Abstract
Objectives
We aimed to understand the immune response among healthcare workers (HCWs) following SARS-CoV-2 infection, and to determine the infection prevalence during the first wave of the pandemic among workers in our hospital.
Methods
Determination of the serological status against SARS-CoV-2 (nucleocapsid) was offered to all HCWs. All HCWs with positive SARS-CoV-2 serology were proposed to be included in a longitudinal medical and serological follow-up (anti-spike) for 7 months.
Results
We included 3062 HCWs; 256 (8.4%) were positive for anti-SARS-CoV-2 nucleocapsid IgG. Among them, early decrease in the anti-nucleocapsid antibody index was observed between the first (S1) and second (S2) serology samplings in 208 HCWs (84.2%). The initial anti-nucleocapsid IgG index seemed to be related to the HCWs’ age. Seventy-four HCWs were included in the 7-month cohort study. Among them, 69 (90.5%) had detectable anti-spike IgG after 7 months and 24 (32.4%) reported persistent symptoms consistent with post-acute COVID-19 syndrome diagnosis.
Conclusion
The prevalence of serological positivity among HCWs was 6.7%. Infection should be followed by vaccination because of antibody decrease.
Keywords: SARS-CoV-2, COVID-19, Healthcare workers, Vaccination, Anti-spike IgG
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by the human pathogen severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a severe disease that has resulted in widespread morbidity and mortality worldwide. Healthcare workers (HCWs) are at high risk of infection due to close contact with SARS-CoV-2-infected patients [1]. Studies of active and past infections, defined as the presence of SARS-CoV-2 immunoglobulin G (IgG) antibodies, reported that the risk for HCWs comes from work-related exposure (patients and coworkers), as well as from the communities in which they live [2]. Prompt identification of cases by real-time reverse-transcriptase polymerase chain reaction (RT-PCR) screening at hospitals is crucial to avoid new infections and implementation of isolation and quarantine measures of HCWs [3]. Previous studies conducted among HCWs found a cumulative prevalence of SARS-CoV-2 infections ranging from 11% to ˃ 40% [[4], [5]]. Prevalence varies according to the timing within the pandemic, location, country, and type of HCWs. The presence of IgG indicates recent or past contact with SARS-CoV-2. Measurable IgG for SARS-CoV-2 antigens develop after most, but not all, SARS-CoV-2 infections [[2], [6], [7], [8]]. Serological responses against SARS-CoV-2 are usually detectable within 1 to 3 weeks after disease onset [[9], [10]]. SARS-CoV-2 IgG titers change over time [11]. The spike (S) protein and nucleocapsid (N) are the major coronavirus antigens that induce immunoglobulin (Ig) production [12]. Antibodies against the N protein, which are the main target for serological diagnosis, are often induced at a relatively higher level than others [[12], [13]].
We established the Saint-Joseph Hospital Healthcare Worker SARS-CoV-2 Serology Cohort Study in April-May 2020 to understand the immune response following SARS-CoV-2 infection and to determine the infection prevalence following the first wave of the pandemic among workers in our hospital.
2. Methods
The study was conducted at the Paris Saint Joseph Hospital Group from April to November 2020. Paris Saint Joseph hospital is a 894-bed tertiary-care teaching hospital, located in Paris and sub-area, France, and employing 3612 HCWs.
2.1. Study population and sample collection
Determination of the serological status against SARS-CoV-2 (Np) (twice, with a 30-day interval between the first (S1) and second sample (S2)) was proposed to all HCWs. The invitation targeted physicians, nurses, hospital assistants, and hospital administrative staff.
Following informed consent, participants completed a questionnaire that covered sociodemographic information and details related to SARS-CoV-2 infection, including the date of PCR testing, date of symptom onset, and a description of symptoms (ageusia, anosmia, asthenia, dry cough, diarrhea, fever, headache, chest pain, myalgia, shivers) (1-month cohort study).
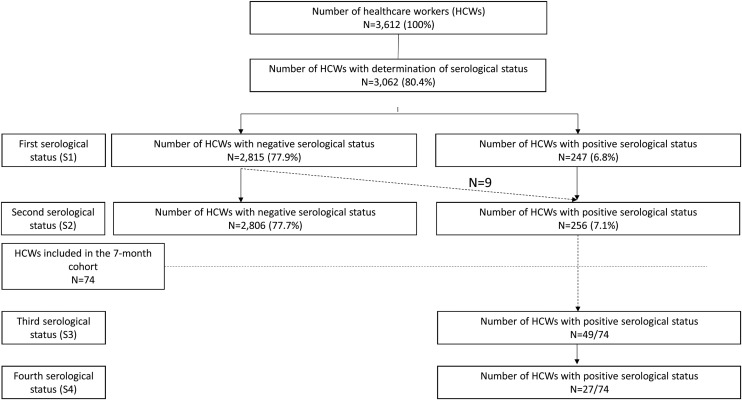
All HCWs with positive SARS-CoV-2 serology were proposed to be included in a longitudinal medical and serological follow-up (day 90 (S3)–day 210 (S4)) (Fig. 1 , 7-month cohort study).
