Abstract
Objective:
South Africa has used antenatal HIV surveys for HIV surveillance in pregnant women since 1990. We assessed South Africa’s readiness to transition to programme data based antenatal HIV surveillance with respect to PMTCT uptake, accuracy of point-of-care rapid testing (RT) and selection bias with using programme data in the context of the 2017 antenatal HIV survey.
Methods:
Between 1 October and 15 November 2017, the national survey was conducted in 1,595 public antenatal facilities selected using stratified multistage cluster sampling method. Results of point-of-care RT were obtained from medical records. Blood samples were taken from eligible pregnant women and tested for HIV using immunoassays (IA) in the laboratory. Descriptive statistics were used to report on: PMTCT uptake; agreement between HIV point-of-care RT and laboratory-based HIV-1 IA; and selection bias associated with using programme data for surveillance.
Results:
PMTCT HIV testing uptake was high (99.8%). The positive percent agreement (PPA) between RT and IA was lower than the World Health Organization (WHO) benchmark (97.6%) at 96.3% (95% confidence interval (CI): 95.9%–96.6%). The negative percent agreement was above the WHO benchmark (99.5%), at 99.7% (95% CI: 99.6%–99.7%) nationally. PPA markedly varied by province (92.9%–98.3%). Selection bias due to exclusion of participants with no RT results was within the recommended threshold at 0.3%.
Conclusion:
For the three components assessed, South Africa was close to meeting the WHO standard for transitioning to routine RT data for antenatal HIV surveillance. The wide variations in PPA across provinces should be addressed.
Keywords: Pregnant women, Surveillance, Test agreement, PMTCT, Rapid testing, South Africa
Introduction
South Africa has the biggest HIV epidemic in the world with 7.9 million people living with HIV in 2017 (Simbayi et al., 2017). HIV surveillance data provides key strategic information for effective and focused response to HIV. In the past decades, antenatal HIV sentinel surveillance surveys have been used to provide HIV prevalence data needed to monitor the HIV epidemic at national and sub-national levels.
World Health Organization (WHO) recommends, where countries have near universal antenatal care (ANC) attendance and prevention of mother-to-child transmission (PMTCT) coverage, and the socio-demographic and HIV testing data is rigorously captured in routine recording and reporting tools, the utility of routine programme data for antenatal surveillance should be assessed as this will enable to optimize the use of existing resources for surveillance (WHO, 2015a; WHO, 2013).
This (WHO) guideline recommends that five elements of the PMTCT programme be assessed before transitioning to PMTCT programme data based antenatal surveillance. These are: (1) PMTCT HIV testing uptake (2) agreement between point-of-care (POC) rapid testing (RT) and laboratory based immunoassays (IA); (3) potential selection bias due to missing and undocumented RT results; (4) quality of routinely collected PMTCT data and (5) the state of quality assurance (QA) practice (WHO, 2013).
South Africa has high antenatal service coverage (94%) (National Department of Health (NDOH), 2017). However, findings from a national study conducted in 2015 to assess the 4th and 5th WHO-recommended core components of the PMTCT programme showed major gaps in data quality, record keeping and rapid test QA procedures (Nsibande et al., 2018). With regards to agreement between HIV POC RT and laboratory-based HIV-1 IA, a few sub-national level studies, that have been conducted to compare RT with laboratory-based IA tests, have shown routine PMTCT RT may under perform in the field when administered by nurses, compared to laboratory based IA testing (Bassettet al., 2011; Moodleyet al., 2008). However, none of these studies were adequately powered to provide estimates that can be generalized at national level.
This study assessed South Africa’s readiness to transition to programme data based antenatal HIV surveillance with respect to three of the five WHO-recommended core components of the PMTCT programme i.e. PMTCT uptake, agreement between HIV POC RT and laboratory-based HIV-1 IA and selection bias with using programme data. This assessment was piggy backed onto the 2017 antenatal survey (the antenatal survey objectives and data collection procedures are briefly discussed in the section below – detailed discussion of the survey methods can be found in the main report (Woldesenbet et al., 2018a)). Two questions, i.e. participant’s uptake of antenatal HIV testing and participant’s HIV rapid test result, were added onto the antenatal survey questionnaire for the purpose of this assessment. The two WHO-recommended components, quality of routinely collected PMTCT data and the state of QA practice, were not assessed in this study as a national evaluation had been conducted prior to this survey (in 2017) to assess these two components (Nsibande et al., 2018).
Methodology
Study setting
South Africa has conducted National Antenatal Sentinel HIV Prevalence Surveys since 1990 (NDOH, 2015). Between 1990 and 2015, the survey was conducted annually to track HIV prevalence among reproductive age women. In 2017, four new indicators- HIV incidence, knowledge of HIV status, antiretroviral (ARV) drug levels, and viral load status- were introduced into the survey in order to provide estimates of new HIV infections and measure the coverage and effectiveness of the antiretroviral therapy(ART) programme, in addition to an assessment of the country’s readiness to use routine program data for HIV surveillance.
Study design
Cross sectional design. As part of the 2017 South African Antenatal Sentinel HIV Survey, this study compared individual-level agreement between the most recent POC RT carried out as part of ANC and laboratory-based HIV-1 immunoassays (IA) performed in the 2017 antenatal survey (both tests were performed on the same individuals).
