ABSTRACT
Linezolid plays a crucial role in the treatment of infections caused by multiresistant Gram-positive bacteria. The poxtA gene not only confers oxazolidinone and phenicol resistance but also decreases susceptibility to tetracycline. In this study, we investigated structural changes in mobilizable poxtA-carrying plasmids in enterococci which occurred during conjugation experiments using S1-PFGE (pulsed-field gel electrophoresis), Southern blot hybridization, and whole-genome sequencing (WGS) analysis. Two poxtA-carrying strains were identified in Enterococcus faecalis E006 and Enterococcus lactis E843, respectively. E. faecalis E006 contains the 121,520-bp conjugative plasmid pE006-121 and the 19,832-bp mobilizable poxtA-carrying plasmid pE006-19, while E. lactis E843 contains the 171,930-bp conjugative plasmid pE843-171 and the 27,847-bp mobilizable poxtA-carrying plasmid pE843-27. Moreover, both poxtA-carrying plasmids were mobilized by their respective conjugative plasmid in enterococci by plasmid fusion; one was generated by homologous recombination in E. faecalis through an identical 864-bp homologous region in the plasmids of the parental strain, while another was generated by an IS1216E-mediated plasmid integration in E. lactis, involving a replicative transposition.
IMPORTANCE Until now, all the poxtA genes described in enterococci, including E. faecalis, E. faecium, and E. hirae, are plasmid-borne, suggesting that plasmids play an important role in the dissemination of the poxtA gene among enterococci. This study showed that the mobilizable poxtA-carrying plasmid could transfer with the help of conjugative plasmid in enterococci via plasmid fusion, with one generated by homologous recombination in E. faecalis, and another by replicative transposition in E. lactis. During both the fusion events, the poxtA-carrying plasmids changed from nonconjugative to conjugative, leading to the generation and enhanced dissemination of the larger phenicol-oxazolidinone-tetracycline resistance-encoding plasmids in enterococci.
KEYWORDS: enterococci, linezolid resistance, poxtA, plasmid integration
INTRODUCTION
Linezolid is a one of the last therapeutic options for the treatment of clinical infections caused by MDR Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE) and penicillin-resistant Streptococcus pneumoniae (PRSP). Florfenicol is a broad-spectrum phenicol drug exclusively used in animals to control respiratory tract infections (1, 2). However, the emergence of cross-resistance to linezolid and florfenicol poses a serious challenge to both human and veterinary medicine. To date, at least seven acquired linezolid/florfenicol resistance genes, belonging to three different groups, have been reported, including cfr, cfr(B), cfr(C), cfr(D), cfr(E), optrA, and poxtA (3–10). The cfr gene and its variants code for 23S rRNA methylases, while the optrA and poxtA genes encode the ribosome-protective proteins of the ABC-F family. The cfr gene confers resistance to phenicols, lincosamides, oxazolidinones (linezolid but not tedizolid), pleuromutilins, and streptogramin A (the so-called PhLOPSA phenotype). It was first described in Staphylococcus sciuri (recently reclassified as Mammaliicoccus sciuri) and thereafter detected in various Gram-positive and Gram-negative bacteria. Unlike cfr, the optrA gene confers resistance to tedizolid, a novel oxazolidinone, in addition to linezolid and phenicol resistance. It was first identified in Enterococcus faecalis and E. faecium (4) but has also been detected in various other Gram-positive bacteria (11). The poxtA gene, which confers decreased susceptibility to tetracycline in addition to phenicol and oxazolidinone resistance, has recently been identified in MRSA and enterococci (9–14). More recently, poxtA2 was detected in Enterococcus gallinarum (15).
The prevalence of the poxtA gene has been investigated in enterococci from food-producing animal farms in China, Italy, and Korea, as well as in linezolid-resistant Enterococcus spp. (LRE) from patients in Ireland, Spain, and France (13, 16–22). In China, Lei et al. reported that the poxtA gene was present in 5.5% (19/345) of enterococci isolated from 36 food-producing animal farms across 12 provinces in 2017 (16). However, in our previous study, the poxtA gene was present in 57.9% (66/114) of florfenicol-resistant enterococci isolated from two swine farms in Henan Province in 2018, possibly the result of widespread use of florfenicol and doxycycline in these swine farms (13). In Italy, Fioriti et al. reported the poxtA gene with the prevalence of 20.7% (30/145) in florfenicol-resistant enterococci isolated from swine fecal samples (17). In Korea, 156 linezolid-resistant isolates were detected in 5,482 enterococci isolated from food-producing animals during 2008 to 2018, and 23.1% of these (36/156) had the poxtA gene (18). In addition, Kim et al. reported the poxtA gene with a prevalence of 2.5% (8/327) in enterococci isolated from food-producing animals and meat in Korea in 2018 (19). In Ireland, 15 poxtA-carrying enterococci were identified in 154 LRE which were recovered from patients in 14 hospitals between June 2016 and August 2019 (20). In Spain, clinical LRE isolates from different hospitals were submitted to the Spanish Reference Laboratory from 2015 to 2018, and 6.2% of them (6/97) were poxtA-positive (21). In France, out of 466 LRE received by the National Reference Centre for Enterococci from French hospitals between 2016 and 2020, 47 (10.1%) were poxtA-positive (22).
Previously, all of the poxtA genes described in enterococci have been plasmid-borne, suggesting that plasmids play an important role in the dissemination of the poxtA gene among enterococci (22, 23). In this study, two mobilizable poxtA-carrying plasmids were identified in E. faecalis and E. lactis, respectively. In addition, two fusion events involving homologous recombination and replicative transposition were investigated, which transferred the poxtA gene from mobilizable plasmids into conjugative plasmids. Consequently, continuous monitoring of poxtA-mediated linezolid resistance in enterococci is urgently needed.
