Abstract
B-cell-depleting agents are among the most commonly used drugs to treat haemato-oncological and autoimmune diseases. They rapidly induce a state of peripheral B-cell aplasia with the potential to interfere with nascent vaccine responses, particularly to novel antigens. We have examined the relationship between B-cell reconstitution and SARS-CoV-2 vaccine responses in two cohorts of patients previously exposed to B-cell-depleting agents: a cohort of patients treated for haematological B-cell malignancy and another treated for rheumatological disease. B-cell depletion severely impairs vaccine responsiveness in the first 6 months after administration: SARS-CoV-2 antibody seroprevalence was 42.2% and 33.3% in the haemato-oncological patients and rheumatology patients, respectively and 22.7% in patients vaccinated while actively receiving anti-lymphoma chemotherapy. After the first 6 months, vaccine responsiveness significantly improved during early B-cell reconstitution; however, the kinetics of reconstitution was significantly faster in haemato-oncology patients. The AstraZeneca ChAdOx1 nCoV-19 vaccine and the Pfizer BioNTech 162b vaccine induced equivalent vaccine responses; however, shorter intervals between vaccine doses (<1 m) improved the magnitude of the antibody response in haeamto-oncology patients. In a subgroup of haemato-oncology patients, with historic exposure to B-cell-depleting agents (>36 m previously), vaccine non-responsiveness was independent of peripheral B-cell reconstitution. The findings have important implications for primary vaccination and booster vaccination strategies in individuals clinically vulnerable to SARS-CoV-2.
Keywords: SARS-CoV-2, vaccination, rituximab, CD20 depletion, haematological malignancy, rheumatoid arthritis
B cell depleting drugs including rituximab are commonly used to treat haematological malignancy and autoimmune diseases but may impair the immunological response to vaccination. This study investigates SARS-CoV-2 vaccine responses in individuals with haematological and rheumatological disease with previously exposure to B cell depleting agents. Vaccination within the first 6 months of B cell depletion is associated with significant impairment of vaccine responsiveness; however, rheumatology and haemato-oncological patients display different kinetics of B cell reconstitution corresponding to differential vaccine responsiveness over time.
Graphical Abstract
Graphical Abstract.
Introduction
Biologic, anti-CD20 B-cell-depleting agents are among the most commonly used immunotherapeutics in the treatment of haematological malignancy and autoimmune diseases [1–3]. These agents rapidly induce peripheral B-cell aplasia, with the kinetics of subsequent B-cell reconstitution dependent on factors including the underlying disease and concurrent immunosuppressive treatments. Generally, a detectable B-cell population returns between 6 and 9 months after treatment; however, B-cell aplasia can be prolonged in some individuals [4, 5] and persistent in those individuals treated with anti-CD19 CAR-T cell therapy [6].
The impact of anti-CD20 B-cell depletion on vaccine responsiveness has been considered with respect to vaccines designed to prevent invasive bacterial disease and influenza. Increased peripheral B-cell numbers are associated with better serological responses to influenza vaccine in patients previously treated with rituximab [7], suggesting B-cell reconstitution is important in overall vaccine responsiveness. However, vaccine responsiveness to pure polysaccharide vaccines and polysaccharide conjugate vaccines are diminished at 6 months following treatment with rituximab [8, 9]. Although responses to protein antigens such as tetanus toxoid appear better preserved following CD20 depletion [8], there is no consensus on the optimum time to vaccinate individuals following B-cell depletion. Furthermore, these data do not explicitly inform upon when a nascent immune response to a novel antigen can be made following CD20 depletion because vaccine-induced augmentation of existing memory responses cannot be excluded. Vaccine responsiveness to SARS-CoV-2 offers an opportunity to study this process clearly.
Patients with secondary immunodeficiencies are at significantly increased risk of morbidity and mortality from COVID-19 [10]. Although CD8+ T-cell immunity may compensate for impaired humoral immunity in patients with haematological malignancy infected with SARS-CoV-2 [11], there is also evidence that persistent infection can occur in individuals with humoral immunodeficiency that only resolves following treatment with exogenous antibody, indicating humoral immunity is non-redundant in some patients [12–14]. In healthy individuals, there is strong evidence to suggest that SARS-CoV-2 seropositivity offers protection against future SARS-CoV-2 infection [15–17] and the vast majority of healthy individuals seroconvert following natural infection or vaccination [18].
