Abstract
Introduction:
Manganese and lead have been cross-sectionally associated with adverse respiratory outcomes in childhood but there is limited data on their combined effects starting in utero. We examined associations between in utero exposure to metals and childhood respiratory symptoms.
Methods:
We assessed 633 mother-child dyads enrolled in the Programming Research in Obesity, Growth, Environment, and Social Stressors (PROGRESS) birth cohort in Mexico City. Blood manganese (BMn) and lead (BPb) were measured in mothers at 2nd and 3rd trimester. Ever wheeze, current wheeze and asthma diagnosis were ascertained at 4–5 and 6–7 year visits through the International Study of Asthma and Allergies in Childhood survey. Logistic mixed model regression was used to assess the association between prenatal metals and respiratory outcomes in children across the 4–5 and 6–7 year visits. Covariates included mother’s age, education and asthma, environmental tobacco smoke, child’s sex and assessment time.
Results:
In adjusted models, higher 2nd trimester BPb had a significant association with elevated odds of ever wheeze (Odds Ratio (OR): 1.97, 95% CI: 1.05, 3.67). BMn at 2nd trimester was associated with decreased (OR: 0.06, 95% CI: 0.01, 0.35) odds of current wheeze. We did not find any statistically significant associations with 3rd trimester blood metals.
Conclusion:
Prenatal exposure to Pb was associated with higher odds of ever wheeze while Mn was negatively associated with odds of current wheeze. These findings underscore the need to consider prenatal metal exposure, including low exposure levels, in the study of adverse respiratory outcomes.
Introduction
Wheezing in childhood is associated with substantial morbidity and health care utilization in Latin America (Asher et al., 2007; Bloom et al., 2013; Gold et al., 2013), and is a risk factor for asthma development (Gern et al., 1999). The fetal environment has been shown to be a previously unrecognized contributor to disease risk (Hanson and Gluckman, 2011; Hanson et al., 2019) including lung disease (Heyob et al., 2019; Lai et al., 2019; Melen and Guerra, 2017; Rosa et al., 2018). Lung development begins prenatally through a carefully coordinated sequence of events that are vulnerable to disruption by even relatively low-dose environmental exposures, which can alter the maturation of organ systems and impact developmental trajectories which affect the respiratory system later in life (Pinkerton and Joad, 2006). During the first half of gestation, the development of bronchi occurs along with the branching of the airways. Throughout the second half of gestation, the alveoli develop and the lungs continue to mature in number, size, and the complexity of the alveoli years after birth (De Luca et al., 2010). Alteration of fetal programming and dysfunctional remodeling of lung cells may occur when the fetus is exposed to noxious stimuli. In utero environmental exposures may thus alter fetal lung development, predisposing the fetus to future respiratory disease. Identifying early life risk factors that may be amenable to intervention, can provide mechanistic information to better elucidate the link between environmental exposures and childhood respiratory disease and inform the timing these interventions.
An understudied but ubiquitous perinatal risk factor are metals. Globally, millions of people are exposed to manganese (Mn) and lead (Pb) from naturally occurring and anthropogenic sources like pesticides, sewage discharge, fertilizers, industrial waste contaminating air, drinking water, diet and soil and other occupational sources (EPA, 2003; Lin et al., 2021; Mazari-Hiriart et al., 2019; Nriagu, 1988; Pantic et al., 2018). While Mn is an essential nutrient, it has been shown to be toxic at elevated levels and interact with other co-ocurring metal exposures like Pb (Sanders et al., 2015). Additionally in Mexico, the site of our study, traditional lead-glazed ceramics used in food preparation and storage have been identified as important sources of Pb exposure (Avila et al., 1991; Pantic et al., 2018; Tellez-Rojo et al., 2019). Metals are drivers of oxidative stress (OS), either by catalyzing redox reactions (i.e. Fenton reaction), replacing cofactors in enzymatic reactions, or binding to sulfhydryl groups inactivating anti-oxidant enzymes (Jomova and Valko, 2011). Inflammation secondary to these metals during the prenatal and early postnatal periods may disturb progenitor cell differentiation and alter lung structure resulting in both reduced function after birth and increased susceptibility to lung disease in adulthood (Cao et al., 2016).
There is also epidemiological evidence linking Mn and Pb exposure to respiratory outcomes in children and adults, mostly in cross-sectional studies (Kim et al., 2019; Pesce et al., 2021; Shaheen et al., 2004). Prenatal but not concurrent blood Pb was associated with increased risk for atopic sensitization to common aeroallergens in children at 5 years of age in Poland; this study suggested even low levels of in utero Pb exposure may be implicated in the process of allergic sensitization (Jedrychowski et al., 2011). Personal air Mn levels were associated with greater odds of report of asthma and asthma medication use in a cross-sectional study of adolescents living in Brescia, Italy (Rosa et al., 2016). Cross-sectional exposure to Pb has also been associated with lung function deficits in children (Little et al., 2017; Madrigal et al., 2018; Zheng et al., 2013). Furthermore, susceptibility to in utero metal exposure has also been shown to vary by fetal sex (Kasten-Jolly and Lawrence, 2017; Signes-Pastor et al., 2019; Singh et al., 2018; Winterbottom et al., 2019). Data from both animal and human studies also demonstrates lung development differs by sex (Ishak et al., 2014; Torday and Nielsen, 1987), but the environmental exposures that may contribute to these differences are not well understood.
Therefore, we leveraged longitudinal data from the Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS) birth cohort study in Mexico City to address these research gaps. We assessed the associations between 2nd and 3rd trimester blood Mn (BMn) and Pb (BPb) levels and wheeze/asthma outcomes at ages 4–7 years. We hypothesized that higher prenatal metals exposure would be associated with increased risk of childhood wheeze/asthma. We also examined potential sex differences.
Methods
Study Participants
Between July 2007 and February 2011, the PROGRESS study recruited pregnant women receiving prenatal care at Mexican Social Security System (IMSS) clinics. The inclusion criteria included the following: < 20 weeks pregnant, ≥18 years, no history of kidney or heart disease, telephone access, and intention to reside in Mexico City for a minimum of three years. Additional inclusion criteria details have been described elsewhere (Burris et al., 2013). The Icahn School of Medicine at Mount Sinai, Harvard T.H. Chan School of Public Health and the Mexico National Institute of Public Health institutional review boards approved the study protocols. Women provided written informed consent.
Respiratory outcomes
The International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire, which has been validated in Spanish (Asher et al., 2007) was administered during the 4–5 and 6–7 year study visits. Caregiver’s yes/no responses to “Has your child ever had wheezing or whistling in the chest at any time in the past?”, “Has your child had wheezing or whistling in the chest in the last 12 months?”, and “Has your child ever had asthma in their life?” were used to define ever wheeze, current wheeze, and asthma diagnosis, respectively.
