Abstract
Objective
To identify drug classes and individual selective serotonin reuptake inhibitors (SSRIs) with high rates of remission and low risk of adverse events in the treatment of panic disorder with or without agoraphobia.
Design
Systematic review and network meta-analysis.
Data sources
Embase, Medline, and ClinicalTrials.gov from inception to 17 June 2021.
Eligibility criteria for study selection
Randomised controlled trials that included adults aged ≥18 years with a diagnosis of panic disorder, compared drugs used to treat the panic disorder, and measured the outcomes of interest, including remissions, dropouts, and adverse events.
Methods
Risk of bias in the included studies was assessed using the revised Cochrane risk of bias tool for randomised trials. Direct meta-analyses were performed using random effects models. A two stage network meta-analysis with surface under the cumulative ranking curve (SUCRA) was used to estimate the comparative efficacy of drug classes and individual SSRIs.
Results
87 studies including a total of 12 800 participants and 12 drug classes were eligible for inclusion. Almost all the studies (86/87) had some concern or were at high risk of bias. Network meta-analysis of remission with consistent results indicated that tricyclic antidepressants, benzodiazepines, monoamine oxidase inhibitors, SSRIs, and serotonin-noradrenaline reuptake inhibitors (SNRIs) were associated with significantly higher remission rates than placebo, with risk ratios of 1.39 (95% confidence interval 1.26 to 1.54), 1.47 (1.36 to 1.60), 1.30 (1.00 to 1.69), 1.38 (1.26 to 1.50), and 1.27 (1.12 to 1.45), respectively. SUCRAs identified benzodiazepines (84.5%, mean rank=2.4), tricyclic antidepressants (68.7%, 3.8), and SSRIs (66.4%, 4.0) as the top three best treatments for remission. However, tricyclic antidepressants, benzodiazepines, and SSRIs were also significantly associated with increased risk of adverse events compared with placebo, with risk ratios of 1.79 (1.47 to 2.19), 1.76 (1.50 to 2.06), and 1.19 (1.01 to 1.41), respectively. Consistency assumption of adverse events was upheld but could still be present on removal of studies with high percentages of women participants and those with agoraphobia. A SUCRA cluster ranking plot considering both remission and adverse events among all drug classes indicated that SSRIs were associated with high remission and low risk of adverse events. Among individual SSRIs, sertraline and escitalopram provided high remission with an acceptable risk of adverse events.
Conclusion
The findings suggest that SSRIs provide high rates of remission with low risk of adverse events for the treatment of panic disorder. Among SSRIs, sertraline and escitalopram were associated with high remission and low risk of adverse events. The findings were, however, based on studies of moderate to very low certainty levels of evidence, mostly as a result of within study bias, inconsistency, and imprecision of the findings reported.
Systematic review registration
PROSPERO CRD42020180638.
Introduction
The lifetime prevalence of the common psychological problem known as panic disorder is between 1% and 5%.1 2 Panic disorder is characterised by recurrent and unexpected panic attacks associated with several comorbid psychiatric and non-psychiatric conditions2 such as anxiety, depression, and cardiovascular diseases3 4 and impairment of social, work, and family functioning.5 Agoraphobia is a strong fear or anxiety provoked by real or anticipated exposure to a wide range of situations and is often associated with panic disorder. Successive revisions of the Diagnostic and Statistical Manual of Mental Disorders (DSM-III, DSM-III-R, and DSM-IV) provide similar definitions of panic disorder, refined with each new edition; but in the fifth revision (DSM-5) panic disorder and agoraphobia have been defined individually.
Several drug treatments are available for panic disorder, including tricyclic antidepressants, benzodiazepines, selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors, and serotonin-noradrenaline reuptake inhibitors (SNRIs).6 Findings from previous systematic reviews and direct meta-analyses suggest these treatments are more effective in reducing panic symptoms than placebo, but tricyclic antidepressants and benzodiazepines are linked with a significantly higher risk of adverse events.7 8 As a result several guidelines recommend SSRIs as the primary treatment owing to their preferable long term safety over benzodiazepines and tricyclic antidepressants.6 9 10 Nevertheless, it remains unclear which SSRI is most efficacious and is associated with the lowest risk of adverse events given the limited availability of direct comparisons between SSRIs and other drug classes.
Network meta-analysis is used to indirectly synthesise evidence on all possible treatments using direct comparisons with common comparators. Although several systematic reviews and direct meta-analyses of panic disorder have been published over the past decade, a network meta-analysis that simultaneously estimates relative efficacy and safety between commonly available drug treatments (eg, tricyclic antidepressants, benzodiazepines, SSRIs, SNRIs, monoamine oxidate inhibitors) has not been undertaken. We therefore conducted a systematic review and network meta-analysis to identify which drug classes—tricyclic antidepressants, benzodiazepines, β blockers, monoamine oxidase inhibitors, noradrenaline and dopamine reuptake inhibitor (eg, buspirone), SSRIs, SNRIs, noradrenaline reuptake inhibitors, and noradrenergic and specific serotonergic antidepressants provided high benefits (remission) and low risk (adverse events) for the treatment of panic disorder. Although guidelines usually recommend SSRIs as the preferred drugs for the treatment of panic disorder, evidence to indicate the most appropriate SSRI is inadequate; thus, we also undertook a network meta-analysis to compare individual SSRIs (eg, fluoxetine, fluvoxamine, paroxetine, sertraline, escitalopram, and citalopram).
Methods
This systematic review and network meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA).11 12
Relevant studies were identified from Medline through PubMed and Embase from inception to 17 June 2021. The search terms were constructed according to population (eg, panic disorder, panic) and intervention domains (eg, benzodiazepine, SSRI, SNRI). Supplementary appendix 1 provides details of the search terms and search strategies. For searching in PubMed, we used the filter function to select only randomised controlled trials and phase I-IV clinical trials. ClinicalTrials.gov was also searched to identify ongoing trials. In addition, to identify other relevant studies we reviewed the reference lists of included studies and previous meta-analyses.
Randomised controlled trials published in any language were eligible that included adults aged ≥18 years with panic disorder diagnosed in accordance with the Feighner criteria,13 DSM third (and revised third) to fifth revisions, or the International Statistical Classification of Diseases and Related Health problems; compared any pairs of interventions involving drugs for the treatment of panic disorder, with no treatment (eg, placebo or waitlist); and had any of the outcomes of remission, dropout, anxiety symptoms, depressive symptoms, quality of life, and adverse events. We excluded studies that compared different dosages of the same drugs, such as alprazolam 0.5 mg/day v 1 mg/day, did not report sufficient data necessary for pooling (eg, patient numbers by treatment and outcome groups in contingency tables for dichotomous outcomes or mean and standard deviation values by treatment groups for continuous outcomes), or had concurrent psychotherapy with drug interventions. For multi-arm studies comparing different dosages of the same drug with other drugs or placebo, we only used data from the arm with a common or recommended dosage. Two reviewers (NC and KC) independently selected studies based on titles and abstracts; if eligibility was still undetermined, they reviewed full study texts. Discrepancies between reviewers were resolved by consensus with a third researcher (TA).
Interventions of interest
Interventions of interest for panic disorder included tricyclic antidepressants, SSRIs, monoamine oxidase inhibitors, SNRIs, noradrenergic and specific serotonergic antidepressant (eg, mirtazapine), noradrenaline reuptake inhibitors (eg, reboxetine), benzodiazepines, noradrenaline and dopamine reuptake inhibitor (eg, buspirone), and β adrenergic receptor blocking agents.
Outcomes of interest
The primary outcomes were remission, defined as no panic attack for at least one week at the end of study, and dropout, defined as patients who discontinued treatment or withdrew before the end of the study owing to adverse events, protocol violation, or a lack of treatment efficacy. The secondary outcomes were anxiety and depression symptom scores and any adverse events (eg, sedation, fatigue or weakness, malaise, ataxia, slurred speech, cognitive impairment, sleep problems, sexual dysfunctions, tachycardia, palpitations, dry mouth, diarrhoea, constipation, nausea or vomiting, gastrointestinal problems, chest pain, nervousness, headache, lack of coordination, blurred vision, difficulty with urination, menstrual irregularity, change in appetite, change in body weight, upper respiratory tract infection, irritability, agitation, paraesthesia, diaphoresis, tremor, anxiety, depressive symptoms, asthenia, and orthostatic hypotension). Anxiety and depression symptom scores were originally measured by the tools specified in the studies included, such as the Hamilton rating scale for anxiety, clinical anxiety scale, Hamilton rating scale for depression, and Montgomery-Åsberg depression rating scale. These validated tools have been accepted and commonly used for measuring anxiety and depression symptoms.14 15 16 17 Given the limited number of studies that reported health related quality of life, this was not considered as an outcome (see review protocol registered with PROSPERO, CRD42020180638).
Data extraction
Two reviewers (NC and KC) independently extracted data on study characteristics (eg, setting, design, study period), participant characteristics (eg, mean age, percentage of women, duration of panic disorder), duration of treatment, and outcome measurements. Data used for pooling were extracted as means and standard deviations or continuous outcomes, and number of participants across treatments for dichotomous outcomes. When data were insufficient, we contacted the corresponding authors of the studies.
Risk of bias of individual studies
Two reviewers (NC and KC) independently assessed risk of bias using the revised Cochrane risk of bias tool for randomised trials (RoB 2.0).18 Five domains were assessed: bias arising from the randomisation process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome, and bias in selection of reported results.
We graded each risk of bias domain as low, some concern, or high risk. If all the domains for a study were low risk we judged the overall risk of bias as low. In addition, if multiple domains were some concern, we judged the overall risk of bias as high.18 Disagreements between the two reviewers were resolved by a third researcher (TA) through consensus.