RESULTS
Linezolid resistance mediated by plasmids in enterococci was transferable.
The florfenicol- and linezolid-resistant E. faecalis E006 (ST692) and E. lactis E843 were poxtA-positive. Conjugation assays showed that the poxtA gene in E. faecalis E006 and E. lactis E843 could be transferred to the recipient strains E. faecalis JH2-2 (ST8) and E. faecium GE-1 (ST515), respectively. The corresponding transconjugants were designated E. faecalis E006×JH2-2-TC1 (ST8) and E. faecium E843×GE-1-TC1 (ST515), respectively. Antimicrobial susceptibility testing (AST) results for the donor strains, their transconjugants, and the recipient strains are shown in Table 1. Both transconjugants displayed elevated MICs of the respective antimicrobial agents compared with those of the recipient strain. S1-PFGE (pulsed-field gel electrophoresis) showed that there was a single plasmid in both the transconjugant E. faecalis E006×JH2-2-TC1 and E. faecium E843×GE-1-TC1, but two plasmids in their parental strains (Fig. 1). Moreover, the size of the plasmids in the transconjugants was almost equivalent to that of one of the plasmids in their parental strains. Southern blot hybridization confirmed that the poxtA gene was located on the smaller plasmid in the parental strains and on the single plasmid in the transconjugants (Fig. 1). To investigate how the poxtA gene could be transferred from the small plasmid in the parental strain to the larger plasmid in the transconjugants, whole-genome DNA sequencing was performed for all four stains (E. faecalis E006, E. lactis E843, transconjugants E. faecalis E006×JH2-2-TC1, and E. faecium E843×GE-1-TC1). In addition, the larger plasmids in E. faecalis E006×JH2-2-TC1 and E. faecium E843×GE-1-TC1 could further be transferred to the recipient strains E. faecalis E533 (ST69) and E. faecalis JH2-2 at frequencies of 1.7 × 10−9 and 1.4 × 10−6, respectively, indicating that the poxtA-carrying plasmids can change from mobilizable to conjugative and become self-transferable.
TABLE 1.
The antimicrobial susceptibilities of the donor strains, transconjugants, and recipient strains used in this studya
| Strains | MICs (mg/L) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RIF | FUS | FFC | LZD | TZD | TET | GEN | VAL | LIN | ERY | |
| E. faecalis | ||||||||||
| Donor E006 | <1 | 2 | 128 | 16 | 4 | 128 | >128 | >128 | >128 | >128 |
| Recipient JH2-2 | >128 | >128 | 2 | 2 | 1 | 2 | 32 | >128 | 16 | <1 |
| Transconjugant E006×JH2-2-TC1 | >128 | >128 | 64 | 16 | 4 | <1 | >128 | >128 | >128 | >128 |
| E. faecium | ||||||||||
| Donor E843 | 16 | 2 | 128 | 4 | 1 | 128 | >128 | 32 | >128 | >128 |
| Recipient GE-1 | >128 | >128 | 1 | 1 | 0.5 | 64 | 8 | <1 | 16 | 2 |
| Transconjugant E843×GE-1-TC1 | >128 | 128 | 8 | 4 | 1 | 128 | >128 | 16 | >128 | >128 |
RIF, rifampicin; FUS, fusidic acid; FFC, florfenicol; LZD, linezolid; TZD, tedizolid; TET, tetracycline; GEN, gentamicin; VAL, valnemulin; LIN, lincomycin; ERY, erythromycin.
FIG 1.
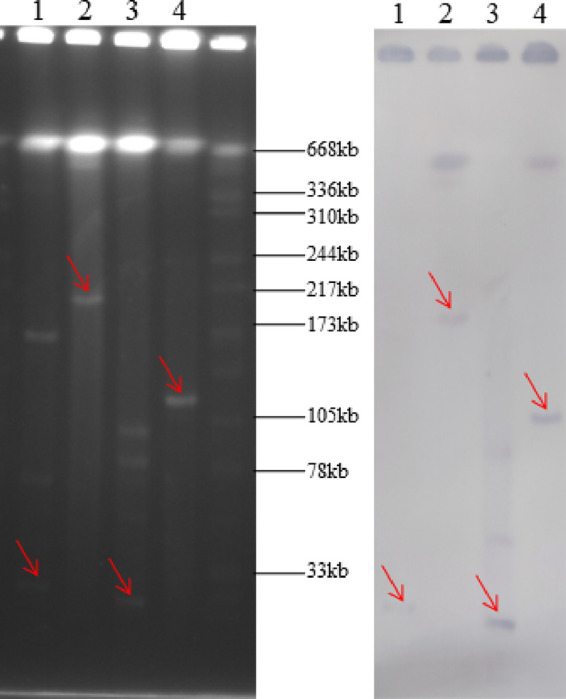
Detection of poxtA-carrying plasmids in E. faecalis E006, E. lactis E843 and their transconjugants by S1-PFGE (left) and Southern hybridization (right) with poxtA specific probe. Lane 1, E. lactis E843; lane 2, transconjugant E. faecium E843×GE-1-TC1; lane 3, E. faecalis E006; lane 4, transconjugant E. faecalis E006×JH2-2-TC1.
Genetic basis of transmission of linezolid resistance in E. faecalis.
