Key Points
Question
Among extremely preterm infants born at US academic medical centers between 2013 and 2018, what were mortality, in-hospital morbidity, and 2-year neurodevelopmental outcomes?
Findings
In this observational study based on a prospective registry of 10 877 infants born at 22-28 weeks’ gestational age in 2013-2018 in 19 US academic medical centers, survival to discharge occurred in 78.3% and was significantly improved compared with a historical rate of 76.0% among infants born in 2008-2012. Among infants born at less than 27 weeks’ gestational age who survived to follow-up assessment at 2 years, 49.9% had been rehospitalized and severe neurodevelopmental impairment occurred in 21.2%.
Meaning
Among extremely preterm infants born at US academic medical centers from 2013 to 2018, survival to discharge significantly improved compared with infants born in 2008-2012, but among those born at less than 27 weeks’ gestational age, rehospitalization and neurodevelopmental impairment at 2 years were common.
Abstract
Importance
Despite improvement during recent decades, extremely preterm infants continue to contribute disproportionately to neonatal mortality and childhood morbidity.
Objective
To review survival, in-hospital morbidities, care practices, and neurodevelopmental and functional outcomes at 22-26 months’ corrected age for extremely preterm infants.
Design, Setting, and Participants
Prospective registry for extremely preterm infants born at 19 US academic centers that are part of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. The study included 10 877 infants born at 22-28 weeks’ gestational age between January 1, 2013, and December 31, 2018, including 2566 infants born before 27 weeks between January 1, 2013, and December 31, 2016, who completed follow-up assessments at 22-26 months’ corrected age. The last assessment was completed on August 13, 2019. Outcomes were compared with a similar cohort of infants born in 2008-2012 adjusting for gestational age.
Exposures
Extremely preterm birth.
Main Outcomes and Measures
Survival and 12 in-hospital morbidities were assessed, including necrotizing enterocolitis, infection, intracranial hemorrhage, retinopathy of prematurity, and bronchopulmonary dysplasia. Infants were assessed at 22-26 months’ corrected age for 12 health and functional outcomes, including neurodevelopment, cerebral palsy, vision, hearing, rehospitalizations, and need for assistive devices.
Results
The 10 877 infants were 49.0% female and 51.0% male; 78.3% (8495/10848) survived to discharge, an increase from 76.0% in 2008-2012 (adjusted difference, 2.0%; 95% CI, 1.0%-2.9%). Survival to discharge was 10.9% (60/549) for live-born infants at 22 weeks and 94.0% (2267/2412) at 28 weeks. Survival among actively treated infants was 30.0% (60/200) at 22 weeks and 55.8% (535/958) at 23 weeks. All in-hospital morbidities were more likely among infants born at earlier gestational ages. Overall, 8.9% (890/9956) of infants had necrotizing enterocolitis, 2.4% (238/9957) had early-onset infection, 19.9% (1911/9610) had late-onset infection, 14.3% (1386/9705) had severe intracranial hemorrhage, 12.8% (1099/8585) had severe retinopathy of prematurity, and 8.0% (666/8305) had severe bronchopulmonary dysplasia. Among 2930 surviving infants with gestational ages of 22-26 weeks eligible for follow-up, 2566 (87.6%) were examined. By 2-year follow-up, 8.4% (214/2555) of children had moderate to severe cerebral palsy, 1.5% (38/2555) had bilateral blindness, 2.5% (64/2527) required hearing aids or cochlear implants, 49.9% (1277/2561) had been rehospitalized, and 15.4% (393/2560) required mobility aids or other supportive devices. Among 2458 fully evaluated infants, 48.7% (1198/2458) had no or mild neurodevelopmental impairment at follow-up, 29.3% (709/2419) had moderate neurodevelopmental impairment, and 21.2% (512/2419) had severe neurodevelopmental impairment.
Conclusions and Relevance
Among extremely preterm infants born in 2013-2018 and treated at 19 US academic medical centers, 78.3% survived to discharge, a significantly higher rate than for infants born in 2008-2012. Among infants born at less than 27 weeks’ gestational age, rehospitalization and neurodevelopmental impairment were common at 2 years of age.
This prospective observational study assesses mortality, in-hospital morbidity, care practices, and 2-year outcomes among US infants born at 22-28 weeks’ gestational age in 2013-2018 and compares these outcomes with those of a similar cohort of infants born in 2008-2012.
Introduction
Mortality and most short-term morbidities among extremely preterm infants have declined in recent decades,1,2,3,4,5 but ongoing review of medical and neurodevelopmental outcomes is needed as care strategies and technologies continue to evolve. Published reports of trends in neurodevelopmental outcomes among extremely preterm infants who survive differ, highlighting challenges to interpretation due to variation in age at assessment, assessment instruments, and other factors.6,7,8,9,10 Moreover, additional postdischarge milestones including functional and health-related outcomes are increasingly recognized as important for children born extremely preterm and their families.10,11,12,13,14
The Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network tracks in-hospital outcomes for infants born before 29 weeks’ gestational age at network centers. At 22-26 months’ corrected age,15 the neurological and developmental status of those born before 27 weeks are assessed by certified examiners. Since 1991, the network has periodically reported extremely preterm infant in-hospital mortality and morbidity outcomes and longer-term developmental outcomes. Network investigators have not previously published a comprehensive update on both short-term and postdischarge follow-up outcomes. This study was conducted to assess mortality, in-hospital morbidities, and care practices for infants born in 2013-2018 and neurodevelopmental, functional, and health-related outcomes at 22-26 months’ corrected age for infants born in 2013-2016.
Methods
The Neonatal Research Network’s extremely preterm infant registry enrolls infants born weighing 401 g to 1000 g and/or born between 22 weeks 0 days’ and 28 weeks 6 days’ gestation at network hospitals. Trained research coordinators prospectively collect maternal and neonatal data from birth until discharge home, transfer, death, or 120 days. For infants transferred or still hospitalized at 120 days, vital status is collected until 1 year of age. Surviving infants born before 27 weeks are eligible for comprehensive follow-up assessment at 22-26 months’ corrected age. The institutional review board at each participating hospital approved participation in the registry. Waiver of consent for enrollment in the extremely preterm infant registry was granted at most affiliated hospitals, but parental consent was required at 5 hospitals (4 written, 1 oral). Most hospitals required written parental consent for participation in the follow-up study, but 5 hospitals allowed participation under waiver of consent.
Infants born at each of 19 network centers between January 1, 2013, and December 31, 2018, at gestational ages of 22-28 weeks and enrolled in the registry were included in the present study. Postdischarge outcomes assessed at the 2-year follow-up visit were available for infants born between January 1, 2013, and December 31, 2016. The final in-hospital outcome was determined on December 20, 2019; the final 2-year assessment was completed on August 13, 2019.
Maternal demographic, pregnancy, and delivery information and infant characteristics were recorded. Gestational age was determined by best obstetric estimate or, if unavailable, by neonatal estimate. Maternal race and ethnicity were self-identified from a list of options; race and ethnicity were included because they are known to be associated with differences in care and outcomes in preterm infants.16 Infants with birth weights below the 10th percentile for gestational age17 were classified as small for gestational age. Clinical interventions and morbidities during the birth hospitalization were recorded for infants surviving longer than 12 hours.
Outcomes
Infant survival was recorded to 12 hours and to discharge or 1 year if still hospitalized. Morbidities recorded included necrotizing enterocolitis (modified Bell stage ≥IIA18,19), early-onset sepsis and/or meningitis (≤72 hours), late-onset sepsis and/or meningitis (>72 hours), intracranial hemorrhage (grade III or IV20), cystic periventricular leukomalacia, retinopathy of prematurity, and bronchopulmonary dysplasia. Bronchopulmonary dysplasia was defined using the optimal definition (definition 15) from Jensen et al.21 This definition classifies bronchopulmonary dysplasia based on the mode of support received at 36 weeks’ postmenstrual age or at discharge home if earlier: no bronchopulmonary dysplasia is classified as no support or breathing room air; grade 1, nasal cannula at a flow rate of 2 L/min or lower; grade 2, nasal cannula at a flow rate higher than 2 L/min or noninvasive positive airway pressure; and grade 3, invasive mechanical ventilation. Active treatment was defined as intubation, surfactant therapy, respiratory support, chest compressions, epinephrine, volume resuscitation, blood pressure support, and/or parenteral nutrition.22
The 2-year study visit included structured interviews with children’s primary caretakers to review medical history, rehospitalizations, and medical equipment use. A standardized neurologic examination and the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III)23 were administered by certified examiners who completed annual training to ensure interrater reliability.24 Bayley-III cognitive, language, and motor composite scores are normalized to a mean of 100 (SD, 15), with lower scores indicating a higher degree of impairment. Severity of motor impairment was determined by the Gross Motor Function Classification System (GMFCS) of Palisano et al.25 Mild cerebral palsy was defined as GMFCS level 1, moderate as GMFCS level 2 or 3, and severe as GMFCS level 4 or 5.
Mild or no neurodevelopmental impairment (NDI) was defined as a Bayley-III cognitive composite score of 85 or higher, a Bayley-III motor composite score of 85 or higher, and GMFCS level 0 or 1. Moderate NDI was defined as a Bayley-III cognitive composite score or motor composite score of 70 to 84 or GMFCS level 2 or 3. Severe NDI was defined as a Bayley-III cognitive composite score or motor composite score lower than 70, GMFCS level 4 or 5, bilateral blindness, or bilateral severe functional hearing impairment.6,8
The following outcomes were determined at the 2-year assessment by history from caregivers and/or medical records: rehospitalization after initial discharge and reason for hospitalization; feeding assistance required; need for supportive medical equipment, such as apnea monitor, oxygen, or other respiratory support; and need for mobility aid, such as braces, walker, or adaptive stroller or wheelchair.
Statistical Analysis
Descriptive statistics were used to summarize maternal characteristics, neonatal characteristics at birth, and survival for all infants; in-hospital treatments and morbidities for infants surviving more than 12 hours; and outcomes at follow-up for infants evaluated. Survival was analyzed separately for actively treated infants. Follow-up outcomes were included if infants underwent neurologic examination and/or Bayley-III assessment at 18-30 months’ corrected age, allowing for 4 months outside the targeted age.
Regression models were used to assess whether outcomes differed across gestational ages; reported P values were adjusted for the covariates listed below. Logistic, generalized logit, and linear models were used to assess whether binary proportions, categorical outcomes with more than 2 levels, or means differed by gestational age, with statistical significance determined by Wald χ2 or F tests. Quantile regression was used to examine differences in medians across gestational ages for outcomes with highly skewed distributions, with statistical significance determined by a likelihood ratio χ2 test. Models assessing survival, morbidities, and treatments included gestational age, study center, small for gestational age, sex, multiple gestation, and maternal race and ethnicity. Covariates known to affect outcomes were chosen a priori and entered in models simultaneously. Study center was entered as a fixed effect in all models. Because of the role of socioeconomic factors in postdischarge child development, maternal education was added to models examining outcomes at follow-up and was entered with a category for missing/unknown data to minimize loss of observations from the models. Each other covariate was missing for less than 1% of the cohort. In total, less than 2% of observations were excluded from most models (maximum of 6% from 2 models) due to missing covariates or outcomes. Unless otherwise indicated, reported P values indicate whether there were differences in outcomes by gestational age overall with all gestational age group comparisons considered together. Tests of overall differences by gestational age are reported in the text and in table footnotes. Comparisons between 2 proportions at different gestational ages were reported as risk (prevalence) differences with 95% Miettinen-Nurminen confidence intervals and P values by χ2 test indicating statistical significance for the pairwise comparison. P values, all 2-sided, were adjusted as described above. A P < .05 was considered significant. Analyses were performed using SAS version 9.4 (SAS Institute Inc). Because of the potential for type I error due to multiple comparisons, findings for the analyses should be interpreted as exploratory.
