Abstract
Objectives
The mRNA-based COVID-19 vaccines are now employed globally and have shown high efficacy in preventing SARS-CoV-2 infection. However, less is known about the vaccine efficacy in immune-suppressed individuals. This study sought to explore whether humoral immunity to the COVID-19 vaccine BNT162b2 is altered in RA patients treated with Janus kinase inhibitors by analysing their antibodies titre, neutralization activity and B cell responses.
Methods
We collected plasma samples from 12 RA patients who were treated with Janus kinase inhibitors and received two doses of the BNT162b2 vaccine, as well as 26 healthy individuals who were vaccinated with the same vaccine. We analysed the quantity of the anti-spike IgG and IgA antibodies that were elicited following the BNT162b2 vaccination, the plasma neutralization capacity and the responsiveness of the B-lymphocytes. We used ELISA to quantify the antibody titres, and a plasma neutralization assay was used to determine the virus neutralization capacity. Alteration in expression of the genes that are associated with B cell activation and the germinal centre response were analysed by quantitative PCR.
Results
Reduced levels of anti-spike IgG antibodies and neutralization capacity were seen in the RA patients who were treated with JAK inhibitors in comparison with healthy individuals. Furthermore, B cell responsiveness to the SARS-CoV-2 spike protein was reduced in the RA patients.
Conclusion
RA patients who are treated with JAK inhibitors show a suppressed humoral response following BNT162b2 vaccination, as revealed by the quantity and quality of the anti-spike antibodies.
Keywords: COVID-19, SARS-CoV-2, rheumatoid arthritis, Janus kinase inhibitors, antibodies, B cells, vaccines
Rheumatology key messages
Rheumatoid arthritis patients treated with Janus kinase inhibitors show reduced humoral immunity following BNT162b2 vaccination.
Antibody titres in the plasma of rheumatoid arthritis patients are reduced.
Plasma samples from vaccinated rheumatoid arthritis patients show reduced neutralization activity.
Introduction
The employment of the mRNA-based COVID-19 vaccines has changed the landscape of the COVID-19 pandemic, and it has been able to efficiently reduce the infection rate in regions with high percentages of vaccinees [1]. Despite the success of these vaccines, several factors can impair successful eradication of SARS-CoV-2. One of these factors is the emergence of variants of concern, and several studies have demonstrated reduced sensitivity of the B.1.1.7/501Y.V1 variant, the B.1.351/501Y.V2 variant, the P.1 variant and the B.1.617 variant to the mRNA-based vaccines [2]. An additional concern arises from the possibility that the vaccine will ultimately show reduced efficacy in individuals with altered immune responses and in immunocompromised individuals [3], which will enable SARS-CoV-2 to continue circulating and will impair the eradication of SARS-CoV-2.
RA is a systemic inflammatory disorder, affecting about 1% of the general population [4]. RA patients suffer from severe joint inflammation and damage, which can lead to disability and increased mortality, primarily due to a higher rate of cardiovascular disorders [5]. Recent progress in the understanding of the disease pathogenesis and the implementation of biologic medicines for the treatment of RA patients has significantly improved disease outcomes [6]. Janus kinase (JAK) inhibitors are the newest drug class approved for the treatment of RA patients [7]. Tofacitinib targets JAK-1 and JAK-3, baricitinib targets JAK-1 and JAK-2, and upadacitinib targets only JAK-1. JAK inhibitors are characterized by a rapid onset of action, have the same or better efficacy in comparison with existing biologic medicines, and have an acceptable safety profile [7]. Being administered orally, JAK inhibitors are becoming increasingly popular for the treatment of patients with moderately severe and severe RA [7, 8].
In the clinical trials conducted by Pfizer and BioNTech, the COVID-19 vaccine BNT162b2 showed 95% efficacy in preventing infection [9]. The high protection capacity of these vaccines was later confirmed by real-life data that demonstrated a sharp decrease in COVID-19 cases, hospitalization, and COVID-related deaths in regions with a high vaccination rate [1]. The RNA-based COVID-19 vaccines can also protect from a number of variants of concern, but with a slightly lower efficacy [2]. For example, vaccine effectiveness measurements preformed in Qatar showed that BNT162b2 vaccine efficacy against the B.1.1.7 variant was 89.5% (14 or more days after the second dose), and that its effectiveness against infection with the B.1.351 variant was 75.0% [10]. A few studies have also tested the efficacy of the mRNA-based vaccines in immunocompromised individuals and in individuals with dysregulated immune responses. For example, Binu et al. have tested the effectiveness of the BNT162b2 COVID-19 vaccine in patients with cirrhosis, a chronic disease that is characterized by immune dysregulation and which was previously associated with vaccine hyporesponsiveness [11]. Despite the tendency of these individuals to demonstrate lower vaccine-responsiveness, the mRNA-based COVID vaccine seems to be protective in cirrhosis patients, and a significant reduction in COVID-19-related hospitalization or death was seen in these individuals [11]. An additional study evaluated the serologic status and safety of the BNT162b2 vaccine in patients receiving active treatment for cancer [12]. This study uncovered a pronounced lag in antibody production compared with the rate in non-cancer controls; however, in most patients the antibody titres reached the desired levels after the second dose of the vaccine [12]. Finally, a recent study demonstrated that the kinetics of the vaccine-induced humoral immune response differs between patients with RA and healthy individuals [13]. Further characterization of COVID-19 vaccine efficacy in RA patients is required for determining whether these individuals are at higher risk for SARS-CoV-2 infection.
