Key Points
Question
Is a 5-day strategy of antibiotics superior to a 10-day strategy for treatment of nonsevere pneumonia in young children demonstrating early clinical response?
Findings
In this randomized clinical trial of 380 children with community-acquired pneumonia, a 5-day strategy resulted in similar treatment response with fewer antibiotic days compared with a 10-day strategy. For the primary composite outcome, the 5-day strategy was associated with a 69% probability of a more desirable outcome and a significantly lower abundance of antibiotic resistance genes.
Meaning
Among young children responding to initial therapy, a 5-day antibiotic strategy was superior to a 10-day strategy for treatment of nonsevere pneumonia.
This randomized clinical trial compares a short (5-day) vs standard (10-day) antibiotic treatment strategy for community-acquired pneumonia in young children.
Abstract
Importance
Childhood community-acquired pneumonia (CAP) is usually treated with 10 days of antibiotics. Shorter courses may be effective with fewer adverse effects and decreased potential for antibiotic resistance.
Objective
To compare a short (5-day) vs standard (10-day) antibiotic treatment strategy for CAP in young children.
Design, Setting, and Participants
Randomized double-blind placebo-controlled clinical trial in outpatient clinic, urgent care, or emergency settings in 8 US cities. A total of 380 healthy children aged 6 to 71 months with nonsevere CAP demonstrating early clinical improvement were enrolled from December 2, 2016, to December 16, 2019. Data were analyzed from January to September 2020.
Intervention
On day 6 of their originally prescribed therapy, participants were randomized 1:1 to receive 5 days of matching placebo or 5 additional days of the same antibiotic.
Main Outcomes and Measures
The primary end point was the end-of-treatment response adjusted for duration of antibiotic risk (RADAR), a composite end point that ranks each child’s clinical response, resolution of symptoms, and antibiotic-associated adverse effects in an ordinal desirability of outcome ranking (DOOR). Within each DOOR rank, participants were further ranked by the number of antibiotic days, assuming that shorter antibiotic durations were more desirable. Using RADAR, the probability of a more desirable outcome was estimated for the short- vs standard-course strategy. In a subset of children, throat swabs were collected between study days 19 and 25 to quantify antibiotic resistance genes in oropharyngeal flora.
Results
A total of 380 children (189 randomized to short course and 191 randomized to standard course) made up the study population. The mean (SD) age was 35.7 (17.2) months, and 194 participants (51%) were male. Of the included children, 8 were Asian, 99 were Black or African American, 234 were White, 32 were multiracial, and 7 were of unknown or unreported race; 33 were Hispanic or Latino, 344 were not Hispanic or Latino, and 3 were of unknown or unreported ethnicity. There were no differences between strategies in the DOOR or its individual components. Fewer than 10% of children in either strategy had an inadequate clinical response. The short-course strategy had a 69% (95% CI, 63-75) probability of a more desirable RADAR outcome compared with the standard-course strategy. A total of 171 children were included in the resistome analysis. The median (range) number of antibiotic resistance genes per prokaryotic cell (RGPC) was significantly lower in the short-course strategy compared with the standard-course strategy for total RGPC (1.17 [0.35-2.43] vs 1.33 [0.46-11.08]; P = .01) and β-lactamase RGPC (0.55 [0.18-1.24] vs 0.60 [0.21-2.45]; P = .03).
Conclusions and Relevance
In this study, among children responding to initial treatment for outpatient CAP, a 5-day antibiotic strategy was superior to a 10-day strategy. The shortened approach resulted in similar clinical response and antibiotic-associated adverse effects, while reducing antibiotic exposure and resistance.
Trial Registration
ClinicalTrials.gov Identifier: NCT02891915.
Introduction
Community-acquired pneumonia (CAP), one of the most common serious infections in childhood, is responsible for approximately 1.5 million ambulatory visits in children annually in the US.1 Professional society guidelines recommend 10 days of antibiotic therapy for outpatient CAP but acknowledge that shorter courses of therapy may be equally effective.2 Limiting antibiotic use to the shortest possible effective duration is critical to reducing adverse effects of antibiotic treatment and the spread of antimicrobial resistance.3,4 Several randomized clinical trials have demonstrated noninferiority of shorter treatment durations in children,5,6 including a recent trial in Canada7 that found that 5 days of amoxicillin therapy was noninferior to 10 days for treatment of CAP in children younger than 10 years. However, these studies were designed as noninferiority efficacy trials and did not comprehensively consider potential antibiotic-associated adverse effects, an important patient-centered outcome that impacts patient experience, treatment adherence, and efficacy. None of the aforementioned studies examined potential associations between treatment duration and its impact on the reservoir of antibiotic resistance genes in the respiratory tract microbiome (ie, the resistome).2,8
In this study, we compared a short- vs standard-course treatment strategy for childhood CAP, hypothesizing that a short-course strategy would be superior to a standard-course strategy, resulting in similar clinical outcomes with fewer antibiotic-associated adverse effects and fewer days of antibiotic exposure. We also examined associations between treatment strategy and antibiotic resistome patterns at the end of treatment.