Description of the 2017 ANC survey
The South African Antenatal HIV Sentinel Survey is a cross-sectional survey, conducted every 1–2 years, to measure HIV prevalence among pregnant women attending ANC at public health care facilities in South Africa (Woldesenbet et al., 2018a). The 2017 survey had a planned enrolment of 36,015 pregnant women, aged 15–49 years, from 1,595 public facilities selected from across all 52 districts of South Africa. Public facilities for the survey were selected using stratified multistage cluster sampling method and probability proportional to size (PPS) sampling method.
Data collection
Data collection for the survey was conducted between 1 October and 15 November 2017. Data were collected by health workers as they provided routine ANC services during the survey period. The data collection procedures included obtaining written informed consent, a brief interview, data abstraction from medical records, and collection of a blood specimen from each consecutive pregnant women attending any ANC visit (either for first or follow-up ANC visit) during the survey period. Demographic and clinical information extracted from medical records of enrolled women included age of the woman, HIV status (per POC RT) at first-ANC-visit or enrolment in the survey, HIV status prior to the current pregnancy for those initiated on ART prior to pregnancy, and timing of ART initiation. For participants receiving routine HIV testing on the day of the survey, the HIV status (per POC RT) data were extracted immediately after POC RT was performed, and the specimen for IA was collected on the same visit. For participants who did not receive HIV testing on the day of the survey (including participants already on ART prior to pregnancy) the last HIV test result was extracted from the medical record. A blood specimen was taken from each woman regardless of prior knowledge of HIV status or ART history, and tested for HIV and viral load in the laboratory. Detailed description of site selection criteria, sampling of women, the data collection procedures and training provided to nurses prior to data collection is presented elsewhere (Woldesenbet et al., 2018a).
Specimen testing for HIV
Figure 1a and b shows the HIV testing algorithm used for the POC RT and the laboratory-based IA tests respectively.
Figure 1.
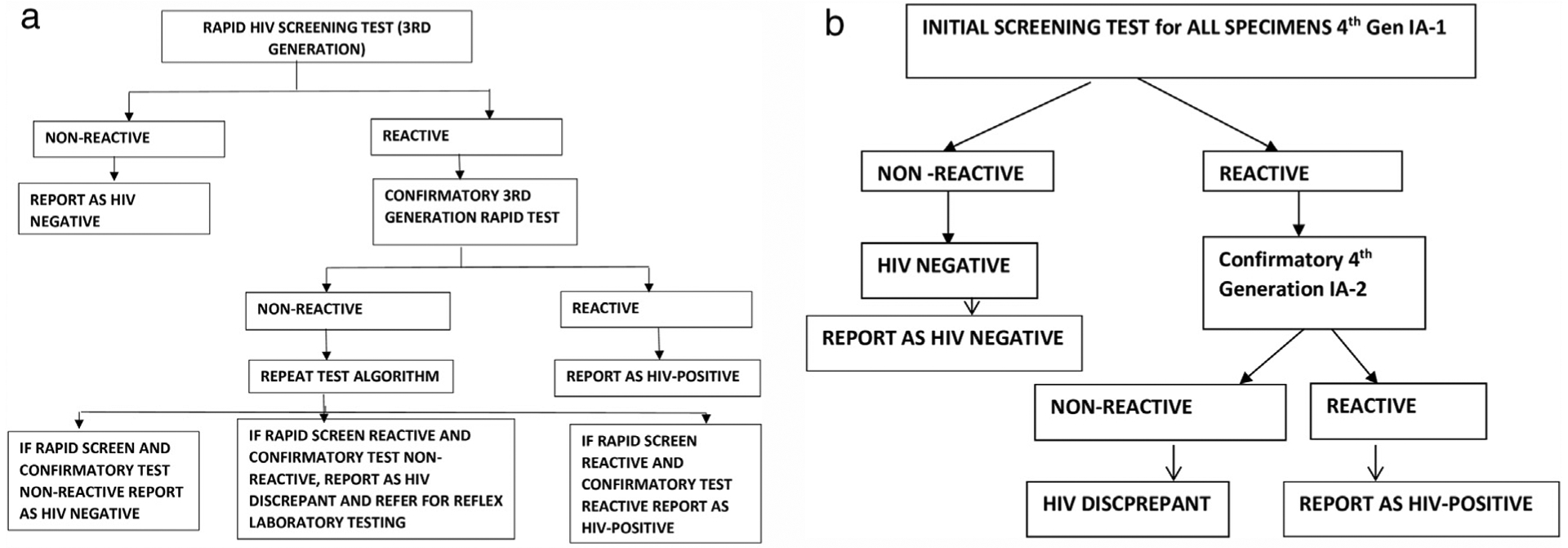
(a) HIV testing algorithm for point-of-care routine rapid testing, South Africa. (b) The laboratory HIV testing algorithm for the Antenatal Sentinel HIV Survey, 2017, South Africa.