However, absent serological responses to SARS-CoV-2 natural infection and vaccination have been reported in patients with secondary immunodeficiency and rheumatological diseases treated with rituximab ([19], AS manuscript in preparation). This occurs in the wider context of diminished serological responses to SARS-CoV-2 vaccinations across varied diseased and immunosuppressed cohorts [20]. The aim of this study was to investigate the relationship between B-cell reconstitution and SARS-CoV-2 vaccine responsiveness in patients with prior exposure to anti-CD20 B-cell-depleting therapies.
Methods
Patients undergoing routine lymphocyte immunophenotyping for the purposes of monitoring B-cell reconstitution following exposure to B-cell-depleting agents (i.e. rituximab or obinutuzumab) to treat underlying haemato-oncological or rheumatological disease, were eligible for this study. In the haematology cohort, active chemotherapy was defined as any patient receiving B-cell-depleting chemotherapy during or within 1 month of completion of the vaccine schedule and any patient receiving a BTK inhibitor or PI3K inhibitor. Excess plasma from ethylenediaminetetraacetic acid samples sent for immunophenotyping was tested for the presence of SARS-CoV-2 antibodies; the use of excess sample for additional laboratory testing and assay development and the gathering of contemporaneous clinical data was approved by the South Birmingham Research Ethics Service (REC 2002/201). Antibody responses from healthy health care workers following vaccination were collected either as part of the COCO/PITCH study (REC 20/HRA/1817) or a separate University of Birmingham led study (REC 20\\NW\\0240).
Laboratory studies were undertaken by the University of Birmingham Clinical Immunology Service which delivers a UKAS accredited diagnostic immunology service for NHS hospitals across the West Midlands. Routine peripheral blood lymphocyte subset (CD3, CD4, CD8, CD19, CD56/16) enumeration was performed using six-colour flow cytometry (BD Trucount, Beckon Dickinson, Oxford, UK). SARS-CoV-2 antibody status following vaccination was determined using a human IgG/A/M anti-SARS-CoV-2 ELISA (MK654, The Binding Site, Birmingham, UK), which measures the total antibody against the SARS-CoV-2 spike glycoprotein. The development, verification, and validation of this assay has been published elsewhere [21]; the output of this ELISA is reported as the IgGAM ratio – a ratio of the sample OD 450 nm divided by that of a calibrator that runs at the assay cut-off. Samples producing an IgGAM ratio greater than 1.0 are therefore considered seropositive. During validation, a sensitivity of 98.6% (95% confidence interval [CI]: 92.6–100%) and specificity of 98.3% (95% CI: 96.4–99.4%) was demonstrated [21]. Serological responses to vaccination are presented as a seroprevalence and the median IgGAM ratio of seropositive results, which provides a semi-quantitative assessment of the magnitude of the antibody response.
The results of lymphocyte immunophenotyping and antibody testing were collated with clinical data including SARS-CoV-2 vaccine received, interval between vaccine dosing and timing, and extent of previous rituximab exposure. Data were analysed using Graph Pad Prism 9.0 (GraphPad Software, San Diego, CA, USA). Continuous variables were analysed using the two-tailed Mann–Witney U test.
Results
The vaccine responses of 116 patients with previous exposure to anti-CD20 B-cell-depleting agents were measured; the demographics of the study population are given in Table 1. Eighty patients had received treatment for underlying haemato-oncological disease and 36 patients had received treatment for underlying rheumatological disease. The median interval between last administration of anti-CD20 B-cell-depleting agent was 483 days overall and significantly longer in haemato-oncology patients than rheumatology patients (714 days vs 302 days, P = 0.0002). Disease-specific characteristics of the haemato-oncology and rheumatology cohorts are given in Supplementary Tables 1 and 2. All patients included in this study had received two doses of a SARS-CoV-2 vaccine approved for use in the UK between December 2020 and April 2021 (i.e. Pfizer- Tozinameran or AstraZeneca - Vaxzeveria). Median interval between administration of the second dose of vaccine and patient sampling for this study was 46.5 days. No patients were receiving immunoglobulin replacement therapy at the time of this study, excluding the possibility of passive transfer of anti-spike antibodies. Overall seropositivity following vaccination in this cohort was 62.9% and the median magnitude of the antibody response, quantified by the IgGAM ratio in seropositive individuals, was 2.98 (Table 1). By comparison, in a cohort of 36 healthy, age-matched controls (median age 66 years, IQR 61–73 years, 49% vaccinated with Pfizer, 51% vaccinated with AstraZeneca) seropositivity following vaccination was 97.2% with a median IgGAM ratio in seropositive individuals of 4.87.
