SUMMARY
The spread of biofilms on medical implants represents one of the principal triggers of persistent and chronic infections in clinical settings, and it has been the subject of many studies in the past few years, with most of them focused on prosthetic joint infections. We review here recent works on biofilm formation and microbial colonization on a large variety of indwelling devices, ranging from heart valves and pacemakers to urological and breast implants and from biliary stents and endoscopic tubes to contact lenses and neurosurgical implants. We focus on bacterial abundance and distribution across different devices and body sites and on the role of environmental features, such as the presence of fluid flow and properties of the implant surface, as well as on the interplay between bacterial colonization and the response of the human immune system.
KEYWORDS: biofilms, fluid flow, immune response, medical implants, microbial contamination
INTRODUCTION
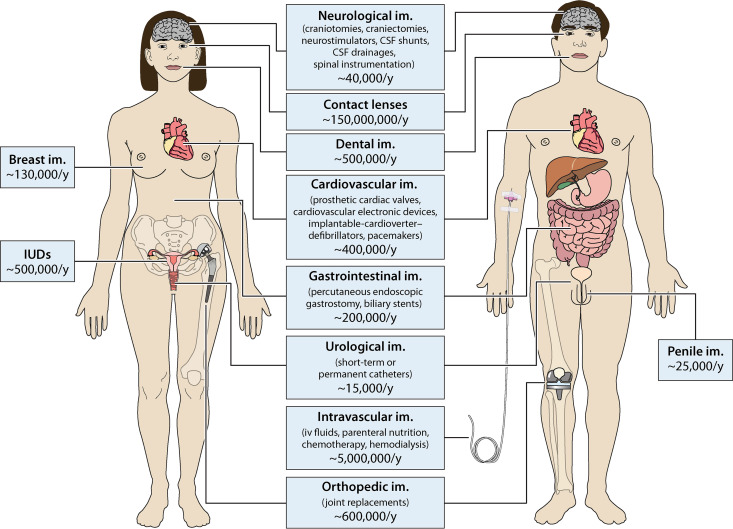
Medical implants are artificial devices partly or entirely inserted into the human body and intended to remain after the procedure for diagnostic, therapeutic, and rehabilitation purposes. The wealth of functions they offer is continually expanding and evolving and demand for such implants is expected to increase due to the aging population (1–4). It was estimated that, only in 2018, the U.S. market of medical devices alone reached about 90 billion USD, while the forecast market for 2019 to 2025 would predict a compound annual growth rate (CAGR) of 6.3%, reaching by 2025 the market value of about 140 billion USD (5). Intravascular devices are the most employed, with an estimate of approximately 5,000,000 devices implanted in a single year only in the United States, followed by orthopedic implants (∼600,000/year), dental implants (∼500,000/year), and cardiovascular devices (∼400,000/year) (Fig. 1) (6). The use of medical devices ameliorated the treatment of multiple pathologies and, ultimately, patient quality of life. Unfortunately, device-associated nosocomial infections, often related to biofilm formation (7–10), still represent a significant health concern worldwide (11–14), with substantial clinical and economic consequences. In fact, compared to planktonic cells of the same species, bacteria within a biofilm are up to 1,000 times more resistant to antimicrobial agents (15), thus becoming the primary cause of persistent and chronic infections, which in turn affect the well-being of individuals and increase the treatment costs for the national health systems (16, 17).
FIG 1.
Most used medical devices. The figure depicts the most common medical devices, their distribution within the human body, and the number of implants employed per year (/y) in the United States (6, 402, 403). Implants commonly used in both women and men are listed in the middle, while sex-specific implants are reported at the side of each figure. The graphic work was done using Smart Servier Medical Art.
Biofilms are surface-associated microbial colonies, embedded in a self-secreted matrix of extracellular polymeric substances (EPS). EPS consists of many types of polymers, including polysaccharides, extracellular nucleic acids (eDNA), proteins, amyloids, and amphiphilic surfactants. Being prevalent on most wet surfaces in nature (18–20), the communal lifestyle of biofilms favors the emergence of properties substantially different from the ones exhibited by planktonic cells, mainly due to the presence of the extracellular matrix (21). The sessile mode of growth, by keeping the cells in proximity, mediates communication between bacteria (22), fosters horizontal gene transfer (23) and promotes the sharing of metabolic products within the biofilm community (24). A remarkable property in terms of biofilm survival is the increased resistance to phagocytosis (25) and biocides (26), which, in a clinical context, implies resistance to host defense mechanisms (27, 28) and treatments with antibiotics and antimicrobials (29, 30). The biofilm matrix can retard the diffusion of solutes (31, 32) and of some antibiotics (33) with an efficiency that strongly depends on the specific interaction between EPS components and antibiotic molecules (34, 35). Moreover, although many antibiotics can penetrate the EPS, bacterial cells inside the biofilm are often protected due to their slower metabolism, which makes them less susceptible to the effects of antimicrobials. An additional cause of the increased antibiotic tolerance in biofilms is the presence of dormant cells, which can be less susceptible to chemical attacks (36, 37). These cells, called persisters, can survive antibiotic treatments in the absence of specific resistance mechanisms (38, 39), and their existence is intrinsically related to the physicochemical biofilm heterogeneity that promotes the formation of phenotypical niches (40, 41).
An essential aspect of the biofilm-associated infection treatment is the microbiological diagnosis (42). The conventional method is to perform microbial cultures using tissue samples or fluids close to the infection site. However, a more effective approach, which can be applied when the implant is removed, is the sonication of the device to dislodge the biofilm from the surface and disperse the cells in sonicate fluid (43). The sonicate fluid culture has a higher sensitivity compared to standard tissues samples since in biofilm-associated infections cells are retained in the matrix and its dispersion increases their detectability (44, 45). Indeed, the use of conventional culture methods is not always indicative of biofilm growth: a negative result may not necessarily indicate the absence of bacterial infection but could be due to the slow proliferation rate of microbes within a biofilm. Thus, advances in sequencing methods, such as 16S rRNA gene sequencing (46, 47) or metagenomic sequencing (48–50), have begun to identify pathogens and to provide data describing their composition and function across multiple sites in the human body (51, 52). Several independent culture techniques could be employed (53). Denaturing gradient gel electrophoresis (PCR-DGGE) uses a polyacrylamide gel containing linearly increasing concentrations of a denaturing agent to separate amplicons of ribosomal DNA having the same size, but different sequences (54, 55). Terminal restriction fragment length polymorphism (T-RFLP) is based on detecting differences within the pattern obtained with a single restriction enzyme cut on the 16S rRNA gene. T-RFLP can identify the dominant community members comprising at least 1% of the total (56); both DGGE and T-RFLP can assess the community structure and variation over time or space. Fluorescent in situ hybridization (FISH) probes target large taxonomic groups and can quantify microbial communities even within complex environmental samples (57). Direct 16S rRNA gene sequencing can identify a single bacterium species; however, in recent years, thanks to the development of next-generation high-throughput sequencing, it is possible to analyze a PCR mix, including hundreds of different rRNAs from a bacteria community (or the rRNA gene internal transcribed spacer [ITS] regions for yeast), to identify the different bacteria present and their relative quantities within this community (48, 49). This identification step is crucial for undertaking efficacious therapeutic treatments.