Percentages in the current cohort were compared with percentages reported by Stoll et al1 for births at 22-28 weeks’ gestational age in Neonatal Research Network centers during 2008-2012, when outcomes were reported for the earlier cohort. Risk differences adjusted for gestational age and 95% confidence intervals were estimated for overall proportions using binomial models.26 Differences in proportions for infants in 1 gestational age group were unadjusted. The outcome definitions were the same in the 2 studies with 2 exceptions. First, in the current study, no assumption was made when an infant’s final vital status was unknown, while in the study by Stoll et al,1 these infants were assumed to have survived to discharge (44 infants [0.5%]). Second, early- and late-onset sepsis and/or meningitis included sepsis and/or meningitis in the current cohort, but the reported proportions in the cohort of Stoll et al1 did not include meningitis; this difference had minimal impact because few infants had meningitis alone.
Results
Study Population
We studied 10 877 infants born between January 1, 2013, and December 31, 2018, at Neonatal Research Network study centers with gestational ages of 22-28 weeks. The median maternal age was 29 (IQR, 24-33) years; 26.5% (n = 2886) of infants were multiples, 49.0% (n = 5327) were female and 51.0% (n = 5550) were male, 9.3% (n = 1016) were small for gestational age, and 5.5% (603/10 868) had major birth defects (Table 1). Overall, 88.1% (9571/10 867) of mothers received corticosteroids, 75.0% (8135/10 847) received antibiotics, and 79.4% (8622/10 858) received magnesium sulfate. Although 64.8% (7043/10 867) of infants were born by cesarean delivery overall, only 3.1% (17/549) of infants at 22 weeks and 39.7% (430/1083) at 23 weeks were born by cesarean delivery.
Table 1. Maternal and Infant Characteristics and Antenatal Therapies for Infants Born at 22-28 Weeks’ Gestational Age.
| Characteristicsa | Gestational age, wk, 2013-2018 | Gestational age, wk, 2008-2012b | Adjusted difference (95% CI)c | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 22 (n = 550) | 23 (n = 1083) | 24 (n = 1398) | 25 (n = 1604) | 26 (n = 1836) | 27 (n = 1987) | 28 (n = 2419) | 22-28 (n = 10 877) | 22-28 (n = 8877) | ||
| Maternal characteristics | ||||||||||
| Age, median (IQR), y | 28 (23-32) | 28 (23-32) | 28 (24-33) | 29 (24-33) | 29 (24-33) | 29 (24-33) | 29 (24-33) | 29 (24-33) | ||
| Education, No./total (%)d | ||||||||||
| Less than high school | 61/376 (16.2) | 143/859 (16.6) | 191/1135 (16.8) | 235/1272 (18.5) | 273/1514 (18.0) | 268/1580 (17.0) | 339/1931 (17.6) | 1510/8667 (17.4) | ||
| High school diploma | 116/376 (30.9) | 238/859 (27.7) | 379/1135 (33.4) | 363/1272 (28.5) | 433/1514 (28.6) | 443/1580 (28.0) | 557/1931 (28.8) | 2529/8667 (29.2) | ||
| Trade or technical school or some college | 99/376 (26.3) | 242/859 (28.2) | 309/1135 (27.2) | 345/1272 (27.1) | 440/1514 (29.1) | 459/1580 (29.1) | 509/1931 (26.4) | 2403/8667 (27.7) | ||
| College degree or more | 100/376 (26.6) | 236/859 (27.5) | 256/1135 (22.6) | 329/1272 (25.9) | 368/1514 (24.3) | 410/1580 (25.9) | 526/1931 (27.2) | 2225/8667 (25.7) | ||
| Public medical insurancee | 312/547 (57.0) | 594/1078 (55.1) | 814/1394 (58.4) | 894/1598 (55.9) | 1064/1830 (58.1) | 1121/1978 (56.7) | 1393/2417 (57.6) | 6192/10 842 (57.1) | ||
| Hispanic or Latino ethnicity, No./total (%) | 80/544 (14.7) | 169/1070 (15.8) | 228/1390 (16.4) | 267/1585 (16.8) | 311/1821 (17.1) | 297/1971 (15.1) | 381/2395 (15.9) | 1733/10 776 (16.1) | ||
| Race, No./total (%) | ||||||||||
| American Indian or Alaska Native | 5/522 (1.0) | 6/1048 (0.6) | 14/1364 (1.0) | 12/1549 (0.8) | 21/1777 (1.2) | 30/1941 (1.5) | 37/2354 (1.6) | 125/10 555 (1.2) | ||
| Asian | 19/522 (3.6) | 39/1048 (3.7) | 50/1364 (3.7) | 59/1549 (3.8) | 66/1777 (3.7) | 92/1941 (4.7) | 113/2354 (4.8) | 438/10 555 (4.1) | ||
| Black | 251/522 (48.1) | 455/1048 (43.4) | 594/1364 (43.5) | 602/1549 (38.9) | 689/1777 (38.8) | 744/1941 (38.3) | 870/2354 (37.0) | 4205/10 555 (39.8) | ||
| Native Hawaiian or Other Pacific Islander | 7/522 (1.3) | 2/1048 (0.2) | 6/1364 (0.4) | 4/1549 (0.3) | 9/1777 (0.5) | 11/1941 (0.6) | 7/2354 (0.3) | 46/10 555 (0.4) | ||
| White | 233/522 (44.6) | 530/1048 (50.6) | 679/1364 (49.8) | 850/1549 (54.9) | 961/1777 (54.1) | 1044/1941 (53.8) | 1300/2354 (55.2) | 5597/10 555 (53.0) | ||
| >1 Race | 7/522 (1.3) | 16/1048 (1.5) | 21/1364 (1.5) | 22/1549 (1.4) | 31/1777 (1.7) | 20/1941 (1.0) | 27/2354 (1.1) | 144/10 555 (1.4) | ||
| Characteristics of pregnancy and delivery, No./total (%) | ||||||||||
| Prenatal care (≥1 visit) | 516/547 (94.3) | 1034/1081 (95.7) | 1326/1394 (95.1) | 1522/1599 (95.2) | 1759/1832 (96.0) | 1918/1984 (96.7) | 2323/2410 (96.4) | 10398/10 847 (95.9) | 8444/8834 (95.6) | 0.3 (−0.3 to 0.9) |
| Multiple gestation | 181 (32.9) | 329 (30.4) | 326 (23.3) | 369 (23.0) | 509 (27.7) | 513 (25.8) | 659 (27.2) | 2886 (26.5) | 2311 (26.0) | 0.3 (−0.9 to 1.5) |
| Chorioamnionitis documented in medical record | 127/549 (23.1) | 246/1082 (22.7) | 257/1396 (18.4) | 263/1602 (16.4) | 251/1831 (13.7) | 230/1986 (11.6) | 219/2415 (9.1) | 1593/10 861 (14.7) | ||
| Hypertension | ||||||||||
| Existing before pregnancy | 34/545 (6.2) | 104/1077 (9.7) | 177/1382 (12.8) | 227/1583 (14.3) | 266/1807 (14.7) | 344/1962 (17.5) | 351/2388 (14.7) | 1503/10 744 (14.0) | ||
| During but not before pregnancy | 15/545 (2.8) | 42/1077 (3.9) | 112/1382 (8.1) | 191/1583 (12.1) | 257/1807 (14.2) | 333/1962 (17.0) | 486/2388 (20.4) | 1436/10 744 (13.4) | ||
| Insulin-dependent diabetes | 18/542 (3.3) | 35/1077 (3.2) | 59/1385 (4.3) | 69/1588 (4.3) | 84/1818 (4.6) | 116/1964 (5.9) | 129/2388 (5.4) | 510/10 762 (4.7) | 443/8836 (5.0) | −0.1 (−0.7 to 0.5) |
| Antenatal corticosteroidsf | 177/548 (32.3) | 877/1083 (81.0) | 1266/1398 (90.6) | 1448/1601 (90.4) | 1699/1834 (92.6) | 1851/1986 (93.2) | 2253/2417 (93.2) | 9571/10 867 (88.1) | 7506/8858 (84.7) | 3.3 (2.5 to 4.2) |
| Antenatal antibiotics | 282/548 (51.5) | 808/1078 (75.0) | 1105/1394 (79.3) | 1233/1601 (77.0) | 1389/1833 (75.8) | 1517/1981 (76.6) | 1801/2412 (74.7) | 8135/10 847 (75.0) | 6249/8819 (70.9) | 4.5 (3.2 to 5.7) |
| Magnesium sulfate | 137/548 (25.0) | 761/1081 (70.4) | 1141/1396 (81.7) | 1301/1602 (81.2) | 1555/1832 (84.9) | 1697/1985 (85.5) | 2030/2414 (84.1) | 8622/10 858 (79.4) | ||
| Cesarean delivery | 17/549 (3.1) | 430/1083 (39.7) | 912/1394 (65.4) | 1096/1601 (68.5) | 1356/1836 (73.9) | 1438/1986 (72.4) | 1794/2418 (74.2) | 7043/10 867 (64.8) | 5548/8871 (62.5) | 2.4 (1.2 to 3.7) |
| Infant characteristics | ||||||||||
| Sex, No. (%) | ||||||||||
| Female | 263 (47.8) | 533 (49.2) | 671 (48.0) | 800 (49.9) | 869 (47.3) | 984 (49.5) | 1207 (49.9) | 5327 (49.0) | 4273/8873 (48.2) | |
| Male | 287 (52.2) | 550 (50.8) | 727 (52.0) | 804 (50.1) | 967 (52.7) | 1003 (50.5) | 1212 (50.1) | 5550 (51.0) | 4600/8873 (51.8) | −0.9 (−2.3 to 0.5) |
| Birth weight, median (IQR), g | 480 (431-528) | 575 (520-637) | 660 (585-730) | 760 (660-843) | 860 (745-965) | 970 (840-1090) | 1103 (950-1240) | 810 (640-1014) | ||
| Small for gestational age, No. (%)g | 38 (6.9) | 62 (5.7) | 110 (7.9) | 170 (10.6) | 184 (10.0) | 193 (9.7) | 259 (10.7) | 1016 (9.3) | 656/8873 (7.4) | 2.4 (1.6 to 3.2) |
| Major birth defect, No. (%)h | 21/550 (3.8) | 42/1083 (3.9) | 63/1396 (4.5) | 109/1603 (6.8) | 109/1835 (5.9) | 101/1985 (5.1) | 158/2416 (6.5) | 603/10 868 (5.5) | 331/8876 (3.7) | 1.8 (1.2 to 2.4) |
| Actively treated at birth, No. (%)i | 201 (36.5) | 958 (88.5) | 1369 (97.9) | 1589 (99.1) | 1827 (99.5) | 1976 (99.4) | 2400 (99.2) | 10320 (94.9) | ||
Characteristic distributions differed significantly across gestational age for maternal age, maternal race, multiple gestation, chorioamnionitis, hypertension existing before pregnancy, hypertension during but not before pregnancy, insulin-dependent diabetes, antenatal corticosteroids, antenatal antibiotics, magnesium sulfate, cesarean delivery, infant birth weight, small for gestational age, presence of birth defect, and active treatment at birth; adjusted P < .01 for all except P = .02 for insulin-dependent diabetes, adjusting for study center.
