Abstract
Vasculitis and other autoimmune conditions are known complications of tumour necrosis factor alpha (TNF-α) inhibitor use. By definition, TNF-α inhibitor induced vasculitis is a secondary systemic vasculitis. However, its phenotype is varied and can present as an isolated vasculitic neuropathy. This presents a diagnostic challenge as the gold standard for diagnosis of a vasculitic neuropathy is a peripheral nerve biopsy that meets predefined histopathological criteria. Given the poor sensitivity of the peripheral nerve biopsy, it is important that clinicians take a good history and maintain a high index of suspicion, as this is a treatable iatrogenic condition. Here we present a case of adalimumab-induced sensory vasculitic neuropathy, treated according to the Peripheral Nerve Society guideline for non-systemic vasculitic neuropathy, given her disease phenotype.
Keywords: clinical neurophysiology, peripheral nerve disease, unwanted effects / adverse reactions, biological agents, vasculitis
Background
Tumour necrosis factor alpha (TNF-α) inhibitors are commonly used for a variety of inflammatory conditions, and one of the side effects of their use is other autoimmune disease.1 They are known to cause central nervous system2 and peripheral nervous system2 3 autoimmune conditions. They are also known to cause vasculitis, which can manifest in one or more phenotypes concurrently including vasculitic neuropathy.1 4 Given the known positive rechallenge phenomenon,1 it is important to have a high index of suspicion, as these complications represent treatable iatrogenic conditions. Of particular challenge is isolated vasculitic neuropathy, as gold standard diagnosis requires the presence of predefined histopathological features, and the poor sensitivity of peripheral nerve biopsy.5
Case presentation
A 42-year-old woman presented with an 18-month history of progressive pain and paraesthesia in both lower limbs. She had a background of seronegative inflammatory arthritis, bipolar disorder, hypothyroidism and fatty liver disease. At the onset of her sensory symptoms, she was receiving adalimumab monotherapy for her inflammatory arthritis.
Her pain was described as hot needles, tingling and numbness up to the knee on the right, and the mid-shin on the left. On further questioning, her pain had started in the right lower limb, followed by the left lower limb a few months after. She felt unsteady with her eyes closed and on uneven surfaces. She did not have palpitations or night sweats. There were no autonomic features or motor weakness.
She has been under the care of the rheumatology team for seronegative spondyloarthritis since 2013. She had initially been managed with depo-medrone injections and sulfasalazine. She developed back pain in 2015 but no sacroiliitis was found on MRI. Subsequently she went on to develop left medial epicondylar pain and left knee pain in 2017, and briefly presented to a local district general hospital for a rash in her right foot that spontaneously resolved. Retrospective review of photographs suggests that this was non-vasculitic. In 2018 she developed mouth ulcers and was switched to adalimumab.
On examination she had no motor weakness. Her cranial nerve and upper limb examinations were normal. She had impaired pinprick up to the right knee and left mid-shin. Her Romberg’s was positive although formal testing of vibration and proprioception did not show any deficit. Her ankle reflexes were attenuated and her plantars were flexor.
Investigations
Blood tests (table 1) showed a low folate of 4.4, and an atypical P-antineutrophil cytoplasmic antibody (ANCA) pattern not directed against myeloperoxidase (MPO) or proteinase 3 (PR3). The folate level was borderline and not felt to be relevant. The ANCA antibodies had been present since after starting sulfasalazine and before starting adalimumab, and therefore did not fit temporally with the onset of symptoms.
Table 1.
Serum neuropathy screen
| Test | Results | Test | Results |
| FBC | Normal | RF | Normal |
| UE | Normal | ANA | Negative |
| HbA1C | 40 | ANCA | Atypical P-ANCA |
| B12 | Normal | (MPO/PR3 Neg) | |
| Ferritin | Normal | Immunoglobulins | Normal |
| Folate | 4.4 | anti-MAG | Normal |
| Lead | Normal | Electrophoresis | Normal |
| Mercury | Normal | HIV | Negative |
| CK | 26 | Hepatitis B | Negative |
| CRP | 9.1 | Hepatitis C | Negative |
| ESR | Normal | TB Quantiferon | Negative |
ANA, antinuclear antibody; ANCA, antineutrophil cytoplasmic antibody; CK, creatine kinase; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; FBC, full blood count; HIV, human immunodeficiency virus; MAG, myelin associated glycoprotein; RF, rheumatoid factor; TB, tuberculosis; UE, urea & electrolytes.
