Abstract
Background
The objective of the present work was to assess the reactogenicity and immunogenicity of heterologous COVID-19 vaccination regimens in clinical trials and observational studies.
Methods
PubMed, Cochrane Library, Embase, MedRxiv, BioRxiv databases were searched in September 29, 2021. The PRISMA instruction for systemic review was followed. Two reviewers independently selected the studies, extracted the data and assessed risk of bias. The quality of studies was evaluated using the New Castle-Ottawa and Cochrane risk of instrument. The characteristics and study outcome (e.g., adverse events, immune response, and variant of concern) were extracted.
Results
Nineteen studies were included in the final data synthesis with 5 clinical trials and 14 observational studies. Heterologous vaccine administration showed a trend toward more frequent systemic reactions. However, the total reactogenicity was tolerable and manageable. Importantly, the heterologous prime-boost vaccination regimens provided higher immunogenic effect either vector/ mRNA-based vaccine or vector/ inactivated vaccine in both humoral and cellular immune response. Notably, the heterologous regimens induced the potential protection against the variant of concern, even to the Delta variant.
Conclusions
The current findings provided evidence about the higher induction of robust immunogenicity and tolerated reactogenicity of heterologous vaccination regimens (vector-based/mRNA vaccine or vector-based/inactivated vaccine). Also, this study supports the application of heterologous regimens against COVID-19 which may provide more opportunities to speed up the global vaccination campaign and maximize the capacity to control the pandemic.
Keywords: Reactogenicity, Immunogenicity, Vaccine, COVID-19, Prime-boost
Graphical Abstract
1. Introduction
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in a global pandemic in late 2019 [1]. As of 10th Jan 2022, there have been over 3.0 million confirmed cases of COVID-19, with 5,464,532 deaths reported worldwide [2], prompting unprecedented efforts to contain the virus [1], [3], [4]. Covid-19 has not only killed more than five million people worldwide but has also left at least 1.5 million orphans, leading to a dramatic burden on the healthcare system and social security [5]. Globally, vaccination programmes have proved to be safe, effective and save lives [6], [7]. However, most vaccines do not confer 100% protection potential, and it is not known how the current vaccines will prevent future transmission of SARS-CoV-2 [8], given emerging variants [9]. But vaccination is an effective tool to reach herd immunity and to interrupt the spreading of the disease against current and future variants [9], [10]. The SARS-Cov-2 vaccines were developed in different platforms such as inactivated virus, protein subunit, vector-based and mRNA-based vaccines [3]. Heterologous vaccination refers to the use of booster and priming vaccines developed with different platforms. Heterologous vaccination against COVID-19 should be considered under some circumstances. There are some reasons for using heterologous regimen in clinical practices: (1) intermittent supply shortages of vaccines due to limited capacity in vaccine production and logistic challenge of distributing the right vaccines into the right people at the right time; (2) rare but severe adverse events of vector-based vaccines (e.g., thromboembolism in Oxford-AstraZeneca ChAdOx1-S COVID-19 vaccine); (3) emerging SARS-Cov-2 variants lead to demand for alternative second vaccination. Heterologous vaccine regimens were applied for other diseases including tuberculosis, yellow fever, and influenza [11], [12]. Matching two different vaccine platforms could increase efficacy and elicit a strong and long-lasting immune response [13]. Heterologous prime-boost vaccination against SARS-Cov-2 was used in several countries although evidence of safety and robust immunogenicity to support the application of the heterologous regimens was scarce. We conducted this systematic review to investigate and point out the reactogenicity and immunogenicity of heterologous vaccine regimens for preventing COVID-19 disease.
2. Methods
2.1. Eligibility criteria
We included clinical trials and observational studies that examined the reactogenicity and immunogenicity of heterologous regimens of COVID-19 vaccine in healthy adults (uninfected human subjects). We considered published articles in peer-reviewed journals or preprints in English up until September 29, 2021.
3. Search strategy
We searched PubMed, Cochrane Library, Embase, and pre-print servers (Medrxiv and Biorxiv) to identify the relevant studies. Databases were searched with pre-specified keywords including “COVID-19″, “SARS-CoV-2″, “Coronarivus”, “prime-boost”, “vaccine”, “immunization”, “inoculation”, “heterologous”, “mix”, “match”, and “combination”. The complete search strategy is detailed in Supplement file.
3.1. Study selection
After removing exact duplicates, two authors (T.T.N and G.V.V) independently screened the titles and abstracts of the articles to identify the potentially eligible studies. For those selective studies, the two authors independently assessed the full-text articles for eligibility for inclusion in this review. Disagreements between the authors on the inclusion of a given study were resolved by discussion between T.T.N and G.V.V to clarify eligibility. If no consensus was reached, the article was further evaluated by the third author (T.H.T.Q).
3.2. Data extraction and statistical analysis
Following PRISMA guideline, two authors (N.T.T and V.V.G) independently extracted the following data: general study information (authors, year of publication, and location of study), study characteristics, subgroup of study, sample size, description of vaccine, vaccination regimens, reactogenicity, immunology, and information to assess the quality of the study. Because of heterogeneity of quantifications, criteria for positivity vary in different studies, reported outcomes; comparison between trials are impossible for direct meta-analysis. Therefore, we conducted the network meta-analysis using the extracted data for reactogenicity of vaccination.
3.3. Quality assessment
Two reviewers (N.T.T and V.V.G) independently assessed study quality and discussed if disagreements occurred. If no agreement was reached, the study quality was evaluated by an additional reviewer (T.H.T.Q). The Cochrane risk of bias instrument was used to assess the risk of bias for clinical trials. We classified a clinical trial as high risk of bias if at least one category was rated as high risk of bias [14]. The Newcastle-Ottawa quality assessment scale was used to assess the risk of bias for observational studies. We classified observation studies with ≥ 7 stars as low risk of bias, 5 or 6 stars as medium risk of bias, and less than 5 stars as high risk of bias [15] (Table S1).