Here we evaluated the humoral immune responses that are elicited in RA patients who were being treated with JAK inhibitors and who received two doses of the BNT162b2 vaccine.
Methods
Patient recruitment
The study enrolled adults (≥18 years) who fulfilled the 2010 ACR-EULAR classification criteria for RA and who were treated in the Rheumatology Unit, Bnai Zion Medical Center, with JAK inhibitors or TNF-α antagonists. Five RA patients were treated with tofacitinib, four with baricitinib and three patients with upadacitinib tablets. One RA patient was treated with golimumab (s.c.), one with adalimumab (s.c.) and one with certolizumab (tablet). All study participants received two doses of the BNT162b2 vaccine. Controls were recruited from the medical staff or patients who came to the allergy clinic in Bnai Zion Medical Center. The study was approved by the Ethics Committee of the Bnai Zion Medical Center, and all participants gave written informed consent.
Of note, the majority of the individuals who were recruited were females, in accordance with the higher prevalence of RA in women than in men. However, a similar ratio of females to males was found within the healthy individual group and the RA patient group.
Production of SARS-CoV-2 pseudovirions and 293 T-ACE2 infection
SARS-CoV-2 pseudoviruses carrying Wuhan-Hu-1 spike protein were produced by cotransfection of 293 T cells with 2.5 µg of pMD2.G SARS-CoV-2 –SΔ19-opt mixed with 7 µg of pPAX2 and 7 µg pLenti-Luc. At 48 h following the transfection, the supernatants were collected and filtered through a 0.22 mM filter. For SARS-CoV-2 pseudovirions infection, 2 × 104 293 T cells were seeded in a 96-well plate and were incubated with the virus for 48 h. Polybrene (10 mg/ml) was added to increase infectivity [14]. At 48 h post infection, the cells were lysed and the luciferase activity was analysed by a plate reader (Infinite M200 PRO) after incubating the cell lysate with One-Step luciferase reagent (BPS Bioscience, cat #60690–1).
ELISA
ELISA was performed by coating high-binding 96-well plates (Thermo scientific, cat #44–2404-21) with 50 μl per well of a 1 μg/ml of the S1 subunit of the Wuhan-Hu-1 spike in sodium bicarbonate solution overnight at 4°C. Plates were washed 6 times with washing buffer (1 × PBS with 0.05% Tween-20) and incubated with 200 μl/well blocking buffer (1 × PBS with 2% skim milk) for 2 h at room temperature. Plasma samples were then diluted in blocking buffer. The dilution started at 1:102, and 3 additional serial dilutions in blocking buffer were performed. Diluted plasma samples were added to all wells and were incubated for 2 h. Wells were then washed 6 times with washing buffer and incubated with anti-human IgG secondary antibody conjugated to horseradish peroxidase diluted in blocking buffer at 1:10 000 dilution. Plates were developed by the addition of the horseradish peroxidase substrate, TMB (Life technologies, cat #002023). The absorbance was measured using an ELISA microplate reader (Infinite M200 PRO) at 650 nm. For quantitative ELISA, plasma samples were diluted in a 1:1000 ratio. A standard curve was generated by using 0.5–250 ng of a purified IgG/IgA.
Pseudotyped virus neutralization assay
Plasma samples from healthy individuals and from RA patients were diluted in serum-free media (1.0 × 10−1 to 1.0 × 10−5). Plasma samples were incubated with SARS-CoV-2 pseudovirus 1 h at 37°C and then added 2 × 104 293 T-ACE2 cells in a 96-well plate for 48 h at 37°C 5% CO2, after which infectivity/neutralization was checked using a one-step luciferase system (BPS Bioscience, cat #60690–1) and by analysing luciferase activity using a plate reader (Infinite M200 PRO). In order to calculate the relative SARS-CoV-2 neutralization, the luminescence values seen in the 1.0 × 10−4, dilution was set as 1. The half-maximal inhibitory concentrations for plasma samples (NT50) were determined using a four-parameter non-linear regression (GraphPad Prism).