She was also sent for nerve conduction studies (NCS), which showed unrecordable bilateral sural, right peroneal, right medial plantar sensory responses (table 2). Motor nerve studies and electromyography (EMG) of the right peroneus tertius were unremarkable, suggesting a symmetrical sensory axonal polyneuropathy.
Table 2.
Sensory nerve conduction studies showing unrecordable lower limb sensory nerve responses
| Nerve/sites | Lat (ms) |
Amp (μV) |
Dist (mm) | CV (m/s) |
| Right median—Dig II-wrist | ||||
| Wrist | 2.9 | 2 | 150 | 52 |
| Right ulnar—Dig V-wrist | ||||
| Dig V | 2.1 | 1.6 | 115 | 54 |
| Right radial—snuff box | ||||
| Forearm | 1.6 | 10.3 | 100 | 64 |
| Right sural—antidromic | ||||
| Calf | NR | NR | ||
| Left sural—antidromic | ||||
| Calf | NR | NR | ||
| Right superficial peroneal | ||||
| Leg | NR | NR | ||
| Right medial plantar, lateral plantar | ||||
| Medial plantar medial sole | NR | NR |
Amp, amplitude; CV, conduction velocity; Dig, digit; Dist, distance; Lat, latency; NR, not recordable.
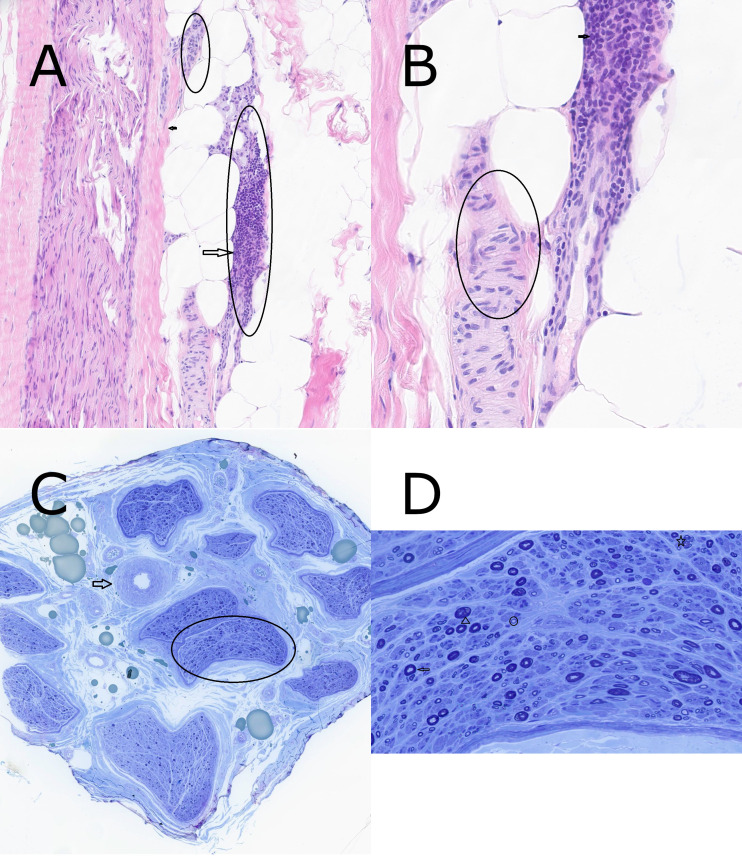
A right sural nerve biopsy was organised (figure 1). The nerve biopsy showed extensive inflammatory infiltrate by lymphocytes in an epineural arteriole wall. The inflammation was active still showing no obvious chronic damage in the vessel wall such as fibrosis. This inflammation is felt to be the aetiology for the axonal loss, the latter which is still active as indicated by Wallerian degeneration and with regeneration as indicated by the regenerative clusters and thinly myelinated axons. Overall, the biopsy results were supportive of a clinical diagnosis of vasculitic neuropathy.
Figure 1.
(A) Section shows normal arteriole (small circle) and extensive inflammation by lymphocytes in adjacent artery wall (large circle). The small arrow indicates nerve fascicle. ×10 H&E stain. (B) Section shows normal artery wall (circle) and dense infiltration by lymphocytes in adjacent arteriole wall (arrow). ×40 H&E stain. (C) Cross section through nerve showing many nerve fascicles arranged in endoneurial compartments surrounded by perineural cells (circle) with normal arteriole (arrow) in the epineurial connective tissue. ×10 toluidine blue stain. (D) Cross section shows normal myelinated axons (arrow), Wallerian degeneration (arrowhead), regenerative cluster (star) and thinly myelinated axon (circle). ×60 toluidine blue stain.
