Key Points
Question
What is the effect of a P2Y12 inhibitor added to anticoagulant therapy on clinical outcomes in non–critically ill patients hospitalized for COVID-19?
Findings
In this bayesian, adaptive, randomized clinical trial that included 562 patients, use of a therapeutic dose of heparin plus a P2Y12 inhibitor, compared with a therapeutic dose of heparin only (usual care), did not increase the odds of improvement in the number of days alive and free of cardiovascular or respiratory organ support within 21 days during the index hospitalization (adjusted odds ratio, 0.83), and the posterior probability of futility (defined as an odds ratio <1.2) was 96%.
Meaning
These findings do not support the addition of a P2Y12 inhibitor to a therapeutic dose of heparin among non–critically ill patients hospitalized for COVID-19.
Abstract
Importance
Platelets represent a potential therapeutic target for improved clinical outcomes in patients with COVID-19.
Objective
To evaluate the benefits and risks of adding a P2Y12 inhibitor to anticoagulant therapy among non–critically ill patients hospitalized for COVID-19.
Design, Setting, and Participants
An open-label, bayesian, adaptive randomized clinical trial including 562 non–critically ill patients hospitalized for COVID-19 was conducted between February 2021 and June 2021 at 60 hospitals in Brazil, Italy, Spain, and the US. The date of final 90-day follow-up was September 15, 2021.
Interventions
Patients were randomized to a therapeutic dose of heparin plus a P2Y12 inhibitor (n = 293) or a therapeutic dose of heparin only (usual care) (n = 269) in a 1:1 ratio for 14 days or until hospital discharge, whichever was sooner. Ticagrelor was the preferred P2Y12 inhibitor.
Main Outcomes and Measures
The composite primary outcome was organ support–free days evaluated on an ordinal scale that combined in-hospital death (assigned a value of −1) and, for those who survived to hospital discharge, the number of days free of respiratory or cardiovascular organ support up to day 21 of the index hospitalization (range, −1 to 21 days; higher scores indicate less organ support and better outcomes). The primary safety outcome was major bleeding by 28 days as defined by the International Society on Thrombosis and Hemostasis.
Results
Enrollment of non–critically ill patients was discontinued when the prespecified criterion for futility was met. All 562 patients who were randomized (mean age, 52.7 [SD, 13.5] years; 41.5% women) completed the trial and 87% received a therapeutic dose of heparin by the end of study day 1. In the P2Y12 inhibitor group, ticagrelor was used in 63% of patients and clopidogrel in 37%. The median number of organ support–free days was 21 days (IQR, 20-21 days) among patients in the P2Y12 inhibitor group and was 21 days (IQR, 21-21 days) in the usual care group (adjusted odds ratio, 0.83 [95% credible interval, 0.55-1.25]; posterior probability of futility [defined as an odds ratio <1.2], 96%). Major bleeding occurred in 6 patients (2.0%) in the P2Y12 inhibitor group and in 2 patients (0.7%) in the usual care group (adjusted odds ratio, 3.31 [95% CI, 0.64-17.2]; P = .15).
Conclusions and Relevance
Among non–critically ill patients hospitalized for COVID-19, the use of a P2Y12 inhibitor in addition to a therapeutic dose of heparin, compared with a therapeutic dose of heparin only, did not result in an increased odds of improvement in organ support–free days within 21 days during hospitalization.
Trial Registration
ClinicalTrials.gov Identifier: NCT04505774
This randomized clinical trial evaluates the benefits and risks of the addition of a P2Y12 inhibitor to anticoagulant therapy compared with anticoagulant therapy only among non–critically ill patients hospitalized for COVID-19.
Introduction
SARS-CoV-2, the virus responsible for COVID-19, results in significant morbidity and mortality.1,2,3 The majority of hospitalized patients are not critically ill and do not require intensive care–level support. Although data from a multiplatform randomized clinical trial demonstrated that a therapeutic dose of heparin increased the number of days alive and free of organ support in non–critically ill patients with COVID-19, 24% of the patients (247/1048) died or received intensive care–level support,4 highlighting the need for additional therapies in this high-risk cohort.
Autopsy and clinical data highlighted the potential role of platelets in the pathogenesis of COVID-19.5,6 Circulating biomarkers reflecting platelet activity (eg, soluble CD40 ligand, P-selectin, thromboxane B2) were found to be independently associated with risk of severe disease, thrombosis, and death in patients with COVID-19.7 Platelet × viral interactions have induced a proinflammatory platelet phenotype in other viral infections such as influenza.8,9 Platelets isolated from patients with COVID-19 were found to be hyperreactive and have induced activation of myeloid and endothelial cells.10,11,12 Taken together, these data suggest that activated platelets may represent a therapeutic target for improved clinical outcomes in patients with COVID-19.
The P2Y12 receptor, a Gi-coupled adenosine diphosphate receptor, plays a pivotal role in platelet activation and aggregation. Platelet inhibition targeting the P2Y12 receptor was effective for the prevention of thrombotic complications in patients with acute coronary syndrome or undergoing percutaneous coronary intervention.13 In addition to their antithrombotic properties, P2Y12 inhibitors, and ticagrelor in particular, have reduced inflammation; other beneficial effects have been reported in experimental models and in clinical inflammatory diseases, including sepsis and acute lung injury.14,15
Accelerating COVID-19 Therapeutic Interventions and Vaccines 4 Acute (ACTIV-4a) is an international, adaptive, randomized clinical trial to evaluate whether P2Y12 inhibition increases organ support–free days evaluated on an ordinal scale that combined in-hospital death and the number of days free of cardiovascular or respiratory organ support in patients hospitalized for COVID-19.