Point-of-care rapid testing
Nurses performed the RT either on the day of the survey or at a prior ANC visit during the current pregnancy. In South Africa, the PMTCT programme is integrated into ANC services. According to the guidelines in place at the time of the survey, all women attending first-ANC-visit in their current pregnancy were to be offered HIV RT unless they were already on ART. For women already initiated on ART prior to pregnancy, the POC RT would have been performed before pregnancy and therefore no POC RT was performed during the survey. As per guidelines, women testing negative at first-ANC-visit in the current pregnancy were meant to be re-tested every 3 months during pregnancy and postpartum period (NDOH, 2016). These tests were performed according to the national guidelines using a serial algorithm as indicated in Figure 1a (NDOH, 2016). After the tests were performed, the nurses documented each result on the patient medical record, and the result was extracted from the medical record to the survey-specific data collection form.
Laboratory-based IA testing
At the seven National Health Laboratory Service (NHLS) regional laboratories selected to participate in the survey, specimens were tested for presence of HIV antibodies and antigens using a serial algorithm that consisted of two fourth generation IA platforms (Figure 1b and Supplementary Table 1). All participating NHLS laboratories were South African National Accreditation System (SANAS) accredited based on ISO15189-2012 and/or had NHLS compliance audit score of >80% and had passed the third NHLS HIV proficiency testing panel for the 2016–2017 period. Standard HIV testing strategies as outlined in the 2016 guideline were used. Plasma specimens that were non-reactive on IA-1 were classified as HIV negative and were not tested further. All specimens that were reactive on IA-1 were further tested using the confirmatory assay (IA-2). If specimens were reactive on IA-2 they were classified as HIV-positive. If IA-2 was non-reactive, the specimen was considered to have a “discrepant” HIV result.
The final IA test results were returned to participants if they were unaware of their HIV status or if there was a discrepancy between the results of the survey-provided laboratory test and the routine rapid test.
Data management
Data collected on the survey-specific data collection forms were captured in district health information system (DHIS) by data clerks. All HIV screening, confirmatory, and final HIV test results were captured into the NHLS laboratory information management system (TrakCare) and exported to Microsoft Excel (2016 version). The laboratory data were then merged with the interview and record review data captured on DHIS, using STATA® 14 (Stata Corporation, College Station, TX) for data cleaning and analysis.
The final database excluded observations for participants outside the age range of 15–49 years, participants with missing laboratory test results, or rapid test results, with rejected or lost specimens, and those with equivocal or unconfirmed (discrepant) HIV test results either in the POC RT or in the IA testing.
Description of outcomes and data analysis
Data analysis took into account the survey design (clustering within health facilities, and stratification by district). Data were post-stratified (benchmark weighted) to the Statistics South Africa (Stats SA) 2017 mid-year population size of women of reproductive age (15–49 years) at provincial level, to adequately represent provinces with fewer ANC clients (Stats-SA, 2017). Given that sites were sampled using PPS, and that the sampling period was fixed, this provided a self-weighted sample at district level. A population-finite correction factor was added, to adjust for the >5% of primary sampling units sampled without replacement from a finite population of about 4,000 public facilities.
Descriptive analyses included description of the survey sites and baseline demographic and clinical characteristics of participants. The following WHO-recommended core components of PMTCT surveillance assessment were measured:
positive percent agreement (PPA) and negative percent agreement (NPA) were defined as the percentage of IA-positive and IA-negative participants who had an HIV-positive and HIV negative result respectively on POC RT. PPA and NPA were reported at national, provincial and district level.
PMTCT HIV testing uptake: defined as participants who accepted routine PMTCT HIV testing offered during antenatal visits over the total eligible antenatal survey participants with PMTCT uptake data, and
Selection bias due to missing data collection forms or RT results was calculated using the following WHO-recommended formula:
A sensitivity analysis was performed restricting the test agreement (NPA and PPA) analysis to ART-naive participants in order to exclude participants who had taken ART for long durations. Stratified analysis was performed to assess differences in PPA and NPA by ANC numbers (large facilities with annual ANC numbers of ≥250 versus facilities with annual ANC numbers of <250 clients), geographical type (rural, urban, and semi-urban), participant age (15–24 years versus 25–49 years), and ANC visit type (first-ANC-visit in current pregnancy versus follow-up visit). Two sided 95% CIs were reported for prevalence, PPA and NPA.
We also characterised the viral load levels (of IA-positive, RT-negative participants) and ART status (of IA-negative, RT-positive participants) of participants with discrepant IA and RT result to assess if very early stage of acute infections or long duration of ART would explain the discrepancy between IA and rapid test results.
Sample size considerations
With the planned sample size for the 2017 survey, it was possible to estimate PPA and NPA between POC RT and laboratory-based IA within a precision of 1.2% and 0.5% respectively, at the provincial level. This precision was calculated assuming expected PPA and NPA of 97% and 99.5% respectively at the province level, with 95% confidence interval (CI), a design effect of 1.5 and 10% error rate. All eligible women who participated in the 2017 antenatal survey, regardless of prior knowledge of HIV status, were included in this analysis.
Results
Thirty-six thousand one hundred twenty-eight (36,128) participants were enrolled in the survey. Sixty-five (0.2%) of the participants fell out of the age range (15–49 years) for inclusion in the survey. RT results were not documented in the data collection form for 2.8% (1,020) of participants, and 2.8% (1,000) of the data collection forms were missing. From clinic records, thirty-four thousand forty-three (34,043) participants had PMTCT data available; 99.8% (33,977) accepted PMTCT HIV testing; 30.6% were HIV-positive; 69.3% HIV-negative and 0.1% inconclusive per the RT result (unweighted data) (Figure 2). IA tests were performed in 93.6%, 93.9%, and 100% of rapid test negative, positive, and inconclusive specimens respectively. About 0.3% of the specimens had a discrepant result on IA test and these were excluded. In the final sample, 31,748 (87.9%) participants with HIV-positive or negative result in both RT and IA test were included in the test agreement analysis.