Flow cytometry analysis of ACE2 protein expression
293T-ACE2 (5 × 104) cells were seeded in a 96-well plate and stained with 2 μg of RBD-Ig [15] for 1 h at 4°C. Cells were washed and stained with PE-conjugated goat anti-human IgG (Jackson ImmunoResearch, Cat #109–116-088, 1:200) for 45 min. Control cells were stained only with PE-conjugated goat anti-human IgG. Staining was analysed by BD LSRFortessa flow cytometer by using FlowJo software v10.7.
Isolation of peripheral blood mononuclear cells and real-time PCR
Blood samples (10 ml) were collected from healthy individuals and from RA patients treated with JAK inhibitors. Samples were processed within 2 h of collection. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Ficoll 400 (Merck, F4375). Isolated PBMCs were counted and seeded at a 4 million cells/ml concentration. For antigen-specific stimulation of the B cells, the S1 subunit of SARS-CoV-2 spike protein was added at a concentration of 25 μg/ml for 48 h. Cells were collected, lysed and analysed for expression of BCL6, AID and IRF4 by quantitative PCR using Sybr Green MasterMix (BioRad) and a BioRad CFX connect Real-Time PCR. Samples were normalized with GAPDH gene amplification and data were analysed using the CFX Manager Software. The primer sequences are shown in Supplementary Table S1, available at Rheumatology online.
FACS staining
Isolated PBMCs were incubated with the following antibodies: anti-human CD20 (Jackson ImmunoResearch), anti-human CD19 (Jackson ImmunoResearch), anti-human CD45 (Jackson ImmunoResearch) and live/dead dye (Thermo scientific). Cells were incubated for 45 min at 4°C and were then washed twice with FACS media. Staining was evaluated using the BD LSRFortessa flow cytometer. Further analysis of the staining results was done using FlowJo software v10.7. Samples were processed within 2 h of collection. The percentage of the B cells in each sample was evaluated from that of the live CD45+ cells.
Statistical analysis
Comparisons of the days post second vaccination, IgG and IgA anti-spike antibodies, NT50 and NT80 between RA patients and healthy controls were performed with Student’s t test. The correlation between the IgG anti-spike titre and NT50 was calculated by using Spearman’s correlation test. A P-value of <0.05 was considered significant.
Results
Anti-spike IgG antibody titres were reduced in RA patients treated with JAK inhibitors
To test the effect of JAK inhibitors on the antibody responses following BNT162b2 vaccination, we recruited 12 RA patients who were treated with JAK inhibitors and received two doses of the BNT162b2, as well as 26 healthy individuals who were vaccinated with the same vaccine. The demographics and the drug treatment for each patient are shown in Table 1. Specifically, five RA patients were treated with tofacitinib, four with baricitinib and 3 with upadacitinib tablets.
Table 1.
Participant demographics
| Patient ID | Age, years | Gender | Treatment | Time post second vaccine dose, days | Patient ID | Age, years | Gender | Treatment | Time post second vaccine dose, days |
|---|---|---|---|---|---|---|---|---|---|
| C1 | 56 | M | T.atozat | 66 | C24 | 50 | F | 85 | |
| C2 | 55 | F | 65 | C25 | 63 | F | 84 | ||
| C3 | 55 | M | 66 | C26 | 34 | F | 43 | ||
| C4 | 37 | F | 58 | C27 | 38 | F | 40 | ||
| C5 | 62 | M | 60 | C28 | 70 | M | 105 | ||
| C6 | 50 | F | 54 | P1 | 62 | F |
|
35 | |
| C7 | 62 | M |
|
60 | P2 | 54 | F |
|
34 |
| C8 | 60 | F | 63 | P3 | 56 | F |
|
27 | |
| C9 | 62 | M | T.tritace | 58 | P6 | 72 | F | T. Xeljanz | 32 |
| C10 | 55 | F | T.amlodypine | 62 | P7 | 65 | F |
|
44 |
| C12 | 50 | F | T.tritace | 77 | P9 | 51 | F | T. Rinvoq | 20 |
| C13 | 37 | F | 77 | P10 | 65 | M |
|
50 | |
| C14 | 51 | F | 76 | P14 | 32 | M |
|
42 | |
| C15 | 57 | F | 66 | P16 | 73 | F | TNF-α antagonist: S.C simfoni | 60 | |
| C16 | 29 | F | 76 | P19 | 73 | F | T. Xeljans | 65 | |
| C18 | 65 | F | 79 | P20 | 75 | F | T. Xeljans | 61 | |
| C19 | 71 | F | Topamax | 76 | P21 | 31 | F |
|
76 |
| C20 | 74 | M | 76 | P21a | 65 | F |
|
75 | |
| C21 | 52 | F | 48 | P22 | 67 | F |
|
54 | |
| C22 | 60 | M | 44 | P23 | 72 | M | T. Rinvoq | 92 | |
| C23 | 43 | F | 44 |
F, female; M, male; T: Tablet.