Data synthesis
When at least three randomised controlled trials compared similar interventions and outcomes, we performed a direct meta-analysis. For continuous outcomes, we estimated relative treatment effects for individual randomised controlled trials using an unstandardised mean difference if studies used the same outcome measures; otherwise we used a standardised mean difference. A risk ratio was estimated for dichotomous outcomes. The unstandardised mean differences, standardised mean differences, and risk ratios were pooled using the Hartung-Knapp restricted maximum likelihood method. Heterogeneity was assessed using the Q test and I2 statistic. If heterogeneity was present (P<0.10 or I2 ≥25%), we explored heterogeneity using meta-regression by including possible sources of heterogeneity (mean age, duration of treatment and disease, percentage of women, and presence of agoraphobia) on a one-by-one basis within the meta-regression. We undertook subgroup analyses if the individual factor decreased the τ2 or I2 values by at least 30% from those of the meta-regression.19
A two stage frequentist network meta-analysis approach was applied.20 For each study we estimated a relative treatment effect (unstandardised and standardised mean differences and risk ratios) along with variance-covariance. Then we pooled these relative treatment effects across studies using a random effect multivariate meta-analysis with a consistency model. To deal with studies with zero events we applied a continuity correction by adding 0.5 to all cells. A common between study variation (τ2) was assumed for all relative treatment comparisons, in which the τ2 was classified as low, low-moderate, moderate-high, and high if it was <0.4, 0.4-0.16, 0.16-0.36, and >0.36, respectively, for binary outcomes.21 22 We ranked the probability of treatments being the best—associated with increasing efficacy and decreasing adverse events—and ranked each by a surface under the cumulative ranking curve (SUCRA). SUCRA is a numerical presentation of the overall treatment ranking, which ranges from 0 (low rank) to 100% (top rank). We constructed a cluster plot of SUCRA values for remission and adverse events to assess benefit and risk simultaneously. In addition, to evaluate the SSRI that provided high remission rate and low risk of adverse events, we produced SUCRA cluster ranking plots for individual SSRIs by remission and adverse events. Sensitivity analyses were performed according to the duration of treatment to remission, dropout, and adverse events to assess treatment efficacy for studies of similar duration (8-12 weeks). In addition, we applied a one stage approach using a mixed effect logistic regression for pooling outcomes with zero events.23
A design-by-treatment model with a global χ2 test was applied to assess the consistency assumption of the network.24 25 If inconsistency was present, we used a loop specific approach to estimate an inconsistency factor. The studies responsible for the inconsistency and their characteristics were then explored. We used comparison adjusted funnel plots to examine small study effects.26 If these plots showed asymmetry, we constructed a contour enhanced funnel plot to explore whether the asymmetry was due to small study effects (most of the small studies showed statistically significant results) or heterogeneity (most of the studies showed both significant and non-significant results). All analyses were performed on the basis of a frequentist approach using Stata version 16. A two sided P value <0.05 was considered statistically significant, except for the Q test, where a P value <0.10 was applied.
The level of certainty of evidence from the results of the network meta-analysis were assessed using the Confidence in Network Meta-Analysis (CINeMA) tool.27 Supplementary appendix 2 provides details on the grading of the level of confidence according to CINeMA.
Patient and public involvement
Patients or the public were not involved in the design, conduct, reporting, or dissemination plans of our research owing to the nature of secondary data capture and complex statistical analysis.
Results
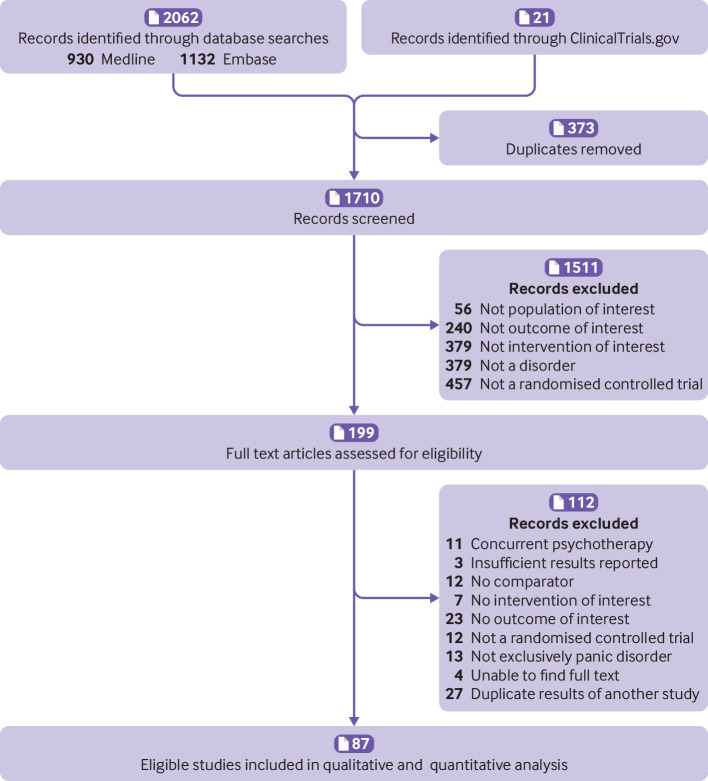
A total of 2019 studies were identified, of which 87 (12 800 participants) met the inclusion criteria (fig 1). Table 1 shows the characteristics of the included studies (see supplementary file for references e1-e87). The mean age of participants was 35.0 years and 63.7% (7819/12 276) were women. Most of the studies (39/87, 44.3%) used the DSM-III-TR (text revision) criteria for diagnosing panic disorder. Eighty three studies (95%) included participants with agoraphobia, and duration of panic disorder was 6.9 years before study commencement. The most common duration of treatment was eight weeks (35%), followed by 12 weeks (19%). A total of 21 comparisons were considered for analysis; most compared benzodiazepines with placebo (n=16 studies) and SSRIs with placebo (n=16), followed by tricyclic antidepressants versus benzodiazepines (n=8), tricyclic antidepressants versus SSRIs (n=8), and SSRIs versus SSRIs (n=6), and tricyclic antidepressants versus placebo (n=5), with the remaining comparisons represented in only a few studies. Fifty two studies reported outcomes for remission, 75 for dropouts, 41 for anxiety symptoms, 22 for depression symptoms, and 54 for adverse events. Quality of life outcomes were not considered as data were only available from seven studies.
Fig 1.
Study selection in review
Table 1a.
Characteristics of included studies—part 1 (see supplementary file for references)
| Studies and drug comparisons | Year | Setting | Diagnostic criteria | Presence of agoraphobia | Duration of treatment (weeks) | Comparison | No of participants | % women | Mean age (years) | Duration of panic disorder (years) | % with agoraphobia |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tricyclic antidepressants v placebo | |||||||||||
| Lydiarde25 | 1993 | Single centre | DSM-III-R | Some | 12 | Desipramine v placebo | 56 | - | - | - | - |
| Mavissakaliane75 | 1995 | - | DSM-III | All | 8 | Imipramine v placebo | 37 | 75 | 36.4 | 10.8 | 100 |
| Caillarde67 | 1999 | Multicentre | DSM-III-R | Some | 8 | Clomipramine v placebo | 118 | 59 | 33.6 | - | - |
| Barlowe64 | 2000 | Multicentre | DSM-III-R | Some | 12 | Imipramine v placebo | 107 | 64 | 35.2 | 6.4 | - |
| Broockse66 | 1998 | Single centre | DSM-III-R | Some | 10 | Clomipramine v placebo | 30 | 43 | 35.4 | 5.5 | 90 |
| Benzodiazepines v placebo | |||||||||||
| Ballengere6 | 1988 | Multicentre | DSM-III | Some | 8 | Alprazolam v placebo | 460 | 68 | 37.1 | 8.8 | 79 |
| Cirauloe15 | 1990 | Single centre | DSM-III | All | 14 | Alprazolam v placebo | 36 | 72 | 36.7 | - | 100 |