Data are from Stoll et al.1 Infants included were restricted to those with birth weights of 401 g to 1500 g, consistent with Neonatal Research Network eligibility criteria during prior years. This criterion primarily restricted eligibility for infants born at 22 weeks’ gestational age. The current cohort includes infants born at 22-28 weeks’ gestational age with any birth weight, including 163 (1.5%) with birth weights less than 401 g and 79 (0.7%) with birth weights greater than 1500 g.
Where possible given data reported by Stoll et al, differences adjusted for gestational age are shown between the proportion reported for infants born at 22-28 weeks’ gestational age in the 2013-2018 cohort vs in 2008-2012 along with associated 95% confidence intervals for the difference. A positive difference indicates that the proportion increased in the 2013-2018 cohort compared with the 2008-2012 cohort; a negative difference indicates that the proportion decreased in the 2013-2018 cohort compared with the earlier cohort.
Maternal education was assessed at the time of delivery.
Public medical insurance may include Medicaid, a state or federally funded program, or insurance obtained through the Affordable Care Act.
Antenatal corticosteroid use was defined as any antenatal corticosteroid given. Of the 9571 infants who received antenatal corticosteroids, information about whether a complete course was given was missing for 23 infants. Of the remaining 9548 infants, 7203 (75.4%) received a complete course and 2345 (24.6%) did not.
Small for gestational age was defined as less than the 10th percentile for sex and age based on Alexander percentiles.17
Major birth defect refers to any syndrome and/or major malformation including chromosomal abnormalities, central nervous system defects, congenital heart defects, gastrointestinal tract defects, genitourinary tract defects, skeletal dysplasias, cystic adenomatoid malformation, inborn errors of metabolism, and other serious or life-threatening birth defects.
Active treatment was defined as intubation, surfactant therapy, respiratory support, chest compressions, epinephrine, volume resuscitation, blood pressure support, or parenteral nutrition.22
Survival
Overall, 9966 (91.6%) infants survived more than 12 hours and 8495 of 10 848 (78.3%) survived to discharge or to 1 year if still hospitalized (Table 2; eFigure 1 in Supplement 1). Survival to discharge increased significantly with gestational age (P < .001) (Table 2; eFigure 2 in Supplement 1). Of live-born infants at 22 weeks, 10.9% (60/549) survived compared with 6.6% (22/334) in 2008-2012 (difference, 4.3%; 95% CI, 0.6%-8.1%; P = .03); at 23 weeks, survival increased significantly to 49.4% (535/1083) from 32.3% (252/779) in 2008-2012 (difference, 17.1%; 95% CI, 12.6%-21.5%; P < .001).1 Survival at 28 weeks was 94.0% (2267/2412). Only 36.5% of infants born at 22 weeks’ gestational age were actively treated, but 88.5% born at 23 weeks, 97.9% born at 24 weeks, and more than 99% born at 25-28 weeks received active treatment. Although only 10.9% of infants live-born at 22 weeks survived to discharge or 1 year, 30.0% (60/200) of those actively treated survived. Among those born at 23 weeks, 49.4% of all live-born infants and 55.8% (535/958) of actively treated infants survived.
Table 2. Survival of Infants Born at 22-28 Weeks’ Gestational Age in 2013-2018 for All Infants and Infants Actively Treated at Birth.
| Survival | No./total (%), by gestational age, in weeks | Adjusted difference (95% CI)b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2013-2018 | 2008-2012a | |||||||||
| 22 | 23 | 24 | 25 | 26 | 27 | 28 | 22-28 | 22-28 | ||
| All infants | ||||||||||
| No. | 550 | 1083 | 1398 | 1604 | 1836 | 1987 | 2419 | 10 877 | 8877 | |
| Survived >12 h | 159 (28.9) | 856 (79.0) | 1298 (92.8) | 1546 (96.4) | 1788 (97.4) | 1943 (97.8) | 2376 (98.2) | 9966 (91.6) | 8034 (90.5) | 0.1 (−0.3 to 0.6) |
| Survived to discharge or 1 yc | 60/549 (10.9) | 535/1083 (49.4) | 972/1391 (69.9) | 1266/1599 (79.2) | 1608/1835 (87.6) | 1787/1979 (90.3) | 2267/2412 (94.0) | 8495/10 848 (78.3) | 6746/8877 (76.0) | 2.0 (1.0 to 2.9) |
| Discharged home | 56/549 (10.2) | 520/1083 (48.0) | 948/1391 (68.2) | 1245/1599 (77.9) | 1582/1835 (86.2) | 1772/1979 (89.5) | 2259/2412 (93.7) | 8382/10 848 (77.3) | ||
| Remained in hospital at 1 y | 4/549 (0.7) | 15/1083 (1.4) | 24/1391 (1.7) | 21/1599 (1.3) | 26/1835 (1.4) | 15/1979 (0.8) | 8/2412 (0.3) | 113/10 848 (1.0) | ||
| Infants actively treated at birth d | ||||||||||
| No. | 201 | 958 | 1369 | 1589 | 1827 | 1976 | 2400 | 10 320 | ||
| Survived >12 h | 159 (79.1) | 856 (89.4) | 1298 (94.8) | 1546 (97.3) | 1788 (97.9) | 1943 (98.3) | 2375 (99.0) | 9965 (96.6) | ||
| Survived to discharge or 1 yc | 60/200 (30.0) | 535/958 (55.8) | 972/1362 (71.4) | 1266/1584 (79.9) | 1608/1826 (88.1) | 1787/1968 (90.8) | 2266/2393 (94.7) | 8494/10 291 (82.5) | ||
| Discharged home | 56/200 (28.0) | 520/958 (54.3) | 948/1362 (69.6) | 1245/1584 (78.6) | 1582/1826 (86.6) | 1772/1968 (90.0) | 2258/2393 (94.4) | 8381/10 291 (81.4) | ||
| Remained in hospital at 1 y | 4/200 (2.0) | 15/958 (1.6) | 24/1362 (1.8) | 21/1584 (1.3) | 26/1826 (1.4) | 15/1968 (0.8) | 8/2393 (0.3) | 113/10 291 (1.1) | ||
Data are from Stoll et al.1 Infants included were restricted to those with birth weights of 401 g to 1500 g, consistent with Neonatal Research Network eligibility criteria during prior years. This criterion primarily restricted eligibility for infants born at 22 weeks’ gestational age. The current cohort includes infants born at 22-28 weeks’ gestational age with any birth weight, including 163 (1.5%) with birth weights less than 401 g and 79 (0.7%) with birth weights greater than 1500 g.
Where possible given data reported by Stoll et al, differences adjusted for gestational age are shown between the proportion reported for infants born at 22-28 weeks’ gestational age in the 2013-2018 cohort vs in 2008-2012 along with associated 95% confidence intervals for the difference. A positive difference indicates that the proportion increased in the 2013-2018 cohort compared with the 2008-2012 cohort; a negative difference indicates that the proportion decreased in the 2013-2018 cohort compared with the earlier cohort.
For infants who transferred or were still hospitalized at 120 days, vital status was collected up to 1 year. The proportion of infants who survived more than 12 hours and the proportion who survived to discharge or 1 year differed significantly across gestational age; adjusted P < .001 for each by Wald χ2 test adjusting for study center, small for gestational age, male sex, multiple gestation, and maternal race and ethnicity. Final vital status was unknown for 18 infants who were still in the hospital at 120 days and 11 infants who were transferred to another facility after 7 to 192 days in the birth hospital, with most transferred after 33 to 58 days. In the 2008-2012 cohort, final vital status was unknown for 44 (0.5%) of 8877 infants. In the prior report, these infants were assumed to have survived to discharge. Without this assumption, survival to discharge would change from 6746 (76.0%) of 8877 to 6702 (75.9%) of 8833.
Active treatment was defined as intubation, surfactant therapy, respiratory support, chest compressions, epinephrine, volume resuscitation, blood pressure support, or parenteral nutrition.22
Treatments and Morbidities During Initial Hospitalization
Delayed umbilical cord clamping or cord milking was documented for 40.6% (1982/4881) of births (Table 3). Treatments including intubation/ventilation, nutrition/hydration, and/or medication were withheld, limited, or withdrawn for 10.1% (1005/9956) of infants at some time after 12 hours of age.
Table 3. In-Hospital Treatments and Outcomes for Infants Surviving More Than 12 Hours After Birth.
