Key Points
Question
Is telomere length associated with mortality and development of specific diseases?
Findings
In this cohort study, UK Biobank data from more than 450 000 individuals found that reduced baseline leukocyte telomere length was associated with increased overall and various disease-specific mortalities. The study identified more than 200 disorders that were significantly overrepresented or underrepresented in participants with shorter leukocyte telomere length.
Meaning
The study findings suggest the relevance of telomere shortening for several diseases and warrant further mechanistic and therapeutic studies.
Abstract
Importance
Telomeres protect DNA from damage. Because they shorten with each mitotic cycle, leukocyte telomere length (LTL) serves as a mitotic clock. Reduced LTL has been associated with multiple human disorders.
Objective
To determine the association between LTL and overall as well as disease-specific mortality and morbidity.
Design, Setting, and Participants
This multicenter, community-based cohort study conducted from March 2006 to December 2010 included longitudinal follow-up (mean [SD], 12 [2] years) for 472 432 English participants from the United Kingdom Biobank (UK Biobank) and analyzed morbidity and mortality. The data were analyzed in 2021.
Main Outcomes and Measures
Hazard ratios (HRs) and odds ratios for mortality and morbidity associated with a standard deviation change in LTL, adjusted for age, sex, body mass index (calculated as weight in kilograms divided by height in meters squared), and ethnicity.
Results
This study included a total of 472 432 English participants, of whom 54% were women (mean age, 57 years). Reduced LTL was associated with increased overall (HR, 1.08; 95% CI, 1.07-1.09), cardiovascular (HR, 1.09; 95% CI, 1.06-1.12), respiratory (HR, 1.40; 95% CI, 1.34-1.45), digestive (HR, 1.26; 95% CI, 1.19-1.33), musculoskeletal (HR, 1.51; 95% CI, 1.35-1.92), and COVID-19 (HR, 1.15; 95% CI, 1.07-1.23) mortality, but not cancer-related mortality. A total of 214 disorders were significantly overrepresented and 37 underrepresented in participants with shorter LTL. Respiratory (11%), digestive/liver-related (14%), circulatory (18%), and musculoskeletal conditions (6%), together with infections (5%), accounted for most positive associations, whereas (benign) neoplasms and endocrinologic/metabolic disorders were the most underrepresented entities. Malignant tumors, esophageal cancer, and lymphoid and myeloid leukemia were significantly more common in participants with shorter LTL, whereas brain cancer and melanoma were less prevalent. While smoking and alcohol consumption were associated with shorter LTL, additional adjustment for both factors, as well as cognitive function/major comorbid conditions, did not significantly alter the results.
Conclusions and Relevance
This cohort study found that shorter LTL was associated with a small risk increase of overall mortality, but a higher risk of mortality was associated with specific organs and diseases.
This cohort study examines the association between leukocyte telomere length and overall as well as disease-specific mortality and morbidity.
Introduction
Telomeres are DNA structures at the end of chromosomes that protect them from damage and instability.1 In most cells, telomeres shorten with each cell cycle; thus, telomere shortening indicates the proliferative history of the cell.2 When telomeres become critically short, cells enter senescence cell cycle arrest or undergo apoptosis.2 Telomere attrition is largely associated with age and genetic determinants but is modulated by host-related genetic (such as male sex) as well as lifestyle factors (eg, smoking, physical activity, and stress).3 Leukocyte telomere length (LTL) serves as a biomarker for telomere length of the individual organism, and shortening of LTL has been associated with a broad range of pathologies, including lung, liver, hematologic, and cardiovascular diseases, as well as multiple cancer entities.4,5,6 Despite that, to our knowledge, the prognostic value of LTL remains to be comprehensively characterized,7 as most studies have either relatively small sample sizes, short follow-up duration, or insufficient power to detect clinically meaningful associations.
Using the well-characterized, community-based UK Biobank (UKB), which comprises the largest data set of directly measured LTL consisting of more than 472 000 participants, we analyzed the association between LTL and overall as well as disease-specific mortality. Additionally, we explored the association between LTL and 1847 phenomewide association study codes (phecodes) available in the data set. The outcomes of this approach suggest that participants with shorter telomeres are predisposed to specific organ dysfunctions, including cardiovascular, digestive, respiratory, musculoskeletal, or hematopoietic diseases. While these data highlight the association of proliferative history of leukocytes with specific human disorders, further studies are needed to dissect the underlying mechanisms.
Methods
Data and Code Availability
The data sets used in this study have not been deposited in a public repository but are available after approval of a reasonable application at https://www.ukbiobank.ac.uk. All participants provided written informed consent for the study. The UKB study has approval from the Northwest Multicenter research ethics committee.
UKB Participants
The UKB is a population-based cohort study conducted in the UK from March 2006 to December 2010 that recruited 502 511 volunteers aged 37 to 73 years at baseline. All participants were registered with the UK National Health Service and attended an initial examination, which was followed by a long-term follow-up that takes place continuously. All participants gave written informed consent for genotyping and data linkage to medical reports. For definition of outcomes and covariates, see eTable 1 in the Supplement.
Ongoing inpatient hospital records beginning in 1996 until May 2021 were used to identify diagnoses according to the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes. Participants who underwent stem cell transplant (Z94.8) before the baseline examination were excluded (n = 158). The UKB receives death notifications (age at death and primary ICD-10 diagnosis associated with death) through linkage to national death registries. End of follow-up was defined as death or end of hospital inpatient data collection in May 2021. Heavy alcohol consumption was defined as more than 60 g of alcohol per day for men and more than 40 g of alcohol per day for women, while moderate alcohol consumption was defined as more than 24 g of alcohol per day for men and more than 12 g of alcohol per day for women. The study has been approved by the UKB access committee (project 47527).
Telomere Measurements
The UKB extracted DNA from peripheral blood leukocytes as part of a cohortwide array genotyping project. Research staff at the University of Leicester (Leicester, England) conducted LTL measurements using the multiplex quantitative polymerase chain reaction (PCR) methods that were masked to phenotypic information. They compared the amplification of a telomere length PCR product (T) against a PCR product (S) of a reference single copy gene to produce a T/S ratio.8 T and S were calculated relative to a calibrator sample (pooled DNA from 20 individuals) that was included in every run. In this study, the loge-transformed z score–adjusted T/S ratio (ie, UKB data field 22 192) was used, which is the relative LTL adjusted for the effect of technical parameters. For the analyses using the first percentile of telomere length, the 1% of the participants with the shortest telomere length per each age/sex group were analyzed.
