Abstract
Background
Bariatric surgical operation is taken into consideration to be the handiest remedy for extreme obesity. Durability is the main requirement for the broad usage of bariatric surgery. According to several factors, the present work tries to match the SG and RYGB techniques.
Methods
This is a retrospective work that studied 200 morbid obese patients randomized and categorized into two groups according to the treatment method: the laparoscopic sleeve gastrectomy (LSG) and LRYGB groups, within the period from 2014 to 2019 and matched weight dissipation, complications, quality of life, and adverse events.
Results
BMI had a mean value of 39.66 ± 3.770 kg/m2 in the RYGB group versus 39.38 ± 3.648 kg/m2. No significant differences were found according to comorbidity, height, and weight. There was no significant difference between the study groups according to complications and morbidity—no recorded unexpected histopathology results in the excised LSG specimens.
Conclusion
There was no significant change in weight dissipation, fluctuations in comorbidities, increase in Quality of Life (QoL), and complications for pathological obesity patients according to the treatment methods of laparoscopic SG (sleeve gastrectomy) and RYGB at 2-years postoperative follow-up.
Keywords: Bariatric surgery, Laparoscopic surgery, Sleeve gastrectomy, Quality of life, Weight loss
Highlights
-
•
A study on 200 morbid obese patients of 2 groups; laparoscopic sleeve gastrectomy (LSG) and LRYGB.
-
•
No significant difference between the study groups according to complications and morbidity.
-
•
No significant difference in Quality of Life at 2-years postoperative follow-up.
-
•
Histopathology results for the examined Sleeve gastrectomy specimen were unremarkable.
-
•
Challenge of psychological or emotional changes should be considered.
1. Introduction
Obesity is the accumulation of overabundant fats [1] related to several chronic complications (e.g., T2DM (Type Two Diabetes Mellitus), hypertension, dyslipidemia, obstructive sleep apnea, osteoarthritis, and gastroesophageal reflux disease) [2]. Pathological obesity and its related complications are a big global challenge and an economic problem in many countries [3].
Although there are numerous treatment options for the morbidly obese by non-surgical weight dissipation methods based on the diet, exercise, drug administration and behavior, and some other therapeutic strategies, e.g., acupuncture [4], these methods cannot affect obesity-associated complications. In some patients, reverse reactions may be faced [5].
Bariatric surgical operation is very beneficial for morbid obesity therapy; this surgery was considered not only a metabolic but also a weight-dissipation operation [6]; it leads to an excellent durable steady weight-dissipation and decreases complications [7].
Bariatric surgical operation is advised for extreme obesity cases with a BMI (≥35 kg/m2) with at least one pathological situation (e.g., T2DM, hypertension, and obstructive sleep apnea) [8].
LRYGB (Laparoscopic Roux-en-Y gastric bypass) is the best and widely used for bariatric surgery due to its effectiveness and durability [9]. Still, this way needs a technicality and a long-term examination [10].
LRYGB has a higher loss in weight in comparison with some limited steps without clinically significant malabsorption [11]. It induces weight dissipation primarily by limiting food intake and dumping effect [12].
LSG (Laparoscopic Sleeve Gastrectomy) was considered to be the primary stage in a dual-step proceeding for high-obese patients’ treatment; now, it was considered as an independent way [13,14].
LSG is demonstrated by taking off about 80% of the side of the stomach in a perpendicular manner, leaving a residual tubular gastric pouch or sleeve [15]. The sleeve gastrectomy operation is technically handier to achieve and more rapid relative RYGB [16].
While one might assume that improved health, weight loss, and increased quality of life (QoL) would improve patients’ mood, a minority of patients may experience severe psychological complications, including depression, alcohol abuse, and suicidality, particularly after a period of 1–2 years post-surgery “honeymoon period.” [17].
In the present work, the prime goal was to match the SG and RYGB techniques according to weight dissipation, complications, life quality, and adverse events.
2. Material and methods
This is a retrospective work that studied 200 morbidly obese patients randomized and categorized into two groups according to the treatment method: the LSG and LRYGB groups.
The technique was illustrated with detail to all cases regarding probable complications and dietary plans after the operation. Informed written consent was obtained from each patient to be included in this work.
The study included cases age ranging from 18 to 65 with a BMI (Body Mass Index) more than 35 (BMI = weight (kg)/[height (m)]2), with at least one comorbidity (hypertension-dyslipidemia-obstructive sleep apnea-T2DM-arthritis) and previous failure of conservative treatment.
For Patients with BMI more than 60, we excluded psychiatric disorder, active gastric ulcer, active substance abuse, GERD (Severe Gastroesophageal Reflux Disease) with a large hiatal hernia, and previous bariatric surgery from the study.
Selected cases for histopathological examination.
The histopathological examination for the excised parts of LSG operations was performed for selected cases; the pathologists retrieved and studied the cases in this work.
The results of this study were reported in accordance with STROCCS reporting statements [18].
