Abstract
Coronavirus disease 2019 (COVID-19) and vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are associated with cardiovascular complications. Here, we report a case of right-sided heart failure caused by constrictive pericarditis that developed after the administration of messenger ribonucleic acid (mRNA) vaccine against SARS-CoV-2. A 70-year-old woman presented with body weight gain, peripheral edema, and dyspnea on effort, which developed over a period of 1 week after the second dose of vaccine. The jugular venous pressure was high with a prominent y descent (Friedreich's sign) and paradoxical increase on inspiration (Kussmaul's sign). The results of IgM and IgG testing specific to SARS-CoV-2 spike and nucleocapsid proteins indicated the presence of mRNA vaccine-induced antibody and were not suggestive of COVID-19 infection. Echocardiography showed pericardial thickening and septal bounce of the interventricular septum. Computed tomography (CT) also showed pericardial thickening compared with the results of the previous CT scan performed 4 months earlier. A diagnosis of right-sided heart failure due to constrictive pericarditis was confirmed on the basis of pressure analysis during cardiac catheterization.
Keywords: Constrictive pericarditis, COVID-19, SARS-CoV-2, Vaccination
Abbreviations: COVID-19, Coronavirus disease 2019; CT, computed tomography; mRNA, messenger ribonucleic acid; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Introduction
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is associated with a wide range of cardiovascular complications such as myocarditis, heart failure, arrhythmias, and venous thromboembolism (Chang et al., 2021). Although SARS-CoV-2 vaccination has been widely adopted to prevent the spread of COVID-19, vaccine-associated cardiovascular complications, such as myocarditis and pericarditis, have been reported (Bozkurt et al., 2021; Pepe et al., 2021). Here, we report a case of right-sided heart failure due to constrictive pericarditis that developed after the administration of messenger ribonucleic acid (mRNA) vaccine against SARS-CoV-2.
Case report
A 70-year-old woman was referred to the Department of Cardiology of Matsushita Memorial Hospital because of body weight gain, peripheral edema, and dyspnea on effort. Nine weeks before this evaluation, the patient had received the first dose of vaccine against SARS-CoV-2 with no adverse effects. The patient was in a normal state of health until approximately 5 weeks before presentation, when weight increase (i.e., from 40 kg to 50 kg) followed by dyspnea on effort developed over a period of 1 week after the second dose of vaccination, which was administered 6 weeks earlier. Oral diuretics were administered, and her weight decreased to 44 kg. Neither pleural effusion nor cardiomegaly was noted on chest radiography. Two weeks later, her body weight decreased to 41 kg and edema almost disappeared, but her N-terminal pro-brain natriuretic peptide (BNP) level was 466 pg/mL (reference value, ≤55 pg/mL). She had a medical history of type 2 diabetes, hypertension, dyslipidemia, and pulmonary fibrosis. She was prescribed azosemide, carvedilol, pitavastatin, ezetimibe, insulin degludec, glimepiride, vildagliptin, and miglitol; the dose for none of these drugs was changed for more than a year. The patient was a past smoker with a 28 pack-year history, did not drink or use illicit drugs, and had no known allergies. The patient had no antecedent trauma to the chest. Her father had myocardial infarction and diabetes.
On examination, her blood pressure was 129/60 mm Hg, pulse was 88 beats per minute, body temperature was 36.3°C, respiratory rate was 18 breaths per minute, and oxygen saturation was 98% while breathing ambient air. The jugular venous pressure was high with a prominent y descent (Friedreich's sign) and paradoxical increase on inspiration (Kussmaul's sign). Cardiac auscultation was unremarkable; no knock sound was audible. There was mild pitting edema in the legs. Electrocardiography showed a normal axis, and no ST-T segment changes were observed. Chest radiography revealed a cardiothoracic ratio of 38% with reduced opacity in both lung fields, findings unchanged from those obtained 3 months earlier. The complete blood cell counts were normal, as were the results of renal and liver function tests, electrolyte balance, C-reactive protein level, and thyroid function test. The glycated hemoglobin level was 7.5%, and the BNP level was 58.5 pg/mL (reference value, ≤18.4). The troponin T result was negative (reference value, ≤0.014). The results of IgM against SARS-CoV-2, IgG specific to SARS-CoV-2 spike protein, and IgG specific to SARS-CoV-2 nucleocapsid protein were negative, positive, and negative, respectively, confirming the presence of vaccine-induced antibody and no COVID-19 infection (Noda et al., 2021).