Fig. 1.
Flow chart of the study.
Post-acute COVID-19 syndrome was defined as the persistence of symptoms and organ damage that stretched beyond the 3-month period after the infection [14].
2.2. Laboratory assays
Anti-SARS-CoV-2 IgG in serum samples was measured using the Abbott Architect i2000 chemiluminescent microparticle immunoassay (CMIA; Abbott, Maidenhead; UK) according to the manufacturer's instructions. During the study, we used two different assays, one dedicated to the semi-quantitative detection of anti-SARS-CoV-2 IgG directed against nucleocapsid proteins and the other to the quantitative detection of anti-SARS-CoV-2 IgG directed against the two receptor-binding domains (RBDs) of the spike-1 protein. They are two-step immunoassays. Paramagnetic microparticles coated with recombinant SARS-CoV-2 nucleocapsid protein or RBD of spike-1 protein are bound by specific anti-SARS-CoV-2 IgG present in the serum. Anti-human IgG acridinium-labeled conjugates are used as the detection antibody. The presence or absence of IgG antibodies to SARS-CoV-2 in the sample is determined by comparing the chemiluminescent RLU in the reaction to the calibrator RLU, which is calculated by the system as an Index (S/C) for the semi-quantitative test or converted to AU/ml for the quantitative test.
Antibody index ≥ 1.40 arbitrary units of the manufacturer were considered positive for anti-SARS-CoV-2 nucleocapsid IgG; those between 0.49–1.39 were considered equivocal (following Abbott Diagnostics Product Information Letter PI1060-2020); and those < 0.49 were considered negative. The positivity threshold for anti-SARS-CoV-2 spike-1 IgG was 50 AU/ml.
2.3. Anti-SARS-CoV-2 ELISpot IFN-γ assay
The ELISpot IFN-γ assay is a reference method to explore T-cell response. ELISpot IFN-γ assays were performed on PBMCs and BAL-collected cells, as previously described [15], using ELISpot IFN-γ-pair-antibodies (Diaclone). Briefly, 1 × 105 PBMCs/well were plated (Merck Millipore, Molsheim, France) in triplicate with medium, phytohemagglutinin (2 μg/mL, Sigma-Aldrich) or SARS-CoV-2-peptide pools (2 μg/mL). Plates were developed with streptavidin-alkaline phosphatase conjugate (Amersham, Freiburg, Germany) and NBT/BCIP substrate (Sigma-Aldrich) and then air-dried for 24 hours before spot-forming cell units (SFC) were read (AID Elispot reader, Autoimmun Diagnostika GmbH, Straßberg, Germany). Results are expressed as the mean SFC × 106 from triplicates after background subtraction. The positivity threshold was set at 50 SFC/106 PBMCs. SARS-CoV-2 overlapping 18-mer-peptides covering the viral nucleocapsid and spike proteins were tested separately.
2.4. Statistical methods
HCW characteristics are presented using means ± standard deviation (SD), medians [interquartile range], and percentages for categorical variables. Percentages were calculated based on documented data (missing data were excluded from percentages). Inter-group comparisons were made using the Mann-Whitney test for quantitative variables and Fisher's exact test for qualitative variables. The statistical analysis was performed using the R software (version 3.2.2). All tests were two-tailed and P values < 0.05 (calculated by Chi2 test, Student's t-test, or Mann-Whitney test) were considered significant.
2.5. Ethics approval
The trial obtained approval from the independent Ethics Committee “Sud Méditérrannée I” on May 5,2020 (2020-A01837-32) and was conducted according to Good Clinical Practices and the Declaration of Helsinki, as last amended. The study was sponsored by the Paris Saint-Joseph Hospital Group.
3. Results
3.1. One-month serological follow-up
Among the 3612 HCWs in the hospital group, 3062 (85%) agreed to participate and were included in the 1-month cohort. After the first two serological tests (S1 and S2), 256 (8.4%) HCWs were positive for anti-SARS-CoV-2 nucleocapsid IgG. This included symptomatic as well as asymptomatic HCWs, and all were asked to participate in the 7-month cohort study.