Table 1:
Patient characteristics and demographics
| Overall | Haematology | Rheumatology | |
|---|---|---|---|
| n | 116 | 80 | 36 |
| Age | 69.0 (60.0–75.8) | 70.0 (60.3–76.0) | 65.0 (59.0–70.75) |
| Female, n (%) | 52 (44.8) | 30 (37.5) | 22 (61.1) |
| Anti-SARS-CoV-2 spike glycoprotein seropositivity, n (%) | 73 (62.9) | 48 (60.0) | 25 (69.4) |
| IgGAM Ratioa | 2.98 (2.21–4.17) | 2.84 (2.11–3.64) | 3.27 (2.20–4.33) |
| Previous PCR proven SARS-CoV-2 infection | 4 (3.4) | 3 (3.8) | 1 (2.8) |
| Vaccination, n (%) | |||
| AstraZeneca ChAdOx1 nCoV-19 | 64 (55.2) | 43 (53.8) | 21 (58.3) |
| Pfizer BioNTech 162b | 47 (40.5) | 32 (40.0) | 15 (41.7) |
| Unknown | 5 (4.3) | 5 (6.3) | 0 (0.0) |
| Median interval between vaccine doses (days)b | 70 (34–83) | 77 (66–86) | 32 (27–53) |
| Median cycles of B-cell-depleting therapy | 6.0 (6.0–14.0) | 6.0 (6.0–18.0) | 6.0 (2.3–10.8) |
| Median interval between last B-cell depletion to second vaccine dose (days) | 483 (276–1134) | 714 (346–1686)c | 302 (256–493) |
| Median interval from second vaccine dose to sampling (days) | 46.5 (35.8–63.3) | 45.0 (34.0–64.0) | 50.0 (40.0–57.0) |
Median and interquartile ranges are provided.
Only seropositive samples included.
Excludes four patients where date of first vaccine dose unknown.
Excludes patients receiving B-cell depletion within 1 month of second dose of vaccination.
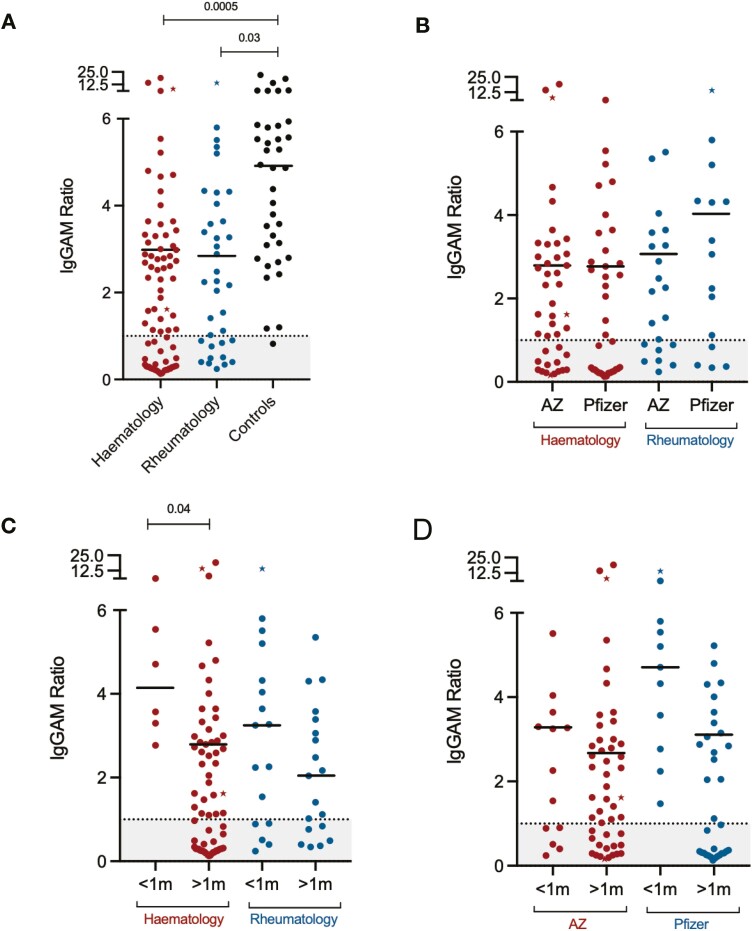
Individuals receiving active chemotherapy for haematological malignancy had significantly lower seroprevalence than those vaccinated following the completion of chemotherapy (27.3% vs 72.4%, P = 0.0003). There was no significant difference in overall anti-SARS-CoV-2 spike glycoprotein seropositivity (60.0% vs 69.4%, P = 0.34) or the magnitude of the antibody response (IgGAM 2.84 vs 3.27, P = 0.45) following vaccination between individuals with underlying haematological and rheumatological disease (Fig. 1A). There was also no significant difference between seropositivity or the magnitude of the antibody responses following vaccination with either the AstraZeneca or Pfizer vaccine in either cohort (Fig. 1B). A shorter interval between vaccination (i.e. <1m between vaccine doses) significantly improved seropositivity (100% vs 56.9%, P < 0.0001) and the magnitude of vaccine responses (IgGAM 4.14 vs 2.79, P = 0.04) in haemato-oncology patients but not in rheumatology patients (Fig. 1C). Seroconversion was significantly greater amongst recipients of the Pfizer vaccination when doses were administered less than 1 month apart (Pfizer vs AZ, 100.0% vs 61.5%, P = 0.02) and trends towards greater antibody levels in vaccine responders were observed when either vaccine was administered using short-interval dosing (Fig. 1D).