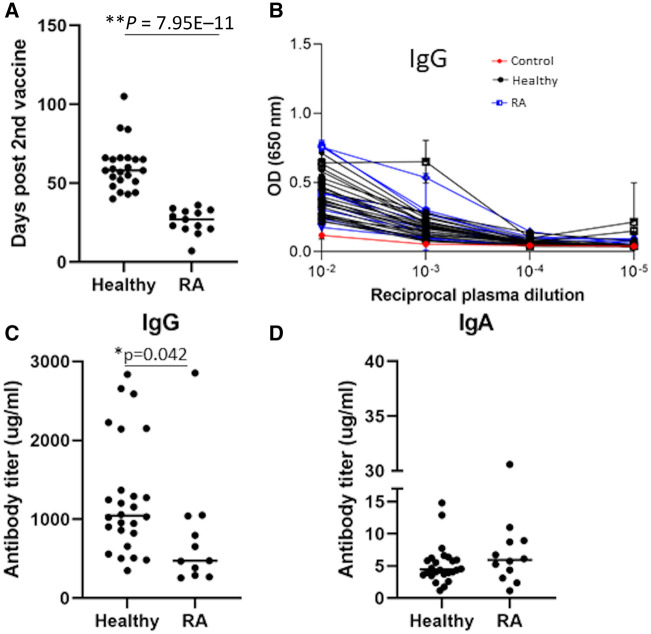
Plasma samples were taken from these individuals after the second dose of the vaccine. The times of plasma collection are depicted in Table 1 and Fig. 1A. Notably, plasma samples from RA patients were collected at a significantly shorter time after the second vaccine dose relative to the healthy controls (Fig. 1A); the plasma sampling time for the RA patients was also closer to the reported peak in antibody titres following BNT162b2 vaccination [16].
Fig. 1.
Measurements of IgG and IgA antibodies that recognize the SARS COV-2 Spike S1 domain
(A) Time (days) post second dose of the vaccine, in which the plasma samples were collected. Statistically significant differences in the time post second vaccine are indicated (Student’s t test, *P < 0.05). (B) The OD values of IgG ELISA against the spike S1 subunit of plasma from healthy individuals and RA patients. The x-axis depicts the plasma dilution. Experiments were done in triplicate and repeated three times. One representative experiment is shown. Mean values and standard errors are shown. The OD value of the control plasma (from an unvaccinated healthy individual) is shown in red. (C) Anti-S1 IgG levels in mg/ml in plasma from healthy individuals and RA patients. Experiments were done in triplicate and repeated three times. One representative experiment is shown. Statistically significant differences in the time post second vaccine are indicated (Student’s t test, *P < 0.05). (D) Anti-S1 IgA levels in mg/ml in plasma from healthy individuals and RA patients. Experiments were done in triplicate and repeated two times. One representative experiment is shown. OD = optical density.
Plasma antibodies were then tested for binding to the SARS-CoV-2 Spike S1 subdomain by a quantitative ELISA. Plasma from unvaccinated healthy individuals did not show any spike-specific antibodies, while spike-specific antibodies were detected in both plasma from healthy vaccinees and in plasma from RA vaccinees (Fig. 1B). Despite the fact that plasma samples from the RA patients were taken closer to the reported peak in antibody titres following BNT162b2 vaccination [16], the levels of anti-spike IgG titres were significantly lower in the JAK inhibitor–treated RA patients in comparison with untreated healthy individuals (Fig. 1B and C). The average anti-IgG levels in the plasma samples from the JAK inhibitor–treated RA patients was 775 mg/ml, while the average anti-IgG levels in the plasma samples from the untreated healthy individuals was 1261 mg/ml. These values are not consistent with the findings reported in a previous article, that the anti-spike IgG titres following two doses were around 48.8–71.4 μg/ml [17]. This difference is most likely a result of different sensitivities of the ELISA kits that were used in each study. In contrast to the IgG levels, no significant changes between these two groups were detected in the anti-spike IgA (Fig 1D; an average of 7.8 mg/ml in the plasma samples from the JAK inhibitor–treated RA patients in comparison with an average of 5.7 mg/ml in the plasma samples from the untreated individuals).