Prenatal metals
Venous whole blood samples were collected at enrollment in royal blue top vacutainer tubes with potassium ethylenediaminetetraacetate (K2EDTA) (Becton-Dickinson and Company, Franklin Lakes, New Jersey). Prenatal metal (μg/dL) levels of Pb and Mn were analyzed using a dynamic reaction cell-inductively coupled plasma mass spectrometer (ICP-DRC-MS) (Elan 6100; PerkinElmer, Norwalk, CT). Further quality control details have been previously described (Mullin et al., 2019; Tamayo et al., 2016). Blood metals were converted from micrograms per deciliter (μg/dL) to micrograms per liter (μg/L) for our study analyses. Additionally, blood metals were natural log transformed due to being right skewed.
Covariates
Mother’s age, maternal education, and environmental tobacco smoke information was collected via questionnaire at enrollment. Child’s sex (male/female) was collected at delivery. Maternal education was collapsed into three categories: < high school, high school, and > high school. Exposure to environmental tobacco smoke was determined based on whether the mothers reported having household members who smoke inside the home during the 2nd or 3rd trimester of pregnancy. Maternal asthma was based on the mother’s report of being medically diagnosed with asthma.
Statistical Analysis
We performed descriptive statistics for dependent and explanatory variables, and Spearman correlations for prenatal metals. We utilized a logistic mixed model regression to assess the association between prenatal metals and each adverse respiratory outcome while accommodating for intra-subject correlated observations. All of the logistic mixed models were performed using the GLIMMIX procedure with a random statement. Three separate adjusted models were generated for each outcome: ever wheeze, current wheeze and asthma diagnosis. In all the mixed models, we co-adjusted for both metals and, and included mother’s age, education and report of ever asthma at enrollment, child’s sex, and environmental tobacco smoke which were selected a priori based on previous literature. We also included a term to account for time of outcome assessment. In order to examine potential metal x sex interactions, we also ran models with the inclusion of product terms for each metal x sex. SAS 9.4 (SAS, Inc., Cary, NC) was used to perform all statistical analyses.
Results
Study Population
Participants attended 1209 visits across the 4–5 and 6–7 year visit. Among the 948 women who enrolled in the study and delivered a live birth, 575 participants attended both the 4–5 and 6–7 year visit, 33 participants attended only the 4–5 year visit, and 26 participants only attended the 6–7 year visit. Table 1 presents information regarding demographic characteristics, prenatal blood metals, and respiratory outcomes. There were 634 women who provided responses about wheeze and asthma diagnosis in their children at either the 4–5 or 6–7 year visit. One participant was excluded from the analysis due to a data error which left 633 participants in the analytical sample. There was 28.1%, 13.7%, and 5.1% of women who reported ‘yes’ to their child experiencing ever wheeze, current wheeze, and asthma diagnosis at either study visit, respectively.
Table 1.
PROGRESS participant characteristics (N = 633).
| n (%) or median (IQR) | |
|---|---|
|
| |
| Mother’s Age (Years) | 27.2 (23.3, 31.2) |
| Environmental Tobacco Smoke | |
| No | 435 (69.2) |
| Yes | 194 (30.8) |
| Maternal Education | |
| Less Than High School | 254 (40.1) |
| High School | 230 (36.3) |
| More Than High School | 149 (23.5) |
| Child’s Sex | |
| Male | 325 (51.3) |
| Female | 308 (48.7) |
| Maternal Asthma Diagnosis | |
| No Asthma | 626 (98.89) |
| Asthma | 7 (1.11) |
| BMn at 2nd Trimester (μg/L) | 13.7 (11.1, 17.0) |
| BPb at 2nd Trimester (μg/L) | 28.8 (19.3, 44.2) |
| BMn at 3rd Trimester (μg/L) | 18.3 (14.8, 22.4)) |
| BPb at 3rd Trimester (μg/L) | 30.6 (20.1, 47.4) |
| Ever Wheeze | |
| No Wheeze | 455 (71.9) |
| Wheeze | 178 (28.1) |
| Current Wheeze | |
| No Wheeze | 546 (86.3) |
| Wheeze | 87 (13.7) |
| Child Asthma Diagnosis | |
| No Asthma | 601 (94.9) |
| Asthma | 32 (5.1) |
The median and interquartile range (IQR) for BMn and BPb at 2nd trimester were 13.7 μg/L (11.1, 17.0) and 28.8 μg/L (19.3, 44.2) respectively. The median IQR for BMn and BPb at 3rd trimester were 18.3 μg/L (14.8, 22.4) and 30.6 μg/L (20.1, 47.4) respectively. 2nd trimester blood metals were weakly correlated with each other (Table 2). The same was true for correlations across all combinations of the 3rd trimester blood metals. However, there were strong correlations between the 2nd and 3rd trimester blood metals, which is why they were not included in the same models. Among the mothers in the study, 40.1% had less than a high school education, 30.8% reported exposure to environmental tobacco during pregnancy, and the median (IQR) age at enrollment was 27.2 (23.3, 31.2) years. The male to female ratio was evenly distributed among the children.
Table 2.
Spearman correlations of metals at 2nd and 3rd trimester among mother-child dyads in Mexico City
| BMn at 2nd Trimester | BPb at 2nd Trimester | BMn at 3rd Trimester | BPb at 3rd Trimester | |
|---|---|---|---|---|
|
| ||||
| BMn at 2nd Trimester | 1 | |||
| BPb at 2nd Trimester | 0.12** | 1 | ||
| BMn at 3rd Trimester | 0.66*** | 0.12** | 1 | |
| BPb at 3rd Trimester | 0.06 | 0.79*** | 0.20*** | 1 |
p < 0.05
p < 0.01
p < 0.001
Multivariable Analysis
Associations between 2nd trimester blood metals and each outcome longitudinally across both 4–5 and 6–7 year visit are shown in Figures 1–3. As shown in Figure 1, higher 2nd trimester BPb levels had a significant association with elevated odds of ever wheeze (Odds Ratio (OR): 1.97, 95% Cl: 1.05, 3.67). Higher BMn at 2nd trimester was associated with decreased (OR: 0.06, 95% Cl: 0.01, 0.35) odds of current wheeze(Figure 2). We did not find any statistically significant associations between prenatal metals and asthma diagnosis (Figure 3) or between 3rd trimester BMn and BPb and any of the respiratory outcomes (Figure 4–5). The adjusted mixed model for blood metals at 3rd trimester and asthma diagnosis did not converge. Numerical results are shown in table form in the supplement. We also did not find evidence of interaction between blood metals and child sex (all interaction p-values >0.20).
Figure 1.