| Variablesa | No./total (%), by gestational age in weeksb | Adjusted difference (95% CI)d | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2013-2018 | 2008-2012c | |||||||||
| 22 (n = 159) | 23 (n = 856) | 24 (n = 1298) | 25 (n = 1546) | 26 (n = 1788) | 27 (n = 1943) | 28 (n = 2376) | 22-28 (n = 9966) | 22-28 (n = 8034) | ||
| Peridelivery interventions | ||||||||||
| Delivery room intubation | 153/159 (96.2) | 818/856 (95.6) | 1123/1298 (86.5) | 1215/1546 (78.6) | 1133/1787 (63.4) | 946/1943 (48.7) | 954/2374 (40.2) | 6342/9963 (63.7) | ||
| Delayed umbilical cord clamping or cord milkinge | 38/86 (44.2) | 146/450 (32.4) | 233/637 (36.6) | 296/756 (39.2) | 344/859 (40.0) | 402/930 (43.2) | 523/1163 (45.0) | 1982/4881 (40.6) | ||
| Interventions during hospitalization | ||||||||||
| Treatments withheld, limited, or withdrawn to limit caref | 57/159 (35.8) | 248/856 (29.0) | 226/1298 (17.4) | 190/1544 (12.3) | 121/1787 (6.8) | 97/1941 (5.0) | 66/2371 (2.8) | 1005/9956 (10.1) | ||
| Surfactant | 158/159 (99.4) | 849/856 (99.2) | 1241/1298 (95.6) | 1407/1546 (91.0) | 1480/1788 (82.8) | 1364/1943 (70.2) | 1458/2376 (61.4) | 7957/9966 (79.8) | ||
| Postnatal corticosteroid (systemic) | 50/154 (32.5) | 328/808 (40.6) | 443/1196 (37.0) | 379/1470 (25.8) | 284/1724 (16.5) | 197/1913 (10.3) | 110/2359 (4.7) | 1791/9624 (18.6) | 971/7936 (12.2) | 4.0 (3.1 to 4.9) |
| Inhaled nitric oxide | 55/159 (34.6) | 172/856 (20.1) | 230/1297 (17.7) | 171/1544 (11.1) | 147/1788 (8.2) | 126/1941 (6.5) | 108/2374 (4.5) | 1009/9959 (10.1) | ||
| Vitamin A | 70/159 (44.0) | 229/848 (27.0) | 309/1275 (24.2) | 340/1531 (22.2) | 370/1758 (21.0) | 279/1910 (14.6) | 202/2315 (8.7) | 1799/9796 (18.4) | ||
| Patent ductus arteriosus medicationg | 36/158 (22.8) | 278/855 (32.5) | 487/1296 (37.6) | 480/1544 (31.1) | 462/1786 (25.9) | 316/1941 (16.3) | 272/2374 (11.5) | 2331/9954 (23.4) | ||
| Patent ductus arteriosus surgery or cardiac catheterization for closure | 20/158 (12.7) | 121/855 (14.2) | 192/1295 (14.8) | 169/1543 (11.0) | 119/1787 (6.7) | 73/1941 (3.8) | 47/2374 (2.0) | 741/9953 (7.4) | ||
| Probioticse | 13/68 (19.1) | 31/391 (7.9) | 41/570 (7.2) | 53/715 (7.4) | 91/837 (10.9) | 89/908 (9.8) | 125/1141 (11.0) | 443/4630 (9.6) | ||
| Receipt of human milk in first 28 dh | 116/159 (73.0) | 711/848 (83.8) | 1140/1282 (88.9) | 1369/1514 (90.4) | 1661/1760 (94.4) | 1820/1928 (94.4) | 2202/2339 (94.1) | 9019/9830 (91.7) | ||
| Parenteral nutrition, median (IQR), d | 15 (7-38) [n = 158] | 25 (12-44) [n = 855] | 25 (14-40) [n = 1297] | 22 (14-35) [n = 1545] | 18 (13-30) [n = 1788] | 15 (11-24) [n = 1938] | 13 (10-20) [n = 2373] | 17 (11-30) [n = 9954] | ||
| Red blood cell transfusion | 156/159 (98.1) | 837/856 (97.8) | 1267/1298 (97.6) | 1471/1544 (95.3) | 1561/1788 (87.3) | 1345/1941 (69.3) | 1170/2374 (49.3) | 7807/9960 (78.4) | ||
| Mechanical ventilation | 159/159 (100.0) | 855/856 (99.9) | 1282/1298 (98.8) | 1476/1545 (95.5) | 1579/1786 (88.4) | 1466/1940 (75.6) | 1571/2367 (66.4) | 8388/9951 (84.3) | 6983/8030 (87.0) | −2.1 (−3.2 to −0.9) |
| Tracheostomy | 1/159 (0.6) | 5/856 (0.6) | 12/1298 (0.9) | 16/1545 (1.0) | 18/1788 (1.0) | 11/1941 (0.6) | 4/2374 (0.2) | 67/9961 (0.7) | ||
| Morbidities during hospitalization | ||||||||||
| NEC stage ≥IIAi | 19/159 (11.9) | 130/854 (15.2) | 150/1298 (11.6) | 178/1544 (11.5) | 144/1787 (8.1) | 140/1941 (7.2) | 129/2373 (5.4) | 890/9956 (8.9) | 825/8032 (10.3) | −1.6 (−2.5 to −0.8) |
| Surgery for NEC | 12/159 (7.5) | 78/854 (9.1) | 82/1298 (6.3) | 78/1544 (5.1) | 48/1787 (2.7) | 47/1941 (2.4) | 41/2373 (1.7) | 386/9956 (3.9) | ||
| Early-onset sepsis and/or meningitisj | 11/158 (7.0) | 38/856 (4.4) | 52/1298 (4.0) | 39/1545 (2.5) | 31/1787 (1.7) | 38/1941 (2.0) | 29/2372 (1.2) | 238/9957 (2.4) | 165/8032 (2.1) | 0.2 (−0.2 to 0.6) |
| Infants surviving >3 d | 133 | 779 | 1216 | 1481 | 1753 | 1913 | 2347 | 9622 | 7738 | |
| Late-onset sepsis and/or meningitisj | 61/132 (46.2) | 319/778 (41.0) | 407/1215 (33.5) | 379/1479 (25.6) | 322/1752 (18.4) | 242/1911 (12.7) | 181/2343 (7.7) | 1911/9610 (19.9) | 1890/7737 (24.4) | −4.9 (−6.0 to −3.7) |
| Cranial sonogram within 28 d | 136/159 (85.5) | 819/855 (95.8) | 1251/1298 (96.4) | 1501/1545 (97.2) | 1752/1788 (98.0) | 1905/1941 (98.1) | 2343/2375 (98.7) | 9707/9961 (97.5) | 7852/8034 (97.7) | −0.2 (−0.6 to 0.3) |
| Intracranial hemorrhage grade III or IVk | 52/136 (38.2) | 298/819 (36.4) | 308/1251 (24.6) | 260/1500 (17.3) | 202/1752 (11.5) | 143/1905 (7.5) | 123/2342 (5.3) | 1386/9705 (14.3) | 1149/7851 (14.6) | −1.0 (−1.9 to −0.1) |
| Shunt for posthemorrhagic hydrocephalus | 3/136 (2.2) | 21/819 (2.6) | 33/1251 (2.6) | 24/1501 (1.6) | 24/1752 (1.4) | 23/1905 (1.2) | 16/2342 (0.7) | 144/9706 (1.5) | ||
| Cranial imaging within 28 d or after 28 d and closest to 36 wk postmenstrual age | 136/159 (85.5) | 821/855 (96.0) | 1255/1298 (96.7) | 1507/1545 (97.5) | 1756/1788 (98.2) | 1913/1941 (98.6) | 2353/2375 (99.1) | 9741/9961 (97.8) | 7876/8034 (98.0) | −0.1 (−0.5 to 0.3) |
| Finding of cystic periventricular leukomalacia | 6/135 (4.4) | 64/820 (7.8) | 103/1254 (8.2) | 96/1505 (6.4) | 70/1755 (4.0) | 57/1911 (3.0) | 48/2351 (2.0) | 444/9731 (4.6) | 352/7872 (4.5) | −0.2 (−0.8 to 0.3) |
| Infants still hospitalized at 28 d | 77 | 609 | 1053 | 1352 | 1655 | 1813 | 2254 | 8813 | ||
| ROP examination | 70/77 (90.9) | 568/609 (93.3) | 1013/1053 (96.2) | 1307/1351 (96.7) | 1626/1655 (98.2) | 1786/1811 (98.6) | 2216/2253 (98.4) | 8586/8809 (97.5) | ||
| ROP | 65/70 (92.9) | 499/567 (88.0) | 871/1013 (86.0) | 922/1307 (70.5) | 944/1626 (58.1) | 752/1786 (42.1) | 660/2216 (29.8) | 4713/8585 (54.9) | 3821/6789 (56.3) | −2.1 (−3.5 to −0.7) |
| Severe ROP (stage ≥3) | 22/70 (31.4) | 216/567 (38.1) | 322/1013 (31.8) | 249/1307 (19.1) | 166/1626 (10.2) | 79/1786 (4.4) | 45/2216 (2.0) | 1099/8585 (12.8) | 839/6789 (12.4) | 0.5 (−0.1 to 1.2) |
| Intervention and/or treatment for ROPl | 19/69 (27.5) | 180/566 (31.8) | 242/1009 (24.0) | 164/1299 (12.6) | 96/1611 (6.0) | 40/1777 (2.3) | 20/2208 (0.9) | 761/8539 (8.9) | ||
| ROP status at discharge, transfer, death, or 120 d | ||||||||||
| Determined, favorable in both eyes | 6/70 (8.6) | 95/564 (16.8) | 255/1003 (25.4) | 403/1284 (31.4) | 589/1588 (37.1) | 685/1736 (39.5) | 859/2088 (41.1) | 2892/8333 (34.7) | ||
| Determined, severe ROP in 1 or both eyes | 13/70 (18.6) | 153/564 (27.1) | 197/1003 (19.6) | 132/1284 (10.3) | 85/1588 (5.4) | 32/1736 (1.8) | 21/2088 (1.0) | 633/8333 (7.6) | ||
| Undetermined in either eye (neither eye with severe ROP) | 51/70 (72.9) | 316/564 (56.0) | 551/1003 (54.9) | 749/1284 (58.3) | 914/1588 (57.6) | 1019/1736 (58.7) | 1208/2088 (57.9) | 4808/8333 (57.7) | ||
| Infants surviving to 36 wk postmenstrual agem | 64 | 559 | 1015 | 1302 | 1634 | 1823 | 2291 | 8688 | 6909 | |
| Evaluation for BPD | ||||||||||
| Oxygen use at 36 wkn | 50/64 (78.1) | 470/557 (84.4) | 769/1009 (76.2) | 837/1296 (64.6) | 822/1629 (50.5) | 722/1814 (39.8) | 637/2272 (28.0) | 4307/8641 (49.8) | 3064/6853 (44.7) | 4.3 (2.8 to 5.8) |
| Mode of support at 36 wko | ||||||||||
| No BPD | 6/64 (9.4) | 46/553 (8.3) | 145/984 (14.7) | 315/1266 (24.9) | 573/1554 (36.9) | 850/1725 (49.3) | 1325/2159 (61.4) | 3260/8305 (39.3) | ||
| Grade 1 | 9/64 (14.1) | 198/553 (35.8) | 358/984 (36.4) | 488/1266 (38.5) | 518/1554 (33.3) | 500/1725 (29.0) | 566/2159 (26.2) | 2637/8305 (31.8) | ||
| Grade 2 | 36/64 (56.3) | 209/553 (37.8) | 316/984 (32.1) | 340/1266 (26.9) | 347/1554 (22.3) | 278/1725 (16.1) | 216/2159 (10.0) | 1742/8305 (21.0) | ||
| Grade 3 | 13/64 (20.3) | 100/553 (18.1) | 165/984 (16.8) | 123/1266 (9.7) | 116/1554 (7.5) | 97/1725 (5.6) | 52/2159 (2.4) | 666/8305 (8.0) | ||
| Assisted ventilation by 36 wk postmenstrual age, median (IQR), dp | ||||||||||
| Total | 93 (81-95) [n = 63] | 81 (69-88) [n = 555] | 71 (57-81) [n = 996] | 57 (43-72) [n = 1291] | 44 (28-61) [n = 1617] | 29 (12-45) [n = 1810] | 17 (6-32) [n = 2268] | 41 (19-62) [n = 8600] | ||
| Invasive only | 65 (50-81) [n = 64] | 51 (36-70) [n = 557] | 37 (23-57) [n = 1003] | 23 (7-40) [n = 1295] | 8 (2-26) [n = 1627] | 2 (0-9) [n = 1812] | 1 (0-5) [n = 2276] | 7 (1-31) [n = 8634] | ||
| Length of hospitalization, median (IQR), d | ||||||||||
| All patients | 26 (7-142) [n = 159] | 121 (18-156) [n = 856] | 115 (82-148) [n = 1298] | 104 (83-129) [n = 1546] | 93 (75-116) [n = 1788] | 80 (65-98) [n = 1943] | 69 (56-85) [n = 2376] | 88 (63-117) [n = 9966] | ||
| Surviving infants only | 156 (135-217) [n = 60] | 143 (122-175) [n = 535] | 127 (108-156) [n = 972] | 110 (93-134) [n = 1266] | 96 (80-118) [n = 1608] | 82 (68-99) [n = 1787] | 70 (57-85) [n = 2267] | 93 (72-122) [n = 8495] | ||
Abbreviations: BPD, bronchopulmonary dysplasia; ROP, retinopathy of prematurity.
Distributions of the variables reported differed significantly across gestational age; adjusted P < .01 for all except adjusted P = .04 for tracheostomy, by Wald χ2, likelihood ratio χ2, or F test, adjusting for study center, small for gestational age, male sex, multiple gestation, and maternal race and ethnicity. Study center could not be included in models fit to vitamin A use and probiotic use because some centers reported no use.
Data are expressed as No./total (%) of infants unless otherwise indicated.
From Stoll et al.1 Infants included were restricted to those with birth weights of 401 g to 1500 g, consistent with Neonatal Research Network eligibility criteria during prior years. This criterion primarily restricted eligibility for infants born at 22 weeks’ gestational age. The current cohort includes infants born at 22-28 weeks’ gestational age with any birth weight, including 163 (1.5%) with birth weights less than 401 g and 79 (0.7%) with birth weights greater than 1500 g.
Where possible given data reported by Stoll et al, differences adjusted for gestational age are shown between the proportion reported for infants born at 22-28 weeks’ gestational age in the 2013-2018 cohort vs in 2008-2012 along with associated 95% confidence intervals for the difference. A positive difference indicates that the proportion increased in the 2013-2018 cohort compared with the 2008-2012 cohort; a negative difference indicates that the proportion decreased in the 2013-2018 cohort compared with the earlier cohort.
