Abstract
The effects of a vasoactive intestinal peptide (VIP) receptor antagonist (VIPhyb) on human glioblastoma cells were characterized. Pituitary adenylate cyclase activating polypeptide (125I-PACAP-27) bound with high affinity to U87, U118, and U373 cells. Specific 125I-PACAP-27 binding to U87 cells was inhibited, with high affinity, by PACAP but not VIP or VIPhyb (IC50 = 10, 1500, and 500 nM, respectively). By reverse transcriptase-polymerase chain reaction (RT-PCR), a major 305bp band was observed indicative of PAC1 receptors. PACAP-27 caused cAMP elevation and the increase in cAMP caused by PACAP-27, was inhibited by the VIPhyb. Also, PACAP-27 caused cytosolic Ca2+ elevation in Fura-2AM loaded U87 cells and the VIPhyb inhibited this increase. Using the MTT growth assay, the VIPhyb was shown to inhibit glioblastoma growth in a concentration-dependent manner. Using a clonogenic assay in vitro, 10 μM VIPhyb significantly inhibited proliferation of U87, U118, and U373 cells. In vivo, 0.4 μg/kg VIPhyb inhibited U87 xenograft proliferation in nude mice. These results suggest that the VIPhyb antagonizes PAC1 receptors on glioblastoma cells and inhibits their proliferation.
Index Entries: PACAP receptors, glioblastoma, VIP antagonist, proliferation
Introduction
Vasoactive intestinal peptide (VIP), a 28 amino acid peptide (Said and Mutt, 1970) and pituitary adenylate cyclase activating polypeptide (PACAP), a 27 or 38 amino peptide (Arimura et al., 1992) are examples of biologically active peptides in the central nervous system (CNS) (Magistretti et al., 1997). Immunoreactive VIP is present in distinct subpopulations of neurons the cerebral cortex, where it can be co-localized with acetylcholine (Magistretti et al., 1988; Bayraktar et al., 1997). VIPelevates cAMPlevels and stimulates adeylate cyclase in the cortex, striatum, hypothalamus, hippocampus, thalamus, and midbrain of the rat (Deschodt-Lanckman et al., 1977; Etgen and Browning, 1983; Quick et al., 1978). Furthermore, VIP causes glycogenolysis in the cortex and vasodilation of isolated cerebral arteries (Magistretti et al., 1981; Duckles and Said; 1982). Other functions attributed to VIP include protection of embryonic mouse spinal cord neurons in culture against electrical blockade (Brenneman and Eiden, 1986) through the secretion of glial factors such as activity-dependent neurotrophic factor (ADNF, Brenneman and Gozes, 1996). Like VIP, PACAP may exhibit neuroprotective properties as well and was shown to rescue cerebellar neurons from apoptotic death (Journot et al., 1997) and to facilitate differentiation of cortical neurons (DiCicco-Bloom et al., 1998).
The actions of VIP and PACAP are mediated by cell-surface receptors. The VPAC1 receptor was initially cloned from the rat and contains 459 amino acid residues and 7 hydrophobic domains (Ishihara et al., 1992). It has an 84% sequence homology with the human VPAC1 receptor and binds VIP as well as PACAP with high affinity. The VPAC2 receptor has been cloned and binds VIP and PACAP with high affinity (Lutz et al., 1993). The rat and human VPAC2 receptor have 459 and 437 amino acids, respectively, and 49% sequence homology with the rat VPAC1 receptor. Both the VPAC1 and VPAC2 receptor activate a guanine nucleotide binding protein (Gs), which stimulates adenylyl cyclase and elevates cAMP. The elevated cAMP activates protein kinase A (PKA), which phosphorylates cellular proteins such as CREB (Magistretti et al., 1997). In turn, a cAMP response element is activated on the 5′ upstream regulatory regions of early response genes such as c-fos. A PAC1 receptor has been cloned that has 495 amino acids (Spengler et al., 1993; Pisegna and Wank, 1993). The PAC1 receptor binds PACAP but not VIP with high affinity and activates both Gs and Gq. Gs activates adenylyl cyclase, whereas Gq activates phospholipase C leading to phosphatidyl inositol (PI) turnover. PI is thus metabolized to inositol-1,4,5-trisphosphate (IP3) and diacylglycerol. IP3 causes release of Ca2+ from the endoplasmic reticulum (ER; Tatsuno et al., 1992). The diacylglycerol released activates PKC, which leads to phosphorylation of cytoplasmic proteins such as MEK and mitogen-activated protein kinase (MAPK). MAPK can enter the nucleus and activate the serumresponse element of early genes such as c-fos. VIP alters the proliferation of cancer cells including neuroblastoma and small cell lung cancer (SCLC) (Wollman et al., 1993; O’Dorisio et al., 1992; Maruno et al., 1993; Moody et al., 1993a).
VIP receptors are antagonized by C-terminal fragments of VIP such as VIP(10–28) or VIP(6–28) (Fishbein et al., 1994); by D-amino acid substituted analogs of VIP or by growth hormone-releasing factor (GRF) such as [4Cl-DPhe6,Leu17]VIP or [AcTyr1,DPhe2] GRF (Waelbroeck et al., 1985; Pandol et al., 1986); and by chimeric peptides such as, Neurotensin 6–11VIP7–28 (VIPhyb) (Gozes et al., 1991a). VIPhyb inhibited the growth of lung, breast, and pancreatic cancer in vitro and in vivo (Moody et al., 1993, Zia et al., 1996, Zia et al., 2000). Here the effects of VIPhyb were investigated on glioblastoma cell lines.
Methods
Glioblastoma cells (U87, U138, and U373) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% heat-inactivated fetal bovine serum (FBS). The cells were cultured in 5%CO2/95% air at 37°C and were used during their exponential growth phase. Routinely the cells, which are adherent, were split 1/10 weekly.
The ability of glioblastoma cells to bind 125I-PACAP-27 and 125I-VIP was investigated. Glioblastoma cells were rinsed three times in SIT medium (DMEM containing 3 × 10−8 M Na2SeO3, 5 μg/mL insulin, and 10 μg/mLtransferrin) and binding studies conducted. Cells in 24-well plates (0.5 × 106) were incubated with 125I-PACAP-27 (0.2 nM) in the presence or absence of a competitor; the receptor-binding medium was SIT containing 1% bovine serum albumin (BSA) and 1 mg/mLbacitracin. After incubation at 37°C for 30 min, free radiolabeled peptide was removed by washing three times in receptor-binding medium. The cells, which contained bound peptide, were dissolved in 0.2 N NaOH and counted in a gamma counter.
VIP receptor subtypes were investigated using reverse transcriptase-polymerase chain reaction (RT-PCR) techniques. Total RNA was isolated using the guanidinium isothiocyanate (GIT) method. The cDNA was made from the RNA by reverse transcriptase. Specific primers for the PAC1 receptor were 5′-CATCCTTGTGCAGAAACTTC-3′ (sense, 1231–1250); 5′-GGTGCTTGAAGTCCACAGCG-3′ (antisense, 1516–1535). One μM primer pair, 100 μM dNTP, 2.5 U Taq polymerase, 2.5 mM MgCl2 and PCR buffer (Life Technologies, Rockville, MD) were added to the cDNA. The PCR was performed on a Perkin-Elmer/Cetus thermal cycler; 35 cycles at 94°C for 30 sec, 62°C for 45 s, and 72°C for 60 sec. Amplification products were separated by electrophoresis (2% agarose in TAE buffer) and visualized by ethidium bromide fluorescence.