Phenomewide Association Study Analysis
The coding for clinical diagnoses in the data set followed the World Health Organization’s ICD-10 coding systems. For each study participant, ICD-10 codes from the electronic health record diagnoses throughout the study period were collated and duplicates removed. We converted the ICD-10 codes to 1847 associated phecodes using the PheWAS package, version 0.99.5.5.9 Using a continuous model, we examined the association of decreased telomere length (per SD) with the development of each phecode.
Primary Care Records
To ensure the validity of the hospital records, we repeated the analyses using the primary care records available for a subset of patients (more information available at https://www.ukbiobank.ac.uk). The CTV3 or Read, version 3, codes were converted to ICD-10 codes. In the confirmatory analysis, we included only phecodes that were diagnosed at least 1 year after the baseline examination.
Analysis of Genetic Proxies for LTL (rs10936599 and rs2736100)
For the first 50 000 participants, genotyping was performed using the UK BiLEVE Axiom array, while the Affymetrix UK Biobank Axiom array was used for the remaining 450 000 participants. Two common genetic proxies for LTL were analyzed (rs10936599 and rs2736100).10
Statistical Analysis
The results of continuous variables are presented as mean (SD) (normal distribution). All categorical variables were displayed as relative (%) frequencies and the corresponding contingency tables. Univariate analysis of variance was used to determine overall differences between the 4 quartiles of telomere length. The primary outcomes of this study were estimates of the probability of overall and cause-specific mortality per SD telomere shortening, adjusted for age, sex, ethnicity, and objectively measured body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) at baseline. Self-reported alcohol use and smoking behavior at baseline were included in further analyses.
For the competing risk analyses, we tabulated the numbers of death per ICD-10 code and compared them with those who were deceased of other causes as well as survivors. Hazard ratios (HRs) or odds ratios per SD of telomere shortening were presented with their corresponding 95% CIs. All multivariable analyses were adjusted for age, sex, ethnicity, and BMI at baseline. To account for major comorbidities, as well as physical and cognitive function, Charlson Comorbidity index11 (CCI) scores and Phenotypic Age (PhenoAge12) were incorporated (eTables 2 and 3 in the Supplement). The PheWAS analysis was performed using the PheWAS R package (R Foundation).9 For PheWAS and mortality analyses, Bonferroni correction was performed to account for multiple testing for the number of the major ICD-10 phecodes (1847) analyzed (P < .05). The reference groups in this association study for analyzing any specific ICD-10 code were participants without the specific ICD-10 phecode. Differences were considered to be statistically significant with a 2-tailed P < .05. The data were analyzed using R version, 4.0.2 (R Foundation for Statistical Computing); SPSS, version 26 (IBM); and Prism, version 8 (GraphPad). eFigures 1, 4, and 5 in the Supplement were generated with Biorender software.
Results
Association of Telomere Length With Overall and Cause-Specific Survival