2.1. Interventions
LSG (Laparoscopic sleeve gastrectomy) technique:
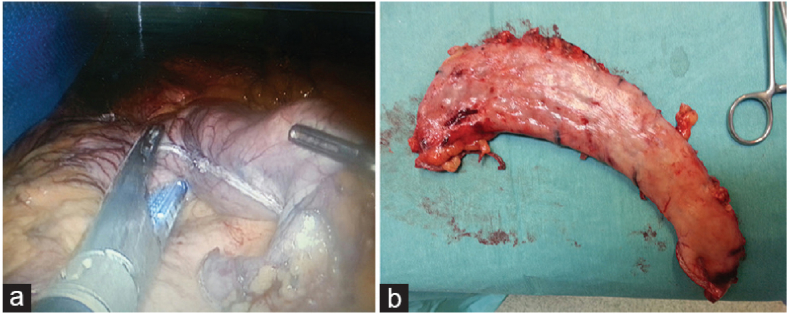
0A 36 Fr bougie was applied over the lower curvature to adjust the gastric tube. Longitudinal amputation of the stomach was performed for about 4–6 cm per pecker of the pylorus to the corner of His. No supportive materials were used, and over-suturing of the basic line was performed only over the bleeding points (see Fig. 1 from a patient's medical file).
Fig. 1.
(a) Resection of the outer part of the stomach using endo-GI stapler during sleeve gastrectomy. (b) Excised part of the stomach after sleeve gastrectomy. GI, gastrointestinal.
Specimens of LSG with any suspicious mucosal lesion were sent to histopathology laboratory for examination.
RYGB (Laparoscopic Roux-en-Y gastric bypass) method:
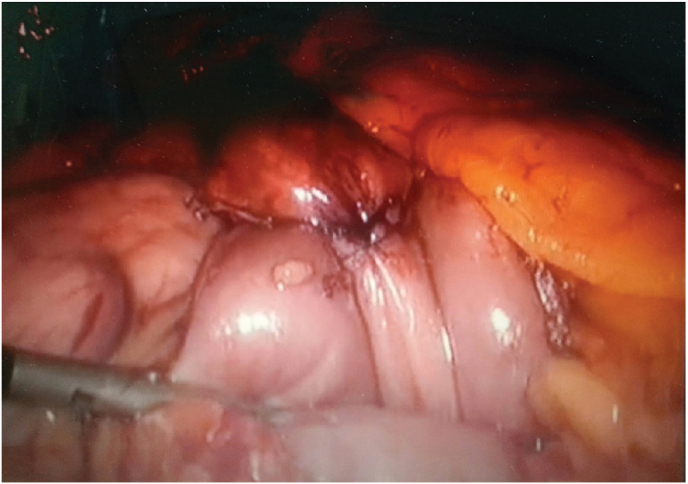
An ante colic and antegastric RYGB become executed with a 150 cm alimentary limb with a linearly or circularly kink (25 mm) gastrojejunostomy in step with the desire of the surgeon. A 50-cm-lengthy biliopancreatic limb was elected (Fig. 2).
Fig. 2.
Gastrojejunostomy (pouch-jejunostomy) during RYGB.
Methylene blue leak examination was routinely done during the operation, and a wide bore drain was applied near the staple line or anastomosis in both techniques.
After surgery, all the cases were periodically assayed on six weeks and after (3- 6-9-12-18-24) months, then annually.
All patients of the two groups underwent surgery under general anesthesia, premedicated with Metoclopramide 10 mg with Ranitidine 150 mg, pre -oxygenated with O2 100% for 5 min, Routinely ‘ramping’ in the 20° to 30° head-up position, then induction of anesthesia with propofol 1.5–2 mg/kg IBW(Ideal Body Weight), suxamethonium 1–1.5 mg/kg ABW(Actual Body Weight), Fentanyl 1 μg/kg ABW, lidocaine 1.5 mg/kg, intubated with rapid sequence induction using conventional laryngoscopy, McCoy laryngoscope (flexible tip blade) and bougie according to Mallampatti score (assessment of severity of difficult airway), maintenance of anesthesia with sevoflurane volatile agent, non-depolarizing muscle relaxant, fentanyl 50 mg/20 min, Ventilator settings were adjusted to maintain SPO2 between 94 and 100%and EtCO2 between 35 and 40 mmHg and. PEEP (Positive end expiratory pressure) of 5 cm H2O has been added to all patients, neuromuscular block has been reversed with Atropine 0.01 mg/kg and Neostigmine 0.04 mg/kg, after recovery pain controlled using multimodal analgesia.
2.2. Outcomes
The prime outcome of this work was weight dissipation that was recorded periodically.
The second outcomes included the change of obesity-related comorbidities as persisted, improved, or resolved and QoL based on GIQLI (Gastrointestinal Quality of Life Index) (consisting of 36 factors; ranging (0–144 points); each element scores from 4 to 0 points; the average record for normal persons is 125.8 points) [19] and the BAROS QoL (Bariatric Analysis and Reporting Outcome System QoL) score (consisting of 5 factors; record ranging (−3 to 3) points; for one factor the score range (1 to −1) and for the rest four factors ranging (0.5 to −0.5)) [20].
