Abstract
Background
This is one of a series of reviews of cervical ripening and labour induction using standardised methodology. Misoprostol administered by the oral and sublingual routes have the advantage of rapid onset of action, while the sublingual and vaginal routes have the advantage of prolonged activity and greatest bioavailability.
Objectives
To determine the effectiveness and safety of misoprostol administered buccally or sublingually for third trimester cervical ripening and induction of labour.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (8 December 2003), the Cochrane Central Register of Controlled Trials (The Cochrane Library, Issue 4, 2003), and bibliographies of relevant papers.
We updated the search of the Cochrane Pregnancy and Childbirth Group's Trials Register on 28 July 2009 and added the results to the awaiting classification section.
Selection criteria
Randomised controlled trials comparing buccal or sublingual misoprostol used for third trimester cervical ripening or labour induction with placebo/no treatment or other methods listed above it on a predefined list of labour induction methods.
Data collection and analysis
A generic strategy was developed to deal with the large volume and complexity of trial data relating to labour induction. Data were extracted onto standardized forms, checked for accuracy, and analysed using RevMan software.
Main results
Three studies (502 participants) compared buccal/sublingual misoprostol respectively with a vaginal regimen (200 µg versus 50 µg) and with oral administration (50 versus 50 µg and 50 versus 100µg).
The buccal route was associated with a trend to fewer caesarean sections than with the vaginal route (18/73 versus 28/79; relative risk (RR) 0.70; 95% confidence interval (CI) 0.42 to 1.15). There were no significant differences in any other outcomes.
When the same dosage was used sublingually versus orally, the sublingual route was associated with less failures to achieve vaginal delivery within 24 hours (12/50 versus 19/50; RR 0.63, 95% CI 0.34 to 1.16), reduced oxytocin augmentation (17/50 versus 23/50; RR 0.74, 95% CI 0.45 to 1.21) and reduced caesarean section (8/50 versus 15/50; RR 0.53, 95% CI 0.25 to 1.14), but the differences were not statistically significant.
When a smaller dose was used sublingually than orally, there were no differences in any of the outcomes.
Authors' conclusions
Based on only three small trials, sublingual misoprostol appears to be at least as effective as when the same dose is administered orally. There are inadequate data to comment on the relative complications and side‐effects. Sublingual or buccal misoprostol should not enter clinical use until its safety and optimal dosage have been established by larger trials.
[Note: The 17 citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
Keywords: Female; Humans; Pregnancy; Cervical Ripening; Administration, Oral; Administration, Sublingual; Labor, Induced; Labor, Induced/methods; Misoprostol; Misoprostol/administration & dosage; Oxytocics; Oxytocics/administration & dosage; Randomized Controlled Trials as Topic
Plain language summary
Buccal or sublingual misoprostol for cervical ripening and induction of labour
Not enough evidence to say if misoprostol administered under the tongue or in the cheek is safe for induction of labour.
Sometimes labour is started artificially (induction) because of concerns for the well‐being of either the baby or the mother. A drug called misoprostol has previously been used either by being put in the mother's vagina or by being swallowed. It is now suggested that placing it under the tongue or in the cheek may be more effective. There were not enough studies to say whether there might be important adverse effects. More research has been called for.
Background
Sometimes clinicians and the woman decide to bring on labour artificially because of safety concerns for the mother or baby. These are balanced against the possible disadvantages of labour induction such as discomfort, conflict with the woman's expectations, cascading interventions and specific complications or side‐effects of the method chosen. This review is one of a series of reviews of methods of labour induction using a standardised protocol. For more detailed information on the rationale for this methodological approach, please refer to the currently published 'generic' protocol (Hofmeyr 2003a). The generic protocol describes how a number of standardised reviews will be combined to compare various methods of preparing the cervix of the uterus and inducing labour.
Misoprostol is an orally active prostaglandin E1 analogue, which is not registered for use in pregnancy. It has been widely used off‐label for termination of pregnancy, labour induction and the management of the third stage of labour. Clinical issues related to its use for labour induction have been covered more fully in the accompanying reviews of oral (Alfirevic 2003) and vaginal (Hofmeyr 2003b) misoprostol for labour induction.
Several routes of administration of misoprostol have been studied, including oral (swallowed), vaginal (inserted into the vagina as a tablet or gel), rectal (inserted into the rectum as a tablet), buccal or sublingual (the tablet held in the cheek or under the tongue respectively). In the absence of pharmacokinetic studies during labour, data from early pregnancy are the best available information on comparative drug profiles following administration by different routes. Zieman 1997 compared vaginal and oral misoprostol administration. Twenty women were randomized. Ten of these women were pregnant and undergoing first‐trimester pregnancy terminations and the last ten were not pregnant. The reported mean peak serum concentrations after 400 µg of misoprostol were 277 +/‐ 124 pg/ml and 165 +/‐ 86 pg/ml in the oral and the vaginal groups respectively (p = 0.03) and the times to peak levels were 34 +/‐ 17 compared with 80 +/‐ 27 minutes in the respective groups (p < 0.001). The prolonged serum concentrations in the vaginal group suggest a longer dosing interval when this route is used.
Danielsson 1999 studied plasma misoprostol levels in 18 women after administration of 200 or 400 µg misoprostol orally or vaginally in the first trimester of pregnancy. Peak levels occurred 30 minutes after oral and one to two hours after vaginal administration.
Tang 2002 compared the pharmacokinetic parameters of four different routes, sublingual, oral, vaginal and vaginal with addition of water, in 40 pregnant women undergoing termination of pregnancy by suction evacuation. The highest peak serum concentration after administration of 400 µg was found in the sublingual group (574.8 +/‐ 250.7 pg/ml) followed by the oral group (287.6 +/‐ 144.3 pg/ml), the vaginal with addition of water group (162.8 +/‐ 57.1 pg/ml) and the vaginal group (125 +/‐ 53.8 pg/ml). The time to peak concentration was shorter in the sublingual and oral routes (26.0 +/‐ 11.5 minutes and 27.5 +/‐ 14.8 minutes respectively).