The ability of VIP to elevate cAMP was determined in glioblastoma cells. U87 cells in 24-well plates were treated with 250 mL of SIT medium containing 1% BSA, 1 mg/mL bacitracin, and 100 μM isobutyl-methyl-xanthine. After 5 min, the reaction was quenched by the addition of an equal volume (0.25 mL) of ethanol. The samples were mixed and frozen at −80°C until assayed. Routinely 25 μL of sample was assayed in the cAMP radioimmunoassay (New England Nuclear, Boston, MA).
The ability of VIPhyb to alter the growth of glioblastoma cells was investigated in vitro using the MTT colorimetric and clonogenic assays. The semiautomated MTT assay measures the reduction of a tetrazolium compound by the tumor cells to formazan. The optical density at 540 nm was determined for 8 wells at each inhibitor concentration. Seeding densities were 5 × 104 cells/well and cells were grown in 96-well plates for 5 d.
Also, the ability of VIP receptor antagonists to inhibit the growth glioblastoma cells was investigated using the clonogenic assay. The base layer consisted of 2 mL of 0.5% agarose in SIT medium containing 5% FBS in 6-well plates (Falcon, Oxnard, CA). The top layer consisted of 2 mL of SIT medium in 0.3% agarose, 0.1% BSA, VIPhyb, and 5 × 104 single viable cells/mL. For each cell line and peptide concentration, triplicate wells were plated. After 2 wk, 1 mL of 0.1% p-iodonitrotetrazolium violet was added and after 16 h at 37°C the plates were screened for colony formation; the number of colonies larger than 50 mm in diameter were counted.
To test the proliferative aspects of VIPhybrid analogs in vivo, nude mice bearing glioblastoma xenografts were utilized. Female athymic Balb/c nude mice, 4–5 wk old, were housed in a pathogenfree temperature controlled isolation room and the diet consisted of autoclaved rodent chow and autoclaved water given ad libitum. U87 cells (1 × 107) were injected into the right flank of each mouse subcutaneously. Palpable tumors were observed in approx 90% of the mice after 2 wk. PBS (100 μL) or VIPhyb (0.1 or 10 μg/d) were injected subcutaneously adjacent to the tumor. The tumor volume (height × width × depth) was determined weekly by calipers and recorded. When the tumor became necrotic, the growth studies were terminated.
Results
Table 1 shows that all glioblastoma cell lines examined bound 125I-PACAP-27 with high affinity. The ratio of specific 125I-PACAP-27 to 125I-VIPwas approx 7/1 suggesting that the PAC1 receptors may predominate in glioblastoma cells. Similar results were obtained using U118 cells (data not shown).
Table 1.
| Cell line | 125I-VIP | 125I-PACAP-27 |
|---|---|---|
| U87 | 486 + 122 | 2825 + 957 |
| U138 | 567 + 147 | 4117 + 447 |
| U373 | 253 + 72 | 1403 + 277 |
The mean specific binding (cpm) + S.D. of 4 determinations is indicated.
The structures of VIP and PACAP-27 are shown below:
PACAP-27 His-Ser-Asp-Gly-Ile-Phe-Thr-Asp-Ser-Tyr-Ser-Arg-Tyr-Arg-Lys-Gln-Met-Ala-Val-Lys-Lys-Tyr-Leu-Ala-Ala-Val-Leu-NH2
VIP His-Ser-Asp-Ala-Val-Phe-Thr-Asp-Asn-Tyr-Thr-Arg-Leu-Arg-Lys-Gln-Met-Ala-Val-Lys-Lys-Tyr-Leu-Asn-Ser-Ile-Leu-Asn-NH2
VIPhyb Lys-Pro-Arg-Arg-Pro-Tyr-Thr-Asp-Asn-Tyr-Thr-Arg-Leu-Arg-Lys-Gln-Met-Ala-Val-Lys-Lys-Tyr-Leu-Asn-Ser-Ile-Leu-Asn-NH2
The specificity of 125I-PACAP-27 binding to U87 cells was investigated. Figure 1 shows that PACAP-27 inhibited 125I-PACAP-27 binding with high affinity (IC50 value of 10 nM). In contrast, VIP was less potent with an IC50 value of 1500 nM. VIPhyb inhibited 125I-PACAP-27 binding with an IC50 value of 500 nM. These results indicate that VIPhyb and VIP inhibit 125I-PACAP-27 binding with moderate affinity.
Fig. 1.
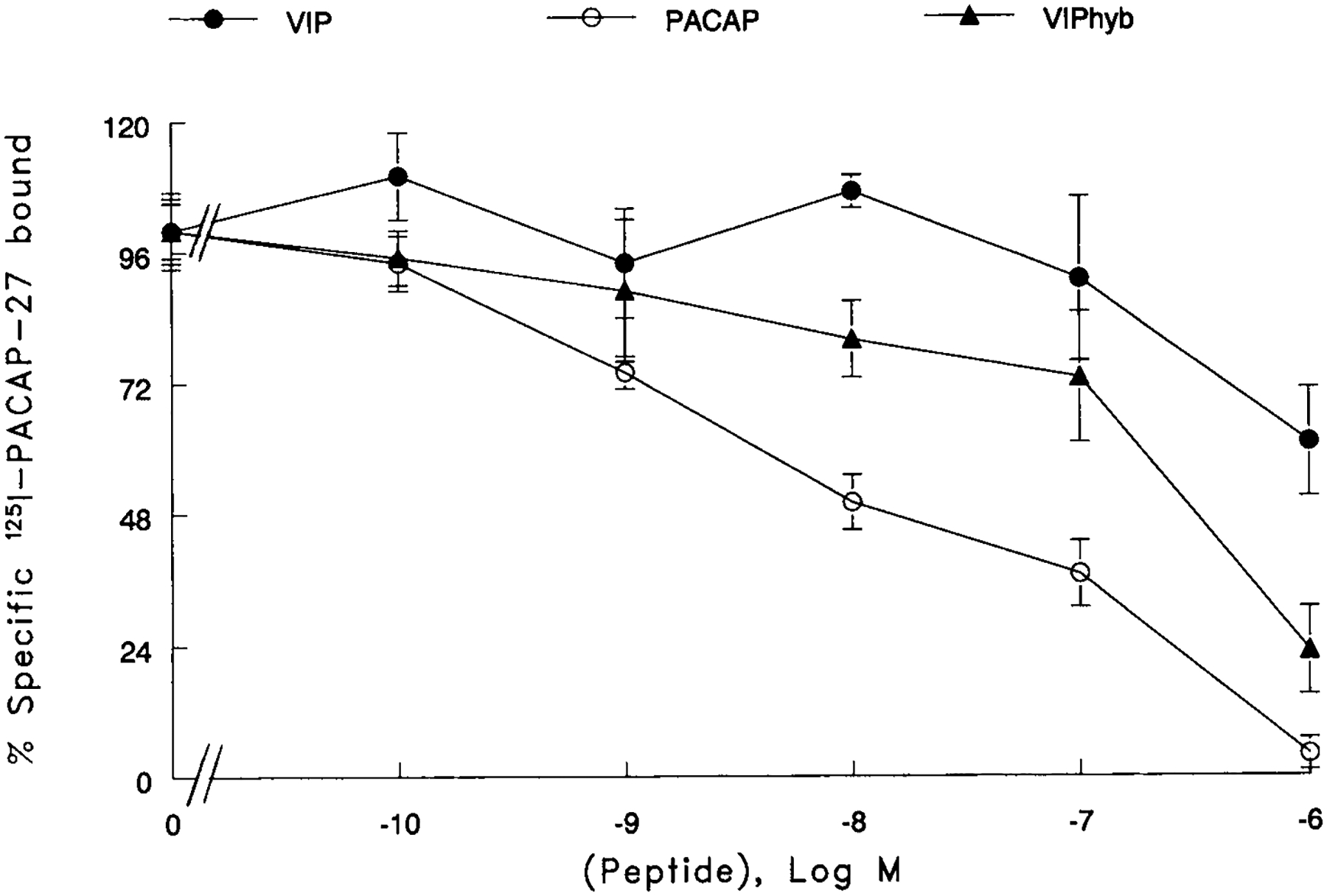
Specificity of 125I-PACAP-27 binding. The ability of varying doses of PACAP (○), VIP (●) and VIPhyb (▲) to inhibited specific 125I-PACAP-27 to U87 cells was determined. The mean value ±S.D. of 3 determinations each repeated in quadruplicate is indicated.