| Kloskoe20 | 1990 | Single centre | DSM-III | Some | 15 | Alprazolam v placebo | 57 | 74 | 37.0 | - | - |
| Tesare54 | 1991 | Single centre | DSM-III | Some | 6 | Alprazolam v placebo | 46 | 37 | 31.3 | 5.8 | 86 |
| Lydiarde24 | 1992 | Multicentre | DSM-III | Some | 6 | Alprazolam v placebo | 54 | - | 37.3 | - | 58 |
| Schweizere44 | 1993 | Multicentre | DSM-III | Some | 6 | Alprazolam v placebo | 194 | 60 | 35.0 | - | - |
| Pecknolde33 | 1994 | Multicentre | DSM-III-R | Some | 6 | Alprazolam v placebo | 139 | 60 | 35.2 | - | 28 |
| Beauclaire9 | 1994 | - | DSM-III | Some | 4 | Clonazepam v placebo | 29 | 59 | 32.8 | 3.0 | 62 |
| Davidsone68 | 1994 | Single centre | DSM-III-R | All | 4 | Adinazolam SR v placebo | 202 | 67 | 35.8 | - | 100 |
| Cartere14 | 1995 | Multicentre | DSM-III-R | All | 4 | Adinazolam v placebo | 315 | - | - | - | 100 |
| Savoldie43 | 1995 | Single centre | DSM-III | Some | 4 | Etizolamv placebo | 30 | 60 | 37.7 | - | - |
| Noyes Jre77 | 1996 | Multicentre | DSM-III | Some | 8 | Alprazolam v placebo | 157 | 65 | 37.0 | 9.1 | 81 |
| Rosenbaume81 | 1997 | Multicentre | DSM-III-R | Some | 9 | Clonazepam v placebo | 137 | 56 | - | 8.9 | 79 |
| Moroze29 | 1999 | Multicentre | DSM-III-R | Some | 6 | Clonazepam v placebo | 438 | 64 | 36.8 | - | 74 |
| Valencae85 | 2000 | Single centre | DSM-IV | All | 6 | Clonazepam v placebo | 24 | 58 | 37.2 | - | 100 |
| Valencae84 | 2003 | Single centre | DSM-IV | All | 6 | Clonazepam v placebo | 34 | 56 | 36.9 | 1.8 | 100 |
| SSRIs v placebo | |||||||||||
| Oehrberge32 | 1995 | Multicentre | DSM-III-R | Some | 12 | Paroxetine v placebo | 120 | 76 | 37.4 | - | 65 |
| Ballengere6 | 1998 | Multicentre | DSM-III-R | Some | 10 | Paroxetine v placebo | 136 | 69.1 | 36.7 | 9.2 | - |
| Berginke65 | 2005 | Single centre | DSM-IV | Some | 9 | Paroxetine v placebo | 19 | 58 | 44.5 | - | - |
| Sheehane48 | 2005 | Multicentre | DSM-IV | Some | 10 | Paroxetine CR v placebo | 889 | 60 | 37.7 | 9.3 | - |
| Hendrikse70 | 2010 | Single centre | DSM-IV | Some | 14 | Paroxetine v waitlist | 29 | 66 | 68.0 | 5.1 | 55 |
| Westernberge60 | 1989 | Single centre | DSM-III-R | Some | 8 | Fluvoxamine v placebo | 40 | - | 37.0 | - | - |
| Blacke11 | 1993 | Multicentre | DSM-III-R | Some | 8 | Fluvoxamine v placebo | 50 | 74 | 36.1 | 4.6 | 88 |
| Hoehn-Sarice18 | 1993 | Single centre | DSM-III-R | Some | 8 | Fluvoxamine v placebo | 37 | 54 | 38.0 | - | 78 |
| Sandmanne82 | 1998 | Single centre | DSM-III-R | Some | 6 | Fluvoxamine v placebo | 46 | 65 | 34.0 | 4.7 | 74 |
| Asnise3 | 2001 | - | DSM-III-R | Some | 8 | Fluvoxamine v placebo | 169 | 68 | 35.4 | 3.1 | - |
| Sharpe83 | 1996 | - | DSM-III-R | Some | 12 | Fluvoxamine v placebo | 57 | 79 | 39.4 | 4.4 | - |
| Michelsone28 | 1998 | Multicentre | DSM-III-R | Some | 10 | Fluoxetine v placebo | 153 | 69 | 36.8 | - | 78 |
| Michelsone27 | 2001 | Multicentre | DSM-IV | Some | 12 | Fluoxetine v placebo | 180 | 62 | - | - | - |
| Pohle35 | 1998 | Multicentre | DSM-III-R | Some | 10 | Sertraline v placebo | 168 | 57 | 37.5 | 9.0 | - |
| Londborge23 | 1998 | Multicentre | DSM-III-R | Some | 12 | Sertraline v placebo | 177 | 47 | 38.8 | - | - |
| Pollacke38 | 1998 | Single centre | DSM-III-R | Some | 10 | Sertraline v placebo | 176 | 65 | 36.4 | 9.9 | 70 |
| SNRIs v placebo | |||||||||||
| Pollacke40 | 1996 | Multicentre | DSM-III-R | Some | 8 | Venlafaxine v placebo | 25 | 20 | 36.7 | - | - |
| Bradwejne12 | 2005 | Multicentre | DSM-IV | Some | 10 | Venlafaxine ER v placebo | 370 | 54 | 38.9 | 8.0 | - |
| Liebowitze21 | 2009 | Multicentre | DSM-IV | Some | 10 | Venlafaxine ER v placebo | 310 | 65 | 36.4 | 6.2 | - |
| Monoamine oxidase inhibitors v placebo | |||||||||||
| Van Vliete57 | 1993 | Single centre | DSM-III-R | Some | 12 | Broforamine v placebo | 30 | 90 | 32.0 | 9.6 | 97 |
| Loerche22 | 1999 | Single centre | DSM-III-R | All | 8 | Moclobemide v placebo | 27 | - | - | - | 100 |
| Benzodiazepines v tricyclic antidepressants | |||||||||||
| Holland (adinazolam)e71 | 1999 | Multicentre | DSM-III-R | Some | 24 | Adinazolam SR v clomipramine | 315 | 63 | 36.2 | - | - |
| Holland (alprazolam)e71 | 1999 | Multicentre | DSM-III-R | Some | 12 | Adinazolam SR v clomipramine | 257 | 64 | 34.8 | - | - |
| Uhlenhuthe56 | 1989 | Single centre | DSM-III | Some | 8 | Alprazolam v imipramine v placebo | 60 | 58 | - | - | 53 |
| Taylore53 | 1990 | Single centre | DSM-III | Some | 8 | Alprazolam v imipramine v placebo | 79 | 73 | 34.7 | 5.6 | 72 |
| Andersche2 | 1991 | Multicentre | DSM-III | Some | 8 | Alprazolam v imipramine v placebo | 123 | 38 | 36.3 | 8.0 | 52 |
| Second Phase Investigatorse1 | 1992 | Multicentre | DSM-III | Some | 8 | Alprazolam v imipramine v placebo | 1122 | 62 | 34.0 | - | 36 |
| Kellere19 | 1993 | Single centre | DSM-III | All | 16 | Alprazolam v imipramine v placebo | 126 | 72 | 33.3 | - | 100 |
| Schweizere46 | 1993 | Single centre | DSM-III | Some | 8 | Alprazolam v imipramine v placebo | 106 | 75 | 33.0 | - | 42 |
| Sheikhe51 | 1999 | Single centre | DSM-III-R | Some | 8 | Alprazolam v imipramine v placebo | 25 | 92 | 61.2 | - | - |
| SSRIs v SSRIs | |||||||||||
| Amoree63 | 1999 | Single centre | DSM-IV | Some | 24 | Fluoxetine v citalopram | 42 | 60 | 37.0 | 5.8 | 69 |
| ClinicalTrials.gove87 | 2010 | Multicentre | DSM-IV | Some | 12 | Paroxetine v sertraline | 319 | 70 | - | - | - |
| Bandelowe8 | 2004 | Multicentre | ICD-10 | Some | 12 | Paroxetine v sertraline | 225 | 63 | 38.9 | 3.9 | 65 |
| Pernae34 | 2001 | Single centre | DSM-IV | Some | 8 | Paroxetine v citalopram | 52 | 65 | 31.3 | - | 79 |
| Marchesie26 | 2006 | Single centre | DSM-IV | Some | 52 | Paroxetine v citalopram | 100 | 69 | 35.4 | 4.2 | 71 |
| Stahle52 | 2003 | Multicentre | DSM-IV | Some | 10 | Escitalopram v citalopram v placebo | 351 | 58 | 37.7 | - | - |
| Tricyclic antidepressants v SSRIs | |||||||||||
| Bystritskye13 | 1995 | Single centre | DSM-III-R | Some | 10 | Desipramine v fluoxetine | 21 | 43 | 37.0 | - | - |
| Amoree62 | 1999 | Single centre | DSM-IV | Some | 24 | Imipramine v fluoxetine | 38 | 47 | 37.1 | 5.6 | 55 |
| Lepolae74 | 2003 | Multicentre | DSM-IV | Some | 26 | Imipramine v sertraline | 207 | 74 | 40.3 | 7.4 | 60 |
| Bakkere5 | 1999 | Single centre | DSM-III-R | Some | 12 | Clomipramine v paroxetine v placebo | 93 | 64 | - | 7.1 | 97 |
| Lecrubiere73 | 1997 | Multicentre | DSM-III-R | Some | 12 | Clomipramine v paroxetine v placebo | 367 | 40 | 34.9 | - | 83 |
| Naire31 | 1996 | Multicentre | DSM-III-R | Some | 8 | Imipramine v fluvoxamine v placebo | 148 | 55 | 34.8 | 7.9 | 47 |
| Bakishe4 | 1996 | - | DSM-III-R | Some | 8 | Imipramine v fluvoxamine v placebo | 35 | 46 | 33.0 | - | 66 |
| Wadee59 | 1997 | Multicentre | DSM-III-R | Some | 8 | Clomipramine v citalopram v placebo | 291 | 70 | - | - | 75 |
Table 1b.
