The Novel COVID-19, caused by SARS-CoV-2, broke out in 2019. It also occurred in various countries around the world. Since then, countries have been working to prevent the spread of COVID-19, whereas efforts to develop vaccines against COVID-19 have simultaneously achieved great success. However, reports of vaccine-related adverse events cannot be ignored.
Since June 2021, cases of myocarditis after the BNT162b2 vaccine (Pfizer/BioNTech) have been reported, mainly in young men after the second dose of the vaccine.1 A report from Israel stated that during a nationwide vaccination campaign involving >5 million residents, conducted from December 2020 to May 2021, the Israeli Ministry of Health recorded 136 definite or probable cases of myocarditis that were timed to coincide with the receipt of the 2 doses of the BNT162b2 messenger RNA (mRNA) vaccine.2 Recently, an increasing number of cases of myocarditis after the mRNA COVID-19 vaccine have been reported, not only with the BNT162b2 vaccine (Pfizer/BioNTech) but also after vaccination with the mRNA-1273 vaccine (Moderna).3, 4, 5 Of the 2,000,287 subjects who received at least 1 dose of a COVID-19 vaccine, 20 were reported with a diagnosis of myocarditis, and 11 of which occurred after receiving the mRNA-1273 vaccine, whereas the other 9 received the BNT162b2 vaccine.4
Myocarditis is a heterogeneous disease with different clinical patterns, etiologies, and treatment responses, reflecting the inflammatory damage of myocardial tissue in the absence of ischemia.3 Common clinical manifestations of COVID-19 vaccination-related myocarditis include chest pain, fever, palpitations, shortness of breath, fatigue, nausea, vomiting, abdominal pain, or abnormal symptoms such as a strong or pounding heartbeat.6 Recent reports of myocarditis after mRNA vaccination may suggest that myocarditis is a rare potential adverse event associated with mRNA vaccination for COVID-19. However, Diaz et al4 noted that while the short time interval between vaccination and the onset of myocarditis and the increased incidence of myocarditis and pericarditis in the study hospitals supported a possible relation, the temporal correlation did not prove causation. Therefore, we conducted this meta-analysis to further investigate the correlation between the mRNA vaccine and myocarditis.
By October 9, 2021, a systematic database search was performed in PubMed, Web of Science, and the Cochrane Library using search terms such as “mRNA COVID-19 vaccine,” “myocarditis,” “adverse event,” “BNT162b2 vaccine,” and “mRNA-1273 vaccine.” A total of 267 publications were preliminarily found, and 5 were finally included in this study (Table 1 ), with a total of 217 cases of myocarditis.1, 2, 3, 4, 5
Table 1.
Basic information of included studies
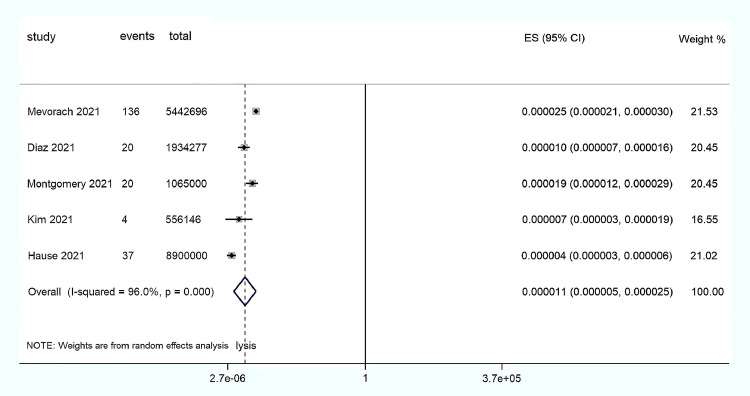
We then used STATA/MP Statistical Software: Release 14 (StataCorp LLC, College Station, Texas) to perform a random-effect meta-analysis for morbidity. Finally, our study found that the incidence of myocarditis was 0.000011 (95% confidence interval 0.000005 to 0.000025) in subjects vaccinated with the mRNA COVID-19 vaccine, which implies an average of 11 cases of myocarditis per 1 million subjects vaccinated with the mRNA COVID-19 vaccine (Figure 1 ). This very low incidence not only indicates the rarity of myocarditis but also suggests a risk of myocarditis after mRNA vaccination.
Figure 1.
Forest map: mRNA vaccine associated with myocarditis. CI = confidence interval; ES = Effect size.
