Abstract
A collection of 105 clinical isolates originally identified as Mycobacterium africanum were characterized using both phenotypic and genotyping methods. The phenotypic methods included routine determination of cultural properties and biochemical tests used to discriminate among the members of the M. tuberculosis complex, whereas genotypic characterization was based on IS6110-restriction fragment length polymorphism (IS6110-RFLP) analysis, IS1081-RFLP analysis, direct repeat-based spacer oligonucleotide typing (spoligotyping), variable number of tandem DNA repeats (VNTR), and the polymorphism of the oxyR, pncA, and mtp40 loci. The results obtained showed that a majority of M. africanum isolates were characterized by a specific spoligotyping pattern that was intermediate between those of M. tuberculosis and M. bovis, which do not hybridize with spacers 33 to 36 and spacers 39 to 43, respectively. A tentative M. africanum-specific spoligotyping signature appeared to be absence of spacers 8, 9, and 39. Based on spoligotyping, as well as the polymorphism of oxyR and pncA, a total of 24 isolates were excluded from the final study (19 were identified as M. tuberculosis, 2 were identified as M. canetti, and 3 were identified as M. bovis). The remaining 81 M. africanum isolates were efficiently subtyped in three distinct subtypes (A1 to A3) by IS6110-RFLP analysis and spoligotyping. The A1 and A2 subgroups were relatively more homogeneous upon spoligotyping than A3. Further analysis of the three subtypes by VNTR corroborated the highly homogeneous nature of the A2 subtype but showed significant variations for subtypes A1 and A3. A phylogenetic tree based on a selection of isolates representing the three subtypes using VNTR and spoligotyping alone or in combination confirmed the subtypes described as well as the heterogeneity of subtype A3.
Described for the first time in 1968, on the basis of observations of a patient from Senegal suffering from pulmonary tuberculosis (1), Mycobacterium africanum is generally responsible for tuberculosis in patients living in or from Sub-Saharan Africa. However, this species has also been observed in European patients for whom no link with Africa could be established. The prevalence of M. africanum varies from country to country. In Senegal the prevalence of M. africanum infections among tuberculosis patients is around 20%, and it ranges from 9% in Casamance to as high as 47% of all M. tuberculosis clinical isolates near the Senegal river (4). In Cameroon, tuberculosis is reportedly mostly caused by M. tuberculosis in the northern part of the country, whereas M. africanum represents about 60% of all pulmonary tuberculosis cases in Yaoundé (11). In contrast to M. tuberculosis and M. bovis, both of which have well-defined phenotypic characters, M. africanum shows an extensive phenotypic heterogeneity, suggesting that M. africanum could indeed be defined as a phenotypic continuum between M. bovis and M. tuberculosis (2). Biogeographical differences linking the West African M. africanum strains to M. bovis and the East African M. africanum isolates to M. tuberculosis have been proposed; however, recent results show that both types of strains could be isolated in a single country, e.g., in Guinea Bissau (10, 13). Thus, the taxonomic status of M. africanum within the M. tuberculosis complex, its phylogenetic link to M. bovis and M. tuberculosis, and its evolutionary origin remain to be understood. In this study, we focused on the phenotypic and genotypic characterization of a collection of 105 clinical isolates identified as M. africanum. The results obtained underline some definite characteristics that distinguish M. africanum from M. tuberculosis and M. bovis.
(The present work was performed as part of the Ph.D. thesis of C. Viana-Niero and I. Filliol.)
MATERIALS AND METHODS
Bacteria and identification.
A collection of 105 clinical isolates previously identified as M. africanum and isolated from patients in Burkina Faso (n = 1), Ivory Coast (n = 15), Senegal (n = 11), Mauritania (n = 2), Benin (n = 2), Burundi (n = 3), Rwanda (n = 3), Cameroon (n = 27), Central African Republic (n = 1), Madagascar (n = 2), and France (n = 38) was used for this investigation. All isolates were sent for reference purposes to the National Reference Laboratory for Mycobacteria at Institut Pasteur, Paris, France, between 1965 and 1998. The bacteria were grown on Löwenstein-Jensen slants, and 4-week-old cultures were characterized by routine cultural properties and biochemical tests that are used to discriminate among the members of the M. tuberculosis complex (1, 2, 7), such as colony morphology, niacin accumulation test, detection of nitrate reductase and urease, growth in presence of thiophene-2-carboxylic acid hydrazide (TCH) and thiosemicarbazone, and resistance to pyrazinamide (PZA).
Genotyping.
DNA was prepared by two methods, depending on the typing method that followed. A simple thermolysis method was enough for PCR-based methodologies. In this case, bacterial colonies were suspended in 150 μl of TE (Tris-HCl, 10 mM; EDTA, 1 mM; pH 8.0) and heat killed for 30 mn at 80°C. After centrifugation, an aliquot of the supernatant was used in PCR experiments. For IS6110-restriction fragment length polymorphism (IS6110-RFLP) and IS1081-RFLP analysis, the bacterial DNA was prepared as follows. About 1 mg of bacteria was resuspended in 5 ml of Middlebrook 7H9 medium supplemented with 1% (vol/vol) Tween and 1 mg of cycloserine per ml, incubated for 18 h at 37°C, heat inactivated at 80°C for 30 min, and centrifuged. The bacterial pellet was resuspended in TE containing 0.5 mg of lysozyme per ml and incubated overnight at 37°C. The wall-deficient cells obtained were lysed for 4 h at 55°C in TE containing 1% (wt/vol) sodium dodecyl sulfate and 0.2 mg of proteinase K per ml, and the DNA was purified using the phenol-chloroform method.
oxyR and pncA polymorphism and mtp40 detection were performed by published PCR-based protocols (3, 5). By studying a specific PCR product of 274 bp (oxyR) (20) or 185 bp (pncA) (5), the polymorphism of a nucleotide at position 285 in the case of oxyR and position 169 in the case of pncA, permits discrimination between M. bovis and M. tuberculosis (A and G, respectively, for oxyR, and G and C, respectively, for pncA).