Methods
Trial Design and Oversight
The ACTIV-4a trial was supported by the National Institutes of Health and was designed to evaluate the effects of antithrombotic therapies in patients hospitalized for COVID-19. The trial, which was coordinated at the NYU Grossman School of Medicine and the University of Pittsburgh, enrolled patients across clinical trial networks. The study received central institutional review board approval for the US sites coordinated by the University of Pittsburgh and central ethics committee approvals in Brazil, Italy, and Spain. After completion of testing anticoagulant therapy with heparins in the initial groups,4,16 this trial randomized patients to a therapeutic dose of heparin plus a P2Y12 inhibitor or a therapeutic dose of heparin only (usual care). Based on previously published data,4 it was strongly recommended that a therapeutic dose with either unfractionated heparin or low-molecular-weight heparin be used for non–critically ill patients with COVID-19.
Written informed consent was obtained from the patient or legal representative if the patient was unable to provide consent. The trial was conducted in accordance with the principles of the Good Clinical Practice guidelines of the International Conference on Harmonization. The trial protocol and statistical analysis plan appear in Supplement 1 and Supplement 2, respectively.
Patients
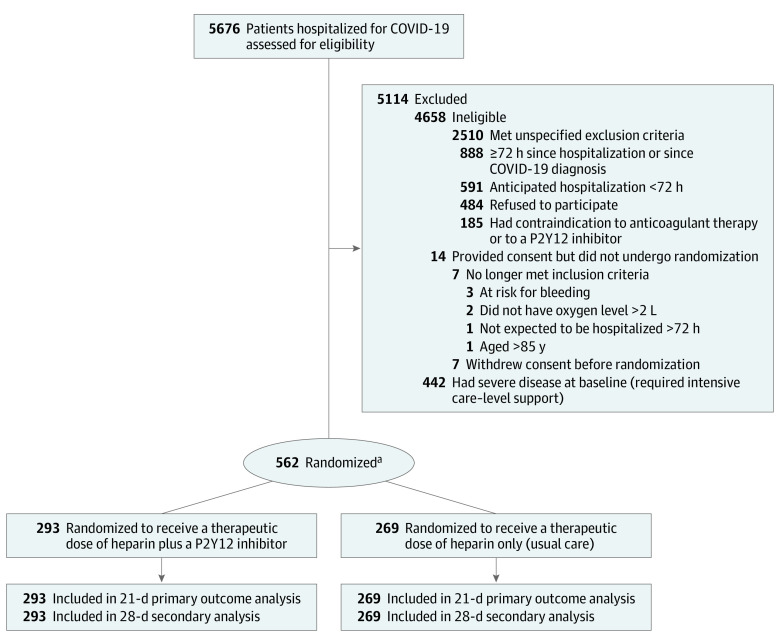
Patients were eligible for the trial if they had laboratory-confirmed SARS-CoV-2 infection and were hospitalized for COVID-19 (Figure 1). The investigators hypothesized that the benefits and risks of a P2Y12 inhibitor may vary according to disease severity. As such, the design of the ACTIV-4a trial prospectively stratified patients into a critically ill (required intensive care–level support) cohort and a non–critically ill cohort (hospitalized but did not require intensive care–level support) at enrollment. The results from the non–critically ill cohort appear in this report.
Figure 1. Eligibility, Randomization, and Follow-up in the ACTIV-4a Trial of P2Y12 Inhibitors in Non–Critically Ill Patients Hospitalized for COVID-19.
ACTIV-4a indicates Accelerating COVID-19 Therapeutic Interventions and Vaccines 4 Acute.
aRandomization was stratified by hospital site and severity of illness.
Intensive care–level support was defined as use of respiratory or cardiovascular organ support (oxygen via high-flow nasal cannula ≥20 L per minute, noninvasive or invasive mechanical ventilation, vasopressors, inotropes, or extracorporeal membrane oxygenation).16 This report describes the results of the analyses for non–critically ill patients hospitalized for COVID-19. The ACTIV-4a trial continues to enroll critically ill patients.
Patients were eligible to be enrolled if they met any 1 of the following criteria: had a D-dimer level that was 2-fold or greater than the upper limit of normal (determined at each hospital site) or were 60 to 84 years of age. If a patient was younger than 60 years of age, he or she could be enrolled if at least 1 of the following criteria were met: had an oxygen requirement greater than 2 L per minute or had hypertension, diabetes, chronic kidney disease (estimated glomerular filtration rate <60 mL/min/1.73 m2), cardiovascular disease, or a body mass index (calculated as weight in kilograms divided by height in meters squared) of 35 or greater. These criteria were chosen to enrich for events based on data from non–critically ill patients.4,16
Patients were ineligible for enrollment if 72 hours or more had elapsed from the hospital admission for COVID-19 or SARS-CoV-2 infection confirmation, if hospital discharge was expected within 72 hours, or if they had a contraindication to P2Y12 inhibitors or a clinical requirement for dual antiplatelet therapy (see the trial protocol in Supplement 1 for detailed eligibility criteria). To account for the differences in race and ethnicity observed during the COVID-19 pandemic, this trial collected self-reported race and ethnicity data from either the patients or their surrogates via fixed categories appropriate to their country and region of residence.
Randomization
Randomization was concealed via a secure IxRS system (Worldwide Clinical Trials). Patients were randomized to receive a therapeutic dose of heparin plus a P2Y12 inhibitor or a therapeutic dose of heparin only (usual care) in an open-label fashion using a 1:1 ratio and permuted block sizes of 2 or 4. Patients were stratified by hospital site and severity of illness using a web-based system (eSocdat, Socar Research).
