Abstract
At the population level we are seeing milder disease during the Omicron wave of the SARS-CoV-2 pandemic. Is this due to the virus or pre-existing immunity, and what should we expect next?
Subject terms: SARS-CoV-2, Viral infection
The Omicron variant of SARS-CoV-2 escapes immunity generated by vaccines and previous infections1,2 yet seems to have some unexpected advantages over the other variants we have experienced so far. Firstly, and most importantly, strong evidence is emerging that the disease induced by infection with Omicron is considerably milder at the population level (Fig. 1a). Secondly, infection with Omicron has been shown to trigger cross-protective immunity to the more pathogenic Delta variant, which together with Omicron’s advantage in transmissibility, may set Delta on the path to extinction3.
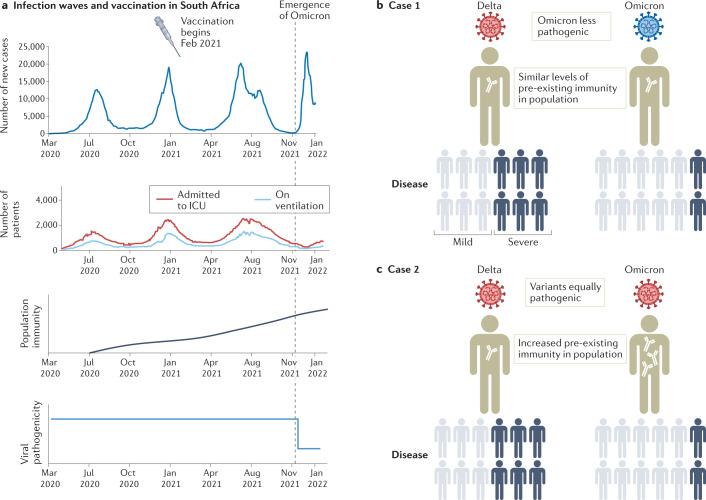
Fig. 1. Delta and Omicron waves in South Africa.
The top graphs indicate the number of infections with SARS-CoV-2 and the numbers of patients with COVID-19 who were admitted to intensive care units (ICUs) or placed on ventilation during the course of the pandemic in South Africa (part a). The lower graphs propose how the relative levels of population immunity and viral pathogenicity may have changed. Data collated from the National Institute for Communicable Diseases in South Africa. Parts b and c illustrate how either reduced Omicron pathogenicity (case 1) or increased levels of pre-existing immunity in a population (case 2) can lead to lower levels of severe disease (indicated by dark blue figures).
But is Omicron infection really milder than Delta (Fig. 1b), or have the populations that Omicron is infecting built up enough immunity so that the disease course will be milder with any variant of SARS-CoV-2 (Fig. 1c)? There is support for both scenarios.
Evidence that the virus itself may cause milder disease comes from in vitro data4 showing that Omicron does not transmit as well as other variants by cell fusion. Such fusion happens through a transmembrane serine protease 2 (TMPRESS2)-dependent process. This is important as it will restrict the virus to the upper respiratory tract where TMPRESS2-independent, cell-free transmission drives infection, and makes it less likely to spread in the lungs, where cell fusion plays a stronger role in viral transmission and the damage from infection is more serious. This observation is supported by studies in Syrian hamsters and human ACE2-expressing mice, which found that Omicron infection causes milder lung pathology5,6. The studies also show that the weight of the infected animals, which decreases by about 10% with Delta infection, does not go down with Omicron infection as much (mice) or at all (hamsters). This is strong evidence for lower pathogenicity, with the caveat that Omicron may be more adapted to infecting human cells and so infects hamsters less well relative to other strains of SARS-CoV-2.
A different line of evidence that the milder pathogenicity is virus based comes from data from a clinical trial of the Janssen (Johnson & Johnson) Ad26.COV2.S vaccine in South Africa7. This study analysed breakthrough infections seen during the Beta, Delta and Omicron infection waves and tracked outcomes of study participants who were hospitalized following infection. Given that all study participants were vaccinated, an assumption can be made that their levels of pre-existing immunity were not so different. During the Beta and Delta infection waves in South Africa, 43% of hospitalized vaccinated trial participants who had breakthrough infections needed supplementary oxygen, and 7–8% needed ventilation. This compared with only 16% and 0.2% of breakthrough infections needing supplementary oxygen and ventilation in the Omicron wave. In addition, the median length of hospitalization for those with breakthrough infections was 5 to 6 days in the Beta and Delta waves, and only 3 days during the Omicron wave. Although the assumption of equivalent immunity may not be completely true because the participants infected in the Omicron wave have had more time to acquire immunity from unreported infections, it will be surprising if such infections would be prevalent enough to account for this effect.