IS6110 fingerprinting was performed using the internationally agreed-upon methodology (22). Labeling and detection were performed using enhanced chemiluminescence kits (ECL direct-test kits; Amersham, Buckinghamshire, United Kingdom). Taxotron software (P. A. D. Grimont, Taxolab, Institut Pasteur) was used to calculate molecular weights of hybridizing bands and to compare the isolates.
The direct repeat-based spacer oligonucleotide typing (spoligotyping) was performed as previously described (14), and the results were scanned and further analyzed using the Gel Compar software (Applied Maths, Kortrijk, Belgium). For phylogenetic reconstruction, the results were documented in the form of a binary code according to the results of hybridization (positive or negative result) for each spacer oligonucleotide probe (n = 43) and entered in an Excel spreadsheet file or in a Recognizer file of the Taxotron software package.
Although variable number of tandem DNA repeats (VNTR) methodology was initially used by Frothingham and Meeker-O'Connell with all of the 11 loci described for M. tuberculosis (6), a recent multicenter study suggested that only 5 VNTR loci (exact tandem repeat A [ETR-A] to ETR-E) were sufficiently discriminant to be retained for studying M. tuberculosis complex organisms (15). Hence, the VNTR typing of the isolates was limited to ETR-A to -E in the present investigation, and was performed as reported earlier (6). The molecular weight determination for PCR fragments were performed using the Taxotron software on images digitized using the Video-Copy system (Bioprobe, Montreuil, France). Once the lengths of the PCR fragments were precisely calculated, the number of copies for each ETR was deduced by a previously published method (15) and documented as a five-digit number representing allele profiles for ETR-A to ETR-E. VNTR results were also entered into Excel spreadsheets and Recognizer files for phylogenetic reconstruction.
The pairwise comparison of strains using spoligotyping and VNTR was performed by calculation of the Jaccard index (12) and by averaging individual distance files for combined numerical analysis as previously described (19). The algorithms chosen were the unweighted pair group using arithmetic average method for generating spoligotyping dendrograms and the neighbor-joining algorithm for phylogenetic purposes (18).
RESULTS AND DISCUSSION
Spoligotyping of the M. africanum collection studied.
Based on the polymorphism of the direct repeat locus of M. tuberculosis complex, spoligotyping permits discrimination between M. tuberculosis, M. bovis, and a rare variant of M. tuberculosis named M. canetti (13, 14, 15, 24). In the present investigation, the spoligotyping results obtained showed that a total of 24 out of 105 isolates taken from an existing collection of M. africanum organisms could be excluded from this study, as 19 isolates showed profiles of M. tuberculosis stricto sensu, 2 showed profiles of M. canetti, and 3 showed profiles that were typical of M. bovis. As discussed below, this species identification based on spoligotyping results was further confirmed by the polymorphism of the oxyR and pncA genes.