Based on the above studies, the oral and sublingual routes have the advantage of rapid onset of action, while the sublingual and vaginal routes have the advantage of prolonged activity and greatest bioavailability. The increased bioavailability is thought to be contributed to by the avoidance of the first pass intestinal‐hepatic circulation with these routes. As clearance of the drug is likely to be rapid irrespective of the route of administration, the prolonged activity of the vaginal and sublingual routes is presumably due to continued absorption over a long period of time. It would therefore be subject to the retention of the tablet in the respective site over a long time.
Clinical trials in the first, second and third trimester of pregnancy have shown that, at equivalent dosage, the vaginal route produces greater clinical efficacy than the oral route. This may in part be due to avoidance of metabolism during the first pass circulation through the liver which occurs with the oral route, as well as slower absorption vaginally. In the third trimester, this increased efficacy has been associated with increased uterine hyperstimulation at vaginal doses exceeding 25 micrograms four‐hourly (Hofmeyr 1999; Hofmeyr 2003b). It has been suggested that this excessive effect might be due to direct effects of vaginal misoprostol on the cervix.
The buccal route for misoprostol in labour third stage management was used in a pilot study of 70 women in 1996 (Hofmeyr 1998). More recently, there has been interest in the sublingual route for labour induction (Shetty 2002), on the assumption that avoidance of the first pass hepatic circulation would yield bioavailability similar to that achieved with the vaginal route. An additional possible advantage was that avoidance of direct cervical effects might reduce the risk of uterine hyperstimulation. In a pilot study of labour induction in 100 women, 50 micrograms of misoprostol sublingually appeared to have better efficacy than the same dose orally, with no demonstrable increase in uterine hyperstimulation (Shetty 2002).
This review will focus on the effectiveness and safety of misoprostol administered buccally or sublingually for cervical ripening and labour induction in the third trimester of pregnancy.
Objectives
To determine, from the best available evidence, the effectiveness and safety of misoprostol administered buccally or sublingually for third trimester cervical ripening and induction of labour.
Methods
Criteria for considering studies for this review
Types of studies
Clinical trials which included some form of random allocation to either group; they reported one or more of the prestated outcomes; reasonable measures were taken to ensure allocation concealment; and violations of allocated management were not sufficient to materially affect outcomes.
Types of participants
Pregnant women due for third trimester induction of labour, carrying a viable fetus. Predefined subgroup analyses (see list below): previous caesarean section or not; nulliparity or multiparity; membranes intact or ruptured, and cervix unfavourable, favourable or undefined. Only those outcomes with data will appear in the analysis tables.
Types of interventions
Buccal or sublingual administration of misoprostol compared with placebo/no treatment or any other method above it on a predefined list of methods of labour induction (see 'Methods of the review').
Types of outcome measures
Clinically relevant outcomes for trials of methods of cervical ripening/labour induction have been prespecified by two authors of labour induction reviews (Justus Hofmeyr and Zarko Alfirevic).
Five primary outcomes were chosen as being most representative of the clinically important measures of effectiveness and complications. Subgroup analyses are limited to the primary outcomes: (1) vaginal delivery not achieved within 24 hours; (2) uterine hyperstimulation with fetal heart rate (FHR) changes; (3) caesarean section; (4) serious neonatal morbidity or perinatal death (e.g. seizures, birth asphyxia defined by trialists, neonatal encephalopathy, disability in childhood); (5) serious maternal morbidity or death (e.g. uterine rupture, admission to intensive care unit, septicemia).
Perinatal and maternal morbidity and mortality are composite outcomes. This is not an ideal solution because some components are clearly less severe than others. It is possible for one intervention to cause more deaths but less severe morbidity. However, in the context of labour induction at term this is unlikely. All these events will be rare, and a modest change in their incidence will be easier to detect if composite outcomes are presented. The incidence of individual components will be explored as secondary outcomes (see below).
Secondary outcomes relate to measures of effectiveness, complications and satisfaction.
Measures of effectiveness: (6) cervix unfavourable/unchanged after 12 to 24 hours; (7) oxytocin augmentation.
Complications: (8) uterine hyperstimulation without FHR changes; (9) uterine rupture; (10) epidural analgesia; (11) instrumental vaginal delivery; (12) meconium stained liquor; (13) Apgar score less than seven at five minutes; (14) neonatal intensive care unit admission; (15) neonatal encephalopathy; (16) perinatal death; (17) disability in childhood; (18) maternal side‐effects (all); (19) maternal nausea; (20) maternal vomiting; (21) maternal diarrhoea; (22) other maternal side‐effects; (23) postpartum haemorrhage (as defined by the trial authors); (24) serious maternal complications (e.g. intensive care unit admission, septicaemia but excluding uterine rupture); (25) maternal death.
Measures of satisfaction: (26) woman not satisfied; (27) caregiver not satisfied.
'Uterine rupture' will include all clinically significant ruptures of unscarred or scarred uteri. Trivial scar dehiscence noted incidentally at the time of surgery will be excluded.
Additional outcomes may appear in individual primary reviews, but will not contribute to the secondary reviews.
While all the above outcomes will be sought, only those with data will appear in the analysis tables.
The terminology of uterine hyperstimulation is problematic (Curtis 1987). In this review we will use the term 'uterine hyperstimulation without FHR changes' to include uterine tachysystole (greater than five contractions per 10 minutes for at least 20 minutes) and uterine hypersystole/hypertonus (a contraction lasting at least two minutes) and 'uterine hyperstimulation with FHR changes' to denote uterine hyperstimulation syndrome (tachysystole or hypersystole with fetal heart rate changes such as persistent decelerations, tachycardia or decreased short‐term variability). However, due to varied reporting there is the possibility of subjective bias in interpretation of these outcomes. Also, it is not always clear from trials if these outcomes are reported in a mutually exclusive manner.
Outcomes will be included in the analysis if: reasonable measures were taken to minimise observer bias; missing data were insufficient to materially influence conclusions; and data were available for analysis according to original allocation.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (8 December 2003).