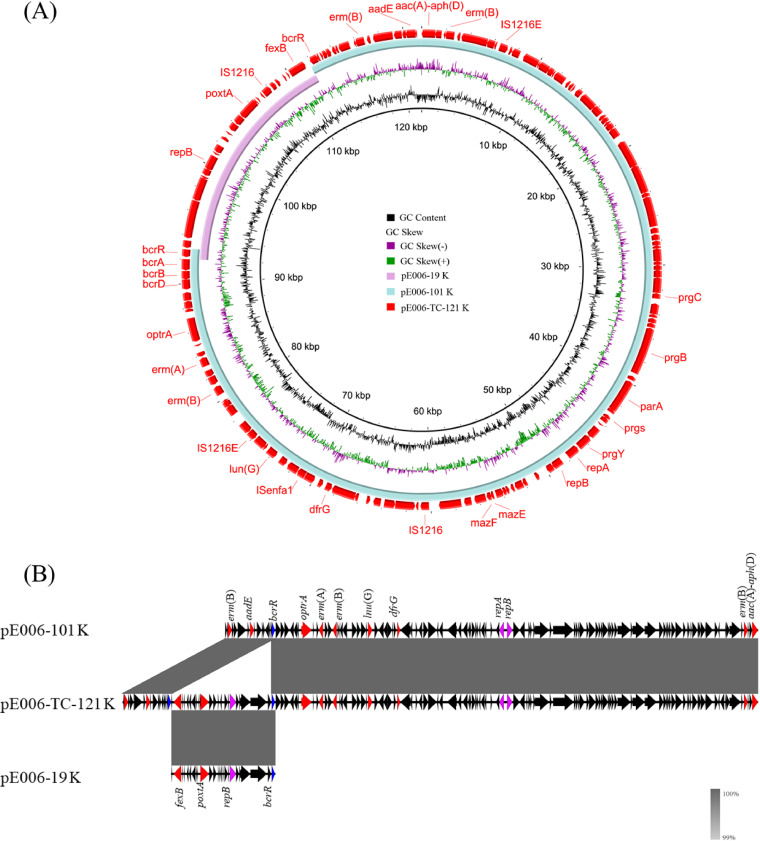
For the two plasmids in E. faecalis E006, the larger plasmid was designated pE006-101 and the smaller one was designated pE006-19. The conjugative plasmid pE006-101 is 101,692 bp in size and harbors three copies of the macrolide-lincosamide-streptogramin B resistance gene erm(B) and one copy of erm(A). In addition, it also carries single copies of the florfenicol/oxazolidinone resistance gene optrA, the lincosamide resistance gene lnu(B), the trimethoprim resistance gene dfrG, the gentamicin-tobramycin-kanamycin resistance gene aac(A)-aph(D), the streptomycin resistance gene aadE, and the bacitracin resistance operon bcrABDR. Moreover, like the previously described optrA-carrying pheromone responsive plasmid pEF10748, pE006-101 is a rep9–type plasmid (pCF10 prototype, the best-studied pheromone responsive plasmid) by plasmid typing, and harbors the essential sex pheromone response genes, such as prgA, prgB, and prgC (Fig. 2A; 24). The mobilizable plasmid pE006-19 is 19,832 bp in size and carries the florfenicol resistance gene fexB, the gene poxtA, and bcrR, which is a part of the bacitracin resistance operon bcrABDR (Fig. 2A).
FIG 2.
Formation of conjugative fusion plasmid pE006-TC-121 co-carrying optrA and poxtA. Alignment of poxtA-carrying mobilizable plasmid pE006-19 and pheromone responsive conjugative plasmid pE006-101 recovered from E. faecalis E006 using the BLAST Ring Image Generator (BRIG) (panel A) and Easyfig (panel B).
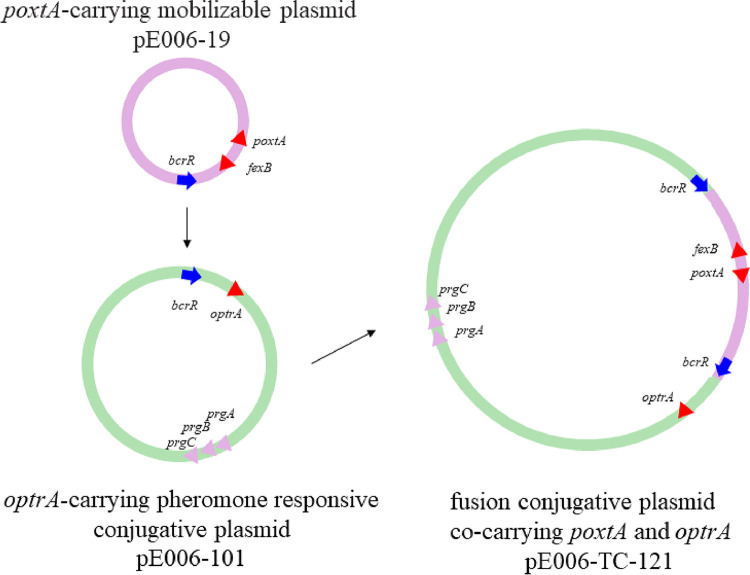
Only one conjugative plasmid, 121,537 bp in size and designated pE006-TC-121, was detected in the transconjugant E006×JH2-2-TC1. Sequence analysis revealed that pE006-TC-121 was obtained through fusion between pE006-101 and pE006-19 (Fig. 2). Detailed analysis of these three plasmids suggested that the mechanism of plasmid fusion was due to homologous recombination involving an 864-bp homologous region (HR) which included bcrR. One copy each of this HR was detected in pE006-101 and pE006-19, but two copies were detected in pE006-TC-121. The HRs in all three plasmids were identical. According to these results, we propose that pE006-101 and pE006-19 underwent homologous recombination at the HR, resulting in the formation of the fusion plasmid pE006-TC-121 (Fig. 3).
FIG 3.
Mechanism of plasmid fusion by homologous recombination. Fusion plasmid was generated by homologous recombination involving an 864-bp HR which included bcrR.
Genetic basis of transmission of linezolid resistance in E. lactis.
