Abstract
Background
International COVID-19 guidelines recommend that health care workers (HCWs) wear filtering facepiece (FFP) respirators to reduce exposure risk. However, there are concerns about FFP respirators causing hypercapnia via rebreathing carbon dioxide (CO2). Most previous studies measured the physiological effects of FFP respirators on treadmills or while resting, and such measurements may not reflect the physiological changes of HCWs working in the emergency department (ED).
Objective
Our aim was to evaluate the physiological and clinical impacts of FFP type II (FFP2) respirators on HCWs during 2 h of their day shift in the ED.
Methods
We included emergency HCWs in this prospective cohort study. We measured end-tidal CO2 (ETCO2), mean arterial pressure (MAP), respiratory rate (RR), and heart rate values and dyspnea scores of subjects at two time points. The first measurements were carried out with medical masks while resting. Subjects then began their day shift in the ED with medical mask plus FFP2 respirator. We called subjects after 2 h for the second measurement.
Results
The median age of 153 healthy volunteers was 24.0 years (interquartile range 24.0–25.0 years). Subjects’ MAP, RR, and ETCO2 values and dyspnea scores were significantly higher after 2 h. Median ETCO2 values increased from 36.4 to 38.8 mm Hg. None of the subjects had hypercapnia symptoms, hypoxia, or other adverse effects.
Conclusion
We did not observe any clinical reflection of these changes in physiological values. Thus, we evaluated these changes to be clinically insignificant. We found that it is safe for healthy HCWs to wear medical masks plus FFP2 respirators during a 2-h working shift in the ED.
Keywords: COVID-19, end-tidal carbon dioxide, filtering facepiece respirators, medical masks, personal protective equipment, physiological effects
Introduction
Health care workers (HCWs) are working on the front lines in the struggle against the COVID-19 pandemic and they have an increased risk of exposure (1). During the SARS pandemic (2003), more than one-fifth of cases were HCWs (2). Recent publications report thousands of COVID-19–infected HCW deaths around the globe (2, 3, 4). The Centers for Disease Control and Prevention and World Health Organization recommend that HCWs in COVID-19–contaminated areas use personal protective equipment (PPE). Medical masks and filtering facepiece (FFP) respirators are commonly used PPE among HCWs (5,6).
Medical masks, also known as surgical masks, provide insufficient protection from airborne pathogens due to their structural properties. On the contrary, FFP type II (FFP2) respirators, which are equivalent to N95 respirators, and FFP type III (FFP3) respirators limit air passage and leakage, providing more effective respiratory protection (> 95% and > 99%, respectively) (7). However, there are concerns about the inadequate gas exchange caused by FFP respirators (8,9). FFP respirators can increase flow resistance, dead space, tidal volume, and finally decrease alveolar ventilation. Rebreathing carbon dioxide (CO2) can cause hypercapnia. Hypercapnic hypoxia can increase anaerobic metabolism, cardiac and renal load, and eventually cause organ dysfunction, putting HCWs with chronic pulmonary, renal, or cardiac diseases at risk (10,11).
Prior studies have reported frequently that medical masks do not have a significant impact on gas exchange or cause clinically important physiological changes (8,12, 13, 14). However, the available data for FFP respirators on HCWs’ physiological values are insufficient and contradictory. In their prospective study with 10 HCWs, Roberge et al. reported that wearing N95 respirators had no significant influence on end-tidal CO2 (ETCO2), oxygen saturation (SatO2), heart rate (HR), or respiratory rate (RR) values after a 1-h walk on a treadmill (15). In contrast, Kim et al. stated that ETCO2, HR, and RR values in 24 subjects with N95 respirators changed substantially after treadmill exercise (16). In studies in which a statistically significant physiological change was found, clinical insignificance in healthy individuals was mostly reported (11,13,15, 16, 17, 18).
The literature indicates that FFP respirators’ influence on physiological values is achieved mainly during treadmill exercise tests or measurements in resting position. However, such measurements might not accurately project HCWs’ physiological changes while working in the emergency department (ED). In this study, we evaluated the physiological and clinical impacts of FFP2 respirators on emergency HCWs during 2 h of their day shift in the ED.