This review presents an overview of biofilms associated with implanted medical devices in different parts of the human body, highlighting the most frequently colonizing microbes, the environments that promote their growth (including the presence of fluid flow and the properties of the implant surface), and the human immunological response. Despite the wealth of research studies in this field, there is a need to collect the most relevant contributions on device-associated biofilms and start pulling the threads together to guide research on this complex and critical problem.
BIOFILM FORMATION AND MICROBIAL COLONIZATION ON MEDICAL DEVICES
Cardiovascular Implants
Implantable cardiac devices are tools for patients with cardiac diseases and are used to prevent heart failure. They comprise pacemakers, implantable cardioverter–defibrillators (ICDs), cardiovascular implantable electronic devices (CIEDs), and prosthetic cardiac valves. The annual number of cardiac devices employed in medicine is constantly increasing (58). Unfortunately, many of them need to be replaced due to malfunctions or infections: it is estimated that the rate of infected devices is currently between 1.2 and 2.4% of the total implanted devices (58–60), with infection rates increasing up to 10-fold after the replacement of the device or after its upgrade (61, 62). Infections occurring within the first 12 months postoperation are predominantly linked to colonization at the time of surgery, and 25% of these infections manifest themselves within the first month following the implantation. Overall, CIED infections are associated with morbidity, mortality (especially when comorbid conditions are present [63]), and substantial health care expenses.
The microbes most frequently identified on the infected device surface or in the skin pockets of cardiovascular implants are coagulase-negative Staphylococcus spp., accounting for almost 60 to 80% of the infections (64, 65), and Staphylococcus aureus, which is identified 11 to 29% of the time (66, 67). Moreover, Enterococcus spp., Streptococcus spp., HACEK (Haemophilus spp., Aggregatibacter spp., Cardiobacterium hominis, Eikenella corrodens, and Kingella spp.), and few fungi, particularly Candida albicans (68–71), have been associated with cardiovascular implants. In cardioverter defibrillators and pacemakers, polymicrobial colonization are identified 1 to 7% of the time, but the composition of these mixed communities was not investigated (70, 71). Patients with possible CIED infection undergo blood cultures to identify the aerobic or anaerobic microbe, since most of the contaminants are methicillin-resistant S. aureus (MRSA); vancomycin is usually the first antibiotic administered. Once the contaminant is identified, a more specific cure is undertaken for 10 to 14 days (65, 72), while the device is replaced. To reduce the chance of infections, antibiotic prophylaxis or antibacterial envelopes covering the device have been employed (73). For example, the use of a polypropylene mesh releasing minocycline and rifampin has been shown to significantly reduce the occurrence of CIED infections (73). Moreover, oral pathogens causing periodontitis, such S. mutans (74, 75), can also reach the heart valve from the bloodstream, causing an infection (76–78). A good oral hygiene in healthy individuals is usually sufficient to avoid these problems, but in patients at high risk of endocarditis an antibiotic prophylaxis is normally suggested before a dental procedure. In this case, amoxicillin is the most frequently utilized antibiotic (79, 80).
Despite the clinical importance of infections associated with cardiovascular implants, very little is known on the growth of biofilms on these devices. Some evidence comes from in vitro studies of biofilm formation from clinical isolates. For instance, Cutibacterium (formerly Propionibacterium) acnes strains—isolated from the surfaces of explanted pacemakers with no clinical signs of infection—can form biofilms, as shown using a microtiter plate assay (81). In addition, cytoplasmic material and a fibrous matrix of eDNA were found via electron microscopy in C. acnes biofilms, suggesting that eDNA may play a role in forming biofilms on pacemakers and other cardiovascular devices (81).
Gastrointestinal Implants
Percutaneous endoscopic gastrostomy (PEG) is the most common access route for enteral nutrition in pediatric and adult populations. However, PEG tubes have been recognized as risk factors for gastrointestinal colonization and biofilm formation by antibiotic-resistant bacteria in long-term care facilities (82, 83). Investigations conducted on PEG tubes always revealed the presence of bacterial and fungal biofilms (84, 85), despite their presence not being the cause for removal. Dautle et al. analyzed the microbiota associated with silicone gastrostomy devices used for long-term (3 to 47 months) enteral nutrition in children (6 months to 17 years) (84). All devices examined showed the presence of biofilms; 24 bacterial species were identified, including Bacillus, Enterococcus, and Staphylococcus species. In that study, scanning electron microscopy (SEM) measurements showed that defects on the surface of PEG tubes provided protected sites for initial attachment and the development of biofilms. In another study (85), PEG tubes removed from 12 patients after 4 to 233 weeks of use were sampled and examined for microbial colonization and biofilm formation. Cultures were positive for fungal contamination in all cases (Candida tropicalis was the species most frequently involved), while the inner walls of the tubes used for more than 12 weeks were always encrusted with a thick biofilm but with variable thicknesses that did not correlate with the PEG age (85).
Obstructive jaundice is a condition that prevents the normal flow of bile to the duodenum. It frequently correlates with symptoms of appetite loss, nausea, recurrent cholangitis, and renal failure; moreover, it is a common indication of pancreatic or periampullary cancer (86). Currently, more than 70% of the patients with obstructive jaundice are treated by biliary stenting in first-line centers receiving the patient under urgent conditions and later referred to specialized high-volume centers for surgery (87). Moreover, due to long waiting lists or the necessity of neoadjuvant chemoradiation therapy, the operation must be postponed by several weeks even in referral centers, and biliary stenting becomes mandatory. During this period, by gaining access to the biliary system, duodenal bacteria can attach to the stent surface and start forming biofilms, playing a critical role in the clogging of biliary stents (88, 89).
Endoscopic gastrointestinal devices are associated with a wide and diverse spectrum of microbial species. Previous works have demonstrated a clear connection between the presence of biliary stents and a dramatic increase in the contamination of bile, which was predominantly characterized by species from the duodenal microbiota such as enterococci (90, 91). In a prospective study, pigtail polyurethane stents and straight polyethylene stents were retrieved from 120 patients (62.5% males; median age, 64 years) with biliary strictures (35% malignant, 65% benign) after 1 to 1,274 days of indwelling time. The occlusion rates of the stents—which significantly increased the risk of cholangitis—were found to be around 11.5% in pigtail stents and 13% in the straight ones. Polymicrobial colonization predominated (95.8% versus 4.2%), with enterococci (79.3%), Enterobacteriaceae (73.7%), and Candida spp. (55.9%) as the leading pathogens among 95 different bacterial and 13 fungal species identified. Interestingly, Candida spp. were more common on stents from patients previously receiving prolonged antibiotic therapy (63% versus 46.7%) (92).