Figure 1:
Serological responses to SARS-CoV-2 immunizations in patients treated with anti-CD20 B-cell-depleting agents. (A) Overall serological response in haemato-oncology, rheumatology patients and healthy controls. (B) Serological response to AstraZeneca and Pfizer vaccines. (C) Serological response to short (<1 m) and longer (>1) dosing intervals in haemato-oncology and rheumatology patients. (D) Serological response to short (<1m) and longer (>1) dosing intervals in recipients of the AstraZeneca and Pfizer vaccines. Points represented as stars represent individuals previously known to have PCR+ SARS-CoV-2 infection. IgGAM ratios less than 1.0 are considered negative and represented by the grey zone on each graph.
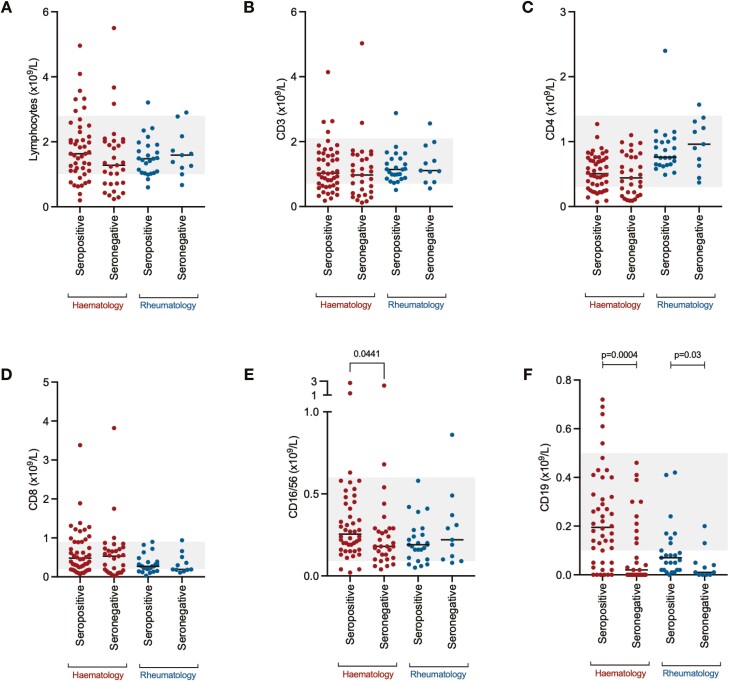
Immunological correlates of the vaccine responsiveness were studied; there were no significant differences in total lymphocytes (Fig. 2A), CD3+-positive lymphocytes (Fig. 2B), CD4+-positive lymphocytes (Fig. 2C), or CD8+-positive lymphocytes (Fig. 2D) between individuals mounting a serological response to vaccine in either patient group. In haemato-oncology patients, the absolute size of the NK cell population was significantly larger in patients who responded to the vaccine; however, the absolute difference was small, and was not replicated in rheumatology patients (Fig. 2E). However, in both haemato-oncology and rheumatology patients, the size of the CD19+ B-cell population was significantly smaller in patients who had not responded to the vaccine (Fig. 2F).
Figure 2:
Lymphocyte and lymphocyte subset enumeration in SARS-CoV-2 vaccine responders and non-responders. (A) Total lymphocyte count, (B) CD3+ T lymphocytes, (C) CD4+ T lymphocytes, (D) CD8+ T lymphocytes, (E) CD16/56+ natural killer cells, (F) CD19+ B lymphocytes. Grey zone represents the normal range for each cell population.