Characteristics of included studies—part 2 (see supplementary file for references)
| Studies and drug comparisons | Year | Setting | Diagnostic criteria | Presence of agoraphobia | Duration of treatment (weeks) | Comparison | No of participants | % women | Mean age (years) | Duration of panic disorder (years) | % with agoraphobia |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SSRIs v benzodiazepines | |||||||||||
| Fisekovice69 | 2005 | Single centre | ICD-10 | Some | 12 | Sertraline v alprazolam | 40 | 55 | 35.8 | - | - |
| Nardie76 | 2011 | Single centre | DSM-IV | Some | 8 | Paroxetine v clonazepam | 120 | 69 | 34.9 | - | 93 |
| Benzodiazepines v β blockers | |||||||||||
| Ravarise41 | 1991 | Single centre | DSM-III | Some | 6 | Alprazolam v propanolol | 29 | 93 | 34.4 | 11.4 | 66 |
| Munjacke30 | 1989 | - | DSM-III | Some | 5 | Alprazolam v propranolol v placebo | 55 | 69 | 31.0 | 6.4 | 25 |
| Monoamine oxidase inhibitors v SSRIs | |||||||||||
| Pinie78 | 2003 | Single centre | DSM-III-R | - | 16 | Moclobemide v Paroxetine | 32 | - | - | - | - |
| Tillere55 | 1999 | - | DSM-III | Some | 8 | Moclobemide v fluoxetine | 366 | 67 | 35.0 | 6 | - |
| Van Vliete86 | 1996 | Single centre | DSM-III-R | Some | 12 | Broforamine v fluvoxamine | 30 | 87 | 35.0 | 9.4 | - |
| SSRIs v SNRIs | |||||||||||
| Pollacke37 | 2007 | Multicentre | DSM-IV | Some | 12 | Paroxetine v venlafaxine v placebo | 464 | 87 | 37.2 | 3.5 | - |
| Pollacke79 | 2007 | Multicentre | DSM-IV | Some | 12 | Paroxetine v venlafaxine v placebo | 465 | 67 | 36.4 | 3.3 | - |
| SSRIs v noradrenaline reuptake inhibitors | |||||||||||
| Bertanie10 | 2004 | Single centre | DSM-IV | Some | 12.8 | Paroxetine v reboxetine | 68 | 68 | 30.8 | - | 66 |
| Seedate47 | 2003 | Single centre | DSM-III | Some | 8 | Citalopram v reboxetine | 17 | 58 | 35.5 | - | - |
| SSRIs+benzodiazepines v SSRIs | |||||||||||
| Goddarde16 | 2001 | Single centre | DSM-IV | Some | 12 | Sertraline plus clonazepam v sertraline | 47 | 55 | 38.2 | 6.8 | 81 |
| Pollacke39 | 2003 | Single centre | DSM-IV | Some | 8 | Paroxetine plus clonazepam v paroxetine | 42 | 60 | - | - | 83 |
| Tricycyclic antidepressants v buspirone v placebo | |||||||||||
| Robinsone42 | 1989 | Single centre | DSM-III | - | 8 | Imipramine v buspirone v placebo | 91 | 67 | 32.7 | - | - |
| Pohle35 | 1989 | Single centre | DSM-III | Some | 8 | Imipramine v buspirone v placebo | 52 | 57 | 30.3 | - | - |
| Sheehane50 | 1990 | - | DSM-III | - | 8 | Alprazolam v buspirone v placebo | 54 | 72 | 35.2 | 11.1 | - |
| Benzodiazepines v buspirone v placebo | |||||||||||
| Schweizere45 | 1988 | - | DSM-III | - | 4 | Clorazepate v buspirone | 16 | 69 | 35.0 | - | - |
| Sheehane49 | 1993 | Single centre | DSM-III-R | Yes | 8 | Alprazolam v buspirone v placebo | 92 | 80 | 36.7 | 15.2 | 17 |
| Other comparisons | |||||||||||
| Woodse61 | 1992 | Single centre | DSM-III-R | Some | 4 | Imipramine plus alprazolam v imipramine | 48 | 54 | 36.2 | 10.0 | 69 |
| Krugere72 | 1999 | Multicentre | DSM-III-R | Some | 8 | Moclobemide v clomipramine | 135 | 59 | 35.5 | 5.7 | - |
| Hirschmanne17 | 2000 | Single centre | DSM-IV | Some | 4 | Fluoxetine plus pindolol v fluoxetine | 25 | 48 | 41.8 | 7.0 | 44 |
| Ribeiroe80 | 2001 | Single centre | DSM-IV | Some | 8 | Fluoxetine v mirtazapine | 27 | 77 | 36.2 | 3.0 | 73 |
| Versianie58 | 2002 | Multicentre | DSM-III-R | Some | 8 | Reboxetine v placebo | 75 | 67 | 35.8 | 1.8 | - |
DSM=Diagnostic and Statistical Manual of Mental Disorders; ICD-10=International Statistical Classification of Diseases, 10th revision; SSRIs=selective serotonin reuptake inhibitors; SNRIs=serotonin–noradrenaline reuptake inhibitors; SR=sustained release; CR=controlled release; ER=extended release.
Risk of bias assessment
Most of the studies had at least some concerns (70%; 61/87) or were at high risk of bias (29%; 25/87); only a single study was considered low risk (supplementary eFigures 1 and 2). Risk of bias was mostly related to insufficient details of randomisation and concealment processes (88%; 77/87 studies) and selection of results reported (91%; 79/87) owing to non-registration or publication of study protocols.
Remissions
Fifty studies (n=9481 participants) reported remission. Supplementary eTable 1 presents the treatment comparisons and data used for pooling in direct meta-analysis and network meta-analysis. The most common treatment comparisons were SSRIs versus placebo (13 studies, 2295 participants), followed by benzodiazepines versus placebo (11/2061).
Results of direct meta-analyses (supplementary eFigure 3) showed significant effects on remission for tricyclic antidepressants, benzodiazepines, paroxetine, sertraline, and venlafaxine compared with placebo, with risk ratios of 1.37 (95% confidence interval 1.27 to 1.47; I2=0%), 1.49 (1.36 to 1.63; I2=13.18%), 1.42 (1.12 to 1.79; I2=55.77%), 1.30 (1.10 to 1.54; I2=0%), and 1.26 (1.08 to 1.48, I2=52.02%), respectively. No significant difference on remission was found between benzodiazepines and tricyclic antidepressants (0.99, 0.94 to 1.04; I2=0%). A meta-regression identified the percentage of women and duration of treatment and panic disorder as potential sources of heterogeneity for venlafaxine versus placebo and paroxetine versus placebo (supplementary eTable 2). A subgroup analysis indicated paroxetine was associated with a significant improvement in remission when the duration of treatment was >10 weeks, with moderate heterogeneity (supplementary eFigure 4).
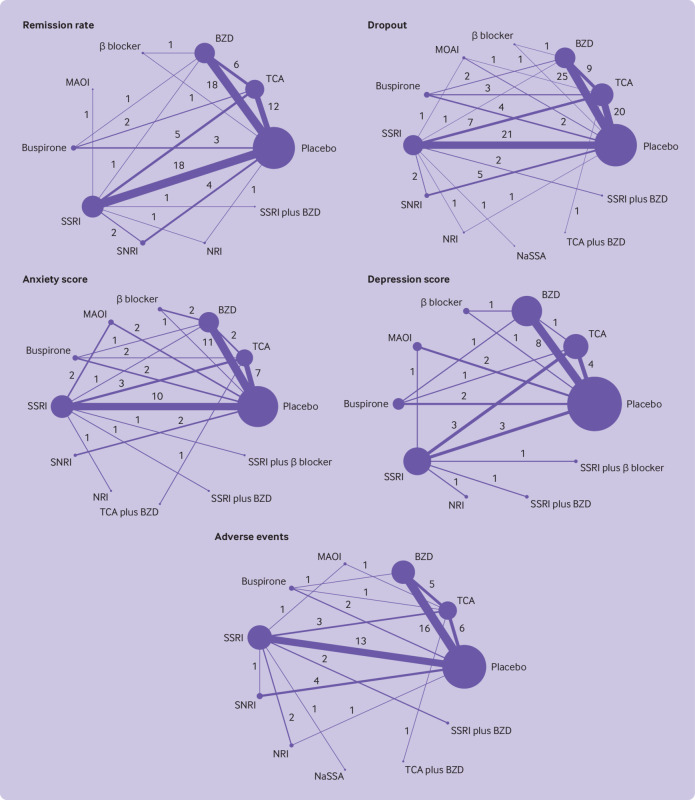
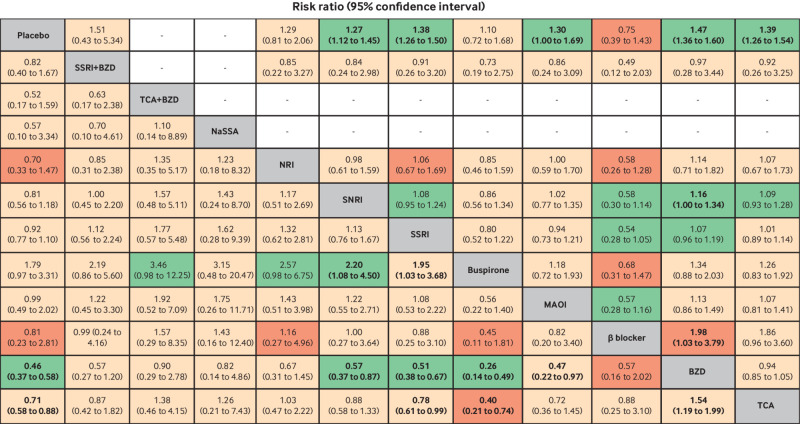
Fifty studies with 10 drug classes were included in a network meta-analysis of remission (fig 2). The global test indicated consistent results (χ2=12.19, P=0.59; τ2=0.09). Relative treatment effects indicated that monotherapy with tricyclic antidepressants, benzodiazepines, monoamine oxidase inhibitors, SSRIs, and SNRIs were associated with significantly higher remission than placebo, with risk ratios of 1.39 (95% confidence interval 1.26 to 1.54), 1.47 (1.36 to 1.60), 1.30 (1.00 to 1.69), 1.38 (1.26 to 1.50), and 1.27 (1.12 to1.45), respectively (fig 3). Only benzodiazepines were associated with significant improvements compared with β blockers and SNRIs, with risk ratios of 1.98 (1.03 to 3.79) and 1.16 (1.00 to 1.34), respectively (fig 3). SUCRAs identified benzodiazepines (84.5%, mean rank=2.4), tricyclic antidepressants (68.7%, mean rank=3.8), and SSRIs (66.4%, mean rank=4.0) as the top three best treatments for remission (supplementary eFigure 5). β blockers and buspirone were ranked worst, with SUCRA values of 9% and 33.2%, respectively.
Fig 2.