Figure 2.
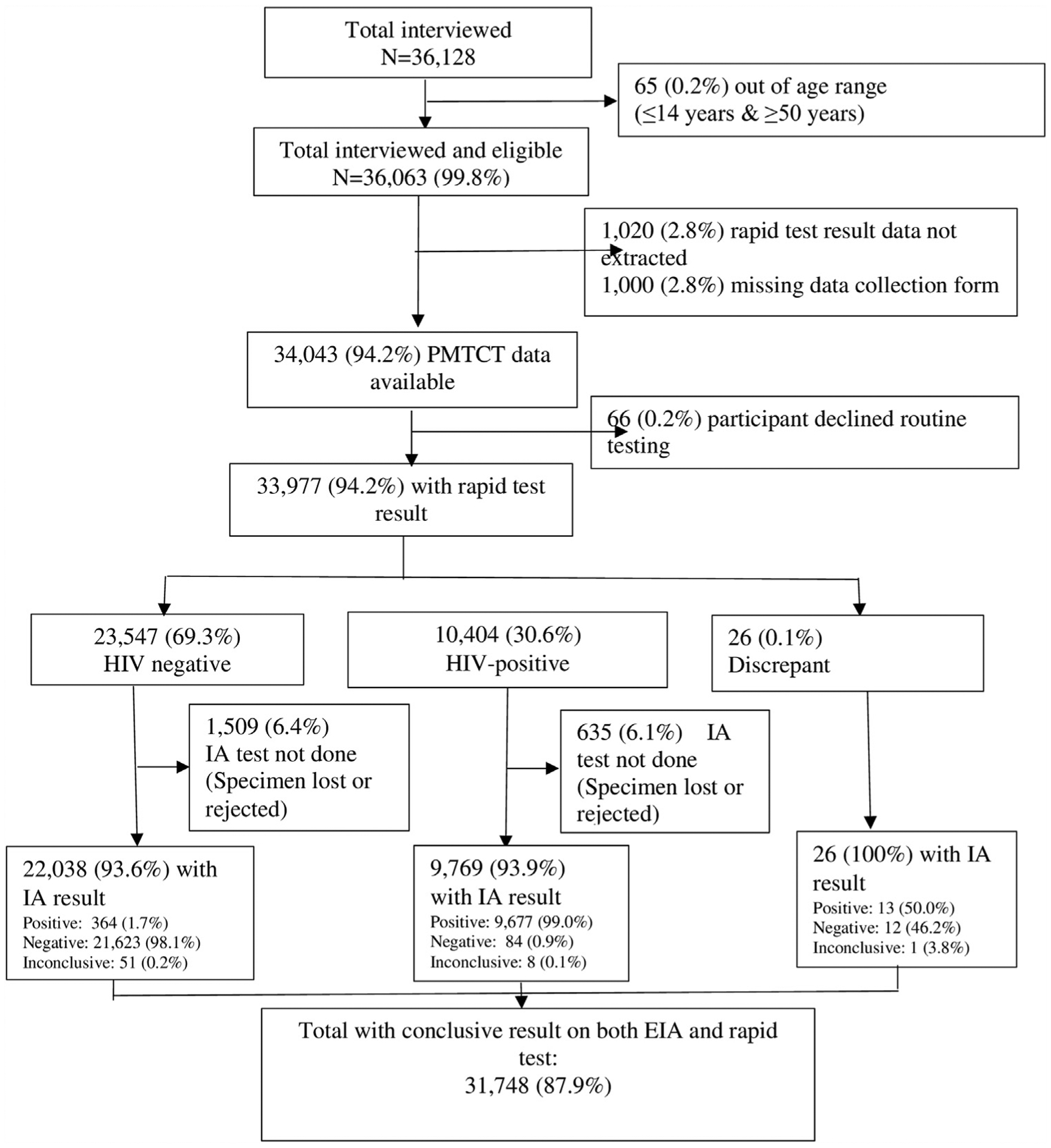
Flow chart of participants tested and excluded from the test agreement analysis: the 2017 Antenatal Sentinel HIV Survey, South Africa.
Demographic and clinical characteristics of participants
The provincial number of participants included in this analysis ranged between 1,497 (4.7%) in Northern Cape to 8,108 (25.5%) in KwaZulu-Natal. The majority (61.2%) of participants were enrolled from urban clinics, while 30.6% and 8.2% of participants were enrolled from rural and peri-urban clinics respectively. The median age of participants was 26 years (IQR: 21–31years). A third of participants (33.3%) were primigravida (data not presented in table).
The specimens for both IA and rapid test were collected on the same day for 67.3% of RT-negative participants; for the remaining 32.7% RT-negative participants, RT was performed in a prior ANC visit during the current pregnancy (data not presented in table).
Of 9,532 RT- positive participants with ART information, 59.3% and 29.2% were already initiated on ART before and after pregnancy, respectively, and 11.3% were ART naïve (data not presented in table).
