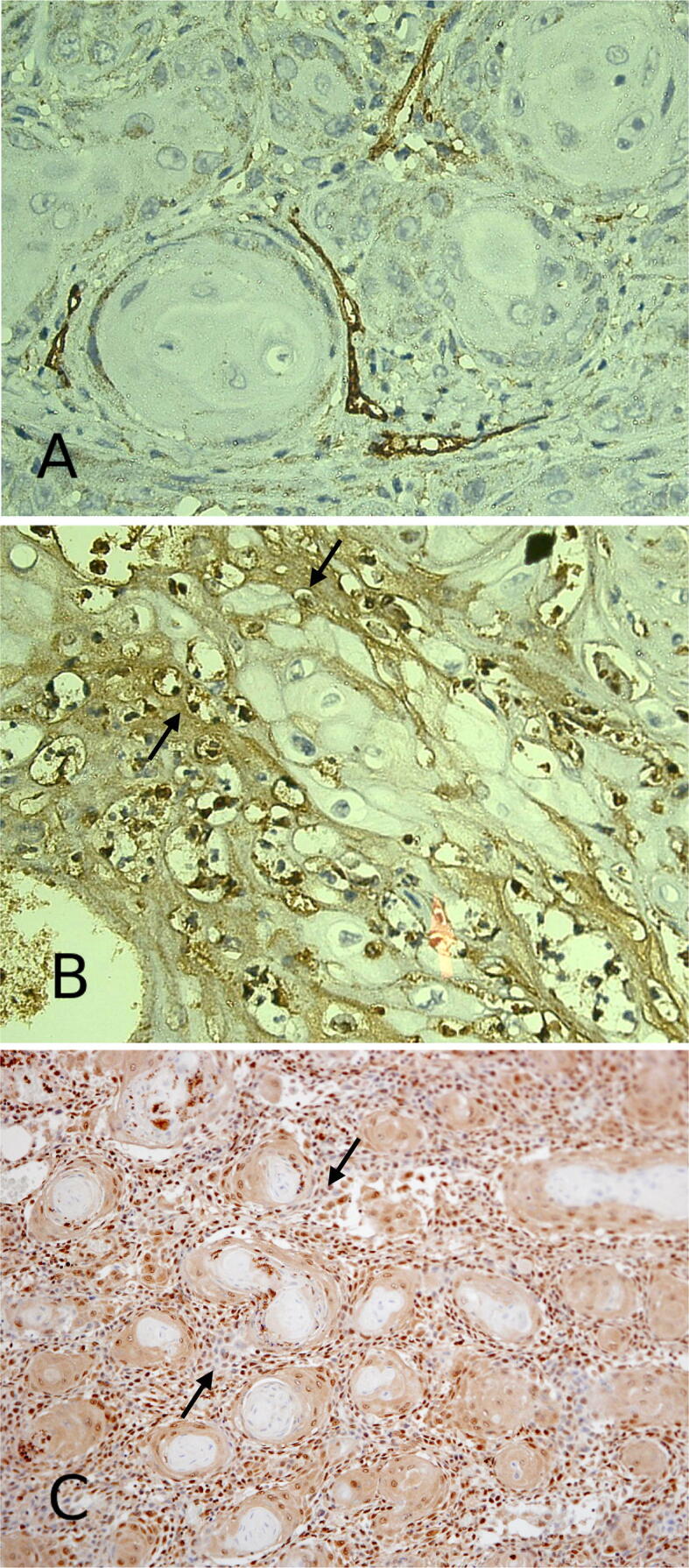
The expression of extracellular matrix proteins MMP-9 (B) and TGF-β (C) (arrows) are seen in OSCC stroma that exhibits CD44 immunostaining (A). Immunoperoxidase stains for CD44 (a), MMP-9 (b) and TGF-β (c) hematoxylin counterstain. (a, b) × 400 (c) × 200.
4. Discussion
Angiogenesis is defined as the process of formation of new blood vessels sprouting out from preexisting ones. It is considered a crucial step in the progression of almost all human malignancies including OSCC (Michael and Taija, 2017). Hence, antiangiogenic therapy may appear to be effective strategy for treatment of angiogenesis-dependent diseases such as cancer (Fallah et al., 2019).
Indeed, many angiogenic factors such as TGF-α, TGF-β, VEGF and matrix metalloproteinase-9 (MMP-9) have been reported (Ferrara, 2011). In the present study, we demonstrated the expression of CD44 in OSCC associated blood vessels. Interestingly, the extracellular matrix proteins MMP-9 and TGF-β were expressed in OSCC that was enriched with CD44 positive blood vessels.
Immunohistochemically, we used CD31 as vascular endothelial cell marker and D2-40 (podoplanin) as lymphatic endothelial cell marker to confirm that OSCC associated endothelial cells were positive with them (Fukunaga, 2005, Liu and Shi, 2012).