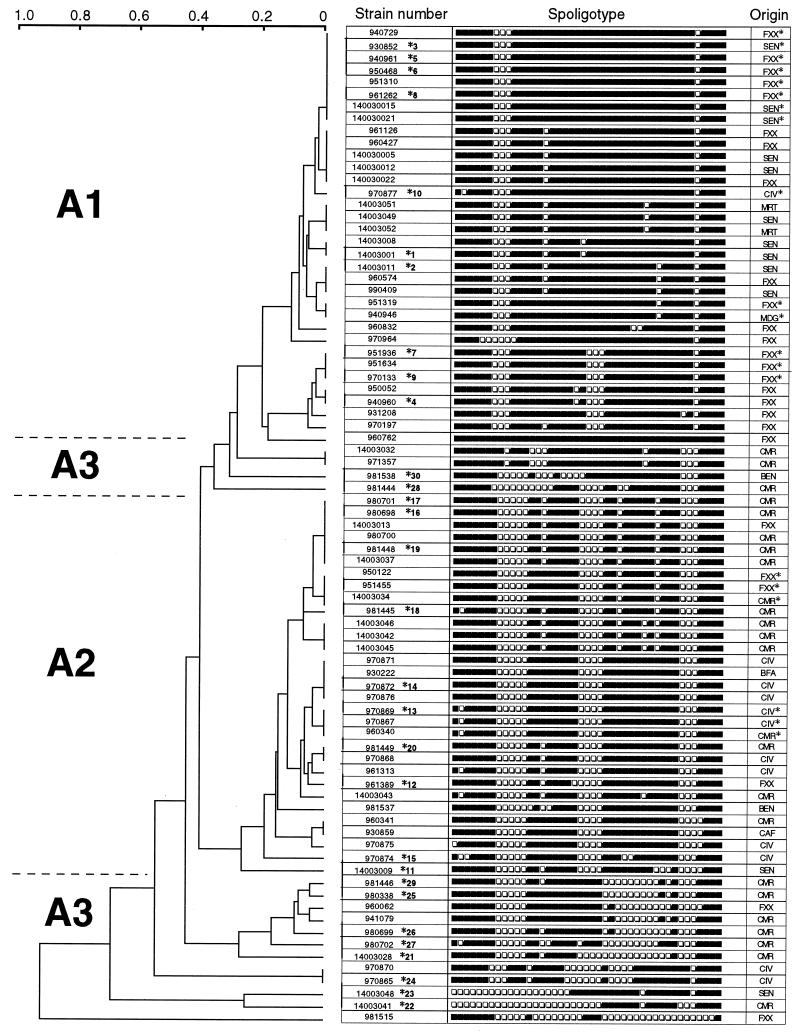
The remaining 81 M. africanum isolates were characterized by a specific spoligotyping pattern that was intermediate between those of M. tuberculosis and M. bovis, which mostly do not hybridize with spacers 33 to 36 (M. tuberculosis) and spacers 39 to 43 (M. bovis). The absence of spacer 39 was a striking spoligotyping signature of all of these M. africanum isolates. Spacers 8 and 9 were also mostly missing (absent in 78 of 81 and 80 of 81 isolates, respectively) (Fig. 1). Thus, a tentative M. africanum-specific spoligotyping signature appeared to be the absence of spacers 8, 9, and 39. Consequently, a clinical isolate showing the absence of spacers 8, 9, and 39 and the presence of spacers 33 to 36 and 40 to 43 may be tentatively identified as M. africanum. It can therefore be concluded that only spacers other from those mentioned above are indeed able to contribute towards the molecular typing of M. africanum, and they further allow classification of the 81 clinical isolates of M. africanum into three distinct subfamilies (Fig. 1 and 2), designated A1 (n = 33), A2 (n = 31), and A3 (n = 17). The A1 and A2 subgroups included relatively homogeneous spoligotypes, whereas group A3 was defined as a heterogeneous group that included isolates that could not be grouped as either A1 or A2.
FIG. 1.
Dendrogram showing the results obtained by spoligotyping of 81 M. africanum clinical isolates of various geographic origins (index, 1-Jaccard; algorithm, unweighted pair group using arithmetic average). Three subgroups (A1 to A3) were defined on the basis of clinical isolates showing a similarity index of ≥0.7. The asterisk followed by a number (from 1 to 30) after some isolate numbers designates the 10 isolates per subgroup (A1 to A3) that were randomly chosen for further analysis using VNTR. Isolates marked by an asterisk under “Origin” show identical spoligotypes between our isolates and those reported in previously published studies (13, 16, 17). Geographic origins, according to ISO code 3166, FXX, metropolitan France; SEN, Senegal; CIV, Ivory Coast; MRT, Mauritania; MDG, Madagascar; CMR, Cameroon; BEN, Benin; BFA, Burkina Faso; CAF, Central African Republic.
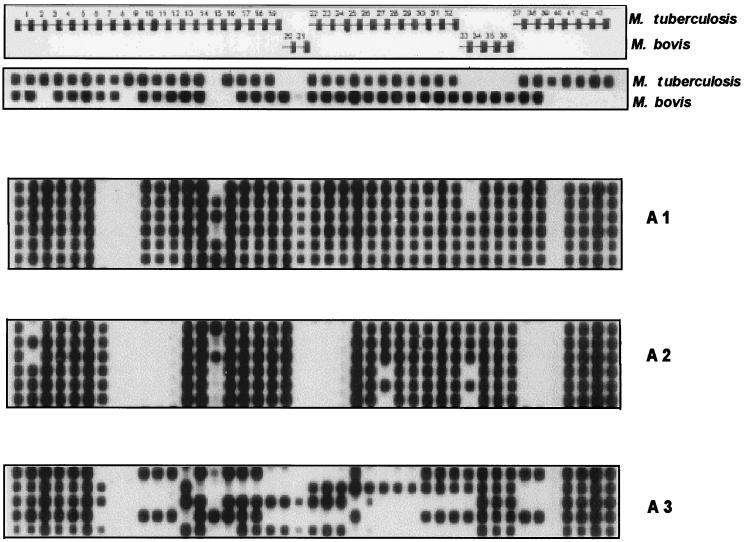
FIG. 2.
Representative spoligotyping patterns of some of the clinical isolates of the three subgroups (A1 to A3) compared to M. tuberculosis and M. bovis, which mostly do not hybridize with spacers 33 to 36 and 39 to 43, respectively. Note that M. africanum isolates show a specific pattern that is intermediate between those of M. tuberculosis and M. bovis. Subgroups A1 and A2 contain isolates with homogeneous spoligotyping patterns, whereas A3 constitutes a very heterogeneous group of isolates. Profiles obtained for type strains of M. tuberculosis and M. bovis are shown at the top.