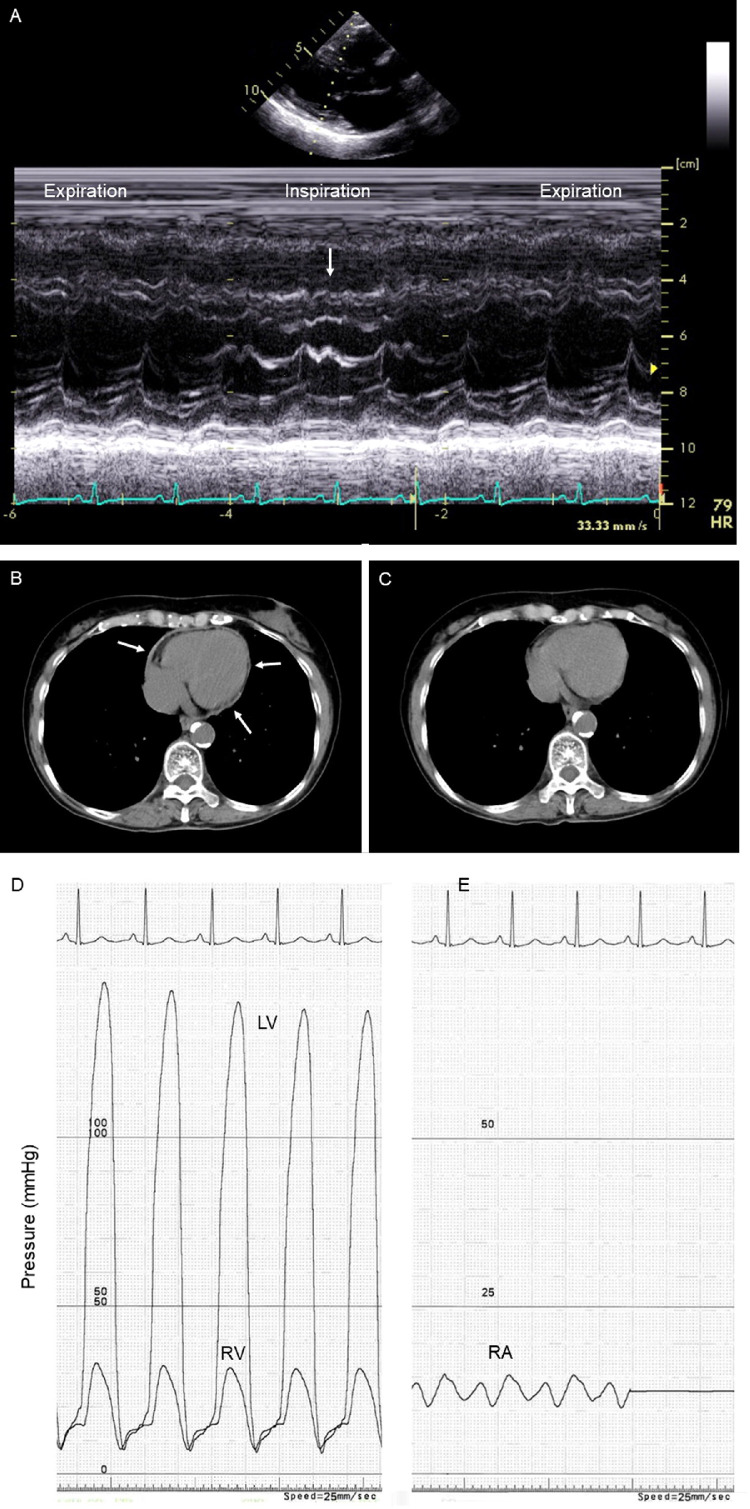
On echocardiography, pericardial thickening and septal bounce (i.e., movement of the interventricular septum to the left ventricle during inspiration) were observed (Figure 1 A). The respiratory variation of the E wave of the mitral flow was 26%, and the respiratory variation of the tricuspid E wave was 18%. The remainder of the echocardiographic examination was unremarkable. Computed tomography (CT) of the chest without the administration of contrast material revealed pericardial thickening compared with the CT scan performed 4 months earlier (Figures 1B and 1C). Although no evidence suggesting myocardial involvement was shown on cardiac magnetic resonance imaging, adhesion of the thickened pericardium to the myocardium was suggested on cine images. No stenosis was found on coronary arteriography, but right heart catheterization showed an increase in end-diastolic filling pressures with a steep y descent on right ventricular pressure tracing, and a prominent x and y descent on right atrial pressure tracing (Figures 1D and 1E). The pulmonary artery pressure was 27/16 mm Hg (mean 21 mm Hg), and the pulmonary capillary wedge pressure was 16 mm Hg.