The findings of this study are consistent with those of Kim et al who demonstrated high expression of CD44 in epithelial ovarian cancer associated endothelial cells as compared to normal tissue (Kim et al., 2018). Moreover, it has been reported that CD44 was involved in blood vessel formation and that tumor and wound angiogenesis were restrained after blocking CD44 function, particularly the specific isoform CD44 v6 with anti-CD44 antibody (Cao et al., 2006).
A variety of settings based on physiological and pathological conditions occurs by the binding of CD44 and its isoforms to numerous extracellular matrix components such as HA and MMPs (Isacke and Yarwood, 2002). It has been demonstrated that the diversity of CD44 function may result from large number of isoforms produced by alternative splicing and their subsequent changeable glycosylation as well as posttranscriptional CD44 modifications (Senbanjo and Chellaiah, 2017). The CD44 initiates the pathways after binding to its immobilized ligand HA, which promotes the angiogenesis in tumors (Misra et al., 2011). Moreover, it has been recognized that CD44-hyaluronate interactions may be more important for migration and differentiation that include endothelial cell tube formation during the process of angiogenesis (Trochon et al., 1996).
In accordance with our findings, Ludwig et al illustrated a strong relationship between CD44 expression and various proangiogenic factors such as VEGF, FGF and TGF-β (Ludwig et al., 2019). Moreover, CD44 was shown to enhance MMP-9 activation and tumor angiogenesis is initiated by activation of TGF-β via a CD44-dependent MMP-9 activation (Qin and Ivan, 2000). In this context, it seems that the cleavage of latent TGF-β in a CD44-dependent manner can promote tumor growth and invasion as a result of induction of angiogenesis that occurs due to remodeling of endothelial pericellular matrix (Qin and Ivan, 2000, Ludwig et al., 2019).
An additional interesting correlation between CD44 and extracellular matrix proteins in which CD44, and matrix metalloproteinase 9 (MMP-9) form a complex on the surface of TA/St mouse mammary carcinoma cells that activates latent transforming growth factor-beta (TGF-β) and is required for tumor invasion (Yu and Stamenkovic, 2004). In addition, Knockdown of CD44 resulted in an inability to form a tubular network and the interaction between CD44 and hyaluronan regulates microvascular endothelial cell tubulogenesis (Olofsson and Porsch, 2014).
In conclusion, CD44 is expressed in OSCC associated endothelial cells as an activation marker associated with proliferation and angiogenesis. Furthermore, CD44 expression is correlated with the extracellular matrix proteins MMP-9 and TGF-β. Therefore, this study provides important new insights into the pivotal role of CD44 in OSCC angiogenesis and we suggest that CD44 might be a useful therapeutic target for anti-angiogenic therapies. However, further investigations are highly recommended to identify the exact mechanism of CD44 in the process of angiogenesis in OSCC.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Ahmed Abdelaziz Mohamed Essa, Email: a.esa@bu.edu.sa, ahmedessa@dent.tanta.edu.eg.
Elsayed Mohamed Deraz, Email: elsayed.deraz@dent.tanta.edu.eg.
References
- Basakran N.S. CD44 as a potential diagnostic tumor marker. Saudi Med. J. 2015;36(3):273–279. doi: 10.15537/smj.2015.3.9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettendorf O., Piffkò J., Bànkfalvi A. Prognostic and predictive factors in oral squamous cell cancer: important tools for planning individual therapy? Oral Oncol. 2004;40(2):110–119. doi: 10.1016/j.oraloncology.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Cao G., Savani R.C., Fehrenbach M., Lyons C., Zhang L., Coukos G., DeLisser H.M. Involvement of endothelial CD44 during in vivo angiogenesis. The Am. J. Pathol. 2006;169(1):325–336. doi: 10.2353/ajpath.2006.060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortesina G., Martone T. Molecular metastases markers in head and neck squamous cell carcinoma: review of the literature. Acta Otorhinolaryngol. Ital. 2006 Dec;26(6):317. [PMC free article] [PubMed] [Google Scholar]
- Desai B., Rogers M.J., Chellaiah M.A. Mechanisms of osteopontin and CD44 as metastatic principles in prostate cancer cells. Mol Cancer. 2007;7(6):18. doi: 10.1186/1476-4598-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallah A., Sadeghinia A., Kahroba H., Samadi A., Heidari H.R., Bradaran B., Zeinali S., Molavi O. Therapeutic targeting of angiogenesis molecular pathways in angiogenesis-dependent diseases. Biomed. Pharmacother. 2019;110:775–785. doi: 10.1016/j.biopha.2018.12.022. [DOI] [PubMed] [Google Scholar]
- Ferrara N. VEGF and the quest from tumor angiogenesis factors. Nat. Rev. Cancer. 2002;2(10):795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis in Cancer Medicine (Holland J. F., ed) 5th Ed., (2000) pp. 132–152, B. C. Decker, Inc., Ontario, Canada.
