Abstract
Recent advances in brain-computer interface technology to restore and rehabilitate neurologic function aim to enable persons with disabling neurologic conditions to communicate, interact with the environment, and achieve other key activities of daily living and personal goals. Here we evaluate the principles, benefits, challenges and future directions of brain-computer interfaces in the context of neurorehabilitation. We then explore the clinical translation of these technologies and propose an approach to facilitating implementation of brain-computer interfaces for persons with neurologic disease.
Keywords: Brain-computer interface, neurorecovery, neurorehabilitation, neurotechnology, brain-machine interface
I. Introduction
The co-evolution of computer technology, bioengineering and neuroscience over the past two decades has enabled the potential for unprecedented advances in facilitating neurorecovery through brain-computer interfaces (BCI). The rapidly expanding BCI technology field and its implications for clinical research and practice is of growing importance for clinicians who seek to deliver optimal care to persons with lasting functional deficits resulting from neurologic disease or neurotrauma.1,2 By enabling restoration or replacement of lost function, BCIs have the potential to improve quality of life by enhancing the autonomy and agency of users, ameliorating isolation, and promoting societal re-integration.3,4 Here, after reviewing the principles, benefits, challenges and opportunities of BCIs in the context of neurorecovery, clinical translation of these technologies is explored, and a practical approach to facilitating access to BCIs for people with neurologic disease in different phases of care is proposed. Here, we use the term “neurorestoration” to refer to the restored function that results immediately from use of a technology (in this case, a BCI). “Neurorehabilitation” is used to refer to the process by which the remaining/intact neural system regains the ability to perform a function. “Neurorecovery” is our more general term for the goal, which is agnostic to approach.
What is a Brain-Computer Interface?
A brain-computer interface (BCI) is a system that translates central nervous system (CNS) signals into command signals for an external or internal device. The historical groundwork for BCI technology was set in the 1800s by the pioneering research of Richard Caton, Adolf Beck and Hans Berger whose discoveries surrounding continuous electrical activity in the brain provided a substrate for the measurement and manipulation of nervous system signals.5,6 These discoveries paved the way for the key non-human primate research and later development and deployment of EEG neurofeedback and the first BCI prototypes.7–12 The term BCI has since come to encompass a broad array of technologies that interface with the nervous system, from cochlear implants to restore hearing to the NeuroPace device, a responsive neurostimulator for the treatment of medically refractory epilepsy.13,14 Broadly construed, BCIs serve to restore or rehabilitate function, with the ultimate aim of improving users’ capacities to communicate, interact with the environment, and achieve other personal goals.15
Restoration of lost function typically entails bypassing a lesion incurred by disease or trauma, with the aim of directly supplanting the function lost. Examples of this include BCI-enabled prosthetic arm control to supplant lost limb function16–22 or BCI-enabled typing or speech to supplant impaired verbal communication capability.23–31 In so doing, such technologies facilitate novel means to perform an activity in a manner that bypasses the lesioned area that is ordinarily engaged in performing that function.
Rehabilitation of function through BCIs typically involves the use of neurofeedback with or without neural stimulation, with the goal of promoting plasticity and enabling re-learning of a lost function.32 Instead of bypassing a deficit-producing lesion, rehabilitative BCIs aim to promote the nervous system’s ability to re-learn previously lost or deteriorated function.33 One example is training on a BCI orthosis system with the goal of ultimately restoring native upper extremity function for patients with hemiparesis after stroke.34–36 Rehabilitative BCI techniques that aim to shape or engage cortical/subcortical/spinal plasticity to facilitate neural re-learning and re-mapping may be coupled with restorative BCI systems and operate synergistically.37
Another emerging role of BCIs is to improve diagnostic precision in disorders of consciousness, thereby illuminating opportunities for neurorehabilitation. BCIs may foreseeably aid in assessing covert responsiveness (i.e., responsiveness that is not detectable on bedside neurologic exam) in persons with disorders of consciousness; such persons are often misdiagnosed with traditional behavioral assessments that rely heavily on intact motor systems or higher-order cognitive abilities to infer level of awareness.38–41 For example, one BCI system has been used to complement assessment of visual fixation;42 by coupling a computer-based visual fixation task with EEG to detect event-related potentials occurring with visual fixation, the system aims to aid in detection of awareness that can sometimes evade bedside behavioral assessment.42,43
Populations commonly cared for by neurologists who may benefit from BCIs include people with syndromes resulting from disconnection of the pathways to peripheral neuromotor targets with resulting severe speech and motor impairments. This includes those who have sustained functional deficits due to stroke, spinal cord injury, traumatic brain injury, motor neuron disease, multiple sclerosis, locked in syndrome, cerebral palsy, and disorders of consciousness.44–46
II. The Components of a BCI: Actuating Cognition
The components of BCIs include the trio of sensor, decoder and effector.47 The BCI sensor serves to detect and record neural data, and a decoder then processes and converts this data into a command signal that is transmitted to an effector to carry out relevant function. Critically, sensory feedback is provided to the user, traditionally in the form of visual feedback.48,49 Auditory50 and haptic feedback approaches51,52 are also being explored.
BCI Sensors: Capturing Intention
BCI sensors primarily detect electrical, hemodynamic or magnetic signals from the central nervous system. Sensors designed to detect electrical signals utilize methods such as scalp-based electroencephalography (EEG), brain surface-based electrocorticography (ECoG), and intracortical microelectrodes. Sensors designed to detect hemodynamic signals utilize methods such as functional magnetic resonance imaging (fMRI) and functional near-infrared spectroscopy (fNIR), which rely on changes in blood oxygenation to localize neural activity of interest.53,54 Magnetoencephalography (MEG) is the primary modality used to sense magnetic brain signals induced by synchronized neural currents.55 Multimodal sensors may combine detection of different signals to enhance performance. Examples of multimodal sensors include combinations of MEG and EEG56, or of EEG and fMRI.57 Sensors can be distinguished by their location (e.g., implanted within deep brain structures, or within cortex, or subdural, epidural, intra- or epicranial, on the scalp, or external to the head), temporal resolution (i.e., sensing speed), spatial resolution (i.e., sensing detail), signal-to-noise ratio, sensor size, and ability to record signals for an extended period of time.58 Electrical sensors, and specifically EEG and MEGs, are the two most common types of BCI sensors used toward the restoration of movement and communication for people with neurologic disease.
Neural Decoding: Translating Neural Information
After neural activity or its proxy is captured and recorded by a sensor, a BCI decoder uses an algorithm to process this information and produce a signal that can be transmitted to an effector to actuate a helpful output. Neural decoding algorithms associate patterns of neural activity with intended user behavior.47,59 While initial neural decoding algorithms relied on linear statistical analyses (such as the Kalman filter, also known as linear quadratic estimation), advances in computational power and artificial intelligence are leading to improvements in BCI performance through machine learning techniques.60,61 These advances in neural decoding hold promise for improved BCI performance by accounting for the variability in neural activity that has historically proved to be a challenge to some BCI’s consistency in inferring intended user action.29
Neural effector and Feedback: Bringing Intention to Action
BCI effectors process signals received by a neural decoder to produce a desired output. Examples of BCI effectors include computer cursors62, robotic orthoses,63–67 exoskeletons68–70, wheelchairs71–73, virtual reality environments (i.e., computer-generated simulations that allow users to interactively practice activities)74–76, artificial voice77,78, flash spellers79–83, or reanimation of one’s own limb.84,85 86–88
Neurofeedback is a critical component of both restoration of function (closed-loop control) as well as rehabilitation of function. The most common neurofeedback strategy has been audiovisual (AV) feedback.89 Real-time AV feedback to subjects has been studied in aiding task-specific training and recovery.90 Neurofeedback strategies also include neuromodulation techniques, such as adaptive deep brain stimulation, and non-invasive brain stimulation, directed at changing the nervous system to achieve improved motor/cognitive/mood aims.91 For example, through haptic or behavioral feedback techniques, persons with limb weakness resulting from stroke may learn to improve ipsilesional mu-rhythm activation and upregulate ipsilesional sensorimotor networks.91–93
A critical component of BCIs is the feedback provided, which “closes the loop” between the device and user. This could come in the form of pure visual feedback, for example a 2-dimensional cursor control on a computer screen or multidimensional robot arm movement, or through visual and sensory feedback during functional electrical stimulation (FES)-induced movement of one’s own arm and wrist.94 This feedback has been shown to influence the tuning properties of individual neurons and cortical networks involved in BCI control, a concept called closed-loop neural adaptation.95 BCI neurofeedback has been shown to change properties of the neurons and groups of neurons involved in BCI control96,97, and the implications of these changes for closed-loop BCI control are being further explored.98 Systems-neuroscience level neurofeedback paradigms are currently being explored to enhance neurorehabilitation.99
III. BCIs in Neurorecovery and Neurorehabilitation
In the context of neurorecovery and neurorehabilitation, a BCI may aim to restore a capacity lost due to injury or disease. Capacities that BCIs may serve to restore include communication, motor function, mobility, autonomic functions (bowel, bladder and sexual functions), hearing (through cochlear implants), and vision (retinal prostheses).