Collected beginning in 2016.
Treatments included intubation/ventilation, nutrition/hydration, and/or medication.
Patent ductus arteriosus medication included indomethacin, ibuprofen, and acetaminophen.
Human milk includes both maternal and donor milk.
Bell18 staging of necrotizing enterocolitis (NEC) as modified by Walsh and Kliegman19: stage IA, suspected NEC without gross hematochezia; stage IB, suspected NEC with gross hematochezia; stage IIA, definite NEC, mildly ill; stage IIB, definite NEC, moderately ill; stage IIIA, advanced NEC, severely ill, bowel intact; stage IIIB, advanced NEC, severely ill, bowel perforated.
Early-onset (≤72 hours) and late-onset (>72 hours) sepsis was defined by positive blood culture and appropriate therapy for 5 or more days or intent to treat if death occurred within 5 days. Early-onset and late-onset meningitis were defined by positive cerebrospinal fluid culture and appropriate therapy for 7 or more days or intent to treat if death occurred within 7 days. In 2008-2012, the reported proportions of early-onset and late-onset sepsis did not include meningitis. In the current cohort, 2 (0.8%) of 238 infants with early-onset sepsis and/or meningitis had meningitis alone. Of the 1911 infants with late-onset sepsis and/or meningitis, 24 (1.3%) had meningitis alone.
Intracranial hemorrhage was determined based on the cranial sonogram with the most severe findings performed within 28 days after birth and was classified by the criteria of Papile et al20: grade I, subependymal hemorrhage; grade II, intraventricular hemorrhage without ventricular dilatation; grade III, intraventricular hemorrhage with ventricular dilatation; grade IV, intraventricular hemorrhage with parenchymal hemorrhage.
Interventions and treatments for ROP included any of retinal ablation (laser and/or cryotherapy), scleral buckle, vitrectomy, and/or bevacizumab or other antivascular endothelial growth factor therapies.
Infants surviving to 36 weeks’ postmenstrual age include infants still in the hospital at 36 weeks (90%) or discharged home or transferred before 36 weeks.
For surviving infants who were discharged or transferred before 36 weeks’ postmenstrual age, BPD was defined as supplemental oxygen use at 36 weeks if known or use at discharge or transfer. Of the 8641 infants evaluated, 7782 (90%) were still in the hospital at 36 weeks’ postmenstrual age, 541 (6%) had been discharged home, and 318 (4%) had been transferred to another hospital after a median of 41 days in the birth hospital.
Based on the optimal definition (definition 15) from Jensen et al.21 This definition classifies BPD based on the mode of support received at 36 weeks’ postmenstrual age or at discharge home if earlier: no BPD, no support or breathing room air; grade 1, nasal cannula at a flow rate of 2 L/min or less; grade 2, nasal cannula at a flow rate of more than 2 L/min or noninvasive positive airway pressure; and grade 3, invasive mechanical ventilation. BPD was undefined for infants transferred prior to 36 weeks’ postmenstrual age unless status at 36 weeks was known. Of the 8305 infants evaluated, 7764 (93%) were still in the hospital at 36 weeks’ postmenstrual age, 538 (6%) had been discharged home, and 3 (<1%) had been transferred to another hospital.
Total includes high-frequency ventilation, conventional ventilation, nasal intermittent positive pressure ventilation, and/or continuous positive airway pressure. Invasive includes high-frequency and/or conventional ventilation.
A total of 84.3% (8388/9951) of infants who survived more than 12 hours underwent mechanical ventilation during the hospitalization, and 79.8% received surfactant; these proportions varied significantly with gestational age (P < .001 for each). Overall, 18.6% (1791/9624) of infants received postnatal corticosteroids for bronchopulmonary dysplasia, 10.1% (1009/9959) of infants received inhaled nitric oxide, and 18.4% (1799/9796) received vitamin A; proportions varied significantly by gestational age (P < .001 for each). Medication was given to 23.4% (2331/9954) of infants for closure of a patent ductus arteriosus, and 7.4% (741/9953) had surgery or cardiac catheterization for patent ductus arteriosus closure. Overall, 78.4% (7807/9960) of infants underwent transfusion with red blood cells; more than 95% of infants born at 22-25 weeks’ gestational age had transfusion, as did 49.3% (7807/9960) of those born at 28 weeks. Probiotics were received by 9.6% (443/4630) of infants, but use varied by center; no infants received probiotics at 12 centers, less than 6% received probiotics at 4 centers, and more than 30% received probiotics at 3 centers. Overall, 91.7% (9019/9830) of infants received human milk in the first 28 days of life. Parenteral nutrition was received for a median of 17 (IQR, 11-30) days. Infants remained in the hospital for a median of 88 (IQR, 63-117) days; those born at 23-25 weeks’ gestation had median hospital stays of 104 to 121 days. The median length of hospitalization for all surviving infants was 93 (IQR, 72-122) days.
The proportion of infants diagnosed as having each morbidity during the birth hospitalization varied significantly by gestational age (P < .001 for each) (Table 3). Necrotizing enterocolitis was diagnosed in 8.9% (890/9956) of infants, and 3.9% (386/9956) received surgery. Early-onset sepsis and/or meningitis was diagnosed in 2.4% (238/9957) of infants. Late-onset sepsis and/or meningitis was diagnosed in 19.9% (1911/9610) of infants surviving more than 72 hours. The overall incidence of severe intracranial hemorrhage was 14.3% (1386/9705); severe intracranial hemorrhage was diagnosed in 38.2% (52/136) of infants born at 22 weeks compared with 5.3% (123/2342) born at 28 weeks (difference, 33.0%; 95% CI, 25.2%-41.4%; P < .001). Cystic periventricular leukomalacia was diagnosed in 4.6% (444/9731) of infants. The overall incidence of retinopathy of prematurity was 54.9% (4713/8585); 12.8% (1099/8585) had severe retinopathy of prematurity (stage ≥3). Retinopathy of prematurity was diagnosed in 92.9% (65/70) of infants born at 22 weeks compared with 29.8% (660/2216) of those born at 28 weeks (difference, 63.1%; 95% CI, 54.4%-67.7%; P < .001). Of the infants who survived to 36 weeks’ postmenstrual age, 49.8% (4307/8641) had bronchopulmonary dysplasia based on continued oxygen use, including more than 75% of infants of 22-24 weeks’ gestational age. Based on the mode of support received at 36 weeks’ postmenstrual age,21 60.7% (5045/8305) of infants had bronchopulmonary dysplasia of grade 1 or higher and 8.0% (666/8305) had grade 3. The distribution of bronchopulmonary dysplasia grade differed by gestational age (P < .001). Infants received respiratory support for a median of 41 days, decreasing significantly from 93 days at 22 weeks to 17 days at 28 weeks.
Compared with the 2008-2012 cohort reported by Stoll et al,1 the incidence of necrotizing enterocolitis in the present cohort was significantly lower (8.9% vs 10.3%; adjusted difference, −1.6%; 95% CI, −2.5% to −0.8%), as was the incidence of late-onset sepsis and/or meningitis (19.9% vs 24.4%; adjusted difference, −4.9%; 95% CI, −6.0% to −3.7%), severe intracranial hemorrhage (14.3% vs 14.6%; adjusted difference, −1.0%; 95% CI, −1.9% to −0.1%), and retinopathy of prematurity (54.9% vs 56.3%; adjusted difference, −2.1; 95% CI, −3.5% to −0.7%). The incidence of bronchopulmonary dysplasia (supplemental oxygen use at 36 weeks’ postmenstrual age) was significantly higher (49.8% vs 44.7%; adjusted difference, 4.3%; 95% CI, 2.8%-5.8%) in the present cohort (Table 3). There was no significant difference between the current cohort and the 2008-2012 cohort in the incidence of early-onset sepsis and/or meningitis, cystic periventricular leukomalacia, or severe retinopathy of prematurity.
Outcomes at 22-26 Months’ Corrected Age for Infants Born in 2013-2016
Among 2930 surviving infants born in 2013-2016 at gestational ages of 22-26 weeks who were eligible for follow-up, 2566 (87.6%) were seen at visits between January 22, 2015, and August 13, 2019, and were included in analysis of outcomes (eFigure 1 in Supplement 1); 2458 infants (83.9%) had all information needed to complete the NDI evaluation. Mothers of children seen at follow-up had an older median age and were more likely to have a college degree and to be of Black or White race compared with those lost to follow-up, and more children seen at follow-up were born at lower gestational ages and with lower birth weights (eTable 1 in Supplement 1).
A total of 48.7% (1198/2458) of the children evaluated had no or mild NDI, 29.3% (709/2419) had moderate NDI, and 21.2% (512/2419) had severe NDI (Figure, A; eTable 2 in Supplement 1). For infants born at 23-26 weeks’ gestation, the proportion with no or mild NDI increased significantly from 31.0% (88/284) at 23 weeks to 58.1% (518/892) at 26 weeks (difference, 27.1%; 95% CI, 20.6%-33.2%; P < .001). Of 29 infants born at 22 weeks’ gestation and evaluated for NDI, 13 (44.8%) had no or mild NDI, 7 (24.1%) had moderate NDI, and 9 (31.0%) had severe NDI. eFigure 3 in Supplement 1 shows a complete accounting of all infants who were eligible for follow-up assessment, including those who died before assessment or were lost to follow-up. The proportions of children who died or who survived with varying degrees of NDI are shown for all children who died or were evaluated (eFigure 4 in Supplement 1) and for the subset of children who were actively treated at birth (Figure, B).
Figure. Neurodevelopmental Impairment at 22-26 Months’ Corrected Age in Children Born at 22-26 Weeks’ Gestational Age During 2013-2016.
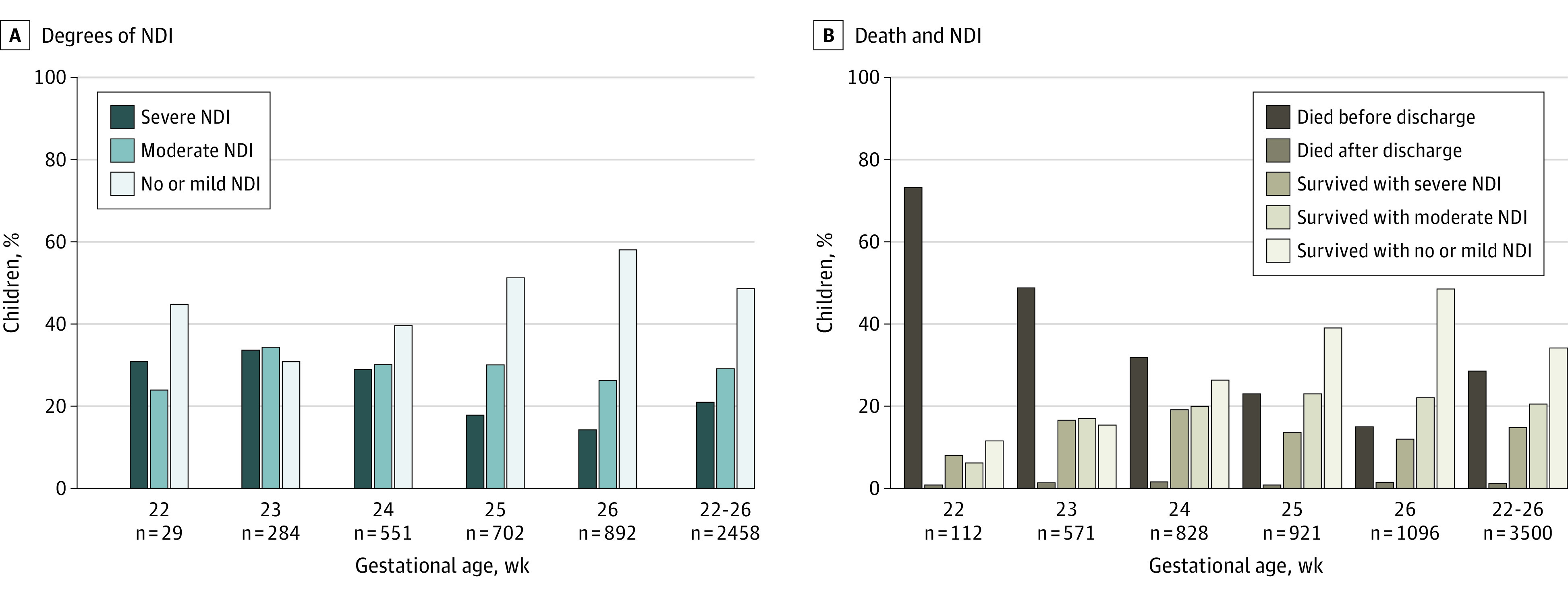
A, Neurodevelopmental impairment (NDI) among 2458 children evaluated at 22-26 months’ corrected age. Mild or no NDI was defined as a Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III) cognitive composite score of 85 or higher, a Bayley-III motor composite score of 85 or higher, and Gross Motor Function Classification System (GMFCS)25 level 0 or 1. Moderate NDI was defined as any of a Bayley-III cognitive composite score or motor composite score of 70 to 84 or GMFCS level 2 or 3. Severe NDI was defined by any of a Bayley-III cognitive composite score or motor composite score less than 70, GMFCS level 4 or 5, bilateral blindness, or severe hearing impairment (see Table 4 footnotes b and d for more details).