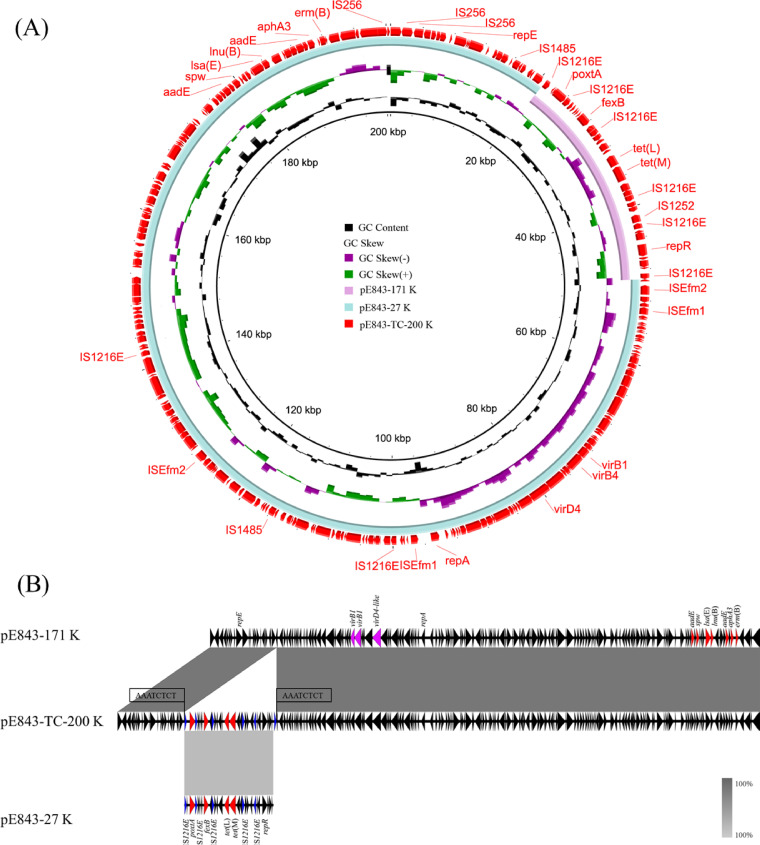
A plasmid fusion event was also observed in E. lactis E843. There are two plasmids in the parental strain, designated pE843-171 and pE843-27, as well as one plasmid in the transconjugant E843×GE-1-TC1, designated pE843-200. Plasmid pE843-171 is 171,930 bp in size and harbors two copies of the streptomycin resistance gene aadE, as well as single copies of the macrolide-lincosamide-streptogramin B resistance gene erm(B), the neomycin-kanamycin resistance gene aphA3, the lincosamide resistance gene lnu(B), the pleuromutilin-lincosamide-streptogramin A (PLSA) resistance gene lsa(E), and the spectinomycin resistance gene spw. All resistance genes were clustered in an ∼14-kb region. pE843-171 was found to be a pLG1-like conjugative plasmid with essential type IV secretion system (T4SS) genes (25), such as virB1, virB4, and virD4 homologues (Fig. 4A). The mobilizable plasmid pE843-27 is 27,847 bp in size and carries the florfenicol resistance genes poxtA and fexB and the tetracycline resistance genes tet(M) and tet(L). In pE843-27, there are a total of six copies of IS1216E, with five of them located in the same orientation (Fig. 4A).
FIG 4.
Formation of conjugative fusion plasmid pE843-TC-200. Alignment of poxtA-carrying mobilizable plasmid pE843-27 and conjugative pLG1-like plasmid pE843-171 recovered from E. lactis E843 using the BLAST Ring Image Generator (BRIG) (panel A) and Easyfig (panel B).
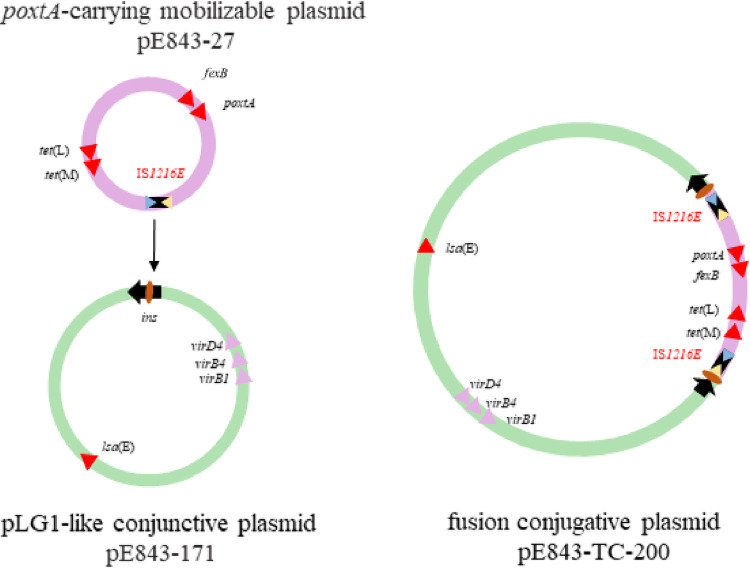
As in E006×JH2-2-TC1, there was only one conjugative plasmid in the transconjugant E843×GE-1-TC1. This plasmid, designated pE843-TC-200, is 200,566 bp in size (Fig. 4A). Based on the detailed sequence analysis, the formation of this fusion plasmid was due to a series of genetic events generated by IS1216E. We propose that IS1216E in the mobilizable plasmid pE843-27 attached to the target site (AAATCTCT) in the pLG1-like plasmid pE843-171; then, the replicative transposition occurred, and pE843-27 was inserted into pE843-171, forming a characteristic 8-bp target site duplication (TSD, 5′-AAATCTCT-3′) and generating an additional copy of IS1216E in the fusion plasmid pE843-200 (Fig. 4 and 5).
FIG 5.