We updated this search on 28 July 2009 and added the results to Studies awaiting classification.
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
The initial search was performed simultaneously for all reviews of methods of inducing labour, as outlined in the generic protocol for these reviews (Hofmeyr 2003a).
In addition, we searched CENTRAL (The Cochrane Library 2003, Issue 4) using the term 'misoprostol'.
Searching other resources
We searched the reference lists of trial reports and reviews by hand.
We did not apply any language restrictions.
Data collection and analysis
A strategy has been developed to deal with the large volume and complexity of trial data relating to labour induction. Many methods have been studied, in many different categories of women undergoing labour induction. Most trials are intervention‐driven, comparing two or more methods in various categories of women. Clinicians and parents need the data arranged by category of woman, to be able to choose which method is best for a particular clinical scenario. To extract these data from several hundred trial reports in a single step would be very difficult. We have therefore developed a two‐stage method of data extraction. The initial data extraction is done in a series of primary reviews arranged by methods of induction of labour, following a standardised methodology. The data are then extracted from the primary reviews into a series of secondary reviews, arranged by category of woman.
To avoid duplication of data in the primary reviews, the labour induction methods have been listed in a specific order, from one to 25. Each primary review includes comparisons between one of the methods (from two to 25) with only those methods above it on the list. Thus, the review of intravenous oxytocin (4) will include only comparisons with intracervical prostaglandins (3), vaginal prostaglandins (2) or placebo (1). Methods identified in the future will be added to the end of the list. The current list is as follows: (1) placebo/no treatment; (2) vaginal prostaglandins; (3) intracervical prostaglandins; (4) intravenous oxytocin; (5) amniotomy; (6) intravenous oxytocin with amniotomy; (7) vaginal misoprostol; (8) oral misoprostol; (9) mechanical methods including extra‐amniotic Foley catheter; (10) membrane sweeping; (11) extra‐amniotic prostaglandins; (12) intravenous prostaglandins; (13) oral prostaglandins; (14) mifepristone; (15) estrogens; (16) estrogens with amniotomy; (17) corticosteroids; (18) relaxin; (19) hyaluronidase; (20) castor oil, bath, and/or enema; (21) acupuncture; (22) breast stimulation; (23) sexual intercourse; (24) homoeopathic methods; (25) buccal or sublingual misoprostol; (26) hypnosis.
The primary reviews are analysed by the following subgroups: (1) previous caesarean section or not; (2) nulliparity or multiparity; (3) membranes intact or ruptured; (4) cervix favourable, unfavourable or undefined.
The secondary reviews will include all methods of labour induction for each of the categories of women for which subgroup analysis has been done in the primary reviews, and will include only five primary outcome measures. There will thus be six secondary reviews of methods of labour induction in the following groups of women: (1) nulliparous, intact membranes (unfavourable cervix, favourable cervix, cervix not defined); (2) nulliparous, ruptured membranes (unfavourable cervix, favourable cervix, cervix not defined); (3) multiparous, intact membranes (unfavourable cervix, favourable cervix, cervix not defined); (4) multiparous, ruptured membranes (unfavourable cervix, favourable cervix, cervix not defined); (5) previous caesarean section, intact membranes (unfavourable cervix, favourable cervix, cervix not defined); (6) previous caesarean section, ruptured membranes (unfavourable cervix, favourable cervix, cervix not defined).
Each time a primary review is updated with new data, those secondary reviews which include data which have changed, will also be updated.
The trials included in the primary reviews were extracted from an initial set of trials covering all interventions used in induction of labour (see above for details of search strategy). The initial data extraction process was conducted centrally. This was co‐ordinated from the Clinical Effectiveness Support Unit (CESU) at the Royal College of Obstetricians and Gynaecologists, UK, in co‐operation with the Pregnancy and Childbirth Group of The Cochrane Collaboration. This process allowed the data extraction process to be standardised across all the reviews.
The trials were initially reviewed on eligibility criteria, using a standardised form and the basic selection criteria specified above. Following this, data were extracted to a standardised data extraction form which was piloted for consistency and completeness. The pilot process involved the researchers at the CESU and previous reviewers in the area of induction of labour.
Information was extracted regarding the methodological quality of trials on a number of levels. This process was completed without consideration of trial results. Assessment of selection bias examined the process involved in the generation of the random sequence and the method of allocation concealment separately. These were then judged as adequate or inadequate using the criteria described in the Cochrane Reviewers' Handbook (Clarke 2000).
Performance bias was regarded as adequate if the subjects, caregivers and assessors were blinded to the group allocation.
Individual outcome data are included in the analysis if they met the prestated criteria in 'Types of outcome measures'. Included trial data were processed as described in the Cochrane Reviewers' Handbook (Clarke 2000). Data extracted from the trials were analysed on an intention‐to‐treat basis (when this is not done in the original report, re‐analysis was performed if possible). Where data were missing, clarification was sought from the original authors. Exclusion of attrition bias was regarded as inadequate if the attrition was such that it might significantly affect the results. This decision rested with the reviewers of primary reviews and is clearly documented. Once missing data become available, they will be included in the analyses.
Once the data had been extracted, they were checked for accuracy, and analysed as above using the Review Manager software (RevMan 2002). For dichotomous data, relative risks and 95% confidence intervals are calculated, and in the absence of heterogeneity, results were pooled using a fixed effect model.
The predefined criteria for sensitivity analysis were adequate exclusion of selection, performance and attrition bias.
Because a wide range of misoprostol dosages and dosing intervals may be used, the included study identifiers for this review will be coded with a prefix to give an approximation of the dosage of misoprostol received in the first six hours, calculated as follows: initial dose + (s x (6 ‐ interval)/4), where 's' is a subsequent dose within six hours, and 'interval' is the interval between the first and subsequent doses given in less than six hours. For example, 50 µg four‐hourly would be code '075'. This approximation is based on the assumption that buccal or sublingual misoprostol is absorbed reasonably consistently over four hours. It is intended as no more than a crude ranking of the various dosage regimens used. A similar approximation is used in the review of vaginal misoprostol for labour induction (Hofmeyr 2003b), but would not be appropriate for oral misoprostol (Alfirevic 2003), which has a high peak and short half‐life in the circulation. This ranking allows readers to view the results ranked in terms of approximate dosage of misoprostol used. It does not exclude the possibility of subgroup analysis by dosage categories, but at present too little is known about the dosage for buccal misoprostol to determine meaningful cut‐off points for subgroups.