Orthopedic Implants
Total joint replacement is a safe and standard procedure that can restore functionality and improve the well-being of patients with hip and knee arthritis (93). Despite its safety, postoperative complications still occur: the most common is prosthetic joint infection (PJI), a major mechanism of failure of the implant that often requires surgical revision. In many cases, if surgical debridement and implant retention are performed or after implant removal with a two-stage procedure, the treatment of PJI fails (94). In the last decade, a new classification has been described based on the pathogenicity and etiology of the infection. PJI can be recognized as (i) an acute infection characterized by early onset and highly virulent bacteria; (ii) a low-grade infection, a chronic type of infection that arises later and is caused by low virulence or small colony-forming bacterial stains; or (iii) a late hematogenous high-grade infection.
In general, the bacterial species found most in PJIs are S. aureus, Staphylococcus epidermidis, and Staphylococcus lugdunensis (95, 96). Several studies identified three different mechanisms of pathophysiology in staphylococcal chronic PJIs: the formation of small colony variants (SCVs) (97), bacterial internalization in osteoblasts (98), and the formation of biofilm (99). Both SCV and biofilm formation have been observed for S. aureus, S. epidermidis, and S. lugdunensis. An acute PJI is usually caused by S. aureus and, in up to 50% of cases, by MRSA strains (93). In particular, the presence of biofilms of S. aureus have been observed directly from the bone cement retrieved during a revision surgery and, in synovial fluid samples, as free-floating biofilm-like aggregates (100, 101). Moreover, polymicrobial communities have been also identified in association with PJIs, comprising P. aeruginosa-E. faecalis-K. pneumoniae, MRSA-MSSA, and MRSE-E. faecalis (93, 95).
Osteomyelitis could occur because of PJIs and biofilm formation; in this case, it is referred to as contiguous-focus osteomyelitis, and it is usually caused by S. aureus (102). Considering that the biofilm’s presence complicates the treatment of osteomyelitis, different approaches have been considered, such as the use of anti-polysaccharide intercellular adhesin (PIA) antibodies to prevent microbial attachment or PIA formation (103, 104) or the coating of medical devices before implantation. Recently, also the prosthesis surface has been investigated to prevent bacterial colonization or biofilm formation (105). Among these approaches, the most studied is silver coating (106, 107), despite its several limitations, especially its toxicity (108). For this reason, different kinds of nanoparticles with antimicrobial abilities, such as silver, copper, quantum dots, and zinc oxide, have been used on prosthesis surfaces to reduce cell viability and bacterial adhesion (109–112). For treating osteomyelitis, Bioactive Glass (BAG-S53P4), a bone substitute with proven antibacterial and bone-bonding properties (113), is used. Furthermore, Franceschini et al. found that an antibacterial hydrogel coating, composed of hyaluronan, poly-d,l-lactide (defensive antibacterial coating [DAC]), could be used as protective biomaterial, in association also with topic antibiotics, to prevent bacterial adhesion and biofilm formation (114).
Studies have shown that one of the biofilm persistence mechanisms involved a crosstalk with myeloid-derived suppressor cells (MDSCs), i.e., immature monocytes and granulocytes that are involved, during inflammation or injury, in the generation of a mature myeloid population (115, 116). During bacterial biofilm infection, MDSCs lose their ability to mature, and they negatively regulate inflammatory mechanisms through their suppressive actions (117, 118). Heim and colleagues found that MDSCs improve bacterial persistence during S. aureus orthopedic biofilm infection via interleukin-10 production (119). As described above, biofilm can protect pathogens that would otherwise be eradicated in their planktonic or free-floating form. For these reasons, the treatment of PJI is very complex because it often involves a two-stage exchange, with implant removal and antibiotic spacer placement followed by systemic antibiotic therapy and delayed reimplantation. To reduce morbidity, antibiotic therapy could be improved to a one-stage exchange, or implant retention may be more feasible. Using a mouse in vivo model, Niska et al. evaluated and compared efficacies of vancomycin-rifampin combination therapy for PJIs and demonstrated an increased efficacy compared to monotherapy (120).
Neurosurgical Implants
Neurosurgical devices comprise neurostimulators, cerebrospinal fluid (CSF) shunts, external ventricular CSF drainage, and external lumbar CSF drainage spinal instrumentation. The rate of infections of these devices ranges, on average, from 3 to 15%, but it can reach even higher numbers with craniectomies and external ventricular CSF drainages (6, 121–123). The consequences of these infections are often devastating for the patient and are associated with an increase in morbidity and mortality (124, 125). Management of this problem is even more difficult if we consider (i) that diagnosis of infection is challenging; (ii) that biofilms are easily formed on the implant surface, requiring prolonged antimicrobial treatment; (iii) that standardized approaches to fight the infections are lacking; and (iv) the impossibility, in some cases, of removing or replacing the implant (126–128). Most of the infections are related to microbes found in skin and mucosal flora or disturbance of the wound healing process. Indeed, preoperative infection prevention protocols have been shown to decrease infection rates (129). Acute infections present themselves within 6 weeks of operation. Since immature biofilms are present at this stage, infections are most frequently treated with prolonged antimicrobial treatment (from 4 to 12 weeks) and shunt removal. Before microbial identification, general treatment can start with intravenous vancomycin plus ceftriaxone, cefepime, or ceftazidime (127). If Gram-positive bacteria are detected, rifampicin is administrated in combination with co-trimoxazole, levofloxacin, moxifloxacin, or doxycycline to avoid the development of resistance (130), whereas fluoroquinolones are the antimicrobial agents most frequently used against Gram-negative bacteria. Whenever chronic infections present themselves more than 6 weeks after the operation, biofilms on the implant surface are usually mature and stable, and to remove these biofilms, antibiotic treatment is performed, along with removal or replacement of the device (127, 128). Overall, the most frequently identified bacteria are Staphylococcus spp., especially S. aureus, which is identified more than 50% of the time; followed by Cutibacterium spp., where C. acnes is isolated 5.4% of the time; and Enterobacter spp. (3.78%); among fungi, Candida spp. are isolated 1.63% of the time (131, 132).
Urological Implants, Nephrostomy Tubes, and Stents
Biofilm formation has been observed in the most used devices in the urogenital tract: short-term or permanent catheters, ureteral stents, nephrostomy tubes, and penile implants (133). In current clinical practice, indwelling catheters are widely used to relieve upper urinary tract obstruction, prevent stricture formation, drain urinary tract leaks, and hinder postsurgical complications (134). In indwelling stents, which are infected in most cases, fever and urinary tract infections (UTIs) are the most common complications, followed by bacteremia and even death (135, 136). Bacterial penetration is probably due to the permissive environment; in fact, these devices meet polysaccharides, ions, and glycoproteins, which form a conditioning film on the implant surface, allowing various planktonic bacteria to adhere and form biofilms (137, 138). This event occurs typically within 24 h of the stent insertion (136, 138). Moreover, encrustation could lead to bacterial biofilm formation. This process involves the deposition of mineral crystals onto the surface and lumen of a ureteral stent. As a result, the stent becomes calcified and loses its tensile strength, raising the risk of stent fracture or ureteral avulsion during removal.