Differential diagnosis
The history suggested an asymmetrical onset with pain as a significant feature despite relatively symmetrical examination findings in clinic. The examination suggested a peripheral sensory neuropathy with no motor involvement and therefore further imaging was unnecessary. Vasculitic neuropathy can also present as a symmetrical neuropathy.5 As a result, the index of suspicion for a vasculitic neuropathy was high from the history provided before the tests were performed.
NCS/EMG should be thought of as an extension of the physical examination performed in clinic and were helpful in confirming the distribution and axonal pattern. This allowed us to proceed to the right sural nerve biopsy which showed extensive inflammation but no evidence of vascular wall damage. Applying the criteria listed in the Peripheral Nerve Society guideline, web supplement 1,6 this meant that the criteria for pathologically definite vasculitic neuropathy could not be satisfied. The patient had predominantly axonal changes, perivascular inflammation and multifocal nerve fibre degeneration (with some features of regeneration) therefore satisfying the criteria for pathologically probable vasculitic neuropathy. This, alongside the high clinical suspicion, enabled a level 2 case definition of vasculitis as defined by the Brighton Collaboration,7 which was originally developed to allow capture of peripheral nerve vasculitis correlated with vaccine administration. A level 1 gold standard histopathological diagnosis was not achieved which meant clinical correlation with the case presentation was still required, but the authors note that the sensitivity of the nerve biopsy has previously been noted to be only around 50%.5
While useful to ensure accurate case definition, the Brighton Collaboration guidelines do not provide aetiological information. Therefore, other possible aetiologies for a vasculitic neuropathy were considered in this case. Sulfasalazine is a potential cause,8 but she had discontinued this medication before symptom onset. Inflammatory conditions can themselves be associated with a vasculitis, but her symptoms were well controlled at the time of presentation. On balance, it was felt that adalimumab was the most likely cause.
Treatment
Although the Peripheral Nerve Society guideline would define this as a secondary systemic vasculitis,5 the clinical phenotype was consistent with an isolated peripheral nervous system vasculitis, mimicking non-systemic vasculitic neuropathy (NSVN). Therefore, the Peripheral Nerve Society guidelines for treatment of NSVN were used for reference,6 bearing in mind the positive rechallenge phenomenon and therefore avoiding the use of other TNF-α inhibitors where mentioned. Her adalimumab was withheld, and she was started on prednisolone 60 mg once a day.
Outcome and follow-up
Her symptoms improved with prednisolone. However, she subsequently went on to develop steroid induced diabetes. A taper of her prednisolone caused a resurgence of symptoms and therefore she received pulsed cyclophosphamide to enable tapering to continue.
Discussion
Vasculitis secondary to TNF-α inhibitors have previously been reported in other case series.1 4 These papers found that the most common manifestation was cutaneous vasculitis. Mean onset of symptoms from initiation of TNF-α ranged from 38 weeks1 to 34.5 months,3 with time to resolution of symptoms post-treatment ranging from 6.9 months3 to 14.8 months.1 We could not find papers specifically addressing isolated vasculitic neuropathy as a result of TNF-α administration.
We noted with interest the advances in diagnosis and management of NSVN5 9 as a distinct clinical entity. By definition, our patient had a secondary systemic vasculitis5 which was further supported by her non-specific p-ANCA positive result,8 despite the latter’s lack of temporal correlation with her symptoms. Furthermore, her p-ANCA positivity and use of adalimumab effectively excludes NSVN as the diagnosis.6
However, we could not help noticing that the clinical phenotype most resembled NSVN, although it remains to be seen if TNF-α inhibitor administration can cause the other proposed subtypes of NSVN. We presume that, like NSVN, isolated vasculitic neuropathy as a result of TNF-α administration can present in a multifocal pattern, late overlapping multifocal pattern and distal symmetrical pattern, thus requiring a high index of suspicion from the treating clinician.5 In addition, this is made more difficult as the sural nerve biopsy is only around 50% sensitive for the condition,5 although it can be supportive of the clinical diagnosis. In this case, it is postulated that the diagnosis could not be achieved histopathologically because the biopsy was performed at an early stage of inflammation.
Tumour necrosis factor was first isolated in 1975. Its name reflects its initial promise as an anticancer therapy, but it has since been shown to be involved in signalling pathways leading to both cell apoptosis and inflammation/survival.10 It is therefore unsurprising that TNF-α inhibitors can both cause and be used to treat autoimmune conditions. It is logical to think that the exact biological mechanisms leading to the phenotypic expression of disease (an NSVN phenotype, for example) underpinning this would depend on genetic susceptibility and its interaction with the environment. More research is needed into characterising the epidemiology of this condition, which may lead to plausible molecular targets for investigation.