- Fukunaga M. Expression of D2–40 in lymphatic endothelium of normal tissues and in vascular tumours. Histopathology. 2005;46(4):396–402. doi: 10.1111/j.1365-2559.2005.02098.x. [DOI] [PubMed] [Google Scholar]
- Hiraga T., Ito S., Nakamura H. Cancer stem-like cell marker CD44 promotes bone metastases by enhancing tumorigenicity, cell motility, and hyaluronan production. Cancer Res. 2013;73(13):4112–4122. doi: 10.1158/0008-5472.CAN-12-3801. [DOI] [PubMed] [Google Scholar]
- Isacke C.M., Yarwood H. The hyaluronan receptor, CD44. Int. J. Biochem. Cell Biol. 2002;34(7):718–721. doi: 10.1016/s1357-2725(01)00166-2. [DOI] [PubMed] [Google Scholar]
- Kim G.H., Won J.E., Byeon Y., Kim M.G., Wi T.I., Lee J.M., Park Y.-Y., Lee J.-W., Kang T.H., Jung I.D., Shin B.C., Ahn H.J., Lee Y.J., Sood A.K., Han H.D., Park Y.-M. Selective delivery of PLXDC1 small interfering RNA to endothelial cells for anti-angiogenesis tumor therapy using CD44-targeted chitosan nanoparticles for epithelial ovarian cancer. Drug Deliv. 2018;25(1):1394–1402. doi: 10.1080/10717544.2018.1480672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Shi G.-P. CD31: beyond a marker for endothelial cells. Cardiovasc. Res. 2012;94(1):3–5. doi: 10.1093/cvr/cvs108. [DOI] [PubMed] [Google Scholar]
- Ludwig N., Szczepanski M.J., Gluszko A., Szafarowski T., Azambuja J.H., Dolg L., Gellrich N.-C., Kampmann A., Whiteside T.L., Zimmerer R.M. CD44 (+) tumor cells promote early angiogenesis in head and neck squamous cell carcinoma. Cancer Lett. 2019;467:85–95. doi: 10.1016/j.canlet.2019.10.010. [DOI] [PubMed] [Google Scholar]
- Maturana-Ramírez A., Espinoza I., Reyes M., Pablo Aitken J., Aguayo F., Hartel S. Rojas-Alcayaga G Higher blood vessel density in comparison to the lymphatic vessels in oral squamous cell carcinoma. Int J Clin Exp Pathol. 2015;8(10):13677–13686. [PMC free article] [PubMed] [Google Scholar]
- Michael P., Taija M. Vascular heterogeneity and specialization in development and disease. Nat. Rev. Mol. Cell Biol. 2017;18(8):477. doi: 10.1038/nrm.2017.36. [DOI] [PubMed] [Google Scholar]
- Misra S., Heldin P., Hascall V.C., Karamanos N.K., Skandalis S.S., Markwald R.R., Ghatak S. Hyaluronan–CD44 interactions as potential targets for cancer therapy. FEBS J. 2011;278(9):1429–1443. doi: 10.1111/j.1742-4658.2011.08071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville B.W., Day T.A. Oral cancer and precancerous lesions. CA Cancer J. Clin. 2002;52(4):195–215. doi: 10.3322/canjclin.52.4.195. [DOI] [PubMed] [Google Scholar]
- Olofsson B., Porsch H. Heldin P Knock-Down of CD44 Regulates Endothelial Cell Differentiation via NFkB-Mediated Chemokine Production. PLoS ONE. 2014;9(3) doi: 10.1371/journal.pone.0090921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y., Ivan S. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14(2):163–176. [PMC free article] [PubMed] [Google Scholar]
- Senbanjo L.T., Chellaiah M.A. CD44: a multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of Cancer cells. Front. Cell Dev. Biol. 2017;5:18. doi: 10.3389/fcell.2017.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. CA Cancer J. Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- Trochon V., Mabilat C., Bertrand P., Legrand Y., Smadja-Joffe F., Soria C., Delpech B., Lu H.e. Evidence of involvement of CD44 in endothelial cell proliferation, migration and angiogenesis in vitro. Int. J. Cancer. 1996;66(5):664–668. doi: 10.1002/(SICI)1097-0215(19960529)66:5<664::AID-IJC14>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Yu Q., Stamenkovic I. Transforming growth factor-beta facilitates breast carcinoma metastasis by promoting tumor cell survival. Clin. Exp. Metastasis. 2004;21(3):235–242. doi: 10.1023/b:clin.0000037705.25256.d3. [DOI] [PubMed] [Google Scholar]