In one of the first papers analyzing encrustation and its relationship with bacterial biofilm, Wollin et al. found a conditioning biofilm layer on indwelling stents derived from 64 patients, half of whom showed encrustation, and 13% had been coated by a bacterial biofilm (139). Though the production of urease can lead to encrustation, the relationship between these two processes is poorly understood. In fact, on one hand, bacterial biofilms may facilitate precipitation of crystals causing encrustation, and on the other hand, encrustation could constitute a niche for bacteria colonization and bacterial biofilm formation (140).
The most common biofilm-forming pathogens found on these devices are E. coli, P. mirabilis, P. aeruginosa, E. faecalis, Staphylococcus spp., and C. tropicalis (141, 142). The latter are considered strong biofilm-forming microbes and are also involved in polymicrobial infections (142), since the capacity to form a biofilm could serve as a shelter for the weak formers. There are several studies on the bacterial adhesion of uropathogens to host cells. For E. coli, a connection between type I pili, in particular its protein FimH, and Tamm-Horsfall protein (THP) was found. This protein is usually responsible for eliminating bacteria, but in permanent stents it becomes an anchor for E. coli via FimH and for P. mirabilis and P. aeruginosa via different adhesion proteins (141). Antibiotic treatment is often not sufficient to eliminate or avoid the formation of bacterial biofilms on ureteral stents (143); thus, the usual approach to eliminate the infection consists of replacement or early removal of the implants (138). Modifications of the surface material or treatment of the surface itself have been investigated as ways to prevent bacterial attachment and biofilm formation (143–145).
Different prevention strategies to avoid both encrustation and biofilm formation are currently under investigation. The most common method is to coat surface devices. The use of silver and silver nanoparticles, antibiotics, bacteriophages, chlorhexidine, triclosan, antimicrobials peptides and enzymes, hydrogel, polytetrafluoroethylene (PTFE), polyzwitterions, and polyethylene glycol (PEG) coatings are the most explored and used techniques (146–149). Moreover, changes in surface topographies have been studied to avoid bacterial attachment (146). Recently, Mosayyebi et al. proposed an innovative approach in which a microfluidic model of the stented and occluded ureter is used to study the effect of stent architecture on wall shear stress (WSS) distribution and encrustation over its surface (150). Measuring the stent thickness and the hole vertex angle, a reduction in the encrustation thickness by ∼90% was detected. Penile implants are primarily used for erectile dysfunction (ED), a disease that affects 20 to 25% of men over 40 and 5 to 10% below this age. ED is predominantly linked to medical conditions such as cardiovascular disease and diabetes (151). Infectious complications are generally infrequent (about 3% of primary surgeries and up to 18% of revision surgeries [152, 153]) but can cause serious consequences from both a clinical and an emotional perspective (151). During the revision surgery, bacteria were found in 70% of cases; among these, the majority are Staphylococcus spp., in particular S. epidermidis (137, 153, 154). Recently, surgeons have improved the prosthesis insertion procedure: leveraging the experience in the orthopedic field, they are implementing the strategy employed for PJIs (151).
Intravascular Devices
Intravascular devices are used for different applications, including intravenous fluids, parenteral nutrition, antibiotic therapy, chemotherapy, and hemodialysis. It has been estimated that over 5 million devices are implanted every year in the United States only (155). Needle-free connectors, such as split septum connectors, Luer activated valves, and Luer valves with positive displacement, were introduced to reduce the risk of needlestick injuries and assist catheter management and nursing care. However, environmental contaminations, lack of disinfection procedures (70% alcohol and treatments for 5 to 60 s are suggested), and poor scrubbing techniques increase the risk of infection (156). Colonization of needle-free connectors causes 50% of catheter-related infections (157). To reduce the infection rate, disinfection protocols should be improved, and the predisposition to contamination of different connectors should be tested (156, 158). Overall, S. aureus, S. epidermis, E. faecalis, P. aeruginosa, and K. pneumoniae are the leading infectious agents in intravascular catheters. Within this environment, C. albicans is again the most frequently isolated fungus (159–161). These microbes are related to bloodstream infection, a major problem that in the United States alone causes almost 200,000 cases of infection per year, resulting in prolonged hospitalization and increased costs (162). The first step to solving this problem is antibiotic therapy. Since the most common source of infections are MRSA strains, vancomycin is often used since it is active against coagulase-negative staphylococci and S. aureus. Alternatively, when P. aeruginosa is detected, ceftazidime or cefepime, fourth-generation cephalosporins, are recommended. When a fungal infection is detected, the use of amphotericin B or fluconazole is recommended (155).
Often, the identified microbes form biofilms along the catheter and display a higher antibiotic tolerance. To achieve a higher local concentration of the drugs, the “lock therapy” could also be undertaken. Here, since most infections in tunneled catheters spread to the lumen, the latter gets filled with the desired drug for a few hours or even days, also intermittently, to eradicate the contaminant (163–165). Ideally, lock solutions should be directed against common pathogens or be targeted to specific bacteria, be able to disrupt a biofilm, be compatible with anticoagulants, have long chemical stability and low toxicity, induce no antibiotic resistance, and be cost-effective. Overall, the main combinations used are vancomycin with the anticoagulant heparin, taurolidine, and minocycline with EDTA, which can disrupt biofilm integrity and synergize the activity of the antibiotic, or ethanol (163, 165, 166). If this strategy fails, the infected catheter should be removed and replaced with a new one.
Breast Implants
Breast implants are used in both postmastectomy breast reconstruction and cosmetic surgical procedures. In addition to other surgical procedures, breast implants also face complications such as hematoma, seroma, infection, altered nipple sensation, asymmetry, scarring, rupture, and capsular contracture (CC) (167). Implant removal or revision is usually caused by CC (168), which is also responsible for breast pain and discomfort and affects the breast aesthetic (169). Moreover, prosthesis revision surgery is often associated with CC recurrence. Several factors may participate in CC etiopathogenesis: (i) surgical procedure, such as the choice of location and incision (170, 171); (ii) genetic predisposition (172); (iii) inflammation resulting from subclinical infection and biofilm formation (167); and (iv) implant texturization (173). Bacteria were found in 85% of breast implants that encountered CC, and biofilms, observed using SEM, were detected in more than half of them (174). Staphylococcus spp. are usually present in breast implants removed for CC, in particular S. epidermidis and S. aureus (174–176). However, several bacteria can survive in the periprosthetic environment and form biofilms, such as C. acnes (a commensal species of the skin and gut), streptococci, Bacillus spp., E. coli, Mycobacterium spp., Corynebacterium spp., and lactobacilli (174, 175, 177). These studies indicate a possible correlation between bacterial colonization, biofilm formation, and CC (178).