PAC1 receptors in glioblastoma cells were characterized by RT-PCR. Figure 2 shows that, a major 305 bp band was observed in all glioblastoma cell lines examined. The cell line, U87 appeared to have more of the PAC1-R PCR product than did the cell lines U138 or U373.
Fig. 2.
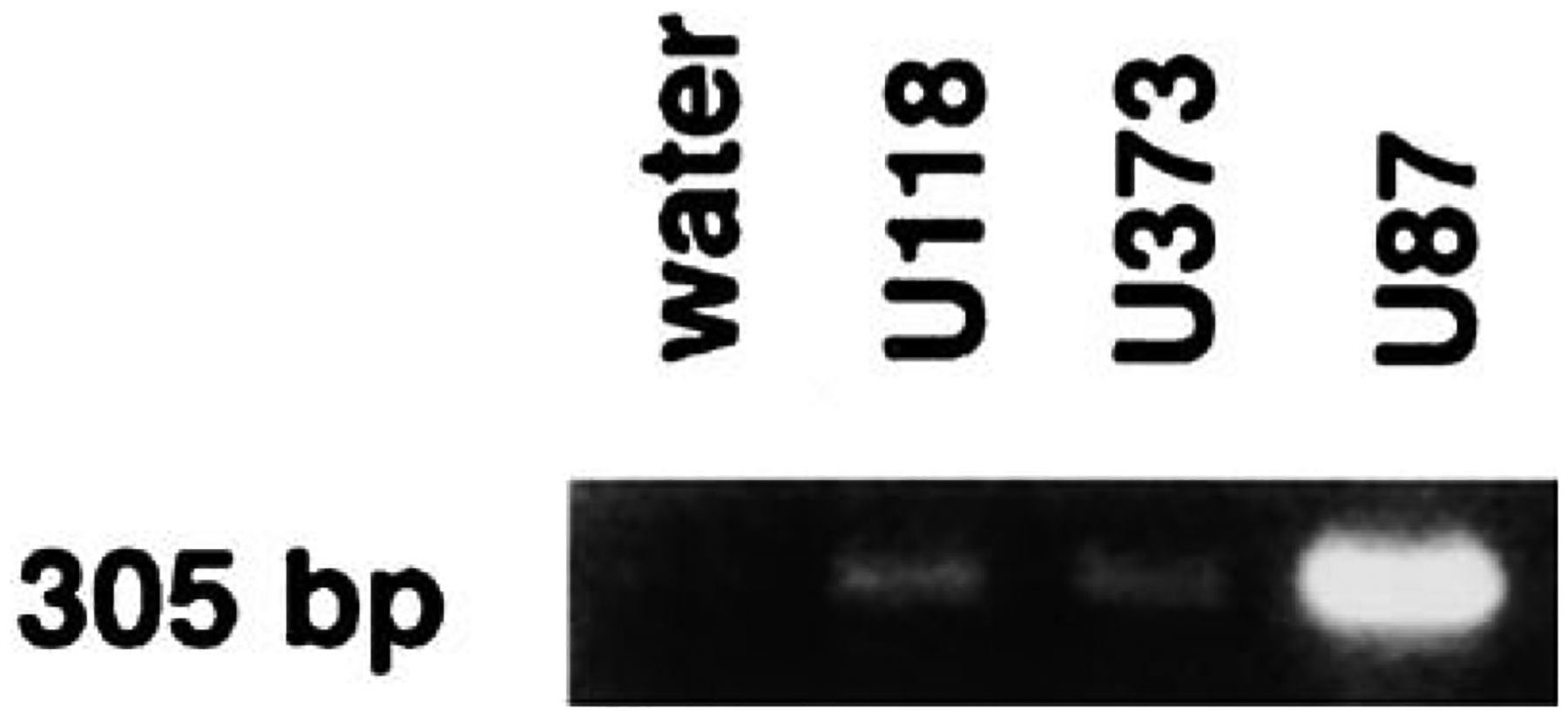
RT-PCR. A major 305 base pair PCR product was observed for U87, U118, and U373 cell lines.
The effects of PACAP-27 on second messenger production were investigated. Figure 3 shows that PACAP-27 caused cAMP elevation in U87 cells in a concentration-dependent manner. PACAP-27 (0.1 nM) had little effect on cAMP but 100 nM PACAP-27 elevated cAMP by 50-fold. The ED50 for PACAP-27 was 10 nM. VIPhyb (10 μM) had little effect on basal cAMP but shifted the PACAP-27 dose-response histogram to the right (ED50 = 50 nM). The results suggest that VIPhyb is a PAC1 receptor antagonist.
Fig. 3.