Among the 81 M. africanum isolates, 26 clinical isolates harbored unique patterns, whereas 55 clinical isolates were grouped into 17 clusters which contained between 2 to 8 clinical isolates. The A1 family (8 clusters including a total of 28 out of 33 patients [Fig. 1]) was found mainly in isolates originating from France (n = 19), Senegal (n = 10), Mauritania (n = 2), Ivory Coast (n = 1), and Madagascar (n = 1). The A2 family (7 clusters including 23 patients) was found mainly in isolates originating from Cameroon (n = 11), Ivory Coast (n = 7), France (n = 2), Central African Republic (n = 1), Burkina Faso (n = 1), and Senegal (n = 1). The A3 family, which consisted of a very heterogeneous group of clinical isolates mainly from Cameroon (10 out of 17 isolates), contained a single cluster of 2 isolates from Ivory Coast. However, one has to be extremely careful when interpreting the geographic origins of the patients, particularly those from France. Indeed, even though the incidence of tuberculosis among African immigrants in France is known to be relatively higher than that among French nationals, the ethnic origin of the patients is not provided in medical records due to ethical considerations.
Phenotypic characteristics.
Within the M. tuberculosis complex, M. tuberculosis and M. africanum are easily differentiated from M. bovis and M. bovis BCG, as the latter organisms are inhibited by 2 μg of TCH per ml but are resistant to PZA. Although M. africanum shares most biochemical characters with M. tuberculosis, it may still be differentiated on the basis of properties such as stimulation of growth by pyruvate and by a number of tests in which it gives a highly variable response (2, 7). The phenotypic characteristics of the collection of M. africanum isolates studied in this investigation showed that 100% of the isolates studied were susceptible to PZA and 79% gave negative results for nitrate reductase, however, the overall response to a number of other tests (e.g., ability to grow in the presence of thiosemicarbazone, accumulation of niacin, and detection of urease) was almost equally divided between positive and negative results. Thus, at the phenotypic level, M. africanum seems to have an intermediate position between the relatively homogeneous M. tuberculosis, and M. bovis (2). However, some important differences were observed when the responses to major biochemical tests were compared among the spoligotyping-defined subgroups A1, A2, and A3; e.g., a positive response to the urease reaction varied from a high of 57% of the isolates in subgroup A1 to a low of 25% in A2, and conversely, a positive response for niacin accumulation varied from a high of 65% in A2 to a low of 38% in A1 and A3. These observations suggested that the phenotypic characteristics may be highly variable among the three subgroups. However, no clear-cut relationship between phenotypic properties and the geographical origins of isolates could be established.
Molecular characteristics.
Further analysis of the M. africanum collection was performed on the basis of the presence of the mtp40 sequence and the study of oxyR and pncA polymorphism. The mtp40 sequence was originally described as being specific for M. tuberculosis and absent in M. bovis (3). Later, it was shown that this sequence was not always present in M. tuberculosis (25). The results obtained in this investigation showed that the presence of this sequence was variable in M. africanum. Out of a total of 105 isolates initially tested, about 45.4% did not harbor the mtp40 sequence, and when the analysis was limited to the 81 isolates that defined the subgroups A1 to A3, no difference in the presence of mtp40 in any particular subgroup was observed (results not shown). The polymorphism of the pseudogene oxyR was studied using the full collection of 105 clinical isolates and permitted identification of 3 isolates with a typical M. bovis profile. This result was further confirmed by the study of the pncA gene in the full collection of isolates, and the three clinical isolates characterized as M. bovis by oxyR typing were indeed found to harbor a G in position 169 upon pncA typing. The remaining 102 isolates showed a characteristic M. tuberculosis profile (data not shown). Thus, our results underline that neither the phenotypic characters, the presence of the mtp40 sequence, or the polymorphism of oxyR and pncA is well suited for characterization of M. africanum.
IS6110- and IS1081-RFLP fingerprinting.
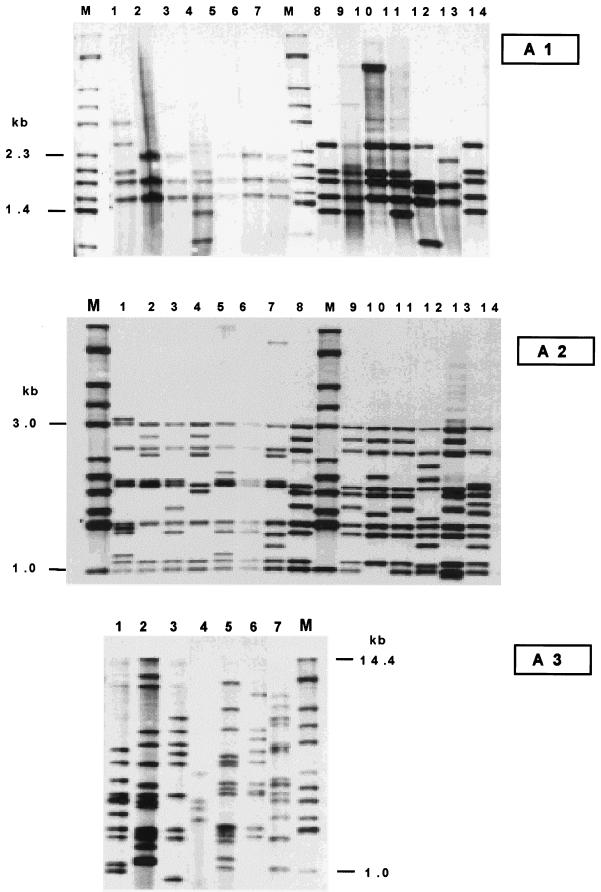
IS6110-RFLP analysis of the 81 selected isolates permitted regrouping of the collection of M. africanum isolates into three distinct subgroups (Fig. 3), and the distribution of strains within the subgroups was equivalent to that defined earlier using spoligotyping (Fig. 2): the A1 subgroup comprised isolates harboring 3 to 5 copies of IS6110 (with common bands within a range of 1.4 to 2.3 kb), the A2 subgroup harbored 9 to 11 copies of IS6110 (with common bands within a range of 1 to 3 kb), and the A3 subgroup was extremely heterogeneous and contained between 4 and 15 copies of IS6110. The fingerprinting of the M. africanum isolates using IS1081-RFLP analysis did not show any significant polymorphism, as most of the isolates had an identical profile of six copies, with only minor variations in the molecular sizes of the bands (results not shown), a result in agreement with previous findings of van Soolingen et al. (23).