Among the 256 included HCWs, the mean age was 38.2 ± 11.9 years, with a sex ratio (M/F) of 0.3. Among them, 116 (45.3%) had RT-PCR performed, of whom 81 (69.8%) had a positive result. Average time between RT-PCR and serology was 31 days. In total, 247 (96.5%) HCWs were positive to the first serological test (S1) for anti-nucleocapsid IgG, of whom 72 (29.1%) reported a history of positive SARS-CoV-2 RT-PCR. After the second serology (S2), nine additional HCWs were detected positive following late infection at the end of the first epidemic wave. Thanks to S2, we observed an early decrease in the anti-nucleocapsid antibody index between the first (S1) and second (S2) serologies for 208 (84.2%) HCWs, which led to offering a longitudinal follow-up to all these HCWs.
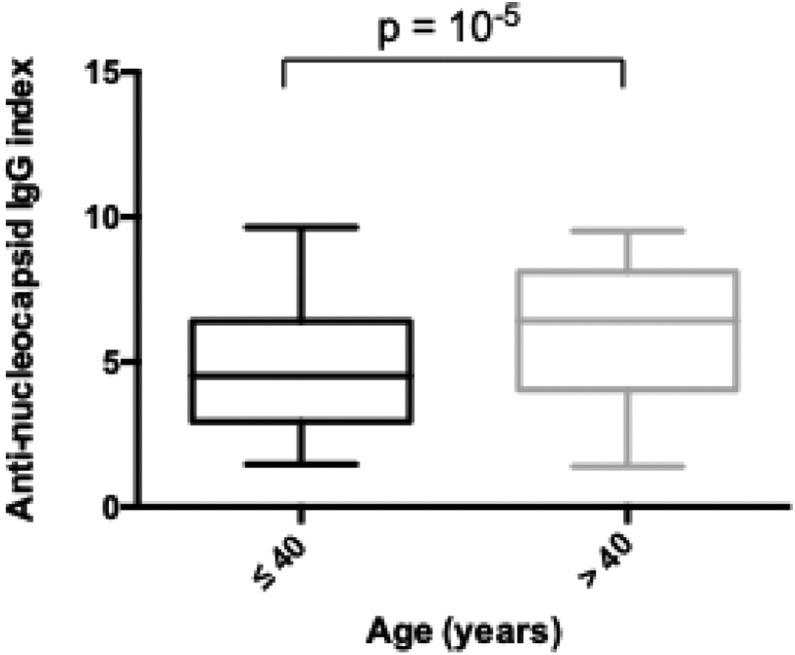
We analyzed the evolution of the anti-nucleocapsid IgG index. There were no factors associated with the increase or decrease of the anti-nucleocapsid IgG index (age, sex, SARS-CoV-2-related infection). However, the initial antibody index seemed to be related to the HCWs’ age (Fig. 2 ). For HCWs ≤ 40 years of age, the median antibody index was 4.52 [2.95–6.48], whereas it was 6.4 [[3], [4], [5], [6], [7]] for HCWs > 40 years of age (P < 0.01 in multivariate analysis), irrespective of disease severity.
Fig. 2.
Anti-nucleocapsid IgG index according to age (n = 256).
3.2. Seven-month medical and serological follow-up
Overall, 74 HCWs (28.9%) agreed to participate in the longitudinal medical and serological study over a period of 7 months and provided ≥ 2 samples for serological testing. HCW characteristics are summarized in Table 1 . The median [IQR] age was 47 [33.2–54.2] years and 82.4% of participants were females. Among the 74 HCWs, 65 (87.8%) recalled self-identified COVID-19-like symptoms and nine (12.2%) HCWs never had any symptom; the positive serology is therefore an incidental finding of previous contact with SARS-CoV-2. Of these 65 HCWs, 39 (52.7%) had a positive SARS-CoV-2 PCR. The most frequently reported symptoms evocative of COVID-19 were headaches, anosmia, ageusia, cough, and fever in respectively 70.8%, 70.8%, 67.7%, 56.9%, and 53.8% of cases.
Table 1.
Baseline cohort demographics for the 74 included patients.