Ticagrelor was the preferred P2Y12 inhibitor; however, clopidogrel and prasugrel were allowed. When clopidogrel was used, a loading dose of 300 mg was encouraged. Additional details of dosing appear in the trial protocol (Supplement 1). The duration of P2Y12 inhibitor treatment was 14 days or until hospital discharge, whichever came first. All non–critically ill patients were recommended to receive a therapeutic dose of heparin based on reported findings in patients with COVID-19.4 This study followed the reporting guidelines of the Consolidated Standards of Reporting Trials.
Outcome Measures
The composite primary outcome was organ support–free days evaluated on an ordinal scale that combined in-hospital death (assigned a value of −1) and, for those who survived to hospital discharge, the number of days free of respiratory or cardiovascular organ support up to day 21 of the index hospitalization. Any death during the index hospitalization through 90 days was assigned the worst outcome (–1). This outcome reflects both use of critical care therapies and survival with a range of −1 to 21 days; higher values indicate less organ support and better outcomes. The primary safety outcome was major bleeding by 28 days as defined by the International Society on Thrombosis and Hemostasis.
A key secondary outcome was major thrombotic events or death by 28 days (a composite outcome of myocardial infarction, pulmonary embolism, ischemic stroke, systemic arterial embolism, and in-hospital death). Secondary efficacy outcomes included all thrombotic events (major thrombotic events plus deep venous thrombosis) or death by 28 days. The secondary safety outcome was a composite of major bleeding or death by 28 days. All reported bleeding and thrombotic events were adjudicated in a blinded fashion by the clinical end point committee using consensus definitions (eMethods 1 in Supplement 3). The full list of the study outcomes appears in eTable 1 in Supplement 3 and in the statistical analysis plan (Supplement 2).
Sample Size Calculation
The design was adaptive in sample size with a range of 200 to 2000 patients. Adaptive analyses were planned monthly with a minimum of 200 patients enrolled between analyses. The operating characteristics were determined through clinical trial simulations. The adaptive sample size yields more than 80% power for an odds ratio (OR) change of 1.25 in the organ support–free days outcome. There is approximately 90% power for an OR of 1.5 with 400 patients per group in the adaptive analyses. An OR of 2 results in more than 90% power for the first interim analysis of 200 patients per group.
Statistical Analysis
The primary analysis population enrolled patients with confirmed COVID-19 randomized to either treatment strategy and analyzed the patients according to the randomly assigned treatment, irrespective of the actual treatment patients received. The overall analysis used a bayesian modeling approach as described in the statistical analysis plan (Supplement 2) and in the trial protocol (Supplement 1). Monthly adaptive analyses of the data were performed by an independent statistical analysis committee using a hierarchical model to borrow information between the critically ill patients and the non–critically ill patients.
Randomization continued until a statistical threshold for superiority (defined as >99% posterior probability of a proportional OR >1, which implies improved outcomes on organ support–free days) or futility (>95% posterior probability of a proportional OR <1.2) was met for each group. An OR less than 1 implies worse outcomes on organ support–free days and inferiority of the new treatment compared with usual care. An OR less than 1.20 was deemed an appropriate clinical threshold for a small clinical effect and for determination of futility by consensus among study leadership, investigators, and funders (eMethods 2 in Supplement 3) and is consistent with prior analyses using this outcome.4,16
The primary analysis used a bayesian cumulative logistic regression model (described in the statistical analysis plan in Supplement 2) that calculated the posterior probability distribution of organ support–free days with a proportional OR for a therapeutic dose of heparin plus a P2Y12 inhibitor compared with a therapeutic dose of heparin only (usual care). The assumption of a proportional effect between treatment with a P2Y12 inhibitor and usual care on the ordinal outcome was not strictly tested in the bayesian analysis because the model is clinically robust to slight variations and the effect on organ support–free days and mortality was consistent.
The results are reported for non–critically ill patients, but a hierarchical model was included that borrowed data from critically ill patients. Specifically, the mean treatment effect in each group was assumed to follow a hierarchical normal distribution with the same mean, which created a dynamic amount of borrowing depending on the similarity across groups. When consistent effects were observed for the groups, the posterior distribution for each intervention group effect was shrunk toward the overall estimate. The primary model adjusted for age, sex, hospital site, respiratory support at enrollment, history of cardiovascular disease, and time (in 2-week intervals).
The primary analysis model incorporated a weakly informative Dirichlet prior distribution for the outcome of organ support–free days. The model was fit using a Markov chain Monte Carlo algorithm in R version 3.5.2 (R Foundation for Statistical Computing) with 500 000 samples from the joint posterior distribution, allowing calculation of the posterior distributions for the proportional ORs, including medians and 95% credible intervals (CrIs), and the posterior probabilities of superiority and futility for a P2Y12 inhibitor compared with usual care.
A post hoc sensitivity analysis was performed without use of dynamic borrowing and after excluding patients who did not receive a therapeutic dose of heparin. Similar bayesian analyses were carried out for the 2-level (death vs survival to hospital discharge), 3-level (death, organ support without death, and no organ support without death), and 4-level (death, invasive mechanical ventilation without death, organ support without invasive mechanical ventilation and without death, and no organ support without death) derivatives of the primary outcome with a cumulative logistic model and a flat Dirichlet prior.
Assessment of the primary outcome, including the interim adaptive analyses, used a bayesian framework. We used a bayesian analysis for the primary outcome and its individual components. Because the trial was stopped for futility and the bayesian analysis is more onerous, we also used frequentist logistic and cumulative logistic models for the outcome of organ support–free days and its components. As noted in eTable 2 in Supplement 3, the results obtained from the bayesian and frequentist models were consistent. Thus, we decided to use the frequentist approach for the secondary outcomes and the subgroup analyses to speed up the dissemination process.
Logistic and cumulative logistic models were used for binary and ordinal outcomes in the secondary and subgroup analyses. Odds ratios and 95% CIs were estimated through a random-effects logistic regression model that accounted for the same covariates in the primary analysis model. For time remaining alive and time remaining alive and free of organ support (the latter as a post hoc analysis), hazard ratios and 95% CIs were estimated through a frailty proportional hazards model that accounted for the same covariates in the primary analysis model.