FIG. 3.
IS6110-RFLP genomic patterns of representative clinical isolates of the three subgroups (A1 to A3) as previously defined using spoligotyping. Note that the A1 subgroup contains significantly fewer copies of IS6110 than the A2 and A3 subgroups. Lanes M, reference strain Mt14323.
VNTR allele determination.
In order to further analyze the collection of M. africanum isolates, 10 clinical isolates of each subgroup (A1 to A3) were randomly selected for VNTR typing, a method that has been recently used to reconstruct the molecular phylogeny of the M. tuberculosis complex (7). The VNTR results showed some interesting differences among the three subgroups previously defined using spoligotyping and IS6110-RFLP analysis (Table 1). The A2 subgroup isolates constituted a strictly homogeneous group without any variability (all isolates characterized by allele 42432 [Table 1]), the A1 subgroup was more heterogeneous with an elevated ETR-A allele number, and the A3 subgroup was very heterogeneous. Strikingly, no allele of the A1 subgroup was found in the A3 subgroup and vice versa (Table 1).
TABLE 1.
Summary of VNTR resultsa
| Subgroup | No. of isolates with the following VNTR allele (ETR-A to ETR-E)
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 64544 | 74545 | 32333 | 64535 | 74535 | 63535 | 74536 | 42432 | 71442 | 72536 | 42232 | 32432 | 42232 | 42422 | |
| A1 | 1 | 1 | 1 | 2 | 3 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| A3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 1 | 1 | 2 | 1 | 1 |
| Total | 1 | 1 | 1 | 2 | 3 | 1 | 1 | 13 | 1 | 1 | 1 | 2 | 1 | 1 |
Phylogeny reconstruction of M. africanum using combined numerical analysis of spoligotyping and VNTR results.
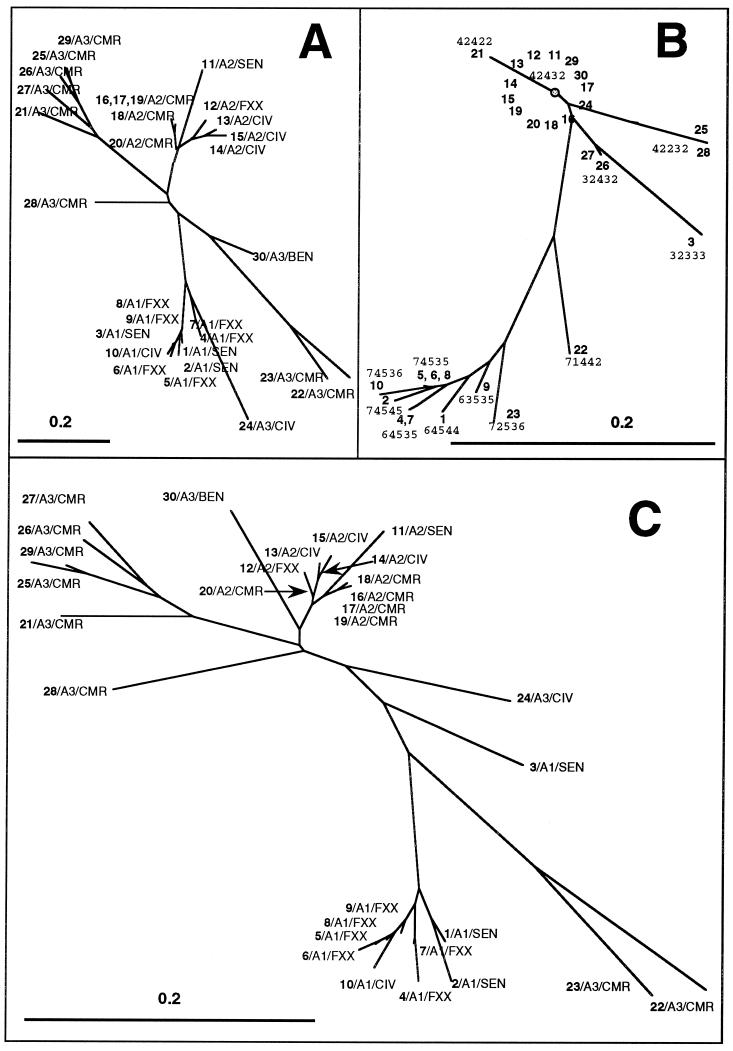
In order to infer potential phylogenetic relationships, neighbor-joining trees based on spoligotyping and VNTR results alone and in combination were built for 30 clinical isolates that were typed by both methods (10 isolates for each of the subgroups, numbered 1 to 10 for A1, 11 to 20 for A3, and 21 to 30 for A2) (Fig. 4). The neighbor-joining spoligotype tree (Fig. 4A) and VNTR tree (Fig. 4B) constructed using the 1-Jaccard index showed some similarities; however, one of the major differences between the two trees was the fact that VNTR was unable to discriminate among the A2 subgroup isolates, which gave identical profiles (allele 42432). A single strain of the A1 subgroup (isolate 930852, identified as number 3 in Fig. 4) gave apparently discrepant results by spoligotyping and VNTR; this clinical isolate, which bore an M. africanum-specific spoligotype, harbored a 32333 VNTR allele (Fig. 4B), which is mainly found in M. tuberculosis stricto sensu (particularly in the Haarlem subfamily of strains [15]). Finally, combined numerical analysis of spoligotyping and VNTR data for all 30 isolates (Fig. 4C) underlined at least three well-defined branches made up of the A1, A2, and A3 subgroups. The biogeographical distribution of strains within the three subgroups was not perfectly homogeneous; however, a relative predominance of isolates from certain countries could be observed for various subtypes (e.g., the A1 subgroup was mainly confined to clinical isolates from Senegal; the A2 subgroup included a majority of isolates from Ivory Coast, Senegal, and Cameroon; and the A3 subgroups isolates were usually from Cameroon).