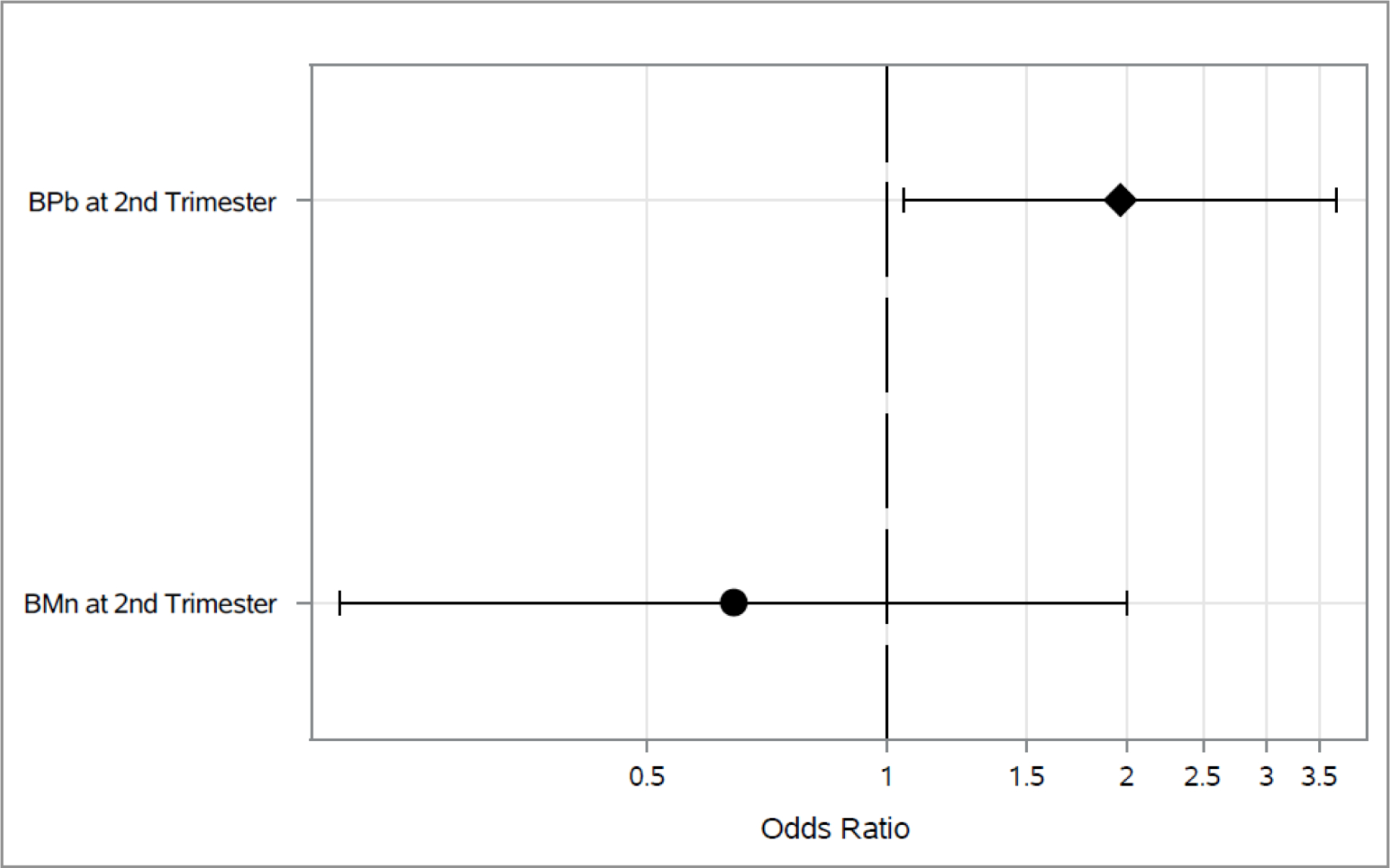
Associations between 2nd trimester BPb and BMn, and odds of ever wheeze across the 4–5 and 6–7 year visits*
*Adjusted for maternal age, education and asthma at enrollment, prenatal environmental tobacco smoke exposure, child’s sex and assessment time.
*Diamond represents p-value <0.05.
Figure 3.
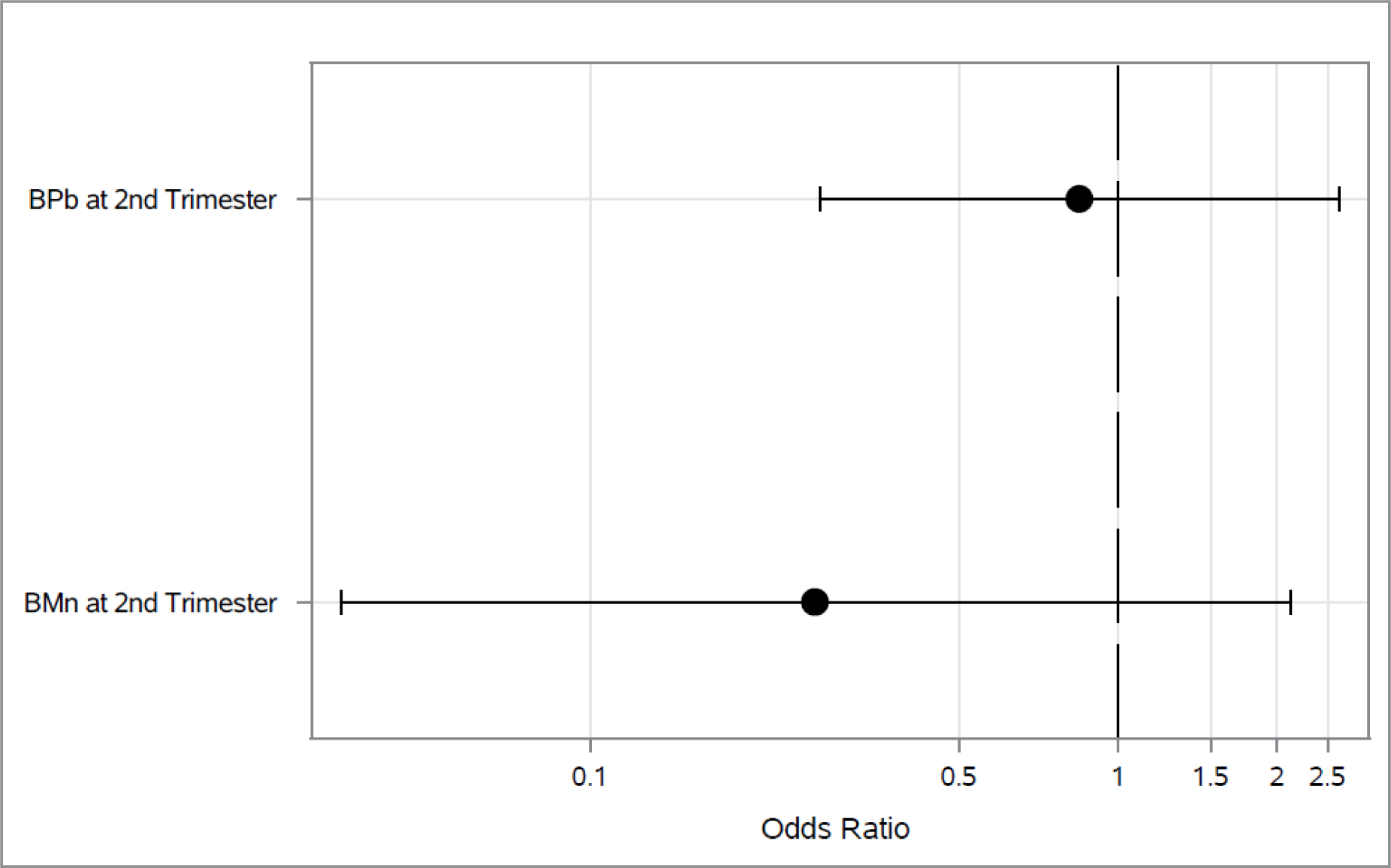
Associations between 2nd trimester BPb and BMn and odds of asthma diagnosis across the 4–5 and 6–7 year visits*
*Adjusted for maternal age, education and asthma at enrollment, prenatal environmental tobacco smoke exposure, child’s sex and assessment time.
