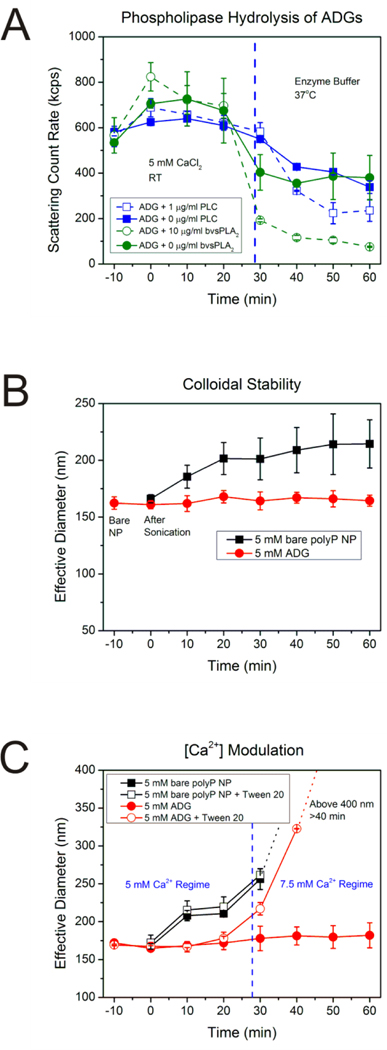
Figure 3.
A: Scattering intensity of ADGs after phospholipase digestion. ADGs were prepared in 5 mM CaCl2 at room temperature (RT) and monitored for stability for 30 min before transferal to an enzyme buffer at 37°C containing either 10 μg/mL sPLA2 from bee venom (bvsPLA2) or 1 μg/mL PLC from C. perfringens. The scattering count rate drops significantly for both 10 μg/mL bvsPLA2 and 1 μg/mL PLC ADG digestions, implying that the phospholipases are inducing hydrolysis of the lipid envelope, leading to degradation and agglomeration of ADGs and precipitation of the polyP NP cargo. B: Stability of ADGs in suspension. The average effective diameter was determined for the bare polyP NP (immediately before adding liposome, t=−10 min), immediately after sonication (t=0 min), and every ten minutes thereafter until 1 h had elapsed. The ADGs do not appreciably change in size over the duration of the experiment. The bare polyP NPs, on the other hand, prepared at the same supersaturation ratio, grow in a power-law manner to a mean effective diameter of approximately 220 nm in 1 h. C: Verification of the ADG encapsulation efficiency by measuring ADG diameter shifts after exposure to detergent and changes in calcium concentration. Liposome solubilization by the non-ionic detergent Tween 20, in conjunction with an increase in the calcium concentration, was exploited to judge the ADG encapsulation efficiency semi-quantitatively. In the absence of Tween 20 and an increase in calcium concentration to 7.5 mM at t=30 min, there is no statistically significant perturbation in the ADG effective diameter (solid red dots). However, increasing the calcium concentration after dissolution of the lipid envelope by detergent exposure allows for the polyP NPs to be exposed to the higher amount of calcium, resulting in an increased effective diameter (hollow red dots).