Methods
Study Population
We conducted a multicenter randomized double-blind placebo-controlled superiority clinical trial evaluating a strategy of 5 days (short) vs 10 days (standard) of oral β-lactam therapy for outpatient treatment of pediatric CAP in individuals demonstrating early clinical improvement. Potentially eligible participants included otherwise healthy children aged 6 to 71 months and diagnosed with uncomplicated CAP in an outpatient clinic, urgent care, or emergency department setting at 1 of 8 study sites and prescribed either amoxicillin, amoxicillin and clavulanate, or cefdinir according to standard of care and independent of study protocol (Figure 1; eMethods in Supplement 2).2 Potentially eligible children were approached for enrollment on days 3 to 6 of their initially prescribed (prestudy) oral β-lactam therapy. Those assessed on day 6 were eligible only if they had not yet received a dose of antibiotic therapy on that day. Children were considered for inclusion if their caregiver reported none of the following at enrollment: subjective fever or documented temperature 38.3 °C or higher in the preceding 24 hours, tachypnea (50 breaths per minute for those younger than 2 years and 40 breaths per minute for those 2 years and older), and severe cough. Participants were enrolled December 2, 2016, to December 16, 2019. Data were analyzed from January to September 2020. Written informed consent was obtained from all caregivers prior to enrollment. The study was approved by the institutional review boards at all participating sites and followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
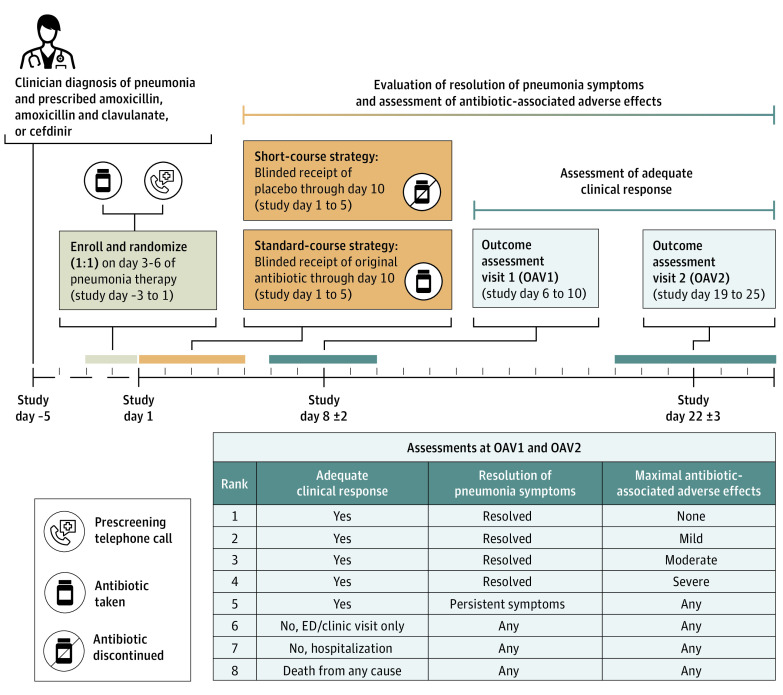
Figure 1. Short-Course Outpatient Therapy of Community Acquired Pneumonia (SCOUT-CAP) Clinical Trial Schema.
SCOUT-CAP was a multicenter randomized double-blind placebo-controlled superiority clinical trial evaluating a short-course (5 days) vs standard-course (10 days) strategy of oral β-lactam therapy for outpatient treatment of pediatric CAP. Participants were enrolled on days 3 to 6 of their initially prescribed (prestudy) oral β-lactam therapy and randomized 1:1 to a strategy of 5 days of matching placebo (short course) or an additional 5 days of their initially prescribed prestudy antibiotic (standard course). Outcome assessment visits (OAV) occurred on study days 6 to 10 (OAV1) and 19 to 25 (OAV2). At these time points, each participant’s clinical outcome was categorized according to a desirability of outcome ranking (DOOR), an 8-level ordinal outcome created from 3 components: adequate clinical response (absence of a medically attended visit, surgical procedure, or receipt of nonstudy antibiotics for persistent or worsening pneumonia after randomization), resolution of symptoms (absence of fever, elevated respiratory rate, and moderate or severe cough), and the presence and severity of antibiotic-associated adverse effects (irritability, vomiting, diarrhea, allergic reaction, stomatitis, or candidiasis).
Enrollment and Outcome Assessment Visits
Following written informed consent, participants were randomized 1:1 to a strategy of 5 days of matching placebo (short course) or an additional 5 days of their initially prescribed prestudy antibiotic (standard course), with stratification by age group (younger than 24 months vs 24 to 71 months), site of initial treatment (emergency department vs outpatient clinic or urgent care), and antibiotic received (Figure 1). Active study drugs were amoxicillin or amoxicillin and clavulanate, both dosed as 80 to 100 mg/kg/d (maximum 2000 mg/d) of the amoxicillin component divided twice daily, or cefdinir, dosed as 12 to 16 mg/kg/d (maximum 600 mg/d) divided twice daily. Matching placebo (taste and appearance) was dosed at the same volume calculated for the active drug. Study day 1 was the first scheduled day of study drug administration. Study staff examined participants and interviewed their caregivers to ensure eligibility and provided caregivers with a daily memory aid to record medication administration, temperature, cough, and prespecified antibiotic-associated adverse effects. Outcome assessment visit (OAV) 1 occurred on study days 6 to 10, and OAV2 occurred on study days 19 to 25; both visits were completed in person.