BCIs may alternatively serve to induce plasticity in neural circuits in order to regain native function after neural injury. The brain’s capacity for structural reorganization was studied and codified by neuropsychologist Donald Hebb, who proposed that “[w]hen an axon of cell A is near enough to excite a cell B and repeatedly or persistently takes part in firing it, some growth process or metabolic change takes place in one or both cells such that A’s efficiency, as one of the cells firing B, is increased…any two cells or systems of cells that are repeatedly active at the same time will tend to become ‘associated,’ so that activity in one facilitates activity in the other.”100 The notion that co-active cells could form stronger synaptic connections in rich learning environments is a principle now known as Hebbian plasticity. The short mantra is that “neurons that fire together wire together.”101 In line with this principle, some BCIs aim to induce patterned recruitment of neurons to strengthen synaptic connections sub-serving functions that have been impaired due to neural damage. Approaches to inducing neural plasticity include repetitive stimulation of a neural pathway, paired stimulation of multiple points in a neural pathway, or closed-loop stimulation, a technique that uses endogenous activity at one point in a pathway to trigger activation of a second point in a neural pathway.102
IV. BCIs in Clinical Translation
How may clinicians prescribe a BCI to a person who might benefit?
In order for a BCI system to enter into clinical use in the field of neurorehabilitation, a series of complex development phases and checkpoints must be met.103,104 The bench to bedside costs of medical device development are substantial.105 From its nascent stages of research design and development, a BCI system must pass through clinical trials to demonstrate safety and, hopefully, efficacy, attain clearance from relevant regulatory bodies (such as the FDA in the United States), and succeed in manufacturing and marketing before finally arriving at clinical implementation. This complex landscape may prove challenging to navigate not only for researchers but also for clinicians interested in providing BCIs to patients in clinical practice.
The FDA has taken a leading role in crafting a roadmap for the regulatory assessment of BCIs, releasing draft guidance in February 2019 on “Implanted Brain-Computer Interface (BCI) Devices for Patients with Paralysis or Amputation – Non-Clinical Testing and Clinical Considerations.”106 The NeuroTech Network (http://www.neurotechnetwork.org) is a non-profit organization that promotes advocacy for, access to, and awareness of neurotechnology products including BCIs for persons with impairments, and provides resources for learning about available categories of neurotechnologies for patients and providers. While clinicaltrials.gov captures most ongoing BCI research in the US, to date there is no unified, routinely updated repository of clinically actionable knowledge of available BCIs for clinicians, patients or surrogates to reference and utilize. Such a resource could serve to increase access to the latest in BCI technology. The emergence of consumer applications may be valuable in their ability to gather considerable capital to advance technology development, but may also confuse consumers regarding the ability of these devices to provide efficacy in a medical context.
Clinicians interested in providing BCIs to persons who might benefit from them should be aware of the following three categories of products, stratified according to stage of development: (1) BCIs approved for clinical use and available through manufacturers (2) BCIs under clinical study and available through active clinical trials (3) BCIs in pre-clinical development.
BCIs approved for clinical use
The category of BCIs approved for clinical use includes deep brain stimulation systems for persons with movement disorders or epilepsy (including NeuroPace, which relies on an implanted ECoG strip electrode to detect epileptogenic activity and responsively deliver electric pulses to preempt impending seizures). While open-loop DBS is FDA-approved, closed-loop DBS systems whose neuromodulatory outputs are determined in part by brain-recorded and decoded signals, are still investigational. The NeuroPace system is estimated to cost 30,000–40,000 USD and is covered by major insurance companies for patients with refractory focal epilepsy who are not candidates for epilepsy surgery.107 The costs of BCIs are inclusive of materials, surgical placement (when required) and support services; there is no uniform policy of reimbursement for BCIs among insurers and government programs.108
Neurotechnologies for neurorecovery that have been approved for clinical use span beyond the class of BCIs, and include myoelectric orthoses for persons who have lost limb function due to amputation or spinal cord injury, wearable robotic exoskeletons, and cochlear implants. While not the primary focus of this review, clinicians should be aware of several of these neurotechnologies given their clinical availability and utility in neurorecovery. The first FDA-approved neuroprosthesis was the NeuroControl Freehand System, which restored hand function in patients with spinal cord injuries; unfortunately, manufacturing of this system is no longer ongoing, despite the technical success of the product and satisfaction of its users.104,109 The DARPA-funded DEKA LUKE arm system was FDA-approved for clinical use is 2014. It uses EMG electrodes to sense user-intended movement and translates these signals into complex movements with multiple degrees of freedom. The cost of the LUKE arm is around 150,000 USD and may be a covered service by the Veterans Affairs (VA) but is not by most insurance companies.110 Other neuromuscular prostheses, including a bone-anchored robotic arm with implanted electrodes in nerves and muscles of the upper arm allowing for bidirectional sensorimotor communication with a prosthetic hand, are in active development and have been integrated into daily use for some patients.111 The ReWalk robotic exoskeleton, manufactured by ARGO Medical Technologies (Israel), Ekso GT, by Ekso Robotics (California), and Indego, by Parker Hannifin (Ohio), are FDA-approved devices that enable user-initiated lower extremity motion through wearable robotic support systems.112,113 The cost of ReWalk is around 80,000 USD and is covered through the VA Choice program for eligible veterans but is not covered by most insurance companies.114 Prerequisites for its use include spinal cord injury; height between 160 cm and 190 cm (5’3– 6’2); weight below 100 kg (220 lbs); healthy bone density; absence of severe spasticity, cognitive conditions that could interfere with the operation of the device, significant contractures, or history of severe neurologic illness other than spinal cord injury.115 Ekso GT was the first exoskeleton FDA-approved for stroke patients.116,117 These products are available through their respective manufacturers and distributors, and through networks of training centers listed online.112,113,115
BCIs under clinical investigation
Most patients using clinical-grade BCIs today are doing so as part of clinical trials. As of May 2019, there are currently 93 brain-computer interface projects with registered clinical trials. Clinicians can visit www.clinicaltrials.gov and search by eligibility criteria, study type and location to find which trial(s) a patient might be suitable for, and contact information for study coordinators to determine patient eligibility and coordinate enrollment. Once eligibility has been determined, study coordinators work with patients and families to orchestrate necessary steps in enrolling.
The category of BCIs under development contains a broad array of investigational technologies. In the context of neurorehabilitation, these include technologies to restore communication, motor function and mobility; to enhance sleep, awareness and cognition; and to restore autonomic functions including bowel, bladder and sexual functions. Patients, families and clinicians should be made aware of BCIs on the horizon which may bear on future approaches to neurorecovery while reinforcing realistic expectations. Knowledge of available and emerging BCIs that may aid in restoration or rehabilitation of function may serve to counter therapeutic nihilism sometimes encountered in settings of acute devastating neurologic injury, and aid in optimally informing decision-making for patients, families and clinicians.118
There are a variety of research groups actively investigating and improving BCI technology in both animal and human research. A milestone in restorative BCI for communication was recently achieved with the application of deep-learning methods to reconstruct neural signals detected by implanted electrocorticography (ECoG) devices into audible language in human subjects.77,78
V. Challenges and Potential Solutions for BCI Translation
Despite significant promise, a variety of scientific, technical, clinical, ethical, and economic steps have limited the clinical translation of BCIs.