| Characteristics | Whole cohort n (%) or median [IQR] |
|---|---|
| Age, years | 47 [33.2–54.2] |
| Gender | |
| Female | 61 (82.4) |
| Male | 13 (17.6) |
| Body mass index (kg/m2) | 23.7 [21.5–26] |
| Risk factors for severe COVID-19 infections | |
| Yes | 20 (27) |
| No | 50 (67.6) |
| Not disclosed | 4 (5.4) |
| COVID-19-like symptoms between March and June 2020 | |
| Yes | 65 (87.8) |
| No | 8 (10.8) |
| Not disclosed | 1 (1.4) |
| COVID-19 symptoms (n = 65) | |
| Headaches | 46 (70.8) |
| Anosmia | 46 (70.8) |
| Ageusia | 44 (67.7) |
| Cough | 37 (56.9) |
| Fever | 35 (53.8) |
| Dyspnea | 26 (40) |
| Diarrhea | 24 (36.9) |
| Arthralgia | 12 (18.4) |
| Previous positive SARS-CoV-2 PCR | 39 (52.7) |
| Post-acute COVID-19 syndrome | 24 (32.4) |
| Asthenia | 12 (16.2) |
| Dyspnea | 10 (13.5) |
| Concentration disorder | 7 (9.5) |
Among the 39 HCWs with a positive SARS-CoV-2 RT-PCR, 37 (94.8%) showed seroconversion, resulting in the appearance of anti-spike and anti-nucleocapsid IgG. Time from the first positive RT-PCR to the first serological test was 33 [25.2–40.75] days. Median [IQR] time from the first to the last sample was 214 [203.5–221] days.
3.2.1. Anti-nucleocapsid and anti-spike IgG evolution
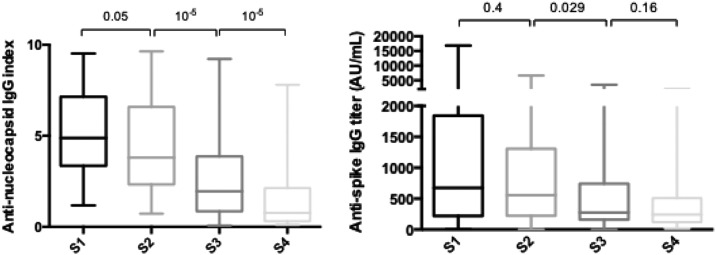
Among the 74 HCWs with a first anti-nucleocapsid IgG-positive sample, 25 (37.9%) were negative at day 90. Increasing age was associated with persistence of a positive serological test at day 90 (S3) (median age 50.7 [41.5–56.7] years vs. 33.5 [27.5–50.3] years; p = 0.0011). However, prior symptoms compatible with COVID-19 and a positive SARS-CoV-2 RT-PCR were not associated with persistence of a positive serological test at day 90. There was no difference in the anti-nucleocapsid antibody index at S1, S2, S3, or S4 between HCWs with post COVID-19 syndrome and those with no persistent symptom. The observed anti-nucleocapsid IgG antibody evolution is presented in Fig. 3A.
Fig. 3.
A. SARS-CoV-2 anti-nucleocapsid and B. Anti-spike IgG antibody evolution for the whole cohort. (S1 = day 0, S2 = month 1 or day 30, S3 = month 3 or day 90, S4 = month 8 or day 210) (n = 74).
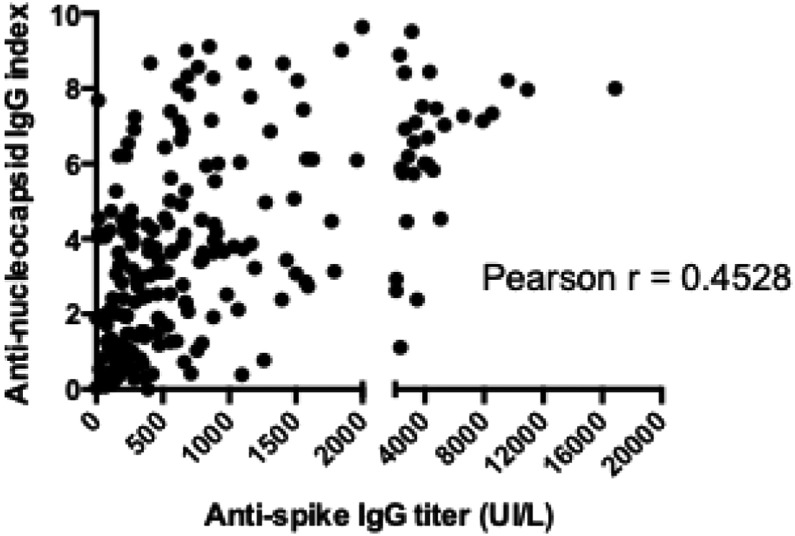
Among the 74 included HCWs, 69 (90.5%) had detectable anti-spike IgG at S4. Of the five (6.7%) for whom serology was negative, four never had positive anti-spike IgG titer and the last was negative as early as S1. Despite a significant reduction between S1 and S2, anti-spike IgG titers remained above the positive threshold for most seropositive HCWs for the duration of the study (up to 210 days, Fig. 3B). No correlation between age, symptomatic nature of the infection, or persistence of symptoms and the initial titer or evolution of the anti-spike IgG antibody titer could be identified. However, there was a weak correlation between the anti-nucleocapsid IgG index and the anti-spike IgG titer (Fig. 4 ).