Figure 1.
Transthoracic echocardiography, computed tomography of the chest, and cardiac catheterization.
M-mode of the parasternal long-axis view shows a septal bounce or transient movement of the interventricular septum to the left ventricle during inspiration (A, arrow). A short-axis image without the administration of intravenous contrast material shows pericardial thickening overlying the whole heart on admission (B, arrows). Note that there was no pericardium thickness on the same level of the heart on computed tomography performed 4 months earlier for the assessment of pulmonary fibrosis (C). The systolic, diastolic, and end-diastolic pressures are 32/8/15 mm Hg in the right ventricle (RV) and 140/8/16 mm Hg in the left ventricle (LV), both of which exhibit similar pressure curves during diastole, known as a “dip-and-plateau” pattern (D). The right atrial (RA) pressure is 15/10 mm Hg (mean 13 mm Hg), which is a “W” or “M” configuration (E).
A diagnosis of right-sided heart failure due to constrictive pericarditis was made. Additional evaluations were performed to clarify the etiology of constrictive pericarditis. For example, the interferon-gamma release assay was negative, and there were no clinical, imaging, or laboratory data suggesting malignancy or autoimmune disease. Pericardiectomy was deferred considering her mild symptoms with oral diuretics. The patient has been doing well for more than 3 months after the diagnosis, although mild dyspnea has persisted.
Discussion
The patient in this report developed right-sided heart failure due to constrictive pericarditis 1 week after the second dose of SARS-CoV-2 mRNA vaccination. On the basis of her clinical course and imaging findings including changes in the pericardium on CT, which was performed at an interval of 4 months, we diagnosed her with a rare complication after SARS-CoV-2 vaccination. The patient has no known history of malignancy, tuberculosis, autoimmune disease, or prior cardiac surgery, which are common causes of constrictive pericarditis.
In general, post-vaccination myocarditis and pericarditis have been reported as rare adverse events after vaccination, such as against smallpox or influenza, and there is increasing evidence for such complications after SARS-CoV-2 vaccination (Bozkurt et al., 2021; Pepe et al., 2021). After SARS-CoV-2 vaccination, myocarditis and pericarditis are likely to develop in young adult and adolescent males, with an estimated rate of 12.6 cases per million doses of 2-dose mRNA vaccines (Bozkurt et al., 2021). The exact mechanism of myocarditis and pericarditis related to SARS-CoV-2 vaccination is currently being investigated, although several mechanisms such as an immune-mediated or hypersensitivity trigger are proposed (Tsilingiris et al., 2022). Our patient had no clinical features indicating developing acute myocarditis or acute pericarditis, but a diagnosis of constrictive pericarditis was confirmed. Of note, few patients with acute myocarditis are asymptomatic, whereas acute pericarditis may develop and spontaneously resolve only with non-specific symptoms. Only a few case reports are available regarding constrictive pericarditis associated with COVID-19 (Diaconu et al., 2021, Beckerman et al., 2019, SeyedAlinaghi et al., 2020 Dec 9). Although the mechanism of mRNA vaccination against the virus remains to be elucidated, to our knowledge, our case report is the first to show a possible association of constrictive pericarditis with SARS-CoV-2 vaccination.
Constrictive pericarditis may be a complication of acute pericarditis. In a series of 500 consecutive patients with a first episode of acute pericarditis, constrictive pericarditis developed in 9 patients (1.8%), among whom only 2 had idiopathic or viral pericarditis (0.48%) (Imazio et al., 2011). It is important to note that the study had a long follow-up period of 6 years, ranging from 2 to 10 years, which is consistent with constrictive pericarditis being a late complication of chronic pericarditis. In our case, no pericardial effusion was observed on CT 4 months before the diagnosis of constrictive pericarditis, which made a diagnosis of chronic pericarditis less likely before this episode. Interestingly, constrictive pericarditis reportedly developed 2 weeks after the initial symptoms in a patient with COVID-19 (SeyedAlinaghi et al., 2020). Constrictive pericarditis related to COVID-19 or vaccines against SARS-CoV-2 may have different features from those of constrictive pericarditis as a late complication of acute pericarditis due to other etiologies. Further studies are warranted to examine the effects of the mRNA vaccine on the pericardium.
