Abstract
Data concerning the combined prognostic role of natriuretic peptide (NP) and troponin in patients with COVID-19 are lacking. The aim of the study is to evaluate the combined prognostic value of NPs and troponin in hospitalized COVID-19 patients. From March 1, 2020 to April 9, 2020, consecutive patients with COVID-19 and available data on cardiac biomarkers at admission were recruited. Patients admitted for acute coronary syndrome were excluded. Troponin levels were defined as elevated when greater than the 99th percentile of normal values. NPs were considered elevated if above the limit for ruling in acute heart failure (HF). A total of 341 patients were included in this study, mean age 68 ± 13 years, 72% were men. During a median follow-up period of 14 days, 81 patients (24%) died. In the Cox regression analysis, patients with elevated both NPs and troponin levels had higher risk of death compared with those with normal levels of both (hazard ratio 2.94; 95% confidence interval 1.31 to 6.64; p = 0.009), and this remained significant after adjustment for age, gender, oxygen saturation, HF history, and chronic kidney disease. Interestingly, NPs provided risk stratification also in patients with normal troponin values (hazard ratio 2.86; 95% confidence interval 1.21 to 6.72; p = 0.016 with high NPs levels). These data show the combined prognostic role of troponin and NPs in COVID-19 patients. NPs value may be helpful in identifying patients with a worse prognosis among those with normal troponin values. Further, NPs’ cut-point used for diagnosis of acute HF has a predictive role in patients with COVID-19.
Cardiac involvement is a prominent feature in COVID-19 and is associated with a worse prognosis.1, 2, 3 The assessment of cardiovascular involvement has a crucial role in defining a prognostic stratification.1, 2, 3, 4, 5 Early increase of markers of myocardial injury, defined as increased troponin levels, has been associated with risk of poor outcome.5, 6, 7, 8 It has also been suggested that the assessment of natriuretic peptides (NPs) might be helpful in predicting outcome in patients with COVID-19.9, 10, 11, 12 However, previous observations have been limited to single-center studies, mostly analyzing only NPs determinations during clinical course without combined troponin evaluation.13, 14, 15, 16 An important question is whether troponin and NPs have similar clinical significances or independent roles. Studies have yielded controversial results to date.9 , 11 , 17 , 18 Particularly, the predictive role of the 2 cardiac biomarkers in combination has not been deeply assessed. Another unresolved issue that remains is the ability of specific NPs cut-off to predict outcome in COVID-19 patients. Indeed, it is not fully explored whether the values used to rule out acute heart failure (HF) might have also a prognostic role in this clinical setting. The aim was to evaluate the prognostic role of early assessment of NPs in combinations with troponin value and to estimate a prognostic performance of NPs’ cut-off levels above the recommended limit for ruling out acute HF.
This is a multicenter, retrospective observational study, enrolling consecutive patients with laboratory-confirmed COVID-19 infection who were referred to 13 Italian cardiology units from March 1, 2020 to April 9, 2020 (list of centers and investigators were previously reported).5 This multicenter registry included patients hospitalized with a laboratory confirmed diagnosis of COVID-19 and high-sensitivity plasma troponin levels (either troponin I or troponin T), and NPs levels measured within 24 hours from COVID-19 diagnosis. Patients hospitalized with a diagnosis of acute coronary syndrome were excluded. Diagnoses of COVID-19 were made by real-time reverse transcriptase–polymerase chain reaction assays of nasal and pharyngeal swabs. Real-time reverse transcriptase–polymerase chain reaction assays of lower respiratory tract aspirates were also performed when indicated. Patients were followed up after the COVID-19 diagnosis, and all causes of in-hospital mortality or discharge were ascertained until April 23, 2020. This study complied with the Declaration of Helsinki and was approved by the ethical committee of Spedali Civili di Brescia, Brescia, Italy, and each recruiting center. As such, a waiver for consent was granted by local ethics committees, provided that informed consent was collected at the follow-up visit for the patients who were still alive. Patients’ data including demographics, medical history, in-hospital clinical course, treatment, and outcomes were extracted from the in-hospital medical records. Clinical characteristics at presentation including fever, cough, dyspnea, breath rate, and heart rate were analyzed. Laboratory measurements on admission and within 24 hours were recorded. Cardiac injury was defined by plasma levels of high sensitivity troponin (either troponin T or troponin I) greater than the 99th percentile of normal values, as per manufacturer indications.19 According to the fourth definition of myocardial infarction, we excluded both type I and type II myocardial infarction. NPs were considered elevated if serum level was above the limit for ruling out acute HF.20 Patients were grouped according to value of concomitant biomarkers as categoric variables. Specifically, they were classified as normal values of both biomarkers (NPs–/Tn–), increased values of NP (NPs+/Tn–), increased value of troponin (NPs–/Tn+), and increased value of both biomarkers (NPs+/Tn+). All laboratory test within 24 hours from admission were recorded. In-hospital acute HF was defined by the occurrence of dyspnea and clinical signs of congestion requiring intravenous diuretic treatment. The presence of congestion was evaluated with physical examination, laboratory biomarkers, chest X-ray, or echocardiography. Clinical history and clinical complications during hospitalization were assessed. The primary end point was all-cause in-hospital mortality. In-hospital cardiovascular and noncardiovascular complications were also recorded.