HIV prevalence was 29.8% (95% CI: 29.1%–30.4%) nationally per the RT result, as opposed to 30.7% (95% CI: 30.1%–31.3%) per the laboratory-based IA test (Table 1). Across provinces, HIV prevalence measured by the RT was consistently lower than the prevalence measured by the IA test. This difference was highest (2.0%) in Mpumalanga and lowest in KwaZulu-Natal (0.4%) and North West (0.4%). However, the confidence intervals of the two estimates overlap in all provinces and nationally showing none of these differences are statistically significant.
Table 1.
Provincial variation of HIV prevalence per point-of-care rapid test and per laboratory-based immunoassay test, the 2017 Antenatal Sentinel HIV Survey, South Africa.
| Provinces | HIV prevalence | |
|---|---|---|
| POC RT % (95% CI) |
IA % (95% CI) |
|
| Eastern Cape | 31.8 (30.2–33.4) |
33.7 (32.2–35.3) |
| Free State | 31.8 (30.1–33.5) |
32.7 (31.1–34.4) |
| Gauteng | 31.2 (29.8–32.7) |
32.2 (30.7–33.6) |
| KwaZulu-Natal | 40.7 (39.5–41.9) |
41.1 (39.9–42.3) |
| Limpopo | 22.6 (20.9–24.3) |
23.4 (21.8–25.1) |
| Mpumalanga | 35.3 (33.4–37.3) |
37.3 (35.4–39.2) |
| Northern Cape | 16.7 (14.8–18.8) |
17.9 (16.0–20.1) |
| North West | 27.3 (25.2–29.5) |
27.7 (25.7–29.8) |
| Western Cape | 15.2 (13.4–17.2) |
15.9 (14.2–17.8) |
| South Africa |
29.8
(29.1–30.4) |
30.7
(30.1–31.3) |
The data presented in this table have been weighted. The denominator for the POC rapid test was n = 33,951 participants with conclusive rapid test result (it excludes rapid test discrepant result but includes participants whose IA tests were lost, rejected, or inconclusive). The denominator for IA was n = 32,716 participants with conclusive IA result (it excludes IA discrepant result but includes participants with missing or discrepant rapid test result. Participants with IA low-positive result were categorized as HIV-positive). IA: immunoassay; POC RT: Point-of-care rapid testing.
Selection bias due to the exclusion of participants with no RT result was 0.3% [(30.8%–30.7%)/30.8%]. Overall, 1.4% (448) of participants had a discrepant result between rapid test and IA test, ranging from 0.8% in Western Cape to 2.5% in Mpumalanga (data not presented in table).
As illustrated in Table 2, the PPA was 96.3% (95% CI: 95.9%–96.6%) nationally, ranging from 92.9% in Northern Cape to 98.3% in KwaZulu-Natal. The NPA was 99.7% (95% CI: 99.6–99.7%) at national level, ranging from 99.2% in North West to 99.8% in Northern Cape, Free State, Gauteng, and Western Cape. The NPA was higher than the PPA in all nine provinces. At the district level, the median PPA and NPA were 96.2% (IQR: 94.1%–97.7%) and 99.7% (IQR: 99.3%–100%), respectively.
Table 2.
Agreement between point-of-care rapid test and laboratory-based immunoassay testing, the 2017 Antenatal Sentinel HIV Survey, South Africa.
| National | Eastern Cape | Free State | Gauteng | KwaZulu Natal | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Laboratory-based testing | |||||||||||||||
| POC testing | HIV+ | HIV− | Total | HIV+ | HIV− | Total | HIV+ | HIV− | Total | HIV+ | HIV− | Total | HIV+ | HIV− | Total |
| HIV+ | 9,677 | 84 | 9,761 | 1,254 | 7 | 1,261 | 849 | 4 | 853 | 1,463 | 6 | 1,469 | 3,270 | 28 | 3,298 |
| HIV− | 364 | 21,623 | 21,987 | 69 | 2638 | 2,707 | 24 | 1,806 | 1,830 | 49 | 3,184 | 3,233 | 56 | 4,754 | 4,810 |
| Total | 10,041 | 21,707 | 31,748 | 1,323 | 2,645 | 3,968 | 873 | 1,810 | 2,683 | 1,512 | 3,190 | 4,702 | 3,326 | 4,782 | 8,108 |
| PPA = 96.3% (95% CI: 95.9%–96.6%) | PPA = 94.8% (95% CI: 93.7%–95.7%) | PPA = 97.3% (95% CI: 96.3%–98.0%) | PPA = 96.8% (95% CI: 95.3%–97.5%) | PPA = 98.3% (95% CI: 97.9%–98.7%) | |||||||||||
| NPA = 99.7% (95% CI: 99.6–99.7%) | NPA = 99.7% (95% CI: 99.5%–99.9%) | NPA = 99.8% (95% CI: 99.6%–99.9%) | NPA = 99.8% (95% CI: 99.6%–99.9%) | NPA = 99.4% (95% CI: 99.2%–99.6%) | |||||||||||
| Limpopo | Mpumalanga | Northern Cape | North West | Western Cape | |||||||||||
| Laboratory-based testing | |||||||||||||||
| POC testing | HIV+ | HIV− | Total | HIV+ | HIV− | Total | HIV+ | HIV− | Total | HIV+ | HIV− | Total | HIV+ | HIV− | Total |
| HIV+ | 537 | 13 | 550 | 985 | 5 | 990 | 247 | 3 | 250 | 543 | 12 | 555 | 529 | 6 | 535 |
| HIV− | 33 | 1,856 | 1,889 | 65 | 1,748 | 1,813 | 19 | 1,228 | 1,247 | 27 | 1,453 | 1,480 | 22 | 2,956 | 2,978 |
| Total | 570 | 1,869 | 2,439 | 1,050 | 1,753 | 2,803 | 266 | 1,231 | 1,497 | 570 | 1,465 | 2,035 | 551 | 2,962 | 3,513 |
| PPA = 94.2% (95% CI: 92.5%–95.6%) | PPA = 93.8% (95% CI: 92.4%–95.0%) | PPA = 92.9% (95% CI: 89.8%–95.1%) | PPA = 95.3% (95% CI: 93.4%–96.6%) | PPA = 96.0% (95% CI: 94.3%–97.2%) | |||||||||||
| NPA = 99.3% (95% CI: 98.9%–99.5%) | NPA = 99.7% (95% CI: 99.2%–99.9%) | NPA = 99.8% (95% CI: 99.4%–99.9%) | NPA = 99.2% (95% CI: 98.5%–99.5%) | NPA = 99.8% (95% CI: 99.7%–99.9%) | |||||||||||
Percent agreement weighted.