Primary analysis were limited to the prespecified outcomes and subgroup analyses. In the event of differences in unspecified outcomes or subgroups being found, these were analysed post hoc, but clearly identified as such to avoid drawing unjustified conclusions.
Results
Description of studies
Four studies were identified. Three were included. A fourth study (Todd 2002), which compared buccal misoprostol with laminaria insertion, was not considered because it was used for first and second trimester termination of pregnancy rather than induction of labour in the third trimester. (Seventeen reports from an updated search in July 2009 have been added to Studies awaiting classification.)
Three studies compared buccal/sublingual misoprostol with another method of misoprostol administration. Two studies compared sublingual with oral misoprostol four‐hourly: 075 Shetty 2002b compared equal doses of misoprostol, 50 µg sublingually versus 50 µg orally. 075 Shetty 2002a compared 50 µg sublingually with 100 µg orally. The third study (200 Carlan 2002) compared buccal misoprostol (initial two doses 200µg then 300µg) with vaginal misoprostol (initial two doses 50µg, then 100µg), for a maximum of six doses. See table of 'Characteristics of included studies' for details.
Risk of bias in included studies
All three studies used computer‐generated random sequence to generate a series of opaque, sealed envelopes. In 200 Carlan 2002, four women in the buccal and one in the vaginal group were found to be in labour and did not receive misoprostol. One woman was excluded after randomisation in the 075 Shetty 2002a for breech presentation, and none in Shetty 2002B (075 Shetty 2002b). None of the trials were blinded, so that performance bias was not effectively excluded. Overall, the studies were of reasonable quality.
Effects of interventions
Three studies with results on 502 participants are included.
Buccal versus vaginal misoprostol (initial doses 200 µg versus 50 µg) Only one study with 152 women analysed was included (200 Carlan 2002). The buccal route was associated with slightly fewer caesarean sections (18/73 versus 28/79; relative risk (RR) 0.70; 95% confidence interval (CI) 0.42 to 1.15), but this difference was not statistically significant. There were no significant differences in any other outcomes. The numbers with instrumental vaginal deliveries and five‐minute Apgar scores below seven were too few for meaningful statistical analysis.
Sublingual versus oral misoprostol Two studies were included (075 Shetty 2002a; 075 Shetty 2002b). When the same dosage was used by both routes, the sublingual route was associated with less failures to achieve vaginal delivery within 24 hours (12/50 versus 19/50; RR 0.63, 95% CI 0.34 to 1.16), less oxytocin augmentation (17/50 versus 23/50; RR 0.74, 95% CI 0.45 to 1.21) and less caesarean section (8/50 versus 15/50; RR 0.53, 95% CI 0.25 to 1.14). However, none of these differences reached statistical significance.
When a smaller dose was used sublingually than orally, there were no differences in any of the outcomes.
Overall, there were also no significant differences between two routes. In both subgroups, the numbers with uterine hyperstimulation with and without fetal heart rate changes and Apgar scores less than seven at five minutes were too few for meaningful statistical analysis.
Discussion
The results of this review are based on only three small trials. They should therefore be interpreted with caution. It is clear from these studies that sublingual misoprostol is an effective route of administration for induction of labour. Although there are no placebo‐controlled trials, the sublingual route was at least as effective as a similar dose orally, and the oral route has be shown to be effective (Alfirevic 2003). There are inadequate data to comment on the relative complications and side‐effects.
Authors' conclusions
Implications for practice.
There are insufficient data on safety for this route to be recommended for clinical use outside of further research protocols. It should be emphasised that the buccal/sublingual route produces greater bioavailability than other routes, and great caution should be exercised in its use. Dosages previously shown to be relatively safe with other routes of administration may not be safe when given buccally or sublingually.
Implications for research.
Pharmacokinetic studies have shown that the buccal or sublingual route of administration is associated with rapid onset (similar to the oral route and more rapid than the vaginal route) and greater bioavailability than other routes. These studies have focussed on the average effectiveness of different routes. In the context of labour induction, however, because of the risks of hyperstimulation, consistency of effect is more important than average effectiveness, as the latter can be adjusted with different dosages. We would therefore recommend that pharmacokinetic studies consider the consistency rather than only the effectiveness of various routes of administration.
More clinical research is need to establish the optimal dosage regimen for the buccal or sublingual route, its relative effectiveness and safety compared with other routes and other methods of labour induction, and its acceptability for women.
[Note: The 17 citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
What's new
| Date | Event | Description |
|---|---|---|
| 28 July 2009 | Amended | Search updated. Seventeen reports added to Studies awaiting classification. |
History
Protocol first published: Issue 2, 2003 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 31 October 2008 | Amended | Converted to new review format. |
Notes
This review conforms to the standardised methodology for reviews of methods of labour induction as described in the generic protocol ‐ see 'Methods for cervical ripening and labour induction in late pregnancy: generic protocol'.
Acknowledgements
Zarko Alfirevic, Jim Neilson and Sonja Henderson; Tony Kelly and Josephine Kavanagh (Clinical Effectiveness Support Unit, Royal College of Obstetricians and Gynaecologists) for contributions to development of the generic protocol for reviews of labour induction.