3. Results
A number of 200 cases were studied in this work during this period, according to the inclusion criteria. Of these 200 patients, 100 underwent RYGB and 100 with SG. Patients age with RYGB had an Average value of 41.30 ± 12.831 Vs. 42.60 ± 13.018 years.
Patients' sex shows that more than half in the RYGB groups were female 65 (65%) vs 52 (52%) in SG group. BMI had an average value of 39.66 ± 3.770 kg/m2 in RYGB group versus 39.38 ± 3.648 kg/m2. No significant differences were found according to comorbidity, height, and weight (see Table 1).
Table 1.
Demographic data for the two studied groups.
| RYGB Group (n = 100) | SG Group (n = 100) | P Value | |
|---|---|---|---|
| Age | 41.30 ± 12.831 | 42.60 ± 13.018 | 0.945 |
| Sex n(%) | |||
| Male | 35 (35.0%) | 48 (48.0%) | 0.085 |
| Female | 65 (65.0%) | 52 (52.0%) | |
| Comorbidity | |||
| HTN | 49 (49.0%) | 51 (51.0%) | 0.888 |
| Type 2 DM | 54 (54.0%) | 51 (51.0%) | 0.777 |
| Dyslipidemia | 68 (68.0%) | 65 (65.0%) | 0.765 |
| Obstructive Sleep Apnea | 67 (67.0%) | 68 (68.0%) | 1.000 |
| Arthritis | 72 (72.0%) | 67 (67.0%) | 0.539 |
| Height (cm) | 173.91 ± 5.109 | 174.67 ± 5.461 | 0.290 |
| Weight (kg) | 119.69 ± 9.515 | 119.20 ± 9.700 | 0.879 |
| BMI (kg/m2) | 39.66 ± 3.770 | 39.38 ± 3.648 | 0.594 |
Fifty-one (51.0%) patients in the SG group and 49 (49.0%) in the RYGB group had HTN. At 24 months after postoperative, total recovery was recorded in 30 (58.8%) of 51 in the SG group relative to 34 (69.4%) of 49 in RYGB group, and there were no significant differences among the study groups (Table 2).
Table 2.
Changes in Comorbidities at 2 yrs.
| HTN | RYGB Group (n = 100) |
SG Group (n = 100) |
P Value |
|---|---|---|---|
| 49/100 | 51/100 | ||
| Remission | 34 (69.4%) | 30 (58.8%) | 0.802 |
| Improved | 10 (20.4%) | 12 (23.5%) | |
| Unchanged | 3 (6.1%) | 5 (9.8%) | |
| Worsened | 2 (4.1%) | 4 (7.8%) | |
| Type 2 DM | 54/100 | 51/100 | |
| Remission | 44 (81.5%) | 41 (80.4%) | 0.903 |
| Improved | 3 (5.6%) | 5 (9.8%) | |
| Unchanged | 4 (7.4%) | 3 (5.9%) | |
| Worsened | 3 (5.6%) | 2 (3.9%) | |
| Dyslipidemia | 68/100 | 65/100 | |
| Remission | 38 (55.9%) | 34 (52.3%) | 0.258 |
| Improved | 24 (35.3%) | 21 (32.3%) | |
| Unchanged | 6 (8.8%) | 10 (15.4%) | |
| Worsened | 0 (0%) | 0 (0%) | |
| Obstructive Sleep Apnea | 67/100 | 68/100 | |
| Remission | 34 (50.7%) | 36 (52.9%) | 0.719 |
| Improved | 29 (43.3%) | 30 (44.1%) | |
| Unchanged | 2 (3.3%) | 2 (2.9%) | |
| Worsened | 2 (3.3%) | 0 (0%) | |
| Arthritis | 72/100 | 67/100 | |
| Remission | 36 (50.0%) | 37 (55.2%) | 0.312 |
| Improved | 24 (33.3%) | 26 (38.8%) | |
| Unchanged | 11 (15.3%) | 4 (6.0%) | |
| Worsened | 1 (1.4%) | 0 (0%) |
At baseline, 51 (51.0%) of 100 in the SG group and 54 (54.0%) of 100 in the RYGB group had DM2. At 2 yrs. Postoperative, total recovery was recorded in 41 (80.4%) of 51 in SG group and 44 (81.5%) of 54 in RYGB group, with no statistical significance among the study groups regarding FBG (Fasting Blood Glucose) (Table 2).
Before surgery, regarding dyslipidemia, there was a 65 (65.0%) in the SG group and 68 (68%) in the RYGB group. Total recovery was recorded in 34 (52.3%) of 65 in the SG group versus 38 (55.9%) of 68 in the RYGB group 2 years postoperative (Table 2).