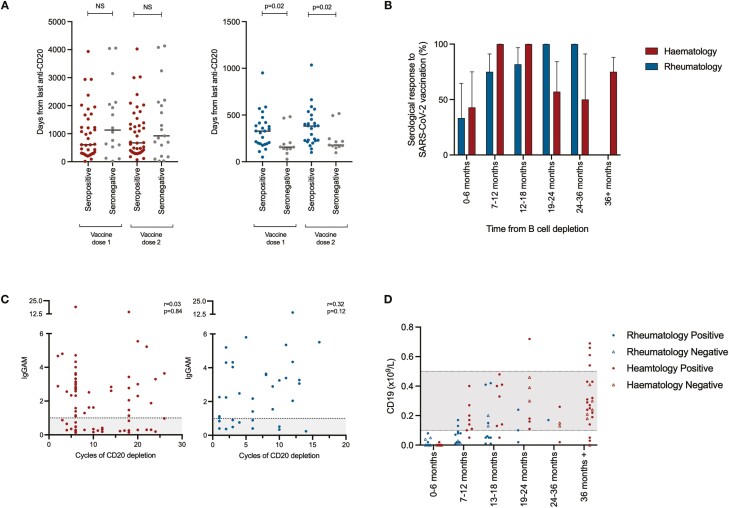
The relationship between the timing of administration of anti-CD20-depleting drug, vaccine administration, and vaccine responsiveness was investigated. In the haemato-oncology cohort, three patients began high-dose chemotherapy following completion of the vaccine schedule (median: 73 days) and all were seropositive. Eleven patients were vaccinated while receiving short-interval, high-dose chemotherapy for leukaemia or lymphoma and universally did not respond to vaccination; these two groups are excluded from this analysis. In haemato-oncology patients, there were no significant differences between vaccine responsiveness and the interval between the administration of last anti-CD20 depletion and vaccine administration (Fig. 3A, left panel), however, in rheumatology patients, seropositivity following vaccination was associated with a significantly longer interval between the last administration of CD20-depleting agents and the administration of SARS-CoV-2 vaccination (Fig. 3A, right panel). In the first 6 months after the administration of an anti-CD20 B-cell-depleting agent, only 42.9% of haemato-oncology patients and 33.3% of rheumatology patients produced a serological response to vaccination (Fig. 3B). In haemato-oncology patients, vaccine responsiveness increased to 100% following this initial 6-month period remaining high up to 18 months from last B-cell depletion. Beyond 18 months, however, haemato-oncology patients appeared less vaccine responsive. In rheumatology patients, progressive increases in overall vaccine responsiveness were observed the greater the interval between last administration of B-cell depletion and vaccine administration. No relationship was observed between total prior exposure to CD20 depletion and the magnitude of the antibody response to vaccination (Fig. 3C).
Figure 3:
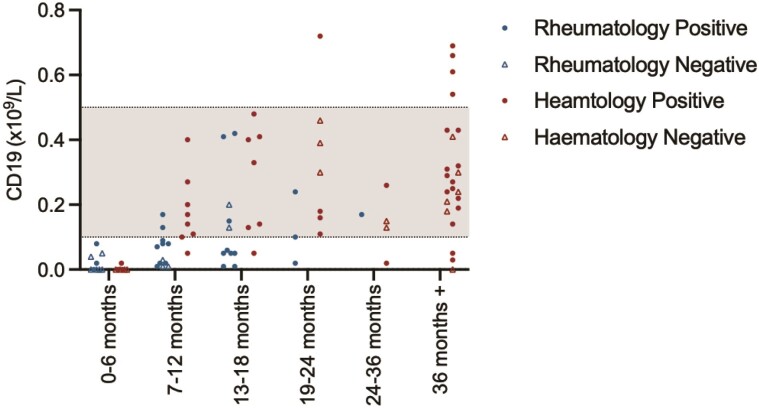
Immune reconstitution following CD20 depletion and vaccine responsiveness. (A) Time between last administration of anti-CD20 B-cell-depleting treatment and vaccine administration (left panel—haemato-oncology patients [red], right panel—rheumatology patients [blue]). (B) Seropositivity following SARS-CoV-2 vaccination with respect to time from last administration of anti-CD20 B-cell depletion. (C) Association between the magnitude of antibody responses and total prior exposure to anti-CD20 B-cell-depleting agents. IgGAM ratios less than 1.0 are considered negative and represented by the grey zone on the graph. (D) B-cell reconstitution and its association with vaccine responsiveness following treatment with anti-CD20 B-cell-depleting agents. Grey zones represent normal range for cellular populations.