Testing was performed for the proportional hazards assumption using 2 approaches: (1) comparison of survival curves and log[-log] curves by treatment and (2) including parameters for covariate × log(time) interaction. Both approaches supported the proportional hazards assumption. There were no missing data for the outcomes. Only the covariate for baseline oxygen had missing values when the missing values were treated as a separate class. For the subgroup analyses, we included a treatment × group interaction in the model and set up contrasts to compare use of a P2Y12 inhibitor with usual care within each subgroup.
All frequentist analyses were run in SAS version 9.4 (SAS Institute Inc) with 2-sided tests at the significance level of .05. Because of the potential for type I error due to multiple comparisons, the findings for the secondary and subgroup analyses should be interpreted as exploratory.
Results
Enrollment
The first patient was randomized on February 26, 2021. Enrollment was discontinued for non–critically ill patients on June 19, 2021, after a planned adaptive analysis demonstrated that the statistical criterion for futility was met (eTable 3 in Supplement 3). At that time, 562 non–critically ill patients were recruited from 60 hospital sites (4 sites in Brazil, 2 sites in Italy, 6 sites in Spain, and 48 sites in the US) and randomized. The primary analysis population consisted of these 562 patients (Figure 1). The date of final 90-day follow-up was September 15, 2021. Randomization in the ACTIV-4a trial continues for critically ill patients with COVID-19.
Patients
The mean age of the patients was 52.7 years (SD, 13.5 years), 41.5% were women, 38.1% were a race other than White, and 46.0% reported Hispanic ethnicity (Table 1). At randomization, 88.4% had been receiving supplemental oxygen (57.0% of whom received >2 L per minute). Concomitant baseline therapies included corticosteroids (64.1%), remdesivir (52.0%), and IL-6 receptor antagonists (2.8%). The 2 randomized groups were well balanced across most characteristics. However, a lower percentage of patients in the P2Y12 inhibitor group than in the usual care group had a history of hypertension. The use of aspirin at baseline was comparable between groups (15.0% in the P2Y12 inhibitor group vs 13.4% in the usual care group). Eighty-seven percent of patients in the P2Y12 inhibitor group and 88% of patients in the usual care group initially received a therapeutic dose of heparin by the end of study day 1.
Table 1. Baseline Characteristics of the Patients.
| Characteristic | Therapeutic dose of heparin plus P2Y12 inhibitor (n = 293) |
Therapeutic dose of heparin only (usual care) (n = 269) |
|---|---|---|
| Age, mean (SD), y | 53.1 (14.1) | 52.3 (12.9) |
| Sex, No. (%) | ||
| Female | 124 (42.3) | 109 (40.5) |
| Male | 169 (57.7) | 160 (59.5) |
| Race, No. (%)a | (n = 269) | (n = 246) |
| American Indian or Alaska Native | 10 (3.7) | 8 (3.3) |
| Asian | 7 (2.6) | 9 (3.7) |
| Black | 68 (25.3) | 67 (27.2) |
| Multiple races | 3 (1.1) | 0 |
| Native Hawaiian or Pacific Islander | 2 (0.7) | 2 (0.8) |
| White | 167 (62.1) | 152 (61.8) |
| Other | 12 (4.5) | 8 (3.3) |
| Hispanic, No./total (%)b | 69/214 (32.2) | 48/193 (24.9) |
| Hospital site location, No. (%) | ||
| Brazil | 67 (22.9) | 70 (26.0) |
| Italy | 1 (0.3) | 1 (0.4) |
| Spain | 44 (15.0) | 41 (15.2) |
| US | 181 (61.8) | 157 (58.4) |
| Enrichment criteriac | ||
| D-dimer level ≥2-fold the ULN, No./total (%) | 105/262 (40.1) | 100/234 (42.7) |
| Age, No. (%) | ||
| ≥60 y | 96 (32.8) | 73 (27.1) |
| <60 y with an oxygen level >2 L/min or ≥1 comorbidityd | 178 (60.8) | 180 (66.9) |
| Body mass indexe | (n = 283) | (n = 265) |
| Median (IQR) | 31.4 (27.1-36.8) | 31.6 (27.5-37.9) |
| >30, No. (%) | 164 (58.0) | 152 (57.4) |
| Cardiovascular disease, No. (%)f | 128 (43.7) | 150 (55.8) |
| Hypertension | 125 (42.7) | 147 (54.9)g |
| Heart failure | 12 (4.1) | 11 (4.1)g |
| Coronary artery disease | 15 (5.1) | 17 (6.3)g |
| Peripheral arterial disease | 1 (0.3) | 2 (0.7) |
| Cerebrovascular disease | 1 (0.3) | 3 (1.1)g |
| Other diseases and chronic conditions, No. (%) | ||
| Diabetes | 72 (24.6) | 73 (27.1) |
| Chronic kidney disease | 11 (3.8) | 11 (4.1) |
| Liver disease | 4 (1.4) | 8 (3.0) |
| Respiratory disease | 56 (19.1) | 37 (13.8) |
| Asthma | 39 (13.3) | 24 (8.9) |
| COPD | 17 (5.8) | 13 (4.8) |
| Baseline treatment, No. (%) | ||
| Glucocorticoids | 192 (65.5) | 168 (62.5) |
| Remdesivir | 164 (56.0) | 128 (47.6) |
| Aspirin | 44 (15.0) | 36 (13.4) |
| Anticoagulant therapy | 31 (10.6) | 39 (14.5) |
| IL-6 inhibitors | 8 (2.7) | 8 (3.0) |
| Respiratory support, No. (%)h | (n = 262) | (n = 238) |
| None | 32 (12.2) | 26 (10.9) |
| Low-flow nasal cannula, L/min | ||
| 0-2 | 80 (30.5) | 77 (32.4) |
| 2-4 | 91 (34.7) | 95 (39.9) |
| >4 | 57 (21.8) | 39 (16.4) |
| High-flow nasal cannula (20-40 L/min) | 2 (0.8) | 1 (0.4) |
| Laboratory values, median (IQR)i | ||
| D-dimer, μg/mL | (n = 262); 1.017 (0.598-1.780) | (n = 234); 1.060 (0.640-2.080) |
| C-reactive protein, mg/L | (n = 237); 93.8 (50.4-153) | (n = 224); 93.0 (57.5-139) |
| Creatinine, mg/dL | (n = 288); 0.84 (0.71-1.00) | (n = 265); 0.85 (0.71-1.07) |
| Hemoglobin, g/dL | (n = 292); 13.6 (12.6-14.6) | (n = 268); 13.7 (12.6-14.9) |
| White blood cell count, ×109/L | (n = 269); 0.86 (0.62-1.20) | (n = 247); 0.91 (0.67-1.30) |
| Platelet count, ×103/μL | (n = 291); 211 (163-274) | (n = 268); 217 (170-281) |
Abbreviations: COPD, chronic obstructive pulmonary disease; ULN, upper limit of normal.