Data were presented stratified by NPs and troponin levels at admission. Sensitivity and specificity were calculated for both cut-offs used to categorize NT-proBNP and BNP. Continuous variables were shown as means and SDs, skewed variables as medians and interquartile ranges, and dichotomous variables as counts and percentages. Comparisons between groups were made, respectively, using ANOVA test for means, Kruskal-Wallis test for medians, and chi-square test (or Fisher's exact test whenever appropriate) for proportions. Cumulative incidence function of death was computed, taking into account hospital discharge as a competing event. Overall and pairwise comparisons of cumulative incidence functions among subgroups were performed by means of Gray test.21
Variables clinically relevant and significantly associated with the risk of death at univariable analysis were tested in a multiple Cox regression model to identify independent risk factors. The hazard ratios (HRs), 95% confidence intervals (CIs) and p values from a Wald test were reported.22 Different multivariable models were compared by likelihood ratio test and their prognostic accuracy was measured by the Harrell's concordance statistics (c-index),23 which is the probability that given 2 randomly selected patients, the survival time predicted by the model is greater for the subject who survived longer. A value of 1 denotes perfect concordance, whereas a value of 0.5 is no better than chance. A 2-tailed p value <0.05 was considered statistically significant. Statistical analyses were performed using SAS statistical software version 9.4 (SAS Institute, Inc., Cary, North Carolina) and Stata version 16.0 (StataCorp LLC, College Station, Texas).
We included 341 patients, aged 68 ± 13 years, 246 were men (72%). Overall, there was a high burden of hypertension (208 patients; 62%), diabetes mellitus (84 patients; 25%), and coronary artery disease (74 patients; 22%). Forty-two patients presented with history of HF (12%), whereas 36 patients (11%) had chronic obstructive pulmonary disease. Blood test at admission showed inflammatory status, with mildly elevated C-reactive protein and d-dimer values, leukocytosis, and anemia. At admission, there was low oxygen saturation and PAO2/FIO2 value and a high respiratory rate, reflecting relevant respiratory impairment (Table 1 ). Patients’ characteristics, vital signs and laboratory measurements, stratified by 2 cardiac biomarkers value are displayed in Table 1 . At admission, 97 patients (28%) had normal values for both biomarkers (NPs–/Tn–), 46 elevation of troponin alone (14%) (NPs–/Tn+), 67 elevation of NPs alone (20%) (NPs+/Tn–), whereas 131 patients (38%) presented with elevated values of both biomarkers (NPs+/Tn+). Relevant differences were observed with respect to demographics, preexisting cardiac disease, and laboratory analyses. In short, patients having both elevated biomarkers were more likely to be older, and with history of HF and/or coronary artery disease, diabetes mellitus, atrial fibrillation, or chronic kidney disease. These patients also presented a lower oxygen saturation and higher lactate and white blood cell count compared with the other groups. No significant differences occurred across groups for lymphocytes count, C-reactive protein, and lactate dehydrogenase. There were also no differences for respiratory rate and mean value of PAO2/FIO2. Data about cardiac and noncardiac complications are shown in Table 2 . Patients with elevated biomarkers were more likely to experience cardiovascular and noncardiovascular complications during clinical course (Table 2).
Table 1.
Demographic and clinical characteristics of the study population at admission stratified by combinations of normal/elevated NPs and troponin levels (n = 341)
| COMBINATION OF NPs AND Troponin LEVELS |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NPs/Tn | N | overall population (n = 341) | N | NPs–/Tn– (n = 97) | N | NPs–/Tn+ (n = 46) | N | NPs+/Tn– (n = 67) | N | NPs+/Tn+ (n = 131) | p value |
| Age (years) | 341 | 68 ± 13 | 97 | 61 ± 13 | 46 | 68 ± 13 | 67 | 67 ± 14 | 131 | 73 ± 10 | <0.001 |
| Gender (male) | 341 | 246 (72%) | 97 | 75 (77%) | 46 | 35 (76%) | 67 | 45 (67%) | 131 | 91 (69%) | 0.401 |
| Body mass index (kg/m2) | 289 | 27.1 ± 5.3 | 86 | 27.4 ± 4.9 | 36 | 27.3 ± 4.7 | 62 | 26.8 ± 5.0 | 105 | 27.0 ± 5.9 | 0.902 |
| Respiratory rate <22 (bpm) | 248 | 101 (41%) | 75 | 32 (43%) | 27 | 11 (41%) | 58 | 25 (43%) | 88 | 33 (37%) | 0.889 |