Mechanism of plasmid fusion by replicative transposition. IS1216E in mobilizable plasmid pE843-27, attached to the target site (AAATCTCT) in the pLG1-like plasmid pE843-171, forming a characteristic 8-bp target site duplication (TSD, AAATCTCT) and generating an additional copy of IS1216E downstream from the fusion plasmid pE843-200.
DISCUSSION
Enterococci are opportunistic pathogens which have become one of the main causes of nosocomial and community-acquired human infections, including septicemia, endocarditis, and urinary tract infections (26). Their intrinsic resistances and ability to acquire resistances through mobile genetic elements (MGEs) have compromised the choice of therapeutic options to treat enterococcal infections. Among MGEs, conjugative plasmids play a key role in the dissemination of resistance genes, as conjugative plasmids are self-transmissible and can mobilize nonconjugative plasmids present in the same enterococcal cell (27).
In Gram-negative bacteria, IS26-mediated replicative transposition and translocation play an important role in mobilizing antimicrobial resistance genes (28, 29). IS1216 in Gram-positive bacteria and IS26 in Gram-negative bacteria belong to the IS6/IS26 family of bacterial insertion sequences and are assumed to exert similar functions. To our knowledge, the genetic environment of the poxtA gene, as described previously, contains IS1216E (23), suggesting its potential role in the dissemination of the poxtA gene. However, how IS1216E promotes the transmission of the poxtA gene is not yet fully explored.
In our previous study, the role of IS1216E-mediated transposition and translocation was shown in the formation of poxtA fusion plasmids (30). By IS1216E-mediated replicative transposition, a fusion poxtA-carrying plasmid was generated through a poxtA-carrying transposon fused into another small plasmid. Moreover, a new, larger fusion plasmid was formed via IS1216E-mediated translocation, generated by the integration of an IS1216E-based transmissible unit into the mobilizable poxtA-carrying plasmid (30). In both cases, the fused poxtA plasmids could be mobilized with the aid of conjugative plasmids in the same strains.
Transmissible plasmids can be classified as conjugative or mobilizable plasmids. In order to realize the transfer of the mobilizable plasmids, at least two processes must be taken into account: (i) the mobilizable plasmids can be mobilized by functions encoded in trans provided by other auxiliary conjugative elements, where a distinct relaxase gene expressing a Mob protein on the majority of mobilizable plasmids targets an oriT and the resulting relaxosome is recruited to the T4SS encoded by a coresident conjugative element (27), as observed in our previous study (30); or (ii) the mobilizable plasmids could be directly fused to conjugative plasmids by IS-mediated replicative transposition or homologous recombination through an identical HR, which caused the mobilizable plasmids to become conjugative, as observed in this study.
Several studies have shown that replicative transposition or homologous recombination played an important role in the evolution of plasmid backbones and the acquisition of antimicrobial resistance genes in Gram-negative bacteria (28, 29, 31–33). To the best of our knowledge, this is the first time that the roles of replicative transposition and homologous recombination were shown in the shaping of poxtA-carrying plasmids from mobilizable to conjugative in enterococci, which are important health care-associated Gram-positive opportunistic pathogens. Because the mobilizable plasmids are not self-transmissible, their transmission usually requires the assistance of conjugative plasmids or other conjugative elements. In this study, replicative transposition and homologous recombination have been shown to participate in changing two mobilizable poxtA-carrying plasmids from nonconjugative to conjugative; as a result, these fused poxtA-carrying plasmids become self-transmissible, which accelerates their transmission.
Pheromone responsive plasmid transfer systems are highly efficient for genetic exchanges that allow antimicrobial resistance genes to be spread within bacterial populations (34). In our previous study, two pheromone-responsive conjugative optrA-carrying plasmids which showed high transfer frequencies of ∼10−2 were identified in porcine E. faecalis isolates (35). In a recent study, pheromone-responsive plasmids were proven to be the predominant plasmids in optrA-carrying E. faecalis clinical isolates, and most of them were conjugative (24). In this study, the conjugative optrA-carrying pheromone-responsive plasmid pE006-101 fused with the mobilizable poxtA-carrying plasmid, suggesting that linezolid resistance co-conferred by both optrA and poxtA genes in the same plasmid could be widely disseminated through sex pheromone plasmids.
In summary, this study showed that mobilizable poxtA-carrying plasmids can transfer through fusion into conjugative plasmids in enterococci. Two fusion events were identified: one through homologous recombination via an identical HR, the other through replicative transposition mediated by IS1216E. In both fusion events, the poxtA-carrying plasmids changed from mobilizable to conjugative, leading to the generation of new plasmids and the potentially enhanced dissemination of the poxtA gene in enterococci.
MATERIALS AND METHODS
Bacterial strains and antimicrobial susceptibility testing.
E. faecalis E006 and E. lactis E843 were isolated from fecal samples during a routine survey of antimicrobial resistance in bacteria from pigs in Henan Province, China, in 2018. Strain species of E843 and E006 were identified by PCR with 16s rRNA primers, followed by sequencing. E. faecalis JH2-2 (RIFr), E. faecium GE-1 (RIFr), and E. faecalis E533 (a strain without plasmids, identified and stored in our lab, TETr) served as recipient strains in transfer experiments. AST was performed using broth microdilution according to the recommendations given in the M100 (31st ed.) of the Clinical and Laboratory Standards Institute (CLSI; 36). S. aureus ATCC 29213 served as the quality control strain.
PCR analysis and transfer experiments.