Another factor potentially implicated in biofilm formation and consequent CC is the breast-implant texture. Although some works did not find a correlation between bacterial colonization and the type of textured implants (175), other studies have shown, both in vitro and in vivo, that biofilm formation is more common on rough surfaces (179, 180). At the same time, smooth prostheses have also been associated with increased biofilm formation (171, 173, 181, 182). Although CC is considered one of the main problems with breast implants, anaplastic large cell lymphoma associated with a breast implant (BIA-ALCL) has also been recently investigated. BIA-ALCL was identified for the first time in 1997 (183); however, it is not known whether its formation derives from the formation of biofilms or the specific texture of the implants. Hu et al. studied the bacterial population present in BIA-ALCL compared to normal patients and found that Ralstonia spp. are mainly associated with tumor capsule specimens compared to nontumor ones (184). However, it was recently reported that BIA-ALCL is primarily associated with macrotextured implants (185). In order to prevent biofilm formation and also capsular contraction, clinicians have improved the surgical procedures by (i) administering intravenous antibiotic prophylaxis, avoiding periareolar incision (170, 186), reducing the permanence of drainage tube, and using nipple shields to prevent spillage of bacteria into the pocket (187); (ii) irrigating the pocket with a triple antibiotic solution or betadine (188); and (iii) using an antibiotic-impregnated mesh during breast reimplants and implementing the “no touch” methods by an introduction sleeve to minimize skin contact (189).
Dental Implants
The oral microbiota is highly complex since the oral cavity is the site of the human body with the most bacteria species able to colonize differently specific niches (i.e., tongue, cheeks, teeth, and gums). Mouth colonization starts at birth, and it changes over time due to aging, tooth appearance or extraction, diet, characteristics of saliva, and the use of antibiotics (190). Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Spirochaetes, and Fusobacteria are among the most representative taxa. Using the methods described above, it is now possible to fully describe the microbial diversity present in the human oral cavity and to assess its composition when health problems arise. Indeed, oral bacteria have been implicated in cardiovascular disease, pneumonia, diabetes, and systemic disease (191–195). Therefore, the prevention and control of oral diseases are essential to prevent these conditions.
Biomaterial surfaces that are employed to restore optimal oral cavity functionality can be colonized by several microbes, often forming stable biofilm structures leading to peri-implantitis (196, 197). Consequences can be severe: gingival health can be compromised; composite resins could be degraded, leading to the possibility of bacterial invasion at the interface between the tooth and the restoration; and tooth enamel can demineralize (198–201). Because of several components present in the saliva, host tissue, and bacterial products, a pellicle is formed around the implant surface within minutes of its placement (202–204), and early colonization of tooth and implants has been shown to be different (205). Microbial colonization occurs immediately, with Gram-positive cocci and rod-like microbes being the primary colonizers (205, 206), producing the basic structure that enables secondary colonization thanks to interbacterial adhesion. Whenever Gram-negative anaerobic bacteria become the main colonizers, the dominant microbial species are Fusobacterium spp., Porphyromonas gingivalis, Eikenella corrodens, Prevotella intermedia, Campylobacter spp., and C. albicans (197, 202, 207, 208). Under these conditions, peri-implantitis may develop in less than a year after implant placement. Antibiotic prophylaxis could avoid this phenomenon (209). Overall, to prevent implant failure, correct planning of treatments, placement of the implant, and follow-up visits are extremely important, along with monitoring the overall health state (i.e., osteoporosis, diabetes, obesity, use of drugs, etc.). In addition, evaluation of surface modifications and different coating possibilities will help to identify materials less prone to pathogen colonization (210, 211).
Contact Lenses
Microbial keratitis is an infection of the cornea which, if not properly treated, could lead to the loss of vision. The main risk factor is the widespread use of contact lenses. Bacterial keratitis is connected to biofilm-forming bacteria and the following are the most frequently identified genera and species: coagulase-negative staphylococci, Cutibacterium spp., Corynebacterium spp., S. aureus, and P. aeruginosa. Fungal infections are more probable in tropical and subtropical climates, and Fusarium spp., Aspergillus spp., and Candida spp. are often isolated. Tiny amoebas have also been implicated in this pathogenesis (Acanthamoeba) (212–215). The origin of the infection is linked to the ability of contact lenses, even cosmetic ones, to induce alterations of the corneal epithelium and carry organisms to the ocular surface (216). Organisms on contact lenses form stable biofilms, primarily the Gram-negative bacterium P. aeruginosa, due to its capacity to survive different stress situations and to adhere to many different surfaces (216, 217). Physical and chemical characteristics, along with the water retention capacity of the lens’ material and its hydrophobicity, are factors influencing the colonization (214, 217). Researchers are constantly looking for new materials able to inhibit this process (218–221). Moreover, the presence of neutrophils can enhance P. aeruginosa biofilms, a stimulation that can be counteracted by treatment with DNase and anionic poly-aspartic acid (222). Of course, as contact lenses are regularly removed, to avoid their contamination, special care should be taken in correctly using not only the lenses themselves but also their cases and the liquid storage solutions. Overall, the storage conditions should provide a clean environment that prevents microbial growth. The latter represents intensive ongoing research (223–227).
Other Implants
Intrauterine devices (IUDs) are effective long-term contraception methods. However, their usage period is between 4 and 5 years unless signs of pelvic inflammatory disease are detected. Indeed, IUDs represent a foreign body that biofilm-forming bacteria or fungi can colonize. The most common microbes associated with IUDs and upper genital tract infections are S. aureus, E. faecalis, E. coli, Streptococcus spp., Actinomyces spp., Prevotella spp., Bacteroides spp., Clostridium spp., and C. albicans (228–231).
Patients requiring mechanical ventilation can develop ventilator-associated pneumonia due to the use of breathing machines. This life-threatening infection is often linked with biofilm formation on the inserted tubes. The principal genera colonizing the oral and respiratory tract of intubated patients are Streptococcus, Neisseria, and Prevotella (232). Many factors contribute to the development of infections; since the tubes prevent the cough reflex, resulting in an inhibition of mucous clearance, infection damages the tracheal epithelium and finally provides an easy entrance for bacteria able to reach the lower respiratory tract and form biofilm around the surface, representing a dangerous reservoir of bacteria potentially able to migrate and develop pneumonia. The main bacteria responsible for this colonization are S. aureus, K. pneumoniae, E. coli, P. aeruginosa, and Acinetobacter baumannii (159), often found in a mixed population. Indeed, polymicrobial communities are identified between 8 and 58% of the time in endotracheal tubes (233). When these bacteria, all high-grade biofilm formers, stably colonize the tube, they are covered with extracellular polymeric substances typical of biofilm growth, which provides higher tolerance to antimicrobials (234).
Finally, cochlear implants are also prone to infection, and the incidence of complications due to this colonization ranges between 1.7 and 4.1% of patients, a problem that can lead to the removal of the implant and even, in severe cases, be fatal. S. aureus and C. albicans are the leading agents of such infections (235–238).
MICROBIAL DISTRIBUTION
A detailed overview of the microorganisms found in implanted medical devices is shown in Table 1. Gastrointestinal and urological implants are devices that can be colonized by the most diverse strains. S. aureus is the main contaminant in urological implants, along with E. faecalis and E. coli, while gastrointestinal implants are most frequently infected by the pan-antibiotic-resistant A. baumannii, a bacterium resistant to desiccation and nutrient deficiency (239), which forms a stable biofilm resistant to many antimicrobials. A. baumannii is currently one of the top microbes against which new antibiotics are being developed (240–243). Microbes colonizing dental implants are quite different from the ones found on other devices (Table 1). The microbiome colonizing the oral tract has been described as having a dynamic “biofilm lifestyle” (204), forming complex communities composed of a different combination of bacterial species (between 500 and 700 different species have been identified as members of the oral microbiome), many of which are still not cultivable (244). Disequilibrium within the microbial community, the host, and the local microenvironment results in advantageous conditions for the growth of Fusobacterium spp., P. gingivalis, Prevotella spp., Selenomonas spp., Staphylococcus spp., and Streptococcus spp. (197, 202, 207, 208, 245, 246).