The UKB data set comprises 472 432 individuals with valid LTL measurements (eFigure 1 in the Supplement). Their telomere length distribution is depicted in eFigure 2A in the Supplement. As expected, higher age, male sex, obesity, alcohol consumption, and smoking were associated with shorter LTL; however, all parameters displayed only a weak association with LTL (eTable 4; eFigure 2, B-D in the Supplement). During the median (IQR) follow-up of 12 (11-13) years, 32 491 participants died (mortality rate, 5.7 per 1000 person-years; eTable 4 in the Supplement). Baseline telomere length was significantly shorter in participants who died within the follow-up period (mean telomere length, −0.23) than in those who survived (mean telomere length, 0.02; P value adjusted for age, sex, BMI, and ethnicity, <.001). Compared with participants in the highest LTL quartile, individuals in the lowest LTL quartile displayed 76% higher mortality (eFigure 3 and eTable 4 in the Supplement). The univariate analysis revealed a significant increase in the overall mortality per SD telomere shortening (HR, 1.27; 95% CI, 1.25-1.29), and while the HR dropped after adjustment for age, it remained highly significant (HR, 1.12; 95% CI, 1.10-1.13). Even after adjustment for age, sex, BMI, and ethnicity, shorter LTLs still conferred a significantly elevated overall death rate (HR, 1.08; 95% CI, 1.07-1.09; eTable 5 in the Supplement). Inclusion of additional covariates had only a negligible association with the HR per SD telomere shortening (eTable 5 in the Supplement).
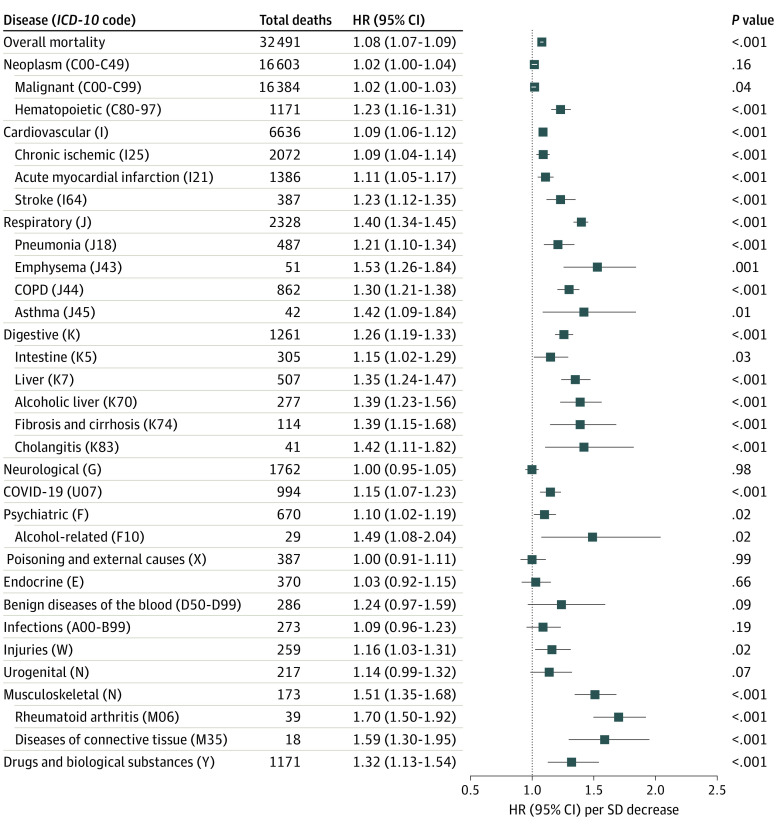
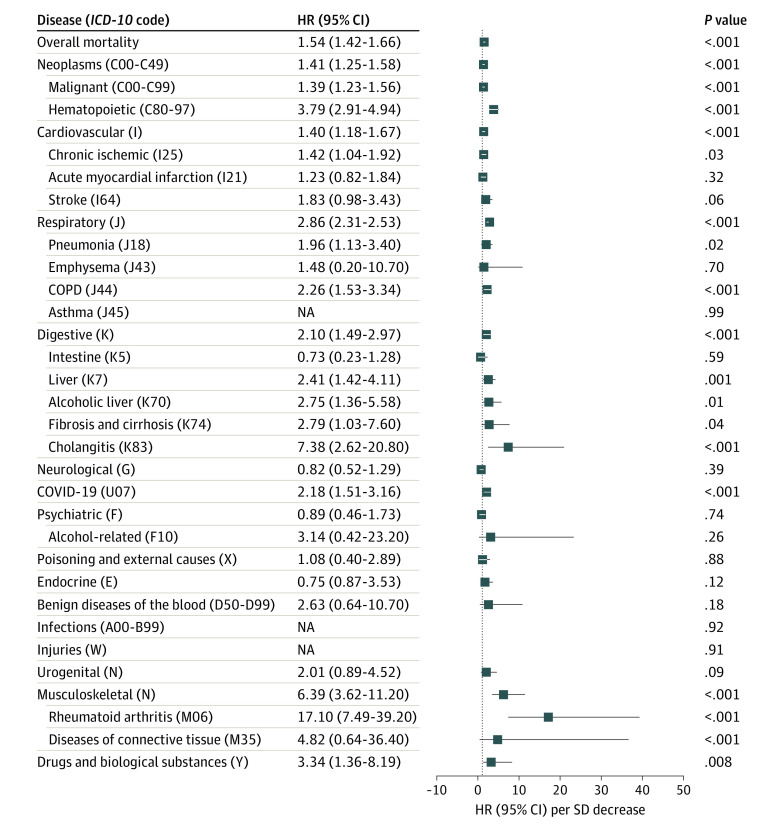
Shorter LTLs were associated with significantly increased organ-specific mortalities in the univariate analysis (eTable 6 in the Supplement). After adjustment for age, sex, BMI, and ethnicity, 1-SD shorter LTL was associated with a higher risk of death of cardiovascular (HR, 1.09; 95% CI, 1.06-1.12), respiratory (HR, 1.40; 95% CI, 1.34-1.45), digestive (HR, 1.26; 95% CI, 1.19-1.33), and musculoskeletal diseases (HR, 1.51; 95% CI, 1.35-1.68) as well as of COVID-19 (HR, 1.15; 95% CI, 1.07-1.23), but neither of cancer nor neurological disorders (Figure 1; eFigure 4 in the Supplement). Among the specific entities, a particularly high HR was seen for mortality because of emphysema, chronic obstructive pulmonary disorder, (alcoholic) liver disease, and rheumatoid arthritis (Figure 1). Additional adjustment for smoking, alcohol consumption, PhenoAge, and CCI score did not significantly change the associations of LTL with overall mortality or mortality because of specific diseases, as well as cancers (eTables 7 and 8 in the Supplement).
Figure 1. Association of Overall and Cause-Specific Mortality With Leukocyte Telomere Shortening Adjusted for Age, Sex, Body Mass Index (BMI), and Ethnicity.
All major International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) categories, as well as selected cause-specific mortalities, were analyzed. Hazard ratios (HRs) per ICD-10 codes were generated from competing risk regression models, accounting for the risk of death through other causes; HRs (95% CI) per SD decrease of telomere length are shown. COPD indicates chronic obstructive pulmonary disease. BMI was calculated as weight in kilograms divided by height in meters squared.
Overrepresentation of Respiratory, Hepatic, and Hematologic Phecodes in Individuals With Shorter LTL
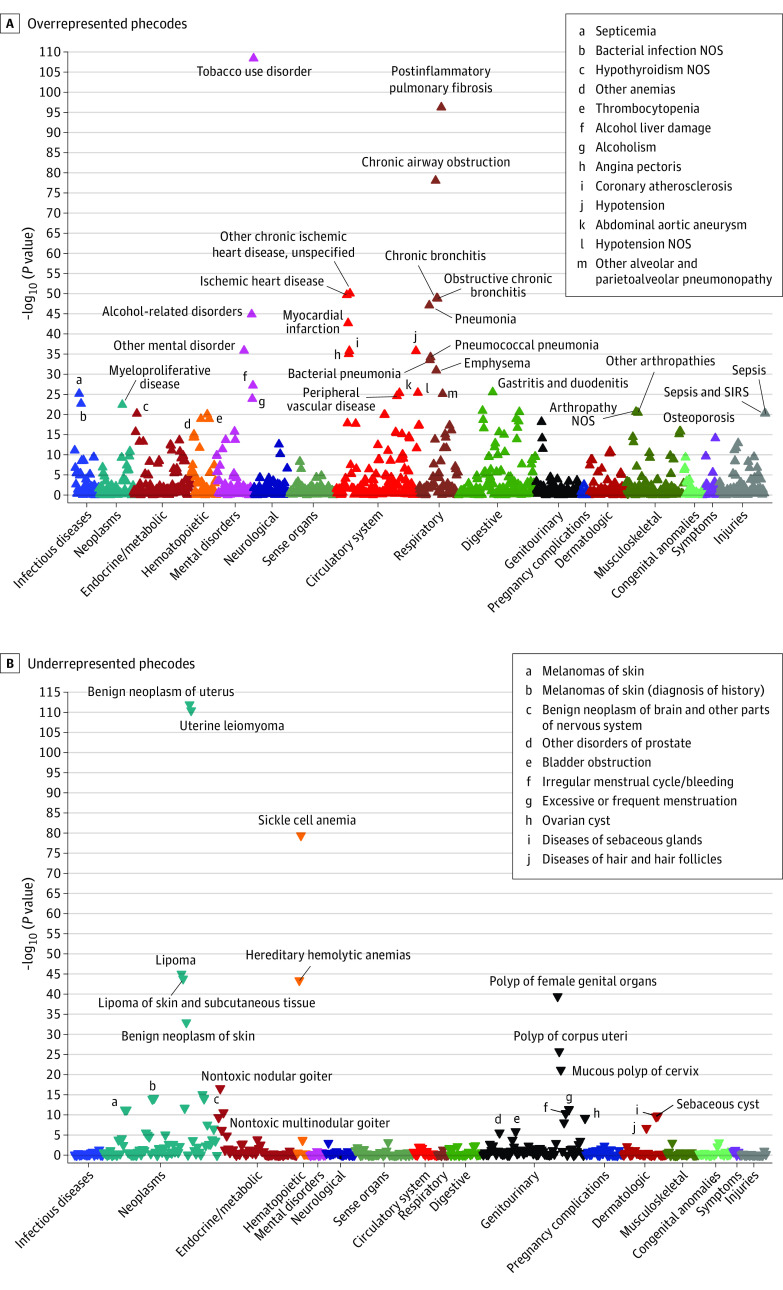
To obtain insight into conditions associated with shorter LTL (per SD telomere shortening), we conducted a multivariable PheWAS analysis. Of 1847 phecodes available in the UKB (eFigure 1 in the Supplement), 214 were significantly overrepresented and 37 underrepresented in participants with shorter LTLs (Figure 2 and Figure 3; eTables 9 and 10 and eFigures 5 and 6 in the Supplement). The results were robust even when considering different methods used for LTL presentation. In particular, a PheWAS analysis using a non–loge-corrected non–z score–corrected T/S ratio shared 230 significantly associated phecodes with the z-transformed depiction that was used in this study.