The presence of the resistance gene poxtA in E. faecalis E006 and E. lactis E843 was determined by PCR using the primers described previously (13). All PCR products were subjected to Sanger sequencing. Conjugation experiments were performed as previously described (37). In brief, the overnight cultures of donors and recipients were mixed at a ratio of 1:4 (200:800 μL), centrifuged, and suspended with 100 μL fresh brain heart infusion (BHI) broth; the mixed bacteria were then plated on BHI agar at 37°C for 18 h. The bacteria grown on the BHI agar were sluiced off with 4 mL BHI broth, and 100 μL of the culture was plated on selective BHI agar. When E. faecalis E006 was used as the donor, E. faecalis JH2-2 was used as the recipient; when E. lactis E843 was used as the donor, E. faecium GE-1 served as the recipient. Transconjugants were selected on BHI agar containing rifampicin (50 mg/L) and florfenicol (10 mg/L) and further confirmed by AST and multilocus sequence typing (MLST) following harmonized protocols (https://pubmlst.org/). The positive transconjugants were E. faecalis E006×JH2-2-TC1 and E. faecium E843×GE-1-TC1, respectively. To assess the self-transferability of the fusion plasmids, further conjugation experiments were performed. When E. faecalis E006×JH2-2-TC1 was used as the donor, E. faecalis E533 (TETr) was used as the recipient, and the transconjugants were selected on BHI agar containing tetracycline (10 mg/L) and florfenicol (10 mg/L). When E. faecium E843×GE-1-TC1 was used as the donor, E. faecalis JH2-2 [VALr, natural resistance due to the presence of lsa(A)] served as the recipient, and the transconjugants were selected on BHI agar containing valnemulin (64 mg/L) and florfenicol (4 mg/L). All transconjugants were further confirmed using the methods described above.
S1-PFGE and Southern blot hybridization.
The genomic DNA was digested with S1 endonuclease (New England Biolabs, Beverly, MA, USA) separated by PFGE as previously described (38). After transfer to Amersham Hybond-N+ membranes (GE Healthcare), the genomic DNA was hybridized with a poxtA probe as described in other studies (30).
WGS and sequence analysis.
To investigate the genetic basis of plasmid alterations in transconjugants, whole-genome DNA of E. faecalis E006 and E. lactis E843, and their transconjugants, E006×JH2-2-TC1 and E843×GE-1-TC1, was sequenced using the Oxford Nanopore and Illumina MiSeq platforms (Shanghai Personal Biotechnology Co. Ltd., China). The Nanopore sequence reads were assembled with HGAP4 and CANU (version 1.6) and corrected by Illumina MiSeq with Pilon (version 1.22). The prediction of open reading frames and their annotation was performed using Glimmer 3.0. The plasmid replicon genotype was identified using PlasmidFinder (https://cge.cbs.dtu.dk/services/PlasmidFinder/). Insertion sequence (IS) elements were identified using ISfinder (https://isfinder.biotoul.fr/). Comparative analysis and plasmid maps were generated by Easyfig and BRIG (39, 40).
Accession number(s).
The sequences of the six plasmids pE006-101, pE006-19, pE006-TC-121, pE843-171, pE843-27, and pE843-TC-200, determined in this study, have been deposited in GenBank under the accession numbers CP082232, CP082233, CP081506, CP082266, CP082268, and CP081503, respectively.
ACKNOWLEDGMENTS
This work was supported by grants from the China Postdoctoral Science Foundation (no. 2018M642751) and the German Federal Ministry of Education and Research (BMBF) under project number 01KI1727D as part of the Research Network of Zoonotic Infectious Diseases.
Contributor Information
Dexi Li, Email: lidexi2006@126.com.
Xiang-Dang Du, Email: xddu@henau.edu.cn.
Tino Polen, Forschungszentrum Jülich.
REFERENCES
- 1.Hashemian SMR, Farhadi T, Ganjparvar M. 2018. Linezolid: a review of its properties, function, and use in critical care. Drug Des Devel Ther 12:1759–1767. doi: 10.2147/DDDT.S164515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Garch F, de Jong A, Simjee S, Moyaert H, Klein U, Ludwig C, Marion H, Haag-Diergarten S, Richard-Mazet A, Thomas V, Siegwart E. 2016. Monitoring of antimicrobial susceptibility of respiratory tract pathogens isolated from diseased cattle and pigs across Europe, 2009-2012: VetPath results. Vet Microbiol 194:11–22. doi: 10.1016/j.vetmic.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Kehrenberg C, Ojo KK, Schwarz S. 2004. Nucleotide sequence and organization of the multiresistance plasmid pSCFS1 from Staphylococcus sciuri. J Antimicrob Chemother 54:936–939. doi: 10.1093/jac/dkh457. [DOI] [PubMed] [Google Scholar]
- 4.Deshpande LM, Ashcraft DS, Kahn HP, Pankey G, Jones RN, Farrell DJ, Mendes RE. 2015. Detection of a new cfr-like gene, cfr(B), in Enterococcus faecium isolates recovered from human specimens in the United States as part of the SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother 59:6256–6261. doi: 10.1128/AAC.01473-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang Y, Dai L, Sahin O, Wu Z, Liu M, Zhang Q. 2017. Emergence of a plasmid-borne multidrug resistance gene cfr(C) in foodborne pathogen Campylobacter. J Antimicrob Chemother 72:1581–1588. doi: 10.1093/jac/dkx023. [DOI] [PubMed] [Google Scholar]