4. Results
4.1. Study selection and description
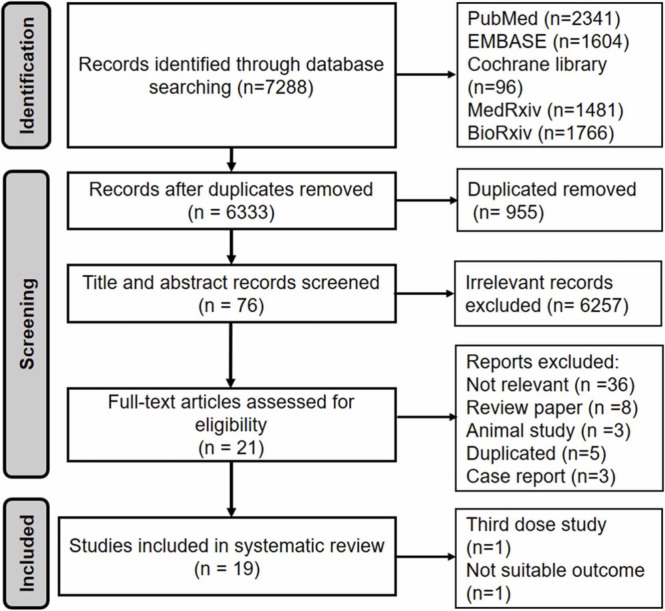
A total of 7288 papers were found based on the search strategy (2341 PubMed, 1604 EMBASE, 96 Cochrane library, 1481 MedRxiv, 1766 BioRxiv). After exclusion of exact duplicates, we screened titles and abstracts of 6333 articles to identify 76 potentially eligible articles. Of 76 articles, 21 articles met eligibility criteria and were included. Of 21 eligible studies, two papers were excluded because one study was third dose study [16] and another did not get the suitable outcome [17]; 19 studies were included in this review with 5 clinical trials and 14 observational studies ( Fig. 1). The description of chosen studies was detailed in Table 1 with all the studies are conducted in 2021 year.
Fig. 1.
Flowchart of the searching protocol and the final articles for systematic review. There studies were excluded because of third dose study [16], not suitable outcome [17], case report [54], [23], [55]. These studies did not get the inclusion criteria although the studies conducted in combination of the COVID—19 vaccines.
Table 1.
Summary of included studies on heterologous COVID-19 vaccination.
| Author | Country | Study design | Vaccine regimens† | Time period after boosting | Mean of age (range of age) | Gender Female (%) | Vaccine regimen | Sample size |
|---|---|---|---|---|---|---|---|---|
| Borobia[22] | Spain | Open label, randomized controlled trial | ChAd/BNT ChAd/no boost | 14 days | 43.98 (18–60 years) | 56.5% | ChAd, BNT | 676 |
| Liu[28] | UK | Single-blind, randomized non-inferiority trial | ChAd/BNT ChAd/ChAd | 28 days | 57.8 (≥50 years) | 45.8% | ChAd, BNT | 830 |
| Shaw[18] | UK | Multi-center, single-blind, randomized non-inferiority trial | ChAd/ BNTBNT/BNTBNT/ChAdChAd/ChAd | 28 days | 57.8 (≥50 years) | 45.8% | ChAd, BNT | 830 |
| Li[26] | China | Randomized, controlled, observer-blinded trial | CoVac/ConvideciaCoVac/CoVac | 28 days | 44.25 | 41% | 299 | |
| Tenbusch[19] | Germany | Non-blinded non-randomized study | ChAd/ChAdChAd/BNTBNT/BNT | 2 weeks | 42.5 (31–55) | 90.2% | ChAd, BNT | 642 |
| Hillus[23] | Germany | Prospective cohort study | ChAd/ChAdChAd/BNTBNT/BNT | 3 weeks | 35 | 66.6% | ChAd, BNT | 380 |
| Groβ[24] | Germany | Prospective cohort study | ChAd/BNT | 14–19 days | 30.5 (25–46 yrs) | 61.5% | ChAd, BNT | 26 |
| Barros[30] | Germany | Prospective cohort study | ChAd/ChAdChAd/BNTBNT/BNT | 17 days | 39 | 75% | ChAd, BNT | 129 |
| Behrens[31] | Germany | Prospective cohort study | ChAd/ChAdChAd/BNT | 16.3 days | 39 | 21.7 | ChAd, BNT | 23 |
| Benning[32] | Germany | Prospective cohort study | ChAd/ChAdChAd/BNTBNT/BNT | 20 days | ChAd/ChAd 55 (33–60 yrs)ChAd/BNT 30 (24–45 yrs)BNT/BNT 45 (33–56 yrs) | 81% | ChAd, BNT | 134 |
| Dimeglio[34] | France | Prospective cohort study | ChAd/ChAdChAd/BNTBNT/BNT | 28 days | 37 (20–55 yrs) | 74% | ChAd, BNT | 132 |
| Fabricius[35] | Germany | Prospective cohort study | BNT/BNTmRNA1273/mRNA1273ChAd/BNTCHAd/mRNA1273ChAd/ChAd | 2 weeks | 44 | 62% | ChAd, mRNA 1273 | 116 |
| Hammerschmidt[20] | Germany | Prospective cohort study | ChAd/ChAdChAd/BNTBNT/BNT | 17 days | NA | 75% | ChAd, BNT | 115 |
| Kant[25] | India | Retrospective cohort study | ChAd/CovaxinChAd/ChAdCovaxin/Covaxin | 3 weeks | ≥ 50 yrs | 49% | ChAd, Covaxin | 98 |
| Normark[29] | Sweden | Prospective cohort study | ChAd/ChAdChAd/mRNA1273 | 30 days | 43 (23–62 yrs) | NA | ChAd, mRNA1273 | 88 |
| Schmidt[33] | UK | Prospective cohort study | ChAd/ChAdChAd/mRNA1273mRNA1273/mRNA1273 | 13 days | 47.1 | 69.9% | ChAd, mRNA1273 | 213 |
| Valiee[21] | France | Prospective cohort study | ChAd/BNTBNT/BNT | 30 days | 34.5 | 69.1% | ChAd, BNT | 197 |
| Yorsaeng[36] | Thailand | Prospective cohort study | CoVac/CoVacCoVac/ChAdChAd/ChAd | 32 days | 41.5 | 62.7% | ChAd, Covac | 214 |
| Schmidt[27] | Prospective cohort study | ChAd/ChAdChAd/mRNA1273mRNA1273/mRNA1273 | 14 days | 54.5 | 65.5% | ChAd, mRNA1273 | 110 |
†Bold text indicates the heterologous regimens.