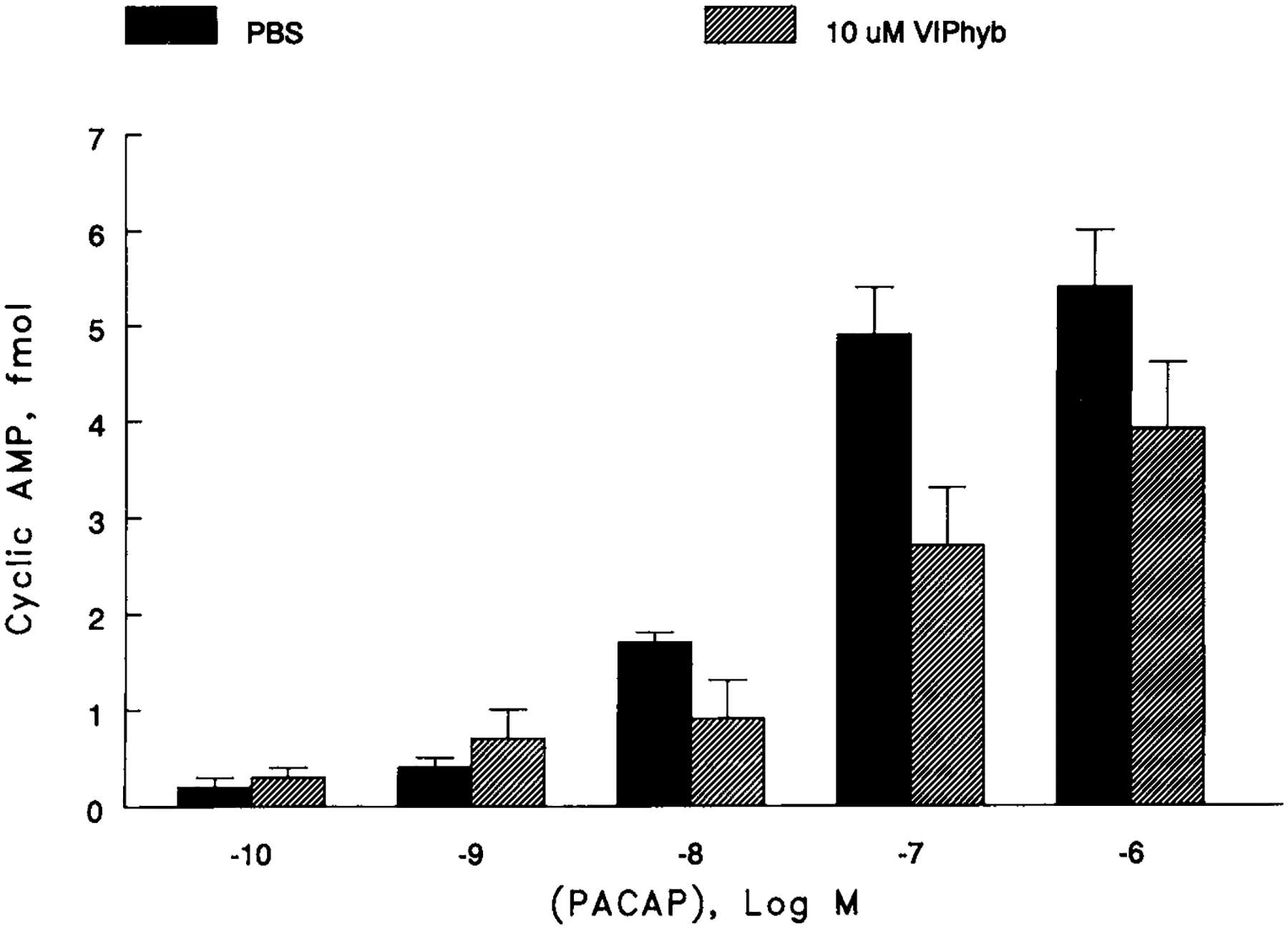
cAMP. The ability of PACAP to elevate the cAMP was determined as a function of concentration in the absence (g) or presence (1) of 10 mM VIPhyb. The mean value + S.D. of 4 determinations is shown.
The effects of PACAP-27 on cytosolic Ca2+ were investigated. Figure 4 shows that PACAP-27 (30 nM) caused cytosolic Ca2+ elevation within seconds after addition to U87 cells. The response was maximal within 15 s and slowly declined returning to basal levels after 1 min. 10 and 30 μM VIPhyb inhibited weakly and strongly, respectively, the increase in cyotoslic Ca2+ caused by 30 nM PACAP. Similar results were obtained using NIH/3T3 cells transfected with PAC1 receptor (data not shown).
Fig. 4.
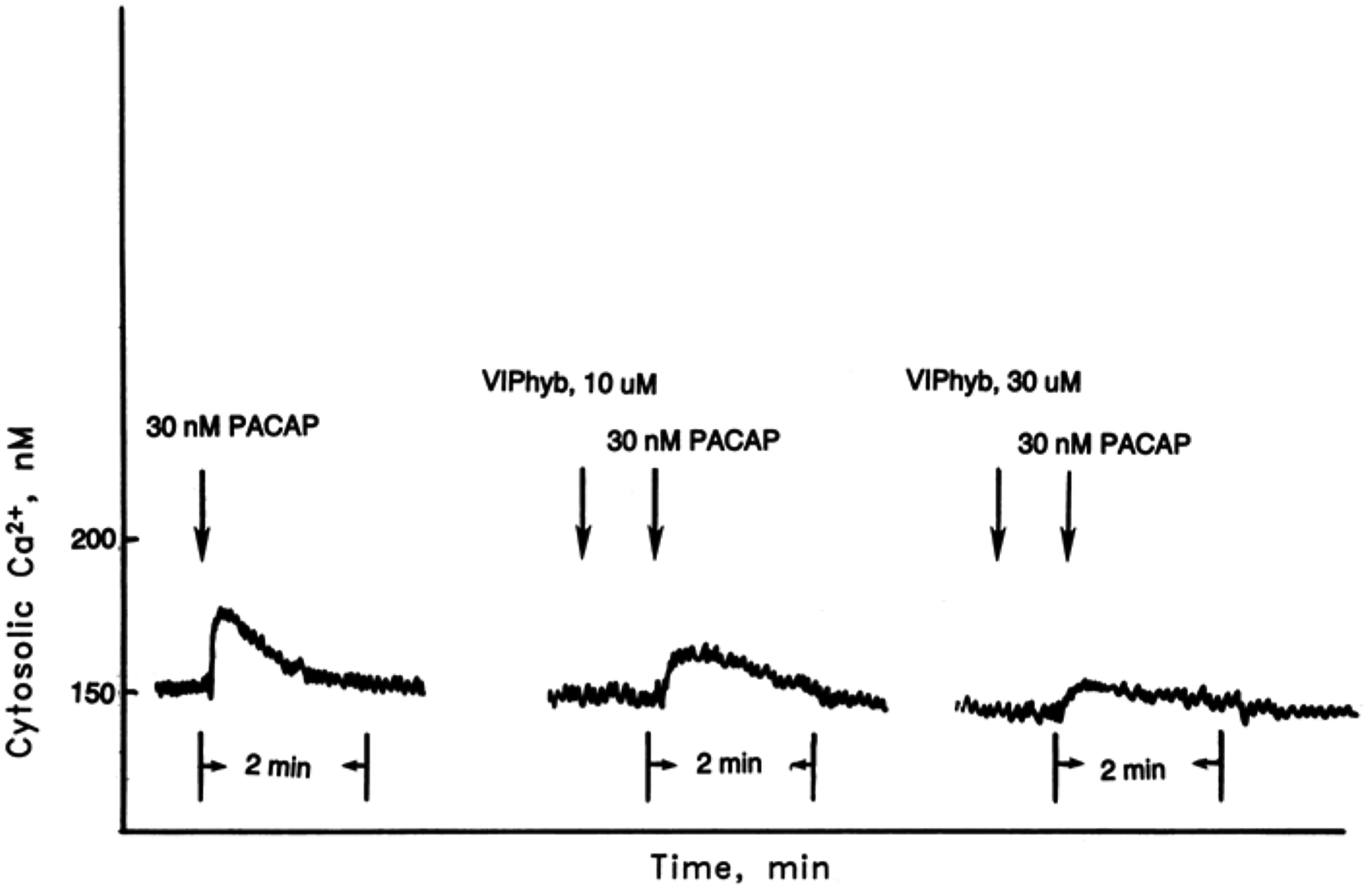
Cytosolic Ca2+. PACAP-27 (30 nM) caused cytosolic Ca2+ elevation using Fura-2 AM loaded U87 cells. The ability of 10 and 30 mM VIPhyb to inhibit the cytosolic Ca2+ increase caused by PACAP-27 was determined. This experiment is representative of two others.