TABLE 1.
Bacterial species and fungi isolated and identified from the most clinically relevant medical devicesa
| Devices | Bacteria |
Fungi | References | |||
|---|---|---|---|---|---|---|
| Most frequently isolated | Frequently isolated | Less frequently isolated | Rarely isolated (all <1%) | |||
| Cardiac devices (∼1.2–2.4%) | Coagulase-negative staphylococci (∼60–80%), S. aureus | S. aureus (11–29%), Streptococcus spp. (∼12%), Enterococcus spp. (∼10%) | Cutibacterium acnes (∼4%), HACEK group (∼2%) (Haemophilus, Aggregatibacter spp., Cardiobacterium hominis, Eikenella corrodens, Kingella spp.) | Cutibacterium avidum, Granulicatella adiacens, S. lugdunensis | Candida spp. (∼2–6%) | 58, 66, 68, 71, 81, 273, 276, 277, 404–408 |
| Gastrointestinal implants (∼11.5 [biliary stents]-100% [gastrostomy devices])b | Enterococci (∼21.5–79.3%), (E. faecalis, E. faecium, E. casseliflavus, E. avium, E. gallinarum), Enterobacteriaceae spp. (∼73.7%) (E. coli, E. cloacae, K. pneumoniae, K. oxytoca), streptococci (∼7–31.5%) (S. anginosus, S. parasanguinis, S. mitis, S. constellatus) | Staphylococci (∼9–11.3%), Pseudomonas spp. (∼4–11%) | Micrococcus spp. (∼2.5%), M. morgana (∼2.5%), Vibrio spp. (∼1.5%) | Hafnia alvei, S. liquefaciens, Fusobacterium spp., S. liquefaciens, S. anaerobius | Candida spp. (∼7.5–55.9%) (C. albicans, C. glabrata, C. kefyr, C. tropicalis) | 79, 86, 88–90 |
| Orthopedic implants (∼0.7–1.1%) | Methicillin-sensitive S. aureus (∼30%) | Methicillin-resistant S. epidermidis (∼16%), methicillin-sensitive S. epidermidis (∼14%) | Methicillin-resistant S. aureus (∼8%), E. faecalis (∼2%), Corynebacterium spp. (∼3%) | S. lugdunensis, Streptococcus agalactiae, Streptococcus pyogenes, S. mitis, Streptococcus pneumoniae, Corynebacterium striatum, E. coli, K. pneumoniae, P. mirabilis, P. aeruginosa, S. marcescens | Candida spp. (∼1–3%) | 93, 95, 96, 100, 247 |
| Neurosurgical implants (∼1.6–25.6%) | Staphylococcus spp. (∼76.2%) (S. aureus) | Coagulase-negative staphylococci (∼20%), Cutibacterium spp. (formerly Propionibacterium spp.) (∼5.4–21%) | Enterobacter spp. (∼3.8%), (E. aerogenes, E. cloacae), Streptococcus spp. (∼1.9%), Klebsiella spp. (∼1.9%) (K. pneumoniae, K. oxytoca), E. coli (∼2.2%), Pseudomonas spp. (∼1.6%) (P. aeruginosa) | Acinetobacter baumannii, S. marcescens, Proteus mirabilis, Providencia stuartii, Morganella morganii | Candida spp. (∼1.6–15%) (C. albicans, C. parapsilosis) | 131, 132 |
| Urological implants (∼3–18%) | Coagulase negative staphylococci (∼70–77%) (S. lugdunensis, S. capitis, S. haemolyticus, S. mitis, S. auricularis), E. faecalis (∼55%), E. coli (∼39%) | Klebsiella spp. (∼30%), (K. pneumoniae, K. oxytoca, K. ornithinolytica, K. planticola), P. aeruginosa (∼27%), E. faecium (∼17%), P. mirabilis (∼12.5%), S. epidermis (∼1–39%) | Propionibacterium spp. (∼5%), E. cloacae (∼3.4%), M. morganii (∼3.3%), E. aerogenes (∼3%), S. aureus (∼2.8%), A. baumannii (∼1.4%), Pantoea agglomerans (∼1.3%), P. vulgaris (∼1.1%), Stenotrophomonas maltophilia (∼1.1%) | B. bronchiseptica, B. cepacia, C. diversus, E. dissolvens, E. kobei, H. alvei, Kluyvera cryocrescens, Providencia stuartii, Providencia rettgeri, Ralstonia pickettii, Raoultella terrigena, S. marcescens, Yersinia rohdei | Candida spp. (∼8–26%) (C. albicans, C. glabrata, C. tropicalis) | 135, 137, 141–143, 153 |
| Intravascular devices | S. aureus (∼>30%), P. aeruginosa (∼>30%) | S. epidermis (∼10–30%), E. faecalis (∼10 to 30%), P. aeruginosa (∼10–30%), K. pneumoniae (∼10–30%) | C. albicans (∼3–8%) | 155, 160–162, 164 | ||
| Breast implants (∼1–2.5%) | S. epidermis (∼19–41%), C. acnes (∼12–25%) | S. aureus (∼5%), E. coli (∼1.5%), Ralstonia spp. (∼1–7%) | Corynebacterium spp., Lactobacillus spp., M. fortuitum, M. chelonae, M. abscessus | 174–176, 178, 184, 409, 410 | ||
| Dental implants | Fusobacterium spp. (∼5–9%) | Campylobacter spp. (∼1–7%), Porphyromonas gingivalis (∼1.8–5%), Prevotella spp. (P. intermedia) (∼1–5%), Tannerella forsythia (∼5%), Eubacterium spp. (∼1–1.9%) | E. corrodens | C. albicans (∼<1%) | 197, 202, 207, 208, 245, 246 | |
| Contact lenses (∼2.4–7%) | Coagulase-negative staphylococci (∼39%) | Cutibacterium spp. (∼25.8%), Corynebacterium spp. | Corynebacterium spp. (∼4.6%), Streptococcus spp. (∼2.8%), Bacillus spp. (∼2.2%), Pseudomonas spp. (∼2%), Micrococcus spp. (∼1.9%), S. aureus (∼1.6%), Pseudomonas spp. (∼2%), S. maltophilia (∼1.5%), Serratia spp. (∼1.1%), Acinetobacter (∼1.1%) spp. | Stomatococcus spp., Planococcus spp., Nocardia spp., Listeria spp., Peptococcus spp., Moraxella spp., Flavobacterium spp., Commons spp., Neisseria spp., Achromobacter spp., Klebsiella spp., Alcaligenes spp., Haemophilus spp., Escherichia coli, Aggregatibacter spp., Sphingobacterium spp., Enterobacter spp., Moraxella spp. | Fusarium spp. (∼1–36%), Aspergillus spp. (∼1–30%) | 214, 411, 412 |
| Other devices: IUDs (∼62%), mechanical ventilation breathing machines (∼2.7–10%), cochlear implants (∼1.7–4.1%) | S. aureus (>30%), Actinomyces spp. (∼10–66%), Prevotella spp. (∼10–43%) | E. faecalis (∼26%), Streptococcus spp. (∼23%), E. coli (∼17%), Bacteroides spp. (∼36%), Clostridium spp. (∼25%), P. aeruginosa (∼30%), Acinetobacter baumannii (∼13–26%) | K. pneumoniae (∼3–9%) | C. albicans (∼1–50%) | 228–231, 234–238 | |
Microbes have been divided into four categories according to the relative isolation frequency reported in the literature. The “most frequently isolated” bacteria are found, according to the literature, more than 30% of the time, the “often isolated” are found between 10 and 30% of the time, the “less frequently isolated” correspond to a frequency between 1 and 10%, and the “rarely isolated” correspond to a frequency of <1%. Since fungi are rarely identified under the conditions considered, these microbes were grouped in a single column. Microbial percentages in intravascular devices were extrapolated from the reported literature. The first columns report also the percentages of infected devices versus the total of the devices used (only when data were available).