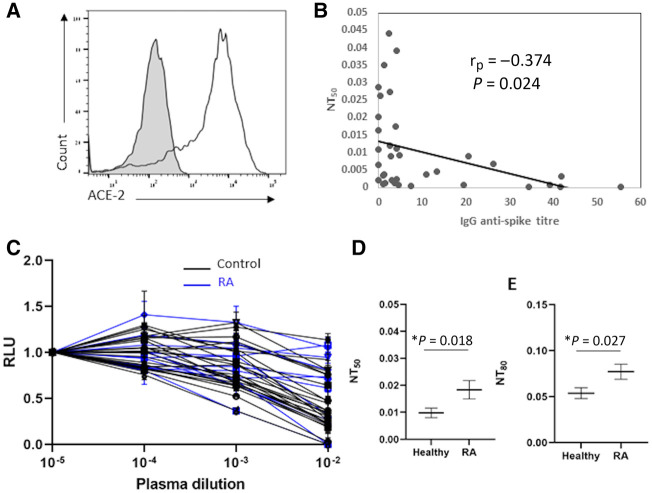
Plasma samples from RA patients treated with JAK inhibitors exhibited reduced neutralization activity, which correlated with the lower IgG antibody titres
After demonstrating that treatment with JAK inhibitors impaired the elicitation of anti-spike antibodies in vaccinated individuals, we next wanted to examine the neutralization capacity of the antibodies that are elicited in JAK inhibitor–treated RA patients. To do this, we used a standard neutralization SARS-Cov-2 assay using SARS-CoV-2 pseudoviruses and 293 T cells expressing ACE2 [18] (Fig. 2A). We found that, in plasma samples from both RA patients and healthy individuals, the levels of the anti-spike IgG antibody titre demonstrated low negative correlation with the plasma neutralization capacity (Fig. 2B) (r = –0.374, P = 0.024). This negative correlation was expected, because increased antibody titres improve the neutralization capacity of plasma samples and therefore reduce the NT50 values. Thus, RA patients treated with JAK inhibitors and healthy controls who developed high antibody titres (>6 mg/ml) showed high neutralization capacity. In contrast, when analysing vaccinees who developed antibody titres that were lower than 6 mg/ml, we found a significantly reduced neutralization capacity among RA patients treated with JAK inhibitors relative to the healthy controls in both NT50 and in NT80 (Fig. 2C–E). These results suggest that humoral immunity to the SARS-CoV-2 vaccine of RA patients treated with JAK inhibitors is reduced in both quantity and quality. Since the plasma samples of the healthy individuals and the RA patients were collected at significantly different time points following vaccination, we also performed an additional comparison, in which samples from similar time points were compared (Supplementary Fig. S1, available at Rheumatology online). In accordance with our previous analysis, significantly lower anti-spike IgG titres were seen in the JAK inhibitor–treated RA patients in comparison with untreated healthy individuals. Additionally, the JAK inhibitor-treated RA patients showed increased NT50 values (Supplementary Fig. S1, available at Rheumatology online).
Fig. 2.
Neutralization activity of plasma samples
(A) FACS staining of 293 T-ACE2 cells. The grey histogram shows the staining of the 293 T-ACE2 cells with secondary antibody only. The empty black histogram depicts the staining anti-ACE2 antibody. Shown is one representative experiment out of three performed. (B) The half-maximal inhibitory concentrations for plasma samples (NT50) plotted against the anti-S1 IgG titre. The y-axis depicts the NT50 values. The x-axis depicts the anti-S1 IgG titre. Dots depict healthy individuals’ and RA patients’ samples. The correlation between the IgG anti-spike titre and NT50 was calculated by using Spearman’s correlation test. One representative experiment out of three is shown. (C) Neutralization assays, comparing the sensitivity of pseudotyped viruses expressing the Wuhan-Hu-1 spike with neutralization by plasma samples. Plasma dilutions are shown in the x-axis. The y-axis depicts the normalized relative luminescence units (RLU). Values were normalized to the RLU values seen with the 1.0 × 10−5 plasma dilution. Black graphs depict the healthy individual samples. Blue graphs depict RA patient samples. Mean values and standard errors are shown; a representative of two independent experiments is shown. (D–E) NT50 mean value and NT80 mean value of control plasma samples and RA patients’ samples. Shown is a summary of two experiments, with mean values and standard errors. Statistically significant differences in the time post second vaccine are indicated (Student’s t test, *P < 0.05).
Moreover, we also analysed the anti-spike IgG titres and neutralization activity of plasma samples from three RA patients treated with TNF-α antagonists. These individuals showed reduced anti-spike IgG titres in comparison with healthy individuals, but no significant difference was seen in their plasma neutralization activity (Supplementary Fig. S2, available at Rheumatology online).