- 6.Pang S, Boan P, Lee T, Gangatharan S, Tan SJ, Daley D, Lee YT, Coombs GW. 2020. Linezolid-resistant ST872 Enteroccocus faecium harbouring optrA and cfr (D) oxazolidinone resistance genes. Int J Antimicrob Agents 55:105831. doi: 10.1016/j.ijantimicag.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Stojković V, Ulate MF, Hidalgo-Villeda F, Aguilar E, Monge-Cascante C, Pizarro-Guajardo M, Tsai K, Tzoc E, Camorlinga M, Paredes-Sabja D, Quesada-Gómez C, Fujimori DG, Rodríguez C. 2019. cfr(B), cfr(C), and a new cfr-like gene, cfr(E), in Clostridium difficile strains recovered across Latin America. Antimicrob Agents Chemother 64:e01074-19. doi: 10.1128/AAC.01074-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Lv Y, Cai J, Schwarz S, Cui L, Hu Z, Zhang R, Li J, Zhao Q, He T, Wang D, Wang Z, Shen Y, Li Y, Feßler AT, Wu C, Yu H, Deng X, Xia X, Shen J. 2015. A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J Antimicrob Chemother 70:2182–2190. doi: 10.1093/jac/dkv116. [DOI] [PubMed] [Google Scholar]
- 9.Antonelli A, D'Andrea MM, Brenciani A, Galeotti CL, Morroni G, Pollini S, Varaldo PE, Rossolini GM. 2018. Characterization of poxtA, a novel phenicol-oxazolidinone-tetracycline resistance gene from an MRSA of clinical origin. J Antimicrob Chemother 73:1763–1769. doi: 10.1093/jac/dky088. [DOI] [PubMed] [Google Scholar]
- 10.Li D, Cheng Y, Schwarz S, Yang M, Du XD. 2020. Identification of a poxtA- and cfr-carrying multiresistant Enterococcus hirae strain. J Antimicrob Chemother 75:482–484. [DOI] [PubMed] [Google Scholar]
- 11.Huang J, Chen L, Wu Z, Wang L. 2017. Retrospective analysis of genome sequences revealed the wide dissemination of optrA in Gram-positive bacteria. J Antimicrob Chemother 72:614–616. doi: 10.1093/jac/dkw488. [DOI] [PubMed] [Google Scholar]
- 12.D'Andrea MM, Antonelli A, Brenciani A, Di Pilato V, Morroni G, Pollini S, Fioriti S, Giovanetti E, Rossolini GM. 2019. Characterization of Tn6349, a novel mosaic transposon carrying poxtA, cfr and other resistance determinants, inserted in the chromosome of an ST5-MRSA-II strain of clinical origin. J Antimicrob Chemother 74:2870–2875. doi: 10.1093/jac/dkz278. [DOI] [PubMed] [Google Scholar]
- 13.Hao W, Shan X, Li D, Schwarz S, Zhang SM, Li XS, Du XD. 2019. Analysis of a poxtA- and optrA-co-carrying conjugative multiresistance plasmid from Enterococcus faecalis. J Antimicrob Chemother 74:1771–1775. doi: 10.1093/jac/dkz109. [DOI] [PubMed] [Google Scholar]
- 14.Brenciani A, Fioriti S, Morroni G, Cucco L, Morelli A, Pezzotti G, Paniccià M, Antonelli A, Magistrali CF, Rossolini GM, Giovanetti E. 2019. Detection in Italy of a porcine Enterococcus faecium isolate carrying the novel phenicol-oxazolidinone-tetracycline resistance gene poxtA. J Antimicrob Chemother 74:817–818. doi: 10.1093/jac/dky505. [DOI] [PubMed] [Google Scholar]
- 15.Baccani I, Antonelli A, Di Pilato V, Coppi M, Di Maggio T, Spinicci M, Villagran AL, Revollo C, Bartoloni A, Rossolini GM. 2021. Detection of poxtA2, a Presumptive poxtA Ancestor, in a Plasmid from a Linezolid-Resistant Enterococcus gallinarum Isolate. Antimicrob Agents Chemother 65:e0069521. doi: 10.1128/AAC.00695-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lei CW, Kang ZZ, Wu SK, Chen YP, Kong LH, Wang HN. 2019. Detection of the phenicol-oxazolidinone-tetracycline resistance gene poxtA in Enterococcus faecium and Enterococcus faecalis of food-producing animal origin in China. J Antimicrob Chemother 74:2459–2461. doi: 10.1093/jac/dkz198. [DOI] [PubMed] [Google Scholar]
- 17.Fioriti S, Morroni G, Coccitto SN, Brenciani A, Antonelli A, Di Pilato V, Baccani I, Pollini S, Cucco L, Morelli A, Paniccià M, Magistrali CF, Rossolini GM, Giovanetti E. 2020. Detection of oxazolidinone resistance genes and characterization of genetic environments in enterococci of swine origin, Italy. Microorganisms 8:2021. doi: 10.3390/microorganisms8122021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Na SH, Moon DC, Kim MH, Kang HY, Kim SJ, Choi JH, Mechesso AF, Yoon SS, Lim SK. 2020. Detection of the phenicol-oxazolidinone resistance gene poxta in Enterococcus faecium and Enterococcus faecalis from food-producing animals during 2008–2018 in Korea. Microorganisms 8:1839. doi: 10.3390/microorganisms8111839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim E, Shin SW, Kwak HS, Cha MH, Yang SM, Gwak YS, Woo GJ, Kim HY. 2021. Prevalence and characteristics of phenicol-oxazolidinone resistance genes in Enterococcus faecalis and Enterococcus faecium isolated from food-producing animals and meat in Korea. Int J Mol Sci 22:11335. doi: 10.3390/ijms222111335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egan SA, Shore AC, O'Connell B, Brennan GI, Coleman DC. 2020. Linezolid resistance in Enterococcus faecium and Enterococcus faecalis from hospitalized patients in Ireland: high prevalence of the MDR genes optrA and poxtA in isolates with diverse genetic backgrounds. J Antimicrob Chemother 75:1704–1711. doi: 10.1093/jac/dkaa075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moure Z, Lara N, Marín M, Sola-Campoy PJ, Bautista V, Gómez-Bertomeu F, Gómez-Dominguez C, Pérez-Vázquez M, Aracil B, Campos J, Cercenado E, Oteo-Iglesias J, Spanish Linezolid-Resistant Enterococci Collaborating Group . 2020. Interregional spread in Spain of linezolid-resistant Enterococcus spp. isolates carrying the optrA and poxtA genes. Int J Antimicrob Agents 55:105977. doi: 10.1016/j.ijantimicag.2020.105977. [DOI] [PubMed] [Google Scholar]