From an engineering standpoint, the ability of implanted sensors to reliably detect and record signals119 may depreciate with time, for a variety of reasons that have been attributed to reactive gliosis120 and inflammation121, micromovement, mechanical breakdown122, or changes in impedance that may progress as a device remains implanted.123–128 The relative contributions of these factors to the decline (which can occur slowly, over many months to years) are not clear. Recent advances in materials chemistry, computational modeling, and nanotechnology, however, enable construction of more durable sensors to withstand the challenges faced by earlier materials.129–131 Coupled with developments in sensor calibration and signal decoding utilizing machine learning techniques, these advances portend more robust, reliable and accurate BCIs in coming years.
Even if an efficacious, safe and beneficial technology is devised, if the target patient population is small or economically disenfranchised, deployment can prove unsustainably expensive, especially given the multidisciplinary team required for implementation and support. The NeuroControl Freehand System, which restored hand function in patients with spinal cord injuries but was discontinued in 1998 despite technical success, is an example of this.104 These barriers are especially high in the case of implanted BCIs. Recognizing these factors and the unmet public health needs that BCIs promise to meet, the FDA’s Center for Devices and Radiological Health (CDRH) has introduced new mechanisms for expedited access, efficient review (including a joint-review mechanism with CMS), and pre-submission assessments to streamline the development cycle and catalyze clinical translation while maintaining safeguards on patient protection.104,106
From a clinical standpoint, many patients and clinicians lack actionable knowledge about BCIs and how they can be applied toward care.132 For clinicians actively caring for patients with BCIs, relevant outcome measures and methods for evaluating performance and patient satisfaction may be opaque. Closer collaboration among engineers, clinicians, patient advocates, and other stakeholders will be instrumental in ensuring that clinical outcome measures are clear, patient priorities are met, risk-benefit balance is optimized, and public-health impact is maximized. Before prescribing a BCI, which might entail referral to a specialty center or consultation with an expert in the relevant domain, clinicians and researchers should ensure realistic expectations among users and caregivers. This should include detailed discussions of the risks, benefits and potential shortcomings of the relevant BCI.133
In the absence of a uniform policy of reimbursement for BCIs among insurers and government programs, the current costs of BCIs may affect their adoption.108,134 In the event that a BCI is not paid for by insurance, patients may be responsible for the costs; some have turned to medical crowdfunding for this purpose, a trend that has raised ethical and social issues.135 Recognizing these obstacles, more uniform reimbursement policies are needed to help ensure that BCIs are affordable and equitably accessible to those in need. Such policies will undoubtedly be easier to develop once BCIs demonstrate a consistent and reliable benefit to even a small group of people, as portrayed for example in the development and deployment of the NeuroPace BCI.136
As BCIs continue to advance in sophistication, ethical questions relating to user agency and responsibility137, decision-making capacity138, shaping of personal identity139, privacy132,140,141, storage and sharing of recorded neural data142, bio-enhancement applications143, access disparities144, and research ethics133,145 are anticipated to grow increasingly prevalent and pertinent. Careful anticipation and evaluation of these issues can help to ensure bioethical resilience across the BCI development lifecycle and foster successful clinical translation into neurorehabilitation practice.146–148
Conclusions
Brain-computer interfaces offer immense potential for neurorecovery and neurorehabilitation for patients with neurologic disease. BCIs may serve to measure and restore capacities lost due to neurologic injury or disease, or to induce plasticity to improve learning and remapping after neural injury. A variety of obstacles and opportunities in successful clinical translation can be identified across the lifecycle of BCI development, from early design and discovery, to clinical trials, regulatory approval and clinical implementation. Clinicians caring for persons with neurologic impairments should be aware of the current landscape of BCIs across various stages of the development lifecycle and understand how to match BCI technologies with eligible patients. Ongoing collaboration between researchers, engineers, clinicians, patient advocates, regulatory bodies, bioethicists, payers and other stakeholders will be essential to ensure that the promises of BCIs for neurorecovery are captured and sustained in this evolving era of novel neurotechnologies.
Figure 1.
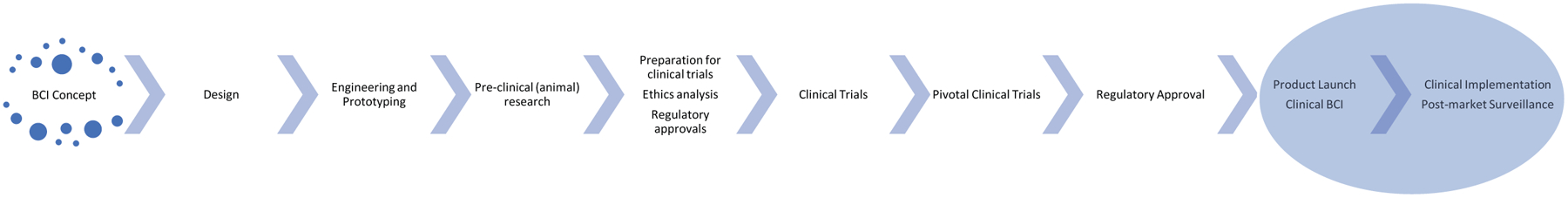
Lifecycle of BCI Development
Figure 2.
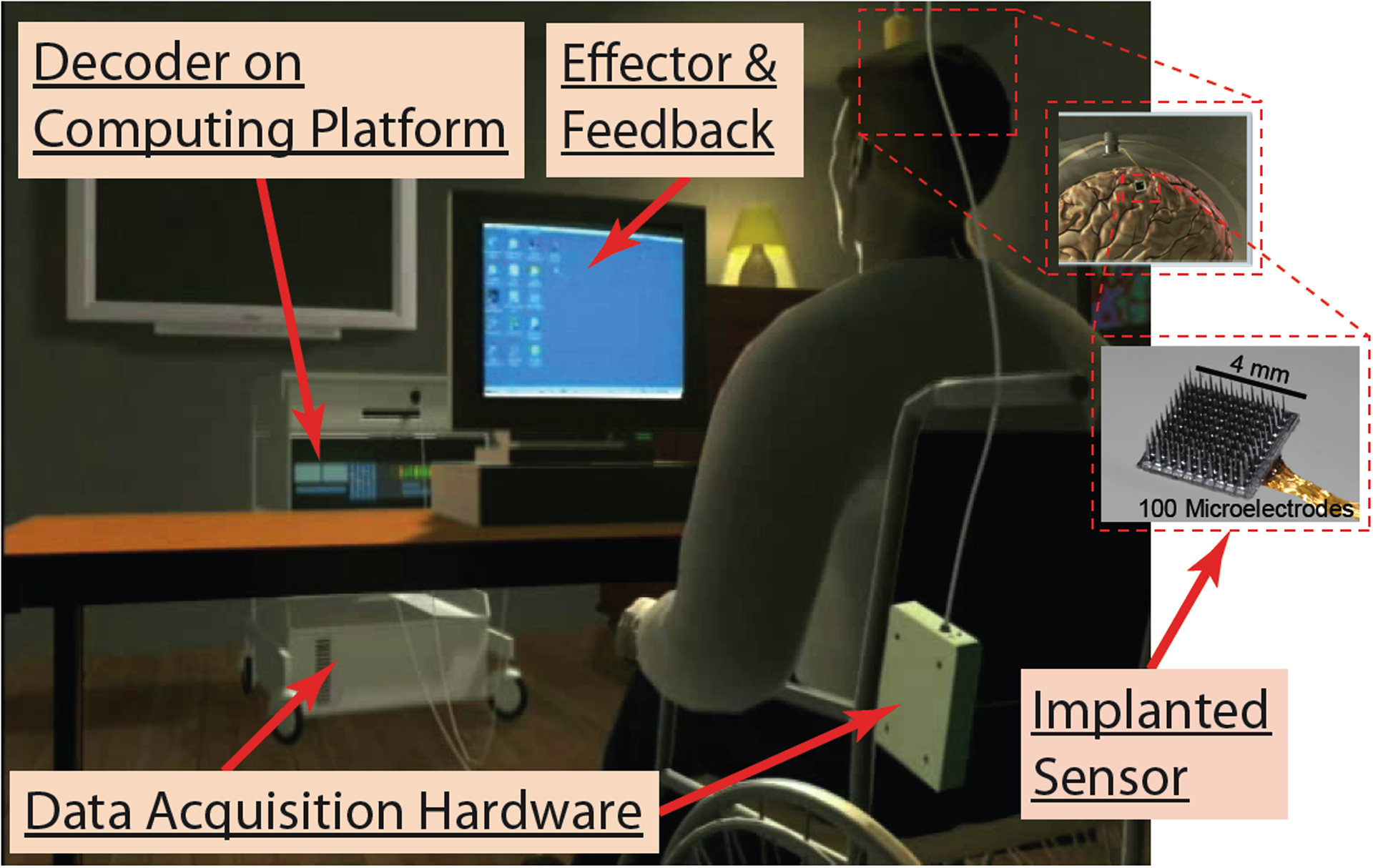
Schematic of BrainGate BCI Setup. This figure depicts the percutaneous and cable-connected system used currently. Percutaneous wireless systems are in clinical testing, and fully implanted systems are in development.