There were no reports of rare myocarditis after non-mRNA vaccination (such as Vaxzevria or Janssen). In contrast, an increasing number of reports cited myocarditis after mRNA vaccination. Why does myocarditis only occur after mRNA vaccination? Several hypotheses have been proposed for this phenomenon. It is speculated from the data reported in preliminary trials of mRNA vaccines in adults that mRNA vaccines may produce very high antibody responses in a small number of young subjects, triggering similar responses in children with multisystem inflammatory syndrome associated with the SARS-CoV-2 infection.6 The COVID-19 mRNA vaccines contain nucleoside-modified mRNA, and it is believed that, in certain genetically predisposed subjects, the immune response to mRNA may not be inhibited and may drive the activation of abnormal innate and acquired immune responses.7 , 8 The immune system may detect genes in the vaccine as antigens, thereby activating proinflammatory cascades and immune pathways that may play a role in the development of myocarditis and become part of the systemic response in some subjects.8 Other hypothesized mechanisms include inducing cytokine expression mediated by anti-idiotypic cross-reactive antibodies in the myocardium and abnormal induction of apoptosis leading to myocardial and pericardium inflammation.6 Although many hypotheses have been proposed in current studies, there is no clear evidence for a specific mechanism of myocarditis after mRNA vaccination, and further studies are needed to prove it.
On June 23, 2021, the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices reviewed the available data and concluded that the benefits of COVID-19 vaccination for subjects and the population outweighed the risks of myocarditis and recommended its continued use in subjects aged 12 years and over.1 Considering the risk of myocarditis in humans, the incidence of myocarditis after vaccination still needs to be monitored intensively. In the context of the current pandemic, vigilance regarding rare adverse events, including myocarditis, after COVID-19 vaccination is warranted, but should not reduce overall confidence in vaccination during the current pandemic.3 Moreover, the Centers for Disease Control and Prevention needs to continue monitoring for adverse events after vaccination, especially myocarditis, to better guide the efficacy and safety assessment of the mRNA COVID-19 vaccine.
This study is a cross-sectional investigation, and we have conducted the Egger test but found no significant publication bias. However, because of the small number of cases of myocarditis, there may be some limitations in the analysis. In addition, because of the high heterogeneity of this study, we adopted the random-effect model. It should also be noted that we failed to conduct subgroup analysis because of the few publications that could be included in the study and the lack of specific age, gender, and other grouping information in some studies. Accordingly, the influence of confounding factors such as age and gender on the results cannot be excluded. If given the opportunity, it is necessary to expand the study to demonstrate a causal relation between the mRNA vaccine and the incidence of myocarditis.
Disclosures
The authors have no conflicts of interest to declare.
Footnotes
The work was supported by the Key Medical Disciplines of Hangzhou (Hangzhou, China).
References
- 1.Hause AM, Gee J, Baggs J, Abara WE, Marquez P, Thompson D, Su JR, Licata C, Rosenblum HG, Myers TR, Shimabukuro TT, Shay DK. COVID-19 vaccine safety in adolescents aged 12-17 years - United States, December 14, 2020-July 16, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1053–1058. doi: 10.15585/mmwr.mm7031e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mevorach D, Anis E, Cedar N, Bromberg M, Haas EJ, Nadir E, Olsha-Castell S, Arad D, Hasin T, Levi N, Asleh R, Amir O, Meir K, Cohen D, Dichtiar R, Novick D, Hershkovitz Y, Dagan R, Leitersdorf I, Ben-Ami R, Miskin I, Saliba W, Muhsen K, Levi Y, Green MS. Keinan-Boker L, Alroy-Preis S. Myocarditis after BNT162b2 mRNA vaccine against COVID-19 in Israel. N Engl J Med. 2021;385:2140–2149. doi: 10.1056/NEJMoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, Loran D, Hrncir D, Herring K, Platzer M, Adams N, Sanou A, Cooper LT., Jr Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6:1202–1206. doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diaz GA, Parsons GT, Gering SK, Meier AR, Hutchinson IV, Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. 2021;326:1210–1212. doi: 10.1001/jama.2021.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim HW, Jenista ER, Wendell DC, Azevedo CF, Campbell MJ, Darty SN, Parker MA, Kim RJ. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. 2021;6:1196–1201. doi: 10.1001/jamacardio.2021.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das BB, Moskowitz WB, Taylor MB, Palmer A. Myocarditis and pericarditis following mRNA COVID-19 vaccination: what do we know so far? Children (Basel) 2021;8:607. doi: 10.3390/children8070607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144:471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caso F, Costa L, Ruscitti P, Navarini L, Del Puente A, Giacomelli R, Scarpa R. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102524. [DOI] [PMC free article] [PubMed] [Google Scholar]