While the evidence for a virus-based effect seems strong, the evidence for pre-existing immunity having a role in milder outcomes is also considerable. Studies looking at neutralizing immunity against Omicron showed that although Omicron can escape this immunity, it is essentially a numbers game. Increased levels of neutralizing antibodies owing to booster vaccines or a combination of previous infection and vaccination counteract viral escape, so that antibody neutralization against Omicron in these groups is more or less equivalent to the neutralization seen against variants such as Delta in those who are fully vaccinated but not boosted1,2. Furthermore, the levels of antibody-mediated immunity required for protection against severe disease are much lower and may be achieved by vaccination alone, even in the absence of boosters8. To this can be added the incremental build-up of immunity in the population from infections by Delta and other variants, combined with the fact that Omicron does not show extensive escape from other immune responses such as T cell-mediated immunity induced by prior vaccinations or infections9. Most strikingly, the unvaccinated have a fivefold higher chance of being admitted to hospital because of Omicron infection relative to people vaccinated with two doses of the Pfizer BNT162b2 mRNA vaccine (see data from the Discovery Health Network in South Africa). Therefore, severe disease is still possible with Omicron infection, but can be largely prevented by vaccination.
Lower viral pathogenicity and higher population immunity do not have to exclude one another. Most likely both play a part in what is by now clear: Omicron leads to less severe disease at the population level. If the viral component is as important as it seems, then the question is, what kind of SARS-CoV-2 variant will we get next? The emergence of another major variant of concern is highly probable as the virus has proven it can evolve to escape immunity in the current climate of infection. So far, the one documented way in which immune-escape variants have been found to evolve is in people who cannot clear the virus because of immunosuppression (see10 and references therein). This leads to a prolonged infection where, in some cases, there are trace levels of anti-SARS-CoV-2 antibody that are not sufficient to clear the virus, but that possibly provide selective pressure for the virus to evolve antibody-escape mutations. Immunosuppression is relatively common in Africa and elsewhere in a subset of people with advanced HIV disease, where poorly suppressed HIV infection over many years leads to severe damage to the immune response. A prolonged SARS-CoV-2 infection because of immunosuppression may persist for months and, surprisingly, may not lead to overt COVID-19 symptoms for much of its course10. The low disease severity during immunosuppression may be explained by the lack of a pathological inflammatory response against the virus. Alternatively, the virus may become attenuated during a prolonged infection — otherwise the host would die and the virus could not persist and ultimately evolve into an immune-escape variant.
Will the trend now be the emergence of SARS-CoV-2 variants with lower pathogenicity, Omicron level or better, or will the next variant reverse this and induce more severe disease? While we have utterly failed to predict the course of the pandemic so far, perhaps it is time to better consider how new variants evolve, keeping in mind that different variants may evolve in different ways, so that we have an idea of what to expect next.
Competing interests
The author declares no competing interests.
Footnotes
Related links
Discovery Health Network in South Africa: https://www.discovery.co.za/corporate/health-insights-vaccines-real-world-effectiveness
References
- 1.Cele S, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2021 doi: 10.1038/s41586-021-04387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rössler A, et al. SARS-CoV-2 Omicron Variant Neutralization in Serum from Vaccinated and Convalescent Persons. N. Engl. J. Med. 2022 doi: 10.1056/NEJMc2119236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan K, et al. Omicron infection enhances neutralizing immunity against the Delta variant. medRxiv. 2021 doi: 10.1101/2021.12.27.21268439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meng B, et al. SARS-CoV-2 Omicron spike mediated immune escape, infectivity and cell-cell fusion. bioRxiv. 2021 doi: 10.1101/2021.12.17.473248. [DOI] [Google Scholar]
- 5.Bentley EG, et al. SARS-CoV-2 Omicron-B.1.1.529 variant leads to less severe disease than Pango B and Delta variants strains in a mouse model of severe COVID-19. bioRxiv. 2021 doi: 10.1101/2021.12.26.474085. [DOI] [Google Scholar]
- 6.McMahan K, et al. Reduced pathogenicity of the SARS-CoV-2 Omicron variant in hamsters. bioRxiv. 2022 doi: 10.1101/2022.01.02.474743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goga A, et al. Breakthrough Covid-19 infections during periods of circulating Beta, Delta and Omicron variants of concern, among health care workers in the Sisonke Ad26.COV2.S vaccine trial, South Africa. medRxiv. 2021 doi: 10.1101/2021.12.21.21268171. [DOI] [Google Scholar]
- 8.Khoury DS, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;7:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 9.Keeton R, et al. SARS-CoV-2 spike T cell responses induced upon vaccination or infection remain robust against Omicron. medRxiv. 2021 doi: 10.1101/2021.12.26.21268380. [DOI] [Google Scholar]
- 10.Cele S, et al. SARS-CoV-2 prolonged infection during advanced HIV disease evolves extensive immune escape. Cell Host Microbe. 2022 doi: 10.1016/j.chom.2022.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]