The ability of VIPhyb to inhibit the growth of glioblastoma cells was investigated. Figure 5 shows that VIPhyb inhibited the growth of U373 cells using the MTT assay. VIPhyb had little effect of proliferation at 1 μM but significantly inhibited proliferation at 3 and 10 μM. Table 2 shows that VIPhyb had little effect on colony number at 0.1 μM and 1 μM VIPhyb significantly inhibited the proliferation of U87 and U373 but not U138 cells. In contrast, 10 μM VIPhyb significantly inhibited the proliferation of U87, U138, and U373 cells. These results indicate that VIPhyb inhibits the growth of glioblastoma cells.
Fig. 5.
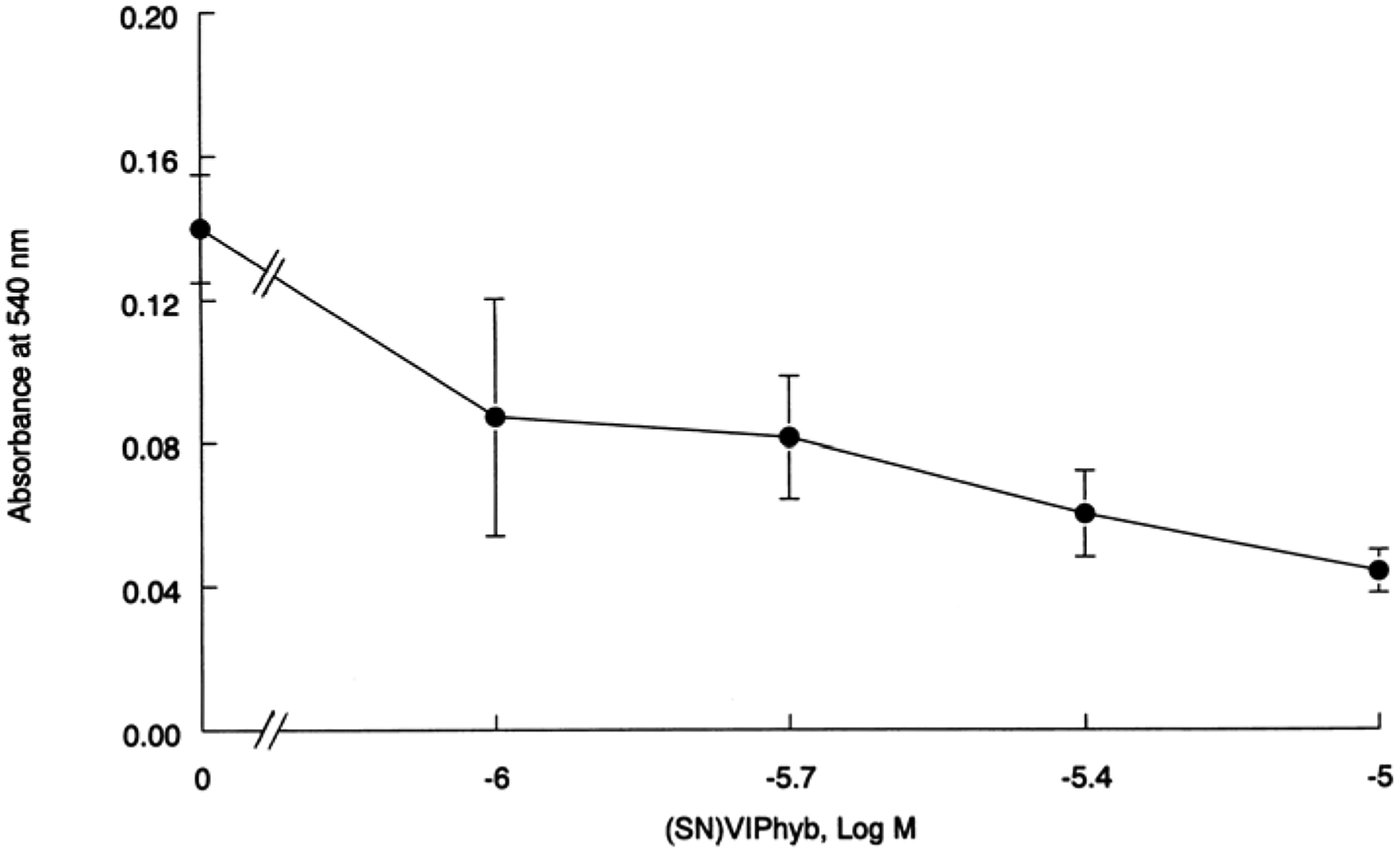
MTT assay. U373 cells were incubated with varying concentrations of VIPhyb. The mean value ± S.D. of 8 determinations is indicated; p < 0.05,* using Student’s t-test.
Table 2.
Clonogenic Assay
| Colony number | |||
|---|---|---|---|
| Addition | U87 | U138 | U373 |
| None | 180 ± 39 | 72 ± 20 | 242 + 42 |
| VIPhyb, 0.1 mM | 104 ± 53 | 55 ± 27 | 241 + 40 |
| VIPhyb, 1 mM | 95 ± 23* | 49 ± 20 | 141 + 38* |
| VIPhyb, 10 mM | 61 ± 26* | 41 ± 7* | 138 + 42* |
The mean value + S.D. of 3 determinations is indicated, p < 0.05, * using the student’s t-test.
The ability of VIPhyb to inhibit the proliferation of glioblastoma cells was investigated in vivo. Figure 6 shows that a palpable mass formed after 2 wk. The tumor grew exponentially and after 4 wk the tumor volume was 2392 mm3. Daily injection of VIPhyb (0.4 mg/kg, s.c.) slowed significantly xenograft poliferation and after 4 wk the tumor volume was 1571 mm3. In contrast, VIPhyb (0.004 mg/kg, s.c.) had little effect on U87 tumor poliferation and after 3 wk the tumor volume was 2464 mm3. These data indicate that VIPhyb inhibits glioblastoma proliferation in vivo.
Fig. 6.