End Points
The primary efficacy end point was the response adjusted for duration of antibiotic risk (RADAR) at OAV1.9 This approach has been used in observational studies examining antibiotic treatment outcomes and retrospectively applied in several clinical trials.10,11,12,13,14 A priori, RADAR assumes that shorter treatment durations are preferable when clinical outcomes are similar. Under this assumption, RADAR was determined using a 2-step process. First, each participant’s clinical outcome was categorized according to a desirability of outcome ranking (DOOR), an 8-level ordinal outcome encompassing 3 components: adequate clinical response, resolution of symptoms, and the presence and severity of antibiotic-associated adverse effects (Figure 1; eMethods in Supplement 2). Next, using actual reported treatment duration (not treatment assignment) for each participant, we ranked the participants’ overall experiences according to the following criteria: (1) when comparing 2 participants with different DOOR ranks, the participant with a more desirable DOOR received a higher (more desirable) rank and (2) when comparing the overall outcomes of 2 participants with identical DOOR ranks, the participant with fewer reported total days of antibiotic received a higher rank. Secondary efficacy end points included RADAR at OAV2 as well as DOOR and its components (adequate clinical response, resolution of symptoms, and antibiotic-associated adverse effects) at OAV1 and OAV2.
Resistome Substudy
For those consenting to participate in an optional resistome substudy, throat swabs were obtained at study visits and stored for later analysis. DNA was extracted from throat swabs at the end of study (OAV2) to classify and quantify antibiotic resistance genes (ARGs). The end point for this prespecified analysis was the quantity of ARGs at OAV2, expressed as resistance genes per prokaryotic cell (RGPC) (eMethods in Supplement 2).15,16
Statistical Analysis
The null hypothesis for this study was no difference in RADAR, defined as the probability of a more desirable RADAR for the short-course strategy (plus one-half the probability of a tied RADAR) of 50%.9 The alternative hypothesis was a 60% probability of a more desirable RADAR for the short-course strategy, a clinically relevant difference as judged by the investigators. A sample size of 360 (180 per arm) was needed to provide 90% power using a 2-sided α = .05 (Wilcoxon Mann-Whitney U test). For the primary analysis, we estimated the probability of a more desirable outcome in the short-course strategy and its 95% CI under the intention-to-treat (ITT) principle. Multiple imputation was used for missing data in the primary ITT analysis. Prespecified analyses were carried out for secondary efficacy end points of RADAR at OAV2 and DOOR at OAV1 and OAV2. Analyses of DOOR and RADAR were also conducted for the complete-case, according-to-protocol, and worst-case analysis sets. Sensitivity analyses of RADAR were performed by increasing the threshold in assigning different ranks owing to differing numbers of days of antibiotic use from a 1-day difference to a 2- to 5-day difference. Risk of inadequate clinical response, persistent symptoms, and presence and severity (mild, moderate, or severe) of antibiotic-associated adverse effects were compared between treatment strategies using the complete-case analysis sets. For the resistome analyses, total and β-lactamase RGPC were compared using 1-sided Wilcoxon rank sum tests. See the eMethods in Supplement 2 for additional analytical details. Results of sensitivity analyses were consistent with the primary analysis and are presented in the eResults in Supplement 2. For the main analysis, 2-sided P values were considered significant at < .05. For resistome analyses, a 1-sided Wilcoxon test was used to assess for differences at α level .05. Analyses were run using SAS version 9.3 (SAS Institute) and R version 3.2 (the R Foundation).
Results
Study Population
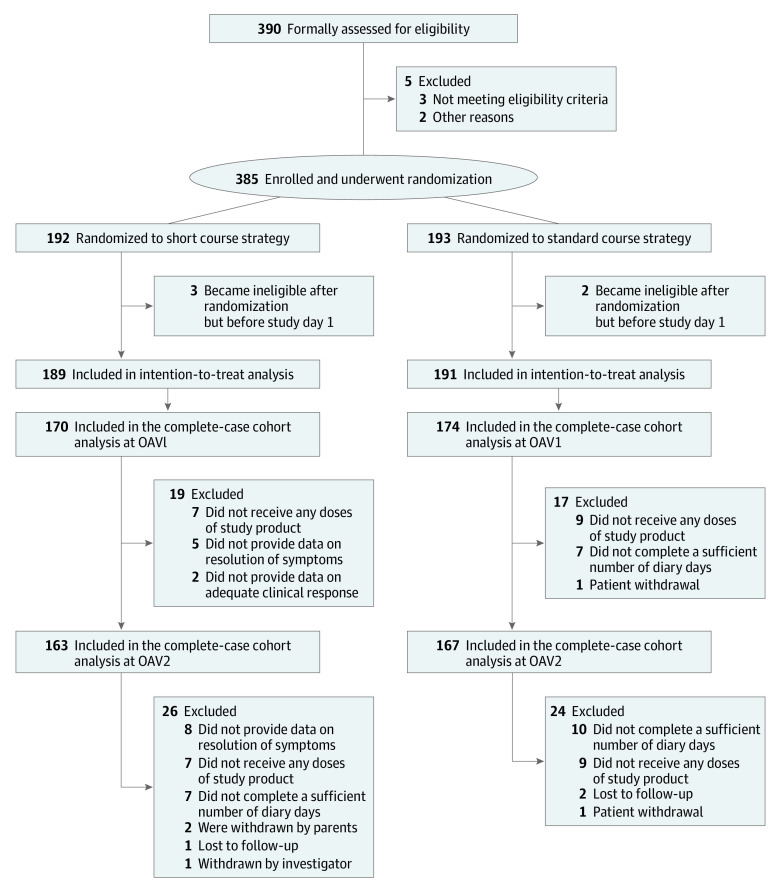