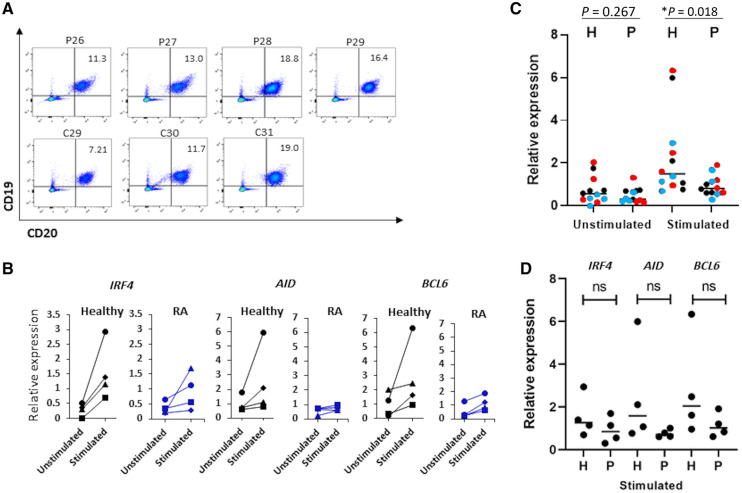
Impaired B cell responsiveness to the SARS-CoV-2 spike in RA patients treated with JAK inhibitors
We next tested whether the reduced titres of anti-spike IgG antibodies in JAK inhibitor–treated patients results from altered activation of the B cells. To do so, PBMCs from RA patients treated with JAK inhibitors and from healthy controls were cultured in vitro for 40 h in the presence of SARS-CoV-2 spike protein. We then used quantitative PCR to quantify the expression of genes associated with B cell activation and the germinal centre response: interferon regulatory factor 4 (IRF4), activation-induced cytidine deaminase (AID) and B cell lymphoma 6 protein (BCL6). As shown in Fig. 3A, the relative proportion of B cells in the PBMCs did not differ between patients and controls, ranging from 7% to 19%. The results in Fig. 3B reveal that expression of the IRF4, AID and BCL6 genes in response to stimulation with SARS-CoV-2 spike protein was reduced in cultures derived from RA patients treated with JAK inhibitors. This is reflected in the individual responses. We observed ≥3-fold increases in IRF4 expression (relative to the unstimulated culture) in all four healthy controls, but only one out of four RA patients, ≥1.5-fold increases in AID expression in three out of four healthy controls, but only one out of four RA patients, and ≥3-fold increases of BCL6 expression in 3 out of 4 healthy controls relative to only two out of four RA patients (Fig. 3B). Finally, in an attempt to evaluate the overall activation state of the B cells, we combined the expression levels of all three genes that were analysed on a single plot. Fig. 3C reveals a significant higher response to stimulation with SARS-CoV-2 spike protein in samples of healthy individuals compared with samples of RA patients (Fig. 3C). These statistically significant changes are not seen when each gene is analysed separately (Fig. 3D).
Fig. 3.
B cell responsiveness to SARS-CoV-2 spike
(A) FACS staining of PBMCs isolated from plasma samples of healthy individuals and RA patients. The percentage of B cells (CD20+, CD19+) was calculated from the CD45+ cell population. Dead cells were excluded from the analysis. (B) Relative expression of BCL6, AID and IRF4 as measured by quantitative PCR using Sybr Green Master Mix. The expression in unstimulated samples and samples that were stimulated with the S1 subunit of the Wuhan-Hu-1 spike is shown. Each line depicts a different individual. Data was normalized to GAPDH gene amplification. The genes that were analysed are depicted in the figure. (C) Dot plot summarizing the relative expression levels of all three genes analysed. BCL6 is represented by red dots, IRF4 by blue, and AID by black dots. Statistically significant differences are indicated (Student’s t test, *P < 0.05). (D) Dot plot summarizing the relative expression levels of BCL6, IRF4 and AID in stimulated samples of healthy individuals and RA patients; ns = not statistically significant.
Discussion
Treat-to-target strategies in the management of patients with RA have cardinally improved disease outcomes during recent decades [8]. The current arsenal of DMARDs used for RA treatment include multiple groups of traditional synthetic and newer biologic medicines [8]. JAK inhibitors, the latest and, probably, the most effective group of drugs for RA treatment, were introduced in 2016 [8]. Inhibition of JAK was shown to affect both the innate and the adaptive immune responses [19, 20]. This includes inhibition of type-I IFN production by dendritic cells, alteration in TCR stimulation, and impairment of Th1 responses [19, 20]. Additionally, B cell–specific alteration has been documented as a result of treatment with JAK inhibitors; examples include impaired differentiation of human B cells into plasmablasts following B cell receptor and type-I IFN stimuli, and reduced secretion of B cell cytokines such as IL-6 [19]. Moreover, since Janus kinases constitutively bind cytokine receptors [21], inhibition of this tyrosine kinase can significantly impair the effect of the cytokines on their target cells [22]. In accordance with that, JAK inhibitors impair the function of many key cytokines (e.g. IL-6, IL-12, IL-23, IFNs and GM-CSF) that use the JAK/STAT pathway to exert their effects on the target cells, which is likely a key factor in the therapeutic effect of these drugs [22]. Due to the efficient modulation of proinflammatory cytokines by JAK inhibitors, there are currently five JAK inhibitors (tofacitinib, baricitinib, peficitinib, upadacitinib and filgotinib) that are approved for treatment of immune-mediated inflammatory diseases (IMIDs) [23]. In this study, we have tested the effect of JAK inhibitors on the humoral immune responses of RA patients compared with those of a control group, following the administration of the second dose of mRNA-based COVID vaccine, BNT162b2.