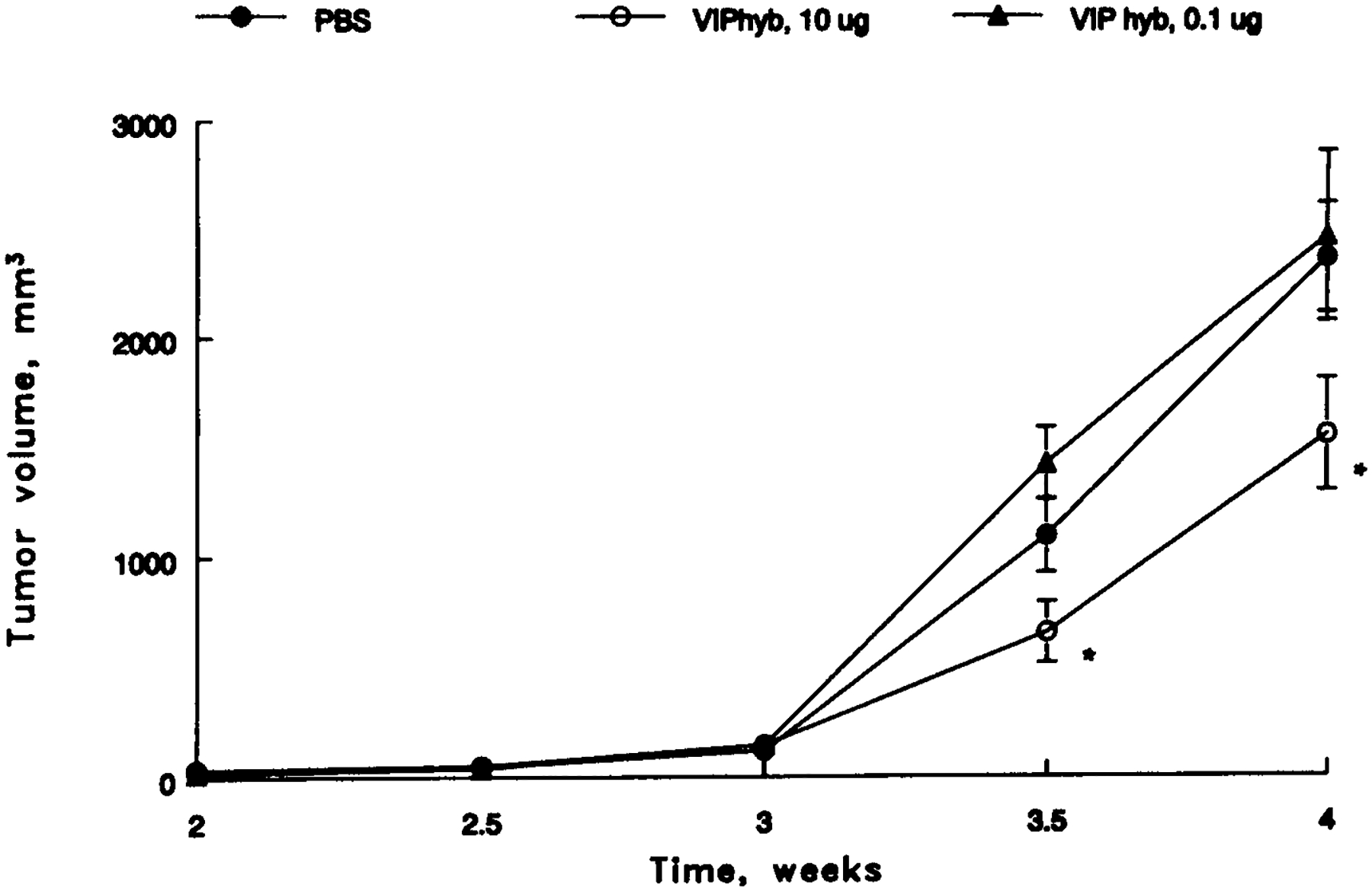
U87 xenografts in nude mice. A palpable mass formed after 1 wk and animals were subsequently injected with 100 mL of PBS daily s.c. (●), 10 μg of VIPhyb daily s.c. (○), and 0.1 μg VIP hyb (▲); p < 0.05, *. The mean value ± S.E. of 5 determinations is indicated.
Discussion
Glioblastoma, multiform, and infiltrative astrocytomas are the most common brain cancers (Levin et al., 1997). Traditionally, glioblastoma patients are treated with surgical resection followed by combination chemotherapy. Because only 25% of the treated patients show a 5-yr-survival, new modes of therapy are needed (Chu and Kramer, 1995). Previously, we found that VIPhyb antagonized VPAC1 receptors in neuroblastoma, breast, lung, and pancreatic cancer cells (Moody et al., 1993a, 1993b; Wollman et al., 1993; Lilling et al., 1995; Zia et al., 1996, 1999; Granoth et al., 2000a, 2000b). VIPhyb inhibited 125I-VIP binding to cancer cells. Also, VIPhyb inhibited the increase in cAMP and c-fos mRNA caused by VIP. Finally, VIPhyb inhibited the proliferation of cancer-cell lines in vitro and in vivo. Here the effects of VIPhyb were investigated on glioblastoma cells.
VIPhyb inhibited binding of 125I-PACAP-27 to glioblastoma cells with an IC50 value of 500 nM. In contrast, PACAP-27 and VIP inhibited 125I-PACAP-27 to glioblastoma cells with IC50 values of 10 and 1500 nM, respectively. Because PACAP binds with approx 1000-fold greater affinity than does VIP, PAC1 receptors may predominate in glioblastoma cells. U87, U138, and U373 cells had RT-PCR products for PAC1 receptors, suggesting that these cells have mRNA for PAC1 receptors. Preliminary data (T. Moody, unpublished) indicate that when NIH/3T3 cells are transfected with PAC1 receptors, 125I-PACAP-27 binds with high affinity and the specific 125I-PACAP-27 binding is inhibited by PACAP, VIPhyb, and VIP with IC50 values of 3, 700, and 1200 respectively. Also, the increase in cAMP, cytosolic Ca2+, and c-fos mRNA caused by PACAP-27 addition to NIH/3T3 cells that are transfected with the PAC1 receptor, was antagonized by the addition of the VIPhyb. Thus, the VIPhyb may be a PAC1 receptor antagonist.
Previously PAC1 receptors were detected in human gliomas (Reubi et al., 2000), including astroglial neoplasms, ependyomas, and oligoden-drogliomas. PACAP-27 or PACAP-38 but not VIP inhibited 125I-PACAP-27 to malignant astroglial membranes binding with high affinity (Robberecht et al., 1994). Also, PACAP-27 and PACAP-38 but not VIP strongly increased adenylyl cyclase activity in astroglial, eppendyoma, and oligodendroglioma membranes. PAC1 receptor mRNA was detected in astroglial neoplasms, glioblastoma, oligodendroglioma, ependymoma, and pituitary tumors but not meningliomas (Vertongen et al., 1995).
In addition to PAC1 receptors, VPAC1 receptors may be present on glioblastoma cells. Preliminary data (T. Moody, unpublished) indicate that VPAC1 receptor PCR products were present in U87, U118, and U373 cells. Further 125I-VIP did bind specifically to U-87, U118, and U-373 cells, although not as well as 125I-PACAP-27. Previously we found that the IC50 for VIPhyb to inhibit specific 125I-VIPbinding to NCI-H1299 lung cancer cells was 500 nM. Also, VIPhyb inhibited the ability of VIP to elevate cAMP and increase c-fos mRNA. Further, VIPhyb inhibited to clonal growth of lung cancer cells and the growth of xenografts in nude mice. Thus VIPhyb is a VPAC1 receptor antagonist using lung cancer cells. In addition, VPAC1 receptors have been identified in human glioma cell line U-343 (Nielsen et al., 1990). 125I-VIP bound with high affinity to U-343 cells and specific 125I-VIP binding was inhibited with moderate affinity by (N-Ac-Tyr1, D-Phe2)GRF(1–29)NH2, a VIP receptor antagonist. 125I-VIP was internalized at 37°C and readily degraded.