Since many of the devices were analyzed after being removed because they were displaying signs of infections, the percentages of the device infected could be overestimated.
Moreover, Staphylococcus spp. are the most frequently isolated; among these, S. aureus is found in essentially all implants. Among the coagulase-negative staphylococci, S. epidermidis, S. haemolyticus, S. lugdunensis, S. capitis, S. mitis, and S. auricularis are the most commonly isolated. Streptococcus spp. are very often found on orthopedic, cardiac, neurosurgical implants, and contact lenses. Candida spp. are predominant among fungal colonizations, an observation that was already reported in 2004 by Kojic and Darauiche (247); among these, C. albicans is the most frequently isolated species. Only contact lenses have been reported to be infected by other fungi: Fusarium spp. and Aspergillus spp. Interestingly, fungal colonization has yet to be reported in breast implants (see Table 1 for details and relative references).
Much is known about the most frequently isolated microbes mentioned above. Indeed, recent outstanding works and reviews have focused on Staphylococcus spp. (in particular S. aureus) (248–256), Acinetobacter spp. (especially A. baumannii) (239–243, 256–259), Enterobacteriaceae such as E. coli and K. pneumoniae (243, 256, 260–263), Pseudomonas spp. (in particular P. aeruginosa and P. fluorescens) (256, 264–267), Enterococcus spp. (256, 268, 269), and C. acnes (270–273).
Some of the “less known” biofilm-forming microbial contaminants mentioned in Table 1 are considered emerging pathogens and much future work will be necessary to understand their biology. Among them, it is worth mentioning Staphylococcus lugdunensis, which is a newly recognized threat whose biofilm biomass has been recently investigated (274). S. lugdunensis is connected with hospital-acquired infections, especially in cases of orthopedic infections and aortic prosthetic valve endocarditis (96, 249, 275–277). Cutibacterium avidum is an “under-recognized” microbe (273), an opportunistic pathogen able to invade axillary regions and wet sites, where it is causing infections difficult to eradicate thanks to its capacity to form biofilms, particularly in total hip arthroplasty or breast implant surgeries (273, 278, 279). While much is known of C. acnes, very little is currently known regarding the biology of C. avidum, and many of its features must be better understood to treat C. avidum biofilm-related infections efficiently. Stenotrophomonas maltophilia is a Gram-negative bacillus and an opportunistic emergent pathogen isolated in gastrointestinal and urological implants (see Table 1) and can cause pneumonia in subjects mechanically ventilated, such as COVID-19 hospitalized patients (280). S. maltophilia can form biofilms that are clinically relevant and more resistant to antibiotic treatment than their planktonic counterparts (281, 282). Finally, special attention on Corynebacterium striatum, a strain usually found on human skin and nasal mucosa and able to cause nosocomial diseases, is needed. This organism can colonize orthopedic implants or catheters due to its strong capacity to adhere to surfaces and form biofilms. In addition, C. striatum is often isolated as a multidrug-resistant strain, so it is considered a concern within the scientific community (283–285).
ENVIRONMENTAL FEATURES
Fluid Flow
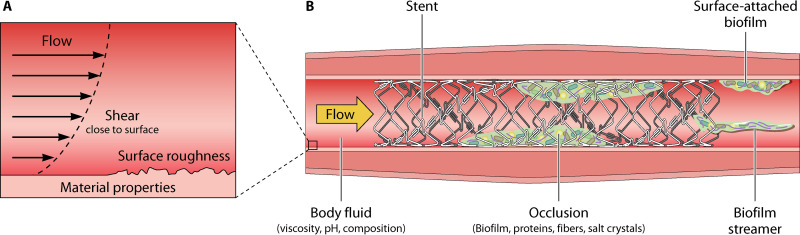
Many environmental factors can shape the development of biofilms on implanted medical devices. A nearly ubiquitous factor is fluid flow, along with the hydrodynamic forces and pressure variations that it generates (Fig. 2A). In many human body systems, including the gut, urinary tract, veins, artery, and eye, liquids are in motion. The shape and compliance of organs and their cyclic contraction movements make fluid flow in the human body complex and nonuniform; the typical range of values are given in Table 2. Medical implants placed in these systems are exposed to flow and are often placed to restore the physiological fluid motion through the systems after surgery or pathological obstructions. Urological catheters, biliary stents, and cerebrospinal fluid drainages are examples of implants placed to favor fluid flow (Fig. 2B), while contact lenses and dental implants are examples of devices exposed to liquids in motion. When considering flow-through medical devices, we must remember that their shape affects the flow and frequently adds complexity to the fluid flow path, as exemplified previously (286) using numerical simulations for the stented ureter. In all of these systems, flow can affect both the transport and the physiology of the small resident organisms, and these can respond to flow with phenotypic and behavioral changes, as extensively discussed earlier (287). Below, we describe some in vitro studies on bacterial-flow and biofilm-flow interactions, performed in different geometries and flow conditions (see Table 3 for details on geometry and flow conditions). These studies shed light on general biophysical phenomena triggered by flow in model systems. Given the wide range of flow velocities and shear stresses at play in the human body (Table 2), these phenomena probably play a role in human infections and colonization of medical devices. However, the complexity of the physical and physiological conditions in the human body and their variability makes it difficult to compare in vitro studies to their in vivo potential realization.
FIG 2.
Nonmicrobiological factors affecting biofilm formation in a medical device. (A) When a moving fluid encounters a solid surface, its speed decreases gradually so that it becomes zero at the surface (i.e., no-slip boundary condition). This variation of the fluid speed in the layer close to the surface generates flow shear. Looking at the surface with a micrometric resolution, we can see the surface roughness, which is due to the material itself and the way material is processed. In addition, solid surfaces can have different material properties, as material mechanical properties and surface charge. Material properties, surface roughness, and flow shear close to the surface potentially influence the accumulation of bacteria or precipitates on the surface. (B) In conducts, fluid properties, such as viscosity and composition, can influence the flow regime within the conduct. When stents or catheters are inserted, occlusions can occur due both to biofilm growth and accumulation of substances transported by the fluid, such as fibers, proteins, and salt crystals. The nature of the precipitates depends on the type of body fluid and its chemical conditions. Biofilms inside the device can grow with different morphologies depending on the local flow conditions and geometrical constraints: surface-attached or as filament suspended in the flow, called streamers. The graphic work was done by modifying elements from Smart Servier Medical Art.