Data and analyses
Comparison 1. Subligual/buccal vs vaginal misoprostol: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.91, 1.63] |
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.80, 2.71] |
| 3 Caesarean section | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.42, 1.15] |
| 5 Serious maternal morbidity or death | 1 | 152 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12‐24 hours | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.24 [0.13, 78.38] |
| 7 Oxytocin augmentation | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.84, 1.34] |
| 10 Epidural analgesia | 1 | 152 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.57, 2.56] |
| 11 Instrumental vaginal delivery | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.10, 1.28] |
| 13 Apgar score < 7 at 5 minutes | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.73 [0.53, 177.64] |
| 14 Neonatal intensive care unit admission | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.54, 2.64] |
| 24 Serious maternal complication | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
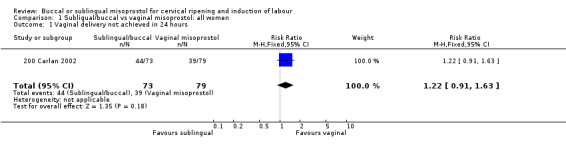
Comparison 1 Subligual/buccal vs vaginal misoprostol: all women, Outcome 1 Vaginal delivery not achieved in 24 hours.
1.2. Analysis.
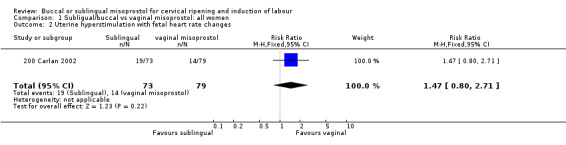
Comparison 1 Subligual/buccal vs vaginal misoprostol: all women, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
1.3. Analysis.
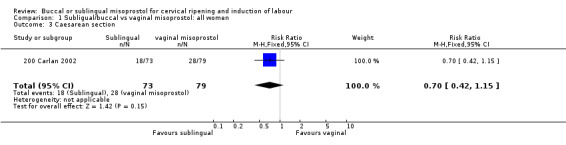
Comparison 1 Subligual/buccal vs vaginal misoprostol: all women, Outcome 3 Caesarean section.
1.5. Analysis.
Comparison 1 Subligual/buccal vs vaginal misoprostol: all women, Outcome 5 Serious maternal morbidity or death.
1.6. Analysis.
Comparison 1 Subligual/buccal vs vaginal misoprostol: all women, Outcome 6 Cervix unfavourable/unchanged after 12‐24 hours.
1.7. Analysis.
Comparison 1 Subligual/buccal vs vaginal misoprostol: all women, Outcome 7 Oxytocin augmentation.
1.10. Analysis.
Comparison 1 Subligual/buccal vs vaginal misoprostol: all women, Outcome 10 Epidural analgesia.
1.11. Analysis.
Comparison 1 Subligual/buccal vs vaginal misoprostol: all women, Outcome 11 Instrumental vaginal delivery.
1.13. Analysis.
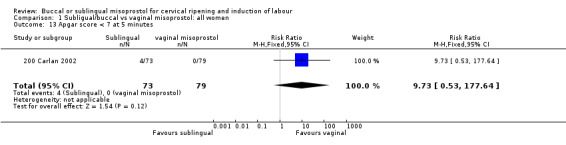
Comparison 1 Subligual/buccal vs vaginal misoprostol: all women, Outcome 13 Apgar score < 7 at 5 minutes.
1.14. Analysis.
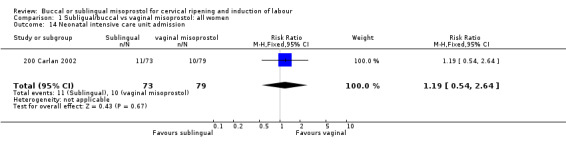
Comparison 1 Subligual/buccal vs vaginal misoprostol: all women, Outcome 14 Neonatal intensive care unit admission.
1.24. Analysis.
Comparison 1 Subligual/buccal vs vaginal misoprostol: all women, Outcome 24 Serious maternal complication.
Comparison 10. Subligual/buccal vs oral misoprostol: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 2 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.68, 1.11] |
| 1.1 Same dose | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.34, 1.16] |
| 1.2 Bigger dose orally | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.73, 1.22] |
| 2 Uterine hyperstimulation with fetal heart rate changes | 2 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.28, 6.96] |
| 2.1 Same dose | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.92] |
| 2.2 Bigger dose orally | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.14, 6.93] |
| 3 Caesarean section | 2 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.57, 1.19] |
| 3.1 Same dose | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.25, 1.14] |
| 3.2 Bigger dose orally | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.63, 1.47] |
| 6 Cervix unfavourable/unchanged after 12‐24 hours | 2 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.03, 1.14] |
| 6.1 Same dose | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.60] |
| 6.2 Bigger dose orally | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.40] |
| 7 Oxytocin augmentation | 2 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.68, 1.07] |
| 7.1 Same dose | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.45, 1.21] |
| 7.2 Bigger dose orally | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.70, 1.16] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [0.49, 12.54] |
| 8.1 Same dose | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Bigger dose orally | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [0.49, 12.54] |
| 10 Epidural analgesia | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.66, 1.29] |
| 10.1 Same dose | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Bigger dose orally | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.66, 1.29] |
| 11 Instrumental vaginal delivery | 2 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.87, 1.88] |
| 11.1 Same dose | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.66, 3.72] |
| 11.2 Bigger dose orally | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.78, 1.86] |
| 13 Apgar score < 7 at 5 minutes | 2 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.68] |
| 13.1 Same dose | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Bigger dose orally | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.68] |
| 14 Neonatal intensive care unit admission | 2 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.44, 1.47] |
| 14.1 Same dose | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.27, 2.55] |
| 14.2 Bigger dose orally | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.39, 1.63] |
10.1. Analysis.
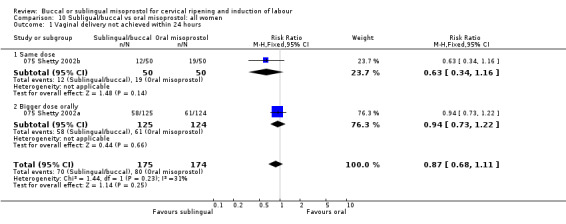
Comparison 10 Subligual/buccal vs oral misoprostol: all women, Outcome 1 Vaginal delivery not achieved within 24 hours.