Sites prescreened children with clinician-diagnosed CAP, and caregivers of potentially eligible children were approached for screening and enrollment. Consent was obtained for 390 children and eligibility was assessed. Of these, 385 met eligibility criteria and were enrolled; 192 were randomized to the short-course strategy and 193 to the standard-course strategy (Figure 2). Five additional participants were deemed ineligible prior to study day 1 and were excluded from the ITT analysis. The final ITT population included 380 participants. The mean (SD) age was 35.7 (17.2) months, and 194 participants (51%) were male. Race and ethnicity data were reported by caregivers at the time of study participation and were used to compare baseline demographic characteristics by treatment strategy. None of the included children were American Indian or Alaskan Native, 8 were Asian, 99 were Black or African American, none were Native Hawaiian or other Pacific Islander, 234 were White, 32 were multiracial, and 7 were of unknown or unreported race; 33 were Hispanic or Latino, 344 were not Hispanic or Latino, and 3 were of unknown or unreported ethnicity. Amoxicillin was the prescribed antibiotic in 345 participants (91%), and 300 (79%) were initially evaluated in outpatient or urgent care settings. Patient characteristics were similar across treatment groups (Table 1).
Figure 2. Short-Course Outpatient Therapy of Community Acquired Pneumonia (SCOUT-CAP) CONSORT Diagram.
Outcome assessment visits (OAV) occurred on study days 6 to 10 (OAV1) and 19 to 25 (OAV2). For the intention to treat analysis, 1 or more desirability of outcome ranking components were imputed for 36 participants at OAV1 and 50 participants at OAV2.
Table 1. Baseline Characteristics, Intention-to-Treat Population.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Course | All individuals | ||
| Short | Standard | ||
| No. | 189 | 191 | 380 |
| Age, mean (SD), mo | 34.6 (16.6) | 36.8 (17.8) | 35.7 (17.2) |
| Age group, mo | |||
| <24 | 55 (29) | 56 (29) | 111 (29) |
| 24-71 | 134 (71) | 135 (71) | 269 (71) |
| Sex | |||
| Female | 95 (50) | 91 (48) | 186 (49) |
| Male | 94 (50) | 100 (52) | 194 (51) |
| Racea | |||
| American Indian or Alaska Native | 0 | 0 | 0 |
| Asian | 4 (2) | 4 (2) | 8 (2) |
| Black or African American | 48 (25) | 51 (27) | 99 (26) |
| Native Hawaiian or other Pacific Islander | 0 | 0 | 0 |
| White | 118 (62) | 116 (61) | 234 (62) |
| Multiracial | 15 (8) | 17 (9) | 32 (8) |
| Unknown or unreported | 4 (2) | 3 (2) | 7 (2) |
| Ethnicitya | |||
| Hispanic or Latino | 15 (8) | 18 (9) | 33 (9) |
| Not Hispanic or Latino | 173 (92) | 171 (90) | 344 (91) |
| Unknown or unreported | 1 (<1) | 2 (1) | 3 (<1) |
| Site of initial diagnosis | |||
| Outpatient or urgent care clinic | 149 (79) | 151 (79) | 300 (79) |
| Emergency department | 40 (21) | 40 (21) | 80 (21) |
| Initial antibiotic treatment | |||
| Amoxicillin | 173 (92) | 172 (90) | 345 (91) |
| Amoxicillin and clavulanate | 10 (5) | 10 (5) | 20 (5) |
| Cefdinir | 6 (3) | 9 (5) | 15 (4) |
Race and ethnicity data were reported by caregivers at the time of study participation and were used to compare baseline demographic characteristics by treatment strategy.
Of those assigned to short-course therapy, 182 (96%) received at least 1 dose of placebo, and 159 (84%) reported taking the expected 10 doses of placebo (range 0-11 doses). Of those assigned to standard-course therapy, 182 (95%) received at least 1 dose of antibiotic, and 152 (80%) reported taking the expected 10 doses (range 0-12 doses).
Adequate Clinical Response, Resolution of Symptoms, and Antibiotic-Associated Adverse Effects
To assign a DOOR rank for each participant, we first evaluated the components of DOOR, including clinical response, resolution of symptoms, and the frequency of antibiotic-associated adverse effects. There were no significant differences between treatment strategies in proportions of inadequate clinical response at OAV1 (1% vs <1%; difference in proportion, 0.5%; 95% CI, −2.4 to 3.7) or OAV2 (1% vs 2%; difference in proportion, −0.5%; 95% CI, −3.9 to 2.8) (Figure 3; eResults in Supplement 2). No participant died or required hospitalization or surgery for persistent or worsening pneumonia.