Fig. 4.
Correlation between the anti-nucleocapsid antibody index and anti-spike antibody titers.
3.2.2. ELISpot IFN-γ assays
Among the 74 HCWs, ELISpot IFN-γ assays were performed for 19 HCWs (including HCWs with symptomatic and asymptomatic infections, positive and negative SARS-CoV-2 RT-PCR, and negative and positive SARS-CoV-2 serology) at the time of the fourth serological test. The effector and effector memory T-cell responses were mainly directed against the spike protein (eight responders) or the N-terminal (five responders), or C terminal portion (four responders) of the protein (Table 2 ).
Table 2.
Description of 19 patients analyzed for cellular and humoral immune response.
| Patient | Age | Symptoms | ELISpot SARS-CoV-2 | Serology | PCR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sampling date | Results | Target protein | Sampling date | Anti-nucleocapsid IgG index | Results | Anti-spike IgG (UA/mL) | Results | Performed | Sampling date | Results | |||
| 1 | 44 | Yes | 12/08/2020 | POS | Spike | 08/12/2020 | 2.12 | POS | 229.1 | POS | Yes | NA | POS |
| 2 | 33 | Yes | 12/08/2020 | POS | Spike | 08/12/2020 | 0.71 | NEG | 159.5 | POS | No | – | – |
| 3 | 40 | Yes | 12/03/2020 | POS | Spike | 08/12/2020 | 3.45 | POS | 97.7 | POS | No | – | – |
| 4 | 56 | No | 12/14/2020 | POS | Spike, Np | 30/11/2020 | 4.39 | POS | 378.5 | POS | Yes | 08/19/2020 | NEG |
| 5 | 33 | Yes | 12/08/2020 | NEG | – | 08/12/2020 | 0.28 | NEG | 285.2 | POS | Yes | 04/01/2020 | POS |
| 6 | 44 | No | 11/23/2020 | NEG | – | 23/12/2020 | 0.53 | NEG | 26.3 | NEG | No | – | – |
| 7 | 53 | Yes | 11/23/2020 | POS | Spike, Np | 16/11/2020 | 1.41 | POS | 304 | POS | Yes | 03/23/2020 | POS |
| 8 | 27 | Yes | 12/07/2020 | NEG | – | 07/12/2020 | 0.39 | NEG | 104 | POS | No | – | – |
| 9 | 33 | Yes | 12/01/2020 | NEG | – | 01/12/2020 | 4.41 | POS | 537.2 | POS | Yes | 09/01/2020 | NEG |
| 10 | 26 | No | 02/01/2020 | NEG | – | 01/12/2020 | 0.54 | NEG | 281.6 | POS | No | – | – |
| 11 | 58 | No | 11/30/2020 | NEG | – | 30/11/2020 | 7.75 | POS | 214.5 | POS | Yes | 07/27/2020 | NEG |
| 12 | 51 | Yes | 11/24/2020 | POS | Spike, Np | 24/11/2020 | 7.77 | POS | 537.2 | POS | Yes | 03/302020 | POS |
| 13 | 49 | Yes | 11/24/2020 | POS | Np | 24/11/2020 | 0.88 | POS | 121.9 | POS | Yes | 03/16/2020 | POS |
| 14 | 34 | Yes | 11/23/2020 | NEG | – | 23/11/2020 | 0.16 | NEG | 117.5 | POS | No | – | – |
| 15 | 63 | No | 11/23/2020 | NEG | – | 23/12/2020 | 4.04 | POS | 17.6 | NEG | No | – | – |
| 16 | 51 | Yes | 12/01/2020 | NEG | – | 01/12/2020 | 0.19 | NEG | 100.3 | POS | Yes | 03/12/2020 | NEG |
| 17 | 57 | No | 12/07/2020 | POS | Spike | 07/12/2020 | 1.91 | POS | 318.5 | POS | Yes | 04/14/2020 | POS |
| 18 | 24 | Yes | 12/14/2020 | NEG | – | 09/11/2020 | 0.32 | NEG | 988.8 | POS | Yes | 08/10/2020 | POS |
| 19 | 52 | Yes | 12/14/2020 | POS | Spike, Np | 14/12/2020 | 0.32 | NEG | 40.5 | NEG | Yes | 09/25/2020 | NEG |
Np: nucleocapsid; POS: positive; NEG: negative.