| Oxygen saturation (%) | 333 | 92 (88–96) | 96 | 93 (89–96) | 45 | 94 (90–96) | 65 | 93 (89–96) | 127 | 91 (87–94) | 0.017 |
| Red blood cell count (× 10^6/μl) | 339 | 4.47 (4.00–4.79) | 96 | 4.63 (4.25–4.84) | 45 | 4.34 (3.99–4.78) | 67 | 4.32 (3.83–4.71) | 131 | 4.40 (3.90–4.77) | 0.018 |
| White blood cell count (per μl) | 340 | 7,050 (5,100–9,920) | 97 | 6,880 (4,970–9,000) | 45 | 6,350 (4,370–8,360) | 67 | 7,220 (5,570–9,185) | 131 | 7,950 (5,410–11,330) | 0.018 |
| Lymphocytes (per μl) | 302 | 905 (663–1,200) | 86 | 900 (700–1,247) | 39 | 980 (660–1,285) | 60 | 923 (694–1,355) | 117 | 888 (620–1,109) | 0.427 |
| CRP (mg/100 ml) | 327 | 59 (18–145) | 94 | 62 (22–147) | 42 | 59 (11–109) | 63 | 58 (17–138) | 128 | 58 (18–149) | 0.816 |
| Lactate dehydrogenase (U/L) | 277 | 372 (257–504) | 84 | 445 (265–552) | 37 | 352 (258–490) | 53 | 328 (239–481) | 103 | 368 (259–491) | 0.328 |
| Serum creatinine (mg/100 ml) | 333 | 1.00 (0.81–1.33) | 96 | 0.90 (0.77–1.08) | 44 | 0.99 (0.84–1.21) | 65 | 1.03 (0.86–1.40) | 128 | 1.10 (0.90–1.68) | <0.001 |
| eGFR (CKD-EPI) ml/min | 290 | 71.25 (46.77–87.05) | 78 | 82.39 (69.69–96.23) | 42 | 70.71 (49.86–86.06) | 49 | 69.00 (44.55–85.50) | 121 | 59.95 (36.96–79.87) | <0.001 |
| ABG test lactate (mmol/L) | 261 | 1.3 (0.9–1.8) | 70 | 1.2 (0.9–1.4) | 34 | 1.1 (0.9–1.5) | 57 | 1.4 (0.9–2.1) | 100 | 1.5 (1.0–2.0) | 0.001 |
| PAO2/FIO2 (mm Hg/%) | 304 | 238 (120–310) | 90 | 231 (104–298) | 38 | 262 (195–310) | 60 | 241 (105–325) | 116 | 229 (133–305) | 0.456 |
| Heart failure | 338 | 58 (17%) | 96 | 0 (0%) | 46 | 3 (7%) | 66 | 13 (18%) | 130 | 42 (32%) | <0.001 |
| Coronary artery disease | 338 | 74 (22%) | 96 | 9 (9%) | 46 | 7 (15%) | 66 | 17 (26%) | 130 | 41 (32%) | 0.001 |
| Atrial fibrillation | 338 | 62 (18%) | 96 | 5 (5%) | 46 | 7 (15%) | 66 | 13 (20%) | 130 | 37 (28%) | <0.001 |
| COPD | 338 | 36 (11%) | 96 | 8 (8%) | 46 | 2 (4%) | 66 | 6 (9%) | 130 | 20 (15%) | 0.128 |
| Diabetes Mellitus | 338 | 84 (25%) | 96 | 17 (18%) | 46 | 8 (17%) | 66 | 16 (24%) | 130 | 43 (33%) | 0.033 |
| Hypertension | 338 | 208 (62%) | 96 | 49 (51%) | 46 | 24 (52%) | 66 | 48 (73%) | 130 | 87 (67%) | 0.010 |
| Chronic kidney disease (eGFR<60 ml/min/m2) | 338 | 63 (19%) | 96 | 2 (2%) | 46 | 7 (15%) | 66 | 15 (23%) | 130 | 39 (30%) | <0.001 |
| Prior ACEi/ARB therapy | 318 | 139 (44%) | 90 | 35 (39%) | 43 | 18 (42%) | 63 | 29 (46%) | 122 | 57 (47%) | 0.683 |
| Prior statin therapy | 321 | 87 (27%) | 92 | 18 (20%) | 43 | 6 (14%) | 63 | 16 (25%) | 123 | 47 (38%) | 0.003 |
ABG = arterial blood gas; ACEi = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; CKD-EPI = chronic kidney disease epidemiology collaboration formula; COPD = chronic obstructive pulmonary disease; CRP = C-reactive protein; eGFR = estimated glomerular filtration rate; FIO2 = fraction of inspired oxygen; PAO2 = oxygen partial pressure at arterial gas analysis; SOFA = sequential organ failure assessment.
Data shown as mean±SD, median (IQR)., or count (%).
Table 2.
In-hospital cardiovascular and no cardiovascular complications stratified according to combinations of normal/elevated NPs and troponin levels (n = 341)
| COMBINATION OF NPs AND Troponin LEVELS |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| NPs/Tn | N | NPs–/Tn– (n = 97) | N | NPs–/Tn+ (n = 46) | N | NPs+/Tn– (n = 67) | N | NPs+/Tn+ (n = 131) | p value |
| Cardiovascular complication | 83 | 11 (13%) | 43 | 8 (19%) | 64 | 19 (30%) | 123 | 50 (41%) | <0.001 |
| Onset of atrial fibrillation | 68 | 4 (6%) | 25 | 4 (16%) | 55 | 9 (16%) | 94 | 18 (19%) | 0.115 |
| Atrial fibrillation at discharge | 64 | 3 (5%) | 18 | 3 (17%) | 50 | 5 (10%) | 79 | 18 (23%) | 0.014 |
| STEMI | 93 | 0 (0%) | 43 | 0 (0%) | 67 | 0 (0%) | 130 | 5 (4%) | 0.089 |
| Heart failure | 83 | 1 (1%) | 43 | 1 (2%) | 63 | 6 (10%) | 118 | 27 (23%) | <0.001 |
| Pulmonary embolism | 93 | 4 (4%) | 43 | 3 (7%) | 67 | 6 (9%) | 130 | 12 (9%) | 0.541 |
| Stroke | 93 | 0 (0%) | 43 | 1 (2%) | 67 | 0 (0%) | 130 | 1 (1%) | 0.359 |
| Major bleeding | 83 | 3 (4%) | 43 | 2 (5%) | 63 | 2 (3%) | 118 | 12 (10%) | 0.198 |
| No cardiovascular complication | 93 | 19 (20%) | 44 | 9 (21%) | 67 | 15 (22%) | 130 | 42 (32%) | 0.147 |
| Sepsis | 93 | 11 (12%) | 43 | 5 (12%) | 67 | 13 (19%) | 128 | 18 (14%) | 0.541 |
| Acute renal insufficiency | 80 | 6 (8%) | 41 | 4 (10%) | 59 | 6 (10%) | 104 | 27 (26%) | 0.002 |
| Multiorgan failure | 76 | 0 (0%) | 38 | 1 (3%) | 59 | 3 (5%) | 103 | 11 (11%) | 0.008 |
NP = natriuretic peptide; Tn = troponin; STEMI = ST-elevation myocardial infarction.