mRNA: messenger RNA; mRNA1273: Vaccine from Moderna company; ChAd: Astrazeneca, vector (Covisheld) vaccine; BNT: Pfizer mRNA vaccine; Convidecia: recombinant adenovirus type-5-vectored vaccine; CoronaVac: inactivated SARS-CoV-2 vaccine (CoVac); Covaxin: inactivated whole virion BBV152 vaccine; NA: not available;
4.2. Risk of bias
Of 5 clinical trials, two studies were high risk of bias. One trial did not blind outcome assessment [18] and another trial was lack of random sequence and blindness of participants and personnel [19]. The remaining 3 clinical trials were low of bias although they did not provide sufficient information to evaluate blindness of participants and personnel, blindness of outcome assessment, or selective reporting (Table S2). Of the 14 observational studies, 12 were low risk of bias. The other two studies were medium because of the unrepresentativeness of the exposed cohort and inadequacy of following up [20], [21] (Table S1).
4.3. Reactogenicity
Of the nine studies reported the safety of vaccine, the local reactions including injection site pain [22], [18], [23], [24], [25], [26], redness [22], [18], [23], [26], pruritus [22], [18], [26], hardness [22], [18], swelling [18], [23], [26], and urticarial [22] were reported. One study conducted in solid organ transplant recipients, and showed the lower incidence of adverse events than healthy controls [27]. Overall, the local adverse events were mild or moderate and receded by several days after boosting. A higher occurrence of solicited injection site was observed in heterologous vaccination than homologous vaccination (Table 1c). The local and systemic events in females were more frequent than males while there was no different in subgroups of age [22], [24].
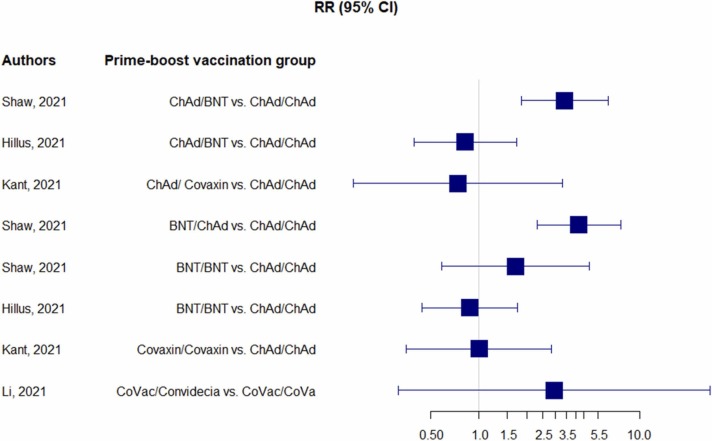
In term of systemic reactions, feverishness [18], [23], [24], [25], [26], fatigue [18], [23], [24], [26], diarrhea [18], [23], myalgia [18], [22], [23], [24], [26], arthralgia [22], [18] 23], malaise [18], [22], [25], chill [18], [22], [24], [27], headache [18], [22], [23], [24], [26] 27], and nausea [18], [22], [23], [26] were reported. No serious adverse events were reported in studies investigated reactogenicity. Although one study reported serious events in 4 participants, it was not related to the vaccination [28]. The result from network meta–analysis showed that the incidence of fever symptom was higher in the heterologous vaccination of vector-based and mRNA vaccine compared to homogenous vaccination. In case of vector-based vaccine and inactivated vaccine, the feverishness was the same between two groups ( Fig. 2). The pyrexia events were managed by administration of antipyretic medication to reduce symptoms within several days post vaccination [25], [18], [23]. Table S3 was detailed systemic reactions through the participants.
Fig. 2.
Forest plot analysis of feverishness event by network meta-analysis in both random clinical trials and observational studies.
4.4. Immunogenicity
4.4.1. Heterologous regimens of vector-based and mRNA-based vaccine
In general, the immunogenicity induced by heterologous ChAd/BNT vaccination was more potent compared to homologous ChAd/ChAd vaccination in most of studies ( Table 2). These findings were similar in both humoral and cellular immune response. Particularly, the anti-spike S antibody response in group vaccinated with heterologous regimens was high [22], [24] [29], [21] and higher than groups vaccinated with homologous regimens [28], [30], [31], [32]. Only two studies showed that the anti-spike S antibody response in group vaccinated with heterologous regimens was the same as groups vaccinated with homologous regimens [33], [23]. Additionally, the receptor-binding domain (RBD) specific antibody was high in two studies [22], [23], [29]. The neutralizing antibody of group vaccinated with heterologous regimens was high [22], [24] 29] and higher than groups vaccinated with homologous regimens [28], [23], [34], [33]. Only two studies showed that neutralizing antibody of heterologous group was the same as groups vaccinated with homologous regimens [32], [35]. In term of cellular response, the T cell response in group receiving heterologous regimens was high [22], [24] [35], [27] and higher than group receiving homologous regimen [28], [23], [30]. Only one study showed the same T cell response between heterologous and homologous regimens [33]. Notably, the B cell response was investigated in one study and showed similar extent in expansion of spike-specific memory B across regimens [30]. While evaluating combination vector-based/mRNA and BNT/BNT vaccination, the immunogenic effect was not consistent across studies. The spike protein Ab level was less than or equal; in contrast to, the T cell response had trend forwards to higher than or equal in heterologous group [28], [23], [30] [32], [34], [21]. Different immunogenicity was not significantly influenced by age or sex [30], [21].
Table 2.