Table 1.
Brain-Computer Interface (BCI) clinical trials actively recruiting.
| Neurologic Condition | BCI Intervention | Location | ClinicalTrials.gov Identifier |
|---|---|---|---|
| Communication | |||
| Locked in syndrome Tetraplegia |
Assistive communication device that enables users to control a text-entry interface using EEG signals to compose messages. | University of Geneva, Campus Biotech Geneva, Switzerland | NCT03213561 |
| Persons with ALS and locked-in syndrome | EEG and/or near-infrared spectroscopy (NIRS) based BCI. NIRS will be used train a classifier to predict “yes” and “no” answering patterns; for patients who can still open eyes, an EEG-controlled BCI for communication will be used. | University of Tuebingen Tubingen, Baden Wuerttemberg, Germany | NCT02980380 |
| Tetraplegia Locked-in Syndrome Brainstem Stroke ALS |
CortiCom system of high-channel ECoG grids to investigate motor imagery and imagined speech as sources for brain-computer interactions | Johns Hopkins Medicine Baltimore, Maryland, United States | NCT03567213 |
| Tetraplegia Locked-in Syndrome ALS |
Placement of BrainGate2 Neural Interface System into motor-related cortex to identify methods and features that could allow for recovery a host of abilities that normally rely on the hands. | Stanford; MGH; Case Western; Providence VA, United States | NCT00912041 |
| Movement Control | |||
| Traumatic Tetraplegia With Cervical Cord Injury | ECoG-enabled motorized exoskeleton | CLINATEC Grenoble, France | NCT02550522 |
| Quadriplegia | Wireless EEG headset to control a virtual keyboard using P300 evoked related potentials (ERPs). | Hopital Raymond Poincare Garches, France | NCT01707498 |
| Chronic Stroke Hemiparesis |
Ipsihand EEG headset designed to use EEG signals from the non-lesioned hemisphere to control a motorized glove worn on the affected hand that moves the according to the type of signal detected. | Washington University in St. Louis Saint Louis, Missouri, United States | NCT03611855 |
| Acute stroke with severe unilateral motor upper extremity hemiparesis | Neuromuscular electrical stimulation (NMES) applied contingent to voluntary activation of primary motor cortex, as detected by a subject-specific EEG patterns extracted with machine learning techniques. | Division of Neurorehabilitation, University Hospital of Geneva Geneva, GE, Switzerland | NCT03379532 |
| Incomplete tetraplegia with injury at level C4–C8 | FES-BCI: Functional electrical stimulation (FES) applied contingent to EEG patterns arising from patient thinking to move hand. | Queen Elizabeth National Spinal Injuries Unit Glasgow, United Kingdom | NCT01852279 |
| Locked in syndrome | ECoG sensing device to control assistive technology with switch signals (such as operating home apparatus or writing text) | University Medical Center Utrecht, Netherlands | NCT02224469 |
| Tetraplegia Spinal Cord Injury |
Blackrock Microsystems NeuroPort Arrays implanted in the motor cortex for long-term neural recording and control of external devices. | University of Pittsburgh Pittsburgh, Pennsylvania, United States | NCT01364480 |
| Spinal Cord Injuries Tetraplegia Quadriplegia |
Neuroport cortical recording array to determine desired grasp patterns for FES; users will be asked to think about holding different shaped objects and corresponding cortical signal patterns will be decoded to match grasp patterns. | Louis Stokes VA Medical Center, Cleveland, OH Cleveland, Ohio, United States |
NCT03482310 |
| ALS SCI Stroke Multiple sclerosis Muscular dystrophies |
ECoG-based wearable hand robotic exoskelaton | University of California San Francisco San Francisco, California, United States |
NCT03698149 |
| Stroke Upper limb impairment |
FES-BCI: Functional electrical stimulation (FES) applied contingent to EEG patterns arising from user thinking to move | Centro de Referencia Estatal de Atención al Daño Cerebral (CEADAC) Madrid, Spain |
NCT03508037 |
| ALS Shoulder trauma |
EEG-based neurofeedback based on motor imaging in therapeutic videogames | Hopital PITIE SALPETRIERE Paris, France |
NCT03545451 |
| Tetraplegia Spinal Cord Injury Brainstem Stroke |
Blackrock Microsystems CRS Arrays will be implanted in the motor cortex and sensory cortex and trained to send user-driven neural signals to devices or displays; microstimulation used to mimic sensory input. | University of Pittsburgh Pittsburgh, Pennsylvania, United States |
NCT01894802 |
| Spinal cord injury, brainstem stroke, muscular dystrophy, amyotrophic lateral sclerosis or other motor neuron disorder with complete or incomplete tetraplegia | Placement of BrainGate2 Neural Interface System into motor-related cortex to identify methods and features that could allow for recovery a host of abilities that normally rely on the hands. | Stanford; MGH; Case Western; Providence VA, United States | NCT00912041 |
| Quadriplegia | Neural Communication System - Placement of Neuroport arrays into posterior parietal cortex with brain-control training of simplified computer or tablet computer environments | University of California Los Angeles Los Angeles, California, United States |
NCT01958086 |
| Quadriplegia | Neural Prosthetic System 2 (NPS2) - Placement of Neuroport arrays into posterior parietal and somatosensory cortexes with brain-control training to perform reach and grasp tasks with sensory feedback via intracortical microstimulation. | Rancho Los Amigos National Rehabilitation Center Downey, California, United States | NCT01964261 |
| Stroke Arm paralysis |
Cortimo Neuromotor Prosthetic to Treat Stroke-Related Paresis - Placement of Blackrock Microsystems Multiport to decode signals to drive activity of wearable arm orthosis | Thomas Jefferson University Philadelphia, Pennsylvania, United States | NCT03913286 |
| Stroke with upper limb deficit | Neurofeedback for Upper-limb Recovery After Stroke (NeuroFB-AVC) - Coupled EEG-fMRI neurofeedback | Rennes University Hospital Rennes, France | NCT03766113 |
| Pain | |||
| Chronic Musculoskeletal Pain Tennis Elbow Lateral Epicondylitis |
Neurofeedback Treatment for Chronic Musculoskeletal Pain - EEG-based neurofeedback of pain-related neuronal oscillation power as a training paradigm for controlling chronic musculoskeletal pain | Center For Sensory-Motor Interaction Aalborg, Denmark | NCT03863847 |
| Cognitive | |||
| Brain Injury | EEG/SSEP evaluation of attentional modulation in different conditions | Hospices Civils de Lyon Lyon, France |
NCT02567201 |
| Mild TBI PTSD |
IASIS System - Transcutaneous electrical stimulation (TES) paired with resting state MEG neurofeedback | VA San Diego Healthcare System, San Diego, CA San Diego, California, United States |
NCT03244475 |
| Attention Deficit Hyperactivity Disorder (ADHD) Autism Spectrum Disorders |
Computer game that uses eye-tracking and EEG to train attention and emotional recognition | Institute of Mental Health Singapore, Singapore | NCT02618135 |
| ADHD | BCI-based cognitive training through P300-based controlled games | Hospices Civils de Lyon Bron, France | NCT03289793 |
| Alzheimer’s disease | EEG-based neurofeedback involving intermittent audiovisual cues to adjust attentional engagement. | Oregon Health & Science University Portland, Oregon, United States |
NCT03790774 |
| Mild Cognitive Impairment | Auditory stimulation during slow wave sleep detected by polysomnography | Northwestern University Chicago, Illinois, United States | NCT02608840 |
References
- 1.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21(10):1371–1383. [DOI] [PubMed] [Google Scholar]