At baseline, 68 (68.0%) in SG group and 67 (67.0%) in RYGB group had Obstructive Sleep Apnea. At 24-month postoperative, A total recovery was reported in 36 (52.9%) of 68 in SG group vs 34 (50.7%) of 67in RYGB group with no statistical significance between the studied techniques (Table 2).
Arthritis was shown in 67 (67.0%) in SG group and 72 (72%) in RYGB group. Total recovery was reported value was 37 (55.2%) of 67 in the SG group in comparison with 36 (50.0%) of 72 in the RYGB group 24 postoperative with no statistical significance between the two studied techniques (Table 2).
Quality of Life (QoL) in the two studied groups within baseline and 24 months was significantly increased. There was no statistical significance between the two studies.
Groups on the GIQLI (SG, 118.15 points, vs RYGB, 120.35 points, and the BAROS QoL record (2.1 for SG vs. 1.96 points for RYGB) (Table 3).
Table 3.
Quality-of-Life comparison for the study groups.
| Baseline |
P Value | After 2 years |
P Value | |||
|---|---|---|---|---|---|---|
| RYGB Group (n = 100) | SG Group (n = 100) | RYGB Group (n = 100) | SG Group (n = 100) | |||
| GIQLI | 101.17 ± 8.788 | 99.37 ± 7.679 | 0.112 | 120.35 ± 8.999 | 118.15 ± 8.362 | 0.245 |
| BAROS score | 0.30 ± 0.359 | 0.30 ± 0.250 | 0.627 | 2.10 ± 0.220 | 1.96 ± 0.335 | 0.546 |
Table 4 show complications and Mortality rate in the two groups and there were no significant differences between them.
Table 4.
Complications and death rate in the study groups.
| Complication | RYGB Group (n = 100) | SG Group (n = 100) | P Value |
|---|---|---|---|
| Leak | 2 (2.0%) | 0 (0.0%) | 0.497 |
| Infection | 5 (5.0%) | 0 (0.0%) | 0.059 |
| Obstruction | 1 (1.0%) | 2 (2.0%) | 1.000 |
| Death | 2 (2.0%) | 0 (0.0%) | 0.497 |
| Morbidity | |||
| Small bowel obstruction | 3 (3.0%) | 0 (0.0%) | 0.246 |
| Internal hernia | 11 (11.0%) | 0 (0.0%) | 0.001* |
| Incisional hernia | 3 (3.0%) | 2 (2.0%) | 1.000 |
| Severe dumping | 2 (2.0%) | 0 (0.0%) | 0.497 |
| Insufficient weight loss | 2 (2.0%) | 6 (6.0%) | 0.279 |
Histopathology examination was performed for the 8 cases of the LSG group; 3 cases showed normal (unremarkable) gastric features and 5 cases showed chronic gastritis. No malignancy was detected in all the examined specimens. All the received cases were shown clinical suspicion of abnormal mucosal lesions.
4. Discussion
Morbid obesity is a prime problem facing the globe which was considered the reason for a lot of comorbidities (e.g., cardiovascular disease, DM2, infertility, metabolic syndrome, and certain cancer types, giving rise to an increase in death rate) [21]. For severe obesity, bariatric surgical treatment is considered as the most successful management way, regarding the surgery type. Due to the variation in hormones and the patient's diet, this effect always takes place even before the beginning of weight dissipation [22].
Bariatric operation is the best way to manage morbid obesity patients. Till now, RYGB was considered the gold standard bariatric operation. However, SG was used recently with a growing rate regardless of the insufficiency of long-term efficacy. The LSG procedure is handier, faster, and might be more secure in comparison with RYGB. However, there are many more available references on the RYGB technique regarding long-time results of clinical and metabolic [23,24].
The auspicious short-time outcomes of LSG have somewhat changed from a two-step procedure to an independent bariatric method. LSG is considered to be less invasive, technically simpler, and operational handier compared with LRYGB. The long-time advantages of LSG include the absence of internal hernias, an intact gastrointestinal tract, and the shortage of malabsorption requiring lifetime observation of dietary status [25,26].
In the current study, the major aim was to compare SG and RYGB techniques regarding fat dissipation, decrease in comorbidities, increase in QoL, and negative events.
The patient's age in the group of RYGB in this study had a mean value of 41.30 ± 12.831 Vs 42.60 ± 13.018 years in LSG group. Patient's sex showed that more than half of patients in the RYGB groups were female 65 (65%) vs 52 (52%) in SG group. BMI had a mean value of 39.66 ± 3.770 kg/m2 in RYGB group versus 39.38 ± 3.648 kg/m2. No significant differences were found according to comorbidity, height, and weight.
In comparison with the study of Sherif [27], which was conducted on 434 patients, their BMI ranged (35 and 60 kg/m 2) with mean age of 42 ± 4.8 years, and 73% of them were women. Cases were assayed at (3–6 - 9 -12- 24) months. They were categorized into two random groups: the LSG group (214 cases) and the LRYGB group (220 patients), no statistical significance between the study groups was recorded regarding age, sex, BMI, and associated comorbidities.