Immunogenicity of heterologous regimens including vector-based and mRNA-based vaccines (ChAd/BNT or ChAd/mRNA-1273).
| Vaccine platform | Studies | Outcomes | ChAd/BNT (Mean (95% CI)) | ChAd/ChAd (Mean (95% CI)) | BNT/BNT (Mean (95% CI)) | Major results |
|---|---|---|---|---|---|---|
| ChAd and BNT | Borobia[22] | Spike protein Ab | 3684.87 BAU/ml (3851.58–4920.85),Ratio was 36.41-fold increase from baseline | ChAd prime only: 101.2 BAU/ml (82.45–124.22) | NA | Heterologous vaccination induced robust response |
| RBD Ab | The number was higher compared with ChAd prime: 7756.68 BAU/ml (7371.53–8161.96) | 99.84 BAU/ml (76.93–129.59) | ||||
| Neutralizing Ab | GMT: increased 45-times, from 41.84 to 1905.69 (1625.65–2233.98) | 41.81 (27.18–64.32) | ||||
| T cell response | IFN-γ significantly increased with GMT: 521.22 pg/ml (422.44–643.09) | 122.67 pg/ml (88.55–169.95) | ||||
| Liu[28] | Spike protein Ab | 12,906 ELU/ml (11,404–14,604) | 1392 ELU/ml (1188–1630) | 14,080 ELU/ml (12,491–15,871) | The higher immunogenicity of mixing vaccination compared with ChAd/ChAd was demonstrated | |
| Neutralizing Ab | Pseudotype virus neutralizing Ab (NT50): 515 (430–617) | 61(50–73) | 574 (475–694) | |||
| T cell response | SFC per million PBMCs: 184 (152–223) | 48 (37–61) | 80 (63–101) | |||
| Hillus[23] | Serum Ab avidity | 100% (88.6–100) | 83% (66.4–92.7) | 90% (74.4–96.5) | The heterologous improved immunogenicity compared with homologous ChAd/ChAd | |
| RDB Ab | 5.6 S/Co (5.1–6.1) | 4.9 S/Co (4.3–5.6) | 5.4 S/Co (4.8–5.9) | |||
| Neutralizing Ab | Reactive neutralizing Ab: 100% (96.1–100) | 88% (71.9–95.0) | 99% (94.6–99.5) | |||
| T cell response | INF-γ concentration: 4762 mIU/ml (IQR: 2723–8403) | 1061 mIU/ml (IQR: 599–2274) | 2026 mIU/ml (IQR 1459–4621) | |||
| Groβ[24] | Spike protein Ab | Median IgG titers increased 135-fold (63.9 U/ ml, 4.27–2005→8815 U/ml, 1206–19,046) | NA | NA | The heterologous ChAd/BNT was potent humoral immune response and elicits T cell reactivity | |
| Neutralizing Ab | Median ACE2 neutralization increased after BNT booster (62%, 32–95–>98%, 89–98) | |||||
| T cell response | 100% participants developed CD4 +T cells and 89% produced CD8 + T cells | |||||
| Barros[30] | Spike protein Ab | Ab IgG: 625.7 RU/mlAb IgA: 3.76 RU/ml | Ab IgG: 160.9 RU/mlAb IgA: 0.87 RU/ml | Ab IgG: 574.1 RU/mlAb IgA: 5.06 RU/ml | Mixing vaccination provided potent higher immune response to ChAd/ChAd group. Boosting with BNT induced higher frequency of T cells response | |
| T cell response | A significant higher T cell response in ChAd/ BNT group | |||||
| B cell response | Similar extent in expansion of spike-specific memory B in all groups | |||||
| Behrens[31] | Spike protein Ab | 611.o RU/ml (SD: 104.5) | 171.9 RU/ml (SD: 121.8) | NA | Study supported for heterologous boost vaccination of individuals with completed homologous ChAd vaccination | |
| Benning[32] | Spike protein Ab | 116.2 9IQR 61.84–170) | 13.09 (IQR 7.03–29.02) | 145.5 (IQR 100–291.1) | Heterologous induced strong and broad humoral response | |
| Neutralizing Ab | Percent of inhibition 96.8 (IQR 96.7–96.9) | 93.5 (88.6–96.7) | 97.0 (96.1–98.0) | |||
| Dimeglio[34] | Neutralizing Ab | 95.4% | 63.6% | 68.2% | ChAd/BNT heteroglous regimen provided a stronger Ab response than either of the homologous regimens | |
| Valiée[21] | Spike protein Ab | 7268.6 (6501.3–8128.3) | NA | 10,734.9 (9141.1–12,589.3) | Applying heterologous second dose of BNT was associated with decrease of Ab response levels compared to homologous BNT vaccination | |
| ChAd, mRNA, BNT | ChAd/mRNA | mRNA/mRNA | ChAd/ChAd | |||
| In these two studies, the mRNA was mRNA 1273-vaccine from Moderna company | Fabricius[35] | Neutralizing Ab | ‘ > 85% participants exhibited stronger neutralizing capacity | ‘> 90% vaccinated individuals exhibited no or medium level neutralization capacity | Heterologus vaccination booster regimens with mRNA could allow enhanced protection against SARS-CoV-2 | |
| T cell response | Peak IFN-γ secretion was significant stronger | |||||
| Normark[29] | Spike protein Ab | 115 time as high as. 128,108 | NA | 4320 | Potent induction of SARS-CoV-2 immune response had trend to be higher in heterologous group | |
| RBD Ab | 125 times as high as, 41,680 | 1224 | ||||
| Neutralizing Ab | Reciprocal serum neutralization titer 20 times as high as | NA | ||||
| In these two studies, the mRNA was either BNT and mRNA1273from Moderna | Schmidt cellular[27] | Humoral response | In transplant recipients, the heterologous regimen led to significant induction in IgG level and neutralizing activity | |||
| T cell response | CD4 T cell levels was reported a significant increase in mixing boosting compared to mRNA boosting | |||||
| Schmidt[33] | Spike protein Ab | 3630 BAU/ml (IQR 3721) | 4932 (IQR 4239) | 404 (IQR 510) | Heterologous strategy led to a strong induction of both antibodies and T cells and approximately tenfold higher than hom0ologous vector vaccination | |
| Neutralizing Ab | Majority of individuals had 100% inhibitory activity | This number was lower | ||||
| T cell response | Median % was 0.17 (IQR 0.13%) | 0.16 (IQR 0.19%) | 0.04 (IQR 0.04%) | |||
cell; Ab: antibody; RBD: receptor binding domain; IQR: presented as median; SD: standard deviation; GMT: Geometric mean titerss; SFC: spot-forming units; PMBC: peripheral blood mononuclear; NA: Not available; CI: confidence interval; mRNA: messenger RNA; mRNA1273: Vaccine from Moderna company; ChAd: Astrazeneca, vector (Covisheld) vaccine; BNT: Pfizer mRNA vaccine
When mRNA vaccine platform derived from different company (Pfizer or Moderna), the immunological activity was consistent in the previous review with the higher immune response being observed in heterologous compared to homologous ChAd/ChAd vaccination (Table 2). These findings supported the mRNA booster vaccination in ChAd prime individuals in order to solve the shortage of vaccine delivery.