The relationship between B-cell reconstitution following B-cell depletion and vaccine responsiveness was explored in more detail (Fig. 3D). B cells were undetectable in the majority of patients in the first 6 months following B-cell depletion. B-cell reconstitution was significantly slower in rheumatology patients compared to haemato-oncology patients: in the 7–12-month window following B-cell depletion, 77.8% of haemato-oncology patients compared to 22.2% of rheumatology patients achieving normal B-cell count (P = 0.005). However, in haemato-oncology patients, long-range secondary immunodeficiency, defined by a failure to respond to SARS-CoV-2 vaccination, may persist for years after cessation of B-cell-depleting therapies and appears independent of peripheral B-cell numbers.
Discussion
SARS-CoV-2 has disproportionately affected patients with secondary immunodeficiencies arising from their underlying conditions and/or treatment. Despite public health measures designed to protect vulnerable individuals [22], mortality rates in these patients are significantly greater than in the general population [10]. Evidence suggests that humoral immunity is an essential component of effective immunological responses and a biomarker of subsequent protection against SARS-CoV-2 [15–17]. Understanding how to optimize vaccination strategies to ensure immunologically vulnerable individuals achieve meaningful serological responses is, therefore, of significant public health importance.
In this study, we have examined the relationship between B-cell reconstitution and SARS-CoV-2 vaccine responsiveness in two cohorts of patients treated with anti-CD20 B-cell-depleting agents. We demonstrate that 37.1% of individuals do not mount a serological response to SARS-CoV-2 vaccination and show the magnitude of the serological response in vaccine responders is diminished compared to healthy controls. The overall non-response rate in our cohort is greater than that observed in the OCTAVE study of varied immunocompromised individuals (≈11%). Furthermore, both the haemato-oncology and rheumatology subgroups of this study had higher non-response rates than their equivalent, unselected subgroups in OCTAVE (Haematological malignancy: 40.0% vs 11.1%, rheumatological disease: 30.6% vs 1.8%), demonstrating the potent effect of B-cell depletion on vaccine responsiveness [19].
Our observations that patients receiving active treatment for leukaemia or lymphoma respond poorly to SARS-CoV-2 vaccination and, that following treatment for high-grade lymphomas, patients responded well to vaccination are consistent with interim analysis from the PROSECO study [23]. A similar study has also looked at vaccine responses exclusively in patients with chronic lymphocytic leukaemia: although the overall response rate following the second vaccination in this cohort was 75%, the majority had not received active treatment and further studies are needed to investigate the relative impact of disease progression and treatment on the development of secondary immunodeficient states.
In patients with immune-mediated inflammatory conditions including rheumatoid arthritis, psoriasis, and psoriatic arthritis, previous studies have shown the detrimental effect of methotrexate on vaccine responsiveness to the Pfizer BioNTech162b2 vaccine; seroconversion rates of approximately 70% are observed in these patients [24, 25]. In our rheumatology cohort, B-cell-depleting therapy reduced vaccine responsiveness to 33.3% in those vaccinated within 6 months of B-cell depletion, consistent with an Austrian study of rheumatoid arthritis patients vaccinated with either the Pfizer or Moderna mRNA vaccines [26]. In that study, the strongest determinant of a serological response to vaccination was the percentage of B cells within the total peripheral lymphocyte population. Concordantly, we observe that vaccine responsiveness significantly improves as B-cell reconstitution proceeds and exceeds 75% after the initial 6 months.