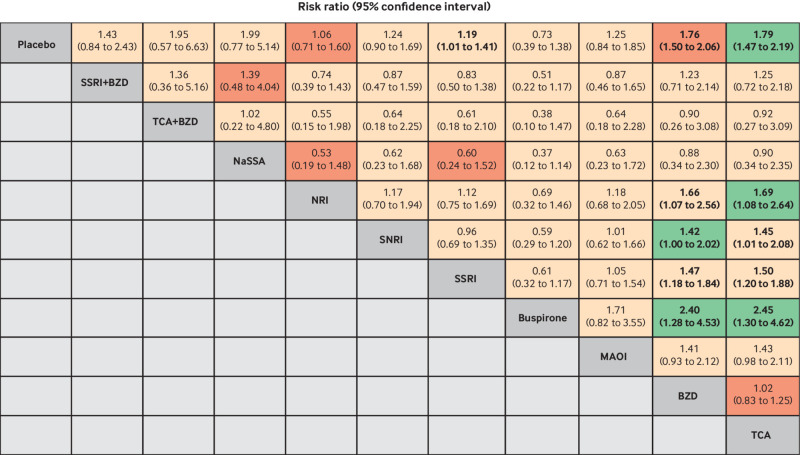
Network maps of outcomes. Size of nodes represents number of participants randomly assigned to treatment comparison. Width of lines represents number of studies comparing the two connected treatments. Number over each line indicates number of studies comparing the two connected treatments. BZD=benzodiazepine; MAOI=monoamine oxidase inhibitor; NaSSA=noradrenergic and specific serotonergic antidepressant; NRI=noradrenaline reuptake inhibitor; SSRI=selective serotonin reuptake inhibitor; SNRI=serotonin-noradrenaline reuptake inhibitor; TCA=tricyclic antidepressant
Fig 3.
Estimation of relative treatment effects on remission (above diagonal line) and dropout (below diagonal line). Results are risk ratios (95% confidence intervals) between each pair of treatments from network meta-analysis. Comparisons are read from right to left. For example, the risk ratio for remission with tricyclic antidepressants (TCAs) compared with benzodiazepines (BZDs) is 0.94 (0.85 to 1.05). For dropout, the effect of TCAs compared with BZDs on the risk of dropout is 1.54 (1.19 to 1.99). Bold font indicates statistical significance. GRADE certainty of evidence of each comparison is: light green=moderate; light red=low; dark red=very low. MAOI=monoamine oxidase inhibitor; NaSSA=noradrenergic and specific serotonergic antidepressant; NRI=noradrenaline reuptake inhibitor; SSRI=selective serotonin reuptake inhibitor; SNRI=serotonin-noradrenaline reuptake inhibitor
A sensitivity analysis for studies with a duration of treatment of 8-12 weeks (38/50) also identified benzodiazepines (81.4%, mean rank=2.5), tricyclic antidepressants (64.9%, mean rank=3.8), and SSRIs (63.7%, mean rank=3.9) as the top three best treatments for remission (supplementary eFigure 6 and eTable 3). The comparison adjusted funnel plot was symmetrical, suggesting an absence of small study effects (supplementary eFigure 7).
Dropouts
A total of 72 studies (10 911 participants) reported the dropout rate. Supplementary eTable 4 shows treatment comparisons and data used for pooling in direct meta-analysis and network meta-analysis. The most common treatment comparisons were benzodiazepines versus placebo (15 studies/2321 participants), followed by SSRIs versus placebo (14/3462).
The results from direct meta-analyses indicated that tricyclic antidepressants and benzodiazepines were associated with a significantly reduced dropout rate than placebo, with risk ratios of 0.42 (95% confidence interval 0.31 to 0.57; I2=71.69%) and 0.70 (0.61 to 0.79; I2=0%), respectively, whereas buspirone was associated with a higher dropout rate than placebo (risk ratio 1.97, 1.03 to 3.76; I2=0%) (supplementary eFigure 8). Tricyclic antidepressants were associated with a higher dropout rate than benzodiazepines, but this was not statistically significant (risk ratio 1.70, 0.97 to 3.00; I2=78.55%). Sources of heterogeneity were identified only for tricyclic antidepressants compared with benzodiazepines, including age, presence of agoraphobia, duration of treatment, and being female; further subgroup analysis was therefore performed. Dropout was found to be significantly higher with tricyclic antidepressants than benzodiazepines in studies with a high percentage of women, mean age of participants <60 years, and treatment duration less than eight weeks (supplementary eTable 5 and eFigure 9).
A network meta-analysis of 72 studies (n=11 263 participants) with 12 interventions was mapped and showed no evidence of inconsistency (global χ2=15.98; P=0.53; τ2=0.35) (fig 2). Benzodiazepines were associated with significantly lower dropout rates than monoamine oxidase inhibitors, buspirone, SSRIs, SNRIs, and placebo, with risk ratios of 0.47 (0.22 to 0.97), 0.26 (0.14 to 0.49), 0.51 (0.38 to 0.67), 0.57 (0.37 to 0.87), and 0.46 (0.37 to 0.58), respectively (fig 3). Typically, buspirone was associated with significantly higher dropout rates than SSRIs and SNRIs, with risk ratios of 1.95 (1.03 to 3.86) and 2.20 (1.08 to 4.50), respectively. In addition, tricyclic antidepressants were associated with significantly lower dropout rates than buspirone, SSRIs, and placebo, with risk ratios of 0.40 (0.21 to 0.74), 0.78 (0.61,0.99) and 0.71 (0.58 to 0.88) respectively, whereas the dropout rate associated with tricyclic antidepressants was significantly higher compared with benzodiazepines (risk ratio 1.54; 1.19 to 1.99). SUCRAs identified benzodiazepines (87.5%, mean rank=2.4) as the top ranked treatment in association with lower dropout rates, followed by benzodiazepines and tricyclic antidepressants combined (74.1%, mean rank=3.8) and noradrenergic and specific serotonergic antidepressant (63.3%, mean rank=5), whereas buspirone and monoamine oxidase inhibitors were ranked worst, with SUCRA values of 4.4% and 34.3%, respectively (supplementary eFigure 10). Sensitivity analysis by study treatment duration of 8-12 weeks (n=48) also suggested that benzodiazepines (94.8%, mean rank=1.5) were top ranked, followed by tricyclic antidepressants (69.3%, mean rank=3.8) and noradrenergic and specific serotonergic antidepressant (66.1%, mean rank=4.1) (supplementary eFigure 11 and eTable 6). The comparison adjusted funnel plot was symmetrical, suggesting no evidence of small study effects (supplementary eFigure 12).
Anxiety scores
Anxiety scores were reported in 39 studies (4112 participants), in which SSRIs versus placebo were the most common comparisons (nine studies, 1884 participants), followed by benzodiazepines versus placebo (seven studies, 771 participants) (supplementary eTable 7). Anxiety scores were measured by the Hamilton scale for anxiety in most studies (36/39) or alternatively the clinical anxiety scale (3/39).
Direct meta-analyses indicated anxiety scores associated with benzodiazepines, tricyclic antidepressants, and paroxetine were significantly lower than those associated with placebo, with corresponding unstandardised mean differences of −4.59 (95% confidence interval −6.39 to −2.80; I2=45.6%), −5.41 (−9.71 to −1.11; I2=73.75%), and −2.32 (−4.09 to −0.55; I2=0%) (supplementary eFigure 13). Duration of panic disorder and percentage of patients with agoraphobia accounted for some heterogeneity in the comparison of benzodiazepines versus placebo (supplementary eTable 8 and eFigure 14).
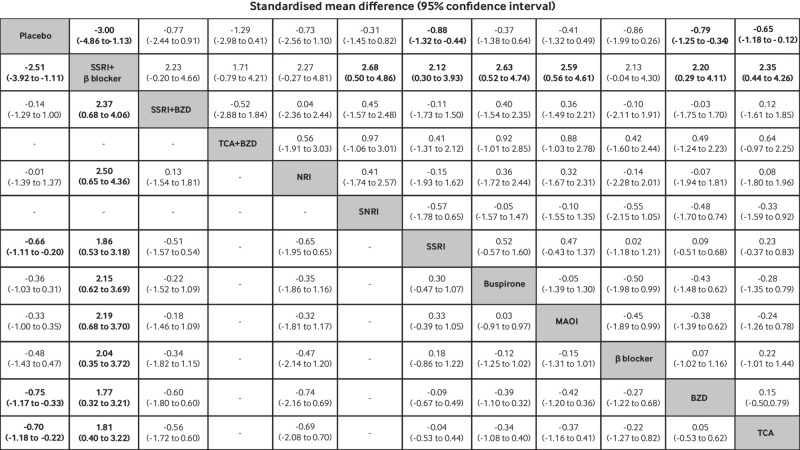
A network meta-analysis of 39 studies with 12 interventions showed no evidence of inconsistency (global χ2=4.63; P=0.97; τ2=0.76) (fig 2). Relative treatment effects by standardised mean differences indicated that tricyclic antidepressants, benzodiazepines, SSRIs, and SSRI plus β blocker were associated with significantly reduced anxiety scores compared with placebo: −0.65 (95% confidence interval −1.18 to −0.12), −0.79 (−1.25 to −0.34), −0.88 (−1.32 to −0.44), and −3.00 (−4.86 to −1.13), respectively (fig 4). In addition, anxiety scores associated with tricyclic antidepressants, benzodiazepines, monoamine oxidase inhibitors, buspirone, SSRIs, and SNRIs were significantly higher than for SSRIs and β blockers combined, with standardised mean differences of 2.35 (0.44 to 4.26), 2.20 (0.29 to 4.11), 2.59 (0.56 to 4.61), 2.63 (0.52 to 4.74), 2.12 (0.30 to 3.93), and 2.68 (0.50 to 4.86), respectively (fig 4). SUCRAs identified SSRIs plus β blockers (97.5%, mean rank=1.3), tricyclic antidepressants plus benzodiazepines (70.9%, mean rank=4.2), and SSRIs (62.9%, mean rank=5.1) as the top ranked treatments associated with lowering anxiety scores, whereas SNRIs and buspirone were ranked worst, with SUCRAs of 31.6% and 33%, respectively (supplementary eFigure 15). The comparison adjusted funnel plot was asymmetrical, with the contour enhanced funnel plot implicating heterogeneity as a contributory factor (supplementary eFigure 16).