Restricting the NPA analysis to ART-naive participants at the time of the IA test did not substantially improve the NPA [99.9% nationally (99.9%–100% across provinces)].
Facilities with ANC client number ≥250 had lower PPA (95.8%; 95% CI: 95.3%–96.2%) compared with facilities with <250 ANC number annually (97.4%, 95% CI: 96.9%–97.8%). The PPA was significantly lower in the younger age group (15–24 years) (95.1%, 95% CI: 94.2%–95.9%) and among first-ANC-visit attendees (95.3%, 95% CI: 94.6%–95.9%) compared with older participants (25–49 years) (96.8%, 95% CI: 96.4%–97.1%) and follow-up visit attendees (96.8%, 95% CI: 96.5%–97.2%) respectively.
NPA did not substantially vary by size of facility, age, or visit type. PPA and NPA did not substantially vary by geographical type.
Of 84 participants with RT-positive, and IA-negative results, 65% (57) were on ART, 21% (18) had not started ART, and 14% (9) had missing response for the ART question at enrolment.
Viral load results were available for 95% (347 of the 364) of participants with IA-positive, RT-negative results. Of the 347 IA-positive, RT-negative participants with viral load data, 31.1% (109) had undetectable viral load (<50 copies/mL), 35.7% (127) had viral load 50–1,000 copies/ml, and 33.2% (111) had viral load above 1,000 copies/ml. More than half (53.1%) of the 364 IA-positive, RT-negative participants were follow-up visit attendees.
Discussion
We aimed to assess South Africa’s readiness to transition to programme data based antenatal HIV surveillance with respect to PMTCT uptake, HIV testing agreement and selection bias in the context of the 2017 ANC HIV survey. Overall we found high PMTCT uptake at 99.8%, high overall agreement between third-generation POC RT and laboratory-based fourth-generation IA test and low selection bias due to the exclusion of participants with no RT result. The WHO-recommended PPA and NPA for transitioning to routine programme data for antenatal surveillance is 97.6% and 99.5% respectively (WHO, 2013). The PPA in this study was very close to this WHO benchmark at national level, with provincial variations. The PPA was lower in large facilities serving 250 or more ANC clients annually. The WHO benchmark for the NPA was met at the national level and in six of the nine provinces. Selection bias due to exclusion of participants with no RT results was within the recommended ±10% WHO threshold.
Altogether, these findings suggest that with strong QA measures in place to improve the PPA in low-performing provinces/facilities, South Africa could be ready to use antenatal POC RT data for tracking HIV prevalence among pregnant women. This would eliminate the need to collect separate blood specimens to determine HIV prevalence and reduce the cost of duplicate testing (WHO, 2015b).
Compared to the PPA (89.6%) and NPA (98.9%) reported in the 2015 South African antenatal survey for six of the nine provinces (unpublished data), the PPA and NPA reported in this study was higher (Goga et al., 2018). The difference could be due to improvements in QA processes following the roll out of the RT QA initiative (RTQII) which was launched in January 2016 and scaled-up in more than 2,000 facilities, between 2016 and 2017, in the 27 high-burden districts of South Africa (Woldesenbet et al., 2018b). QA interventions should continue to be strengthened particularly in facilities with high numbers of ANC clients and in relatively low-performing provinces. The quality of HIV testing services is often constrained by underlying health system factors such as staff shortages, high staff turnover, managerial gaps, and heavy workloads (Jaya et al., 2017). QA interventions should move beyond provision of training and address these underlying factors in order to create an enabling environment for sustainable implementation of QA processes.
Compared to similar studies conducted in Kenya and Brazil, the PPA in this study was high. (Sirengo et al., 2016; Pereira et al., 2017). Rwanda is the only sub-Saharan African country that is ready to transition to PMTCT programme data for antenatal surveillance. Rwanda reported PPA and NPA of 97.5% and 99.9% respectively at the site level (Balisanga et al., 2016).