Figure 3. Inadequate Clinical Response, Persistent Symptoms, and Antibiotic-Associated Adverse Effects, Intention-to-Treat Population at Outcome Assessment Visit 1.
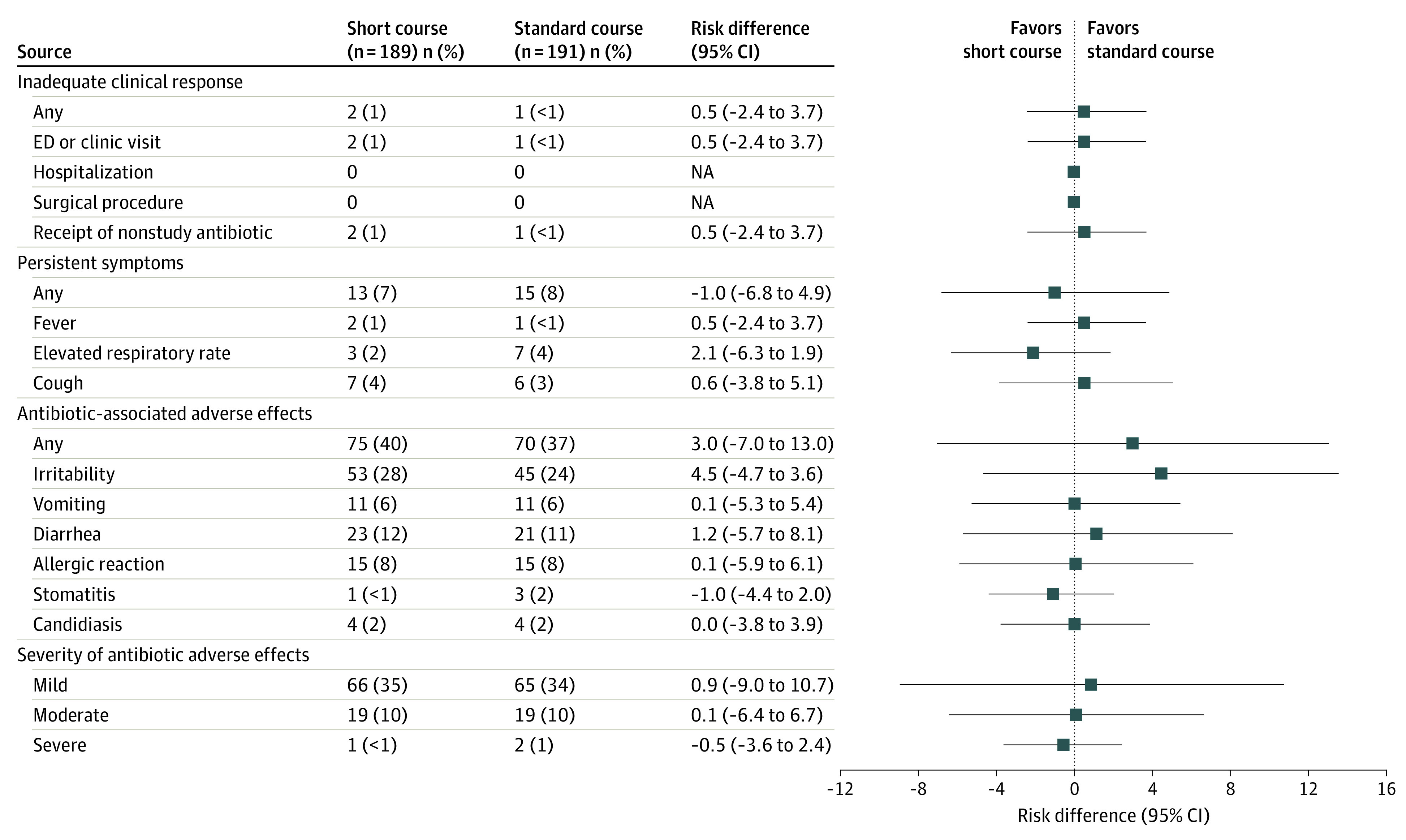
Frequencies (%) by treatment strategy and risk differences (95% CIs) for each component of the desirability of outcome ranking. Risk differences were compared using Fisher exact tests. The Newcombe method with continuity correction was used to compute 95% CIs for risk differences.
There were no significant differences between treatment groups in the number of participants with persistent symptoms at OAV1 or OAV2 (Figure 3; eResults in Supplement 2). At OAV1, 13 participants (7%) in the short-treatment group and 15 (8%) in the standard-treatment group had persistent symptoms (difference in proportion, −1.0%; 95% CI, −6.8 to 4.9). At OAV2, 11 participants (6%) in each group had persistent symptoms (difference in proportion, 0.1%; 95% CI, −5.3 to 5.4).
There were no differences in the proportion of participants reporting antibiotic-associated adverse effects between the 2 strategies (Figure 3; eResults in Supplement 2). At OAV1, 75 children (40%) in the short-course strategy and 70 (37%) in the standard-course strategy reported an antibiotic-associated adverse effect (difference in proportion at OAV1, 3.0%; 95% CI, −7.0 to 13.0. Most adverse effects were mild (eg, irritability and diarrhea), although 20 children in the short-treatment group (11%) and 21 (11%) in the standard-treatment group experienced a moderate to severe adverse effect. At OAV2, 96 children in the short-course strategy (51%) and 92 in the standard-course strategy (48%) reported an antibiotic-associated adverse effect (difference in proportion, 2.6%; 95% CI, −7.7 to 12.9); 36 children in both groups (19%) experienced a moderate to severe adverse effect.