10.2. Analysis.
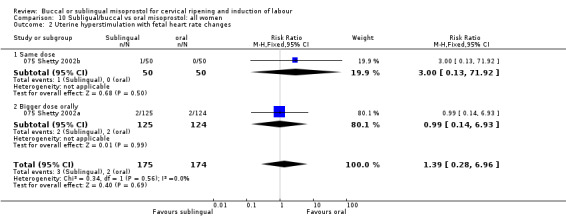
Comparison 10 Subligual/buccal vs oral misoprostol: all women, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
10.3. Analysis.
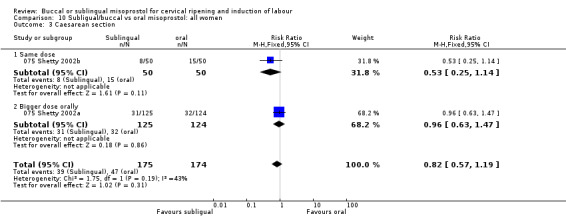
Comparison 10 Subligual/buccal vs oral misoprostol: all women, Outcome 3 Caesarean section.
10.6. Analysis.
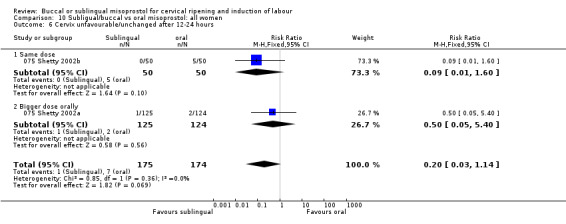
Comparison 10 Subligual/buccal vs oral misoprostol: all women, Outcome 6 Cervix unfavourable/unchanged after 12‐24 hours.
10.7. Analysis.
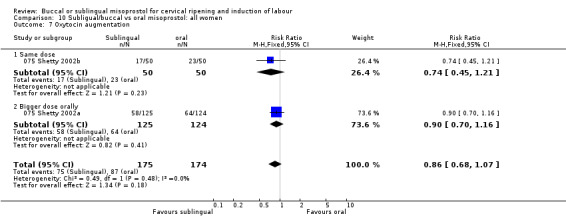
Comparison 10 Subligual/buccal vs oral misoprostol: all women, Outcome 7 Oxytocin augmentation.
10.8. Analysis.
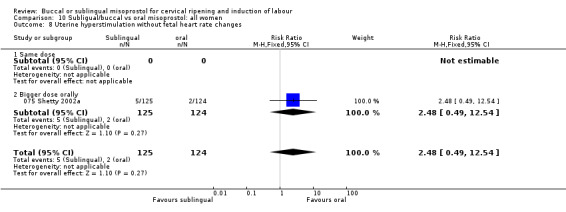
Comparison 10 Subligual/buccal vs oral misoprostol: all women, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
10.10. Analysis.
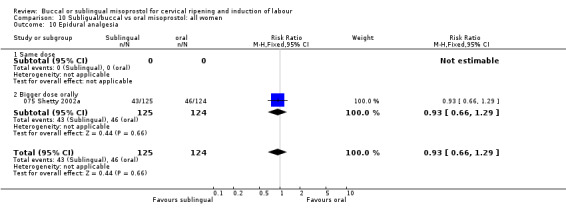
Comparison 10 Subligual/buccal vs oral misoprostol: all women, Outcome 10 Epidural analgesia.
10.11. Analysis.
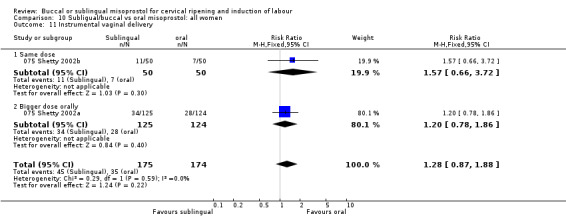
Comparison 10 Subligual/buccal vs oral misoprostol: all women, Outcome 11 Instrumental vaginal delivery.
10.13. Analysis.
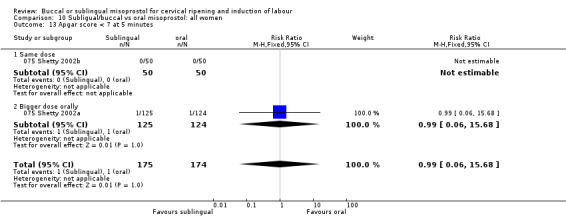
Comparison 10 Subligual/buccal vs oral misoprostol: all women, Outcome 13 Apgar score < 7 at 5 minutes.
10.14. Analysis.
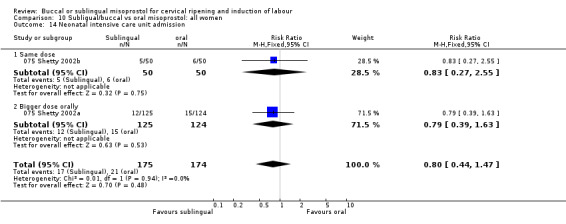
Comparison 10 Subligual/buccal vs oral misoprostol: all women, Outcome 14 Neonatal intensive care unit admission.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
075 Shetty 2002a.
| Methods | Non‐blinded randomised comparative trial. Computer‐generated sequence in sealed opaque envelopes. | |
| Participants | 250 women at term with indications for labour induction. Inclusion criteria: term, parity 0‐5, unfavourable cervix. Exclusion criteria: previous caesarean section, parity > 5. | |
| Interventions | 50 µg misoprostol sublingually every 4 hrs vs 100 µg orally every 4 hrs to maximum of 5 doses. | |
| Outcomes | Main: number of women delivering vaginally within 24 hrs of induction. Secondary: mode of delivery, neonatal outcomes and patient acceptability. | |
| Notes | September 2000 to March 2001. Tertiary level UK hospital. One exclusion from trial after randomisation for undiagnosed breech presentation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