Fig 4.
Estimation of relative treatment effects on anxiety score (above diagonal line) and depression score (below diagonal line). Results are standardised mean differences (95% confidence intervals) between each pair of treatments from network meta-analysis. Comparisons are read from right to left. For example, the standardised mean difference for anxiety score with tricyclic antidepressants (TCAs) compared with benzodiazepines (BZDs) is 0.15 (−0.50 to 0.79). For depression score, the effect of TCAs compared with BZDs on depression score is 0.05 (−0.53 to 0.62). Bold font indicates statistical significance. MAOIs=monoamine oxidase inhibitors; NRI=noradrenaline reuptake inhibitor; SSRI=selective serotonin reuptake inhibitor; SNRI=serotonin-noradrenaline reuptake inhibitor
Depression scores
Depression scores were reported in 21 studies (1453 participants), with benzodiazepines versus placebo as the most common comparison (five studies; 407 participants) (supplementary eTable 9). Depression scores were measured by the Hamilton rating scale for depression and Montgomery-Åsberg depression rating scale. Direct meta-analyses identified that benzodiazepines were associated with lower depression scores than placebo, with unstandardised mean differences of −4.53 (95% confidence interval −6.60 to −2.47; I2=68.3%) (supplementary eFigure 17). The percentage of participants with agoraphobia and treatment duration could represent sources of heterogeneity based on a few studies (supplementary eTable 10 and eFigure 18).
The network meta-analysis of 21 studies included 10 interventions with no evidence of inconsistency (global χ2=3.19; P=0.98; τ2=0.44), (fig 2). Tricyclic antidepressants, benzodiazepines, SSRIs, and SSRIs plus β blockers were significantly associated with lower depression scores than placebo, with standardised mean differences of −0.70 (95% confidence interval −1.18 to −0.22), −0.75 (−1.17 to −0.33), −0.66 (−1.11 to −0.20), and −2.51 (−3.92 to −1.11), respectively (fig 4). In addition, tricyclic antidepressants, benzodiazepines, β blockers, monoamine oxidase inhibitors, buspirone, SSRIs, noradrenaline reuptake inhibitors, and SSRIs plus benzodiazepines were significantly associated with higher depression scores than SSRIs plus β blockers, with standardised mean differences of 1.81 (95% confidence interval 0.40 to 3.22), 1.77 (0.32 to 3.21), 2.04 (0.35 to 3.72), 2.19 (0.68 to 3.70), 2.15 (0.62 to 3.69), 1.86 (0.53 to 3.18), 2.50 (0.65 to 4.36), and 2.37 (0.68 to 4.06), respectively (fig 4). SUCRAs identified SSRIs plus β blockers (99.7%, mean rank=1), benzodiazepines (69.9%, mean rank=3.7), and tricyclic antidepressants (66.4%, mean rank=4.0) as the top ranked treatments associated with lower depression scores, whereas noradrenaline reuptake inhibitors and SSRIs plus benzodiazepines were the worst ranked treatments, with SUCRA values of 27.4% and 29.4%, respectively (supplementary eFigure 19). The comparison adjusted funnel plot was asymmetrical, with the contour enhanced funnel plot indicating that asymmetry might be due to heterogeneity (supplementary eFigure 20).
Adverse events
Fifty two studies (9957 participants) reported adverse events in association with 11 interventions (supplementary eTable 11). From seven direct meta-analyses benzodiazepines, tricyclic antidepressants, and venlafaxine were associated with significantly increased risk of adverse events compared with placebo, with risk ratios of 1.92 (95% confidence interval 1.69 to 2.18; I2=32.7%), 1.51 (1.19 to 1.92; I2=82.5%), and 1.10 (1.02 to 1.18; I2=0%), respectively. In addition, tricyclic antidepressants were associated with non-significantly increased risk of adverse events compared with benzodiazepines (supplementary eFigure 21). Heterogeneity was found, but no source was identified (supplementary eTable 12).
The network meta-analysis of 52 studies initially showed evidence of inconsistency (global χ2=58.64, P<0.001). The two loops of placebo-monoamine oxidase inhibitors-SSRIs and placebo-tricyclic antidepressants-monoamine oxidase inhibitors were identified as sources of inconsistency (supplementary eFigure 22) owing to a high percentage of agoraphobia (75%-97% v 17%-86%) and high percentage of women (69%-90% v 37%-80%). After excluding these studies (see supplementary references e57, e76, e79, e86, e73, e32, e59), the evidence of consistency improved but might still be present (global χ2=20.04, P=0.07; τ2=0.25) (fig 2). Tricyclic antidepressants were significantly associated with increased risk of adverse events compared with buspirone, SSRIs, SNRIs, noradrenaline reuptake inhibitors, and placebo, with risk ratios of 2.45 (95% confidence interval 1.30 to 4.62), 1.50 (1.20 to 1.88), 1.45 (1.01 to 2.08), 1.69 (1.08 to 2.64), and 1.79 (1.47 to 2.19), respectively. Similarly, benzodiazepines also showed significantly higher associations with adverse events compared with buspirone, SSRIs, SNRIs, noradrenaline reuptake inhibitors, and placebo, with risk ratios of 2.40 (95% confidence interval 1.28 to 4.53), 1.47 (1.18 to 1.84), 1.42 (1.00 to 2.02), 1.66 (1.07 to 2.56), and 1.76 (1.50 to 2.06), respectively (fig 5). SSRIs were significantly associated with higher risk of adverse events compared with placebo (risk ratio 1.19, 1.01 to 1.41). SUCRAs identified buspirone (SUCRA=92%, mean rank=1.8) and noradrenaline reuptake inhibitors (73.1%, mean rank=3.7) as the top ranked treatments associated with lower risk of adverse events (supplementary eFigure 23), whereas tricyclic antidepressants (18.4%, mean rank=9.2) and benzodiazepines (20.1%, mean rank=9.0) represented the worst ranked treatments. Sensitivity analysis by treatment duration of 8-12 weeks (n=30/52) also identified buspirone (95.3%, mean rank=1.4) as the top ranked treatment associated with lowest risk of adverse events, followed by noradrenaline reuptake inhibitors (69.2%, mean rank=3.8) (supplementary eFigure 24 and eTable 13). The comparison adjusted funnel plot was slightly asymmetrical, and the contour enhanced funnel plot suggested this might be due to heterogeneity (supplementary eFigure 25).
Fig 5.
Estimation of relative treatment effects on adverse events. Results are risk ratios (95% confidence intervals) between each pair of treatments from network meta-analysis. Comparisons are read from right to left. For example, the risk ratio for adverse events with tricyclic antidepressants (TCAs) compared with benzodiazepines (BZDs) is 1.02 (95% confidence interval 0.83 to 1.25). Bold font indicates statistical significance. GRADE certainty of evidence of each comparison is: light green=moderate; light red=low; dark red=very low. MAOI=monoamine oxidase inhibitor; NaSSA=noradrenergic and specific serotonergic antidepressant; NRI=noradrenaline reuptake inhibitor; SSRI=selective serotonin reuptake inhibitor; SNRI=serotonin-noradrenaline reuptake inhibitor
Clustered ranking plot
The SUCRA clustered ranking plot for remission and adverse events indicated SSRIs as the most efficacious (66.4%) treatment with the least risk of adverse events (58.5%) for panic disorder (fig 6). Other drug classes, such as noradrenaline reuptake inhibitors, monoamine oxidase inhibitors, and SNRIs also showed promising efficacy in remission and acceptability of risk for adverse events. In contrast, buspirone showed low efficacy in remission despite high treatment acceptability.
Fig 6.