- 2.Bockbrader MA, Francisco G, Lee R, Olson J, Solinsky R, Boninger ML. Brain Computer Interfaces in Rehabilitation Medicine. PM R. 2018;10(9S2):S233–S243. [DOI] [PubMed] [Google Scholar]
- 3.Lee MB, Kramer DR, Peng T, et al. Brain-Computer Interfaces in Quadriplegic Patients. Neurosurg Clin N Am. 2019;30(2):275–281. [DOI] [PubMed] [Google Scholar]
- 4.Kogel J, Schmid JR, Jox RJ, Friedrich O. Using brain-computer interfaces: a scoping review of studies employing social research methods. BMC Med Ethics. 2019;20(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coenen A, Fine E, Zayachkivska O. Adolf Beck: a forgotten pioneer in electroencephalography. J Hist Neurosci. 2014;23(3):276–286. [DOI] [PubMed] [Google Scholar]
- 6.Zayachkivska O, Gzhegotsky M, Coenen A. Impact on electroencephalography of Adolf Beck, a prominent Polish scientist and founder of the Lviv School of Physiology. Int J Psychophysiol. 2012;85(1):3–6. [DOI] [PubMed] [Google Scholar]
- 7.Silvoni S, Ramos-Murguialday A, Cavinato M, et al. Brain-computer interface in stroke: a review of progress. Clin EEG Neurosci. 2011;42(4):245–252. [DOI] [PubMed] [Google Scholar]
- 8.Wolpaw JR, Birbaumer N, Heetderks WJ, et al. Brain-computer interface technology: a review of the first international meeting. IEEE Trans Rehabil Eng. 2000;8(2):164–173. [DOI] [PubMed] [Google Scholar]
- 9.Vaughan TM, Heetderks WJ, Trejo LJ, et al. Brain-computer interface technology: a review of the Second International Meeting. IEEE Trans Neural Syst Rehabil Eng. 2003;11(2):94–109. [DOI] [PubMed] [Google Scholar]
- 10.Abiri R, Borhani S, Sellers EW, Jiang Y, Zhao X. A comprehensive review of EEG-based brain-computer interface paradigms. J Neural Eng. 2019;16(1):011001. [DOI] [PubMed] [Google Scholar]
- 11.Leuthardt EC, Schalk G, Roland J, Rouse A, Moran DW. Evolution of brain-computer interfaces: going beyond classic motor physiology. Neurosurg Focus. 2009;27(1):E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Remsik A, Young B, Vermilyea R, et al. A review of the progression and future implications of brain-computer interface therapies for restoration of distal upper extremity motor function after stroke. Expert Rev Med Devices. 2016;13(5):445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mudry A, Mills M. The early history of the cochlear implant: a retrospective. JAMA Otolaryngol Head Neck Surg. 2013;139(5):446–453. [DOI] [PubMed] [Google Scholar]
- 14.Lee B, Zubair MN, Marquez YD, et al. A Single-Center Experience with the NeuroPace RNS System: A Review of Techniques and Potential Problems. World Neurosurg. 2015;84(3):719–726. [DOI] [PubMed] [Google Scholar]
- 15.Chaudhary U, Birbaumer N, Ramos-Murguialday A. Brain-computer interfaces for communication and rehabilitation. Nat Rev Neurol. 2016;12(9):513–525. [DOI] [PubMed] [Google Scholar]
- 16.Cantillo-Negrete J, Carino-Escobar RI, Carrillo-Mora P, Elias-Vinas D, Gutierrez-Martinez J. Motor imagery-based brain-computer interface coupled to a robotic hand orthosis aimed for neurorehabilitation of stroke patients. Journal of healthcare engineering. 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chowdhury A, Raza H, Meena YK, Dutta A, Prasad G. An EEG-EMG correlation-based brain-computer interface for hand orthosis supported neuro-rehabilitation. Journal of neuroscience methods. 2019;312:1–11. [DOI] [PubMed] [Google Scholar]
- 18.Abiri R, Borhani S, Sellers EW, Jiang Y, Zhao X. A comprehensive review of EEG-based brain–computer interface paradigms. Journal of neural engineering. 2019;16(1):011001. [DOI] [PubMed] [Google Scholar]
- 19.Do AH, Wang PT, King CE, Chun SN, Nenadic Z. Brain-computer interface controlled robotic gait orthosis. Journal of neuroengineering and rehabilitation. 2013;10(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu R, Jiang N, Mrachacz-Kersting N, et al. A closed-loop brain–computer interface triggering an active ankle–foot orthosis for inducing cortical neural plasticity. IEEE Transactions on Biomedical Engineering. 2014;61(7):2092–2101. [DOI] [PubMed] [Google Scholar]
- 21.King CE, Dave KR, Wang PT, et al. Performance assessment of a brain–computer interface driven hand orthosis. Annals of biomedical engineering. 2014;42(10):2095–2105. [DOI] [PubMed] [Google Scholar]
- 22.Ang KK, Guan C, Phua KS, et al. Brain-computer interface-based robotic end effector system for wrist and hand rehabilitation: results of a three-armed randomized controlled trial for chronic stroke. Frontiers in neuroengineering. 2014;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brumberg JS, Pitt KM, Mantie-Kozlowski A, Burnison JD. Brain–computer interfaces for augmentative and alternative communication: A tutorial. American journal of speech-language pathology. 2018;27(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brumberg JS, Pitt KM, Burnison JD. A noninvasive brain-computer interface for real-time speech synthesis: The importance of multimodal feedback. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2018;26(4):874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guy V, Soriani M-H, Bruno M, Papadopoulo T, Desnuelle C, Clerc M. Brain computer interface with the P300 speller: usability for disabled people with amyotrophic lateral sclerosis. Annals of physical and rehabilitation medicine. 2018;61(1):5–11. [DOI] [PubMed] [Google Scholar]
- 26.Rabbani Q, Milsap G, Crone NE. The potential for a speech brain–computer interface using chronic electrocorticography. Neurotherapeutics. 2019;16(1):144–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong F, Sahadat MN, Ghovanloo M, Durgin GD. A Stand-Alone Intraoral Tongue-Controlled Computer Interface for People With Tetraplegia. IEEE transactions on biomedical circuits and systems. 2019;13(5):848–857. [DOI] [PubMed] [Google Scholar]
- 28.Rezeika A, Benda M, Stawicki P, Gembler F, Saboor A, Volosyak I. Brain–computer interface spellers: A review. Brain sciences. 2018;8(4):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sellers EW, Donchin E. A P300-based brain–computer interface: initial tests by ALS patients. Clinical neurophysiology. 2006;117(3):538–548. [DOI] [PubMed] [Google Scholar]
- 30.Pandarinath C, Nuyujukian P, Blabe CH, et al. High performance communication by people with paralysis using an intracortical brain-computer interface. Elife. 2017;6:e18554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaudhary U, Xia B, Silvoni S, Cohen LG, Birbaumer N. Brain–computer interface–based communication in the completely locked-in state. PLoS biology. 2017;15(1):e1002593. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Pichiorri F, Mattia D. Brain-computer interfaces in neurologic rehabilitation practice. In: Handbook of Clinical Neurology. Vol 168. Elsevier; 2020:101–116. [DOI] [PubMed] [Google Scholar]
- 33.Huggins JE, Guger C, Ziat M, et al. Workshops of the Sixth International Brain–Computer Interface Meeting: brain–computer interfaces past, present, and future. Brain-computer interfaces. 2017;4(1–2):3–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Remsik A, Young B, Vermilyea R, et al. A review of the progression and future implications of brain-computer interface therapies for restoration of distal upper extremity motor function after stroke. Expert review of medical devices. 2016;13(5):445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kapsalyamov A, Hussain S, Sharipov A, Jamwal P. Brain–computer interface and assist-as-needed model for upper limb robotic arm. Advances in Mechanical Engineering. 2019;11(9):1687814019875537. [Google Scholar]