However, another study by Yang et al. [28] reported that patients’ age in a group of RYGB had an average value of 40.4 ± 9.4 Vs. 41.4 ± 9.3 years in sleeve gastrectomy group, BMI had a mean value of 32.3 ± 2.4 kg/m2 in RYGB group versus 31.8 ± 3.0 kg/m2, the two groups had comparable anthropometric baseline, which includes age, sex, weight, BMI, waist perimeter, diabetes, and the use of medication.
The beneficial impacts of bariatric surgical operation on fat dissipation and comorbidities resulting from obesity are become trusted. In addition, these techniques can also be operated safely with a low death rate and morbidity risk [27]. There are few randomized controlled studies made a comparative study between two of the most usually used bariatric procedures - that is, LRYGB and LSG - with taking into account the weight dissipation and/or comorbidities resulting from obesity in the medium and long time [29].
At two years after surgery, the present study demonstrated that total recovery of HTN was seen in 30 (58.8%) of 51 in the SG group in comparison with 34 (69.4%) of 49 in the RYGB group, with no statistical significance between the studied groups. Total recovery of DM was seen in 41 (80.4%) of 51 in the SG group versus 44 (81.5%) of 54 in the RYGB group with no statistical significance in FBG (Fasting Blood Glucose) between the two groups. Preoperative, 65 (65.0%) in SG group and 68 (68%) in RYGB group had dyslipidemia, total recovery of this disease was recorded in 34 (52.3%) of 65 in the SG group vs 38 (55.9%) of 68 in RYGB group 24 months postoperative. At the same time, total recovery from sleep apnea was recorded in 36 (52.9%) of 68 in SG group in comparison with 34 (50.7%) of 67 in RYGB group with no significant differences. Total recovery of arthritis was seen in 37 (55.2%) of 67 in the SG group and 36 (50.0%) of 72 in the RYGB group 24-months postoperative, with no statistical significance between the studied groups.
According to the obesity-related comorbidities, Sherif [27] observed the recovery rate and development of hypertension, dyslipidemia, DM2, joint pain, obstructive sleep apnea syndrome, and GERD. There turned into a big development in comorbidities in the two groups 1 year postoperative. no statistical significance between the LSG group and LRYGB group regarding the remission of comorbidities or development rate except for the remission of GERD.
In agreement with our findings, similar results were reported by the study group of Maggard et al. [29] on the same subject, even with a lower BMI group, especially the rapid improvement in DM2 after the two operations.
Peterli et al. [23] reported in their study that at 5 yrs. Postoperative, total recovery of diabetes was reported in 16 (61.5%) of 26 in SG group and 19 (67.9%) of 28 in the RYGB group (absolute difference, −0.05%; 94% CI, −0.38%–0.27%; P > 0.98), total recovery of dyslipidemia was reported in 29 (42.6%) of 68 in SG group vs. 33 (62.3%) of 53 in the RYGB group 5 yrs. Postoperative.
Furthermore, in the present study, we found a significant increase in the QoL in the two groups between baseline and 2 years postoperative. Giving rise to the significant increase in GQOLI (SG: 118.15 points, & RYGB:120.35 points and the BAROS QoL score (SG: 2.1 vs RYGB:1.96 points).
In comparison with the study of Peterli et al. [23], in which QoL have a significant increase in the two groups among baseline and 5 years and for GQOLI (SG: 113.7 points, vs RYGB: 117.8 points; absolute difference, −4.34 points; 94.5% CI, −15.09 to 6.42 points; P = 0.41), there was no significance among the two studied groups.
Most importantly, as described by Zhang et al. [30], the trend of QoL appears parallel with %EWL, but the variations among both groups did not have a statistical significance at 5 years postoperative. The total score of the LSG and LRYGB groups are 1.32 ± 0.81 and 1.58 ± 0.72 (P = 0.18), respectively.
Finally, as regard complications and Mortality in the two groups, the current work concluded that there was no statistically significant differences among the studied groups according to complications and morbidity.
Consistent with what has been reported by Zhang et al. [29], the total complication rate was 15.62% (5/31) for LRYGB and 3.24% (1/31) for LSG (P > 0.05).
These results were in harmony with the study of Peterli et al. [23], wherein there has been no statistical significance in complications requiring surgical or endoscopic review within the first 5 yrs. Postoperative.
In regards to Salminen et al. [31], gastric bypass is accompanied by greater weight loss than sleeve gastrectomy over 5 year clinical trial. However, this weight loss wasn't statistically significant. In addition, Gastric bypass is associated with better control of hypertension than sleeve gastrectomy, but these results depended on the type of antihypertensive drug use.
In contrast with Salminen et al., laparoscopic sleeve gastrectomy was associated with more weight loss and better metabolic outcomes than laparoscopic Roux-en-Y gastric bypass over one-year intervals in the Asian population than in the Caucasian population according to Lakdawala et al. [32].