Several of our observations are of direct and immediate relevance to vaccination strategies in vulnerable individuals. First, there was no difference in seropositivity or the magnitude of the antibody response between patients vaccinated the AstraZeneca or Pfizer vaccination. Second, shorter dosing intervals (<1 month) induced significantly better responses in heamato-oncology patients with a trend towards the same pattern of results in rheumatology patients. In particular, seroconversion was significantly greater in recipients of the Pfizer vaccine when delivered at short intervals with trends towards increased antibody responses when either vaccine was used with short interval dosing. This is in contrast to observations from the PITCH study, where, in healthy individuals, extended dosing intervals were associated with higher levels of neutralizing antibody following vaccination [18]. Further studies are necessary to consider the validity of this observation, its underlying immunological mechanisms, and wider impact in other disease cohorts. Third, the percentage of patients mounting humoral immune responses significantly increases 6 months after the last administration of B-cell depletion. Although T-cell responses that may offer some protection against severe COVID-19 have been demonstrated in 58% of patients with immune-mediated inflammatory disease following vaccination [26], further immunizations, delivered at least 6 months after the last administration of B-cell-depleting drugs, may be necessary to optimize long-term protection. An argument can be made to closely monitor immune reconstitution to guide the timing of future immunizations, particularly given our observation that B-cell reconstitution is disease dependent. Further studies may also consider when CD20 depletion can be restarted following vaccination so as not to jeopardize the development of nascent humoral immune responses. Finally, it is interesting to note that vaccine unresponsiveness can persist for years in haemato-oncology patients and, beyond 36 months from last CD20 depletion, appears independent of the absolute size of the peripheral B-cell population. Understanding the immunopathogenesis of persistent secondary immunodeficiency is essential to guiding supportive immunological care for patients, particularly in the context of emergent immunochemotherapies [27].
There are limitations to our study. First, although all patients in this study had prior exposure to anti-CD20-depleting agents, their underlying diagnoses and prior treatments are heterogenous and may represent confounding variables that influence vaccine responsiveness. Second, lymphocyte enumeration was not undertaken at the time of vaccination but at non-fixed timepoints following the second vaccination (median: 46.5 days). Although this time interval is short and immune reconstitution occurs slowly, we cannot exclude the possibility that this may have an effect. Third, this study only considers vaccine responsiveness and not vaccine efficacy; further studies must explore the relationship between these parameters and define the correlates of immunity in vulnerable cohorts, particularly against highly transmissible SARS-CoV-2 variants. This may necessitate the interrogation of vaccine-induced T-cell responses to SARS-CoV-2, which are induced in some, but not all, patients following vaccination in haemato-oncology and rheumatology cohorts but have not been studied herein [20, 26]. Finally, we have only considered biologic anti-CD20 B-cell-depleting agents and not other targeted therapies that specifically interfere with normal B-cell function: for example, Bruton tyrosine kinase inhibitors have also been shown to profoundly inhibit SARS-CoV-2 vaccine responses [28].
In conclusion, we demonstrate vaccine responsiveness is significantly impeded by active chemotherapy for haematological malignancy and in the first 6 months following any B-cell-depleting agent. Based on these observations, additional immunizations may be necessary at least 6 months after B-cell depletion to optimize vaccine responsiveness; monitoring immune reconstitution may facilitate optimal vaccine timing. Further studies are needed to investigate whether shorter vaccine intervals may improve immunological responsiveness in vulnerable individuals. Finally, based on our findings, we also recommend that individuals who do not respond to SARS-CoV-2 vaccinations should be evaluated further for secondary immunodeficiency, particularly if anti-CD20-depleting therapies were last administered 18 months or more prior to vaccination.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the staff of the University of Birmingham Clinical Immunology Service, The Binding Site Ltd and the West Midlands Research Consortium for helping facilitate this work.
Glossary
Abbreviations
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- CAR
chimeric antigen receptor T-cell
- CI
confidence interval
- IgGAM
immunoglobulins G,A,M
Funding
No specific funding was received for this study.
Conflict of interest
M.T.D. reports personal fees from Abingdon Health, outside the submitted work. All other authors declare no competing interests.
Author contributions
A.M.S., S.V., S.S., S.P., M.T.D., and S.B. conceived and managed the study. A.M.S., S.E.F., A.G.R., and M.T.D. provided laboratory leadership and governance for the study including data analysis and interpretation. A.M.S. produced the first draft of the manuscript and revised the manuscript. All other authors recruited patients and/or healthy control into the study. All authors read, amended, and approved the final version of the manuscript.
Data availability
An anonymized data set is available upon reasonable request from the corresponding authors.