Figure 2. Multivariable Phenomewide Association Study Corrected for Age, Sex, Body Mass Index (BMI), and Ethnicity.
Manhattan plot of adjusted log10 (P values) for all phecodes comparing their occurrence per SD telomere shortening. Highlighted are associations results with −log10 P values between 10 and 120. A and B, Upwards/downwards pointing triangles refer to phecodes that are over and/or underrepresented. NOS indicates not otherwise specified; SIRS, systemic inflammatory response syndrome. BMI was calculated as weight in kilograms divided by height in meters squared.
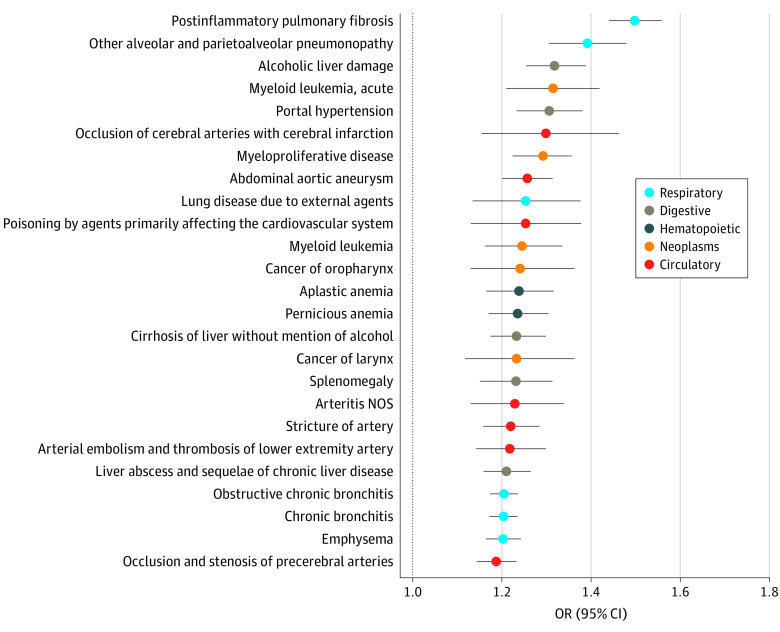
Figure 3. Most Overrepresented Phecodes in Patients With Shorter Telomeres.
Adjusted for age, sex, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), and ethnicity. Odds ratios (ORs) and 95% CIs per SD decrease of telomere length are given. Only phecodes that remained significant after adjustment for multiple testing are displayed. NOS indicates not otherwise specified.
Postinflammatory pulmonary fibrosis constituted the most prominent association that remained highly significant even after adjustment for a reported smoking/drinking behavior, PhenoAge, and CCI score (eFigure 7 in the Supplement). Even after this adjustment, 116 phecodes remained significantly overrepresented, while 38 were underrepresented in individuals with reduced LTL (eTables 11 and 12 in the Supplement). In line with the mortality analyses, respiratory and liver-related phecodes, together with hematologic disorders, were most prominently overrepresented in individuals with shorter LTLs (Figure 3; eFigure 5 in the Supplement). The association between LTL and respiratory disorders was only marginally affected by additional adjustment for smoking and alcohol consumption. Similarly, associations with liver diseases remained significant after correction for drinking and smoking behavior and after exclusion of moderate and heavy drinkers. Multiple cardiovascular phecodes, particularly those associated with arterial disease, were also enriched in participants with shorter LTL (Figure 3; eFigures 5 and 8 in the Supplement). In addition to malignant hematologic disorders, cancers that are associated with smoking and alcohol consumption (ie, cancer of the larynx, [oro]pharynx, and nasal cavities) were significantly associated with short LTLs (Figure 3 and Figure 4).
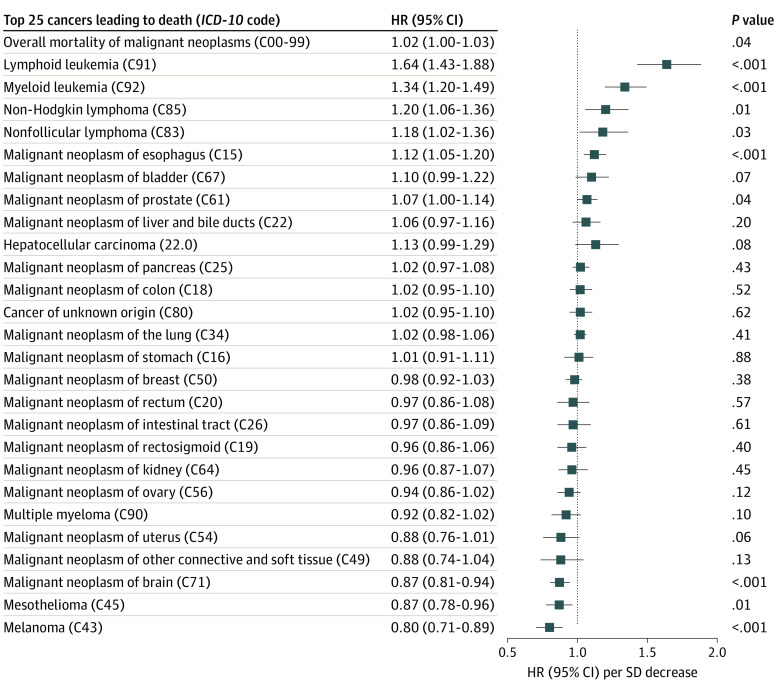
Figure 4. Association Between Telomere Length and the Top 25 Cancers That Lead to Death, Sorted by Descending Hazard Ratio (HR), and Adjusted for Age, Sex, Body Mass Index (BMI), and Ethnicity.