Patient may be referred for a radiological investigation to exclude complications in the early postoperative period or many months later. Radiologists are required to have an understanding of normal anatomy and the complications associated with evey kind of bariatric surgeries to interpret imaging studies correctly.
The Challenge of psychological or emotional changes should be taken into consideration for bariatric surgery. After the first few months of bariatric surgery, patients usually notice that they have settled into this “new normal,” but choosing a bariatric surgery is embarking on a lifelong process of learning about how to handle a new relationship with food and the body, with the need for multidisciplinary support [33].
Bariatric providers are encouraged to educate their patients about potential challenges before this surgery, inquire about the psychological complications afterward, validate the difficult experiences, and connect patients to required resources early in the adjustment period to prevent escalating problems.
The present study revealed only 8% of LSG operations were advised for histopathology examination according to clinician's suspicion of abnormal mucosa, as the followed policy in the hospital recommends selected histopathology examination rather than a routine examination of the SG specimens.
Routine histopathological study for gastrointestinal specimens, including LSG operations, occurs in many tertiary hospitals to confirm the samples and detect unexpected pathological abnormalities [24,34,35]. Histopathological data for SG specimens are insufficient to describe the common histopathological findings leading some authors to support the policy of routine histopathological examination of all SG specimens to detect any pathology that may have an impact on patient management [34,36]. Demirbas et al. [36] studied 253 patients who had undergone SG showing different pathologic findings; H. pylori positivity in 27% of patients, chronic active gastritis in 20.5%, chronic gastritis in 53.4%, and intestinal metaplasia in 2%, whereas unremarkable histopathologic findings were seen in 25.7% of patients.
In contract, this recommendation, Nowak et al. [37], and Al-Tokhy et al. [38] recommend that LSG specimens be subjects to gross pathologic (naked eye) examination in the vast majority of patients because most of the recorded histologic findings had no clinical impact on future treatment, with only a minority of samples being clinically significant.
It is difficult for us to put a recommendation for histopathology examination of LSG due to the minority of the examined cases.
5. Conclusion
Between patients with morbid obesity, there was no statistical significance in weight dissipation, comorbidities, increase quality of life, and negative events between LSG and LRYGB at 2 yrs of follow-up postoperative except for the occurrence of internal rupture on LRYGB group only. The Challenge of psychological or emotional changes (eg. feeling unexpected and isolating) should be considered.
Ethical approval
Ethical approval was obtained from Al-Azhar University.
Funding sources
This study did not receive any funding from public or private sectors.
Author contribution
Study concept or design: MB, SAE, MT, ML, HAA, AE, MM, MBas, YM, AH. Data collection: MB, SAE, MT, AMEH, MSH, EMO, ME, YA, YM, AH. Data interpretation: MB, SAE, MT, YM, ML, HAA, MM, MBas, AH. Literature review: MB, MT, HAA, AE, ML, MBas, AH. Data analysis: MB, AE. Drafting of the paper: ALL. Editing of the paper: ALL. Manuscript revision: ALL.
Registration of research studies
ClinicalTrials.gov number: NCT05145205.
Guarantor
Dr. MB.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Consent
NA.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgements
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2021.103235.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.McMichael A.J., Powles J.W., Butler C.D., Uauy R. Food, livestock production, energy, climate change, and health. Lancet. 2007;370(9594):1253–1263. doi: 10.1016/S0140-6736(07)61256-2. [DOI] [PubMed] [Google Scholar]
- 2.Ma C., Avenell A., Bolland M., Hudson J., Stewart F., Robertson C., et al. Effects of weight-loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ. 2017;359:j4849. doi: 10.1136/bmj.j4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flegal K.M., Kit B.K., Orpana H., Graubard B.I. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. Jama. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Botero A.G., Wenninger M.G., Loaiza D.F. Complications after body contouring surgery in post-bariatric patients. Ann. Plast. Surg. 2017;79(3):293–297. doi: 10.1097/SAP.0000000000001109. [DOI] [PubMed] [Google Scholar]