Materials and Methods
Study Design and Subjects
We conducted this prospective cohort study on emergency care providers at a university hospital after the university's Institutional Review Board (IRB) approved our study protocol (IRB no. 09.2021.66). Undergraduate medical students observe and practice emergency medicine (EM) in the ED during their EM clerkship rotation. EM residents also work in the ED as a requirement of their EM residency program. Healthy undergraduate medical students and EM residents who worked in the emergency care area and volunteered to participate in this study were included for a 6-month period. We excluded subjects who were taking medication for known cardiopulmonary diseases. All subjects signed an informed consent form before participating in this study and this study conformed to the principles of the Declaration of Helsinki.
We used randomization (Research Randomizer, https://www.randomizer.org) to determine the number of measurements per day and each subject's measurement date according to their monthly working schedule. A maximum number of 3 subjects were measured per day, as our resources (i.e., devices, separate rooms, and investigators) were limited. Investigators instructed subjects not to stray from their daily routines in terms of activity, sleep, and nutrition for 24 h before the measurements. The emergency care area where the study was conducted was not a COVID-19 care area and patients with confirmed COVID-19 were transferred to a different area. However, the time between the patient's arrival via ambulance and making a diagnostic confirmation involved risk of exposure. Our institutional PPE guideline, which is responsive to international guidelines, recommends mandatory use of medical masks in areas where there is no risk for COVID-19 exposure and medical mask plus FFP respirators where the risk is present.
We placed subjects in separate rooms for 15 min of resting with medical masks (A&ZMED, OLI-2026 Type IIR). After resting, we carried out the first measurements and data collection at time zero (T0) with medical mask (Figure 1 ). Subsequently, subjects began their day shift in the ED wearing an additional FFP2 respirator without an exhalation valve (FAGO MED, FFP2 NR, EN149:2001+A1:2009, CE 2163). We called the subjects after 2 h of work (T1) for the second measurements wearing medical mask plus FFP2 respirator (Figure 1). One investigator monitored and reported whether subjects took their masks off. We excluded those subjects (n = 2). Every subject was equipped with standard masks (medical masks and FFP respirators) and each had two measurements taken (at T0 and T1).
Figure 1.
Demonstration of a subject wearing medical mask and medical mask plus type II filtering facepiece (FFP2) respirator. (A) Subject wearing medical mask at the first measurement (T0). Nasal prong inserted under the mask. (B) Subject wearing medical mask plus FFP2 respirator at the second measurement (T1). Nasal prong inserted under the mask.
Data Collection
We used noninvasive techniques for all measurements because we were concerned that the invasive procedures might influence physiological values. We measured ETCO2 and RR through nasal prongs and SatO2 with fingertip apparatus via a capnography device (SeaMed PC900B) and blood pressure (BP) and HR with a BP monitor (Omron, M2, HEM-7121-E). We placed nasal prongs under the masks rapidly during measurements to minimize the air leakage. Subjects carried a pedometer (Geonaute, OnWalk 100) and we recorded their step counts during their 2-h work period.
We noted descriptive data, such as comorbidities, COVID-19 history, and smoking status at T0. We evaluated the intensity of subjects’ dyspnea by using a 10-cm-long visual analog scale (VAS) chart (0 = no dyspnea; 10 = extremely strong dyspnea) at T0 and T1. We also asked subjects whether they experienced fatigue, muscular weakness, headaches, drowsiness, or other symptoms at T1.
Statistical Analysis
We reported continuous variables as medians and interquartile ranges (IQRs) and categorical variables as proportions and counts because normality in distribution was not observed via Kolmogorov-Smirnov test. We assessed the significance of the difference between dependent groups by Wilcoxon test and calculated correlations as defined by Spearman. We used SPSS statistical software, version 26.0 (IBM Corp) for statistical analysis and accepted type 1 error as 5%.
Results
The study population consisted of 153 healthy volunteers. Twenty-four EM residents (15.7%) and 129 undergraduate medical students (84.3%) completed the study protocol. The median age was 24.0 years (IQR 24.0–25.0 years). Sixty-five subjects (42.5%) were female and 37 (24.2%) were smokers.
Five subjects (3.3%) reported having one comorbid disease for which they required medication or follow-up (i.e., β-thalassemia, hypothyroidism, asthma, bipolar, and anxiety disorder). Twenty-one subjects (13.7%) had positive COVID-19 history prior to this study. In those 21 subjects, 3 (14.3%) had bilateral pulmonary lesions on computed tomography, 1 (4.8%) was admitted to the hospital, and 18 (85.7%) had received antiviral medication. Eight subjects (5.2%) had no vaccination against COVID-19. Median step count of subjects was 999 (IQR 831–1226) and they reported no adverse effects or symptoms after working with a medical mask plus FFP respirator for 2 h.