Effusive-constrictive pericarditis is an uncommon condition in which disease progression may be faster than that in classical constrictive pericarditis. In an analysis of 15 patients with effusive-constrictive pericarditis (Sagristà-Sauleda et al., 2004), the symptoms developed between 4 days and 26 months before admission; 12 patients had symptoms for less than 3 months. Although no invasive procedures to establish a diagnosis of effusive-constrictive pericarditis (i.e., simultaneous measurement of intrapericardial and intracardiac pressures before and after pericardial drainage) were performed in our case, the possibility was less likely because neither asymptomatic status nor stable hemodynamics without invasive therapy can be observed in patients with effusive-constrictive pericarditis (Yacoub et al., 2021).
In conclusion, this case highlights the importance of acknowledging the rare complication of constrictive pericarditis in association with not only COVID-19 but also administration of vaccines against SARS-CoV-2.
Acknowledgments
None to declare.
Conflict of Interest
None to declare.
Funding Source
None to declare.
Ethical Approval statement
Informed consent was received from the patient, and this case report was approved by the Ethics Committee of Matsushita Memorial Hospital.
References
- Bozkurt B, Kamat I, Hotez PJ. Myocarditis With COVID-19 mRNA Vaccines. Circulation. 2021;144:471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WT, Toh HS, Liao CT, Yu WL. Cardiac Involvement of COVID-19: A Comprehensive Review. Am J Med Sci. 2021;361:14–22. doi: 10.1016/j.amjms.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaconu R, Popescu L, Voicu A, Donoiu I. Subacute effusive-constrictive pericarditis in a patient with COVID-19. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-242443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imazio M, Brucato A, Maestroni S, Cumetti D, Belli R, Trinchero R, Adler Y. Risk of constrictive pericarditis after acute pericarditis. Circulation. 2011;124:1270–1275. doi: 10.1161/CIRCULATIONAHA.111.018580. [DOI] [PubMed] [Google Scholar]
- Noda K, Matsuda K, Yagishita S, Maeda K, Akiyama Y, Terada-Hirashima J, Matsushita H, Iwata S, Yamashita K, Atarashi Y, Watanabe S, Ide N, Yoshida T, Ohmagari N, Mitsuya H, Hamada A. A novel highly quantitative and reproducible assay for the detection of anti-SARS-CoV-2 IgG and IgM antibodies. Sci Rep. 2021;11:5198. doi: 10.1038/s41598-021-84387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe S, Gregory AT, Myocarditis Denniss AR. Pericarditis and Cardiomyopathy After COVID-19 Vaccination. Heart Lung Circ. 2021;30:1425–1429. doi: 10.1016/j.hlc.2021.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagristà-Sauleda J, Angel J, Sánchez A, Permanyer-Miralda G, Soler-Soler J. Effusive-constrictive pericarditis. N Engl J Med. 2004;350:469–475. doi: 10.1056/NEJMoa035630. [DOI] [PubMed] [Google Scholar]
- Beckerman JK, Alarfaj M, Tracy CM, Faiwiszewski AD, Choi AD. Coronavirus disease 2019 (COVID-19)-associated constrictive pericarditis. BMJ Case Rep. 2021;14:e242018. https://doi:10.1136/bcr-2021-242018. [DOI] [PMC free article] [PubMed]
- SeyedAlinaghi S, Ghadimi M, Gharabaghi MA, Ghiasvand F. Constrictive Pericarditis Associated with Coronavirus Disease 2019 (COVID-19): A Case Report. Infect Disord Drug Targets. 2020 Dec 9. doi: 10.2174/1871526520666201209145001. Epub ahead of print. [DOI] [PubMed]
- Tsilingiris D, Vallianou NG, Karampela I, Liu J, Dalamaga M. Potential implications of lipid nanoparticles in the pathogenesis of myocarditis associated with the use of mRNA vaccines against SARS-CoV-2. Metabol Open. 2022;13 doi: 10.1016/j.metop.2021.100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub M, Quintanilla Rodriguez BS, Mahajan K. Constrictive-Effusive Pericarditis. 2021 Jul 28. StatPearls [Internet] Jan 2021 [PubMed] [Google Scholar]