075 Shetty 2002b.
| Methods | Non‐blinded randomised comparative trial. Computer‐generated sequence in sealed opaque envelopes. | |
| Participants | 100 women at term with medical or obstetric indications for induction of labour. Exclusion criteria: previous caesarean section, twins, contraindications to prostaglandins. Inclusion criteria: term, singleton, cephalic, unfavourable cervix, reassuring CTG. | |
| Interventions | 50 µg misoprostol sublingually vs orally every 4 hrs to maximum of 5 doses. | |
| Outcomes | Primary: number of women who delivered vaginally within 24 hrs of induction. Secondary: need for oxytocin, mode of delivery, caesarean section for fetal distress, uterine hyperstimulation, neonatal outcomes acceptability to the women. | |
| Notes | Aberdeen Maternity Hospital, June to September 2000. No exclusions after recruitment. No protocol violations. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
200 Carlan 2002.
| Methods | Non‐blinded randomised comparative trial. Computer‐generated sequence in sealed opaque envelopes. | |
| Participants | 157 pregnant women with indications for induction of labor. Inclusion criteria: singleton, live fetus, cervical score < 7, EFW < 4500 g and gestational age > 24 weeks. Exclusion criteria: vaginal bleeding, non‐reassuring CTG, breech presentation, labour, contractions at least 4 in 20 mins, contraindication to vaginal delivery. Women with previous caesarean section were not excluded. | |
| Interventions | Buccal vs vaginal misoprostol every 6 hrs. Buccal: 1st 2 doses 200 µg, then 300 µg to total 1600 mcg. Vaginal: 1st 2 doses 50 µg then 100 µg to total of 500 µg. | |
| Outcomes | Primary: interval from 1st dose to vaginal delivery. Secondary: incidence of tachysystole. | |
| Notes | Arnold Palmer Hospital, University of South Florida, USA, November 1999 to September 2000. Five women found to be in labour after randomisation (4 buccal, 1 vaginal) did not receive misoprostol. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
CTG: cardiotocography EFW: estimated fetal weight hrs: hours mins: minutes vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Todd 2002 | This study, which compared buccal misoprostol with laminaria insertion, was not included because it was used for first and second trimester termination of pregnancy rather than induction of labour in the third trimester. |
Contributions of authors
G Muzonzini and GJ Hofmeyr prepared the protocol and the review. G Muzonzini is responsible for maintaining the review.
Sources of support
Internal sources
Effective Care Research Unit, University of the Witwatersrand/Fort Hare, East London Hospital Complex, South Africa.
External sources
UNDP/UNFPA/WHO/World Bank (HRP), Switzerland.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
075 Shetty 2002a {published data only}
- Shetty A, Mackie L, Danielian P, Rice P, Templeton A. Sublingual compared with oral misoprostol in term labour induction: a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2002;109:645‐50. [DOI] [PubMed] [Google Scholar]
075 Shetty 2002b {published data only}
- Shetty A, Danielian P, Templeton A. Sublingual misoprostol for the induction of labor at term. American Journal of Obstetrics and Gynecology 2002;186(1):72‐6. [DOI] [PubMed] [Google Scholar]
- Shetty A, Danielian P, Templeton A. Sublingual misoprostol in the induction of labour at term [abstract]. Journal of Obstetrics and Gynaecology 2001;21 Suppl 1:S51. [Google Scholar]
200 Carlan 2002 {published data only}
- Carlan S, Blust D, OBrien W. Buccal versus intravaginal misoprostol administration for cervical ripening. American Journal of Obstetrics and Gynecology 2002;186:229‐33. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Todd 2002 {published data only}
- Todd CS, Soler M, Castleman L, Rogers MK, Blumenthal PD. Buccal misoprostol as cervical preparation for second trimester pregnancy termination. Contraception 2002;65(6):415‐8. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Amador 2007 {published data only}
- Amador LAV, Carmona JCF, Gallego FG, Texido CS, Esteve JLC. Randomized clinical trial of the safety and efficacy of 50 microg sublingual misoprostol versus 25 microg vaginal misoprostol for labor induction at term in pregnant women with diabetes. Progresos de Obstetricia y Ginecologia 2007;50(8):473‐83. [Google Scholar]
Azeem 2006 {published data only}
- Azeem S. Buccal vs. intravaginal misoprostol administration for cervical ripening in induction of labor [abstract]. 49th All India Congress of Obstetrics and Gynaecology; 2006 January 6‐9; Cochin, Kerala State, India. 2006:95.
Bartusevicius 2006 {published data only}
- Bartusevicius A, Barcaite E, Krikstolaitis R, Gintautas V, Nadisauskiene R. Sublingual compared with vaginal misoprostol for labour induction at term: a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2006;113:1431‐7. [DOI] [PubMed] [Google Scholar]
Caliskan 2005 {published data only}
- Caliskan E, Bodur H, Ozeren S, Corakci A, Ozkan S, Yucesoy I. Misoprostol 50 [mu]g sublingually versus vaginally for labor induction at term: a randomized study. Gynecologic and Obstetric Investigation 2005;59(3):155‐61. [DOI] [PubMed] [Google Scholar]
Elhassan 2007 {published data only}
- Elhassan EM, Nasr AM, Adam I. Sublingual compared with oral and vaginal misoprostol for labor induction. International Journal of Gynecology & Obstetrics 2007;97(2):153‐4. [DOI] [PubMed] [Google Scholar]
Esteve 2006 {published data only}
- Esteve JLC, Garcia TJP, Iturralde AS, Ferrer YA, Teixido CS. Randomized, controlled clinical trial to evaluate the safety and efficacy of 25 microg of vaginal misoprostol versus 50 microg of sublingual misoprostol for labor induction [Ensayo clinico, aleatorizado y controlado para evaluar la eficacia y la seguridad de la administracion de 25 mug de misoprostol vaginal frente a 50 mug de misoprostol sublingual para la induccion del trabajo del parto]. Progresos de Obstetricia y Ginecologia 2006;49(7):369‐79. [Google Scholar]
Feitosa 2006 {published data only}
- Feitosa F. Sublingual versus vaginal misoprostol for induction of labor [abstract]. Revista Brasileira de Ginecologia y Obstetricia 2006;28(9):566. [Google Scholar]
Feitosa 2006a {published data only}
- Feitosa FE, Sampaio ZS, Alencar CA, Amorim MM, Passini R. Sublingual vs. vaginal misoprostol for induction of labor. International Journal of Gynecology & Obstetrics 2006;94(2):91‐5. [DOI] [PubMed] [Google Scholar]
Filho 2005 {published data only}
- Filho O, Albuquerque R, Pacheco A, et al. Sublingual versus vaginal misoprostol for labor induction of term pregnancies. Revista Brasileira de Ginecologia e Obstetricia 2005;27(1):24‐31. [Google Scholar]
Lo 2006 {published data only}
- Lo TK, Lau WL, Wong KS, Tang LC. Sublingual misoprostol compared to artificial rupture of membranes plus oxytocin infusion for labour induction in nulliparous women with a favourable cervix at term. Hong Kong Medical Journal 2006;12(5):345‐50. [PubMed] [Google Scholar]
Nassar 2006 {published data only}
- Nassar AH. Sublingual versus vaginal misoprostol for labor induction at term (ongoing trial). ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 March 2006) 2006.