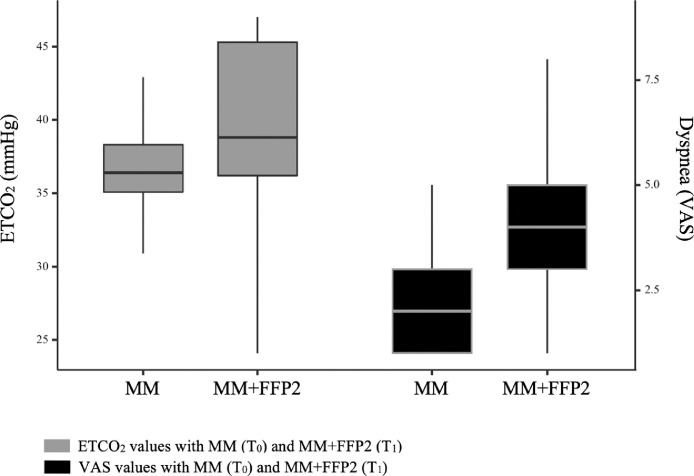
The increase in median mean arterial pressure (MAP), RR, and ETCO2 values and dyspnea scores (VAS) of subjects wearing medical mask plus FFP respirators at T1 was significant (3.1 mm Hg, 1.0 breaths/min, 1.0 mm Hg, and 2.0 cm, respectively) (Figure 2 ). We presented the measurements and comparisons at T0 and T1 in Table 1 . Subjects’ descriptives were not significantly correlated with the changes (Δ = T1 – T0) in measurements between time points. The strongest correlations were moderate correlations observed between ΔETCO2 and ΔHR, ΔETCO2 and ΔVAS, and ΔMAP and ΔHR, and the correlation coefficients (CC) were 0.648, 0.459, and 0.409, respectively (p < 0.001). Other correlations between Δ values of measurements were either weak or statistically insignificant.
Figure 2.
Median ETCO2 values and dyspnea scores with medical mask and medical mask plus FFP2 respirator at 2 time points. ETCO2 = end-tidal carbon dioxide; FFP2 = type II filtering facepiece respirator; MM = medical mask; T = time point; VAS = visual analog scale.
Table 1.
The Comparison of the Measurements at Two Time Points with Medical Mask and Type II Filtering Facepiece Respirator
| Index | Medical Mask, Median (IQR) (n = 153) | Medical Mask + FFP2, Median (IQR) (n = 153) | p Value |
|---|---|---|---|
| MAP, mm Hg | 90.3 (87.7–97.5) | 93.7 (88.5–96.5) | 0.001 |
| HR, beats/min | 82.0 (75.0–92.0) | 86.0 (74.0–92.5) | 0.973 |
| RR, breaths/min | 14.0 (12.0–16.0) | 16.0 (14.0–17.0) | < 0.001 |
| SatO2, % | 99.0 (99.0–100.0) | 99.0 (98.0–99.0) | 0.005 |
| ETCO2, mm Hg | 36.4 (35.1–38.3) | 38.8 (36.2–45.3) | < 0.001 |
| Dyspnea (VAS), cm | 2.0 (1.0–3.0) | 4.0 (3.0–5.0) | < 0.001 |
ETCO2 = end-tidal carbon dioxide; FFP2 = type II filtering facepiece respirator; HR = heart rate; IQR = interquartile range; MAP = mean arterial pressure; RR = respiratory rate; VAS = 1–10 cm visual analog scale.
Discussion
During the COVID-19 pandemic, emergency HCWs are recommended to use FFP2 and FFP3 respirators in the ED to prevent aerosol exposure (5,6). However, FFP respirators with no valves create a sealed air chamber. Rebreathing CO2 in this chamber for a certain amount of time would cause hypercapnic hypoxia. This is a concern for emergency HCWs who work long shifts wearing an FFP respirator.