- 36.Lin DJ, Finklestein SP, Cramer SC. New directions in treatments targeting stroke recovery. Stroke. 2018;49(12):3107–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Micera S, Caleo M, Chisari C, Hummel FC, Pedrocchi A. Advanced Neurotechnologies for the Restoration of Motor Function. Neuron. 2020;105(4):604–620. [DOI] [PubMed] [Google Scholar]
- 38.Lule D, Noirhomme Q, Kleih SC, et al. Probing command following in patients with disorders of consciousness using a brain-computer interface. Clin Neurophysiol. 2013;124(1):101–106. [DOI] [PubMed] [Google Scholar]
- 39.Wang F, He Y, Qu J, et al. A Brain-Computer Interface Based on Three-Dimensional Stereo Stimuli for Assisting Clinical Object Recognition Assessment in Patients With Disorders of Consciousness. IEEE Trans Neural Syst Rehabil Eng. 2019;27(3):507–513. [DOI] [PubMed] [Google Scholar]
- 40.Xiao J, Pan J, He Y, et al. Visual Fixation Assessment in Patients with Disorders of Consciousness Based on Brain-Computer Interface. Neurosci Bull. 2018;34(4):679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie Q, Pan J, Chen Y, et al. A gaze-independent audiovisual brain-computer Interface for detecting awareness of patients with disorders of consciousness. BMC Neurol. 2018;18(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao J, Pan J, He Y, et al. Visual fixation assessment in patients with disorders of consciousness based on brain-computer interface. Neuroscience bulletin. 2018;34(4):679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao J, Xie Q, Lin Q, Yu T, Yu R, Li Y. Assessment of Visual Pursuit in Patients With Disorders of Consciousness Based on a Brain-Computer Interface. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2018;26(6):1141–1151. [DOI] [PubMed] [Google Scholar]
- 44.Chatelle C, Spencer CA, Cash SS, Hochberg LR, Edlow BL. Feasibility of an EEG-based brain-computer interface in the intensive care unit. Clin Neurophysiol. 2018;129(8):1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lech M, Kucewicz MT, Czyzewski A. Human Computer Interface for Tracking Eye Movements Improves Assessment and Diagnosis of Patients With Acquired Brain Injuries. Front Neurol. 2019;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carella T, De Silvestri M, Finedore M, Haniff I, Esmailbeigi H. Emotion Recognition for Brain Machine Interface: Non-linear Spectral Analysis of EEG Signals Using Empirical Mode Decomposition. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference. 2018;2018:223–226. [DOI] [PubMed] [Google Scholar]
- 47.Brandman DM, Cash SS, Hochberg LR. Review: Human Intracortical Recording and Neural Decoding for Brain-Computer Interfaces. IEEE Trans Neural Syst Rehabil Eng. 2017;25(10):1687–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zapała D, Francuz P, Zapała E, et al. The impact of different visual feedbacks in user training on motor imagery control in BCI. Applied psychophysiology and biofeedback. 2018;43(1):23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeunet C, Lotte F, Batail J-M, Philip P, Franchi J-AM. Using recent BCI literature to deepen our understanding of clinical neurofeedback: A short review. Neuroscience. 2018;378:225–233. [DOI] [PubMed] [Google Scholar]
- 50.Schreuder M, Blankertz B, Tangermann M. A new auditory multi-class brain-computer interface paradigm: spatial hearing as an informative cue. PloS one. 2010;5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chatterjee A, Aggarwal V, Ramos A, Acharya S, Thakor NV. A brain-computer interface with vibrotactile biofeedback for haptic information. Journal of neuroengineering and rehabilitation. 2007;4(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lukoyanov M, Gordleeva SY, Pimashkin A, et al. The efficiency of the brain-computer interfaces based on motor imagery with tactile and visual feedback. Human Physiology. 2018;44(3):280–288. [Google Scholar]
- 53.Sitaram R, Caria A, Veit R, et al. FMRI brain-computer interface: a tool for neuroscientific research and treatment. Comput Intell Neurosci. 2007:25487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coyle S, Ward T, Markham C, McDarby G. On the suitability of near-infrared (NIR) systems for next-generation brain-computer interfaces. Physiol Meas. 2004;25(4):815–822. [DOI] [PubMed] [Google Scholar]
- 55.Mellinger J, Schalk G, Braun C, et al. An MEG-based brain-computer interface (BCI). Neuroimage. 2007;36(3):581–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corsi MC, Chavez M, Schwartz D, et al. Integrating EEG and MEG Signals to Improve Motor Imagery Classification in Brain-Computer Interface. Int J Neural Syst. 2019;29(1):1850014. [DOI] [PubMed] [Google Scholar]
- 57.Deshpande G, Rangaprakash D, Oeding L, Cichocki A, Hu XP. A New Generation of Brain-Computer Interfaces Driven by Discovery of Latent EEG-fMRI Linkages Using Tensor Decomposition. Front Neurosci. 2017;11:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hochberg LR, Donoghue JP. Sensors for brain-computer interfaces. IEEE Eng Med Biol Mag. 2006;25(5):32–38. [DOI] [PubMed] [Google Scholar]
- 59.Rao RP. Towards neural co-processors for the brain: combining decoding and encoding in brain-computer interfaces. Curr Opin Neurobiol. 2019;55:142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chiarelli AM, Croce P, Merla A, Zappasodi F. Deep learning for hybrid EEG-fNIRS brain-computer interface: application to motor imagery classification. J Neural Eng. 2018;15(3):036028. [DOI] [PubMed] [Google Scholar]
- 61.Schwemmer MA, Skomrock ND, Sederberg PB, et al. Meeting brain-computer interface user performance expectations using a deep neural network decoding framework. Nature medicine. 2018;24(11):1669–1676. [DOI] [PubMed] [Google Scholar]
- 62.Pandarinath C, Nuyujukian P, Blabe CH, et al. High performance communication by people with paralysis using an intracortical brain-computer interface. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.King CE, Wang PT, Mizuta M, et al. Noninvasive brain-computer interface driven hand orthosis. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference. 2011;2011:5786–5789. [DOI] [PubMed] [Google Scholar]
- 64.Do AH, Wang PT, King CE, Chun SN, Nenadic Z. Brain-computer interface controlled robotic gait orthosis. J Neuroeng Rehabil. 2013;10:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cantillo-Negrete J, Carino-Escobar RI, Carrillo-Mora P, Elias-Vinas D, Gutierrez-Martinez J. Motor Imagery-Based Brain-Computer Interface Coupled to a Robotic Hand Orthosis Aimed for Neurorehabilitation of Stroke Patients. J Healthc Eng. 2018;2018:1624637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chowdhury A, Raza H, Meena YK, Dutta A, Prasad G. An EEG-EMG correlation-based brain-computer interface for hand orthosis supported neuro-rehabilitation. J Neurosci Methods. 2019;312:1–11. [DOI] [PubMed] [Google Scholar]
- 67.Aflalo T, Kellis S, Klaes C, et al. Neurophysiology. Decoding motor imagery from the posterior parietal cortex of a tetraplegic human. Science. 2015;348(6237):906–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Soekadar SR, Witkowski M, Vitiello N, Birbaumer N. An EEG/EOG-based hybrid brain-neural computer interaction (BNCI) system to control an exoskeleton for the paralyzed hand. Biomed Tech (Berl). 2015;60(3):199–205. [DOI] [PubMed] [Google Scholar]