DOOR
Table 2 (OAV1) and the eResults in Supplement 2 (OAV2) display the distribution of DOOR by treatment strategy along with the cumulative risk difference at each rank for the ITT analysis set based on the individual DOOR. At OAV1, 19 participants (10%) in the short-course strategy and 17 (9%) in the standard-course strategy had 1 or more DOOR components imputed. The probability of a more desirable DOOR for the short-course strategy was 0.48 (95% CI, 0.42-0.53), indicating no difference compared with the standard-course strategy. There were no differences in cumulative risk at any DOOR rank. At OAV2, the probability of a more desirable DOOR for the short-course strategy was 0.48 (95% CI, 0.42-0.54).
Table 2. Desirability of Outcome Ranking (DOOR), Intention-to-Treat Population at First Outcome Assessment Visit.
| DOOR descriptiona | Course, No. (%) | Cumulative risk difference (95% CI)b | |
|---|---|---|---|
| Short | Standard | ||
| No. | 189 | 191 | |
| ACR with antibiotic-associated adverse effects | |||
| None (1) | 97 (51) | 107 (56) | −4.7 (−14.9 to 5.7) |
| Mild (2) | 47 (25) | 42 (22) | −1.8 (−10.6 to 7.0) |
| Moderate (3) | 14 (7) | 10 (5) | 0.4 (−7.5 to 8.2) |
| Severe (4) | 0 | 2 (1) | −0.7 (−8.5 to 7.1) |
| Persistent symptoms (5) | 11 (6) | 13 (7) | −1.7 (−8.2 to 4.8) |
| No ACR | |||
| With ED or clinic visit (6) | 2 (1) | 1 (<1) | −1.1 (−7.5 to 5.1) |
| With hospitalization (7) | 0 | 0 | −1.1 (−7.5 to 5.1) |
| Death (8) | 0 | 0 | −1.1 (−7.5 to 5.1) |
Abbreviations: ACR, adequate clinical response; ED, emergency department.
DOOR rank (1) represents the best possible outcome and DOOR rank (8) the worst.
Cumulative risk differences were calculated as follows: for i Σ [1-8], the difference in proportions of participants between treatment strategies with DOOR rank ≤ i was calculated and 95% CIs were estimated using the Newcombe method with continuity correction.
RADAR
When duration of antibiotic treatment was incorporated, the short-course strategy was superior, with an estimated probability of a more desirable RADAR for the short-course strategy of 0.69 (95% CI, 0.63-0.75) at OAV1. At OAV2, the probability of a more desirable RADAR in the short-course strategy was 0.63 (95% CI, 0.57-0.69).
Resistome Analysis
Throat swabs from OAV2 were evaluated for detection of ARGs from 171 participants (87 participants in short-course therapy and 84 participants in standard-course therapy) in the oropharyngeal resistome subgroup. There were no statistically significant differences in age, sex, race and ethnicity, site of diagnosis, or initial antibiotic when comparing frequencies between the 2 treatment strategies. The baseline characteristics of participants who consented to participate in the microbiome study were also similar to those in the overall SCOUT-CAP trial (data not shown). The number of total RGPC and β-lactamase RGPC was significantly lower in the short-course strategy compared with the standard-course strategy (eResults in Supplement 2). The median (range) number of total RGPC was 1.17 (0.35-2.43) for the short-course strategy and 1.33 (0.46-11.08) for the standard-course strategy (P = .01). The median (range) number of β-lactamase RGPC was 0.55 (0.18-1.24) for the short-course strategy and 0.60 (0.21-2.45) for the standard-course strategy (P = .03).
Discussion
In this randomized double-blind placebo-controlled clinical trial including 380 children with outpatient CAP demonstrating early clinical improvement, a 5-day antibiotic strategy was superior to a 10-day strategy. The shortened approach achieved similar clinical response, resolution of symptoms, and antibiotic-associated adverse effects with fewer days of antibiotic therapy. Moreover, there were no differences between treatment groups when examining the individual components of the DOOR. Fewer than 10% of participants in both groups demonstrated inadequate clinical response or persistent symptoms. Antibiotic-associated adverse effects were common, typically mild, and did not differ by treatment strategy. The short-course strategy was associated with a significantly lower frequency of ARGs detected in throat swabs at the end of treatment.
In a smaller randomized trial of 115 children in Israel with clinical and radiographically confirmed outpatient pneumonia, Greenberg et al6 demonstrated noninferiority of 5 days of amoxicillin compared with 10 days. No child in either group experienced treatment failure at 30 days. In a 2-center study in Canada,7 5 days of therapy was found to be noninferior to 10 days among children aged 6 months to 10 years. Five-day courses of antibiotics are already recommended for World Health Organization–defined nonsevere pneumonia,17 as several small randomized trials have demonstrated similar outcomes in children treated with either 3 or 5 days of therapy.5,18,19 Despite potential differences in the populations under study, including pneumococcal conjugate vaccine coverage rates and approaches to pneumonia diagnosis and management, our findings are consistent with these earlier studies. In contrast to these studies, which used noninferiority designs, our study was designed as a superiority trial using a novel design that incorporates both positive (adequate clinical response and resolution of symptoms) and negative (antibiotic-associated adverse effects) aspects of the treatment response as well as antibiotic duration. Further, none of these prior trials explored associations between treatment duration and the oropharyngeal resistome.