This year, Mapelli et al. evaluated the cardiorespiratory effects of facemasks via exercise tests on 12 healthy subjects. They found that subjects had higher mean ETCO2 values with FFP2 respirators (19). Özdemir et al. also noted a significant increase in ETCO2 with FFP respirators in their study in which 12 healthy male HCWs’ physiological variables were monitored for 30 min in resting position (18). Both of these studies reported no subjects with hypercapnia symptoms, hypoxia, or other symptoms (18,19). The increase in ETCO2 due to N95 respirators has also been reported in studies that evaluated specific groups, such as children and pregnant women (20,21). All four studies reported that the increase in ETCO2 values was clinically insignificant and the increased ETCO2 values were within normal reference ranges. Our results are similar to these studies. We found a significant increase of 2.4 mm Hg in ETCO2 values of HCWs after 2 h of working in the ED with medical mask plus FFP respirator (Table 1). In 2006, a healthy intensivist wearing an FFP2 respirator who had hypercapnia during a procedure with an ETCO2 value of 47.25 mm Hg was reported (22). Although we noted 2 subjects with an ETCO2 value of 47.0 mm Hg, no subjects had hypercapnia symptoms, hypoxia, or other symptoms in our study.
Patients with COPD are another specific group who were evaluated for the effects of FFP respirators. Kyung et al. reported increased ETCO2, RR, HR, and decreased SatO2 values in patients with COPD wearing N95 respirators (11). The authors reported more significant changes in physiological variables than what we have found. We evaluated healthy HCWs with a median age of 24.0 years and believe that the difference originates from the difference in the subjects in the two studies.
We found a significant increase in the median values of MAP and RR at T1 with medical mask plus FFP (3.4 mm Hg and 2.0 breaths/min, respectively). These changes did not present any symptoms or adverse events; thus, they may not be clinically significant. Two studies with HCWs wearing FFP respirators reported no difference in MAP, HR, or RR on a treadmill or while resting (15,18). In a systematic review that evaluated 12 studies and 1573 subjects, Shaw et al. noted that subjects wearing FFP respirators had significantly higher HR and RR values compared with subjects wearing no mask when exercising (17). The changes in MAP values were insignificant. The authors added that after studies with a high risk of bias were removed, the difference between wearing FFP and having no mask was no longer significant for HR and RR. They found a minimal decrease (MD) in SatO2 with FFP (MD = –0.3%; 95% confidence interval –0.6% to –0.0%; p = 0.03) (17). Our results suggest that the changes in MAP and RR values are either statistically insignificant or clinically insignificant, and they are compatible with the literature data mentioned above.
In our study, the intensity of subjects’ dyspnea was evaluated by VAS. The median VAS rankings at T0 were 2.0 cm and 4.0 cm with medical mask plus FFP (p < 0.001). Although statistically significant, the clinical significance is debatable because ΔVAS was found to be 2.0 cm. We interpreted the relation between subjects’ dyspnea scores and actual physiological changes. The only significant correlation between the change in VAS and the change in physiological values was between ΔETCO2 and ΔVAS, which was a moderate correlation (CC = 0.459, p < 0.001). A few past studies evaluated dyspnea on 81 subjects and found that N95 respirators significantly increased dyspnea, similar to our results (17). In 2020, Choudhury et al. conducted a study in which 75 HCWs wearing PPE were evaluated for 4 h in a COVID-19 ICU (23). The authors noted that HCWs wearing N95 with an additional PPE kit had significantly increased dyspnea ratings and decreased SatO2. In comparison with our results, they have reported more significant physiological changes and more frequent adverse effects, such as fogging, headache, and difficulty in breathing (100%, 90.67%, and 60%, respectively). The differences in the results may have originated from the different methods of the two studies. In our study, subjects wore only medical mask and FFP2 respirator for 2 h, and no additional PPE was used.
Most of the past publications that studied the physiological effects of FFP respirators have included non-HCW subjects and measured the changes during treadmill exercise or in resting position. However, results obtained with such measurements cannot be generalized to HCWs who work in ED with FFP respirators because a natural ED working environment has its unique physical activity and psychological effects that could change the physiological states of the subjects. These impacts may not be accurately measured with treadmills in laboratory environments. We evaluated HCWs in their natural ED working environments to avoid such measurement limitations. Our results are coherent with the relevant past literature.