Hazard ratios per International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) code were generated from competing risk regression models, accounting for the risk of death through other causes. The HRs (95% CI) per SD decrease of telomere length are shown. BMI is calculated as weight in kilograms divided by height in meters squared.
To avoid biases through the use of inpatient hospital records, we repeated the PheWAS analysis in a subset of UKB participants who were connected to their primary health records using only diagnoses that were reported at least 1 year after the measurement of the telomere length. Despite the limited power, we were able confirm 52 phecodes (eTables 13 and 14 in the Supplement).
To further ensure the robustness of our data, we performed a time-to-event analysis. Notably, the top 10 phecodes that were associated with LTL all remained significant in this assessment (eTables 15 and 16 in the Supplement). Finally, to determine the association of extremely short telomeres, we looked at a subcohort of subjects who fell into the shortest (first) percentile of LTL. While the overall pattern of associated disorders remained similar, extremely short LTL was associated with markedly increased mortality of hematopoietic neoplasms, cholangitis, musculoskeletal diseases (particularly rheumatoid arthritis), and deaths associated with drugs and biological substances (Figure 5).
Figure 5. Association of Overall and Cause-Specific Mortality for the First Percentile of Telomere Length Corrected for Age, Sex, Body Mass Index (BMI), and Ethnicity.
All major International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) categories, as well as selected cause-specific mortalities, were analyzed. Hazard ratios (HRs) per ICD-10 code were generated from competing risk regression models, accounting for the risk of death of other causes. The HRs (95% CI) per SD decrease of telomere length are shown. COPD indicates chronic obstructive pulmonary disease; NA, not applicable.
To dissect the association of well-known genetic variants associated with telomere length, we performed PheWAS analyses for the rs10936599 variant of TERC and the lead single-nucleotide variation for the 5p15.33 (TERT). In this analysis, we found several phecodes that were over and underrepresented that were also associated with direct measured LTL, including sickle cell anemia and postinflammatory pulmonary fibrosis (eFigures 9 and 10 in the Supplement).
Differential Association of Telomere Shortening With Occurrence of Tumors
In line with the mortality analyses described previously that did not reveal a significant association between LTL and the overall cancer death rate, the cancer occurrence was mostly independent on LTL. Among the 25 most common cancers, only esophageal cancer and lymphoid and myeloid leukemia were more common in participants with shorter LTLs (Figure 4). However, brain cancer and melanoma were significantly underrepresented in individuals with shorter LTLs (Figure 4). Longer telomeres were associated with increased risk of benign neoplasms (eFigure 11 in the Supplement).
Discussion
We analyzed the UKB database to explore the relevance of LTL for human health. Given the large number of recruited individuals, the long follow-up period (>5 000 000 person-years), and a precise collection of disease phenotypes, we were able to gain insights and discovered more than 200 phecodes that are significantly associated with LTL.
We confirmed earlier findings that found an association of shorter LTL with modestly increased overall mortality.13,14 Still, the prognostic relevance of LTL seems limited compared with other clinical factors, and it is unlikely that LTL alone will become a meaningful marker for overall mortality. On an organ level, we observed increased mortality of respiratory and digestive disorders as well as hematopoietic neoplasms, which resemble the phenotype caused by genetic defects in telomere maintenance.2 Leukocyte telomere length also associated with several lifestyle-related factors. While the study suggested an association of BMI and smoking with shorter LTL,15,16,17,18 studies on alcohol and LTL are scarce and indicate a complex association that needs further exploration.18 Collectively, the study data extend earlier findings and indicate that organs may vary in their susceptibility to organismal telomere shortening, as reflected by LTL. We found no association between LTL and overall cancer-related and neurological disease–related mortality, contradicting earlier reports.4,5
Among the specific mortality causes, the most significant association was seen for respiratory diseases and persisted after adjustment for smoking. Emphysema-related deaths were particularly overrepresented in individuals with reduced LTL. This association was supported by experimental data that demonstrated that mice with shorter telomeres are susceptible to emphysema development.19 Although chronic obstructive pulmonary disease–related deaths were significantly enriched in participants with reduced LTL, the HR was lower, which aligned with several studies indicating a modest association.13,20 As potential underlying mechanisms, reduced LTL was not only associated with oxidative stress and a higher rate of pneumonia, but was also directly associated with an increase in the susceptibility of participants to an experimentally-induced respiratory infection.14,21,22
We detected a clear association between shorter LTL and excess mortality related to digestive disorders. It was attributed primarily to increased liver-related fatalities and remained significant after adjusting for multiple factors, including alcohol consumption. Although genetic and experimental studies demonstrated the negative association of short telomeres with development of liver disease,23,24 to our knowledge, a direct association with LTL remained to be demonstrated, likely because liver-related death represents less than 5% of overall mortality. A possible explanation is the major role the liver plays in immune and inflammatory responses.25
Among other death causes, a modest but highly significant association between shorter LTL and increased cardiovascular mortality was seen, which is supported by previous reports.26,27 This is unsurprising, because a healthy lifestyle with abundant physical activity attenuates telomere attrition,16,18 while hypertension and insulin resistance promote it.21 The observed surplus mortality from hematopoietic neoplasms in individuals with reduced LTL is plausible because shorter LTL indicates increased leukocyte stress. Moreover, LTL was diminished in participants with acute myeloid leukemia or aggressive non-Hodgkin lymphoma,28,29 for which reduced LTL previously associated with stronger chromosomal aberrations.28
Lastly, we detected a clear association between shorter LTL and death of musculoskeletal diseases, such as rheumatoid arthritis. While previous reports about LTL in rheumatoid arthritis are unequivocal,30,31 increased inflammation was associated with shorter LTL.32,33 This is interesting, because reduced LTL may identify a subset of patients with poorly controlled rheumatoid arthritis and persistent inflammation, as suggested for participants with asthma or periodontitis.33,34
Because of the large data set, we explored the association of LTL with individual phecodes. The study results confirmed a strong association with specific disorders, such as pulmonary fibrosis or aortic aneurysm, that is well documented in the literature.20,35,36 The study data demonstrate that the association with LTL may markedly differ within the same disease group, which is best exemplified by the assessed cancer entities. In these, a positive association between shorter LTL and esophageal/oropharyngeal cancer was seen, which corroborates previous observations and may be associated with a strong negative effect of smoking and alcohol consumption.37,38 Moreover, hematopoietic neoplasms were enriched in people with shorter LTL, which is consistent with a previous report.39 In contrast, but in line with the literature,40 this study revealed a negative association between shorter LTL and occurrence of melanoma or lung cancer, which seems contraintuitive given its association with smoking. Notably, earlier studies were inconclusive, and the association may depend on the histological subtype.41
Finally, a limited number of phecodes are overrepresented in individuals with longer LTL, including various benign neoplasms. Because several of them have been associated with longer telomeres,42,43 it is tempting to hypothesize that increased LTL either protect from the emergence of malignant tumors, possibly because of increased chromosomal stability, or promote benign lesions growth by reduced senescence.