- 69.Bundy DT, Souders L, Baranyai K, et al. Contralesional Brain-Computer Interface Control of a Powered Exoskeleton for Motor Recovery in Chronic Stroke Survivors. Stroke. 2017;48(7):1908–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frolov AA, Mokienko O, Lyukmanov R, et al. Post-stroke Rehabilitation Training with a Motor-Imagery-Based Brain-Computer Interface (BCI)-Controlled Hand Exoskeleton: A Randomized Controlled Multicenter Trial. Front Neurosci. 2017;11:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mohebbi A, Engelsholm SK, Puthusserypady S, Kjaer TW, Thomsen CE, Sorensen HB. A brain computer interface for robust wheelchair control application based on pseudorandom code modulated Visual Evoked Potential. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference. 2015;2015:602–605. [DOI] [PubMed] [Google Scholar]
- 72.Abiyev RH, Akkaya N, Aytac E, Gunsel I, Cagman A. Brain-Computer Interface for Control of Wheelchair Using Fuzzy Neural Networks. Biomed Res Int. 2016;2016:9359868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu Y, Zhou Z, Liu Y, et al. Self-Paced Operation of a Wheelchair Based on a Hybrid Brain-Computer Interface Combining Motor Imagery and P300 Potential. IEEE Trans Neural Syst Rehabil Eng. 2017;25(12):2516–2526. [DOI] [PubMed] [Google Scholar]
- 74.Salisbury DB, Dahdah M, Driver S, Parsons TD, Richter KM. Virtual reality and brain computer interface in neurorehabilitation. Proc (Bayl Univ Med Cent). 2016;29(2):124–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coogan CG, He B. Brain-computer interface control in a virtual reality environment and applications for the internet of things. IEEE Access. 2018;6:10840–10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnson NN, Carey J, Edelman BJ, et al. Combined rTMS and virtual reality brain-computer interface training for motor recovery after stroke. J Neural Eng. 2018;15(1):016009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anumanchipalli GK, Chartier J, Chang EF. Speech synthesis from neural decoding of spoken sentences. Nature. 2019;568(7753):493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chartier J, Anumanchipalli GK, Johnson K, Chang EF. Encoding of Articulatory Kinematic Trajectories in Human Speech Sensorimotor Cortex. Neuron. 2018;98(5):1042–1054 e1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng J, Jin J, Daly I, et al. Effect of a combination of flip and zooming stimuli on the performance of a visual brain-computer interface for spelling. Biomed Tech (Berl). 2019;64(1):29–38. [DOI] [PubMed] [Google Scholar]
- 80.Riccio A, Holz EM, Arico P, et al. Hybrid P300-based brain-computer interface to improve usability for people with severe motor disability: electromyographic signals for error correction during a spelling task. Arch Phys Med Rehabil. 2015;96(3 Suppl):S54–61. [DOI] [PubMed] [Google Scholar]
- 81.Kleih SC, Herweg A, Kaufmann T, Staiger-Salzer P, Gerstner N, Kubler A. The WIN-speller: a new intuitive auditory brain-computer interface spelling application. Front Neurosci. 2015;9:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen X, Wang Y, Nakanishi M, Gao X, Jung TP, Gao S. High-speed spelling with a noninvasive brain-computer interface. Proc Natl Acad Sci U S A. 2015;112(44):E6058–6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cecotti H Spelling with non-invasive Brain-Computer Interfaces--current and future trends. J Physiol Paris. 2011;105(1–3):106–114. [DOI] [PubMed] [Google Scholar]
- 84.Bouton CE, Shaikhouni A, Annetta NV, et al. Restoring cortical control of functional movement in a human with quadriplegia. Nature. 2016;533(7602):247–250. [DOI] [PubMed] [Google Scholar]
- 85.Ajiboye AB, Willett FR, Young DR, et al. Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: a proof-of-concept demonstration. The Lancet. 2017;389(10081):1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sokunbi MO. Using real-time fMRI brain-computer interfacing to treat eating disorders. J Neurol Sci. 2018;388:109–114. [DOI] [PubMed] [Google Scholar]
- 87.Stoeckel LE, Garrison KA, Ghosh S, et al. Optimizing real time fMRI neurofeedback for therapeutic discovery and development. Neuroimage Clin. 2014;5:245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ono T, Shindo K, Kawashima K, et al. Brain-computer interface with somatosensory feedback improves functional recovery from severe hemiplegia due to chronic stroke. Front Neuroeng. 2014;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moghimi S, Kushki A, Marie Guerguerian A, Chau T. A review of EEG-based brain-computer interfaces as access pathways for individuals with severe disabilities. Assistive Technology. 2013;25(2):99–110. [DOI] [PubMed] [Google Scholar]
- 90.Mihara M, Hattori N, Hatakenaka M, et al. Near-infrared spectroscopy-mediated neurofeedback enhances efficacy of motor imagery-based training in poststroke victims: a pilot study. Stroke. 2013;44(4):1091–1098. [DOI] [PubMed] [Google Scholar]
- 91.Sitaram R, Ros T, Stoeckel L, et al. Closed-loop brain training: the science of neurofeedback. Nat Rev Neurosci. 2017;18(2):86–100. [DOI] [PubMed] [Google Scholar]
- 92.Ramos‐Murguialday A, Broetz D, Rea M, et al. Brain–machine interface in chronic stroke rehabilitation: a controlled study. Annals of neurology. 2013;74(1):100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ietswaart M, Johnston M, Dijkerman HC, et al. Mental practice with motor imagery in stroke recovery: randomized controlled trial of efficacy. Brain. 2011;134(5):1373–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wilson RD, Bryden AM, Kilgore KL, et al. Neuromodulation for Functional Electrical Stimulation. Physical medicine and rehabilitation clinics of North America. 2019;30(2):301–318. [DOI] [PubMed] [Google Scholar]
- 95.Green AM, Kalaska JF. Learning to move machines with the mind. Trends Neurosci. 2011;34(2):61–75. [DOI] [PubMed] [Google Scholar]
- 96.Taylor DM, Tillery SIH, Schwartz AB. Direct cortical control of 3D neuroprosthetic devices. Science. 2002;296(5574):1829–1832. [DOI] [PubMed] [Google Scholar]
- 97.Jarosiewicz B, Chase SM, Fraser GW, Velliste M, Kass RE, Schwartz AB. Functional network reorganization during learning in a brain-computer interface paradigm. Proceedings of the National Academy of Sciences. 2008;105(49):19486–19491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shenoy KV, Carmena JM. Combining decoder design and neural adaptation in brain-machine interfaces. Neuron. 2014;84(4):665–680. [DOI] [PubMed] [Google Scholar]
- 99.Sitaram R, Ros T, Stoeckel L, et al. Closed-loop brain training: the science of neurofeedback. Nature Reviews Neuroscience. 2017;18(2):86. [DOI] [PubMed] [Google Scholar]
- 100.Hebb DO. The organization of behavior: a neuropsychological theory. Wiley J; Chapman & Hall; 1949. [Google Scholar]
- 101.Miller KD. Synaptic economics: competition and cooperation in synaptic plasticity. Neuron. 1996;17(3):371–374. [DOI] [PubMed] [Google Scholar]
- 102.Jackson A, Zimmermann JB. Neural interfaces for the brain and spinal cord--restoring motor function. Nat Rev Neurol. 2012;8(12):690–699. [DOI] [PubMed] [Google Scholar]
- 103.Serruya MD. Bottlenecks to clinical translation of direct brain-computer interfaces. Front Syst Neurosci. 2014;8:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bowsher K, Civillico EF, Coburn J, et al. Brain-computer interface devices for patients with paralysis and amputation: a meeting report. J Neural Eng. 2016;13(2):023001. [DOI] [PubMed] [Google Scholar]
- 105.Food, Administration D. Implanted brain-computer interface (BCI) devices for patients with paralysis or amputation---Non-clinical testing and clinical considerations. US Food and Drug Administration. 2019. [Google Scholar]
- 106.Administration FaD. Implanted Brain-Computer Interface (BCI) Devices for Patients with Paralysis or Amputation - Non-clinical Testing and Clinical Considerations Draft Guidance for Industry and Food and Drug Administration Staff 2019.