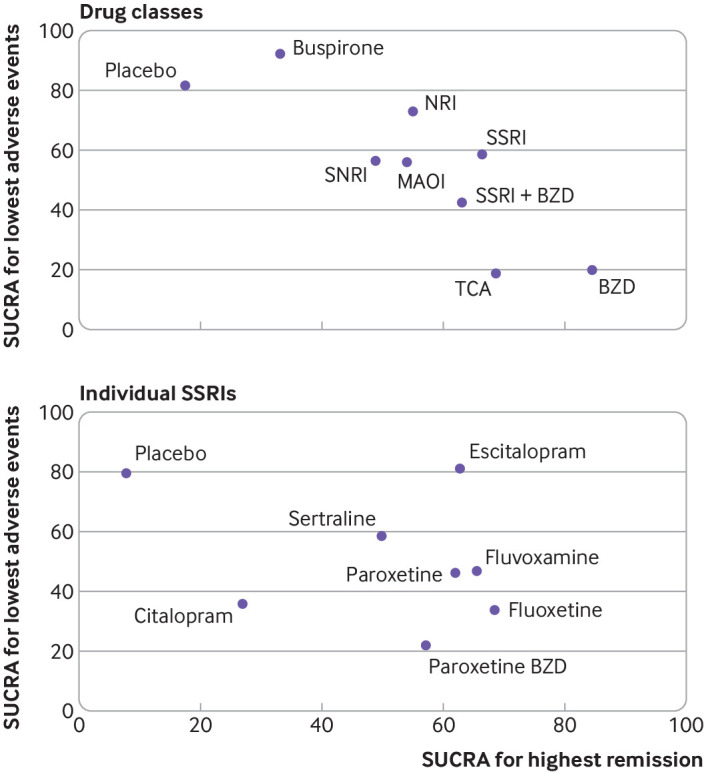
Cluster ranking plot of surface under cumulative ranking curves (SUCRA) of remission and adverse drug events. The plot is based on cluster analysis of SUCRA values. Each plot represents SUCRA values for two outcomes (ie, remission and adverse events). Treatments in the upper right corner are more effective (ie, increased remission rate) and safer (ie, lower risk of adverse events) compared with the other treatments. BZD=benzodiazepine; MAOI=monoamine oxidase inhibitor; NaSSA=noradrenergic and specific serotonergic antidepressant; NRI=noradrenaline reuptake inhibitor; SSRI=selective serotonin reuptake inhibitor; SNRI=serotonin-noradrenaline reuptake inhibitor; TCA=tricyclic antidepressant
Since the clustered ranking plot identified SSRIs as the treatment with the best balance between remission and risk of adverse events and given current clinical practice recommendation of SSRIs as the preferred treatment for panic disorder, we further explored the performance of individual SSRIs for balancing benefit and risk. Supplementary eAppendix 3 provides details on data analysis, tables, and SUCRA values of remission rates and adverse events. According to SUCRA and clustered ranking plots for individual SSRIs, sertraline and escitalopram represented the most efficacious agents with the lowest risk of adverse events. Fluvoxamine, paroxetine, and fluoxetine indicated favourable efficacy but higher risk of adverse events, whereas citalopram showed minimal efficacy in remission and high risk of adverse events (fig 6).
Evaluation of level of evidence from network meta-analysis
The CINeMA tool was used to assess levels of evidence from network meta-analysis for remissions, dropouts, and adverse events for drug classes and individual SSRIs (supplementary eTables 14-18). For drug classes, four out of nine treatment comparisons for SSRIs were graded with moderate confidence, compared with three out of nine for benzodiazepines and two out of nine for tricyclic antidepressants for the outcome of remission. For dropout, levels of evidence in all and nearly all treatment comparisons of noradrenergic and specific serotonergic antidepressants (11/11) and benzodiazepines combined with tricyclic antidepressants (10/11) were low, whereas 36% of level of evidence for treatment comparisons of benzodiazepines (4/11) were moderate. For adverse events, most of the treatment comparisons for buspirone (8/10), noradrenaline reuptake inhibitors (7/10), SSRIs (9/10), and benzodiazepines (7/10) were graded as low confidence.
For individual SSRIs, in remission outcome, levels of evidence were mostly low for treatment comparisons of fluoxetine (5/7), fluvoxamine (6/7), and sertraline (6/7), whereas all treatment comparisons with escitalopram were graded as low levels of evidence. For adverse drug events, nearly all treatment comparisons for sertraline (7/8) and escitalopram (8/8) were graded with low levels of confidence.
Discussion
Our network meta-analysis identified 11 current drug classes for the treatment of panic disorder, highlighting benzodiazepines, tricyclic antidepressants, and SSRIs as the highest ranked treatments for remission based on SUCRA values. Although benzodiazepines were associated with the lowest probability of dropout, they were also associated with the highest risk of adverse events. Overall, SSRIs provided high benefit (remission) with low risk of adverse events. Across individual SSRIs, sertraline and escitalopram were identified as the most efficacious agents with low risk of adverse events.
Comparison with other studies
Multiple options exist for the treatment of panic disorder; however, previous evidence has been mainly limited to placebo comparisons.8 28 Previous systematic reviews and meta-analyses reported benzodiazepines as the most efficacious in reducing panic attack frequency and anticipatory anxiety symptoms.8 Despite this clear evidence of benefit, benzodiazepines have not been recommended as the primary treatment option for panic disorder owing to potential adverse events (sedation, impaired memory and cognitive function, and increased risk of falling, especially in elderly people) and long term risks of dependency and withdrawals.29 This was consistent with our findings of associated risks.
SSRIs as a drug class have been recommended as the primary treatment for panic disorder. However, evidence or comparative studies to identify the most efficacious individual drug has been insufficient.6 30 31 32 In our study we specifically evaluated each SSRI to determine the most suitable as treatment for panic disorder. Our findings suggest that fluoxetine, fluvoxamine, paroxetine, and sertraline were more efficacious than placebo for achieving remission. SSRIs combined with β blockers, were associated with reduced anxiety symptoms and were top ranked for improving both anxiety and depression symptoms. SSRIs were not considered to be the best treatment for dropout, especially compared with benzodiazepines, which might be associated with a delayed onset of action that can take several weeks.33 When the benefits of remission and low adverse events are considered against potential agitation and early dropout, SSRIs were considered the best treatment option.33 Sertraline and escitalopram in particular offered the best balance between benefit (remission) and risk (adverse events) and were the top ranked among individual SSRIs for the treatment of panic disorder. The findings for escitalopram, however, were based on a single study and as such should be interpreted with caution.34 There are concerns about using escitalopram at supratherapeutic dose (30 mg/day) as it has been shown to increase the risk of QT prolongation.35 However, the risk of serious arrythmias is rare when escitalopram is prescribed at treatment dosage (10-20 mg/day).36
For other antidepressants, SNRIs, when compared with placebo, were more efficacious in attaining remission, similar for dropout, and had significantly more associated adverse events. However, SNRIs were ranked lower than SSRIs for achieving remission and adverse events and might represent a second line treatment option for panic disorder. Tricyclic antidepressants were found to be statistically superior to placebo for dropout, remission, anxiety, and depression scores but worse for adverse events. Despite their efficacy, tricyclic antidepressants are associated with adverse effects, which are further exacerbated when combined with benzodiazepines. As such, the clinical use of tricyclic antidepressants alone or combined with benzodiazepines is limited because of the extent of the associated adverse effects.
For additional treatment options not included in previous meta-analyses, monoamine oxidase inhibitors were found to be significantly superior to placebo for remission but were not different from placebo for other outcomes. However, monoamine oxidase inhibitors have severely limited use in real world practice given their adverse effects profile, increased risk of hypertensive crisis, and multiple drug interactions.37 Noradrenaline reuptake inhibitors did not show much effect compared with placebo, although some guidelines have recognised their use as a potential treatment option for panic disorder.10 Given the limited evidence available and lack of demonstrable benefit, noradrenaline reuptake inhibitors might not be a suitable treatment for panic disorder. Evidence is also insufficient to recommend the use of noradrenergic and specific serotonergic antidepressants given their associated high rate of drowsiness and weight gain, and this is in line with current recommendations from the American Psychological Association.32 38 Given the low quality of the studies with identified adverse effects, high dropout rate, and no demonstrable efficacy, buspirone should not be currently considered a viable treatment option for panic disorder.
Strengths and limitations of this study
Our study had several strengths. Our network meta-analysis considered a wide range of drug classes and agents. Both positive (remission) and negative (adverse events) outcomes were considered as part of the SUCRA and cluster ranking plot. Therefore, our findings represent treatments that provided high remission with low risk of adverse events.
Some limitations, however, could not be avoided. Most of the studies included are more than 20 years old and are affected by poor study methodology or reporting. Therefore, the overall risk of bias for all studies included some concerns to high risk, except for a single study, which had a low risk of bias. In addition, consistency assumption might be violated for network meta-analysis of adverse events, even excluding the studies with high inconsistency factors. Risk of bias and inconsistency were considered in the grading of evidence, which represented a low to moderate level for certainty of evidence. Moreover, most of the studies were of short duration (<12 weeks); thus the effects of long term treatment require further investigation and the assessment of only short term effects might overemphasise the therapeutic effects of some drugs such as benzodiazepines without considering the potential harms of dependency and difficulties associated with discontinuation.
The comparison adjusted funnel plots were asymmetrical for some outcomes, which were more likely due to between treatment effect heterogeneity across studies rather than because of small study effects or missing studies. Noticeably for adverse events, the asymmetry in the funnel suggested that the four smaller studies were skewed towards more harmful effects. These studies also represented high treatment effects of benefit outcomes for remission, dropouts, and anxiety scores, suggesting a negative correlation between high benefits and high risk of adverse events.
Heterogeneity was also present for some pooled relative treatment effects. Although potential sources of heterogeneity were explored, in some instances they were not identified owing to missing data in some studies. In addition, seven studies had single zero cells for dropout and one study for adverse events, necessitating a continuity correction through the addition of 0.5 to all cells to allow the estimation of effect measures in the two stage network meta-analysis. A sensitivity analysis was also performed by applying a one stage approach, which has been reported as a more valid method for dealing with zero cells23; results were consistent between the one stage and the two stage approaches used in this study (supplementary eTable 19).
Furthermore, we excluded studies that compared concurrent psychotherapy with drug interventions. Therefore, the efficacy of combined psychotherapy with pharmacotherapy could not be estimated in our study. Lastly, in our network meta-analysis, some comparisons were represented by lower numbers of studies or patients, or both, therefore limiting the precision of the network meta-analysis findings. A predictive interval plot was, however, considered based on current findings reported and any future additional studies.
Policy implications
Further studies should consider direct comparisons of more recent SSRIs in a randomised controlled trial design. Although escitalopram was rated highly, this was based on only a single trial and further evidence is required to confirm this finding. In addition, the comparison between benefits and harms used in our study was based on the findings from the cluster ranking plots, which failed to sufficiently consider any weightings to the correlation and actual magnitude of the treatment benefits and associated harms. As such, further investigations on the individual weightings of associated risk and benefit assessment would prove beneficial, although these were beyond the scope of the current study. Furthermore, an economic evaluation to determine the cost-benefit of sertraline and escitalopram as the drugs of choice would inform policy makers beyond the clinical risk benefit determination. As our study focused on short term outcomes, exploration of long term outcomes associated with SSRIs would also be helpful.