Nassar 2007 {published data only}
- Nassar AH, Awwad J, Khalil AM, Abu‐Musa A, Mehio G, Usta IM. A randomised comparison of patient satisfaction with vaginal and sublingual misoprostol for induction of labour at term. BJOG: an international journal of obstetrics and gynaecology 2007;114(10):1215‐21. [DOI] [PubMed] [Google Scholar]
Parisaei 2005 {published data only}
- Parisaei MP, Erskine KJE. Comparison of sub‐lingual misoprostol with standard regime vaginal prostaglandin E2 gel for the induction of labour after term rupture of membranes [abstract]. Journal of Obstetrics and Gynaecology 2005;25 Suppl 1:S69. [Google Scholar]
Parisaei 2008 {published data only}
- Parisaei M, Erskine KJ. Is expensive always better? Comparison of two induction agents for term rupture of membranes. Journal of Obstetrics and Gynaecology 2008;28(3):290‐3. [DOI] [PubMed] [Google Scholar]
Penaranda 2002 {published data only}
- Penaranda W, Arrieta O, Yances B. Active management of childbirth with sublingual misoprostol: a controlled clinical trial in the Hospital de Maternidad Rafael Calvo [Manejo activo del alumbramiento con misoprostol sublingual: un estudio clinico controlado en al hospital de maternidad rafael calvo de cartagen]. Revista Colombiana de Obstetricia y Ginecologia 2002;53(1):87‐92. [Google Scholar]
Russell 2007 {published data only}
- Russell Z, O'Leary T, Destefano K, Deutsch A, Carlan S. Buccal versus vaginal misoprostol administration for cervical ripening. American Journal of Obstetrics and Gynecology 2007;197(6 Suppl 1):S37, Abstract no: 85. [Google Scholar]
Wolf 2005 {published data only}
- Wolf SB, Sanchez‐Ramos L, Kaunitz AM. Sublingual misoprostol for labor induction: a randomized clinical trial. Obstetrics & Gynecology 2005;105:365‐71. [DOI] [PubMed] [Google Scholar]
Additional references
Alfirevic 2003
- Alfirevic Z. Oral misoprostol for induction of labour (Cochrane Review). The Cochrane Library 2003, Issue 1. [DOI] [PubMed] [Google Scholar]
Clarke 2000
- Clarke M, Oxman AD, editors. Cochrane Reviewers' Handbook 4.1 [updated June 2000]. In: Review Manager (RevMan) [Computer program]. Version 4.1 Oxford, England: The Cochrane Collaboration, 2000.
Curtis 1987
- Curtis P, Evans S, Resnick J. Uterine hyperstimulation. The need for standard terminology. Journal of Reproductive Medicine 1987;32:91‐5. [PubMed] [Google Scholar]
Danielsson 1999
- Danielsson KG, Marions L, Rodriguez A, Spur BW, Wong PY, Bygdeman M. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstetrics & Gynecology 1999;93:275‐80. [DOI] [PubMed] [Google Scholar]
Hofmeyr 1998
- Hofmeyr GJ, Nikodem VC, Jager M, Gelbart BR. A randomised controlled trial of oral misoprostol in the third stage of labour. British Journal of Obstetrics and Gynaecology 1998;105:971‐5. [DOI] [PubMed] [Google Scholar]
Hofmeyr 1999
- Hofmeyr GJ, Gulmezoglu AM, Alfirevic Z. Misoprostol for induction of labour: a systematic review. British Journal of Obstetrics and Gynaecology 1999;106:798‐803. [DOI] [PubMed] [Google Scholar]
Hofmeyr 2003a
- Hofmeyr GJ, Alfirevic Z, Kelly T, Kavanagh J, Thomas J, Brocklehurst P, et al. Methods for cervical ripening and labour induction in late pregnancy: generic protocol (Protocol for a Cochrane Review). The Cochrane Library 2003, Issue 1. [Google Scholar]
Hofmeyr 2003b
- Hofmeyr GJ, Gulmezoglu AM. Vaginal misoprostol for cervical ripening and labour induction in late pregnancy (Cochrane Review). The Cochrane Library 2003, Issue 1. [DOI] [PubMed] [Google Scholar]
RevMan 2002 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.1 for Windows. Oxford, England: The Cochrane Collaboration, 2000.
Shetty 2002
- Shetty A, Danielian P, Templeton A. Sublingual misoprostol for the induction of labour at term. American Journal of Obstetrics and Gynecology 2002;186:72‐6. [DOI] [PubMed] [Google Scholar]
Tang 2002
- Tang OS, Schweer H, Seyberth HW, Lee SW, Ho PC. Pharmacokinetics of different routes of administration of misoprostol. Human Reproduction 2002;17:332‐6. [DOI] [PubMed] [Google Scholar]
Zieman 1997
- Zieman M, Fong SK, Benowitz NL, Banskter D, Darney PD. Absorption kinetics of misoprostol with oral or vaginal administration. Obstetrics & Gynecology 1997;90:88‐92. [DOI] [PubMed] [Google Scholar]