Limitations
While the PheWAS analysis is well suited to identify many LTL-associated conditions, the missing temporal information may introduce reverse causation bias. To address that, we validated the top hits in a time-to-event assessment. The UKB is not an entirely representative population sample, as 94% of participants are White, high-income British individuals.44 Moreover, outcomes based on ICD-10 codes may have some degree of misclassification or underdiagnosis.
Conclusions
This cohort study, which to our knowledge is the largest study of telomere length, found that LTL is associated with specific human diseases and organ manifestations. In particular, individuals with short LTL were susceptible to similar disorders as participants with inherited telomere diseases, suggesting that LTL may contribute to specific illnesses irrespectively of the biology of different genetic variants. However, an association study cannot distinguish between causes and consequences. Shorter LTL may indicate a stressed bone marrow rather than per se predisposing to hematologic disorders. Further studies are needed to identify the underlying mechanisms and to delineate the relative contribution of genetic vs lifestyle-related factors.
eTable 1. Definitions of Outcomes and Covariates
eTable 2. Charlson Comorbidity index
eTable 3. PhenoAge
eFigure 1. Overview of the analyzed cohort
eFiugre 2. Telomere length distribution (A) and correlation of telomer length with age (B), daily alcohol consumption (C) and smoking displayed as pack years (PY)(D)
eTable 4. Characteristics of the UK Biobank cohort, stratified by telomere length
eFigure 3. Overall survival stratified by quartiles of telomer length, corrected for age, sex, BMI and ethnicity
eTable 5. Cox regression models for overall mortality per SD telomere shortening
eTable 6. Univariable Association of overall and cause-specific mortality per SD telomere shortening
eTable 7. Association of overall and cause-specific mortality per SD telomere shortening adjusted for PhenoAge, sex, BMI, ethnicity, smoking in the form of pack years (PY), daily alcohol consumption and Charlson Comorbidity index
eTable 8. Association between telomere length and the top 25 malignancies leading to death, sorted by descending HR and adjusted for PhenoAge, sex, BMI, ethnicity, smoking in the form of pack years (PY), daily alcohol consumption and Charlson Comorbidity index
eFigure 4. Bonferroni significant hazard ratios for cause-specific mortality associated with telomer shortening, adjusted for age, sex, BMI and ethnicity
eFigure 5. Bonferroni significant PheCodes (increased red; decreased green) associated with telomer shortening, adjusted for age, sex, BMI and ethnicity
eFigure 6. Circos plot for the Top25 Bonferroni significant phecodes increased with shorter telomere length, adjusted for age, sex, BMI and ethnicity
eFigure 7. Multivariable linear PheWAS corrected for PhenoAge, sex, BMI, ethnicity, Charlson Comorbidity index , daily alcohol consumption and smoking in the form of pack years (PY)
eFigure 8. Top 25 significant cardiovascular PheCodes increased by decreasing telomer length
eFigure 9. Manhattan plot for the minor allele of rs10936599 variant of TERC
eFigure 10. Manhattan plot for the lead SNP for the 5p15.33 (TERT)
eFigure 11. Top 10 significant PheCodes related to benign neoplasms decreased by decreasing telomere length
eTable 9. Bonferroni-significant PheCodes positively associated with decreasing telomere length in a multivariable analysis adjusted for age, sex, BMI and ethnicity (Hospital Admission Codes)
eTable 10. Bonferroni-significant PheCodes negatively associated with decreasing telomere length in a multivariable analysis adjusted for age, sex, BMI and ethnicity (Hospital Admission Codes)
eTable 11. Bonferroni-significant PheCodes positively associated with decreasing telomere length in a multivariable analysis adjusted for PhenoAge, sex, BMI, ethnicity, daily alcohol consumption, smoking in the form of pack years (PY) and Charlson Comorbidity Index
eTable 12. Bonferroni-significant PheCodes negatively associated with decreasing telomere length in a multivariable analysis adjusted for PhenoAge, sex, BMI, ethnicity, daily alcohol consumption, smoking in the form of pack years (PY) and Charlson Comorbidity Index
eTable 13. Bonferroni-significant PheCodes positively associated with decreasing telomere length in a multivariable analysis adjusted for age, sex, BMI and ethnicity (Primary Care Codes)
eTable 14. Bonferroni-significant PheCodes negatively associated with decreasing telomere length in a multivariable analysis adjusted for age, sex, BMI and ethnicity (Primary Care Codes)
eTable 15. Time to event analysis for the Top10 PheCodes positively associated with shorter telomere length, corrected for age, sex, BMI and ethnicity
eTable 16. Time to event analysis for the Top10 PheCodes negatively associated with shorter telomere length, corrected for age, sex, BMI and ethnicity
References
- 1.Blackburn EH, Epel ES, Lin J. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350(6265):1193-1198. doi: 10.1126/science.aab3389 [DOI] [PubMed] [Google Scholar]
- 2.Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361(24):2353-2365. doi: 10.1056/NEJMra0903373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weischer M, Bojesen SE, Nordestgaard BG. Telomere shortening unrelated to smoking, body weight, physical activity, and alcohol intake: 4,576 general population individuals with repeat measurements 10 years apart. PLoS Genet. 2014;10(3):e1004191. doi: 10.1371/journal.pgen.1004191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eitan E, Hutchison ER, Mattson MP. Telomere shortening in neurological disorders: an abundance of unanswered questions. Trends Neurosci. 2014;37(5):256-263. doi: 10.1016/j.tins.2014.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willeit P, Willeit J, Mayr A, et al. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304(1):69-75. doi: 10.1001/jama.2010.897 [DOI] [PubMed] [Google Scholar]
- 6.Rode L, Nordestgaard BG, Bojesen SE. Peripheral blood leukocyte telomere length and mortality among 64,637 individuals from the general population. J Natl Cancer Inst. 2015;107(6):djv074. doi: 10.1093/jnci/djv074 [DOI] [PubMed] [Google Scholar]