4.4.2. Heterologous regimen of vector-based and inactivated vaccine
Because of emergency vaccine program, the heterologous vaccine regimens combined different vaccine platforms in different orders (e.g., vector-based prime/inactivated boost or inactivated prime/vector-based boost). Although the consideration of anxiety, safety, and efficacy was raised, the heterologous vaccine strategies were demonstrated the higher antibody response compared to inactivated prime-boost vaccine. Particularly, the spike protein antibody was higher in heterologous vaccination group compared to homologous vaccination of vector-based vaccine [25] or homologous vaccination of inactivated vaccine [36]. The neutralizing antibody response and RBD antibody response were higher in participants vaccinated with heterologous vaccination than participants vaccinated with homologous vaccination [26]. In contrast, the RBD antibody was lower among group vaccinated with heterologous regimen than group vaccinated with homologous regimen of vector-based vaccine [25]. Moreover, the T cell response was reported in covaxin/covaxin and ChAd/ChAd groups with the forward higher response was in ChAd/ChAd vaccination [25] ( Table 3). On the other hand, while evaluating the IgG1/IgG4 response of CoVac and Convidecia vaccine, the heterologous group induced more potent response than homologous group [26]. Interestingly, the combination of vector and inactivated virus vaccine could offer potent immune memory in individuals, this might be the effect of immunodominance hierarchy which focusing to the insert in inactivated vaccine group [37], [38].
Table 3.
Immunogenicity in different prime/boost regimen with vector and inactivated vaccine type in included studies.
| Studies | Type of vaccine | Spike protein Ab | RBD Ab | Neutralizing Ab | T cell response | Major results |
|---|---|---|---|---|---|---|
| Kant[25] | ChAd/ Covaxin | 1145 (520.7–2520) | 1866 (1003–3472) | NA | Immunization with combination of an vector-based type and inactivated whole virus vaccine elicited better immunogenicity | |
| Covaxin/ Covaxin | 742.4 (485.8–1134) | 710 (461–1092) | 39.4 (IQR 33.87–49.27) | |||
| ChAd/ChAd | 353.7 (219.9–568.9) | 2260 (1881–2716) | 47.66 (IQR 40.03–55.02) | |||
| Li[26] | CoVac/Convidecia | NA | 941.8 (663.9–1336.1) | 49.6 (35.1–70.2) | IgG1/IgG4: 24.4 (17.7–33.6) | Heterologous prime-boost regiment with ChAd after priming with CoronaVac induced significantly immunogenic than homogenous boost with CoronaVac |
| CoVac/CoVac | 154.1 (116.3–203.3) | 10.6 (8.3–13.5) | IgG1/IgG4: 3.8 (3.1–4.6) | |||
| CoVac/CoVac/Convidecia | 3090.1 (2636.1–3622.3) | 150.3 (128.6–175.7) | IgG1/IgG4: 42.4 (35.6–50.6) | |||
| CoVac/CoVac/CoVac | 369.0 (304.2–447.5) | 35.3 (29.4–42.4) | IgG1/IgG4: 6.1 (5.2–7.1) | |||
| Yorsaeng[36] | CoVac/ChAd | 797.2 U/ml (598.7–1062) | NA | NA | NA | ChAd boosting after CoVac priming provided better antibody response than two doses of CoVac |
| CoVac/ CoVac | 96.4 U/ml (76.1–122.1) | |||||
| ChAd/ ChAd | 818.1 U/ml (662.5–1010) |
ChAd: Astrazeneca, vector (Covisheld); Convidecia: recombinant adenovirus type-5-vectored vaccine; CoronaVac: inactivated SARS-CoV-2 vaccine (CoVac); Covaxin: inactivated whole virion BBV152 vaccine
4.4.3. Effect of heterologous regimens on SAR-Cov-2variants
The heterologous regimens showed more potent immunogenicity against the variant of concern α, β, and γ. The antibody responses against the Delta variant in heterologous regimens were higher than homologous regimen [24], [20], [25]. The greater immune response was reported in combination between vector-based/mRNA vaccine and vector-based/inactivated vaccine [25] ( Table 4 ). These studies reported the evaluation of geometric mean titerss of neutralizing antibody against the variants. The spread of new variant also raised a huge concern due to the reduction of vaccine protection or efficacy of drug therapy. Therefore, the superior immunogenicity of heterologous vaccine regimens supported the application of the heterologous regimens in the nationwide vaccine programs.
Table 4.