Data shown as median (IQR)., or count (%).
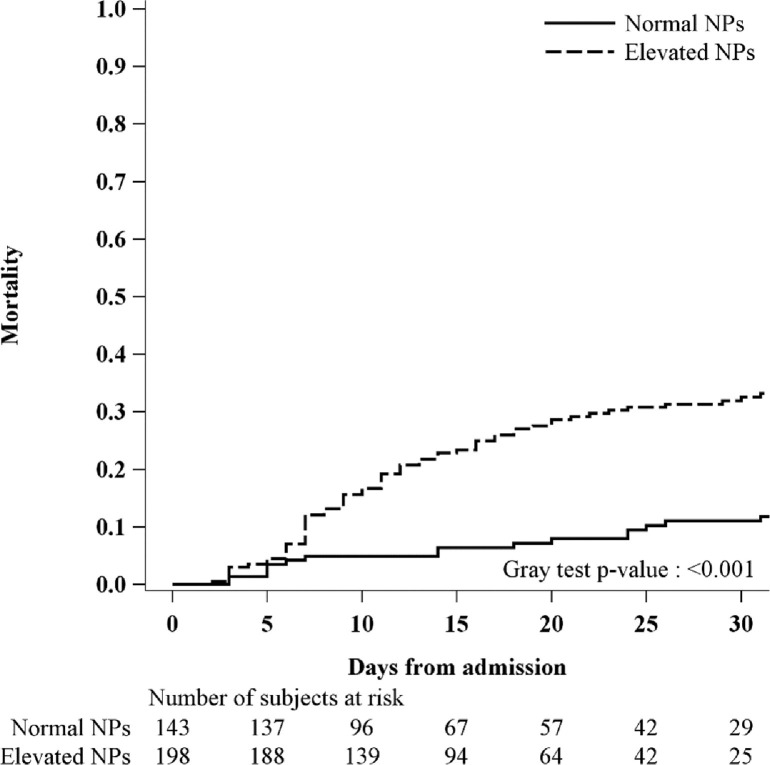
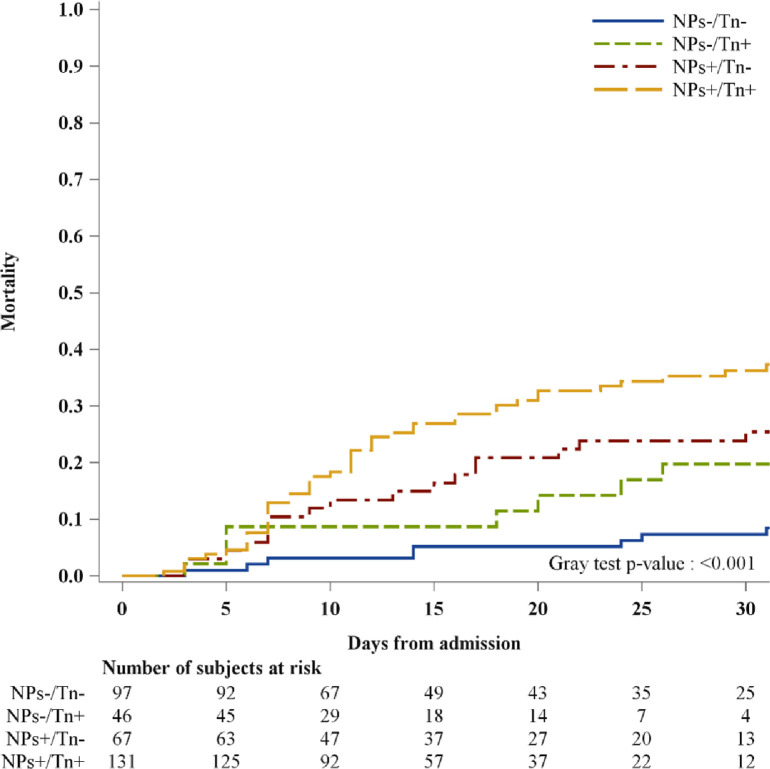
Overall, higher rates of cardiovascular adverse events were more frequently seen in patients having both cardiac biomarkers elevations (p <0.001) (Table 2). Particularly, this group more frequently presented with atrial fibrillation at discharge (p = 0.01) and HF during hospitalization (p <0.001). These events were less likely to occur in patients with elevated NPs but normal troponin levels than those patients with normal values of both biomarkers. Noncardiac complications occurred with similar rates across all different groups, except for acute renal failure (p = 0.002) and multiorgan failure (p = 0.008) that occurred more frequently in those with elevations of troponin and NPs (Table 2). Over a median in-hospital stay (interquartile range) of 14 (9 to 24) days, 81 patients died (23.8%). Of those, 65 patients had elevated NPs levels at admission (80%), 55 had elevated troponin levels (67%) and 47 had both (58%) (Figure 1 ). Patients having normal levels of both biomarkers showed a lower-case fatality rate (8%). Interestingly, among patients with increased values of 1 biomarker (NPs–/Tn+ or NPs+/Tn–), the case fatality rate was higher in patients with only elevated NPs (27%) than those patients having only elevated troponin levels (17%). The cumulative incidence for in-hospital mortality according to combinations of NPs and troponin levels is depicted in Figure 2 . High levels of both biomarkers identified patients with high risk of death (elevated vs normal levels of both biomarkers, p <0.001). To assess the potential independent prognostic role of both biomarkers, we performed a multivariable Cox regression of NPs/Tn combination on in-hospital mortality, adjusting for age, gender, oxygen saturation, HF history, and chronic kidney disease. The presence of elevated levels of both biomarkers was associated with a threefold increase of in-hospital mortality compared with patients with normal values of both biomarkers (NPs/Tn+: HR 2.94; 95% CI 1.31 to 6.64; p = 0.009) (Table 3 ). This risk was similarly increased in patients with NPs value elevations and troponin normal value (NPs+/Tn–: HR 2.86; 95% CI 1.21 to 6.72; p = 0.016), whereas there was a twofold increase in patients having high troponin levels and normal NPs value (HR 2.08; 95% CI 0.77 to 5.65; p = 0.15).