There was no statistical association between response and age or presence of symptoms. There was, however, a correlation with the serology for the same monitoring point (both anti-nucleocapsid IgG and ELISpot negative or positive in 14/19 cases), with two subjects with negative serology but a positive ELISpot and three with a negative ELISpot but positive serology, i.e. a correlation of 73.7%. For anti-spike IgG, the correlation was only 10/19 (52.6%).
4. Discussion
COVID-19 is a major global healthcare challenge. The disease spectrum varies widely, ranging from asymptomatic or with only mild symptoms to severe disease and death. Understanding the temporal profile by which circulating antibody classes are produced following SARS-CoV-2 infection is essential for the interpretation and clinical application of serological test results. After symptom onset or a positive RT-PCR, all included HCWs were tested positive for SARS-CoV-2 IgG within 21 to 28 days regardless of their age, gender, risk factors, or symptoms but there were variations between individuals. In our hospital, the prevalence of serological positivity among healthcare workers was 6.8%.
Despite our relatively young population, with a median age of 47 years, we observed an association between longer anti-nucleocapsid IgG half-lives and increasing age. This may be related to boosting of cross-reactivity with other human coronaviruses [16]. Although IgM immunoglobulins are expected to be the first class detected following infection by SARS-CoV-2, as supported by several studies [[13], [17]], other studies have paradoxically demonstrated IgG responses preceding the IgM response [[18], [19]]. This surprising discrepancy is likely related to cross-reactivity with pre-existing immunity to human coronaviruses. A/pauci-symptomatic and symptomatic patients produced specific SARS-CoV-2 antibodies during the acute phase of the disease, but it is difficult to conclude whether there is a correlation between SARS-CoV-2 IgG kinetics and COVID-19 clinical symptoms, as there was an imbalance between asymptomatic and symptomatic cases. Other studies reported that asymptomatic individuals become seronegative in the early convalescent phase faster than symptomatic individuals, presumably due to a weaker immune response to SARS-CoV-2 infection in the asymptomatic population [13].
We also showed that SARS-CoV-2 anti-nucleocapsid IgG indexes start to decrease early (1 month after infection), and their duration is significantly correlated with the initial antibody titers. This motivated the second cohort study with the longitudinal follow-up of HCWs previously infected with SARS-CoV-2. Indeed, individuals with low titers of SARS-CoV-2 IgG may be more likely to become seronegative as early as 3 months for some of them. Approximately 6 months later, a progressive decline of IgG values was observed; in particular, almost one half of patients had a significant reduction of anti-nucleocapsid IgG below the threshold of positivity. Conversely, as demonstrated in a previous study, long-term persistence of anti-spike IgG was demonstrated in 93.4% of HCWs [20]. The cellular response also weakened over time and was positive in only 42% of cases at 7 months. Our data are consistent with those of previous studies showing seroconversion of SARS-CoV-2 antibodies in almost all patients within two weeks after diagnosis [21]. Data concerning the long-term persistence and levels of anti-SARS-CoV-2 antibodies over time are scarce. Most studies investigating the kinetics of SARS-CoV-2 antibodies have been limited to approximately 40 days after symptom onset [[22], [23]], showing the trend and the rapid increase of IgG titers during the acute phase of COVID-19. However, a recent study that analyzed the long-term kinetics of neutralizing anti-SARS-CoV-2 antibodies suggested that anti-spike IgG are durable [24], with only a modest decline in titers months after symptom onset, and almost all individuals appeared to remain positive for anti-spike IgG 5 to 7 months after COVID-19 infection [[25], [26]]. However, the progressive decrease in titers of anti-spike antibodies raises the question of the need for future booster vaccinations.
Conversely, other studies indicated that although anti-spike IgG can last for more than 6 months after SARS-CoV-2 infection, there is still a significant reduction in antibody titers [27], which possibly predicts the progressive loss of protective antibody response within several months after SARS-CoV-2 infection [28]. We observed a progressive decline of both anti-nucleocapsid and anti-spike IgG titers in our study. However, a significant reduction of IgG titers only concerned anti-nucleocapsid IgG until negativation according to the interpretation criteria and even undetectable in some cases, whereas anti-spike IgG titers consistently remained above the positivity threshold. The cellular response evaluated in 18 patients only showed 42% positivity at month 8. This result shows that the long-term immune memory to SARS-CoV-2 of a/pauci-symptomatic patients is weak, as previously described [29]. It also suggests that protection against re-infection is not complete in this context, in favor of appropriate vaccination even in this population. However, patients with symptomatic COVID-19 in the first wave of infections are at significantly lower risk of positive PCR test later on [[3], [30]]. In our study, two cases of recurrence (new SARS-CoV-2 RT-PCR positivity) were diagnosed during the investigation of a cluster suggesting transient carriage rather than re-infection due to the absence of symptoms.