Limitations
This study has several limitations. First, our results were measured via noninvasive techniques. Invasive measurements are more accurate, however, we were concerned that the invasive procedures might influence physiological values. Second, we measured the intensity of subjects’ dyspnea via VAS, which is a subjective measure by its nature (24). This subjectivity in VAS, as in other dyspnea scales, might have influenced our results. Third, all of the subjects included in this study were young and healthy HCWs, and they were monitored for 2 h. Therefore, our results cannot be generalized to all emergency HCWs and more than 2-h working periods in the ED. Fourth, nasal prong placement under the masks for measurements caused air leakage. This could affect ETCO2 measurements. We inserted the nasal prongs swiftly to minimize the air leakage. Fifth, we did not measure subjects without masks due to our institutional PPE guidelines. Measuring baseline values with medical masks can be considered a limitation. However, measuring baseline values with no masks was not among our goals. In addition, past studies reported that the impact of medical masks on gas exchange is insignificant (8,12, 13, 14). Last, physiological parameters between T0 and T1 were not measured. Continuous monitoring of vital parameters would make more significant contributions to the literature, but we assumed that it would interfere with the HCWs’ working environment.
Conclusions
The use of medical mask plus FFP2 respirator for 2 h caused statistically significant increases in HCWs’ ETCO2, MAP, and RR values and dyspnea scores. However, we did not observe any subject experiencing hypercapnia symptoms, hypoxia, or other adverse events. We determined these changes were clinically insignificant. We found that it is safe for healthy HCWs to wear medical mask plus FFP2 respirators during a 2-h working shift in the ED. Future studies in which emergency HCWs from all age groups and with comorbid diseases are analyzed for more extended periods in real ED environments are needed for more generalizable results.
Article Summary
1. Why is this topic important?
Emergency health care workers (HCWs) have an increased risk of exposure during the COVID-19 pandemic. Guidelines recommend HCWs wear filtering facepiece (FFP) respirators to reduce this risk. Rebreathing CO2 with FFP respirators may cause physiological and clinical impacts on emergency HCWs who work long shifts in the emergency department (ED). Most studies measured the physiological effects of FFP respirators on treadmills or while resting. However, such measurements may not accurately project the physiological changes in emergency HCWs.
2. What does this study attempt to show?
This study attempts to evaluate the physiological and clinical impacts of type II FFP (FFP2) respirators on emergency HCWs during 1 h of their day shift in the ED.
3. What are the key findings?
The physiological values (i.e., mean arterial pressure, respiratory rate, and end-tidal carbon dioxide) and dyspnea scores of 153 healthy HCWs were significantly higher with FFP2 respirators. None of the subjects had hypercapnia symptoms, hypoxia, or other adverse effects.
4. How is patient care impacted?
The changes in physiological parameters did not cause any clinical reflection. We found that it is safe for healthy HCWs to wear FFP2 respirators during a 2-h working shift in the ED.
Acknowledgments
The authors would like acknowledge Ozge Onur, MD and Haldun Akoglu, MD for their technical assistance.
References
- 1.Juang PSC, Tsai P. N95 Respirator cleaning and reuse methods proposed by the inventor of the N95 mask material. J Emerg Med. 2020;58:817–820. doi: 10.1016/j.jemermed.2020.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartoszko JJ, Farooqi MAM, Alhazzani W, Loeb M. Medical masks vs N95 respirators for preventing COVID-19 in healthcare workers: a systematic review and meta-analysis of randomized trials. Influenza Other Respir Viruses. 2020;14:365–373. doi: 10.1111/irv.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erdem H, Lucey DR. Healthcare worker infections and deaths due to COVID-19: a survey from 37 nations and a call for WHO to post national data on their website. Int J Infect Dis. 2021;102:239–241. doi: 10.1016/j.ijid.2020.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization; August 17, 2021. Weekly epidemiological update on COVID-19. PublishedAccessed August 22, 2021. Available at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—17-august-2021. [Google Scholar]