Figure 1.
Cumulative incidence function for intrahospital mortality stratifying patients according to NPs level at admission.
Figure 2.
Cumulative incidence function for intrahospital mortality stratifying patients according to NPs and Tn levels at admission.
Table 3.
Univariable and multivariable Cox regression model for intrahospital mortality
| Univariable | Multivariable (n = 332) |
|||||
|---|---|---|---|---|---|---|
| Level/Units | HR (95% CI) | p Value | HR (95% CI) | p Value | ||
| NPs/Tn levels (vs NPs–/Tn–) | NP+/Tn+ | 5.35 (2.52 to 11.36) | <0.001 | 2.94 (1.31 to 6.64) | 0.009 | |
| NP+/Tn– | 3.48 (1.51 to 8.00) | 0.003 | 2.86 (1.21 to 6.72) | 0.016 | ||
| NP–/Tn+ | 2.73 (1.02 to 7.29) | 0.045 | 2.08 (0.77 to 5.65) | 0.150 | ||
| Age (years) | +5 | 1.38 (1.24 to 1.54) | <0.001 | 1.31 (1.16 to 1.49) | <0.001 | |
| Gender | M versus F | 1.18 (0.70 to 1.99) | 0.544 | 1.40 (0.83 to 2.39) | 0.211 | |
| Oxygen saturation | +5% | 0.84 (0.76 to 0.93) | 0.001 | 0.80 (0.72 to 0.89) | <0.001 | |
| Respiratory rate | ≥22 versus <22 | 1.25 (0.73 to 2.15) | 0.421 | |||
| Red blood cell count | +0.5 × 10^6/μl | 0.86 (0.73 to 1.01) | 0.073 | |||
| White blood cell count | +1,000 U/µl | 1.02 (0.98 to 1.05) | 0.393 | |||
| Lymphocytes count | +100 U/µl | 0.92 (0.86 to 0.97) | 0.005 | |||
| CRP | +10 mg/L | 1.01 (0.99 to 1.03) | 0.247 | |||
| Lactate dehydrogenase | +1,000 mg/100 ml | 1.17 (1.01 to 1.35) | 0.033 | |||
| Serum creatinine | +1 mg/100 ml | 1.09 (1.00 to 1.20) | 0.063 | |||
| eGFR (CKD-EPI) ml/min | +10 ml/min | 0.86 (0.79 to 0.94) | 0.001 | |||
| ABG test lactate | +1 mmol/L | 1.32 (1.20 to 1.46) | <0.001 | |||
| PAO2/FIO2 | +50 mm Hg/% | 0.92 (0.82 to 1.02) | 0.097 | |||
| Heart failure | Yes versus No | 2.51 (1.57 to 4.03) | <0.001 | 1.35 (0.80 to 2.30) | 0.264 | |
| Coronary artery disease | Yes versus No | 2.10 (1.31 to 3.35) | 0.002 | |||
| Atrial fibrillation | Yes versus No | 2.20 (1.34 to 3.60) | 0.002 | |||
| Chronic Obstructive Pulmonary Disease | Yes versus No | 2.05 (1.15 to 3.65) | 0.015 | |||
| Diabetes | Yes versus No | 1.50 (0.93 to 2.41) | 0.099 | |||
| Hypertension | Yes versus No | 1.51 (0.94 to 2.42) | 0.087 | |||
| Chronic kidney disease | Yes versus No | 2.53 (1.58 to 4.05) | <0.001 | 1.59 (0.94 to 2.68) | 0.081 | |
| Prior ACEi/ARB therapy | Yes versus No | 1.20 (0.76 to 1.88) | 0.433 | |||
| Prior statin therapy | Yes versus No | 1.95 (1.22 to 3.12) | 0.005 | |||
ABG = arterial blood gas; ACEi = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; CKD-EPI = chronic kidney disease epidemiology collaboration formula; CRP = C-reactive protein; eGFR = estimated glomerular filtration rate; FIO2 = fraction of inspired oxygen; NT-proBNP = N-terminal fragment of the prohormone brain natriuretic peptide; PAO2 = oxygen partial pressure at arterial gas analysis.
Other significant prognostic factors were oxygen saturation (HR 0.80; 95% CI 0.72 to 0.89; p <0.001) and age (HR 1.31; 95% CI 1.16 to 1.49; p <0.001). History of HF and chronic kidney disease were associated with high risk of in-hospital death in the univariable analysis (HR 2.51; 95% CI 1.57 to 4.03; p <0.001 and HR 2.53; 95% CI 1.58 to 4.05; p <0.001, respectively) but not in the multivariable analysis (HR 1.35; 95% CI 0.8 to 2.3; p = 0.264 and HR 1.59; 95% CI 0.94 to 2.68; p = 0.081, respectively). The multivariable model, including troponin/NPs, was significantly different (likelihood ratio test p = 0.03) with respect to the model, including clinical variables only (age, gender, oxygen saturation, HF, and chronic kidney disease). After the addition of NPs/Tn to the clinical model, there was a slight increase in discrimination with the c-statistic rising from 0.754 (95% CI 0.693 to 0.814) to 0.769 (95% CI 0.709 to 0.830). We also examined the interaction of age with the NPs/Tn combination by adding product terms to the multivariable regression model. An age cut-point of 80 years was selected. Results indicate that the prognostic role of NPs/Tn on in-hospital mortality does not differ significantly between patients over 80 years and younger patients (heterogeneity test p = 0.372).