- 7.Haycock PC, Burgess S, Nounu A, et al. ; Telomeres Mendelian Randomization Collaboration . Association between telomere length and risk of cancer and non-neoplastic diseases: a mendelian randomization study. JAMA Oncol. 2017;3(5):636-651. doi: 10.1001/jamaoncol.2016.5945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Codd V, Denniff M, Swinfield C, et al. A major population resource of 474,074 participants in UK Biobank to investigate determinants and biomedical consequences of leukocyte telomere length. medRxiv. Posted March 24, 2021. doi: 10.1101/2021.03.18.21253457 [DOI]
- 9.Carroll RJ, Bastarache L, Denny JC. R PheWAS: data analysis and plotting tools for phenome-wide association studies in the R environment. Bioinformatics. 2014;30(16):2375-2376. doi: 10.1093/bioinformatics/btu197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Codd V, Nelson CP, Albrecht E, et al. ; Cardiogram Consortium . Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45(4):422-427, e1-e2. doi: 10.1038/ng.2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 12.Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). 2018;10(4):573-591. doi: 10.18632/aging.101414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rode L, Bojesen SE, Weischer M, Vestbo J, Nordestgaard BG. Short telomere length, lung function and chronic obstructive pulmonary disease in 46,396 individuals. Thorax. 2013;68(5):429-435. doi: 10.1136/thoraxjnl-2012-202544 [DOI] [PubMed] [Google Scholar]
- 14.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361(9355):393-395. doi: 10.1016/S0140-6736(03)12384-7 [DOI] [PubMed] [Google Scholar]
- 15.Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366(9486):662-664. doi: 10.1016/S0140-6736(05)66630-5 [DOI] [PubMed] [Google Scholar]
- 16.Cherkas LF, Hunkin JL, Kato BS, et al. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med. 2008;168(2):154-158. doi: 10.1001/archinternmed.2007.39 [DOI] [PubMed] [Google Scholar]
- 17.Müezzinler A, Zaineddin AK, Brenner H. Body mass index and leukocyte telomere length in adults: a systematic review and meta-analysis. Obes Rev. 2014;15(3):192-201. doi: 10.1111/obr.12126 [DOI] [PubMed] [Google Scholar]
- 18.Latifovic L, Peacock SD, Massey TE, King WD. The influence of alcohol consumption, cigarette smoking, and physical activity on leukocyte telomere length. Cancer Epidemiol Biomarkers Prev. 2016;25(2):374-380. doi: 10.1158/1055-9965.EPI-14-1364 [DOI] [PubMed] [Google Scholar]
- 19.Alder JK, Guo N, Kembou F, et al. Telomere length is a determinant of emphysema susceptibility. Am J Respir Crit Care Med. 2011;184(8):904-912. doi: 10.1164/rccm.201103-0520OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duckworth A, Gibbons MA, Allen RJ, et al. Telomere length and risk of idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease: a mendelian randomisation study. Lancet Respir Med. 2021;9(3):285-294. doi: 10.1016/S2213-2600(20)30364-7 [DOI] [PubMed] [Google Scholar]
- 21.Demissie S, Levy D, Benjamin EJ, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5(4):325-330. doi: 10.1111/j.1474-9726.2006.00224.x [DOI] [PubMed] [Google Scholar]
- 22.Helby J, Nordestgaard BG, Benfield T, Bojesen SE. Shorter leukocyte telomere length is associated with higher risk of infections: a prospective study of 75,309 individuals from the general population. Haematologica. 2017;102(8):1457-1465. doi: 10.3324/haematol.2016.161943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plentz RR, Park YN, Lechel A, et al. Telomere shortening and inactivation of cell cycle checkpoints characterize human hepatocarcinogenesis. Hepatology. 2007;45(4):968-976. doi: 10.1002/hep.21552 [DOI] [PubMed] [Google Scholar]
- 24.Rudolph KL, Chang S, Millard M, Schreiber-Agus N, DePinho RA. Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science. 2000;287(5456):1253-1258. doi: 10.1126/science.287.5456.1253 [DOI] [PubMed] [Google Scholar]
- 25.Strnad P, Tacke F, Koch A, Trautwein C. Liver—guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 2017;14(1):55-66. doi: 10.1038/nrgastro.2016.168 [DOI] [PubMed] [Google Scholar]
- 26.Fitzpatrick AL, Kronmal RA, Gardner JP, et al. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007;165(1):14-21. doi: 10.1093/aje/kwj346 [DOI] [PubMed] [Google Scholar]
- 27.Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227. doi: 10.1136/bmj.g4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartmann U, Brümmendorf TH, Balabanov S, Thiede C, Illme T, Schaich M. Telomere length and hTERT expression in patients with acute myeloid leukemia correlates with chromosomal abnormalities. Haematologica. 2005;90(3):307-316. [PubMed] [Google Scholar]
- 29.Widmann TA, Herrmann M, Taha N, König J, Pfreundschuh M. Short telomeres in aggressive non-Hodgkin’s lymphoma as a risk factor in lymphomagenesis. Exp Hematol. 2007;35(6):939-946. doi: 10.1016/j.exphem.2007.03.009 [DOI] [PubMed] [Google Scholar]
- 30.Steer SE, Williams FMK, Kato B, et al. Reduced telomere length in rheumatoid arthritis is independent of disease activity and duration. Ann Rheum Dis. 2007;66(4):476-480. doi: 10.1136/ard.2006.059188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prescott J, Karlson EW, Orr EH, Zee RYL, De Vivo I, Costenbader KH. A prospective study investigating prediagnostic leukocyte telomere length and risk of developing rheumatoid arthritis in women. J Rheumatol. 2016;43(2):282-288. doi: 10.3899/jrheum.150184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Donovan A, Pantell MS, Puterman E, et al. ; Health Aging and Body Composition Study . Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PLoS One. 2011;6(5):e19687. doi: 10.1371/journal.pone.0019687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masi S, Salpea KD, Li K, et al. Oxidative stress, chronic inflammation, and telomere length in patients with periodontitis. Free Radic Biol Med. 2011;50(6):730-735. doi: 10.1016/j.freeradbiomed.2010.12.031 [DOI] [PubMed] [Google Scholar]