A plausible hypothesis is that the 5-day strategy would result in fewer antibiotic-associated adverse effects compared with the standard-course strategy. In this study, antibiotic-associated adverse effects, such as irritability and diarrhea, were common in both strategies, and there were no differences in the presence or severity of adverse effects across groups. Most antibiotic-associated adverse effects were mild, although approximately 20% of participants experienced moderate or severe adverse effects by OAV2. The study was not powered to examine differences between rare antibiotic-associated adverse effects.
Our results suggest that a small reduction (5 days) in exposure to β-lactam therapy is associated with fewer ARGs in the respiratory microbiota. We do not know how persistent these differences in the resistome are, and the clinical relevance of this finding is incompletely understood. Nevertheless, these data support our a priori assumption that shorter antibiotic durations are more desirable when clinical outcomes are similar because of reduced antibiotic selective pressure, which is associated with the prevalence of antibiotic resistance.
Our study supports a 5-day treatment strategy over longer antibiotic courses for previously healthy young children with outpatient CAP who demonstrate early clinical improvement. Providing the shortest duration of antibiotics necessary to effectively treat an infection is a central tenet of antimicrobial stewardship and a convenient and cost-effective strategy for caregivers. With an estimated 1.5 million ambulatory visits for CAP annually,1 reducing treatment from 10 to 5 days for outpatient CAP could result in a reduction of up to 7.5 million antibiotic days in the US each year. Current guidelines recommending longer courses of therapy for uncomplicated outpatient CAP warrant reexamination.
Strengths
Strengths of this study include a double-blind superiority design and the methodological approach. Rather than reducing treatment efficacy to a binary response, the ordinal DOOR recognizes finer gradations of outcome success or failure and enables a more holistic evaluation of participants’ treatment experiences (benefits and harms).20 This methodology is flexible so that other measures of treatment efficacy, such as caregiver or clinician preferences,7 could be added in future studies. For RADAR, duration of antibiotic use was used to break ties in the ordinal DOOR under the a priori assumption that shorter durations of antibiotics are preferred when clinical outcomes are similar. This assumption underlies the core principles of antimicrobial stewardship that antibiotics should be prescribed for the minimum duration necessary to effectively treat the infection and that less antibiotic exposure reduces selective pressure and antibiotic resistance.
Limitations
This study had limitations. Microbiologic testing, such as blood culture, and chest radiography were not routinely performed as part of study protocol as national guidelines discourage use of these diagnostic tests for pneumonia in the outpatient setting.2 Although all enrolled participants had clinician-confirmed pneumonia and received antibiotics, it is almost certain that some did not have bacterial pneumonia. This is a common challenge in both clinical practice and pneumonia treatment trials, owing to the lack of highly sensitive bacterial diagnostics for childhood pneumonia. Thus, the pragmatic design of our study mirrors real-world practices around diagnosis and empirical antibiotic use for outpatient pediatric CAP. Future studies are needed, perhaps incorporating viral testing and/or biomarkers, to test the efficacy of a watchful waiting strategy vs a strategy of empirical antibiotic therapy. The population under study was limited to relatively healthy children younger than 6 years with outpatient CAP who demonstrated clinical improvement during the first 5 days of antibiotic therapy. Thus, the conclusions of our study may not extend to children with underlying conditions, those with more severe pneumonia, and those who do not demonstrate early improvement. It is also possible that characteristics of the children enrolled in the trial differed from those who were eligible but not enrolled. Additionally, 10% of participants had data imputed for 1 or more DOOR components. However, results for complete-case, according-to-protocol, and worst-case analyses were consistent with the primary analyses, suggesting missing data did not influence our key conclusions.
Conclusions
A 5-day course of guideline-recommended antibiotics is a safe and effective approach for treating young children with uncomplicated outpatient CAP who demonstrate early clinical improvement. Implementation of this strategy is encouraged to optimize treatment efficacy, lessen unnecessary antibiotic use, and reduce the prevalence of antibiotic resistance genes among colonizing oropharyngeal flora.