SI conversion factors: To convert creatinine to μmol/L, multiply by 88.4; D-dimer to nmol/L, multiply by 5.476.
Self-reported from either the patients or their surrogates via fixed categories appropriate to their country and region.
Excludes patients in Brazil.
Criteria chosen to enrich for events in non–critically ill patients.
Comorbidities included oxygen level greater than 2 L/min, diabetes, hypertension, chronic kidney disease, cardiovascular disease, or body mass index of 35 or greater.
Calculated as weight in kilograms divided by height in meters squared.
Data were systematically collected using predefined questions.
The denominator is 268.
Home oxygen therapy used to support patients with asthma, chronic obstructive pulmonary disease, bronchiectasis, interstitial lung disease, lung cancer, pulmonary hypertension, and tuberculosis.
The normal ranges differed by hospital site because each site was allowed to determine the ranges for the assays.
Initial adherence to the protocol-assigned P2Y12 inhibitor was observed in 285 of 293 patients (98.3%) receiving at least 1 dose of either ticagrelor (63%) or clopidogrel (37%) by the end of study day 1 (eTable 2 in Supplement 3). Of patients receiving clopidogrel, 89.5% received a loading dose in accordance with the trial protocol recommendations. The median duration of study drug treatment was 6 days (IQR, 4-8 days). The median percentage of index hospital days during which patients received a study drug after randomization was 100% (IQR, 86%-100%). In the usual care group, no patient received a P2Y12 inhibitor.
Primary Outcome and Individual Components
Among the 562 patients, the median number of organ support–free days was 21 days (IQR, 20-21 days) in the P2Y12 inhibitor group and 21 days (IQR, 21-21 days) in the usual care group. The adjusted OR for the effect of a P2Y12 inhibitor on organ support–free days was 0.83 (95% CrI, 0.55-1.25; yielding a posterior probability of futility of 96% and a posterior probability of inferiority of 81%; Figure 2 and Table 2). Compared with usual care, the posterior probability that P2Y12 inhibitors increased survival to hospital discharge was 23% (estimated OR, 0.75 [95% CrI, 0.33-1.56]). There was an 89% probability of futility that use of a P2Y12 inhibitor led to a higher probability of survival to hospital discharge. The frequentist cumulative logistic model showed similar results to the bayesian analysis (eTable 4 in Supplement 3).
Figure 2. Effect of Randomization to a P2Y12 Inhibitor on the Number of Days Not Requiring Respiratory or Cardiovascular Organ Support.
The number of days not requiring respiratory or cardiovascular organ support (eg, oxygen via high-flow nasal cannula ≥20 L per minute, noninvasive or invasive mechanical ventilation, vasopressors, inotropes, or extracorporeal membrane oxygenation) as horizontally stacked proportions of patients in the 2 treatment groups, with the following possible outcomes: in-hospital death with or without organ support (dark red, the worst possible outcome, corresponding to a score of −1 on the ordinal scale); survival with organ support (red-to-blue gradient shading based on the number of days alive without organ support, corresponding to a score of 0-21 on the ordinal scale); and survival until hospital discharge without organ support (dark blue, the best possible outcome, corresponding to a score of 21 on the ordinal scale).
Table 2. Primary Outcome of 21-Day Organ Support–Free Days and Individual Components.
| Therapeutic dose of heparin plus P2Y12 inhibitor (n = 293) |
Therapeutic dose of heparin only (usual care) (n = 269) |
Absolute difference (95% CI) | Adjusted OR (95% CrI)a |
Probability of futility, % | Probability of superiority, % | Probability of inferiority, % | |
|---|---|---|---|---|---|---|---|
| Composite primary outcome b | |||||||
| Organ support–free days, median (IQR) | 21 (20-21) | 21 (21-21) | 0.83 (0.55 to 1.25)c | 96.2 | 18.6 | 81.4 | |
| Components of the primary outcome | |||||||
| Alive and free of organ support | 218 (74.4) | 211 (78.4) | 0.78 (0.51 to 1.17)d | 98.2 | 11.1 | 89.9 | |
| Alive with organ support | 57 (19.5) | 47 (17.5) | |||||
| Death | 18 (6.1) | 11 (4.1) | |||||
| Survival to hospital discharge | 275 (93.9) | 258 (95.9) | −2 (−6 to 2) | 0.75 (0.33 to 1.56) | 89.3 | 22.7 | 77.3 |
Abbreviations: CrI, credible interval; OR, odds ratio.