FIG. 4.
Phylogenetic trees, constructed using the neighbor-joining method and the calculation of the 1-Jaccard index, for a collection of M. africanum isolates representative of subtypes A1, A2, and A3. (A) Spoligotyping-derived tree; (B) VNTR-derived tree; (C) combined numerical analysis based on spoligotyping and VNTR results. Each branch shows three levels of information; strain number (in boldface), showing the 10 isolates selected from each subgroup, which are numbered 1 to 10 for A1, 11 to 20 for A2, and 21 to 30 for A3; the major subgroups A1 to A3; and the geographic origin according to ISO code 3166 as described in the legend to Fig. 1.
We also looked for identical spoligotypes between our isolates and those reported in previously published studies (13, 16, 17), and we found that a total of 20 isolates from our study, representing five clusters and a single isolate (isolates marked by an asterisk immediately after their geographic origin according to ISO code 3166 in Fig. 1 [for ISO 3166 codes, see http://www.din/de/gremien/nas/nabd/iso3166ma]), clustered with a total of 40 previously identified isolates (detailed results not shown). The most prevalent cluster from our study, which contained eight isolates (three isolates from Senegal and five from metropolitan France; cluster at the top in Fig. 1) was also the most frequent in previous studies and clustered with a total of 33 other isolates, the majority from Senegal and Guinea Bissau, suggesting a clonal origin of this spoligotype. Whether this distribution was related to the specific history of tuberculosis spread in the respective countries remains to be investigated, and this investigation would benefit from an extensive study of additional M. africanum isolates from various geographical regions.
Significance of M. africanum genotyping in a probable evolutionary scenario for the M. tuberculosis complex.
The results obtained demonstrate that phenotypic and/or genotypic tests specifically developed to diagnose M. tuberculosis or M. bovis infections may not be well suited for M. africanum identification. To a certain point, spoligotyping gives the most specific answer, since the simultaneous absence of spacers 8, 9, and 39 appears to be characteristic of M. africanum clinical isolates. The absence of these three spacers had previously been reported for strains from Guinea Bissau (group B isolates) (13), and in our opinion, it may serve as a diagnostic test for confirmation of M. africanum stricto sensu. If confirmed in other studies, this would corroborate the existence of a specific M. africanum type among the M. tuberculosis complex, as initially proposed by Castets et al. (1) and whose true significance was later questioned by David et al. (2). Considering the above criteria for the identification of M. africanum developed in our study, we reinvestigated the strains involved in the first outbreak of multi-drug-resistant (MDR) tuberculosis strains in France, diagnosed during the period 1989 to 1992. These strains were initially identified as M. bovis on the basis of phenotypic tests (dysgonic colonies, negative results for niacin accumulation and nitrate reductase, growth inhibition by TCH, and resistance to PZA); however, a later genotypic analysis assigned them to M. tuberculosis on the basis of a high copy number of IS6110, the presence of mtp40, M. tuberculosis alleles for pncA and oxyR genes, and spoligotypes with spacers 40 to 43 (8). By the time of this genomic investigation of these unusual strains, no characteristic genotype of M. africanum was yet described and the taxonomic status of the species was still questioned. A reexamination of these MDR strains showed a genotypic profile characteristic of M. africanum group A2. Thus, in the light of our results as well as those described by others (13), these strains can be confidently considered to be true M. africanum. They represent the first outbreak due to MDR strains of M. africanum described in the literature. The index case originated from Brazil, indicating that despite the high prevalence of the species in African countries, M. africanum has also disseminated to other continents.