Conclusion
Our findings suggest that SSRIs offer important benefit with low risk for drug treatment of panic disorder. When individual agents were explored, sertraline and escitalopram were associated with high remission and low risk of adverse events when compared with other SSRIs. The findings should be interpreted with caution, however, as the results were based on evidence with moderate to very low levels of certainty.
What is already known on this topic
Several guidelines recommend selective serotonin reuptake inhibitors (SSRIs) as the primary treatment for panic disorder
It is unclear which SSRI is most efficacious because of limited evidence of direct comparisons amongst SSRIs and between SSRIs and other drug classes
What this study adds
SSRIs were associated with high rate of remission and low risk of adverse events in the treatment of panic disorder, and among individual SSRIs, sertraline and escitalopram provided high remission with low risk of adverse events
Serotonin-noradrenaline reuptake inhibitors, monoamine oxidase inhibitors, and noradrenaline reuptake inhibitors showed promising efficacy in remission and acceptability in risk of adverse events
The findings were represented by moderate to very low levels of certainty, suggesting cautious consideration of the use of evidence
Web extra.
Extra material supplied by authors
Supplementary information: additional material
Contributors NC and TA (principal researchers) wrote the protocol and submitted it to PROSPERO and searched for eligible studies. NC, KC, and TA selected the studies, extracted data, and assessed risk of bias. All authors were involved in data analysis and writing of the manuscript and approved the final version of the manuscript. NC and TA are responsible for the overall content as guarantors and accept full responsibility for the work and conduct of the study, access to the data, and controlled the decision to publish. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: None.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead authors (NC and TA) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: The findings from this study will be disseminated to relevant patients and public communities through social media such as the Facebook page of the Department of Family Medicine, Ramathibodi Hospital and on the hospital’s TV channel.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Not required.
Data availability statement
No additional data available.
References
- 1. Bandelow B, Michaelis S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci 2015;17:327-35. 10.31887/DCNS.2015.17.3/bbandelow [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Jonge P, Roest AM, Lim CC, et al. Cross-national epidemiology of panic disorder and panic attacks in the world mental health surveys. Depress Anxiety 2016;33:1155-77. 10.1002/da.22572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen YH, Hu CJ, Lee HC, Lin HC. An increased risk of stroke among panic disorder patients: a 3-year follow-up study. Can J Psychiatry 2010;55:43-9. 10.1177/070674371005500107 [DOI] [PubMed] [Google Scholar]
- 4. Gomez-Caminero A, Blumentals WA, Russo LJ, Brown RR, Castilla-Puentes R. Does panic disorder increase the risk of coronary heart disease? A cohort study of a national managed care database. Psychosom Med 2005;67:688-91. 10.1097/01.psy.0000174169.14227.1f [DOI] [PubMed] [Google Scholar]
- 5. Sherbourne CD, Sullivan G, Craske MG, et al. Functioning and disability levels in primary care out-patients with one or more anxiety disorders. Psychol Med 2010;40:2059-68. 10.1017/S0033291710000176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence. Generalised anxiety disorder and panic disorder in adults: management Clinical guideline. [CG113] NICE, 2011. www.nice.org.uk/guidance/cg113 [PubMed]
- 7. Bighelli I, Trespidi C, Castellazzi M, et al. Antidepressants and benzodiazepines for panic disorder in adults. Cochrane Database Syst Rev 2016;9:CD011567. 10.1002/14651858.CD011567.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Breilmann J, Girlanda F, Guaiana G, et al. Benzodiazepines versus placebo for panic disorder in adults. Cochrane Database Syst Rev 2019;3:CD010677. 10.1002/14651858.CD010677.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Psychiatric Association . Practice Guideline for the Treatment of Patients with Panic Disorder. 2nd ed. APA, 2009. [Google Scholar]
- 10. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol 2014;28:403-39. 10.1177/0269881114525674 [DOI] [PubMed] [Google Scholar]
- 11. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777-84. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264-9, W64. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 13. Feighner JP, Robins E, Guze SB, Woodruff RA, Jr, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Arch Gen Psychiatry 1972;26:57-63. 10.1001/archpsyc.1972.01750190059011. [DOI] [PubMed] [Google Scholar]
- 14. Maier W, Buller R, Philipp M, Heuser I. The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J Affect Disord 1988;14:61-8. 10.1016/0165-0327(88)90072-9 [DOI] [PubMed] [Google Scholar]
- 15. Müller MJ, Himmerich H, Kienzle B, Szegedi A. Differentiating moderate and severe depression using the Montgomery-Asberg depression rating scale (MADRS). J Affect Disord 2003;77:255-60. 10.1016/S0165-0327(02)00120-9 [DOI] [PubMed] [Google Scholar]
- 16. Reynolds WM, Kobak KA. Reliability and validity of the Hamilton Depression Inventory: A paper-and-pencil version of the Hamilton Depression Rating Scale Clinical Interview. Psychol Assess 1995;7:472-83 10.1037/1040-3590.7.4.472 . [DOI] [Google Scholar]
- 17. Westhuis D, Thyer BA. Development and Validation of the Clinical Anxiety Scale: A Rapid Assessment Instrument for Empirical Practice. Educ Psychol Meas 1989;49:153-63 10.1177/0013164489491016 . [DOI] [Google Scholar]
- 18. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 19. West SL, Gartlehner G, Mansfield AJ, et al. Table 7 Summary of common statistical approaches to test for heterogeneity. Comparative Effectiveness Review Methods: Clinical Heterogeneity. Agency for Healthcare Research and Quality, 2010. [PubMed] [Google Scholar]
- 20. Liu Y, Wang W, Zhang AB, Bai X, Zhang S. Epley and Semont maneuvers for posterior canal benign paroxysmal positional vertigo: A network meta-analysis. Laryngoscope 2016;126:951-5. 10.1002/lary.25688 [DOI] [PubMed] [Google Scholar]
- 21. da Costa BR, Juni P. Systematic reviews and meta-analyses of randomized trials: principles and pitfalls. Eur Heart J 2014;35:3336-45. 10.1093/eurheartj/ehu424 [DOI] [PubMed] [Google Scholar]
- 22. Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol 2012;41:818-27. 10.1093/ije/dys041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu C, Furuya-Kanamori L, Zorzela L, Lin L, Vohra S. A proposed framework to guide evidence synthesis practice for meta-analysis with zero-events studies. J Clin Epidemiol 2021;135:70-8. 10.1016/j.jclinepi.2021.02.012 [DOI] [PubMed] [Google Scholar]
- 24. Jackson D, Boddington P, White IR. The design-by-treatment interaction model: a unifying framework for modelling loop inconsistency in network meta-analysis. Res Synth Methods 2016;7:329-32. 10.1002/jrsm.1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 2012;3:98-110. 10.1002/jrsm.1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chaimani A, Higgins JPT, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One 2013;8:e76654. 10.1371/journal.pone.0076654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nikolakopoulou A, Higgins JPT, Papakonstantinou T, et al. CINeMA: An approach for assessing confidence in the results of a network meta-analysis. PLoS Med 2020;17:e1003082. 10.1371/journal.pmed.1003082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bighelli I, Castellazzi M, Cipriani A, et al. Antidepressants versus placebo for panic disorder in adults. Cochrane Database Syst Rev 2018;4:CD010676. 10.1002/14651858.CD010676.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bruce SE, Vasile RG, Goisman RM, et al. Are benzodiazepines still the medication of choice for patients with panic disorder with or without agoraphobia? Am J Psychiatry 2003;160:1432-8. 10.1176/appi.ajp.160.8.1432 [DOI] [PubMed] [Google Scholar]
- 30. Ballenger JC, Davidson JR, Lecrubier Y, et al. Consensus statement on panic disorder from the International Consensus Group on Depression and Anxiety. J Clin Psychiatry 1998;59(Suppl 8):47-54. [PubMed] [Google Scholar]
- 31. Bighelli I, Castellazzi M, Cipriani A, et al. Antidepressants versus placebo for panic disorder in adults. Cochrane Database Syst Rev 2018;4:CD010676. 10.1002/14651858.CD010676.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. American Psychiatric Association . Practice Guideline for the Treatment of Patients with Panic Disorder. 3rd ed. APA, 2009. [Google Scholar]
- 33. American Psychiatric Association . Practice Guideline for the Treatment of Patients with Major Depressive Disorder. 3rd ed. APA; 2010. [Google Scholar]
- 34. Stahl SM, Gergel I, Li D. Escitalopram in the treatment of panic disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2003;64:1322-7. 10.4088/JCP.v64n1107 [DOI] [PubMed] [Google Scholar]
- 35. Lam RW. Antidepressants and QTc prolongation. J Psychiatry Neurosci 2013;38:E5-6. 10.1503/jpn.120256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hasnain M, Howland RH, Vieweg WVR. Escitalopram and QTc prolongation. J Psychiatry Neurosci 2013;38:E11. 10.1503/jpn.130055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sub Laban T, Saadabadi A. Monoamine Oxidase Inhibitors (MAOI): StatePearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK539848/. [PubMed]
- 38. Ribeiro L, Busnello JV, Kauer-Sant’Anna M, et al. Mirtazapine versus fluoxetine in the treatment of panic disorder. Braz J Med Biol Res 2001;34:1303-7. 10.1590/S0100-879X2001001000010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: additional material
Data Availability Statement
No additional data available.