Specificity and sensibility for the chosen cut-off values of NPs were analyzed, namely 300 pg/ml for NT-proBNP and 100 pg/ml for BNP. NPs cut-off showed an optimal balance of both sensitivity and specificity. Particularly, the prognostic value of NT-proBNP cut-off showed 85% of sensitivity and 48% of specificity, whereas they were 77% and 48% for BNP cut-off, respectively.
This study reinforces previous observations highlighting that early detection of elevated biomarkers of cardiac injury or dysfunction is common and is associated with adverse outcome in hospitalized patients with COVID-19. Particularly, the increase of both biomarkers was an important predictor of in-hospital mortality irrespective of a previous HF, chronic renal disease, and oxygen saturation on admission. NPs elevation also carries prognostic information in patients considered at low risk according to normal troponin values, highlighting an additional predictive power of NPs in this setting. The high prevalence of NPs elevations was in keeping with previous observational European studies,9 , 11 , 12 whereas our rate was higher compared with the Chinese series.13, 14, 15, 16, 17 These discrepancies may likely be related to the higher prevalence of elderly with their cardiac co-morbidities in the European population. In agreement with findings from other European studies, in our population, there was high prevalence of cardiovascular disease; 17% of patients were affected by HF, 25% by atrial fibrillation, and 33% by coronary artery disease.
Although the prognostic role of NPs has been reported,9, 10, 11, 12 its additive value compared with troponin levels in patients with COVID-19 remains poorly explored. Indeed, previous data lead to contradictory results. Our analysis showed an association of NPs with high-risk mortality even when troponin was within normal levels. This trend is consistent with the previous Italian report.9 Conversely, the Spanish COVID-19 registry highlighted a different trend wherein normal troponin levels combined with high NPs identified a lower risk of death.24 Different prognostic significance may likely be related to a different stage of COVID-19 disease and the burden of underlying cardiovascular disease. Indeed, similar to previous reports,24 our cohort showed about 20% of in-hospital mortality along with high cardiovascular background and pulmonary compromise. However, previous reports have separately explored the prognostic impact of cardiac biomarkers in combination on multivariable model.11 , 12 , 24
In the present study, to maximize the knowledge on their predictive role in patients with COVID-19, the cardiac biomarkers were analyzed through different combinations at multivariable model. NPs preserve prognostic utility even after adjustment for history of HF, chronic renal failure, and age, emphasizing the useful NPs assessment irrespectively from these factors. NPs prognostic role was also confirmed in patients presenting with normal troponin levels. Indeed, NPs appears to be more consistent than troponin in identifying high-risk patients, irrespectively of combined troponin level. In interpreting these data, it is important to underline a different pathophysiologic significance of the 2 biomarkers; NPs reflect left ventricular wall stress, whereas troponin reflects myocardial injury. A peculiar hemodynamic state has been recently described in mechanically ventilated patients with ARDS due to COVID-19, highlighting a crucial role of increased LV filling pressures, high-output state, and high right atrial pressure.25 Patients who exhibited both elevations of biomarkers remain to be at threefold risk of in-hospital death. Adding high levels of 2 cardiac biomarkers on top of a multivariable clinical model, there was a greater improvement in discrimination of clinical model. Again, these findings may reflect a magnitude of co-existing hemodynamic impairment and cardiac impairment-related inflammatory of pneumonia disease. It is notable that NPs elevations were considered as levels above the limit for ruling out HF in the acute setting. Although previous reports have reported high levels of NPs irrespective of clinical signs of HF,18 there is no research concerning whether NPS cut-point upper limit of acute HF predicted the outcome of COVID-19 patients. Caro-Codón et al18 showed about 50% of patients with NT-proBNP above the recommended cut-off values for ruling in acute HF in the absence of clinical criteria for acute HF, suggesting a potential relation of this cut-point and COVID-19 disease. For the first time, we showed that NPs cut-points recommended for acute HF diagnosis20 may also be used as a threshold to identify adverse outcomes over several individual predictors in patients with COVID-19. This may highlight that a finding of elevated NPs in COVID-19 disease is not specific for HF diagnosis, and careful clinical evaluation remains the key for prognostic assessment.
The major limitation of our study concerns cardiac biomarkers measurement. Specifically regarding NPs, each hospital measured BNP or NT-pBNP according to laboratory policy. As such, we considered NPs as categoric variables above or below the cut-off to rule-out HF in the acute setting, without the possibility to assess a relation between different NPs values and outcome. However, using the ESC guidelines cut-off, we also demonstrated their reliable prognostic role in COVID-19 setting. Similar to NPs, troponin value was also considered as a categoric variable because different troponin assays have been used at each hospital. Cardiac biomarkers were not systematically assessed at hospital admission. As such, they might have been requested especially in more unstable patients or according to clinician judgment, limiting our knowledge to the whole population. We considered patients with available cardiac biomarker assessment within 24 hours and limited on admission. Two biomarkers were measured according to physicians’ decision. Because echocardiographic parameters were not systematically collected, we do not have additional information on myocardial involvement beyond cardiac biomarkers. During the COVID-19 outbreak emergency, there were multiple logistic limitations which influenced data collection and limited the multivariate analysis. Finally, this is an observational retrospective study with the intrinsic limits of retrospective analysis.