B, Death and NDI at 22-26 months’ corrected age among children born at 22-26 weeks’ gestational age who were actively treated at birth. Children born at 22-26 weeks’ gestational age were eligible for a follow-up assessment at 22-26 months’ corrected age. Proportions are shown for the 3500 children actively treated at birth who had died by 22-26 months’ corrected age or were seen at follow-up and evaluated for NDI. Active treatment was defined as intubation, surfactant therapy, respiratory support, chest compressions, epinephrine, volume resuscitation, blood pressure support, or parenteral nutrition22 (see Table 4 footnotes b and d for more details).
Mean Bayley-III cognitive, language, and motor composite scores differed significantly overall by gestational age (P < .001 for each) (Table 4; eFigures 5-7 in Supplement 1). The proportion of children with cognitive composite scores of less than 70 decreased from 25.8% (8/31) for those born at 22 weeks to 8.3% (75/905) for those born at 26 weeks (difference, −17.5%; 95% CI, −35.1% to −5.2%; P < .001). Twenty-five percent (607/2431) of children had Bayley-III language composite scores of less than 70, with higher proportions among those born at 23 and 24 weeks. Seven of 30 children (23.3%) born at 22 weeks had a motor composite score of less than 70, while the proportions were 29.1% (81/278) for those born at 23 weeks and 9.6% (85/882) for those born at 26 weeks. Among children born at 22, 23, or 24 weeks’ gestational age, mean cognitive, language, and motor scores were significantly lower for those born at 23 weeks compared with those born at 24 weeks (P < .01 for each), but statistically significant differences were not found between scores for children born at 22 weeks’ gestational age compared with those born at 23 or 24 weeks (Table 4; eFigures 5-7 in Supplement 1). The proportion of children with language and motor scores of less than 70 was significantly higher for children born at 23 weeks’ gestational age compared with those born at 24 weeks (P = .006 and P = .03, respectively), but the proportion with cognitive scores of less than 70 did not differ among children born at 22, 23, or 24 weeks (P = .20).
Table 4. Outcomes at 22-26 Months’ Corrected Age for Children Born at 22-26 Weeks’ Gestational Age in 2013-2016.
| Variables | No./total (%), by gestational age in weeksa | |||||
|---|---|---|---|---|---|---|
| 22 (n = 31) | 23 (n = 292) | 24 (n = 567) | 25 (n = 733) | 26 (n = 943) | 22-26 (n = 2566) | |
| Bayley-III scoresb | ||||||
| Cognitive composite score | ||||||
| No. evaluated | 31 | 285 | 559 | 712 | 905 | 2492 |
| Mean (SD) | 80.0 (16.4) | 79.7 (16.8) | 82.0 (15.7) | 86.3 (15.1) | 88.4 (14.6) | 85.3 (15.6) |
| Median (IQR) | 85 (65-90) | 80 (70-90) | 85 (70-95) | 90 (80-95) | 90 (80-95) | 85 (75-95) |
| <70 | 8 (25.8) | 68 (23.9) | 117 (20.9) | 89 (12.5) | 75 (8.3) | 357 (14.3) |
| 70-84 | 6 (19.4) | 82 (28.8) | 145 (25.9) | 163 (22.9) | 207 (22.9) | 603 (24.2) |
| ≥85 | 17 (54.8) | 135 (47.4) | 297 (53.1) | 460 (64.6) | 623 (68.8) | 1532 (61.5) |
| Language composite score | ||||||
| No. evaluated | 31 | 282 | 544 | 696 | 878 | 2431 |
| Mean (SD) | 78.6 (19.0) | 75.0 (17.8) | 77.6 (17.7) | 82.3 (17.4) | 84.4 (16.7) | 81.1 (17.6) |
| Median (IQR) | 79 (71-94) | 74 (62-89) | 79 (65-89) | 83 (71-94) | 86 (74-97) | 83 (71-94) |
| <70 | 7 (22.6) | 111 (39.4) | 177 (32.5) | 151 (21.7) | 161 (18.3) | 607 (25.0) |
| 70-84 | 11 (35.5) | 74 (26.2) | 155 (28.5) | 214 (30.7) | 241 (27.4) | 695 (28.6) |
| ≥85 | 13 (41.9) | 97 (34.4) | 212 (39.0) | 331 (47.6) | 476 (54.2) | 1129 (46.4) |
| Motor composite score | ||||||
| No. evaluated | 30 | 278 | 539 | 697 | 882 | 2426 |
| Mean (SD) | 76.6 (18.4) | 76.7 (17.8) | 80.2 (16.5) | 85.7 (15.5) | 88.6 (15.0) | 84.4 (16.4) |
| Median (IQR) | 84 (70-91) | 79 (64-88) | 82 (70-91) | 88 (79-97) | 91 (82-97) | 88 (76-94) |
| <70 | 7 (23.3) | 81 (29.1) | 123 (22.8) | 87 (12.5) | 85 (9.6) | 383 (15.8) |
| 70-84 | 8 (26.7) | 87 (31.3) | 151 (28.0) | 175 (25.1) | 181 (20.5) | 602 (24.8) |
| ≥85 | 15 (50.0) | 110 (39.6) | 265 (49.2) | 435 (62.4) | 616 (69.8) | 1441 (59.4) |
| Cerebral palsyc | ||||||
| None | 16/31 (51.6) | 197/290 (67.9) | 432/566 (76.3) | 627/730 (85.9) | 826/938 (88.1) | 2098/2555 (82.1) |
| Mild | 8/31 (25.8) | 41/290 (14.1) | 73/566 (12.9) | 56/730 (7.7) | 65/938 (6.9) | 243/2555 (9.5) |
| Moderate | 4/31 (12.9) | 27/290 (9.3) | 40/566 (7.1) | 24/730 (3.3) | 27/938 (2.9) | 122/2555 (4.8) |
| Severe | 3/31 (9.7) | 25/290 (8.6) | 21/566 (3.7) | 23/730 (3.2) | 20/938 (2.1) | 92/2555 (3.6) |
| GMFCS leveld | ||||||
| 0 | 12/31 (38.7) | 145/289 (50.2) | 336/565 (59.5) | 536/731 (73.3) | 731/937 (78.0) | 1760/2553 (68.9) |
| 1 | 11/31 (35.5) | 83/289 (28.7) | 151/565 (26.7) | 132/731 (18.1) | 146/937 (15.6) | 523/2553 (20.5) |
| 2 or 3 | 5/31 (16.1) | 39/289 (13.5) | 56/565 (9.9) | 39/731 (5.3) | 39/937 (4.2) | 178/2553 (7.0) |
| 4 or 5 | 3/31 (9.7) | 22/289 (7.6) | 22/565 (3.9) | 24/731 (3.3) | 21/937 (2.2) | 92/2553 (3.6) |
| Visione | ||||||
| Normal in both eyes | 22/31 (71.0) | 204/289 (70.6) | 460/566 (81.3) | 629/731 (86.0) | 856/938 (91.3) | 2171/2555 (85.0) |
| Corrective lenses/other abnormality but not blind in either eye | 5/31 (16.1) | 75/289 (26.0) | 94/566 (16.6) | 91/731 (12.4) | 74/938 (7.9) | 339/2555 (13.3) |
| Blind in 1 eye | 1/31 (3.2) | 2/289 (0.7) | 3/566 (0.5) | 1/731 (0.1) | 0/938 (0.0) | 7/2555 (0.3) |
| Blind in both eyes | 3/31 (9.7) | 8/289 (2.8) | 9/566 (1.6) | 10/731 (1.4) | 8/938 (0.9) | 38/2555 (1.5) |
| Hearingf | ||||||
| No functional impairment | 29/30 (96.7) | 271/285 (95.1) | 544/560 (97.1) | 707/724 (97.7) | 907/929 (97.6) | 2458/2528 (97.2) |
| Functional impairment | 1/30 (3.3) | 14/285 (4.9) | 16/560 (2.9) | 17/724 (2.3) | 22/929 (2.4) | 70/2528 (2.8) |
| Cochlear implant or aid | 0/30 (0.0) | 8/285 (2.8) | 13/561 (2.3) | 16/722 (2.2) | 27/929 (2.9) | 64/2527 (2.5) |
| Medical history and functional outcomes | ||||||
| Had a hospitalization after discharge | 20/31 (64.5) | 171/290 (59.0) | 311/567 (54.9) | 360/732 (49.2) | 415/941 (44.1) | 1277/2561 (49.9) |
| No. of hospitalizations, median (IQR) | 3 (2-4) | 2 (1-3) | 2 (1-3) | 2 (1-3) | 1 (1-3) | 2 (1-3) |
| Oral motor skills | ||||||
| Independently feeds self | 20/31 (64.5) | 208/290 (71.7) | 436/567 (76.9) | 622/732 (85.0) | 804/941 (85.4) | 2090/2561 (81.6) |
| Dependent oral feeding | 5/31 (16.1) | 30/290 (10.3) | 50/567 (8.8) | 45/732 (6.1) | 57/941 (6.1) | 187/2561 (7.3) |
| Limited oral feeding | 5/31 (16.1) | 38/290 (13.1) | 59/567 (10.4) | 51/732 (7.0) | 62/941 (6.6) | 215/2561 (8.4) |
| No oral feeding | 1/31 (3.2) | 14/290 (4.8) | 22/567 (3.9) | 14/732 (1.9) | 18/941 (1.9) | 69/2561 (2.7) |
| Current medical equipment | ||||||
| Apnea monitor | 0/31 (0.0) | 8/290 (2.8) | 9/567 (1.6) | 8/732 (1.1) | 14/941 (1.5) | 39/2561 (1.5) |
| Oxygen | 3/31 (9.7) | 33/290 (11.4) | 33/567 (5.8) | 36/732 (4.9) | 30/941 (3.2) | 135/2561 (5.3) |
| Ventilator or continuous positive airway pressure | 0/31 (0.0) | 9/290 (3.1) | 10/567 (1.8) | 14/732 (1.9) | 10/941 (1.1) | 43/2561 (1.7) |
| Gastrostomy or other tube feeding | 6/31 (19.4) | 52/290 (17.9) | 81/567 (14.3) | 75/732 (10.2) | 80/941 (8.5) | 294/2561 (11.5) |
| Tracheostomy | 1/31 (3.2) | 19/290 (6.6) | 29/567 (5.1) | 23/732 (3.1) | 22/941 (2.3) | 94/2561 (3.7) |
| Pulse oximeter | 3/31 (9.7) | 42/290 (14.5) | 57/567 (10.1) | 48/732 (6.6) | 41/941 (4.4) | 191/2561 (7.5) |
| Any (≥1) of above | 7/31 (22.6) | 71/290 (24.5) | 106/567 (18.7) | 99/732 (13.5) | 99/941 (10.5) | 382/2561 (14.9) |
| Multiple (≥2) of above | 3/31 (9.7) | 44/290 (15.2) | 58/567 (10.2) | 46/732 (6.3) | 41/941 (4.4) | 192/2561 (7.5) |
| Mobility aids/supportive equipment | ||||||
| Adapted stroller or wheelchair | 1/31 (3.2) | 16/290 (5.5) | 24/566 (4.2) | 17/732 (2.3) | 20/941 (2.1) | 78/2560 (3.0) |
| Braces/orthotics | 7/31 (22.6) | 60/290 (20.7) | 88/566 (15.5) | 80/732 (10.9) | 93/941 (9.9) | 328/2560 (12.8) |
| Walker | 4/31 (12.9) | 24/290 (8.3) | 23/566 (4.1) | 22/732 (3.0) | 21/941 (2.2) | 94/2560 (3.7) |
| Stander | 1/31 (3.2) | 10/290 (3.4) | 14/566 (2.5) | 19/732 (2.6) | 12/941 (1.3) | 56/2560 (2.2) |
| Corner chair or other adaptive seat | 1/31 (3.2) | 9/290 (3.1) | 7/566 (1.2) | 7/732 (1.0) | 5/941 (0.5) | 29/2560 (1.1) |
| Any (≥1) of above | 10/31 (32.3) | 77/290 (26.6) | 103/566 (18.2) | 91/732 (12.4) | 112/941 (11.9) | 393/2560 (15.4) |
| Multiple (≥2) of above | 3/31 (9.7) | 32/290 (11.0) | 32/566 (5.7) | 35/732 (4.8) | 29/941 (3.1) | 131/2560 (5.1) |
Abbreviations: Bayley-III, Bayley Scales of Infant and Toddler Development, Third Edition; GMFCS, Gross Motor Function Classification System.