*Diamond represents p-value <0.05.
Figure 2.
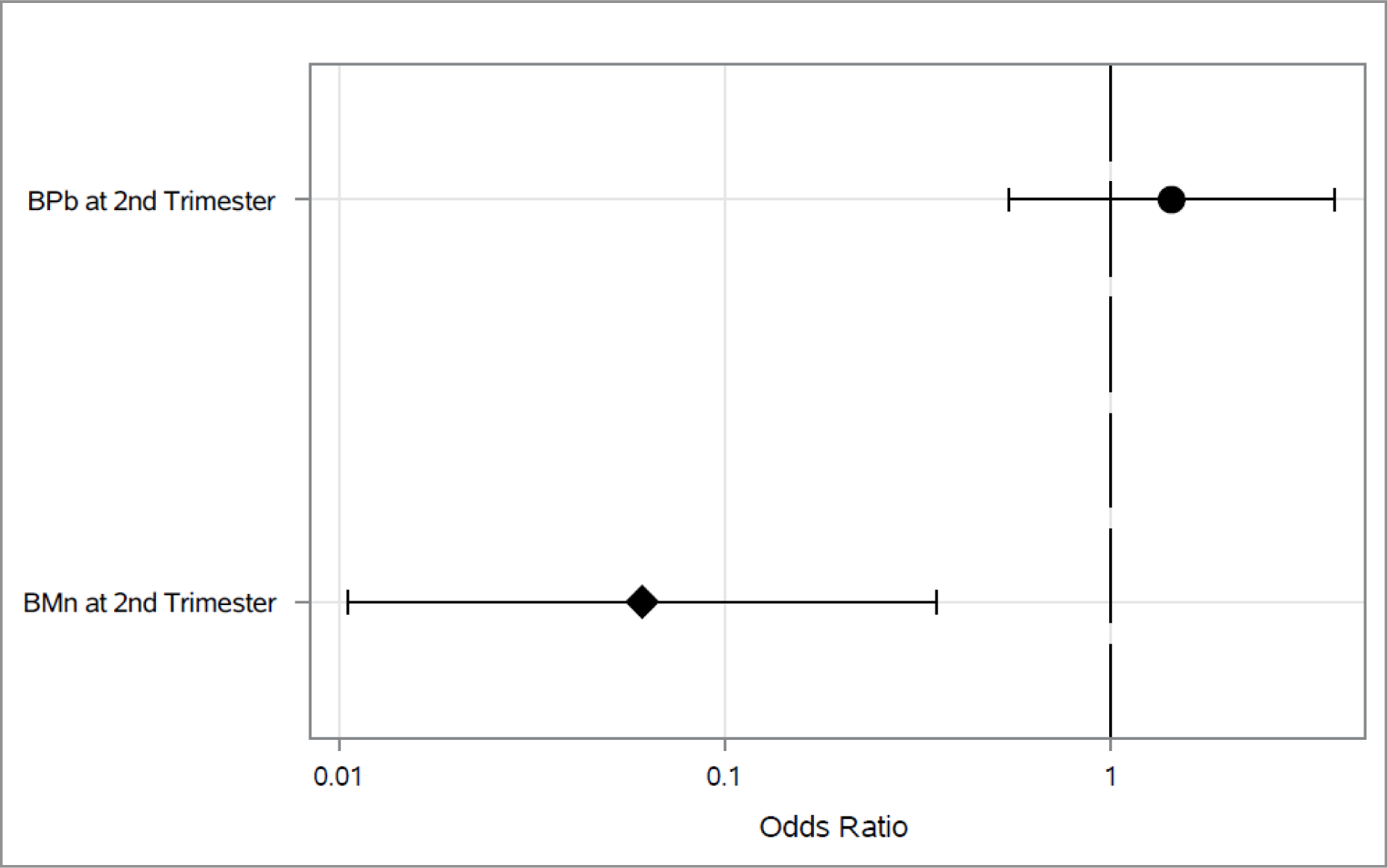
Associations between 2nd trimester BPb and BMn and odds of current wheeze across the 4–5 and 6–7 year visits*
*Adjusted for maternal age, education and asthma at enrollment, prenatal environmental tobacco smoke exposure, child’s sex and assessment time.
*Diamond represents p-value <0.05.
Figure 4.