Patient’s perspective.
I would like to express my gratitude to the team as, without their excellent care, I would not have received my diagnosis and subsequent treatment. Although the possibility of anti-TNF complications had been mentioned to me previously, these had been discounted, quite understandably, by the rheumatology team as being unlikely, due to their rarity. By the time I saw the neurology team in late 2020, I had been experiencing symptoms of peripheral neuropathy for well over a year. By this time, I had numbness in my feet, along with pins and needles in my right foot. They suggested a sural nerve biopsy, which ultimately led to my diagnosis of vasculitis. Although being diagnosed with something like this is hardly pleasant, it was at least a relief to know finally what was causing my symptoms. I have found the treatment to be almost worse than the disease itself, not least because I also suffer from bipolar disorder, so taking a high dose of steroids has had to be managed carefully. I have also now developed steroid-induced type 2 diabetes, but can only hope that this will resolve itself as my dose is reduced. Last week I underwent my sixth dose of cyclophosphamide, which has helped with the symptoms, but which obviously has other nasty side effects. It has been hard trying to manage these while also maintaining a decent attendance record at work. I am extremely lucky to have an understanding employer, as well as a very supportive family. The whole process has made me feel rather low, particularly with having other comorbidities. This has been rendered more difficult because of the precarities around the COVID-19 pandemic. However, the team’s care has been invaluable, and I feel extremely grateful for their help.
Learning points.
TNF-α inhibitors can cause other autoimmune conditions including vasculitis.
Sural nerve biopsy is only 50% sensitive in achieving a histopathological diagnosis of isolated peripheral nervous system vasculitis, but can be supportive in the right clinical setting.
A good history and examination are key to ensure investigations are used in an effective and efficient manner.
Footnotes
Twitter: @azzam9963
Contributors: NS drafted the manuscript, edited the figures and tables, and edited the final manuscript. AI prepared the figure and highlights, and drafted the pathology sections of the manuscript and figure legend. TA prepared the neurophysiology tables. PS conceptualised the manuscript and made significant revisions. All authors commented on the final version of the manuscript and approved it for publication.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Ramos-Casals M, Brito-Zerón P, Muñoz S, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine 2007;86:242–51. 10.1097/MD.0b013e3181441a68 [DOI] [PubMed] [Google Scholar]
- 2.Stübgen J-P. Tumor necrosis factor-alpha antagonists and neuropathy. Muscle Nerve 2008;37:281–92. 10.1002/mus.20924 [DOI] [PubMed] [Google Scholar]
- 3.Tsouni P, Bill O, Truffert A, et al. Anti-TNF alpha medications and neuropathy. J Peripher Nerv Syst 2015;20:397–402. 10.1111/jns.12147 [DOI] [PubMed] [Google Scholar]
- 4.Sokumbi O, Wetter DA, Makol A, et al. Vasculitis associated with tumor necrosis factor-α inhibitors. Mayo Clin Proc 2012;87:739–45. 10.1016/j.mayocp.2012.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins MP, Hadden RD. The nonsystemic vasculitic neuropathies. Nat Rev Neurol 2017;13:302–16. 10.1038/nrneurol.2017.42 [DOI] [PubMed] [Google Scholar]
- 6.Collins MP, Dyck PJB, Gronseth GS, et al. Peripheral nerve society guideline on the classification, diagnosis, investigation, and immunosuppressive therapy of non-systemic vasculitic neuropathy: executive summary. J Peripher Nerv Syst 2010;15:176–84. 10.1111/j.1529-8027.2010.00281.x [DOI] [PubMed] [Google Scholar]
- 7.Hadden RDM, Collins MP, Živković SA, et al. Vasculitic peripheral neuropathy: case definition and guidelines for collection, analysis, and presentation of immunisation safety data. Vaccine 2017;35:1567–78. 10.1016/j.vaccine.2015.11.047 [DOI] [PubMed] [Google Scholar]
- 8.Radić M, Martinović Kaliterna D, Radić J. Drug-induced vasculitis: a clinical and pathological review. Neth J Med 2012;70:12–17. [PubMed] [Google Scholar]
- 9.Collins MP, Dyck PJB, Hadden RDM. Update on classification, epidemiology, clinical phenotype and imaging of the Nonsystemic vasculitic neuropathies. Curr Opin Neurol 2019;32:684–95. 10.1097/WCO.0000000000000727 [DOI] [PubMed] [Google Scholar]
- 10.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer 2009;9:361–71. 10.1038/nrc2628 [DOI] [PubMed] [Google Scholar]