VPAC2 receptors have been identified in T98G cells (Vertongen et al., 1996). Specific 125I-VIP binding to T98G cells was inhibited with high affinity by VIP, PACAP-27, and PACAP-38. By RT-PCR VPAC2, but not VPAC1 or PAC1 receptor mRNA, was detected in T98G cells. Surprising VIP, PACAP-38, and PACAP-27 inhibited the growth of T98G cells. Preliminary data (T. Moody, unpublished) indicate that using NIH/3T3 cells transfected with VPAC2 receptors, specific 125I-VIP binding was inhibited by VIPhyb with an IC50 value of 1000 nM and VIPhyb inhibited the increase in cAMP caused by VIP. These results suggest that VIPhyb may be a VPAC2 receptor antagonist.
A particular advantage of VIPhyb is that it is a broad-spectrum VIP receptor antagonist, which blocks PAC1, VPAC1, and VPAC2 receptors. Previously, it was found that substance Pantagonists such as (D-Arg1, D-Pro2, D-Trp7,9, Leu11)substance P blocks gastrin-releasing peptide receptors in lung cancer cells (Bepler et al., 1988; Woll et al., 1988). Further substance P antagonists block neuromedin B receptors and bombesin receptor subtype-3, substance P receptors also block vasopressin receptors (Moody et al., 1992; Sethi et al., 1992; Mantey et al., 1997). For tumors that have multiple peptide growth-factor receptors, a broad spectrum peptide receptor antagonist may strongly inhibit cancer proliferation. In contrast, nonpeptide receptor antagonists such as PD165929 block only neuromedin B receptors (Eden et al., 1996). Nonpeptide antagonists for VIP and PACAP remain unknown.
Recently we found that VIPhyb enhances the cytotoxicity of chemotherapeutic agents in cancer cells (Moody et al., unpublished). In breast cancer cells VIPhyb decreased the IC50 for doxorubicin and taxol to kill MCF-7 breast cancer cells two- to four-fold in vitro. Also, VIPhyb potentiated the ability of taxol to inhibit MCF-7 xenograft growth in nude mice. These results suggest that in the presence of VIPhyb, less chemotherapeutic agent is needed to kill cancer cells.
In summary VIPhyb inhibits the proliferation of glioblastoma cells and functions as a PAC1 receptor antagonist. It remains to be determined if VIPhyb will be a therapeutic agent for patients with cancer.
Acknowledgments
The authors thank Amy Guzzone for technical assistance.
References
- Arimura A (1992) Pituitary adenylate cyclase activating polypeptide (PACAP): discovery and current status of research. Reg. Peptides 37, 287–303. [PubMed] [Google Scholar]
- Ashur-Fabian O, Giladi E, Brenneman DE, and Gozes I (1997) Identification of VIP/PACAP receptors on astrocytes using antisense oligodeoxynucleotides. J. Mol. Neurosci 9, 211–222. [DOI] [PubMed] [Google Scholar]
- Bayraktar T, Staiger JF, Acsady L, Cozzari C, Freund TF, and Ailles K (1997) Co-localization of vasoactive intestinal polypeptide, gamma-aminobutyric acid and choline acetyltransferase in neocortical interneurons of the adult rat. Brain Res. 757, 209–217. [DOI] [PubMed] [Google Scholar]
- Bepler G Zeymer U Mahmoud S, Fiskum G, Palaszynski E, Totsch M, et al. (1988) Substance P analogues function as bombesin receptopr antagonists and inhibit small cell lung cancer clonal growth. Peptides 9, 1367–1372. [DOI] [PubMed] [Google Scholar]
- Brenneman DE and Eiden LE (1986) Vasoactive intestinal peptide and electrical activity influence neuronal survival. Proc. Natl. Acad. Sci. USA 83, 1159–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman DE and Gozes I (1996) Afemtomolar-acting neuroprotective peptide. J. Clin. Invest 97, 2299–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschodt-Lanckman M, Robberecht P, and Christophe J (1977) Characterization of VIP-sensitive adenylate cyclase activity in guinea pig brain. FEBS Lett. 83, 76–80. [DOI] [PubMed] [Google Scholar]
- DiCicco-Bloom E, Lu N, Pintar JE, and Zhang J (1998) The PACAP ligand/receptor system regulates cerebral cortical neurogenesis. Ann. NY Acad. Sci 865, 274–289. [DOI] [PubMed] [Google Scholar]
- Duckles SP and Said SI (1982) Vasoactive intestinal peptide as a neurotransmitter in the cerebral circulation. Eur. J. Pharmacol 78, 371–374. [DOI] [PubMed] [Google Scholar]
- Eden JM, Hall MD, Higginbottom M, Horwell DC, Howson W, Hughes J, et al. (1996) PD165929-The first high affinity non-peptide neuromedin B (NMB) receptor selective antagonist. Bioorg. Med. Chem. Lett 6, 2617–2623. [Google Scholar]
- Etgen AM and Browning ET (1983) Activators of cyclic adenosine 3′-5′-monophosphate accumulation in rat hippocampal slices: action of vasoactive intestinal peptide (VIP). J. Neurosci 3, 2487–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein JA, Coy DH, Hocart SJ, Jiang NY, Mrozinski JE Jr., Mantey SA, and Jensen RT (1994) A chimeric VIP-PACAP analogue but not VIP pseudopeptides function as VIP receptor antagonists. Peptides 15, 95–100. [DOI] [PubMed] [Google Scholar]
- Gottschall PE, Tatsumo I, and Arimura A (1994) Regulation of interleukin-6 (IL-6) secretion in primary cultured rat astrocytes: synergism of interleukin-1 (IL-1) and pituitary adenylate cyclase activating polypeptide (PACAP). Brain Res. 637, 197–203. [DOI] [PubMed] [Google Scholar]
- Gozes I, McCune SK, Jacobson L, Warren D, Moody TW, Fridkin M, and Brenneman DE (1991) An antagonist to vasoactive intestinal peptide affects cellular functions in the central nervous system. J. Pharm. Exp. Ther 257, 959–966. [PubMed] [Google Scholar]
- Granoth R, Fridkin M, Rubinraut S, and Gozes I (2000a). VIP-derived sequences modified by N-terminal steryl moiety induces cell death: The human keratinocyte as a model. FEBS Lett. 475, 71–77. [DOI] [PubMed] [Google Scholar]
- Granoth R, Fridkin M, and Gozes I (2000b). VIP and the potent analog, stearyl-Nle(17)VIP induce proliferation of keratinocytes. FEBS Lett. 475, 78–83. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Shigemoto R, Mori K, Takahashi K, and Nagata S (1992) Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron 8, 811–819. [DOI] [PubMed] [Google Scholar]
- Journot L, Villalba M, and Bockaert J (1997) PACAP-38 protects cerebellar granule cells from apoptosis. Ann. NY Acad. Sci 865, 100–110. [DOI] [PubMed] [Google Scholar]