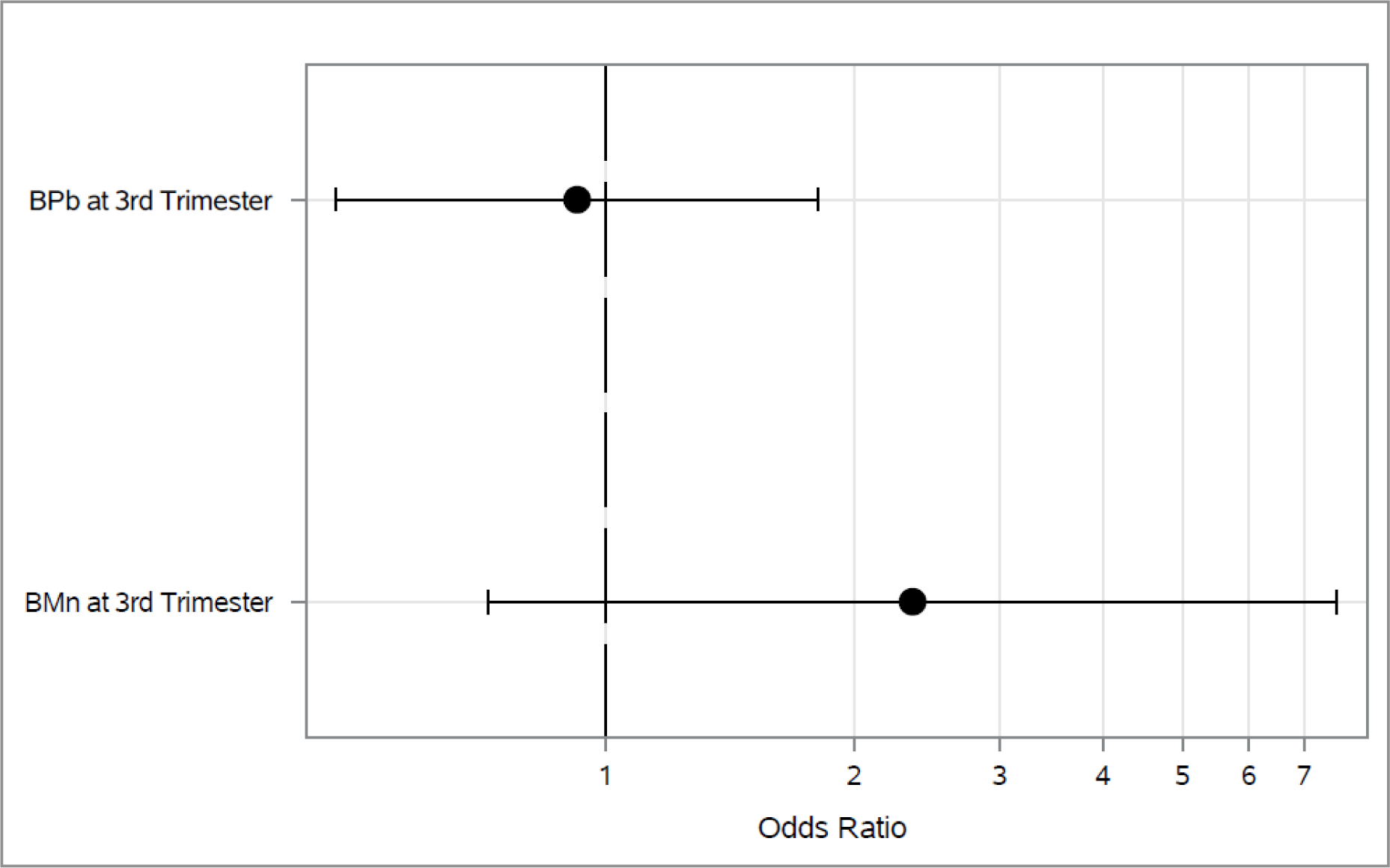
Associations between 3rd trimester BPb and BMn and odds of ever wheeze across the 4–5 and 6–7 year visits*
*Adjusted for maternal age, education and asthma at enrollment, prenatal environmental tobacco smoke exposure, child’s sex and assessment time.
*Diamond represents p-value <0.05.
Figure 5.
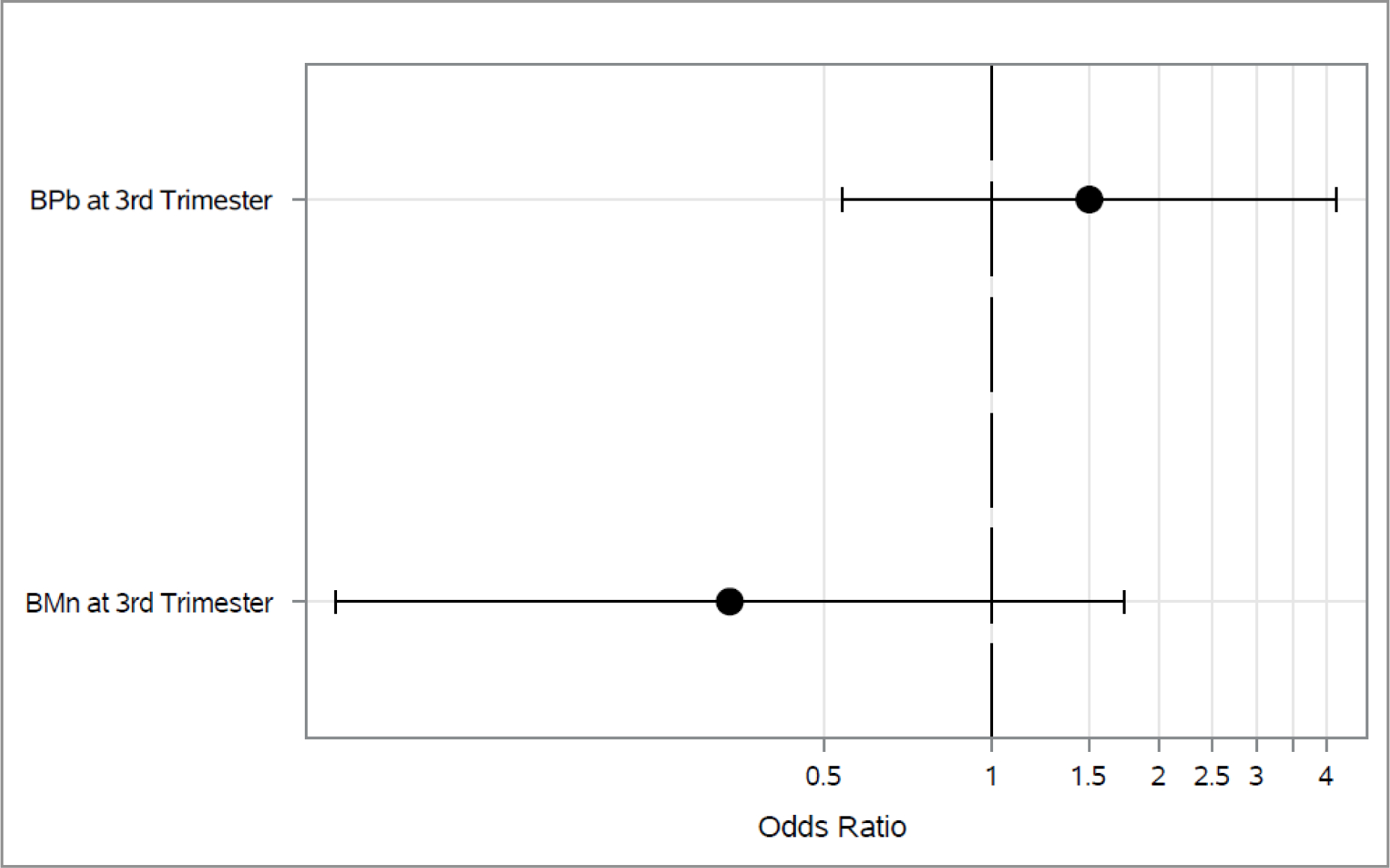
Associations between 3rd trimester BPb and BMn and odds of current wheeze across the 4–5 and 6–7 year visits*
*Adjusted for maternal age, education and asthma at enrollment, prenatal environmental tobacco smoke exposure, child’s sex and assessment time.