Immunogenicity in different variants of concern in reported studies.
| Study | Type of vaccine | Outcomes | Type of variant | |||
|---|---|---|---|---|---|---|
| Hillus[23] | ChAd/BNT | The Geometric mean of 50% inhibitory dose (95% CI) | α (B.1.1.7) | β (B,1.351) | γ (B.1.1.28.1) | δ (B.1.617.2) |
| 956.6 (835.6–1095) | 417.1 (349.3–498.2) | NA | NA | |||
| ChAd/ChAd | 212.5 (131.2–344.4) | 48.5 (28.4–82.8) | ||||
| BNT/BNT | 369.2 (310.7–438.6) | 72.4 (60.5–86.5) | ||||
| Groβ[24] | ChAd/BNT | Neutralizing activities median titer of serum samples | 2744 (209.8–8985) | 1297 (252–6523) | NA | 1309 (150–13,252) |
| BNT/BNT | Compared to ChAd/BNT group | Lower ( p < 0.001) | Lower ( p < 0.05) | Lower (not significant) | ||
| Barros[30] | ChAd/BNT | Neutralization capacity of Ab | All participants | All participants | All but two participants | NA |
| ChAd/ChAd | Increased in some individuals | No effect | No effect | |||
| Behrens[31] | ChAd/BNTWith ChAd/ChAd | 50% neutralization titers (NT50) | ChAD/BNT induced higher levels against all type of variants compared to ChAd/ChAd group.NT50 ≥ 100 in 85% of vaccines in delta variant | |||
| Hammeschmidt[20] | ChAd/BNT with ChAd/ChAd | Surrogate virus neutralization tests | NA | NA | NA | ChAd/BNT vaccination induced ninefold increase in neutralizing titers compared to ChAd/ ChAd group. |
| Fabricius[35] | ChAd/mRNA | Mean neutralization capacity individuals | 87% | 85% | 71% | NA |
| mRNA/mRNA | 76% | 73% | 56% | |||
| ChAd/ChAd | 48% | 57% | 15% | |||
| Normark[29] | ChAd/mRNA | Neutralizing Ab | Induced Ab could neutralize the β variant | |||
| ChAd/ChAd | Did not induce potent Ab against this variant | |||||
| Kant[25] | ChAd/covaxin | Geometric mean titers with 95% confidence interval (CI) | 396.1 (199.1–788) | 151 (80.21–284.3) | NA | 241.2 (74.99–775.9) |
| ChAd/ChAd | 122.7 (59.36–253.7) | 48.43 (19.71–119) | 51.99 (19.65–137.6) | |||
| Covaxin/Covaxin | 112.4 (76.56–164.9) | 52.09 (34.9–77.73) | 54.37 (27.26–108.4) | |||
NA: Not available; CI: confidence interval
5. Discussion
The present work evaluated reactogenicity and immunogenicity of SARS-CoV-2 heterologous vaccination regimens in comparison to homologous vaccination regimens to provide scientific evidence in determination of vaccine strategy for the pandemic. In regard to the reactogenicity, the local and systematic reactions were well tolerated and there were no severe events occurring by vaccination. There was inconsistent between the total results of adverse reactions as equal reactions [22], [23], higher reactions [18], [32], [25], [26] in mixing vaccination group. The explanation for more frequency of systemic reactogenicity in heterologous groups might be the higher reactions in young age group with ChAd and BNT [39], [40] or the variety in interval of prime-boost vaccination time [41]. In contrast, after the second dose vaccination, the adverse events in individuals immunized with the vector-based vaccine was more frequent compared to mRNA vaccines [42]. The systemic events were generally less frequent in individuals receiving heterologous regimen than individuals receiving homologous regimen [27], [43]. However, in solid transplant recipients, the systemic events in individuals receiving heterologous regimen was more frequent than individuals receiving homologous regimen. In term of heterologous regimen of vector-based vaccine and inactivated vaccine, the similar document was cited of the slightly higher incidences of injection-site and systemic reactions. One study reported differences between male and female in reactogenicity because of stronger immune response among females than males [44]. The remaining studies did not observe the difference in subgroup analysis.
Immunological data suggested that either heterologous regimens of vector-based/mRNA or vector-based/ inactivated vaccine might be highly effective in preventing COVID-19. These findings were observed in the study conducted on animal [45]. The mechanism for this action could be that using different platforms has induced protection from different pathways. The mRNA vaccine elicited extremely high neutralizing and binding antibody titers while the vector-based vaccine produced polyclonal antibodies [46], [47]. Additionally, the enhancer immunogenic effect might be related to the different natural immune response activated by the inactivated vaccine and the vector-based vaccine [37], [48]. Furthermore, the differences in innate immunity after the first and second dose of vaccination and the potential role of trained innate cells might partially explain the improvement of immunogenicity in heterologous injection [49]. Notably, the combination of different vaccine platforms increased the cellular immunity responses while the second dose of ChAd failed to improve the cellular response obtained after an initial dose [39]. Another benefit of the heterologous approach was to prevent the development of immunity by the virus against a particular viral vector-based vaccine [13]. These results were consistent with the previous studies in animal models, that the heterologous of ChAd and BNT induced IgG specific titers and robust T cell helper responses in mice [45]. A combination of inactivated virus vaccine with other platform vaccine as adenovirus vectored could improve neutralizing antibody and T cell response [50]. Furthermore, heterologous immunization strategy with adenovirus vectored vaccine followed by inactivated/recombinant subunit/mRNA vaccine vaccination increased levels of neutralizing antibodies and promoted the modulation of antibody responses [51].
With emerging variants of concern, current evidence indicated that the higher immune response in heterologous regimens compared to homogenous regimens against the current type of variants (α, β, γ, δ). The efficacy of heterologous regimens against variants was observed in both humoral and cellular immune response and thus was suggested as a suitable strategy to contain emerging SAR-CoV-2 variants [52]. Besides that, some studies have been conducted to evaluate the third dose application, the result were consistent about the robust immunogenic effect in heterologous vaccination [26], [16].
In contrast to the previous articles, we conducted a systematic study has been updated the status of heterologous strategy for not only prime vector/ boost mRNA vaccination but also the prime/boost vector/ inactivated vaccination [41], [53]. Moreover, this is the first time the studies about matching vaccine was assessed by powerful tools for systematic study as Risk of bias 2 from Cochrane assessment for clinical trials and NewCastle- Ottawa assessment scales for cohort studies. We also used more source of data from printed and preprinted papers to entirely evaluated the matching vaccination. Additionally, the consideration for immunogenicity against the variants have been pointed out as the evidence for potent immune response of mixing vaccination. Thus, this systematic review was essential, important to give comprehensive, completed evaluation of heterologous vaccine strategy. Because of the variety of outcome quantified numbers, the network meta-analysis used the same outcome of previous review, however the method to calculate was different and this result was more intuitive to evaluate the reactogenicity. Besides that, the local and systemic reactions were assessed and pointed out to demonstrate the effect of heterologous or homogenous vaccination to the participants in studies (Table S3, S4).