- Levin VA, Leibel SA, and Gutin PH (1997) Neoplasms of the central nervous system, in Cancer Principles and Practice of Oncology (DeVita VT Jr., Hellman S, and Rosenberg SA, eds.), Lippincott-Raven Publishers, Philadelphia New York, pp. 2022–2082. [Google Scholar]
- Lilling G, Wollman Y, Goldstein MN, Rubinraut S, Fridkin M, Brenneman DE, and Gozes I (1995) Inhibition of human neuroblastoma growth by a specific VIP antagonist. J. Mol. Neurosci 5, 231–239. [DOI] [PubMed] [Google Scholar]
- Lutz EM, Sheward WJ, West KM, Morrow JA, and Harmar AJ (1993) The VIP2 receptor: molecular characterization of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett. 334, 3–8. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Morrison JH, Shoemaker WJ, Sapin V, and Bloom FE (1981) Vasoactive intestinal polypeptide induces glycogenolysis in mouse cortical slices: a possible regulatory mechanism for the local control of energy metabolism. Proc. Natl. Acad. Sci. USA 78, 6535–6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ and Morrison JH (1988) Noradrenaline- and vasoactive intestinal peptide containing neuronal systems in neocortex: Functional convergence with contrasting morphology. Neuroscience 24, 367–378. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Cardinaux JR, and Martin JL (1998) VIP and PACAP in the CNS: regulators of glial energy metabolism and modulators of glutamatergic signaling. Ann. NY Acad. Sci. USA 865, 213–225. [DOI] [PubMed] [Google Scholar]
- Mantey SA, Weber HC, Sainz E, Akeson M, Ryan RR, Pradhan TK, et al. (1997) Discovery of a high affinity radioligand for the human orphan receptor, bombesin receptor subtype 3, which demonstrates that it has a unique pharmacology compared with other mammalian bombesin receptors. J. Biol. Chem 272, 26,062–26, 071. [DOI] [PubMed] [Google Scholar]
- Maruno K and Said SI (1993) Human small cell lung carcinoma inhibition by vasoactive intestinal peptide (VIP) and helodermin (Hd). Biomed. Res 13, 373–375. [DOI] [PubMed] [Google Scholar]
- Moody TW, Mahmoud S, Staley JS, Naldini L, Cirillo D, South V, et al. (1989) Human glioblastoma cell lines have neuropeptide receptors for bombesin/GRP. J. Mol. Neurosci 1, 235–242. [PubMed] [Google Scholar]
- Moody TW, Staley J, Zia F, Coy DH, and Jensen RT (1992) Neuromedin B receptors are present on small cell lung cancer cells. J. Pharmacol. Exp. Therap 263, 311–317. [PubMed] [Google Scholar]
- Moody TW, Zia F, Goldstein A, Naylor P, Sarin E, Brenneman D, et al. (1993a) VIP analogues inhibit small cell lung cancer growth. Biomed. Res 13, 131–136. [Google Scholar]
- Moody TW, Zia F, Brenneman D, Fridkin M, Davidson A, and Gozes I (1993b) A VIP antagonist inhibits the growth of non-small cell lung cancer. Proc. Natl. Acad. Sci. USA 90, 4345–4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen FC, Gammertoft S, Westermark B, and Fahrenkrug J (1990) High affinity receptors for vasoactive intestinal peptide on a human glioma cell line. Peptides 11, 1225–1231. [DOI] [PubMed] [Google Scholar]
- O’Dorisio MS, Fleshman DJ, Qualman SJ, and O’Dorisio TM (1992) Vasoactive intestinal peptide: autocrine growth factor in neuroblastoma. Reg. Pept 37, 213–226. [DOI] [PubMed] [Google Scholar]
- Pandol SJ, Dharmsathaphorn K, Schoeffield MS, Vale W, and Rivier J (1986) Vasoactive intestinal peptide receptor antagonist [4Cl-D-Phe6, Leul7] VIP. Am. J. Physiol 250 (4 Pt 1), G553–G557. [DOI] [PubMed] [Google Scholar]
- Pisegna JR and Wank SA (1993) Molecular cloning and functional expression of the pituitary adenylate cyclase-activating polypeptide type I receptor. Proc. Natl. Acad. Sci. USA 90, 6345–6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick M, Iverson LL, and Bloom SR (1978) Effect of vasoactive intestinal peptide (VIP) and other peptides on cAMP accumulation in rat brain. Biochem. Pharmacol 27, 2209–2213. [DOI] [PubMed] [Google Scholar]
- Reubi JC (2000). In vitro evaluation of VIP/PACAP receptors in healthy and diseased human tissues: Clinical implication. New York Acad. Sci 921, 1–25 [DOI] [PubMed] [Google Scholar]
- Robberecht P, Woussen-Colle MC, Vertongen P, DeNeef P, Hou X, Slamon I, and Brotchi J (1994) Expression of pituitary adenylated cyclase activating polypeptide (PACAP) receptors in human glial cell tumors. Peptides 15, 661–665. [DOI] [PubMed] [Google Scholar]
- Said SI and Mutt V (1970) Polypeptide with broad biological activity: Isolation from the small intestine. Science 69, 1217–1218. [DOI] [PubMed] [Google Scholar]
- Sethi T, Langdon S, Smyth J, and Rozengurt E (1992) Growth of small cell lung cancer cells: Stimulation by multiple neuropeptides and inhibition by a broad spectrum antagonist in vitro and in vivo. Cancer Res. 52, 2737s–2742s. [PubMed] [Google Scholar]
- Shaffer MM, Carney DN, Korman LY, Lebovic GS, and Moody TW (1987) High affinity binding of VIP to human lung cancer cell lines. Peptides 8, 1101–1106. [DOI] [PubMed] [Google Scholar]
- Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, and Journot L (1993) Differential signal transduction by five splice variants of the PACAP receptor. [DOI] [PubMed]
- Vertongen P, Camby I, Darro F, Kiss R, and Robberecht P (1996) VIP and pituitary adenylate cyclase activating polypeptide (PACAP) have an antiproliferative effect on the T98G human glioblastoma cell line through interaction with VIP2 receptors. Neuropeptides 30, 492–496. [DOI] [PubMed] [Google Scholar]
- Waelbroeck M, Robberecht P, Coy DH, Camus JC, De Neef P, and Christophe J (1985) Interaction of growth hormone-releasing factor (GRF) and 14 GRF analogs with vasoactive intestinal peptide (VIP) receptors of rat pancreas. Discovery of (N-Ac-Tyr1, D-Phe2)-GRF (1–29)-NH2 as a VIP antagonist. Endocrinology 116, 2643–2649. [DOI] [PubMed] [Google Scholar]
- Woll PJ and Rozengurt E (1988) D-Arg1, D-Phe5, D-Trp7,9, Leu11 substance P, a potent bombesin antagonist in murine Swiss 3T3 cells, inhibit the growth of human small cell lung cancer. Proc. Natl. Acad. Sci. USA 85, 1859–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman Y, Liling G, Goldstein MN, Fridkin M, and Gozes I (1993) Vasoactive intestinal peptide: A growth promoter in neuroblastoma cells. Brain Res. 624, 339–341. [DOI] [PubMed] [Google Scholar]