Adjusted for age, sex, enrollment epoch, cardiovascular disease (composite of hypertension, heart failure, coronary artery disease, peripheral artery disease, cerebrovascular disease), baseline respiratory support, and hospital site (modeled within parent country). An OR greater than 1 indicates a benefit from treatment.
Evaluated on an ordinal scale consisting of survival to hospital discharge and days free of organ support to day 21. Probabilities of benefit (proportional OR >1), inferiority (proportional OR <1), and futility (proportional OR <1.2) were computed from the posterior distribution of the proportional OR for a P2Y12 inhibitor compared with usual care. The model incorporated dynamic borrowing from 200 critically ill patients. The mean treatment effect in each group was assumed to follow a hierarchical normal distribution with the same mean, which created a dynamic amount of borrowing, depending on the similarity across groups. When consistent effects were observed for the groups, the posterior distribution for each intervention group effect was shrunk toward the overall estimate.
Effect of a P2Y12 inhibitor on organ support–free days without dynamic borrowing from critically ill patients yielded an adjusted OR of 0.79 with a 95% CrI of 0.52 to 1.19 and a posterior probability of futility of 97.7%.
Examines the primary outcome as a 3-category ordinal outcome.
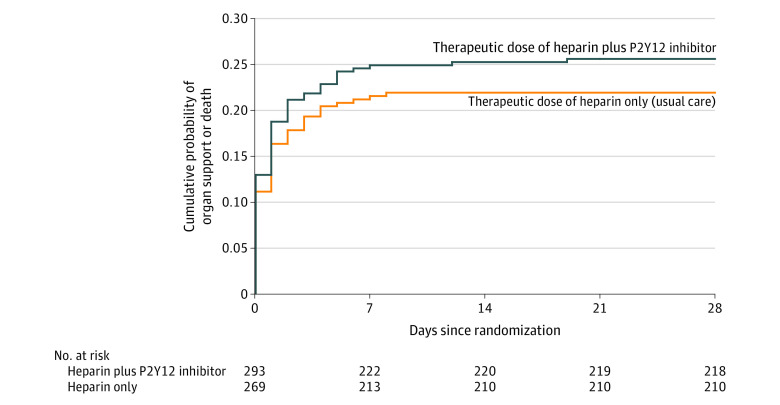
Overall, 75 patients (26%) in the P2Y12 inhibitor group and 58 patients (22%) in the usual care group died or required respiratory or cardiovascular organ support during the first 28 days (adjusted hazard ratio, 1.19 [95% CI, 0.84-1.68], P = .34; Figure 3).
Figure 3. Effect of Randomization to a P2Y12 Inhibitor on the Time to the Event for the Composite Outcome of Organ Support or Death in Non–Critically Ill Patients With COVID-19.
Kaplan-Meier curve for the time to event of respiratory or cardiovascular organ support or death. All patients were followed up until death or 28 days. The time to organ support or death in patients assigned to a P2Y12 inhibitor vs usual care was not significantly different (26% vs 22%, respectively; adjusted hazard ratio, 1.19 [95% CI, 0.84-1.68], P = .34). The time to the event was compared post hoc using Cox regression.
The primary safety outcome of major bleeding occurred in 6 patients (2.0%) in the P2Y12 inhibitor group and in 2 patients (0.7%) in the usual care group (adjusted OR, 3.31 [95% CI, 0.64-17.2], P = .15; eTable 5 in Supplement 3).
Sensitivity and Subgroup Analyses
After excluding 69 patients who did not receive a therapeutic dose of anticoagulant therapy, the estimated OR for the effect of a P2Y12 inhibitor on organ support–free days was 0.72 (95% CrI, 0.46-1.14; yielding a posterior probability of futility of 98%; eTable 6 in Supplement 3). In prespecified subgroup analyses, the treatment effect did not vary significantly by age, sex, race, amount of oxygen support at enrollment, or hospital site preference for ticagrelor or clopidogrel (eTable 7 in Supplement 3). The P value was .04 for the region × treatment effect interaction.
Secondary Outcomes
Thrombotic and major bleeding results appear in Table 3. The key secondary outcome of major thrombotic events or in-hospital death was not significantly different between randomized groups and occurred in 18 patients (6.1%) in the P2Y12 inhibitor group and in 12 patients (4.5%) in the usual care group (adjusted OR, 1.42 [95% CI, 0.64-3.13]; Table 3 and eTable 8 in Supplement 3). The analysis incorporating deep venous thrombosis had similar results.
Table 3. Secondary Outcomes at 28 Days and Individual Components.
| Outcome | No. (%) | Absolute difference, % (95% CI) |
Odds ratio (95% CI)a | ||
|---|---|---|---|---|---|
| Therapeutic dose of heparin plus P2Y12 inhibitor (n = 293) |
Therapeutic dose of heparin only (usual care) (n = 269) |
Unadjusted | Adjustedb | ||
| Secondary outcomes | |||||
| Major thrombotic eventc or in-hospital death | 18 (6.1) | 12 (4.5) | 1.6 (−2.1 to 5.3) | 1.40 (0.66 to 2.97) | 1.42 (0.64 to 3.13) |
| Any thrombotic eventd or in-hospital death | 20 (6.8) | 12 (4.5) | 2.3 (−1.5 to 6.1) | 1.57 (0.75 to 3.28) | 1.60 (0.73 to 3.50) |
| Major bleeding evente or in-hospital death | 18 (6.1) | 10 (3.7) | 2.4 (−1.1 to 5.9) | 1.69 (0.77 to 3.75) | 1.80 (0.79 to 4.10) |
| Components of the secondary outcomes | |||||
| In-hospital death | 13 (4.4) | 8 (3.0) | |||
| Major thrombotic eventc | 7 (2.4) | 5 (1.9) | |||
| Any thrombotic eventd | 9 (3.1) | 5 (1.9) | |||
| Major bleeding evente | 6 (2.0) | 2 (0.7) | 1.3 (−0.6 to 3.2) | 2.65 (0.53 to 13.4) | 3.31 (0.64 to 17.2) |
An odds ratio less than 1 corresponded with a treatment benefit (eg, treatment was associated with fewer adverse events).