- 22.Dejoies L, Sassi M, Schutz S, Moreaux J, Zouari A, Potrel S, Collet A, Lecourt M, Auger G, Cattoir V. 2021. Genetic features of the poxtA linezolid resistance gene in human enterococci from France. J Antimicrob Chemother 76:1978–1985. doi: 10.1093/jac/dkab116. [DOI] [PubMed] [Google Scholar]
- 23.Schwarz S, Zhang W, Du XD, Krüger H, Feßler AT, Ma S, Zhu Y, Wu C, Shen J, Wang Y. 2021. Mobile Oxazolidinone resistance genes in gram-positive and gram-negative bacteria. Clin Microbiol Rev 34:e0018820. doi: 10.1128/CMR.00188-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou J, Tang Z, Yan J, Liu H, Chen Y, Zhang D, Zhao J, Tang Y, Zhang J, Xia Y. 2020. Dissemination of linezolid resistance through sex pheromone plasmid transfer in Enterococcus faecalis. Front Microbiol 11:1185. doi: 10.3389/fmicb.2020.01185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laverde Gomez JA, van Schaik W, Freitas AR, Coque TM, Weaver KE, Francia MV, Witte W, Werner G. 2011. A multiresistance megaplasmid pLG1 bearing a hylEfm genomic island in hospital Enterococcus faecium isolates. Int J Med Microbiol 301:165–175. doi: 10.1016/j.ijmm.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Arias CA, Murray BE. 2012. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 10:266–278. doi: 10.1038/nrmicro2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramsay JP, Firth N. 2017. Diverse mobilization strategies facilitate transfer of non-conjugative mobile genetic elements. Curr Opin Microbiol 38:1–9. doi: 10.1016/j.mib.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 28.He S, Hickman AB, Varani AM, Siguier P, Chandler M, Dekker JP, Dyda F. 2015. Insertion sequence IS26 reorganizes plasmids in clinically isolated multidrug-resistant bacteria by replicative transposition. mBio 6:e00762-15. doi: 10.1128/mBio.00762-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harmer CJ, Moran RA, Hall RM. 2014. Movement of IS26-associated antibiotic resistance genes occurs via a translocatable unit that includes a single IS26 and preferentially inserts adjacent to another IS26. mBio 5:e01801-14. doi: 10.1128/mBio.01801-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shan X, Li XS, Wang N, Schwarz S, Zhang SM, Li D, Du XD. 2020. Studies on the role of IS1216E in the formation and dissemination of poxtA-carrying plasmids in an Enterococcus faecium clade A1 isolate. J Antimicrob Chemother 75:3126–3130. doi: 10.1093/jac/dkaa325. [DOI] [PubMed] [Google Scholar]
- 31.Chen K, Dong N, Chan EW, Chen S. 2019. Transmission of ciprofloxacin resistance in Salmonella mediated by a novel type of conjugative helper plasmids. Emerg Microbes Infect 8:857–865. doi: 10.1080/22221751.2019.1626197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie M, Yang X, Xu Q, Ye L, Chen K, Zheng Z, Dong N, Sun Q, Shu L, Gu D, Chan EW, Zhang R, Chen S. 2021. Clinical evolution of ST11 carbapenem resistant and hypervirulent Klebsiella pneumoniae. Commun Biol 4:650. doi: 10.1038/s42003-021-02148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moran RA, Hall RM. 2019. B/O plasmid R16 from 1956 carries an In1-like class 1 integron embedded in a complex region containing parts of the Acinetobacter baumannii AbaR resistance island. Plasmid 105:102432. doi: 10.1016/j.plasmid.2019.102432. [DOI] [PubMed] [Google Scholar]
- 34.Sterling AJ, Snelling WJ, Naughton PJ, Ternan NG, Dooley JSG. 2020. Competent but complex communication: the phenomena of pheromone-responsive plasmids. PLoS Pathog 16:e1008310. doi: 10.1371/journal.ppat.1008310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shang Y, Li D, Shan X, Schwarz S, Zhang SM, Chen YX, Ouyang W, Du XD. 2019. Analysis of two pheromone-responsive conjugative multiresistance plasmids carrying the novel mobile optrA locus from Enterococcus faecalis. Infect Drug Resist 12:2355–2362. doi: 10.2147/IDR.S206295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clinical and Laboratory Standards Institute (CLSI). 2021. Performance standards for antimicrobial susceptibility testing; 31st informational supplement. CLSI supplement M100 (31st ed). Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 37.Li XS, Dong WC, Wang XM, Hu GZ, Wang YB, Cai BY, Wu CM, Wang Y, Du XD. 2014. Presence and genetic environment of pleuromutilin-lincosamide-streptogramin A resistance gene lsa(E) in enterococci of human and swine origin. J Antimicrob Chemother 69:1424–1426. doi: 10.1093/jac/dkt502. [DOI] [PubMed] [Google Scholar]
- 38.Di Sante L, Morroni G, Brenciani A, Vignaroli C, Antonelli A, D'Andrea MM, Di Cesare A, Giovanetti E, Varaldo PE, Rossolini GM, Biavasco F. 2017. pHTβ-promoted mobilization of non-conjugative resistance plasmids from Enterococcus faecium to Enterococcus faecalis. J Antimicrob Chemother 72:2447–2453. doi: 10.1093/jac/dkx197. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan MJ, Petty NK, Beatson SA. 2011. Easyfig: a genome comparison visualizer. Bioinformatics 27:1009–1010. doi: 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA. 2011. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 12:402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]