- 5.Li J., Lai D., Wu D. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy to treat morbid obesity-related comorbidities: a systematic review and meta-analysis. Obes. Surg. 2016;26(2):429–442. doi: 10.1007/s11695-015-1996-9. [DOI] [PubMed] [Google Scholar]
- 6.Sundbom M. Laparoscopic revolution in bariatric surgery. World J. Gastroenterol.: WJG. 2014;20(41):15135. doi: 10.3748/wjg.v20.i41.15135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang S.H., Stoll C.R., Song J., Varela J.E., Eagon C.J., Colditz G.A. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149(3):275–287. doi: 10.1001/jamasurg.2013.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serrot F.J., Dorman R.B., Miller C.J., Slusarek B., Sampson B., Sick B.T., Leslie D.B., Buchwald H., Ikramuddin S. Comparative effectiveness of bariatric surgery and nonsurgical therapy in adults with type 2 diabetes mellitus and body mass index< 35 kg/m2. Surgery. 2011;150(4):684–691. doi: 10.1016/j.surg.2011.07.069. [DOI] [PubMed] [Google Scholar]
- 9.Jammu G.S. vols. 201–213. Springer; Cham: 2018. Comparison of results of mini-gastric bypass to sleeve gastrectomy and roux-en-Y gastric bypass. Technique of conversion of failed sleeve gastrectomy to MGB. (Essentials of Mini‒One Anastomosis Gastric Bypass). [Google Scholar]
- 10.Suter M., Donadini A., Romy S., Demartines N., Giusti V. Laparoscopic Roux-en-Y gastric bypass: significant long-term weight loss, improvement of obesity-related comorbidities and quality of life. Ann. Surg. 2011;254(2):267–273. doi: 10.1097/SLA.0b013e3182263b66. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y., Zhao H., Cao Z., Sun X., Zhang C., Cai W., et al. A randomized clinical trial of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy for the treatment of morbid obesity in China: a 5-year outcome. Obes. Surg. 2014;24(10):1617–1624. doi: 10.1007/s11695-014-1258-2. [DOI] [PubMed] [Google Scholar]
- 12.Karamanakos S.N., Vagenas K., Kalfarentzos F., Alexandrides T.K. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double-blind study. Ann. Surg. 2008;247(3):401–407. doi: 10.1097/SLA.0b013e318156f012. [DOI] [PubMed] [Google Scholar]
- 13.Chopra A., Chao E., Etkin Y., Merklinger L., Lieb J., Delany H. Laparoscopic sleeve gastrectomy for obesity: can it be considered a definitive procedure? Surg. Endosc. 2012;26(3):831–837. doi: 10.1007/s00464-011-1960-2. [DOI] [PubMed] [Google Scholar]
- 14.Helmiö M., Victorzon M., Ovaska J., Leivonen M., Juuti A., Peromaa-Haavisto P., et al. Comparison of short-term outcome of laparoscopic sleeve gastrectomy and gastric bypass in the treatment of morbid obesity: a prospective randomized controlled multicenter SLEEVEPASS study with 6-month follow-up. Scand. J. Surg. 2014;103(3):175–181. doi: 10.1177/1457496913509984. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen N.T., Varela J.E. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat. Rev. Gastroenterol. Hepatol. 2017;14(3):160. doi: 10.1038/nrgastro.2016.170. [DOI] [PubMed] [Google Scholar]
- 16.Peterli R., Wölnerhanssen B.K., Peters T., Vetter D., Kröll D., Borbély Y., et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. Jama. 2018;319(3):255–265. doi: 10.1001/jama.2017.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sockalingam S., Hawa R., Wnuk S., Santiago V., Kowgier M., Jackson T., et al. Psychosocial predictors of quality of life and weight loss two years after bariatric surgery: results from the Toronto Bari-PSYCH study. Gen. Hosp. Psychiatr. 2017 Jul 1;47:7–13. doi: 10.1016/j.genhosppsych.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Mathew G., Agha R., for the STROCSS Group Strocss 2021: strengthening the Reporting of cohort, cross-sectional and case-control studies in Surgery. Int. J. Surg. 2021;96:106165. doi: 10.1016/j.ijsu.2021.106165. [DOI] [PubMed] [Google Scholar]
- 19.Eypasch E., Williams J.I., Wood‐Dauphinee S., Ure B.M., Schmulling C., Neugebauer E., Troidl H. Gastrointestinal Quality of Life Index: development, validation, and application of a new instrument. Br. J. Surg. 1995;82(2):216–222. doi: 10.1002/bjs.1800820229. [DOI] [PubMed] [Google Scholar]
- 20.Moorehead M.K., Ardelt-Gattinger E., Lechner H., Oria H.E. The validation of the moorehead-ardelt quality of life questionnaire II. Obes. Surg. 2003;13(5):684–692. doi: 10.1381/096089203322509237. [DOI] [PubMed] [Google Scholar]
- 21.Toghaw P., Matone A., Lenbury Y., De Gaetano A. Bariatric surgery and T2DM improvement mechanisms: a mathematical model. Theor. Biol. Med. Model. 2012;9:16. doi: 10.1186/1742-4682-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubino F., Kaplan L.M., Schauer P.R., Cummings D.E. Diabetes Surgery Summit Delegates the Diabetes Surgery Summit consensus conference: recommendations for the evaluation and use of gastrointestinal surgery to treat type 2 diabetes mellitus. Ann. Surg. 2010;251(3):399–405. doi: 10.1097/SLA.0b013e3181be34e7. [DOI] [PubMed] [Google Scholar]