Adjusted for age, sex, enrollment epoch, cardiovascular disease (composite of hypertension, heart failure, coronary artery disease, peripheral artery disease, cerebrovascular disease), and baseline respiratory support. Study country and hospital site were treated as nested random effects.
Includes pulmonary embolism, myocardial infarction, ischemic stroke, and other arterial or venous thromboembolism events.
Includes major thrombotic events and deep vein thrombosis.
Defined by the International Society on Thrombosis and Hemostasis as fatal bleeding, symptomatic bleeding in a critical area or organ (such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular or pericardial, or intramuscular with compartment syndrome), bleeding causing a decrease in hemoglobin level of 2 g/dL or greater, or bleeding leading to transfusion of 2 units or greater of whole blood or red cells.
Discussion
In this open-label, international, multicenter, randomized, clinical trial of non–critically ill patients hospitalized for confirmed COVID-19, use of a P2Y12 inhibitor combined with a therapeutic dose of heparin did not increase the odds of improvement in organ support–free days over 21 days during hospitalization. Major bleeding complications were infrequent and not significantly different between treatment groups.
The results of this trial refute the hypothesis that a strategy of a P2Y12 inhibitor, when added to a therapeutic dose of heparin, would benefit non–critically ill patients hospitalized for COVID-19. This hypothesis was based, in part, on studies that reported an association between increased platelet activity and severity of disease. The Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial found that the use of another platelet antagonist, aspirin, was not significantly associated with improved survival or reduced risk of progression to invasive mechanical ventilation or death.17 Use of both a P2Y12 inhibitor (clopidogrel or ticagrelor) and cyclooxygenase 1 (target of aspirin) are important in the pathogenesis of cardiovascular disease. It remains uncertain whether other platelet-mediated thromboinflammatory pathways may be better therapeutic targets in patients with COVID-19.18 For example, P-selectin is released from activated platelets and mediates platelet interactions with leukocytes and endothelial cells. Preliminary data suggest a pathogenic role for P-selectin in the treatment of COVID-19.19,20
The results of a previously reported multiplatform randomized clinical trial demonstrated that a therapeutic dose of heparin reduced the need for organ support and improved survival when given early during hospitalization to non–critically ill patients with COVID-19.4 The lack of an observed clinical benefit with the antiplatelet intervention in non–critically ill patients with COVID-19 may have been found because the addition of a P2Y12 inhibitor confers no additional benefit beyond that provided by a therapeutic dose of heparin. Even though bleeding risk is generally increased when combining antiplatelet and anticoagulant therapies,21,22 the absolute excess major bleeding risk observed in the current trial was low.
Whether combination therapy with a therapeutic dose of an anticoagulant and an antiplatelet had any effect on alveolar or other intraorgan hemorrhage is unknown. It remains possible that use of a P2Y12 inhibitor as a sole antithrombotic agent may improve outcomes in patients with COVID-19, given that this trial tested only the combination of a P2Y12 inhibitor with anticoagulant therapy, which was most frequently a therapeutic dose of heparin. In addition, the potential for benefit with a longer treatment duration or at an earlier stage of illness (before hospitalization) cannot be ruled out. The ACTIV-4a randomized clinical trial continues to evaluate P2Y12 inhibitors in critically ill patients with COVID-19, a population in whom higher-dose anticoagulant therapy was not found to be superior to a prophylactic dose of anticoagulant therapy16 and is therefore not routinely recommended.
Limitations
This study has several limitations. First, this trial has an open-label design, which may have introduced bias in the ascertainment of thrombotic and bleeding events. However, the primary outcome of organ support–free days and death is linked to the medical record and was chosen to reduce bias. In addition, a consistency of effect was seen for efficacy outcomes related to thrombosis.
Second, many patients in the P2Y12 inhibitor group received clopidogrel. It is possible that the effect of P2Y12 inhibitors on non–critically ill patients with COVID-19 varies according to the type of P2Y12 inhibitor used. However, there was no evidence of a meaningful difference in the treatment effect according to hospital site proclivity for use of clopidogrel or ticagrelor (eTable 8 in Supplement 3). Although the use of P2Y12 inhibitors appears more harmful in the patients enrolled in the US (P = .04 for interaction), this is likely a spurious finding due to multiple subgroup comparisons.
Third, the median duration of administration of a P2Y12 inhibitor was 6 days. It is possible that initiating therapy earlier in the disease course (eg, prior to hospitalization) or a longer treatment duration may have detected a treatment benefit.
Fourth, the reported rates of major bleeding were low but these types of rates can be subject to reporting bias. The rates of minor but clinically significant bleeding were not recorded.
Conclusions
Among non–critically ill patients hospitalized for COVID-19, the use of a P2Y12 inhibitor in addition to a therapeutic dose of heparin, compared with a therapeutic dose of heparin only, did not result in an increased odds of improvement in organ support–free days within 21 days during hospitalization.