Data are expressed as No./total (%) of children assessed at 22-26 months’ corrected age unless otherwise indicated. Corrected age represents age of child based on expected date of delivery, calculated by subtracting the number of weeks before 40 weeks of gestation at which birth occurred from chronological age.15 To minimize the number of comparisons, differences in Bayley-III composite scores by gestational age were tested for the mean scores only. Similarly, differences by gestational age were not examined for each item in the current medical equipment and mobility aids/supportive equipment groups, but “any of above” was compared across gestational ages. Vision data were analyzed as normal in both eyes vs the other categories combined. For the purpose of assessing whether the proportion of infants with a cochlear implant or hearing aid differed by gestational age, infants born at 22 and 23 weeks’ gestational age were combined. Differences by gestational age were significant for most outcomes; adjusted P < .001 except for adjusted P = .12 for hearing impairment, adjusted P = .93 for cochlear implant or hearing aid, and adjusted P = .29 for median number of hospitalizations after discharge, adjusting for study center, small for gestational age, male sex, multiple gestation, maternal race and ethnicity (Black, Hispanic, White, or other), and maternal education assessed at the time of delivery (less than high school degree, high school degree, more than high school degree, or unknown).
Each Bayley-III composite score is standardized to a mean of 100 (SD, 15).The cognitive composite score range is 55 to 145, the language composite score range is 47 to 153, and the motor composite score range is 46 to 154, with lower scores representing more impaired outcomes.
Mild cerebral palsy was defined as GMFCS level 1, moderate cerebral palsy as GMFCS level 2 or 3, and severe cerebral palsy as GMFCS level 4 or 5.25
The GMFCS levels are defined as follows: level 0, walks independently, with normal and fluent gait; level 1, walks 10 steps independently, but with some gait abnormalities; level 2, maintains floor sitting but may need to use hands for support to maintain balance, creeps on stomach or crawls on hands and knees with reciprocal leg movement, may pull to stand and take steps holding onto furniture; level 3, maintains floor sitting when the low back is supported, rolls and creeps forward on stomach or may crawl with or without reciprocal leg movements; level 4, has head control but trunk support is required for floor sitting, can roll to supine, may roll to prone; and level 5, is unable to maintain antigravity head and trunk postures in prone and sitting positions, requires adult assistance to roll. Higher levels indicate greater limitation.
Vision in each eye was recorded as normal, having been prescribed corrective lenses, other abnormality, blind (consistent with refraction of 20/200 or less) with some functional vision, or no useful vision.
No functional hearing impairment was defined as being able to follow oral directions given during the assessment, with or without amplification. Functional hearing impairment was defined as permanent hearing loss that did not permit a child to understand directions and communicate, with or without amplification.
The proportion of children with cerebral palsy of any severity was 17.9% (457/2555) and decreased significantly from 48.4% (15/31) among those born at 22 weeks to 11.9% (112/938) among those born at 26 weeks (difference, −36.5%; 95% CI, −53.4% to −19.9%; P < .001) (Table 4). Most of the affected children had mild cerebral palsy, but 2.1% to 9.7% had severe cerebral palsy, varying inversely with gestational age. Overall, 1.5% (38/2555) of children had blindness in both eyes; 2.5% (64/2527) used hearing aids or cochlear implants.
A total of 49.9% (1277/2561) of children were rehospitalized after initial discharge. The 1277 children who were rehospitalized had 2980 admissions. Respiratory disease was the most common reason for readmission (54.0%), followed by surgery (15.4%) and infection (9.3%) (eTable 3 in Supplement 1).
By 22-26 months’ corrected age, 81.6% (2090/2561) of children were feeding themselves independently; this proportion varied significantly with gestational age (P < .001) (Table 4). Oral feeding requiring more than occasional assistance was reported for 7.3% (187/2561) of children. Limited oral feeding requiring some food by an alternate route or no oral feeding was reported for 11.1% (284/2561).
At 22-26 months’ corrected age, 14.9% (382/2561) of children were using an apnea monitor, oxygen, ventilator or continuous positive airway pressure, feeding tube, tracheostomy, and/or pulse oximeter, with the proportion significantly decreasing from 22.6% (7/31) of those born at 22 weeks’ gestational age to 10.5% (99/941) of those born at 26 weeks (difference, −12.1%; 95% CI, −29.4% to −0.6%; P < .001) (Table 4). The most common assistive device used at 2 years’ corrected age (11.5% [294/2561]) was a feeding tube (including gastrostomy). Oxygen was used by 5.3% (135/2561) of infants, and 1.7% (43/2561) required a ventilator or continuous positive airway pressure. In addition, 15.4% (393/2560) of children required use of a mobility aid or other supportive equipment.
Discussion
Among extremely preterm infants born in 2013-2018 and treated at 19 US academic medical centers, 78.3% survived to discharge, a significantly higher rate than for infants born in 2008-2012.1 Rates of mortality and in-hospital morbidities were higher among those born at earlier gestational ages. Among infants born at less than 27 weeks’ gestational age, rehospitalization and severe NDI were common at 2 years.
With improving survival of children born extremely preterm, there is increasing focus on broader outcomes including neurodevelopment. Although it is important to evaluate these findings with respect to other reports, comparing neurodevelopmental outcome studies is fraught with significant challenges. Cohort inclusion criteria including gestational age, birth years, and other factors may differ, as may assessment instruments or versions of instruments. Similarly, clinical and sociodemographic factors and definitions of NDI vary by study, further confounding comparisons.27
Such factors may result in substantial differences in reported rates of NDI and severe NDI. Nevertheless, these findings are similar to those reported by Synnes et al.7 Their report from a more mature cohort (up to 29 weeks’ gestational age) reported slightly lower overall NDI and severe NDI rates; however, rates of severe NDI at 24 weeks (about 30%), 25 weeks (about 20%), and 26 weeks (about 18%) were consistent with our findings. The EPICure 2 study followed up children born before 26 weeks’ gestational age in the United Kingdom in 2006 to age 2.5 to 3 years and reported a 19% severe NDI rate but used a definition restricted to developmental quotients more than 3 SDs below the mean.10 The Swedish EXPRESS study followed up children born before 27 weeks’ gestational age in 2004-2007, reporting that 58% had some level of disability at 30 months’ corrected age. Only 11% were reported to have severe disability, but the definition used included Bayley-III composite cognitive, language, or motor scores more than 3 SDs below the mean.28 Kono et al4 presented 3-year outcomes from the Neonatal Research Network of Japan for children born before 25 weeks’ gestational age in 2008-2012. They reported an NDI rate of 38.1% among those with neurodevelopmental follow-up using a developmental test standardized for Japanese children, but the follow-up rate was only 60.6%.
Composite end points such as NDI and disability at toddler age are commonly used outcomes for clinical studies in neonatology. Contemporary early neurodevelopmental outcome data are also used in parent counseling at the time of threatened extremely preterm delivery and in the neonatal intensive care unit (NICU) during discussions regarding long-term transitions. However, these composite outcomes may be confusing to parents, conflate multiple end points of varying value to parents, and frame discussions in a negative way.12,29,30 Parents may be more interested in understanding “real-life” outcomes to develop a personalized and functional picture for the future, yet these outcomes are inconsistently reported. Information regarding post-NICU rehospitalizations, feeding, equipment, and medication needs may inform both complex home care preparations and health-related quality-of-life considerations and thus may be more meaningful for families.14,31 Hospitalization after NICU discharge was common, with nearly half of all children being rehospitalized at least once by 22 to 26 months, consistent with previous reports.32,33,34 Profound functional neurosensory impairments were rare for the cohort overall, including blindness and severe functional hearing impairment. The rate of severe cerebral palsy was also low.