The performance of the RT (the PPA) in this study could have been partially affected by the lower sensitivity of the third-generation RT to detect infections during the window period as compared to the 4th-generation IA test used in the survey (Tan et al., 2016). Analysis of the virologic status of RT-negative, IA-positive participants showed that although all RT–negative, IA-positive participants were ART naïve, a significant proportion of these participants had undetectable (<50 copies/ml) (31.1%) or low (50–1,000 copies/ml) viral load (35.7%). These participants may represent participants who were at the early stage of acute infection or elite controllers. Nonetheless, discrepancies in results were also observed among participants with high viral loads; therefore, the low performance of RT is likely to be attributable to both inaccuracies in diagnostic procedures and the lower sensitivity of RT for recent infection.
The high NPA in this study gives reassurance that fewer women would be inaccurately diagnosed as HIV-positive. In the universal test and treat era, this is of high importance as false-positive diagnosis leads to an inappropriate initiation of ART. In this study there were few IA negative individuals (0.3%, 57) who were misdiagnosed and incorrectly placed on treatment.
The actual date when the RT was performed was not documented for HIV-positive participants in this survey. Because of this, the PPA and NPA analysis was not restricted to specimens drawn on the same day. Seroconversion could have occurred in between RT performed in previous visit and the IA test, which may result in under estimation of the performance (PPA) of the RT. Similar method has been used in other studies assessing agreement between RT and IA tests and the utility of this data for surveillance (Pereira et al., 2017; Balisanga et al., 2016).
The performance of both RT and IA in this study was unlikely to be affected by exposure to pre-exposure prophylaxis (PrEP) as the roll out of PrEP in South Africa at the time of the survey was focused on key populations. The algorithm of the laboratory-based IA test performed in this study did not include a confirmatory test (such as Western blot), however, western blot test was performed in a subsample (n = 1722) of IA positive samples which showed a very small percentage of true HIV negative samples (0.1%) would be incorrectly diagnosed as HIV-positive with the IA test.
The other two components not assessed during the 2017 survey are equally important for routine programme data based surveillance. These are availability of high quality PMTCT data and QA practice. The quality of RT data in this study may not represent quality of data in the routine system as additional support (training and supervision) was provided during the survey to ensure the collection of good quality data. A study conducted prior to this survey to assess quality of PMTCT data and QA practice in South Africa reported large gaps in both the quality of PMTCT HIV testing data and QA practice including lack of standardised data recording system, and poor compliance to quality control and test assay procedures (Nsibande et al., 2018). It is crucial to address these gaps in order to ensure availability of accurate and valid RT data for surveillance.
In conclusion, for the three WHO-recommended components assessed in this study, South Africa was close to meeting the WHO standard for transitioning to routine rapid HIV testing data for antenatal surveillance. In the studies conducted to assesses the other two WHO-recommended components, quality of PMTCT data was reported to be low and gaps in QA procedures were identified (Nsibande et al., 2018). Strong measures should be put in place to address the gaps in data quality and QA procedures as well as the wide variations in PPA across provinces.
Supplementary Material
Acknowledgements
We thank Mr. Calle Hedberg and his team, from Health Information system Programme (HISP), for managing data capturing, validation and part of data cleaning; and Ms. Beverley Singh (NICD) and Mr. Henry Julius (NICD) for providing technical support in all laboratory aspects of the survey.
Our appreciation goes to the provincial coordinators: Mr. Z. Merile (Eastern Cape), Ms. M. Nophale, Ms M. Mothibi (Free State), Dr. B. Ikalafeng (Gauteng), N. Mbana, Ms. N. Moodley, Ms O. Mhlongo (KwaZulu-Natal), Ms. F. Ngobeni, Ms. M. Monwa (Limpopo), Mr. J. Sigudla, Dr. T. Mona (Mpumalanga), Mr. M. Khumalo (Northern Cape), Ms. M. Motswasele, Ms. N. Mangonyane (North West), Ms. V. Mudaly, Mr. M. Pere (Western Cape) and their teams who coordinated the survey in their respective provinces and districts. Sincerest gratitude is also extended to the National Health Laboratory Services (NHLS) and NICD testing laboratories and coordinators: Ms. B. Singh, Ms Z. Brukwe (NICD, Gauteng), Ms H. Vilakazi, Mr P. Moyeni, Mr B Tembe (Eastern Cape), Ms H. Potgieter, Mr M. D. Morobadi, Ms M. Nkonyane (Free State), Ms I. Chetty, Mr M. Ellapen (Inkosi Albert Luthuli Hospital, University of KwaZulu-Natal), Ms. R. Diokana, Mr J. Ngwenya (Dr George Mukari, Limpopo and North West), Mr I. Mofokeng (Rob Ferreira Nelspruit, Mpumalanga), and Ms. T. Stander, Ms L.Wilsdorf (Tygerberg, Western Cape and Northern Cape).
Funding source
This project has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) [under the terms of cooperative agreement=5 NU2GGH001631], World Health organization (WHO), South African Medical Research Council (SAMRC), National Department of Health (NDoH), and National Institute for Communicable Diseases (NICD).
Footnotes
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the funding agencies.
Ethical approval
Ethical approval was obtained from the University of the Witwatersrand Human Research Ethics Committee (Medical) and the nine provincial health research ethics committees. The study was also reviewed in accordance with the United States (US) Centers for Disease Control and Prevention (CDC) human research protection office and determined to be research but CDC investigators did not interact with human subjects or have access to identifiable data or specimens for research purposes.