- 107.Responsive Neurostimulation for the Treatment of Refractory Focal Epilepsy. Blue Cross Blue Shield of Massachusetts. https://www.bluecrossma.com/common/en_US/medical_policies/716%20Responsive%20Neurostimulation%20for%20the%20Treatment%20of%20Refractory%20Focal%20Epilepsy%20prn.pdf. Accessed May 15, 2019, 2019. [Google Scholar]
- 108.Prospectus: Amendment No. 3 to F-1 Registration ReWalk Robotics Ltd; Securities and Exchange Commission. https://www.sec.gov/Archives/edgar/data/1607962/000119312514321636/d724635df1a.htm. Published 2014. Accessed. [Google Scholar]
- 109.Bowsher K, Civillico E, Coburn J, et al. Brain–computer interface devices for patients with paralysis and amputation: a meeting report. Journal of neural engineering. 2016;13(2):023001. [DOI] [PubMed] [Google Scholar]
- 110.Biello PNH Vet Becomes First Fitted With Two ‘LUKE’ Arms. NHPR. New Hampshire Public Radio Web site. https://www.nhpr.org/post/nh-vet-becomes-first-fitted-two-luke-arms#stream/0. Published 2018. Accessed March, 2019. [Google Scholar]
- 111.Ortiz-Catalan M, Mastinu E, Sassu P, Aszmann O, Brånemark R. Self-Contained Neuromusculoskeletal Arm Prostheses. The New England Journal of Medicine. 2020;382(18):1732–1738. [DOI] [PubMed] [Google Scholar]
- 112.Fritz H, Patzer D, Galen SS. Robotic exoskeletons for reengaging in everyday activities: promises, pitfalls, and opportunities. Disability and rehabilitation. 2019;41(5):560–563. [DOI] [PubMed] [Google Scholar]
- 113.Gardner AD, Potgieter J, Noble FK. A review of commercially available exoskeletons’ capabilities. Paper presented at: 2017 24th International Conference on Mechatronics and Machine Vision in Practice (M2VIP)2017. [Google Scholar]
- 114.Bissolotti L, Nicoli F, Picozzi M. Domestic Use of the Exoskeleton for Gait Training in Patients with Spinal Cord Injuries: Ethical Dilemmas in Clinical Practice. Frontiers in neuroscience. 2018;12:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.ReWalk. ReWalk https://rewalk.com/training-centers/. Published 2019. Accessed.
- 116.Weber LM, Stein J. The use of robots in stroke rehabilitation: A narrative review. NeuroRehabilitation. 2018;43(1):99–110. [DOI] [PubMed] [Google Scholar]
- 117.Rojek A, Mika A, Oleksy Ł, Stolarczyk A, Kielnar R. Effects of Exoskeleton Gait Training on Balance, Load Distribution, and Functional Status in Stroke: A Randomized Controlled Trial. Frontiers in Neurology. 2020;10:1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pratt AK, Chang JJ, Sederstrom NO. A Fate Worse Than Death: Prognostication of Devastating Brain Injury. Crit Care Med. 2019;47(4):591–598. [DOI] [PubMed] [Google Scholar]
- 119.Slutzky MW, Flint RD. Physiological properties of brain-machine interface input signals. Journal of neurophysiology. 2017;118(2):1329–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Salatino JW, Ludwig KA, Kozai TD, Purcell EK. Glial responses to implanted electrodes in the brain. Nature biomedical engineering. 2017;1(11):862–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ravikumar M, Sunil S, Black J, et al. The roles of blood-derived macrophages and resident microglia in the neuroinflammatory response to implanted intracortical microelectrodes. Biomaterials. 2014;35(28):8049–8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wellman SM, Eles JR, Ludwig KA, et al. A materials roadmap to functional neural interface design. Advanced functional materials. 2018;28(12):1701269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hochberg LR, Donoghue JP. Sensors for brain-computer interfaces. IEEE Engineering in Medicine and Biology Magazine. 2006;25(5):32–38. [DOI] [PubMed] [Google Scholar]
- 124.Sillay KA, Ondoma S, Wingeier B, et al. Long-Term Surface Electrode Impedance Recordings Associated with Gliosis for a Closed-Loop Neurostimulation Device. Annals of neurosciences. 2018;25(4):289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Parker RA, Davis TS, House PA, Normann RA, Greger B. The functional consequences of chronic, physiologically effective intracortical microstimulation. In: Progress in brain research. Vol 194. Elsevier; 2011:145–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Martini ML, Oermann EK, Opie NL, Panov F, Oxley T, Yaeger K. Sensor modalities for brain-computer interface technology: a comprehensive literature review. Neurosurgery. 2020;86(2):E108–E117. [DOI] [PubMed] [Google Scholar]
- 127.Thompson C, Patel PR, Chestek CA, Li W, Purcell EK. Toward guiding principles for the design of biologically-integrated electrodes for the central nervous system. Journal of Neural Engineering. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Das R, Moradi F, Heidari H. Biointegrated and Wirelessly Powered Implantable Brain Devices: A Review. IEEE Transactions on Biomedical Circuits and Systems. 2020. [DOI] [PubMed] [Google Scholar]
- 129.Silva GA. A New Frontier: The Convergence of Nanotechnology, Brain Machine Interfaces, and Artificial Intelligence. Front Neurosci. 2018;12:843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Scaini D, Ballerini L. Nanomaterials at the neural interface. Curr Opin Neurobiol. 2018;50:50–55. [DOI] [PubMed] [Google Scholar]
- 131.Hong G, Yang X, Zhou T, Lieber CM. Mesh electronics: a new paradigm for tissue-like brain probes. Curr Opin Neurobiol. 2018;50:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Letourneau S, Zewdie ET, Jadavji Z, Andersen J, Burkholder LM, Kirton A. Clinician awareness of brain computer interfaces: a Canadian national survey. Journal of NeuroEngineering and Rehabilitation. 2020;17(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hendriks S, Grady C, Ramos KM, et al. Ethical Challenges of Risk, Informed Consent, and Posttrial Responsibilities in Human Research With Neural Devices: A Review. JAMA neurology. 2019;76(12):1506–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shih JJ, Krusienski DJ, Wolpaw JR. Brain-computer interfaces in medicine. Mayo Clin Proc. 2012;87(3):268–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Young MJ, Scheinberg E. The Rise of Crowdfunding for Medical Care: Promises and Perils. JAMA. 2017;317(16):1623–1624. [DOI] [PubMed] [Google Scholar]
- 136.Judson TJ, Dhruva SS, Redberg RF. Evaluation of technologies approved for supplemental payments in the United States. Bmj. 2019;365:l2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Young M Brain-Computer Interfaces and the Philosophy of Action. AJOB neuroscience. 2020;11(1):4. [DOI] [PubMed] [Google Scholar]
- 138.Bernat JL. Medical Decision Making by Patients in the Locked-in Syndrome. Neuroethics. 2018:1–10. [Google Scholar]
- 139.Gilbert F, Cook M, O’Brien T, Illes J. Embodiment and estrangement: results from a first-in-human “intelligent BCI” trial. Science and engineering ethics. 2019;25(1):83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Agarwal A, Dowsley R, McKinney ND, et al. Protecting Privacy of Users in Brain-Computer Interface Applications. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2019;27(8):1546–1555. [DOI] [PubMed] [Google Scholar]
- 141.Kögel J, Jox RJ, Friedrich O. What is it like to use a BCI?–insights from an interview study with brain-computer interface users. BMC Medical Ethics. 2020;21(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Greely HT, Grady C, Ramos KM, et al. Neuroethics guiding principles for the NIH BRAIN initiative. The Journal of Neuroscience. 2018;38(50):10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shook JR, Giordano J. Neuroethics beyond normal: Performance enablement and self-transformative technologies. Cambridge Quarterly of Healthcare Ethics. 2016;25(1):121–140. [DOI] [PubMed] [Google Scholar]
- 144.Eaton ML, Illes J. Commercializing cognitive neurotechnology—the ethical terrain. Nature biotechnology. 2007;25(4):393–397. [DOI] [PubMed] [Google Scholar]
- 145.Klein E Informed consent in implantable BCI research: identifying risks and exploring meaning. Science and engineering ethics. 2016;22(5):1299–1317. [DOI] [PubMed] [Google Scholar]
- 146.Nijboer F, Clausen J, Allison BZ, Haselager P. The Asilomar Survey: Stakeholders’ Opinions on Ethical Issues Related to Brain-Computer Interfacing. Neuroethics. 2013;6:541–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Young MJ. Bioenhancements and the telos of medicine. Med Health Care Philos. 2015;18(4):515–522. [DOI] [PubMed] [Google Scholar]
- 148.Salles A, Bjaalie JG, Evers K, et al. The Human Brain Project: Responsible Brain Research for the Benefit of Society. Neuron. 2019;101(3):380–384. [DOI] [PubMed] [Google Scholar]