Trial protocol
eMethods
eResults
The DMID 14-0079 Study Team
Data sharing statement
References
- 1.Kronman MP, Hersh AL, Feng R, Huang YS, Lee GE, Shah SS. Ambulatory visit rates and antibiotic prescribing for children with pneumonia, 1994-2007. Pediatrics. 2011;127(3):411-418. doi: 10.1542/peds.2010-2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradley JS, Byington CL, Shah SS, et al. ; Pediatric Infectious Diseases Society and the Infectious Diseases Society of America . The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. doi: 10.1093/cid/cir531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rice LB. The Maxwell Finland Lecture: for the duration-rational antibiotic administration in an era of antimicrobial resistance and clostridium difficile. Clin Infect Dis. 2008;46(4):491-496. doi: 10.1086/526535 [DOI] [PubMed] [Google Scholar]
- 4.Spellberg B, Bartlett JG, Gilbert DN. The future of antibiotics and resistance. N Engl J Med. 2013;368(4):299-302. doi: 10.1056/NEJMp1215093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ginsburg AS, Mvalo T, Nkwopara E, et al. Amoxicillin for 3 or 5 Days for chest-indrawing pneumonia in Malawian children. N Engl J Med. 2020;383(1):13-23. doi: 10.1056/NEJMoa1912400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg D, Givon-Lavi N, Sadaka Y, Ben-Shimol S, Bar-Ziv J, Dagan R. Short-course antibiotic treatment for community-acquired alveolar pneumonia in ambulatory children: a double-blind, randomized, placebo-controlled trial. Pediatr Infect Dis J. 2014;33(2):136-142. doi: 10.1097/INF.0000000000000023 [DOI] [PubMed] [Google Scholar]
- 7.Pernica JM, Harman S, Kam AJ, et al. Short-course antimicrobial therapy for pediatric community-acquired pneumonia: the SAFER Randomized Clinical Trial. JAMA Pediatr. 2021;175(5):475-482. doi: 10.1001/jamapediatrics.2020.6735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright GD. The antibiotic resistome: the nexus of chemical and genetic diversity. Nat Rev Microbiol. 2007;5(3):175-186. doi: 10.1038/nrmicro1614 [DOI] [PubMed] [Google Scholar]
- 9.Evans SR, Rubin D, Follmann D, et al. Desirability of outcome ranking (DOOR) and response adjusted for duration of antibiotic risk (RADAR). Clin Infect Dis. 2015;61(5):800-806. doi: 10.1093/cid/civ495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doernberg SB, Tran TTT, Tong SYC, et al. ; Antibacterial Resistance Leadership Group . Good studies evaluate the disease while great studies evaluate the patient: development and application of a desirability of outcome ranking endpoint for Staphylococcus aureus bloodstream infection. Clin Infect Dis. 2019;68(10):1691-1698. doi: 10.1093/cid/ciy766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans SR, Knutsson M, Amarenco P, et al. Methodologies for pragmatic and efficient assessment of benefits and harms: application to the SOCRATES trial. Clin Trials. 2020;17(6):617-626. doi: 10.1177/1740774520941441 [DOI] [PubMed] [Google Scholar]
- 12.Lodise TP, Rosenkranz SL, Finnemeyer M, et al. The emperor’s new clothes: prospective observational evaluation of the association between initial vancomycin exposure and failure rates among adult hospitalized patients with methicillin-resistant Staphylococcus aureus bloodstream infections (PROVIDE). Clin Infect Dis. 2020;70(8):1536-1545. doi: 10.1093/cid/ciz460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Duin D, Lok JJ, Earley M, et al. ; Antibacterial Resistance Leadership Group . Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis. 2018;66(2):163-171. doi: 10.1093/cid/cix783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Duin D, Arias CA, Komarow L, et al. ; Multi-Drug Resistant Organism Network Investigators . Molecular and clinical epidemiology of carbapenem-resistant Enterobacterales in the USA (CRACKLE-2): a prospective cohort study. Lancet Infect Dis. 2020;20(6):731-741. doi: 10.1016/S1473-3099(19)30755-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong Y. Btrim: a fast, lightweight adapter and quality trimming program for next-generation sequencing technologies. Genomics. 2011;98(2):152-153. doi: 10.1016/j.ygeno.2011.05.009 [DOI] [PubMed] [Google Scholar]
- 16.Yin X, Jiang XT, Chai B, et al. ARGs-OAP v2.0 with an expanded SARG database and hidden Markov models for enhancement characterization and quantification of antibiotic resistance genes in environmental metagenomes. Bioinformatics. 2018;34(13):2263-2270. doi: 10.1093/bioinformatics/bty053 [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization . Revised WHO classification and treatment of pneumonia in children at health facilities: evidence summaries. Accessed March 1, 2021. https://apps.who.int/iris/bitstream/handle/10665/137319/9789241507813_eng.pdf?sequence=1 [PubMed]
- 18.Pakistan Multicentre Amoxycillin Short Course Therapy (MASCOT) pneumonia study group . Clinical efficacy of 3 days versus 5 days of oral amoxicillin for treatment of childhood pneumonia: a multicentre double-blind trial. Lancet. 2002;(360)9336:835-841. doi: 10.1016/S0140-6736(02)09994-4 [DOI] [PubMed] [Google Scholar]
- 19.Agarwal G, Awasthi S, Kabra SK, Kaul A, Singhi S, Walter SD; ISCAP Study Group . Three day versus five day treatment with amoxicillin for non-severe pneumonia in young children: a multicentre randomised controlled trial. BMJ. 2004;328(7443):791. doi: 10.1136/bmj.38049.490255.DE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans SR, Bigelow R, Chuang-Stein C, et al. Presenting risks and benefits: helping the data monitoring committee do its job. Ann Intern Med. 2020;172(2):119-125. doi: 10.7326/M19-1491 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eMethods
eResults
The DMID 14-0079 Study Team
Data sharing statement