The overall survival for infants of 22-28 weeks’ gestational age has continued to increase since 2008-2012,1 even though more infants born at 22 weeks were being actively treated in this more recent cohort. The survival of infants live-born at 22 weeks is similar to more recently reported survival among infants born at 22 weeks from the Vermont Oxford Network.35 Higher survival for infants born at 22 weeks has been reported from Japan4 and Sweden.5
Strengths of this study include the large number of infants with high follow-up rates and rigorous prospective data collection including neurodevelopmental assessments by certified evaluators. This study reports survival both for all live-born infants and for only those actively treated. In addition, it reports often overlooked 2-year neurodevelopmental and functional outcomes of importance to families.29,30
Limitations
This study has several limitations. First, the Neonatal Research Network cohort is hospital-based rather than population-based. Although large and diverse, both in terms of geography and sociodemographics, this cohort does not represent the entire US preterm population but, rather, a selected population from academic medical centers. Second, the 2-year end points presented provide only a limited window into the future. Individual prediction of later childhood outcomes at 2 years has limitations.36 The complexity of developmental and cognitive milestones increases throughout childhood and adolescence, and engagement of both parents and children in health-related quality-of-life and other outcome assessments should continue through later childhood and into adulthood.14,31,37
Conclusions
Among extremely preterm infants born in 2013-2018 and treated at 19 US academic medical centers, 78.3% survived to discharge, a significantly higher rate than for infants born in 2008-2012. Among infants born at less than 27 weeks’ gestational age, rehospitalization and severe neurodevelopmental impairment were common at 2 years.
eTable 1. Characteristics of Surviving Children Born at 22-26 Weeks’ GA Who Were Lost to Follow-up Versus Seen at 22-26 Months’ Corrected Age Follow-up
eTable 2. Death and Neurodevelopmental Impairment at 22-26 Months’ Corrected Age in Children Born at 22-26 Weeks’ GA, 2013-2016
eTable 3. Primary Causes for Hospitalizations Since Discharge in Children Born at 22-26 Weeks’ GA, 2013-2016
eFigure 1. Participant Flow Diagram
eFigure 2. Proportion of Infants Who Survived to Discharge or 1 Year by Gestational Age at Birth
eFigure 3. Status at 22-26 Months’ Corrected Age for 4344 Children Born at 22-26 Weeks’ Gestational Age Who Were Eligible for a Follow-up Assessment
eFigure 4. Death and Neurodevelopmental Impairment at 22-26 Months’ Corrected Age in Children Born at 22-26 Weeks’ Gestational Age
eFigure 5. Bayley-III Cognitive Composite Scores by Gestational Age at Birth
eFigure 6. Bayley-III Language Composite Scores by Gestational Age at Birth
eFigure 7. Bayley-III Motor Composite Scores by Gestational Age at Birth
Nonauthor Collaborators. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network
References
- 1.Stoll BJ, Hansen NI, Bell EF, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314(10):1039-1051. doi: 10.1001/jama.2015.10244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horbar JD, Edwards EM, Greenberg LT, et al. Variation in performance of neonatal intensive care units in the United States. JAMA Pediatr. 2017;171(3):e164396. doi: 10.1001/jamapediatrics.2016.4396 [DOI] [PubMed] [Google Scholar]
- 3.Lee SK, Beltempo M, McMillan DD, et al. ; Evidence-Based Practice for Improving Quality Investigators . Outcomes and care practices for preterm infants born at less than 33 weeks’ gestation: a quality-improvement study. CMAJ. 2020;192(4):E81-E91. doi: 10.1503/cmaj.190940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kono Y, Yonemoto N, Nakanishi H, Kusuda S, Fujimura M. Changes in survival and neurodevelopmental outcomes of infants born at <25 weeks’ gestation: a retrospective observational study in tertiary centres in Japan. BMJ Paediatr Open. 2018;2(1):e000211. doi: 10.1136/bmjpo-2017-000211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norman M, Hallberg B, Abrahamsson T, et al. Association between year of birth and 1-year survival among extremely preterm infants in Sweden during 2004-2007 and 2014-2016. JAMA. 2019;321(12):1188-1199. doi: 10.1001/jama.2019.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Younge N, Goldstein RF, Bann CM, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Survival and neurodevelopmental outcomes among periviable infants. N Engl J Med. 2017;376(7):617-628. doi: 10.1056/NEJMoa1605566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Synnes A, Luu TM, Moddemann D, et al. ; Canadian Neonatal Network and the Canadian Neonatal Follow-up Network . Determinants of developmental outcomes in a very preterm Canadian cohort. Arch Dis Child Fetal Neonatal Ed. 2017;102(3):F235-F234. doi: 10.1136/archdischild-2016-311228 [DOI] [PubMed] [Google Scholar]
- 8.Adams-Chapman I, Heyne RJ, DeMauro SB, et al. ; Follow-up Study of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Neurodevelopmental impairment among extremely preterm infants in the Neonatal Research Network. Pediatrics. 2018;141(5):e20173091. doi: 10.1542/peds.2017-3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheong JLY, Anderson PJ, Burnett AC, et al. ; Victorian Infant Collaborative Study Group . Changing neurodevelopment at 8 years in children born extremely preterm since the 1990s. Pediatrics. 2017;139(6):e20164086. doi: 10.1542/peds.2016-4086 [DOI] [PubMed] [Google Scholar]
- 10.Moore T, Hennessy EM, Myles J, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ. 2012;345:e7961. doi: 10.1136/bmj.e7961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marlow N, Doyle LW, Anderson P, et al. ; International Neonatal Consortium . Assessment of long-term neurodevelopmental outcome following trials of medicinal products in newborn infants. Pediatr Res. 2019;86(5):567-572. doi: 10.1038/s41390-019-0526-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janvier A, Farlow B, Baardsnes J, Pearce R, Barrington KJ. Measuring and communicating meaningful outcomes in neonatology: a family perspective. Semin Perinatol. 2016;40(8):571-577. doi: 10.1053/j.semperi.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 13.Saigal S, Ferro MA, Van Lieshout RJ, Schmidt LA, Morrison KM, Boyle MH. Health-related quality of life trajectories of extremely low birth weight survivors into adulthood. J Pediatr. 2016;179(Dec):68-73. doi: 10.1016/j.jpeds.2016.08.018 [DOI] [PubMed] [Google Scholar]
- 14.McAndrew S, Acharya K, Westerdahl J, et al. A prospective study of parent health-related quality of life before and after discharge from the neonatal intensive care unit. J Pediatr. 2019;213(Oct):38-45. doi: 10.1016/j.jpeds.2019.05.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engle WA; American Academy of Pediatrics Committee on Fetus and Newborn . Age terminology during the perinatal period. Pediatrics. 2004;114(5):1362-1364. doi: 10.1542/peds.2004-1915 [DOI] [PubMed] [Google Scholar]
- 16.Travers CP, Carlo WA, McDonald SA, et al. ; Generic Database and Follow-up Subcommittees of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Racial/ethnic disparities among extremely preterm infants in the United States from 2002 to 2016. JAMA Netw Open. 2020;3(6):e206757. doi: 10.1001/jamanetworkopen.2020.6757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163-168. doi: 10.1016/0029-7844(95)00386-X [DOI] [PubMed] [Google Scholar]
- 18.Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis: therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1-7. doi: 10.1097/00000658-197801000-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33(1):179-201. doi: 10.1016/S0031-3955(16)34975-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529-534. doi: 10.1016/S0022-3476(78)80282-0 [DOI] [PubMed] [Google Scholar]
- 21.Jensen EA, Dysart K, Gantz MG, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants: an evidence-based approach. Am J Respir Crit Care Med. 2019;200(6):751-759. doi: 10.1164/rccm.201812-2348OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rysavy MA, Li L, Bell EF, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Between-hospital variation in treatment and outcomes in extremely preterm infants. N Engl J Med. 2015;372(19):1801-1811. doi: 10.1056/NEJMoa1410689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayley N. Bayley Scales of Infant and Toddler Development, Third Edition. Harcourt Assessment; 2006. [Google Scholar]
- 24.Newman JE, Bann CM, Vohr BR, Dusick AM, Higgins RD; Follow-up Study Group of Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Improving the Neonatal Research Network annual certification for neurologic examination of the 18-22 month child. J Pediatr. 2012;161(6):1041-1046. doi: 10.1016/j.jpeds.2012.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214-223. doi: 10.1111/j.1469-8749.1997.tb07414.x [DOI] [PubMed] [Google Scholar]
- 26.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199-200. doi: 10.1093/aje/kwi188 [DOI] [PubMed] [Google Scholar]
- 27.Haslam MD, Lisonkova S, Creighton D, et al. ; Canadian Neonatal Network and the Canadian Neonatal Follow-up Network . Severe neurodevelopmental impairment in neonates born preterm: impact of varying definitions in a Canadian cohort. J Pediatr. 2018;197(Jun):75-81. doi: 10.1016/j.jpeds.2017.12.020 [DOI] [PubMed] [Google Scholar]
- 28.Serenius F, Källén K, Blennow M, et al. ; EXPRESS Group . Neurodevelopmental outcome in extremely preterm infants at 2.5 years after active perinatal care in Sweden. JAMA. 2013;309(17):1810-1820. doi: 10.1001/jama.2013.3786 [DOI] [PubMed] [Google Scholar]
- 29.Jaworski M, Janvier A, Lefebvre F, Luu TM. Parental perspectives regarding outcomes of very preterm infants: toward a balanced approach. J Pediatr. 2018;200:58-63. doi: 10.1016/j.jpeds.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 30.Daly M. Parental perspective on neonatal outcomes. BMJ Paediatr Open. 2019;3(1):e000404. doi: 10.1136/bmjpo-2018-000404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bangma JT, Kwiatkowski E, Psioda M, et al. Assessing positive child health among individuals born extremely preterm. J Pediatr. 2018;202(Nov):44-49. doi: 10.1016/j.jpeds.2018.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamarche-Vadel A, Blondel B, Truffer P, et al. ; EPIPAGE Study Group . Re-hospitalization in infants younger than 29 weeks’ gestation in the EPIPAGE cohort. Acta Paediatr. 2004;93(10):1340-1345. doi: 10.1111/j.1651-2227.2004.tb02934.x [DOI] [PubMed] [Google Scholar]
- 33.DeMauro SB, Jensen EA, Bann CM, et al. Home oxygen and 2-year outcomes of preterm infants with bronchopulmonary dysplasia. Pediatrics. 2019;143(5):e20182956. doi: 10.1542/peds.2018-2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rysavy MA, Colaizy TT, Bann CM, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . The relationship of neurodevelopmental impairment to concurrent early childhood outcomes of extremely preterm infants. J Perinatol. 2021;41(9):2270-2278. doi: 10.1038/s41372-021-00999-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ehret DEY, Edwards EM, Greenberg LT, et al. Association of antenatal steroid exposure with survival among infants receiving postnatal life support at 22 to 25 weeks’ gestation. JAMA Netw Open. 2018;1(6):e183235. doi: 10.1001/jamanetworkopen.2018.3235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor GL, Joseph RM, Kuban KCK, et al. Changes in neurodevelopmental outcomes from age 2 to 10 years for children born extremely preterm. Pediatrics. 2021;147(5):e2020001040. doi: 10.1542/peds.2020-001040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marlow N. Is survival and neurodevelopmental impairment at 2 years of age the gold standard outcome for neonatal studies? Arch Dis Child Fetal Neonatal Ed. 2015;100(1):F82-F84. doi: 10.1136/archdischild-2014-306191 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Characteristics of Surviving Children Born at 22-26 Weeks’ GA Who Were Lost to Follow-up Versus Seen at 22-26 Months’ Corrected Age Follow-up
eTable 2. Death and Neurodevelopmental Impairment at 22-26 Months’ Corrected Age in Children Born at 22-26 Weeks’ GA, 2013-2016
eTable 3. Primary Causes for Hospitalizations Since Discharge in Children Born at 22-26 Weeks’ GA, 2013-2016
eFigure 1. Participant Flow Diagram
eFigure 2. Proportion of Infants Who Survived to Discharge or 1 Year by Gestational Age at Birth
eFigure 3. Status at 22-26 Months’ Corrected Age for 4344 Children Born at 22-26 Weeks’ Gestational Age Who Were Eligible for a Follow-up Assessment
eFigure 4. Death and Neurodevelopmental Impairment at 22-26 Months’ Corrected Age in Children Born at 22-26 Weeks’ Gestational Age
eFigure 5. Bayley-III Cognitive Composite Scores by Gestational Age at Birth
eFigure 6. Bayley-III Language Composite Scores by Gestational Age at Birth
eFigure 7. Bayley-III Motor Composite Scores by Gestational Age at Birth
Nonauthor Collaborators. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network