The present study has several strengths. This review followed the PRISMA construction for conducting the assessment. Although the direct meta-analysis was impossible, the network meta-analysis was carried out to evaluate the reactogenicity of heterologous vaccination in term of fever symptoms which frequently occurred by COVID-19 vaccines. The risk of bias was assessed separately for clinical trials and observational studies by using Cochrane assessment and NewCastle- Ottawa assessment scales.
This systematic review was subjected some limitations. We cannot compare directly between studies because of the diversity of quantitative methods for antibody responses. Therefore, the work has lack of direct meta-analysis result. The interval of prime-boost injections varied between studies and has been not pointed out because of complication and the supplier contradiction.
6. Conclusion
The systematic review provided assessment and evidence about the higher induction of robust immunogenicity and tolerated reactogenicity of heterologous regimens (vector-based/mRNA vaccine or vector-based/inactivated vaccine). The heterologous vaccination regimens might be an effective tool to contain the COVID-19 pandemic and the emergence of new variants. A future studies should investigate the efficacy and effectiveness of heterologous vaccination regimens.
Funding
This research received no external funding.
CRediT authorship contribution statement
TTN, THTQ, TMT, HTN, TKV and GVV involved in conceptualization; TTN, TMT, and GVV performed literature search and screen. TTN, THTQ, TMT, TKV and GVV performed quality assessment and analysis; TTN, THTQ, TMT, HNP, HTN and GVV were responsible for methodology and software; TTN, THTQ and GVV wrote the first draft. TTN, TKV and GVV supervised and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Declaration of conflicting interests
The authors declare no conflict of interest.
Acknowledgments
We thank everyone’s efforts to combat COVID-19.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.biopha.2022.112650.
Appendix A. Supplementary material
Supplementary material
.
Data availability
The datasets used for the analysis in the present study are available from the corresponding author on reasonable request.
References
- 1.Van Vo, G., et al. SARS-CoV-2 (COVID-19): beginning to understand a new virus. Adv. Exp. Med Biol. 2021;1321:3–19. doi: 10.1007/978-3-030-59261-5_1. [DOI] [PubMed] [Google Scholar]
- 2.WHO, Coronavirus disease (COVID-19) Dashboard. [cited 2021 November 04], 2021.
- 3.Nguyen T.T., et al. Microneedles enable the development of skin-targeted vaccines against coronaviruses and influenza viruses. Pharm. Dev. Technol. 2021:1–12. doi: 10.1080/10837450.2021.2008967. [DOI] [PubMed] [Google Scholar]
- 4.Vo V.G., et al. Additional diagnostic testing of the 2019 novel coronavirus (SARS-CoV-2) Mol. Cell Toxicol. 2020:1–3. doi: 10.1007/s13273-020-00096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor L. Covid-19: 1.5 million children have been orphaned by pandemic, study estimates. Br. Med. J. 2021;374:n1871. doi: 10.1136/bmj.n1871. [DOI] [PubMed] [Google Scholar]
- 6.Pilishvili T., et al. Effectiveness of mRNA Covid-19 Vaccine among U.S. Health Care Personnel. N. Engl. J. Med. 2021;385(25) doi: 10.1056/NEJMoa2106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rotshild V., et al. Comparing the clinical efficacy of COVID-19 vaccines: a systematic review and network meta-analysis. Sci. Rep. 2021;11(1):22777. doi: 10.1038/s41598-021-02321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson R.M., et al. Challenges in creating herd immunity to SARS-CoV-2 infection by mass vaccination. Lancet. 2020;396(10263):1614–1616. doi: 10.1016/S0140-6736(20)32318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karim S.S.A., Karim Q.A. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398(10317):2126–2128. doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singanayagam, A., et al., Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. The Lancet Infectious Diseases. [DOI] [PMC free article] [PubMed]
- 11.Marín-Hernández D., Nixon D.F., Hupert N. Heterologous vaccine interventions: boosting immunity against future pandemics. Mol. Med. 2021;27(1):54. doi: 10.1186/s10020-021-00317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoai Ta, Q.T., Vo V.G., Jawad M. COVID-19 and compromised tuberculosis control efforts: Highlighting the need for integration of community pharmacies into the national tuberculosis programme. Res. Soc. Adm. Pharm. 2021;17(4):823–825. doi: 10.1016/j.sapharm.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kunal S., et al. Mix and match COVID-19 vaccines: potential benefit and perspective from India. Post. Med J. 2021 doi: 10.1136/postgradmedj-2021-140648. [DOI] [PubMed] [Google Scholar]
- 14.Higgins J.P.T., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells G.A., D O′Connell B.S., Peterson J., Welch V., Losos M., Tugwell P. Ottawa Health Research Institute; Ottawa: 2012. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-randomized Studies in Meta-analysis. [Google Scholar]
- 16.Bonelli M., et al. Additional heterologous versus homologous booster vaccination in immunosuppressed patients without SARS-CoV-2 antibody seroconversion after primary mRNA vaccination: a randomized controlled trial. medRxiv. 2021 doi: 10.1136/annrheumdis-2021-221558. 2021.09.05.21263125. [DOI] [PubMed] [Google Scholar]
- 17.Gram M.A., et al. Vaccine effectiveness when combining the ChAdOx1 vaccine as the first dose with an mRNA COVID-19 vaccine as the second dose. medRxiv. 2021 2021.07.26.21261130. [Google Scholar]
- 18.Shaw R.H., et al. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet. 2021;397(10289):2043–2046. doi: 10.1016/S0140-6736(21)01115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tenbusch M., et al. Heterologous prime–boost vaccination with ChAdOx1 nCoV-19 and BNT162b2. Lancet Infect. Dis. 2021;21(9):1212–1213. doi: 10.1016/S1473-3099(21)00420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammerschmidt S.I., et al. Neutralization of the SARS-CoV-2 Delta variant after heterologous and homologous BNT162b2 or ChAdOx1 nCoV-19 vaccination. Cell Mol. Immunol. 2021:1–2. doi: 10.1038/s41423-021-00755-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vallée A., et al. An immunogenicity report for the comparison between heterologous and homologous prime‐boost schedules with chadox1–s and bnt162b2 vaccines. J. Clin. Med. 2021;10:17. doi: 10.3390/jcm10173817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borobia A.M., et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398(10295):121–130. doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hillus D., et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir. Med. 2021 doi: 10.1016/S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groß R., et al. Heterologous ChAdOx1 nCoV-19 and BNT162b2 prime-boost vaccination elicits potent neutralizing antibody responses and T cell reactivity. medRxiv. 2021 doi: 10.1016/j.ebiom.2021.103761. 2021.05.30.21257971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kant R., et al. Serendipitous COVID-19 vaccine-mix in Uttar Pradesh, India: safety and immunogenicity assessment of a heterologous regime. medRxiv. 2021 [Google Scholar]