*Diamond represents p-value <0.05.
Discussion
We found that higher 2nd trimester maternal BPb was associated with higher odds of ever wheeze in children at age 4–7 years. In contrast, BMn was associated with lower odds of current wheeze. We did not find any association between blood metals at 2nd trimester and asthma diagnosis or between 3rd trimester metals and any outcome. We also did not find any evidence of effect modification by sex. While previous studies demonstrated associations between postnatal and cross-sectional exposure to metals and respiratory outcomes (Kim et al., 2019; Little et al., 2017; Madrigal et al., 2018; Pesce et al., 2021; Shaheen et al., 2004; Zheng et al., 2013), there is limited evidence regarding prenatal exposure. In a study among children in France, prenatal BMn but not BPb was associated with greater risk of asthma or atopic diseases (Pesce et al., 2021). The duration of atopic dermatitis was positively correlated with cord blood Pb levels (p=0.002) among children in Seoul (Kim et al., 2019). However, umbilical cord Pb and Mn was not associated with wheeze among children in the United Kingdom (Shaheen et al., 2004).
Pb and Mn can cross the placental barrier which indicates that fetal lung development could be impaired by maternal exposure during pregnancy (Claus Henn et al., 2017). In utero exposure to Pb is associated with lower birth weight for gestational age (Rodosthenous et al., 2017) and preterm birth (Ashrap et al., 2020), which are risk factors for later lung dysfunction and alterations in lung development (Briana and Malamitsi-Puchner, 2013; Smith et al., 2010). BPb has been associated with increased bronchial hyperresponsiveness (Min et al., 2008). It has also been shown to affect T-dependent immune response and alter immunoglobulin levels (Min et al., 2008; Wang et al., 2017; Wells et al., 2014). Factors that impact airway growth in early development seem to cause physiological effects on the lungs that can be persistent, like wheeze-like illnesses and the in utero environment is an important determinant of bronchial responsiveness (Stick, 2000). We also found associations only with exposure to metals during the second trimester of pregnancy which coincides with the pseudoglandular and canalicular stages of lung development when conducting airways, terminal bronchioles, pre-acinar blood vessels, primitive alveoli, type 1 and 2 cells and surfactant synthesis are being developed (Kajekar, 2007). The developing fetus may be particularly vulnerable to oxidative stress during this time period because fetal antioxidant capabilities do not increase until the third trimester (Hsu et al., 2015). Mid-gestation has also been identified as a potential window of susceptibility to other oxidative exposures like air pollution. In a Boston birth cohort, air pollution exposure during mid-gestation was associated with higher risk of asthma at age 6 years (Hsu et al., 2015). Similarly, a nationwide birth cohort from Taiwan found a significant association between air pollution exposure during gestational weeks 6–22 and incident asthma (Jung et al., 2019).
We found that prenatal BMn was associated with lower odds of childhood wheeze. Unlike Pb which has no known nutritional value, Mn is an essential metal in trace amounts, and there is no established cut-off for Mn toxicity (Chung et al., 2015). The Mn levels in our study were low compared to Mn levels in other child studies (Chung et al., 2015; Claus Henn et al., 2017) which may have facilitated Mn being beneficial rather than toxic to our study population. A study which followed pregnant CD rats and fetuses exposed to MnSO4 aerosol demonstrated that inhaled Mn did not affect tissue Mn levels in the fetus compared to the control group (Dorman et al., 2005). Mn is eliminated primarily in the bile after it enters circulation. Ambient Mn may be trapped in the lungs where there is not an efficient pulmonary removal process for excess Mn (Andersen et al., 1999; Park et al., 2014). The primary metal exposure route was likely gastrointestinal rather than respiratory but further research is needed on the source of Mn in this population. Further, we cannot assess the correlation between BMn levels and respiratory tract Mn levels, which may differ if there are significant ambient sources of Mn. Our results are consistent with Mn exposure levels being within the homeostatic range (i.e. low enough to maintain it as a nutrient) given that higher Mn levels reduced wheezing risk. While excess Mn in the lungs has the potential to contribute to respiratory issues, Mn at homeostatic levels is well known to in facilitate the functionality of the antioxidant enzyme, Mn superoxide dismutase (MnSOD) (Sachdeva et al., 2019). Due to inflammation and oxidative stress being associated with asthma development, MnSOD’s role in protecting against oxidative stress and inflammation suggests it can support the prevention of respiratory outcomes (Holley et al., 2011).
There is a limited number of studies that examined sex differences in the association between metals and respiratory outcomes. Among them, the metal exposure analyzed was either mercury or cadmium. Cord blood mercury exposure was associated with persistent cough in males but not females at the 4-year follow-up visit in Spain (Carrasco et al., 2021). In this same study, the association between cord blood mercury exposure, wheeze and severe wheezing at 4-years old was slightly stronger in males though none of the results were statistically significant (Carrasco et al., 2021). Among Korean children, exposure to mercury in males at 7–8 years demonstrated an increased risk of incident asthma in contrast to females (Kim et al., 2015). However, the p-value for the interaction was not significant. Among children and adolescents in the United States, the association between urinary cadmium and mid-exhalation forced expiratory flow rate, suggested that cadmium may affect the respiratory system to a greater degree in males more than females (Madrigal et al., 2018). As gender-associated risk disparities exist in lung disease (Pinkerton et al., 2015), sex-specific models will be important at later life stages and in examining objective phenotype measures like lung function.
This study has several strengths. Our analyses included a large sample size of participants with longitudinal data collected rigorously over several years. We were able to assess associations between exposure to several metals and our outcomes. We used a well-validated instrument to assess respiratory outcomes (Mallol et al., 2010) (Mallol et al., 2000). We used mixed model logistic regression which enabled us to assess the association between prenatal metals and wheeze and asthma diagnosis across two study visits in the same multivariate model. This study also has some limitations. Respiratory symptoms were reported by caregivers which might subject to recall bias; however, epidemiological studies frequently use caregiver-reported wheeze (Akinbami et al., 2009; Mallol et al., 2010). Furthermore, the ISAAC is a standardized questionnaire that has been validated internationally and specifically in Spanish-speaking populations (Mallol et al., 2010) Future work should examine more definitive respiratory outcomes like lung function as these children continue to be followed. We only examined Pb and Mn exposure at two time points in pregnancy and we cannot rule out the impact of these exposures at other time points in the postnatal period. As with any observational study, we cannot rule out residual confounding due to unmeasured factors that may influence wheezing in childhood. PROGRESS is composed of urban, low-income families and our results may translate to other disadvantaged populations who face similar exposures to metals.