In our study, as in the previous one, a significant proportion of caregivers (32.4%) reported persistence of symptoms falling within the definition of post-acute COVID-19 syndrome [[31], [32]]. No correlation between the kinetics of anti-spike or anti-nucleocapsid IgG could be demonstrated. A systematic study of sequelae after recovery from acute COVID-19 is required to develop an evidence-based multidisciplinary team approach to manage these patients and to inform research priorities.
Our study had several limitations. First, we did not study the seroconversion date post-symptom onset or after a positive RT-PCR. Second, we did not test the neutralizing activities of the anti-SARS-CoV-2 nucleocapsid or spike antibodies and do not know whether or not their presence confers protective immunity. Finally, we could not analyze the association between immune response and clinical course due to the imbalance between symptomatic and asymptomatic cases.
5. Conclusion
This study confirms literature data on the early decrease in anti-N and slower decrease in anti-S antibodies with persistence of antibodies at a certain level. Our study results confirm that infection should be followed by vaccination because of antibody decrease.
Funding
No Funding.
Contribution of authors
All authors participated in the study, reviewed the manuscript and validated the submission. BP: study design, data collection, manuscript preparation, reviewing; IE: data collection; GPDP: data collection, reviewing; HD: data collection; AB: data collection; AG: data collection, manuscript preparation, reviewing; NC: data collection; PP: study design; AM: data collection; GH: study design, manuscript preparation, reviewing; JL: study design; SL: study design; ALM: study design, data collection, manuscript preparation, reviewing.
Disclosure of interest
The authors declare that they have no competing interest.
Availability of data and material
Data are available.
Consent to participate
All included healthcare workers agreed to participate.
Consent for publication
All authors validated the submission.
Ethics approval
The trial obtained approval from the independent Ethics Committee “Sud Méditérrannée I” on May 5, 2020 (2020-A01837-32) and was conducted according to Good Clinical Practices and the Declaration of Helsinki, as last amended. The study was sponsored by the Paris Saint-Joseph Hospital Group.
Acknowledgments
Pr. Gilles Chatelier (methodologist); Morgane Pauillat, Mathilde Herr
DRC: Estelle PLAN, Audrey FELS, Julien FOURNIER, and Dr Hélène BEAUSSIER
The authors would like to thank the T4/T8 department of the Immunology Department of the Pitié Salpêtrière Hospital, Paris.
The authors would like to thank all colleagues who agreed to participate in the study.
References
- 1.Chou R., Dana T., Buckley D.I., Selph S., Fu R., Totten A.M. Epidemiology of and risk factors for coronavirus infection in health care workers: a living rapid review. Ann Intern Med. 2020;173:120–136. doi: 10.7326/M20-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fafi-Kremer S., Bruel T., Madec Y., Grant R., Tondeur L., Grzelak L., et al. Serologic responses to SARS-CoV-2 infection among hospital staff with mild disease in eastern France. EBioMedicine. 2020;59:102915. doi: 10.1016/j.ebiom.2020.102915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lumley S.F., O’Donnell D., Stoesser N.E., Matthews P.C., Howarth A., Hatch S.B., et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384:533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Basteiro A.L., Moncunill G., Tortajada M., Vidal M., Guinovart C., Jiménez A., et al. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun. 2020;11:3500. doi: 10.1038/s41467-020-17318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houlihan C.F., Vora N., Byrne T., Lewer D., Kelly G., Heaney J., et al. Pandemic peak SARS-CoV-2 infection and seroconversion rates in London frontline health-care workers. Lancet Lond Engl. 2020;396:6–7. doi: 10.1016/S0140-6736(20)31484-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deeks J.J., Dinnes J., Takwoingi Y., Davenport C., Spijker R., Taylor-Phillips S., et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev. 2020;6 doi: 10.1002/14651858.CD013652. [CD013652] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National SARS-CoV-2 Serology Assay Evaluation Group Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison. Lancet Infect Dis. 2020;20:1390–1400. doi: 10.1016/S1473-3099(20)30634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wellinghausen N., Plonné D., Voss M., Ivanova R., Frodl R., Deininger S. SARS-CoV-2-IgG response is different in COVID-19 outpatients and asymptomatic contact persons. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2020;130:104542. doi: 10.1016/j.jcv.2020.104542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiang F., Wang X., He X., Peng Z., Yang B., Zhang J., et al. Antibody detection and dynamic characteristics in patients with Coronavirus Disease 2019. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020;71:1930–1934. doi: 10.1093/cid/ciaa461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.To K.K.-W., Tsang O.T.-Y., Leung W.-S., Tam A.R., Wu T.-C., Lung D.C., et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Legros V., Denolly S., Vogrig M., Boson B., Siret E., Rigaill J., et al. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell Mol Immunol. 2021 doi: 10.1038/s41423-020-00588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhama K., Sharun K., Tiwari R., Dadar M., Malik Y.S., Singh K.P., et al. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccines Immunother. 2020;16:1232–1238. doi: 10.1080/21645515.2020.1735227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo L., Ren L., Yang S., Xiao M., Chang D., Yang F., et al. Profiling early humoral response to diagnose novel Coronavirus Disease (COVID-19) Clin Infect Dis Off Publ Infect Dis Soc Am. 2020;71:778–785. doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wyllie D., Mulchandani R., Jones H.E., Taylor-Phillips S., Brooks T., Charlett A., et al. SARS-CoV-2 responsive T cell numbers are associated with protection from COVID-19: A prospective cohort study in keyworkers. Infectious Diseases (except HIV/AIDS) 2020 doi: 10.1101/2020.11.02.20222778. [DOI] [Google Scholar]
- 16.Ma Z., Li P., Ji Y., Ikram A., Pan Q. Cross-reactivity towards SARS-CoV-2: the potential role of low-pathogenic human coronaviruses. Lancet Microbe. 2020;1:151. doi: 10.1016/S2666-5247(20)30098-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isa M.B., Martínez L., Giordano M., Passeggi C., de Wolff M.C., Nates S. Comparison of immunoglobulin G subclass profiles induced by measles virus in vaccinated and naturally infected individuals. Clin Diagn Lab Immunol. 2002;9:693–697. doi: 10.1128/cdli.9.3.693-697.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long Q., Deng H., Chen J., Hu J., Liu B., Liao P., et al. Antibody responses to SARS-CoV-2 in COVID-19 patients: the perspective application of serological tests in clinical practice. MedRxiv. 2020 doi: 10.1101/2020.03.18.20038018. [DOI] [Google Scholar]
- 19.Lee Y.-L., Liao C.-H., Liu P.-Y., Cheng C.-Y., Chung M.-Y., Liu C.-E., et al. Dynamics of anti-SARS-Cov-2 IgM and IgG antibodies among COVID-19 patients. J Infect. 2020;81:55–58. doi: 10.1016/j.jinf.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breathnach A.S., Riley P.A., Cotter M.P., Houston A.C., Habibi M.S., Planche T.D. Prior COVID-19 significantly reduces the risk of subsequent infection, but reinfections are seen after eight months. J Infect. 2021 doi: 10.1016/j.jinf.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao Y., Ling Y., Chen Y.-Y., Tian D., Zhao G.-P., Zhang X.-H., et al. Dynamic anti-spike protein antibody profiles in COVID-19 patients. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2021;103:540–548. doi: 10.1016/j.ijid.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long Q.-X., Liu B.-Z., Deng H.-J., Wu G.-C., Deng K., Chen Y.-K., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 23.Okba N.M.A., Müller M.A., Li W., Wang C., GeurtsvanKessel C.H., Corman V.M., et al. Severe acute respiratory syndrome Coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26:1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021:371. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marot S., Malet I., Leducq V., Zafilaza K., Sterlin D., Planas D., et al. Rapid decline of neutralizing antibodies against SARS-CoV-2 among infected healthcare workers. Nat Commun. 2021;12:844. doi: 10.1038/s41467-021-21111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wajnberg A., Amanat F., Firpo A., Altman D.R., Bailey M.J., Mansour M., et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X., Lu S., Li H., Wang Y., Lu Z., Liu Z., et al. Viral and antibody kinetics of COVID-19 patients with different disease severities in acute and convalescent phases: a 6-month follow-up study. Virol Sin. 2020;35:820–829. doi: 10.1007/s12250-020-00329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang L., Yang B., Jiang N., Fu W., He X., Zhou Y., et al. Three-month follow-up study of survivors of coronavirus disease 2019 after discharge. J Korean Med Sci. 2020;35:418. doi: 10.3346/jkms.2020.35.e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long Q.-X., Tang X.-J., Shi Q.-L., Li Q., Deng H.-J., Yuan J., et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 30.Hanrath A.T., Payne B.A.I., Duncan C.J.A. Prior SARS-CoV-2 infection is associated with protection against symptomatic reinfection. J Infect. 2020 doi: 10.1016/j.jinf.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chopra V., Flanders S.A., O’Malley M., Malani A.N., Prescott H.C. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2020 doi: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnold D.T., Hamilton F.W., Milne A., Morley A.J., Viner J., Attwood M., et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 2020 doi: 10.1136/thoraxjnl-2020-216086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available.