- 26.Li J., et al. Heterologous prime-boost immunization with CoronaVac and Convidecia. medRxiv. 2021 2021.09.03.21263062. [Google Scholar]
- 27.Schmidt T., et al. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am. J. Transpl. 2021 doi: 10.1111/ajt.16818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X., et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398(10303):856–869. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Normark J., et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 Vaccination. N. Engl. J. Med. 2021;385(11):1049–1051. doi: 10.1056/NEJMc2110716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barros-Martins J., et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat. Med. 2021;27(9):1525–1529. doi: 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Behrens G.M., et al. SARS-CoV-2 delta variant neutralisation after heterologous ChAdOx1-S/BNT162b2 vaccination. Lancet. 2021;398(10305):1041–1042. doi: 10.1016/S0140-6736(21)01891-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benning L., et al. Heterologous ChAdOx1 nCoV-19/BNT162b2 prime-boost vaccination induces strong humoral responses among health care workers. Vaccines. 2021;9:8. doi: 10.3390/vaccines9080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt T., et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat. Med. 2021;27(9):1530–1535. doi: 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dimeglio C., et al. Heterologous ChAdOx1-S/BNT162b2 vaccination: neutralizing antibody response to SARS-CoV-2. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fabricius D., et al. mRNA vaccines enhance neutralizing immunity against SARS-CoV-2 variants in convalescent and ChAdOx1-primed subjects. Vaccines. 2021;9:8. doi: 10.3390/vaccines9080918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yorsaeng R., et al. Immune response elicited from heterologous SARS-CoV-2 vaccination: Sinovac (CoronaVac) followed by AstraZeneca (Vaxzevria) medRxiv. 2021 2021.09.01.21262955. [Google Scholar]
- 37.Kardani K., Bolhassani A., Shahbazi S. Prime-boost vaccine strategy against viral infections: Mechanisms and benefits. Vaccine. 2016;34(4):413–423. doi: 10.1016/j.vaccine.2015.11.062. [DOI] [PubMed] [Google Scholar]
- 38.Liao Y., et al. Intensified antibody response elicited by boost suggests immune memory in individuals administered two doses of SARS-CoV-2 inactivated vaccine. Emerg. Microbes Infect. 2021;10(1):1112–1115. doi: 10.1080/22221751.2021.1937328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramasamy M., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020:396. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polack F.P., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiu, N.C., et al., To mix or not to mix? A rapid systematic review of heterologous prime–boost covid-19 vaccination. Expert Review of Vaccines, 2021. [DOI] [PMC free article] [PubMed]
- 42.Walsh E.E., et al. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N. Engl. J. Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ou M.T., et al. Safety and reactogenicity of 2 doses of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 2021;105(10):2170–2174. doi: 10.1097/TP.0000000000003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCartney P.R. Sex-based vaccine response in the context of COVID-19. J. Obstet. Gynecol. Neonatal Nurs. 2020;49(5):405–408. doi: 10.1016/j.jogn.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spencer A.J., et al. Heterologous vaccination regimens with self-amplifying RNA and adenoviral COVID vaccines induce robust immune responses in mice. Nat. Commun. 2021;12(1):2893. doi: 10.1038/s41467-021-23173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sadarangani M., Marchant A., Kollmann T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021;21(8):475–484. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barrett J.R., et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat. Med. 2021;27(2):279–288. doi: 10.1038/s41591-020-01179-4. [DOI] [PubMed] [Google Scholar]
- 48.Lu S. Heterologous prime-boost vaccination. Curr. Opin. Immunol. 2009;21(3):346–351. doi: 10.1016/j.coi.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palgen J.-L., et al. Optimize prime/boost vaccine strategies: trained immunity as a new player in the game. Front. Immunol. 2021;12:554. doi: 10.3389/fimmu.2021.612747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang J., et al. Boosting with heterologous vaccines effectively improves protective immune responses of the inactivated SARS-CoV-2 vaccine. Emerg. Microbes Infect. 2021;10(1):1598–1608. doi: 10.1080/22221751.2021.1957401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He Q., et al. Heterologous prime-boost: breaking the protective immune response bottleneck of COVID-19 vaccine candidates. Emerg. Microbes Infect. 2021;10(1):629–637. doi: 10.1080/22221751.2021.1902245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duarte-Salles T., Prieto-Alhambra D. Heterologous vaccine regimens against COVID-19. Lancet. 2021;398(10295):94–95. doi: 10.1016/S0140-6736(21)01442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ho T.-C., et al. The effects of heterologous immunization with prime-boost COVID-19 vaccination against SARS-CoV-2. Vaccines. 2021;9:10. doi: 10.3390/vaccines9101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ostadgavahi A.T., et al. Heterologous immunization with Covishield and Pfizer vaccines against SARS-CoV-2 elicits a robust humoral immune response. J. Infect. Dev. Ctries. 2021;15(5):653–656. doi: 10.3855/jidc.15368. [DOI] [PubMed] [Google Scholar]
- 55.Lyski, Z.L., et al., Immunogenicity of Pfizer mRNA COVID-19 vaccination followed by J&J adenovirus COVID-19 vaccination in two CLL patients. medRxiv, 2021. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The datasets used for the analysis in the present study are available from the corresponding author on reasonable request.