In our study, we showed that in patients with COVID-19 infection, early assessment of NPs and troponin may better identify those patients with higher risk of in-hospital mortality. Specifically, NPs showed prognostic role in identifying patients with poor outcome in those with normal troponin value. Importantly, this occurred irrespective of age and history of HF and chronic renal disease. Thus, the same NPs cut-point usually used for acute HF diagnosis may be used in identifying the need for aggressive management and hemodynamic monitoring. As such, cardiac biomarkers information, which can be measured at admission, may have a prognostic role in identifying different risk profiles.
Disclosures
Prof Piergiuseppe Agostoni reported nonfinancial support from Menarini, Novartis, and Boehringer; grants from Daiichiò Sankyo and Bayer; and grants and nonfinancial support from Actelion outside the submitted work. Prov Pietro Ameri reported having received speaker and advisor honoraria from Novartis, AstraZeneca, Vifor, Daiichi Sankyo, Boehringer Ingelheim, Pfizer, GlaxoSmithKline, and Merck, Sharp & Dohme and nonfinancial support from Actelion outside the submitted work. Dr Valentina Carubelli received consulting honoraria from CVie Therapeutics Limited, Servier, and Windtree Therapeutics outside the submitted work. Dr. Riccardo Maria Inciardi received speaker and advisor honoraria from Daiichi-Sankyo, Boehringer Ingelheim. Prof Sergio Leonardi reported grants and personal fees from AstraZeneca and personal fees from BMS/Pfizer, Novo Nordisk, and Chiesi outside the submitted work. Prof Carlo Mario Lombardi received speaker and advisor honoraria from Novartis and Astra Zeneca. Dr Andrea Mortara reports personal consulting honoraria from Novartis, Servier, Astra Zeneca for participation to advisory board meetings and receives grants from Novartis and Niccomo for research trials. Prof Massimo Piepoli reported having received research grants and speaking fees from Novartis, Servier, and TRX and nonfinancial support from Vifor outside the submitted work. Prof Michele Senni reported personal fees from Novartis, Abbott, Merck, Bayer, Boehringer, Vifor, and AstraZeneca outside the submitted work. Prof Marco Metra reported personal consulting honoraria from Abbott Vascular, Amgen, Bayer, Edwards Therapeutics, Servier, Vifor Pharma, and Windtree Therapeutics for participation to advisory board meetings and executive committees of clinical trials. All other authors have nothing to disclose.
References
- 1.Inciardi RM, Adamo M, Lupi L, Cani DS, Di Pasquale M, Tomasoni D, Italia L, Zaccone G, Tedino C, Fabbricatore D, Curnis A, Faggiano P, Gorga E, Lombardi CM, Milesi G, Vizzardi E, Volpini M, Nodari S, Specchia C, Maroldi R, Bezzi M, Metra M. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in northern Italy. Eur Heart J. 2020;41:1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323:1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 3.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei JF, Huang FY, Xiong TY, Liu Q, Chen H, Wang H, Huang H, Luo YC, Zhou X, Liu ZY, Peng Y, Xu YN, Wang B, Yang YY, Liang ZA, Lei XZ, Ge Y, Yang M, Zhang L, Zeng MQ, Yu H, Liu K, Jia YH, Prendergast BD, Li WM, Chen M. Acute myocardial injury is common in patients with COVID-19 and impairs their prognosis. Heart. 2020;106:1154–1159. doi: 10.1136/heartjnl-2020-317007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lombardi CM, Carubelli V, Iorio A, Inciardi RM, Bellasi A, Canale C, Camporotondo R, Catagnano F, Dalla Vecchia LA, Giovinazzo S, Maccagni G, Mapelli M, Margonato D, Monzo L, Nuzzi V, Oriecuia C, Peveri G, Pozzi A, Provenzale G, Sarullo F, Tomasoni D, Ameri P, Gnecchi M, Leonardi S, Merlo M, Agostoni P, Carugo S, Danzi GB, Guazzi M, La Rovere MT, Mortara A, Piepoli M, Porto I, Sinagra G, Volterrani M, Specchia C, Metra M, Senni M. Association of troponin levels with mortality in Italian patients hospitalized with coronavirus disease 2019: results of a multicenter study. JAMA Cardiol. 2020;5:1274–1280. doi: 10.1001/jamacardio.2020.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandoval Y, Januzzi JL, Jaffe AS. Cardiac troponin for assessment of myocardial injury in COVID-19: JACC review topic of the week. J Am Coll Cardiol. 2020;76:1244–1258. doi: 10.1016/j.jacc.2020.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah P, Doshi R, Chenna A, Owens R, Cobb A, Ivey H, Newton S, Mccarley K. Prognostic value of elevated cardiac troponin I in hospitalized Covid-19 patients. Am J Cardiol. 2020;135:150–153. doi: 10.1016/j.amjcard.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lippi G, Lavie CJ. Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63:390–391. doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stefanini GG, Chiarito M, Ferrante G, Cannata F, Azzolini E, Viggiani G, De Marco A, Briani M, Bocciolone M, Bragato R, Corrada E, Gasparini GL, Marconi M, Monti L, Pagnotta PA, Panico C, Pini D, Regazzoli D, My I, Kallikourdis M, Ciccarelli M, Badalamenti S, Aghemo A, Reimers B, Condorelli G. Humanitas COVID-19 Task Force. Early detection of elevated cardiac biomarkers to optimise risk stratification in patients with COVID-19. Heart. 2020;106:1512–1518. doi: 10.1136/heartjnl-2020-317322. [DOI] [PubMed] [Google Scholar]