Competing interest
None.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ijid.2019.11.005.
References
- Balisanga H, Mutagoma M, Remera E, Kayitesi C, Kayirangwa E, Dee J, et al. HIV surveillance in Rwanda: readiness assessment to transition from antenatal care-based to prevention of mother-to-child transmission program-based HIV surveillance. Int J Infect Dis 2016;52:62–7. [DOI] [PubMed] [Google Scholar]
- Bassett IV, Chetty S, Giddy J, Reddy S, Bishop K, Lu Z, et al. Screening for acute HIV infection in South Africa: finding acute and chronic disease. HIV Med 2011;12(1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goga A, Nsibande D, Cheyip M, Pillay Y. Antenatal survey versus routine PMTCT HIV test results in at provincial and district levels in six provinces of South Africa Unpublished. 2018.
- Jaya Z, Drain PK, Mashamba-Thompson TP. Evaluating quality management systems for HIV rapid testing services in primary healthcare clinics in rural KwaZulu-Natal, South Africa. PLoS One 2017;12(8)e0183044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodley D, Moodley P, Ndabandaba T, Esterhuizen T. Reliability of HIV rapid tests is user dependent. South Afr Med J 2008;98(9):707–9. [PubMed] [Google Scholar]
- National Department of Health (NDOH), Statistics South Africa (Stats SA), South African Medical Research Council (SAMRC) and ICF. South African demograhic and health survey 2016: key indicators report statistics South Africa. Pretoria, South Africa and Rockville Maryland, USA: Available from: https://www.statssa.gov.za/publications/Report%2003-00-09/Report%2003-00-092016.pdf. [cited 12/08/2018]. 2017. [Google Scholar]
- NDOH. The National Antenatal Sentinel HIV prevalence Survey, South Africa, 2013. Civitas Building, Corner Struben and Thabo Sehume Street, Pretoria: National Department of Health; 2015. Available from: https://www.health-e.org.za/wp-content/uploads/2016/03/Dept-Health-HIV-High-Res-7102015.pdf. [cited 16/4/2017]. [Google Scholar]
- NDOH. National HIV testing services Policy. 2016. Available from: http://www.hst.org.za/sites/default/files/HTS%20Policy%2028%20July%20final%20copy.pdf. [cited 8/4/2018].
- Nsibande D, Ngandu N, Puren A, Woldesenbet S, Maduna V, Chirinda W, et al. Assessment of the utility of prevention-of-mother-to-child HIV transmission program data for HIV sentinel surveillance among pregnant women in South Africa Unpublished. MRC; 2018. [Google Scholar]
- Pereira GFM, Sabido M, Caruso A, Benzaken AS. Transitioning from antenatal surveillance surveys to routine HIV testing: a turning point in the mother-to-child transmission prevention programme for HIV surveillance in Brazil. BMC Infect Dis 2017;17(1):469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simbayi LC, Zuma K, Zungu N, Moyo S, Marinda E, Jooste S, et al. South African National HIV Prevalence, Incidence, Behaviour and Communication Survey. Cape Town: HSRC Pres; 2017. in press. [Google Scholar]
- Sirengo M, Rutherford GW, Otieno-Nyunya B, Kellogg TA, Kimanga D, Muraguri N, et al. Evaluation of Kenya’s readiness to transition from sentinel surveillance to routine HIV testing for antenatal clinic-based HIV surveillance. BMC Infect Dis 2016;16:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stats- SA. Mid-year population estimates. Statistical release P0302. Available from: https://www.statssa.gov.za/publications/P0302/P03022017.pdf. [cited 23/8/2018]. [Google Scholar]
- Tan WS, Chow EP, Fairley CK, Chen MY, Bradshaw CS, Read TR. Sensitivity of HIV rapid tests compared with fourth-generation enzyme immunoassays or HIV RNA tests. AIDS 2016;30(12):1951–60. [DOI] [PubMed] [Google Scholar]
- WHO. Guidelines for assessing the utility of data from prevention of mother-to-child transmission (PMTCT) programmes for HIV sentinel surveillance among pregnant women. UNAIDS/WHO Working Group on Global HIV/AIDS and STI Surveillance; 2013. Available from: www.who.int/about/licensing/copyright_-form/en/index.html. [cited 10/12/2019]. [PubMed] [Google Scholar]
- WHO. Consolidated strategic information guideline. Available from:. 2015. www.who.int/about/licensing/copyright_form/en/index.html.
- WHO. Conducting HIV surveillance based on routine programe data among pregnant women attending antenatal clinics. 2015. Available from: www.who.int. [cited 8/1/2019].
- Woldesenbet SA, Kufa T, Lombard C, Manda S, Ayalew K, Cheyip M, et al. The 2017 National Antenatal Sentinel HIV Survey, South Africa. National Department of Health; 2018. [Google Scholar]
- Woldesenbet S, Kalou M, Mhlongo D, Kufa T, Makhanya M, Adelekan A, et al. Reportof a 2-year phased implementation of a continuous quality improvement program for HIV rapid testing in South Africa Available at: http://www.nicd.ac.za/wp-content/uploads/2019/07/Antenatal_survey-report_24July19.pdf. NICD; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