Conclusion
Prenatal exposure to Pb was associated with higher odds of ever wheeze while Mn was associated with decreased odds of current wheeze in childhood. Our results highlight the need to consider prenatal metal exposure and the role metal toxicity may play in programming respiratory disease starting prenatally. Additionally, future studies should investigate Mn as a nutrient that may assist in respiratory disease prevention. Enhanced knowledge of ubiquitous chemical risk factors can inform intervention strategies that may impact lung function or prevent the development and persistence of symptoms in childhood.
Supplementary Material
Acknowledgments:
This work was supported by the National Institute of Environmental Health Sciences grant, R00ES027496 (Rosa MJ, PI). The PROGRESS project has been supported by the following grants; R01ES014930, R01ES013744, R24ES028522, P30ES023515 (Wright RO, PI) and R01ES021357 (Baccarelli A and Wright RO, MPI). We are grateful to the PROGRESS participants and staff at the National Institute of Public Health/Ministry of Health of Mexico and the National Institute of Perinatology. We also thank the ABC (American British Cowdray Medical Center) in Mexico for providing some of the needed research facilities.
Footnotes
Conflict of interest
The authors declare that they have no known conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akinbami LJ, et al. , 2009. Status of Childhood Asthma in the United States, 1980–2007. Pediatrics. 123, S131–S145. [DOI] [PubMed] [Google Scholar]
- Andersen ME, et al. , 1999. Pharmacokinetic data needs to support risk assessments for inhaled and ingested manganese. Neurotoxicology. 20, 161–71. [PubMed] [Google Scholar]
- Asher MI, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys (vol 368, pg 733, 2006) Lancet, 370 (2007) 1128–1128 [DOI] [PubMed] [Google Scholar]
- Ashrap P, et al. , 2020. Maternal blood metal and metalloid concentrations in association with birth outcomes in Northern Puerto Rico. Environ Int. 138, 105606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila MH, et al. , 1991. Lead-Glazed Ceramics as Major Determinants of Blood Lead Levels in Mexican Women. Environmental Health Perspectives. 94, 117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B, et al. , 2013. Summary health statistics for U.S. children: National Health Interview Survey, 2012. Vital Health Stat 10. 1–81. [PubMed] [Google Scholar]
- Briana DD, Malamitsi-Puchner A, 2013. Small for gestational age birth weight: impact on lung structure and function. Paediatr Respir Rev. 14, 256–62. [DOI] [PubMed] [Google Scholar]
- Burris HH, et al. , 2013. Association between birth weight and DNA methylation of IGF2, glucocorticoid receptor and repetitive elements LINE-1 and Alu. Epigenomics. 5, 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, et al. , 2016. Early-life Exposure to Widespread Environmental Toxicants and Health Risk: A Focus on the Immune and Respiratory Systems. Ann Glob Health. 82, 119–31. [DOI] [PubMed] [Google Scholar]
- Carrasco P, et al. , 2021. Pre and postnatal exposure to mercury and respiratory health in preschool children from the Spanish INMA Birth Cohort Study. Sci Total Environ.782, 146654. [DOI] [PubMed] [Google Scholar]
- Chung SE, et al. , 2015. Maternal Blood Manganese and Early Neurodevelopment: The Mothers and Children’s Environmental Health (MOCEH) Study. Environ Health Perspect. 123, 717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus Henn B, et al. , 2017. Maternal and Cord Blood Manganese Concentrations and Early Childhood Neurodevelopment among Residents near a Mining-Impacted Superfund Site. Environ Health Perspect. 125, 067020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca G, et al. , 2010. Fetal and early postnatal life roots of asthma. J Matern Fetal Neonatal Med. 23 Suppl 3, 80–3. [DOI] [PubMed] [Google Scholar]
- Dorman DC, et al. , 2005. Maternal-fetal distribution of manganese in the rat following inhalation exposure to manganese sulfate. Neurotoxicology. 26, 625–32. [DOI] [PubMed] [Google Scholar]
- EPA US, Health Effects Support Document for Manganese (No. EPA 822-R-03–003) Office of Water (4304T); Health and Ecological Criteria Division, Washington D.C., 2003. [Google Scholar]
- Gern JE, et al. , 1999. Early life origins of asthma. J Clin Invest. 104, 837–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold LS, et al. , 2013. Level of asthma control and healthcare utilization in Latin America. Allergy. 68, 1463–1466. [DOI] [PubMed] [Google Scholar]
- Hanson M, Gluckman P, 2011. Developmental origins of noncommunicable disease: population and public health implications. Am J Clin Nutr. 94, 1754S–1758S. [DOI] [PubMed] [Google Scholar]
- Hanson MA, et al. , 2019. DOHaD - the challenge of translating the science to policy. J Dev Orig Health Dis. 10, 263–267. [DOI] [PubMed] [Google Scholar]
- Heyob KM, et al. , 2019. Maternal high-fat diet alters lung development and function in the offspring. 317, L167–L174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley AK, et al. , 2011. Manganese superoxide dismutase: guardian of the powerhouse. Int J Mol Sci. 12, 7114–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HH, et al. , 2015. Prenatal Particulate Air Pollution and Asthma Onset in Urban Children. Identifying Sensitive Windows and Sex Differences. Am J Respir Crit Care Med. 192, 1052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishak N, et al. , 2014. Does lung development differ in male and female fetuses? Exp Lung Res. 40, 30–9. [DOI] [PubMed] [Google Scholar]
- Jedrychowski W, et al. , 2011. Intrauterine exposure to lead may enhance sensitization to common inhalant allergens in early childhood: a prospective prebirth cohort study. Environ Res. 111, 119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomova K, Valko M, 2011. Advances in metal-induced oxidative stress and human disease. Toxicology. 283, 65–87. [DOI] [PubMed] [Google Scholar]
- Jung CR, et al. , 2019. Fine particulate matter exposure during pregnancy and infancy and incident asthma. J Allergy Clin Immunol. 143, 2254–2262.e5. [DOI] [PubMed] [Google Scholar]
- Kajekar R, 2007. Environmental factors and developmental outcomes in the lung. Pharmacol Ther. 114, 129–45. [DOI] [PubMed] [Google Scholar]
- Kasten-Jolly J, Lawrence DA, 2017. Sex-specific effects of developmental lead exposure on the immune-neuroendocrine network. Toxicol Appl Pharmacol. 334, 142–157. [DOI] [PubMed] [Google Scholar]