- 6.Interim Infection Prevention and Control Recommendations for Healthcare Personnel During the Coronavirus Disease 2019 (COVID-19) Pandemic. Centers for Disease Control and Prevention. Accessed August 22, 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html#print
- 7.Matuschek C, Moll F, Fangerau H, et al. Face masks: benefits and risks during the COVID-19 crisis. Eur J Med Res. 2020;25:32. doi: 10.1186/s40001-020-00430-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shein SL, Whitticar S, Mascho KK, Pace E, Speicher R, Deakins K. The effects of wearing facemasks on oxygenation and ventilation at rest and during physical activity. PLoS One. 2021;16 doi: 10.1371/journal.pone.0247414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watts S. Discover. September 14, 2020. Why It feels like you can't breathe inside your face mask—and what to do about it.https://www.discovermagazine.com/health/why-it-feels-like-you-cant-breathe-inside-your-face-mask-and-what-to-do PublishedAccessed October 26, 2021. Available at. [Google Scholar]
- 10.Chandrasekaran B, Fernandes S. Exercise with facemask; are we handling a devil's sword?" - a physiological hypothesis. Med Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.110002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyung SY, Kim Y, Hwang H, Park JW, Jeong SH. Risks of N95 face mask use in subjects with COPD. Respir Care. 2020;65:658–664. doi: 10.4187/respcare.06713. [DOI] [PubMed] [Google Scholar]
- 12.Shaw K, Butcher S, Ko J, Zello GA, Chilibeck PD. Wearing of cloth or disposable surgical face masks has no effect on vigorous exercise performance in healthy individuals. Int J Environ Res Public Health. 2020;17(21):8110. doi: 10.3390/ijerph17218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epstein D, Korytny A, Isenberg Y, et al. Return to training in the COVID-19 era: the physiological effects of face masks during exercise. Scand J Med Sci Sports. 2021;31:70–75. doi: 10.1111/sms.13832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Person E, Lemercier C, Royer A, Reychler G. [Effect of a surgical mask on six minute walking distance] Rev Mal Respir. 2018;35:264–268. doi: 10.1016/j.rmr.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Roberge RJ, Coca A, Williams WJ, Powell JB, Palmiero AJ. Physiological impact of the N95 filtering facepiece respirator on healthcare workers. Respir Care. 2010;55:569–577. [PubMed] [Google Scholar]
- 16.Kim JH, Benson SM, Roberge RJ. Pulmonary and heart rate responses to wearing N95 filtering facepiece respirators. Am J Infect Control. 2013;41:24–27. doi: 10.1016/j.ajic.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 17.Shaw KA, Zello GA, Butcher SJ, Ko JB, Bertrand L, Chilibeck PD. The impact of face masks on performance and physiological outcomes during exercise: a systematic review and meta-analysis. Appl Physiol Nutr Metab. 2021;46:693–703. doi: 10.1139/apnm-2021-0143. [DOI] [PubMed] [Google Scholar]
- 18.Ozdemir L, Azizoglu M, Yapici D. Respirators used by healthcare workers due to the COVID-19 outbreak increase end-tidal carbon dioxide and fractional inspired carbon dioxide pressure. J Clin Anesth. 2020;66 doi: 10.1016/j.jclinane.2020.109901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mapelli M, Salvioni E, De Martino F, et al. "You can leave your mask on": effects on cardiopulmonary parameters of different airway protection masks at rest and during maximal exercise. Eur Respir J. 2021;58(3) doi: 10.1183/13993003.04473-2020. [DOI] [PubMed] [Google Scholar]
- 20.Roberge RJ, Kim JH, Powell JB. N95 respirator use during advanced pregnancy. Am J Infect Control. 2014;42:1097–1100. doi: 10.1016/j.ajic.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goh DYT, Mun MW, Lee WLJ, Teoh OH, Rajgor DD. A randomised clinical trial to evaluate the safety, fit, comfort of a novel N95 mask in children. Sci Rep. 2019;9:18952. doi: 10.1038/s41598-019-55451-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fletcher SJ, Clark M, Stanley PJ. Carbon dioxide re-breathing with close fitting face respirator masks. Anaesthesia. 2006;61:910. doi: 10.1111/j.1365-2044.2006.04767.x. [DOI] [PubMed] [Google Scholar]
- 23.Choudhury A, Singh M, Khurana DK, et al. Physiological effects of N95 FFP and PPE in healthcare workers in COVID intensive care unit: a prospective cohort study. Indian J Crit Care Med. 2020;24:1169–1173. doi: 10.5005/jp-journals-10071-23671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delgado DA, Lambert BS, Boutris N, et al. Validation of digital visual analog scale pain scoring with a traditional paper-based visual analog scale in adults. J Am Acad Orthop Surg Glob Res Rev. 2018;2(3):e088. doi: 10.5435/JAAOSGlobal-D-17-00088. [DOI] [PMC free article] [PubMed] [Google Scholar]