References
- 1. Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002, 346, 235–42. [DOI] [PubMed] [Google Scholar]
- 2. Edwards JC, Szczepanski L, Szechinski J, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 2004, 350, 2572–81. [DOI] [PubMed] [Google Scholar]
- 3. Vitolo U, Trněný M, Belada D, et al. Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J Clin Oncol 2017, 35, 3529–37. [DOI] [PubMed] [Google Scholar]
- 4. Anolik JH, Friedberg JW, Zheng B, et al. B cell reconstitution after rituximab treatment of lymphoma recapitulates B cell ontogeny. Clin Immunol 2007, 122, 139–45. [DOI] [PubMed] [Google Scholar]
- 5. Thiel J, Rizzi M, Engesser M, et al. B cell repopulation kinetics after rituximab treatment in ANCA-associated vasculitides compared to rheumatoid arthritis, and connective tissue diseases: a longitudinal observational study on 120 patients. Arthritis Res Ther 2017, 19, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhoj VG, Arhontoulis D, Wertheim G, et al. Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy. Blood 2016, 128, 360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eisenberg RA, Jawad AF, Boyer J, et al. Rituximab-treated patients have a poor response to influenza vaccination. J Clin Immunol 2013, 33, 388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bingham CO, 3rd, Looney RJ, Deodhar A, et al. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum 2010, 62, 64–74. [DOI] [PubMed] [Google Scholar]
- 9. Nazi I, Kelton JG, Larché M, et al. The effect of rituximab on vaccine responses in patients with immune thrombocytopenia. Blood 2013, 122, 1946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shields AM, Burns SO, Savic S, Richter AG.. COVID-19 in patients with primary and secondary immunodeficiency: The United Kingdom experience. J Allergy Clin Immunol 2021, 147, 870–5.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bange EM, Han NA, Wileyto P, et al. CD8(+) T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat Med 2021, 27, 1280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buckland MS, Galloway JB, Fhogartaigh CN, et al. Treatment of COVID-19 with remdesivir in the absence of humoral immunity: a case report. Nat Commun 2020, 11, 6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hueso T, Pouderoux C, Péré H, et al. Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19. Blood 2020, 136, 2290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McKemey E, Shields AM, Faustini SE, et al. Resolution of persistent COVID-19 after convalescent plasma in a patient with B cell aplasia. J Clin Immunol 2021, 41, 926–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hanrath AT, Payne BAI, Duncan CJA.. Prior SARS-CoV-2 infection is associated with protection against symptomatic reinfection. J Infect 2021, 82, e29–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lumley SF, O’Donnell D, Stoesser NE, et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med 2021, 384, 533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shields AM, Faustini SE, Kristunas CA, et al. COVID-19: seroprevalence and vaccine responses in UK dental care professionals. J Dent Res 2021, 100, 1220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Payne RP, Longet S, Austin SA, et al. Immunogenecity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell 2021, 184, 5699–714.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spiera R, Jinich S, Jannat-Khah D.. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS- CoV-2 vaccination in patients with rheumatic diseases. Ann Rheum Dis 2021, 80, 1357–9. [DOI] [PubMed] [Google Scholar]
- 20. Kearns P, Siebert S, Willicombe M, et al. Examining the Immunological Effects of COVID-19 Vaccination in Patients with Conditions Potentially Leading to Diminished Immune Response Capacity – The OCTAVE Trial. Available at SSRN: https://ssrn.com/abstract=3910058 or http://dx.doi.org/102139/ssrn3910058 (1 September 2021, date last accessed). 2021.
- 21. Cook AM, Faustini SE, Williams LJ, et al. Validation of a combined ELISA to detect IgG, IgA and IgM antibody responses to SARS-CoV-2 in mild or moderate non-hospitalised patients. J Immunol Methods 2021, 494, 113046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. UK Government. Guidance on protecting people who are clinically extremely vulnerable from COVID-19. Public Health England. https://www.gov.uk/government/publications/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-covid-19/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-covid-192021 (1 September 2021, date last accessed). [Google Scholar]
- 23. Lim SH, Campbell N, Johnson M, et al. Antibody responses after SARS-CoV-2 vaccination in patients with lymphoma. Lancet Haematol 2021, 8, e542–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haberman RH, Herati R, Simon D, et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis 2021, 80, 1339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mahil SK, Bechman K, Raharja A, et al. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID-19 vaccine BNT162b2: a cohort study. Lancet Rheumatol 2021, 3, e627–e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mrak D, Tobudic S, Koblischke M, et al. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann Rheum Dis 2021, 80, 1345–50. [DOI] [PubMed] [Google Scholar]
- 27. Patel SY, Carbone J, Jolles S.. The expanding field of secondary antibody deficiency: causes, diagnosis, and management. Front Immunol 2019, 10, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parry H, McIlroy G, Bruton R, et al. Antibody responses after first and second Covid-19 vaccination in patients with chronic lymphocytic leukaemia. Blood Cancer J 2021, 11, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
An anonymized data set is available upon reasonable request from the corresponding authors.