Immunocompromised patients, children under the age of 16 and pregnant women were excluded from the COVID-19 vaccine clinical trials [9]. Soon after the completion of these clinical trials, several groups have tested the efficacy of the mRNA-based COVID-19 vaccines in individuals with impaired immune responses or in immunocompromised individuals [12, 24, 25]. A recent study has tested the efficacy of mRNA-based vaccines in various immunocompromised conditions, including solid-organ transplant, haematological malignancies, solid tumours being treated with systemic or radiation therapy over the past 12 months, autoimmune or chronic inflammatory conditions being treated over the past 12 months, and HIV infection [25]. Seropositivity was significantly lower among immunocompromised patients with solid-organ transport, autoimmune conditions, haematological malignancies, and solid tumours [25]. In addition, several reports have indicated severe SARS-CoV-2 infection in vaccinated individuals with solid-organ transplants [26]. To boost the response in these individuals, the administration of three doses of mRNA-based vaccines is currently being tested [24].
In this study, we provided an in-depth analysis of the antibody responses elicited by the BNT162b2 vaccine in RA patients treated with JAK inhibitors. We observed a reduced IgG anti-spike antibody titre in plasma from RA patients in comparison with plasma from healthy controls. Interestingly, this was also associated with the reduced neutralization activity of their plasma samples against SARS-CoV-2 pseudoviruses. The underlying reason for the reduced IgG titres needs further evaluation. The reduced neutralization could be a direct result of the low IgG titres. Alternatively, it is also possible that impaired B cell responses in the RA patients prevents the elicitation of antibodies with high neutralization activity. It was previously demonstrated that JAK inhibitor treatment has important consequences in B cell activation and function [19]. This is partly due to the fact that JAK-3 is constitutively associated with CD40 [27]. Triggering of CD40 is crucial for numerous B cell functions, such as B cell survival, differentiation and isotype switching [19, 27]. The importance of JAK-3 in the development and function of B cells was also demonstrated using JAK-3 knockout mice, with which it was shown that JAK-3 promotes Ig gene rearrangement [27].
Vaccine efficacy in RA patients has been the focus of several studies that tested influenza virus vaccine efficacy [28]. Kapetanovic et al. compared the serological response to influenza vaccine in patients with RA treated with TNF blockers and/or MTX. It was demonstrated that treatment that included MTX alone had a significantly better serological response to influenza vaccination in comparison with those receiving TNF blockers treatment or TNF blockers in combination with other DMARDs [29]. Interestingly, our finding that JAK inhibitor treatment reduces the anti-spike IgG titres is in accordance with a previous report that showed that tofacitinib treatment of RA patients reduces influenza virus antibody titres following vaccination [30]. Our data also suggest that the effect of JAK inhibitors on vaccine efficacy should be further tested in other diseases in which JAK inhibitors are used, such as myelofibrosis and Crohn’s disease. Finally, based on the impaired antibody responses we observed in RA patients, it seems that additional boosts of the BNT162b2 vaccine will be beneficial for these individuals.
Funding: This work has been supported by the ISF Corona (grant no. 2030904).