Trial protocol
Statistical analysis plan
eMethods 1. Endpoint definitions
eMethods 2. Investigators and collaborators and funding agencies
eResults. Site participation in the non-critically ill group P2Y12 inhibitor trial, additional analyses, and eTables
eTable 1. Study outcomes
eTable 2. P2Y12 inhibitor administered and dosage used
eTable 3. Results of the planned adaptive analysis of 395 non-critically ill participants with COVID-19 on June 4, 2021
eTable 4. Frequentist results of endpoint models compared to Bayesian results
eTable 5. Confirmed ISTH major bleeding events
eTable 6. Sensitivity analyses of the primary outcome among all non-critically ill participants
eTable 7. P2Y12 inhibitor moderator analysis of primary outcome
eTable 8. Confirmed thrombotic events occurring during index hospitalization
eReferences
Nonauthor collaborators
Data sharing statement
References
- 1.Nguyen NT, Chinn J, Nahmias J, et al. Outcomes and mortality among adults hospitalized with COVID-19 at US medical centers. JAMA Netw Open. 2021;4(3):e210417. doi: 10.1001/jamanetworkopen.2021.0417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324(8):799-801. doi: 10.1001/jama.2020.13372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Challen R, Brooks-Pollock E, Read JM, Dyson L, Tsaneva-Atanasova K, Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ. 2021;372(n579):n579. doi: 10.1136/bmj.n579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawler PR, Goligher EC, Berger JS, et al. ; ATTACC Investigators; ACTIV-4a Investigators; REMAP-CAP Investigators . Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. 2021;385(9):790-802. doi: 10.1056/NEJMoa2105911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taus F, Salvagno G, Canè S, et al. Platelets promote thromboinflammation in SARS-CoV-2 pneumonia. Arterioscler Thromb Vasc Biol. 2020;40(12):2975-2989. doi: 10.1161/ATVBAHA.120.315175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rapkiewicz AV, Mai X, Carsons SE, et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrett TJ, Lee AH, Xia Y, et al. Platelet and vascular biomarkers associate with thrombosis and death in coronavirus disease. Circ Res. 2020;127(7):945-947. doi: 10.1161/CIRCRESAHA.120.317803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koupenova M, Corkrey HA, Vitseva O, et al. The role of platelets in mediating a response to human influenza infection. Nat Commun. 2019;10(1):1780. doi: 10.1038/s41467-019-09607-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdulnour RE, Gunderson T, Barkas I, et al. Early intravascular events are associated with development of acute respiratory distress syndrome: a substudy of the LIPS-A clinical trial. Am J Respir Crit Care Med. 2018;197(12):1575-1585. doi: 10.1164/rccm.201712-2530OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manne BK, Denorme F, Middleton EA, et al. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136(11):1317-1329. doi: 10.1182/blood.2020007214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett TJ, Cornwell M, Myndzar K, et al. Platelets amplify endotheliopathy in COVID-19. Sci Adv. 2021;7(37):eabh2434. doi: 10.1126/sciadv.abh2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hottz ED, Azevedo-Quintanilha IG, Palhinha L, et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136(11):1330-1341. doi: 10.1182/blood.2020007252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamran H, Jneid H, Kayani WT, et al. Oral antiplatelet therapy after acute coronary syndrome: a review. JAMA. 2021;325(15):1545-1555. doi: 10.1001/jama.2021.0716 [DOI] [PubMed] [Google Scholar]
- 14.Thomas MR, Storey RF. Effect of P2Y12 inhibitors on inflammation and immunity. Thromb Haemost. 2015;114(3):490-497. [DOI] [PubMed] [Google Scholar]
- 15.Lancellotti P, Musumeci L, Jacques N, et al. Antibacterial activity of ticagrelor in conventional antiplatelet dosages against antibiotic-resistant gram-positive bacteria. JAMA Cardiol. 2019;4(6):596-599. doi: 10.1001/jamacardio.2019.1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goligher EC, Bradbury CA, McVerry BJ, et al. ; REMAP-CAP Investigators; ACTIV-4a Investigators; ATTACC Investigators . Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med. 2021;385(9):777-789. doi: 10.1056/NEJMoa2103417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.RECOVERY Collaborative Group . Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. Published online November 17, 2021. doi: 10.1016/S0140-6736(21)01825-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrett TJ, Bilaloglu S, Cornwell M, et al. Platelets contribute to disease severity in COVID-19. J Thromb Haemost. 2021;19(12):3139-3153. doi: 10.1111/jth.15534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowenstein CJ, Solomon SD. Severe COVID-19 is a microvascular disease. Circulation. 2020;142(17):1609-1611. doi: 10.1161/CIRCULATIONAHA.120.050354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman JD, Vani AK, Aleman JO, Weintraub HS, Berger JS, Schwartzbard AZ. The changing landscape of diabetes therapy for cardiovascular risk reduction: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(15):1856-1869. doi: 10.1016/j.jacc.2018.07.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnes GD. Combining antiplatelet and anticoagulant therapy in cardiovascular disease. Hematology Am Soc Hematol Educ Program. 2020;2020(1):642-648. doi: 10.1182/hematology.2020000151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez F, Harrington RA. Management of antithrombotic therapy after acute coronary syndromes. N Engl J Med. 2021;384(5):452-460. doi: 10.1056/NEJMra1607714 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eMethods 1. Endpoint definitions
eMethods 2. Investigators and collaborators and funding agencies
eResults. Site participation in the non-critically ill group P2Y12 inhibitor trial, additional analyses, and eTables
eTable 1. Study outcomes
eTable 2. P2Y12 inhibitor administered and dosage used
eTable 3. Results of the planned adaptive analysis of 395 non-critically ill participants with COVID-19 on June 4, 2021
eTable 4. Frequentist results of endpoint models compared to Bayesian results
eTable 5. Confirmed ISTH major bleeding events
eTable 6. Sensitivity analyses of the primary outcome among all non-critically ill participants
eTable 7. P2Y12 inhibitor moderator analysis of primary outcome
eTable 8. Confirmed thrombotic events occurring during index hospitalization
eReferences
Nonauthor collaborators
Data sharing statement