- 23.Peterli R., Wölnerhanssen B.K., Peters T., Vetter D., Kröll D., Borbély Y., et al. Effect of laparoscopic sleeve gastrectomy vs. Laparoscopic roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319(3):255–265. doi: 10.1001/jama.2017.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baheeg M., El-Din M.T., Labib M.F., Elgohary S.A., Hasan A. Long-term durability of weight loss after bariatric surgery; a retrospective study. International Journal of Surgery Open. 2021 Jan 1;28:37–40. doi: 10.1016/j.ijso.2020.12.008. [DOI] [Google Scholar]
- 25.Sarela A.I., Dexter S.P., O′Kane M., Menon A., McMahon M.J. Long-term follow-up after laparoscopic sleeve gastrectomy: 8-9-year results. Surg. Obes. Relat. Dis. 2012;8(6):679–684. doi: 10.1016/j.soard.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 26.Helmiö M., Victorzon M., Ovaska J., Leivonen M., Juuti A., Jaser N., et al. SLEEVEPASS: a randomized prospective multicenter study comparing laparoscopic sleeve gastrectomy and gastric bypass in the treatment of morbid obesity: preliminary results. Surg. Endosc. 2012;26(9):2521–2526. doi: 10.1007/s00464-012-2225-4. [DOI] [PubMed] [Google Scholar]
- 27.Sherif T.M. Prospective comparative study between laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy in the management of morbid obesity and its comorbidities. The Egyptian Journal of Surgery. 2016 Apr 1;35(2):83. http://www.ejs.eg.net/text.asp?2016/35/2/83/182769 Available from: [Google Scholar]
- 28.Yang J., Wang C., Cao G., Yang W., Yu S., Zhai H., et al. Long-term effects of laparoscopic sleeve gastrectomy versus Roux-en-Y gastric bypass for the treatment of Chinese type 2 diabetes mellitus patients with body mass inde×28-35 kg/m2. BMC Surg. 2015;15:88. doi: 10.1186/s12893-015-0074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maggard-Gibbons M., Maglione M., Livhits M., Ewing B., Maher A.R., Hu J., Li Z., Shekelle P.G. Bariatric surgery for weight loss and glycemic control in non morbidly obese adults with diabetes: a systematic review. Jama. 2013 Jun 5;309(21):2250–2261. doi: 10.1001/jama.2013.4851. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y., Zhao H., Cao Z., Sun X., Zhang C., Cai W., et al. A randomized clinical trial of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy for the treatment of morbid obesity in China: a 5-year outcome. Obes. Surg. 2014;24(10):1617–1624. doi: 10.1007/s11695-014-1258-2. [DOI] [PubMed] [Google Scholar]
- 31.Salminen P., Helmiö M., Ovaska J., Juuti A., Leivonen M., Peromaa-Haavisto P., et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: the SLEEVEPASS randomized clinical trial. Jama. 2018 Jan 16;319(3):241–254. doi: 10.1001/jama.2017.20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lakdawala M.A., Bhasker A., Mulchandani D., Goel S., Jain S. Comparison between the results of laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass in the Indian population: a retrospective 1-year study. Obes. Surg. 2010 Jan;20(1):1–6. doi: 10.1007/s11695-009-9981-9. [DOI] [PubMed] [Google Scholar]
- 33.Roerig J.L., Steffen K. Psychopharmacology, and bariatric surgery. Eur. Eat Disord. Rev. 2015;23:463–469. doi: 10.1002/erv.2396. [DOI] [PubMed] [Google Scholar]
- 34.Al Saady R., Ejeckam G. Histopathological findings in laparoscopic sleeve gastrectomy specimens. Qatar Med. J. 2019 Jul 25;2019(1):5. doi: 10.5339/qmj.2019.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasan A., Nafie K., Aldossary M.Y., Ismail A., Monazea K., Baheeg M., et al. Unexpected histopathology results following routine examination of cholecystectomy specimens: how big and how significant? Annals of Medicine and Surgery. 2020 Dec 1;60:425–430. doi: 10.1016/j.amsu.2020.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demirbas B.T., Erdim A., Celikel C., Akbas G., Cingi A. Is it necessary to send the sleeve gastrectomy specimens to pathology? Surg. Laparosc. Endosc. Percutaneous Tech. 2019 Apr 1;29(2):117–119. doi: 10.1097/SLE.0000000000000607. [DOI] [PubMed] [Google Scholar]
- 37.Nowak K., Di Palma A., Chieu K., Quereshy F., Jackson T., Okrainec A., Serra S., Chetty R. Histologic and cost-benefit analysis of laparoscopic sleeve gastrectomy specimens performed for morbid obesity. Arch. Pathol. Lab Med. 2021 Mar 1;145(3):365–370. doi: 10.5858/arpa.2020-0084-OA. [DOI] [PubMed] [Google Scholar]
- 38.Al-Tokhy A.A., Morsi A.E., Elias A.A. Evaluation of the importance of histopathology of all gastric remnants following sleeve gastrectomy. Al-Azhar Assiut Medical Journal. 2018 Jul 1;16(3):296. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.