- Kim J, et al. , 2019. Prenatal Exposure to Lead and Chromium is Associated with IL-13 Levels in Umbilical Cord Blood and Severity of Atopic Dermatitis: COCOA Study. Immune Netw. 19, e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KN, et al. , 2015. Low-level Mercury Exposure and Risk of Asthma in School-age Children. Epidemiology. 26, 733–9. [DOI] [PubMed] [Google Scholar]
- Lai PY, et al. , 2019. Adverse early-life environment impairs postnatal lung development in mice. Physiol Genomics. 51, 462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PD, et al. , 2021. Diet and erythrocyte metal concentrations in early pregnancy-cross-sectional analysis in Project Viva. Am J Clin Nutr.114, 540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little BB, et al. , 2017. Blood lead levels, pulmonary function and agility in Polish schoolchildren. Ann Hum Biol. 44, 723–728. [DOI] [PubMed] [Google Scholar]
- Madrigal JM, et al. , 2018. Association of heavy metals with measures of pulmonary function in children and youth: Results from the National Health and Nutrition Examination Survey (NHANES). Environ Int. 121, 871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallol J, et al. , 2000. Prevalence of asthma symptoms in Latin America: the International Study of Asthma and Allergies in Childhood (ISAAC). Pediatr Pulmonol. 30, 439–44. [DOI] [PubMed] [Google Scholar]
- Mallol J, et al. , 2010. Regional Variation in Asthma Symptom Prevalence in Latin American Children. Journal of Asthma. 47, 644–650. [DOI] [PubMed] [Google Scholar]
- Mazari-Hiriart M, et al. , 2019. Challenges and Opportunities on Urban Water Quality in Mexico City. Frontiers in Environmental Science. 7. [Google Scholar]
- Melen E, Guerra S, 2017. Recent advances in understanding lung function development. F1000Res. 6, 726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min JY, et al. , 2008. Blood lead levels and increased bronchial responsiveness. Biol Trace Elem Res. 123, 41–6. [DOI] [PubMed] [Google Scholar]
- Mullin AM, et al. , 2019. Maternal blood arsenic levels and associations with birth weight-for-gestational age. Environ Res. 177, 108603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nriagu JO, 1988. A silent epidemic of environmental metal poisoning? Environ Pollut. 50, 139–61. [DOI] [PubMed] [Google Scholar]
- Pantic I, et al. , 2018. Children’s Blood Lead Concentrations from 1988 to 2015 in Mexico City: The Contribution of Lead in Air and Traditional Lead-Glazed Ceramics. Int J Environ Res Public Health. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park RM, et al. , 2014. Respiratory manganese particle size, time-course and neurobehavioral outcomes in workers at a manganese alloy production plant. Neurotoxicology. 45, 276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce G, et al. , 2021. Foetal exposure to heavy metals and risk of atopic diseases in early childhood. Pediatr Allergy Immunol. 32, 242–250. [DOI] [PubMed] [Google Scholar]
- Pinkerton KE, et al. , 2015. Women and Lung Disease. Sex Differences and Global Health Disparities. Am J Respir Crit Care Med. 192, 11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkerton KE, Joad JP, 2006. Influence of air pollution on respiratory health during perinatal development. Clin Exp Pharmacol Physiol. 33, 269–72. [DOI] [PubMed] [Google Scholar]
- Rodosthenous RS, et al. , 2017. Prenatal lead exposure and fetal growth: Smaller infants have heightened susceptibility. Environment International. 99, 228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa MJ, et al. , 2016. Association between personal exposure to ambient metals and respiratory disease in Italian adolescents: a cross-sectional study. BMC Pulm Med. 16, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa MJ, et al. , 2018. Evidence establishing a link between prenatal and early-life stress and asthma development. Curr Opin Allergy Clin Immunol. 18, 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva K, et al. , 2019. Environmental Exposures and Asthma Development: Autophagy, Mitophagy, and Cellular Senescence. Front Immunol. 10, 2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders AP, et al. , 2015. Perinatal and Childhood Exposure to Cadmium, Manganese, and Metal Mixtures and Effects on Cognition and Behavior: A Review of Recent Literature. Curr Environ Health Rep. 2, 284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen SO, et al. , 2004. Umbilical cord trace elements and minerals and risk of early childhood wheezing and eczema. Eur Respir J. 24, 292–7. [DOI] [PubMed] [Google Scholar]
- Signes-Pastor AJ, et al. , 2019. Prenatal exposure to metal mixture and sex-specific birth outcomes in the New Hampshire Birth Cohort Study. Environ Epidemiol. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G, et al. , 2018. Effects of developmental lead exposure on the hippocampal methylome: Influences of sex and timing and level of exposure. Toxicol Lett. 290, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LJ, et al. , 2010. Normal development of of the lung and premature birth. Paediatr Respir Rev. 11, 135–42. [DOI] [PubMed] [Google Scholar]
- Stick S, 2000. Pediatric origins of adult lung disease. 1. The contribution of airway development to paediatric and adult lung disease. Thorax. 55, 587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamayo YOM, et al. , 2016. Longitudinal associations of age and prenatal lead exposure on cortisol secretion of 12–24 month-old infants from Mexico City. Environ Health. 15, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez-Rojo MM, et al. , 2019. [National report of blood lead levels and lead-glazed ceramics use in vulnerable children.]. Salud Publica Mex. 61, 787–797. [DOI] [PubMed] [Google Scholar]
- Torday JS, Nielsen HC, 1987. The sex difference in fetal lung surfactant production. Exp Lung Res. 12, 1–19. [DOI] [PubMed] [Google Scholar]
- Wang IJ, et al. , 2017. Lead exposure, IgE, and the risk of asthma in children. J Expo Sci Environ Epidemiol. 27, 478–483. [DOI] [PubMed] [Google Scholar]
- Wells EM, et al. , 2014. The relationship of blood lead with immunoglobulin E, eosinophils, and asthma among children: NHANES 2005–2006. International Journal of Hygiene and Environmental Health. 217, 196–204. [DOI] [PubMed] [Google Scholar]
- Winterbottom EF, et al. , 2019. Prenatal arsenic exposure alters the placental expression of multiple epigenetic regulators in a sex-dependent manner. Environ Health. 18, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng GN, et al. , 2013. Association between lung function in school children and exposure to three transition metals from an e-waste recycling area. Journal of Exposure Science and Environmental Epidemiology. 23, 67–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