In our opinion, designation of some of the human M. tuberculosis isolates as M. africanum may indeed provide significant phylogenetic evolutionary information and may represent one of the missing links between bovine and human tuberculosis. Indeed, according to Sreevatsan et al. (21), major genetic group I isolates, including M. africanum and M. bovis, that bear a CTG codon at position 463 of katG and a ACC at position 95 of gyrA are probably ancestors of M. tuberculosis stricto sensu. Recently, based on the polymorphism at position 203 of katG, Frothingham et al. (7) further subdivided group I into subgroups IA and IB (ACT and ACC, respectively); subgroup IA contained both M. bovis and M. africanum isolates (which also include the M. africanum type strain ATCC 25420 from Senegal), and subgroup IB contained only M. africanum isolates. That study concluded that multiple genetically distinct strains may have converged toward an M. africanum phenotype (7). We suggest that the true M. africanum isolates (group B in the study of Källenius et al. [13]), as well as some of the isolates of the major genetic group I as defined previously by Sreevatsan et al. (21) and apparently identifiable as group C in the study of Källenius et al. (this group is characterized by the absence of spacers 29 to 32, and 34 [13]), could belong to a unique branch of the M. tuberculosis evolutionary tree that may have in common a recent ancestral M. bovis-like origin. This hypothesis is indirectly supported by the high copy number of the ETR-A allele (between 4 and 7) in M. africanum, which is reportedly closer to M. bovis than to M. tuberculosis (7).
Another study performed with 44 M. africanum isolates from Sierra Leone and Uganda gave essentially similar results for some isolates (group 4 in this study) that usually lacked spacers 8, 9 and 39, representing the true M. africanum isolates (17). However, in contrast to the case for some of the M. tuberculosis complex isolates that were characterized by the single absence of spacer 39 (group 5) in that study (17), we did not find M. africanum strains lacking only a single spacer in the present investigation. Some of these isolates missing spacer 39 were also part of a previous study (9); however, due to the differences in methodologies, we were unable to conclude whether these isolates represented true M. africanum isolates. In this connection, the presence of an isolate probing positively with all the 43 spacers in our study (isolate 960762 from metropolitan France) (Fig. 1) is unusual, and its status as M. africanum or M. tuberculosis would be worth reconsidering.
In conclusion, it is tempting to speculate that the differences observed between West and East African isolates (a low copy number of IS6110 in the west instead of a high copy number in the east) may correspond to the A1 and A2 subgroups shown in our study; however, further investigations will be necessary to support this hypothesis. Our molecular classification of M. africanum from West Africa into three distinct subgroups is based on results with three independent markers, i.e., IS6110-RFLP analysis, spoligotyping, and VNTR. This correlation is indirect evidence of the robustness of the branching obtained. The results are also strengthened by the long period of recruitment of isolates (33 years), which precludes any sampling bias due to potential existence of predominant genotypes (low apparent diversity), which may sometimes be observed in localized settings with high tuberculosis prevalence and a shorter recruitment of cases. Consequently, in a probable evolutionary scenario for M. tuberculosis complex strains, M. africanum may represent one of the missing links between bovine and human tuberculosis and may be the immediate ancestor of M. tuberculosis. These results suggest that a better understanding of phylogenetic relationships among the members of the M. tuberculosis complex would require a deeper insight into the M. africanum organisms.
ACKNOWLEDGMENTS
We thank A. Varnerot for her excellent technical help.
The TB and Mycobacteria unit, Institut Pasteur, Guadeloupe, is grateful to “Délégation Générale au Réseau International des Instituts Pasteur et Instituts Associés,” Institut Pasteur, Paris, and Fondation Française Raoul Follereau, Paris, France, for financial support.
REFERENCES
- 1.Castets M, Boisvert H, Grumbach F, Brunel M, Rist N. Les bacilles tuberculeux de type african: note préliminaire. Rev Tuberc Pneumol. 1968;32:179–184. [PubMed] [Google Scholar]
- 2.David H L, Jahan M T, Jumin A, Grandry J, Lehmann E H. Numerical taxonomy of Mycobacterium africanum. Int J Syst Bacteriol. 1978;28:467–472. [Google Scholar]
- 3.del Portillo P, Murillo L A, Patarroyo M A. Amplification of a species-specific DNA fragment of Mycobacterium tuberculosis and its possible use in diagnosis. J Clin Microbiol. 1991;29:2163–2168. doi: 10.1128/jcm.29.10.2163-2168.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diop S, de Medeiros D, de Medeiros G, Baylet R, Sankalé M. Incidence et répartition géographique de Mycobacterium africanum au sénégal. Bull Soc Med Afr Noire Lang Franç. 1976;21:50–56. [PubMed] [Google Scholar]