- 10.Pranata R, Huang I, Lukito AA, Raharjo SB. Elevated N-terminal pro-brain natriuretic peptide is associated with increased mortality in patients with COVID-19: systematic review and meta-analysis. Postgrad Med J. 2020;96:387–391. doi: 10.1136/postgradmedj-2020-137884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calvo-Fernández A, Izquierdo A, Subirana I, Farrè N, Vila J, Duràn X, García-Guimaraes M, Valdivielso S, Cabero P, Soler C, Garcia-Ribas C, Rodriguez C, Llagostera M, Mojòn D, Vicente M, Solé-Gonzàlez E, Sànchez-Carpintero A, Tevar C, Marrugat J, Vaquerizo B. Markers of myocardial injury in the prediction of short-term COVID-19 prognosis. Rev Esp Cardiol (Engl Ed) 2021;74:576–583. doi: 10.1016/j.rec.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arcari L, Luciani M, Cacciotti L, Musumeci MB, Spuntarelli V, Pistella E, Martolini D, Manzo D, Pucci M, Marone C, Melandri S, Ansalone G, Santini C, Martelletti P, Volpe M, De Biase L. Incidence and determinants of high-sensitivity troponin and natriuretic peptides elevation at admission in hospitalized COVID-19 pneumonia patients. Intern Emerg Med. 2020;15:1467–1476. doi: 10.1007/s11739-020-02498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao L, Jiang D, Wen XS, Cheng XC, Sun M, He B, You LN, Lei P, Tan XW, Qin S, Cai GQ, Zhang DY. Prognostic value of NT-probnp in patients with severe COVID-19. Respir Res. 2020;21:83. doi: 10.1186/s12931-020-01352-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han H, Xie L, Liu R, Yang J, Liu F, Wu K, Chen L, Hou W, Feng Y, Zhu C. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J Med Virol. 2020;92:819–823. doi: 10.1002/jmv.25809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin JJ, Cheng X, Zhou F, Lei F, Akolkar G, Cai J, Zhang XJ, Blet A, Xie J, Zhang P, Liu YM, Huang Z, Zhao LP, Lin L, Xia M, Chen MM, Song X, Bai L, Chen Z, Zhang X, Xiang D, Chen J, Xu Q, Ma X, Touyz RM, Gao C, Wang H, Liu L, Mao W, Luo P, Yan Y, Ye P, Chen M, Chen G, Zhu L, She ZG, Huang X, Yuan Y, Zhang BH, Wang Y, Liu PP, Li H. Redefining cardiac biomarkers in predicting mortality of inpatients with COVID-19. Hypertension. 2020;76:1104–1112. doi: 10.1161/HYPERTENSIONAHA.120.15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng P, Ke Z, Ying B, Qiao B, Yuan L. The diagnostic and prognostic role of myocardial injury biomarkers in hospitalized patients with COVID-19. Clin Chim Acta. 2020;510:186–190. doi: 10.1016/j.cca.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caro-Codón J, Rey JR, Buño A, Iniesta AM, Rosillo SO, Castrejon-Castrejon S, Rodriguez-Sotelo L, Martinez LA, Marco I, Merino C, Martin-Polo L, Garcia-Veas JM, Martinez-Cossiani M, Gonzalez-Valle L, Herrero A, López-de-Sa E, Merino JL, Investigators CARD-COVID. Characterization of NT-proBNP in a large cohort of COVID-19 patients. Eur J Heart Fail. 2021;23:456–464. doi: 10.1002/ejhf.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mueller C. Biomarkers and acute coronary syndromes: an update. Eur Heart J. 2014;35:552–556. doi: 10.1093/eurheartj/eht530. [DOI] [PubMed] [Google Scholar]
- 20.Mueller C, Mcdonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, Coats AJS, Metra M, Mebazaa A, Ruschitzka F, Lainscak M, Filippatos G, Seferovic PM, Meijers WC, Bayes-Genis A, Mueller T, Richards M, Januzzi JL., Jr Heart Failure Association of the European Society of Cardiology. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019;21:715–731. doi: 10.1002/ejhf.1494. [DOI] [PubMed] [Google Scholar]
- 21.Dignam JJ, Kocherginsky MN. Choice and interpretation of statistical tests used when competing risks are present. J Clin Oncol. 2008;26:4027–4034. doi: 10.1200/JCO.2007.12.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wald A. Sequential tests of statistical hypotheses. Ann Math Statist. 1945;16:117–186. [Google Scholar]
- 23.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and aeducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Almeida Junior GLG, Braga F, Jorge JK, Nobre GF, Kalichszterin M, Faria PMP, Bussade B, Penna GL, Alves VO, Pereira MA, Gorgulho PC, Faria MRDSE, Drumond LE, Carpinete FBS, Neno ACLB, Neno ACA. Prognostic value of troponin-T and B-type natriuretic peptide in patients hospitalized for COVID-19. Arq Bras Cardiol. 2020;115:660–666. doi: 10.36660/abc.20200385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caravita S, Baratto C, Di Marco F, Calabrese A, Balestrieri G, Russo F, Faini A, Soranna D, Perego GB, Badano LP, Grazioli L, Lorini FL, Parati G, Senni M. Haemodynamic characteristics of COVID-19 patients with acute respiratory distress syndrome requiring mechanical ventilation. An invasive assessment using right heart catheterization. Eur J Heart Fail. 2020;22:2228–2237. doi: 10.1002/ejhf.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]