- 34.Belsky DW, Shalev I, Sears MR, et al. Is chronic asthma associated with shorter leukocyte telomere length at midlife? Am J Respir Crit Care Med. 2014;190(4):384-391. doi: 10.1164/rccm.201402-0370OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cronkhite JT, Xing C, Raghu G, et al. Telomere shortening in familial and sporadic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178(7):729-737. doi: 10.1164/rccm.200804-550OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Courtwright AM, El-Chemaly S. Telomeres in Interstitial Lung Disease: The Short and the Long of It. Ann Am Thorac Soc. 2019;16(2):175-181. doi: 10.1513/AnnalsATS.201808-508CME [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Risques RA, Vaughan TL, Li X, et al. Leukocyte telomere length predicts cancer risk in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2649-2655. doi: 10.1158/1055-9965.EPI-07-0624 [DOI] [PubMed] [Google Scholar]
- 38.Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA. The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20(6):1238-1250. doi: 10.1158/1055-9965.EPI-11-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohyashiki JH, Sashida G, Tauchi T, Ohyashiki K. Telomeres and telomerase in hematologic neoplasia. Oncogene. 2002;21(4):680-687. doi: 10.1038/sj.onc.1205075 [DOI] [PubMed] [Google Scholar]
- 40.Nan H, Qureshi AA, Prescott J, De Vivo I, Han J. Genetic variants in telomere-maintaining genes and skin cancer risk. Hum Genet. 2011;129(3):247-253. doi: 10.1007/s00439-010-0921-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanchez-Espiridion B, Chen M, Chang JY, et al. Telomere length in peripheral blood leukocytes and lung cancer risk: a large case-control study in Caucasians. Cancer Res. 2014;74(9):2476-2486. doi: 10.1158/0008-5472.CAN-13-2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walsh KM, Codd V, Smirnov IV, et al. ; ENGAGE Consortium Telomere Group . Variants near TERT and TERC influencing telomere length are associated with high-grade glioma risk. Nat Genet. 2014;46(7):731-735. doi: 10.1038/ng.3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bataille V, Kato BS, Falchi M, et al. Nevus size and number are associated with telomere length and represent potential markers of a decreased senescence in vivo. Cancer Epidemiol Biomarkers Prev. 2007;16(7):1499-1502. doi: 10.1158/1055-9965.EPI-07-0152 [DOI] [PubMed] [Google Scholar]
- 44.Bycroft C, Freeman C, Petkova D, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203-209. doi: 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Definitions of Outcomes and Covariates
eTable 2. Charlson Comorbidity index
eTable 3. PhenoAge
eFigure 1. Overview of the analyzed cohort
eFiugre 2. Telomere length distribution (A) and correlation of telomer length with age (B), daily alcohol consumption (C) and smoking displayed as pack years (PY)(D)
eTable 4. Characteristics of the UK Biobank cohort, stratified by telomere length
eFigure 3. Overall survival stratified by quartiles of telomer length, corrected for age, sex, BMI and ethnicity
eTable 5. Cox regression models for overall mortality per SD telomere shortening
eTable 6. Univariable Association of overall and cause-specific mortality per SD telomere shortening
eTable 7. Association of overall and cause-specific mortality per SD telomere shortening adjusted for PhenoAge, sex, BMI, ethnicity, smoking in the form of pack years (PY), daily alcohol consumption and Charlson Comorbidity index
eTable 8. Association between telomere length and the top 25 malignancies leading to death, sorted by descending HR and adjusted for PhenoAge, sex, BMI, ethnicity, smoking in the form of pack years (PY), daily alcohol consumption and Charlson Comorbidity index
eFigure 4. Bonferroni significant hazard ratios for cause-specific mortality associated with telomer shortening, adjusted for age, sex, BMI and ethnicity
eFigure 5. Bonferroni significant PheCodes (increased red; decreased green) associated with telomer shortening, adjusted for age, sex, BMI and ethnicity
eFigure 6. Circos plot for the Top25 Bonferroni significant phecodes increased with shorter telomere length, adjusted for age, sex, BMI and ethnicity
eFigure 7. Multivariable linear PheWAS corrected for PhenoAge, sex, BMI, ethnicity, Charlson Comorbidity index , daily alcohol consumption and smoking in the form of pack years (PY)
eFigure 8. Top 25 significant cardiovascular PheCodes increased by decreasing telomer length
eFigure 9. Manhattan plot for the minor allele of rs10936599 variant of TERC
eFigure 10. Manhattan plot for the lead SNP for the 5p15.33 (TERT)
eFigure 11. Top 10 significant PheCodes related to benign neoplasms decreased by decreasing telomere length
eTable 9. Bonferroni-significant PheCodes positively associated with decreasing telomere length in a multivariable analysis adjusted for age, sex, BMI and ethnicity (Hospital Admission Codes)
eTable 10. Bonferroni-significant PheCodes negatively associated with decreasing telomere length in a multivariable analysis adjusted for age, sex, BMI and ethnicity (Hospital Admission Codes)
eTable 11. Bonferroni-significant PheCodes positively associated with decreasing telomere length in a multivariable analysis adjusted for PhenoAge, sex, BMI, ethnicity, daily alcohol consumption, smoking in the form of pack years (PY) and Charlson Comorbidity Index
eTable 12. Bonferroni-significant PheCodes negatively associated with decreasing telomere length in a multivariable analysis adjusted for PhenoAge, sex, BMI, ethnicity, daily alcohol consumption, smoking in the form of pack years (PY) and Charlson Comorbidity Index
eTable 13. Bonferroni-significant PheCodes positively associated with decreasing telomere length in a multivariable analysis adjusted for age, sex, BMI and ethnicity (Primary Care Codes)
eTable 14. Bonferroni-significant PheCodes negatively associated with decreasing telomere length in a multivariable analysis adjusted for age, sex, BMI and ethnicity (Primary Care Codes)
eTable 15. Time to event analysis for the Top10 PheCodes positively associated with shorter telomere length, corrected for age, sex, BMI and ethnicity
eTable 16. Time to event analysis for the Top10 PheCodes negatively associated with shorter telomere length, corrected for age, sex, BMI and ethnicity
Data Availability Statement
The data sets used in this study have not been deposited in a public repository but are available after approval of a reasonable application at https://www.ukbiobank.ac.uk. All participants provided written informed consent for the study. The UKB study has approval from the Northwest Multicenter research ethics committee.