- 5.Espinosa de los Monteros L E, Galan C, Gutierrez M, Samper S, Garcia-Martin J F, Martin C, Dominguez L, de Rafael L, Baquero F, Gomez-Mampaso E, Blazquez J. Allele-specific PCR method based on pncA and oxyR sequences for distinguishing Mycobacterium bovis from Mycobacterium tuberculosis intraspecific M. bovis pncA polymorphism. J Clin Microbiol. 1998;36:239–242. doi: 10.1128/jcm.36.1.239-242.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frothingham R, Meeker-O'Connell W A. Genetic diversity in the Mycobacterium tuberculosis complex based on variable numbers of tandem DNA repeats. Microbiology. 1998;144:1189–1196. doi: 10.1099/00221287-144-5-1189. [DOI] [PubMed] [Google Scholar]
- 7.Frothingham R, Strickland P L, Bretzel G, Ramaswamy S, Musser J M, Williams D L. Phenotypic and genotypic characterization of Mycobacterium africanum isolates from West Africa. J Clin Microbiol. 1999;37:1921–1926. doi: 10.1128/jcm.37.6.1921-1926.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutierrez M C, Galan J C, Blazquez J, Bouvet E, Vincent V. Molecular markers demonstrate that the first described multi-drug resistant Mycobacterium bovis outbreak was due to Mycobacterium tuberculosis. J Clin Microbiol. 1999;37:971–975. doi: 10.1128/jcm.37.4.971-975.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas W H, Bretzel G, Amthor B, Schilke K, Krommes G, Rüsch-Gerdes S, Sticht-Groh V, Bremer H J. Comparison of DNA fingerprint patterns of isolates of Mycobacterium africanum from East and West Africa. J Clin Microbiol. 1997;35:663–666. doi: 10.1128/jcm.35.3.663-666.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffner S E, Svenson S B, Norberg R, Dias F, Ghebremichael S, Källenius G. Biochemical heterogeneity of Mycobacterium tuberculosis complex isolates in Guinea-Bissau. J Clin Microbiol. 1993;31:2215–2217. doi: 10.1128/jcm.31.8.2215-2217.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huet M, Rist N, Boube G, Potier D. Etude bactériologique de la tuberculose au Cameroun. Rev Tuberc Pneumol. 1971;35:413–426. [PubMed] [Google Scholar]
- 12.Jaccard P. Nouvelles recherches sur la distribution florale. Bull Soc Vaud Sci Nat. 1908;44:223–270. [Google Scholar]
- 13.Källenius G, Koivula T, Ghebremichael S, Hoffner S E, Norberg R, Svensson E, Dias F, Marklund B, Svenson S B. Evolution and clonal traits of Mycobacterium tuberculosis in Guinea-Bissau. J Clin Microbiol. 1999;37:3872–3878. doi: 10.1128/jcm.37.12.3872-3878.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J D A. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kremer K, Van Soolingen D, Frothingham R, Haas W H, Hermans P W M, Martin C, Palittapongarnpim P, Plikaytis B B, Riley L W, Yakrus M A, Musser J M, van Embden J D A. Comparison of methods based on different molecular epidemiological markers for typing of Mycobacterium tuberculosis strains: interlaboratory study of discriminatory power and reproducibility. J Clin Microbiol. 1999;37:2607–2618. doi: 10.1128/jcm.37.8.2607-2618.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niang M N, Goguet de la Salmoniere Y, Samb A, Hane A A, Cisse M F, Gicquel B, Perraut R. Characterization of M. tuberculosis strains from West African patients by spoligotyping. Microbes Infect. 1999;1:1189–1192. doi: 10.1016/s1286-4579(99)00243-9. [DOI] [PubMed] [Google Scholar]
- 17.Niemann S, Richter E, Rüsch-Gerdes S. Differentiation among members of the Mycobacterium tuberculosis complex by molecular and biochemical features: evidence for two pyrazinamide-susceptible subtypes of M. bovis. J Clin Microbiol. 2000;38:152–157. doi: 10.1128/jcm.38.1.152-157.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 19.Sola C, Horgen L, Devallois A, Rastogi N. Combined numerical analysis based on the molecular description of Mycobacterium tuberculosis by four-repetitive sequence-based DNA typing systems. Res Microbiol. 1998;149:349–360. doi: 10.1016/s0923-2508(98)80440-3. [DOI] [PubMed] [Google Scholar]
- 20.Sreevatsan S, Escalante P, Pan X, Geillies II D A, Siddiqui S, Khalaf C N, Kreiswirth B N, Bifani P, Adams L G, Ficht T, Perumaalla V S, Cave M D, van Embden J D, Musser J M. Identification of a polymorphic nucleotide in oxyR specific for Mycobacterium bovis. J Clin Microbiol. 1996;34:2007–2010. doi: 10.1128/jcm.34.8.2007-2010.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sreevatsan S, Pan X, Stockbauer K, Connell N, Kreiswirth B, Whittam T, Musser J. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc Natl Acad Sci USA. 1997;97:9869–9874. doi: 10.1073/pnas.94.18.9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Embden J D A, Cave M D, Crawford J T, Dale J W, Eisenach K D, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick T M, Small P M. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Soolingen D, Hermans P W, de Haas P E, van Embden J D. Insertion element IS1081-associated restriction fragment length polymorphisms in Mycobacterium tuberculosis complex species: a reliable tool for recognizing Mycobacterium bovis BCG. J Clin Microbiol. 1992;30:1772–1777. doi: 10.1128/jcm.30.7.1772-1777.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Soolingen D, Hoogenboezem T, de Haas P E W, Hermans P W M, Koedam M A, Teppema K S, Brennan P J, Besra G S, Portaels F, Top J, Schouls L M, van Embden J D A. A novel pathogenic taxon of the Mycobacterium tuberculosis complex, Canetti: characterization of an exceptional isolate from Africa. Int J Syst Bacteriol. 1997;47:1236–1245. doi: 10.1099/00207713-47-4-1236. [DOI] [PubMed] [Google Scholar]
- 25.Weil A, Plikaytis B B, Butler W R, Woodley C L, Shinnick T M. The mtp 40 gene is not present in all strains of Mycobacterium tuberculosis. J Clin Microbiol. 1996;34:2309–2311. doi: 10.1128/jcm.34.9.2309-2311.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]