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
All data will be made available upon reasonable request.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
References
- 1.Rossman H, Shilo S, Meir T. et al. COVID-19 dynamics after a national immunization program in Israel. Nat Med 2021;27:1055–61. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z, Schmidt F, Weisblum Y. et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature 2021;592:616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore J. Experts discuss COVID-19 – variants and vaccine efficacy, immunosuppressed patients, and more. JAMA 2021;325:1711–2. [DOI] [PubMed] [Google Scholar]
- 4.Uhlig T, Loge JH, Kristiansen IS, Kvien TK.. Quantification of reduced health-related quality of life in patients with rheumatoid arthritis compared to the general population. J Rheumatol 2007;34:1241–7. [PubMed] [Google Scholar]
- 5.Smolen JS, Aletaha D.. Patients with rheumatoid arthritis in clinical care. Ann Rheum Dis 2004;63:221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JM, Kim HY.. Pathogenesis of rheumatoid arthritis. J Korean Med Assoc 2010;53:853–19. [Google Scholar]
- 7.Harrington R, al Nokhatha SA, Conway R.. Jak inhibitors in rheumatoid arthritis: an evidence-based review on the emerging clinical data. J Inflamm 2020;13:519–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burmester GR, Pope JE.. Novel treatment strategies in rheumatoid arthritis. Lancet 2017;389:2338–48. [DOI] [PubMed] [Google Scholar]
- 9.Polack FP, Thomas SJ, Kitchin N. et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, Yassine HM. et al. Protection afforded by the BNT162b2 and mRNA-1273 COVID-19 vaccines in fully vaccinated cohorts with and without prior infection, medRxiv, doi: 10.1101/2021.07.25.21261093, 26 July 2021, preprint: not peer reviewed. [DOI] [Google Scholar]
- 11.John B. V, Deng Y, Scheinberg A. et al. Association of BNT162b2 mRNA and mRNA-1273 vaccines with COVID-19 infection and hospitalization among patients with cirrhosis. JAMA Intern Med 2021;181:1306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goshen-Lago T, Waldhorn I, Holland R. et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol 2021;7:1507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubbert-Roth A, Vuilleumier N, Ludewig B. et al. Anti-SARS-CoV-2 mRNA vaccine in patients with rheumatoid arthritis. Lancet Rheumatol 2021;3:470–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abe A, Miyanohara A, Friedmann T.. Polybrene increases the efficiency of gene transfer by lipofection. Gene Ther 1998;5:708–11. [DOI] [PubMed] [Google Scholar]
- 15.Chaouat A, Achdout H, Kol I. et al. SARS-CoV-2 receptor binding domain fusion protein efficiently neutralizes virus, bioRxiv, doi: 10.1101/2021.04.18.440302, 19 April 2021, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grupel D, Gazit S, Schreiber L. et al. Kinetics of SARS-CoV-2 anti-s IgG after BNT162b2 vaccination. Vaccine 2021;39;5337–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richards NE, Keshavarz B, Workman LJ. et al. Comparison of SARS-CoV-2 antibody response by age among recipients of the BNT162b2 vs the mRNA-1273 vaccine. JAMA Netw Open 2021;4:e2124331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ou X, Liu Y, Lei X. et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun 2020;11:1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moura RA, Fonseca JE.. JAK inhibitors and modulation of B cell immune responses in rheumatoid arthritis. Front. Med 2021;7:1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kubo S, Nakayamada S, Sakata K. et al. Janus kinase inhibitor baricitinib modulates human innate and adaptive immune system. Front Immunol 2018;9:1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamaoka K, Saharinen P, Pesu M. et al. The Janus kinases (Jaks). Genome Biol 2004;5:253–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A.. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis 2013;72:ii111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho Lee Y, Gyu Song G.. Comparative efficacy and safety of tofacitinib, baricitinib, upadacitinib, filgotinib and peficitinib as monotherapy for active rheumatoid arthritis. J Clin Pharm Ther 2020;45:674–81. [DOI] [PubMed] [Google Scholar]
- 24.Kamar N, Abravanel F, Marion O. et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med 2021;385:661–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haidar G, Agha M, Lukanski A. et al. Immunogenicity of COVID-19 vaccination in immunocompromised patients: an observational, prospective cohort study interim analysis, medRxiv, doi: 10.1101/2021.06.28.21259576, 30 June 2021, preprint: not peer reviewed. [DOI] [Google Scholar]
- 26.Wadei HM, Gonwa TA, Leoni JC. et al. COVID-19 infection in solid organ transplant recipients after SARS-CoV-2 vaccination. Am J Transplant 2021;21:3496–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dillon SR, Schlissel MS.. Partial restoration of B cell development in Jak-3–/– mice achieved by co-expression of IgH and Eμ-myc transgenes. Int Immunol 2002;14:893–904. [DOI] [PubMed] [Google Scholar]
- 28.Abdelahad M, Ta E, Kesselman MM, Demory Beckler M.. A review of the efficacy of influenza vaccination in autoimmune disease patients. Cureus 2021;13:e15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapetanovic MC, Saxne T, Nilsson JÅ, Geborek P.. Influenza vaccination as model for testing immune modulation induced by anti-TNF and methotrexate therapy in rheumatoid arthritis patients. Rheumatol 2007;46:608–11. [DOI] [PubMed] [Google Scholar]
- 30.Winthrop KL, Silverfield J, Racewicz A. et al. The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis. Ann Rheum Dis 2016;75:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data will be made available upon reasonable request.