TABLE 2.
Flow velocities and shear stresses measured in different body fluids and human organsa
| Fluid | Organ/Device | Flow velocity (m s−1) | Shear stress (Pa) | Reference(s) |
|---|---|---|---|---|
| Urine | Bladder-urethra | 0–0.3 | 0–0.4 | 413 |
| Ureter | 0–0.48 | 0–0.5 | 414 | |
| Blood | Blood vessels | 0.5–4.3 | 415 | |
| Microcirculation | 0.06–1.2 | 1.3–1.6 | 415, 416 | |
| Saliva | Unstimulated average flow | 0.018–0.024 | 417 | |
| Around orthodontic brackets | 0.069–0.18 | 329 | ||
| Bile | Bile duct | 0–0.2 | 418, 419 | |
| Mucus | Airway surface | (2–7) × 10–5 | 420 |
Several body fluids are considered, and the values characterizing their flow in different organs found in the literature are reported.
TABLE 3.
Flow velocities and shear stresses measured in in vitro studies of bacterial-flow and biofilm-flow interactions, performed using different geometries and flow conditions
| Geometry | Effect(s) of flow | Bacterium/bacteria | Flow velocity (μm s−1) | Shear rate (s–1) | Reference(s) |
|---|---|---|---|---|---|
| Microfluidic straight channel | Cell accumulation in high shear | B. subtilis, P. aeruginosa | 0–104 | 0–50 | 288 |
| Increased residence time | P. aeruginosa | 750–105 | 50–104 | 297 | |
| Upstream swimming motility | E. coli | 0.1–60 0–600 |
290
291 |
||
| Upstream twitching motility |
P. aeruginosa
X. fastidiosa M. mobile |
0–90 0–700 |
0.01–10 |
292
421 294 |
|
| Catch bonds |
S. aureus
E. coli S. epidermidis |
2.5–10 2.5–10 1–10 |
298, 300 298 299 |
||
| Increased virulence upon surface attachment | P. aeruginosa | 301 | |||
| Mechanosensing | P. aeruginosa | 302, 303 | |||
| Shape biofilm architecture |
E. coli
V. cholerae |
2–2,000 |
306
307 |
||
| Microfluidic curved surface | Preferential attachment to specific surface regions | P. aeruginosa, E. coli | 150–2,000 | 289 | |
| Biofilm streamer formation |
P. aeruginosa
S. aureus |
103 6–2 × 104 |
308, 309, 312 313 |
||
| Interplay between flow and biofilm matrix composition | P. aeruginosa | 150–200 | 314 | ||
| Microfluidic flow network | Increased surface colonization capability |
P. aeruginosa
P. aeruginosa and P. mirabilis |
16–250 2–2 × 104 |
295
296 |
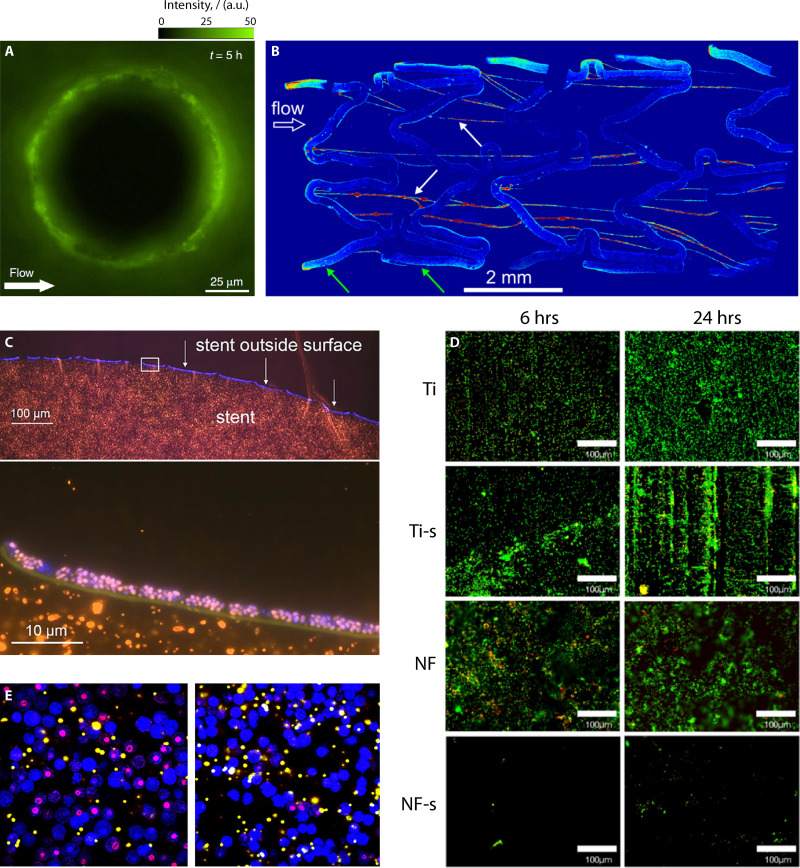
Bacteria in bulk fluid are transported by flow, which exerts hydrodynamic forces on their body and, under certain conditions, affects their spatial distribution. In narrow channels, fluid shear preferentially accumulates motile bacteria in the low-velocity, high-shear regions close to the sidewalls, creating a depletion in cell concentration by up to 70% in the central region of the channel and favoring bacterial surface colonization, as shown for both B. subtilis and P. aeruginosa (288). The same type of interaction promotes the formation of colonization hot spots on curved surfaces (such as pillars; Fig. 3A), creating a heterogeneous bacterial distribution on the surface, with potential effects on the subsequent biofilm development (289). These effects depend only on the morphology and motility of bacteria and are independent of other traits or physiological conditions.
FIG 3.
Mosaic of representative images. (A) Image of fluorescent P. aeruginosa bacteria attached to a 100-μm pillar in the presence of flow. Modified from Secchi et al. (289). (B) Maximum intensity projection image of biofilm streamers formed flowing a suspension of fluorescent P. aeruginosa in a bare-metal stent. Modified from Drescher et al. (312). (C) Bacterial colonization of a biliary polyethylene stent in a patient with bile duct stenosis, visualized by fluorescence in situ hybridization (FISH). Modified from Yan and Bassler (92). (D) Fluorescence images of E. coli on titanium (Ti), silanized titanium (Ti-s), nanoflower-coated (NF), and nanoflower-coated surfaces after silanization (NF-s). Modified from Montgomerie and Poat (333) with permission of Elsevier. (E) Confocal images (X63 magnification) of bone marrow-derived macrophage phagocytosis of fluorescent microspheres (yellow-white) and cell death with propidium iodide stain (red-purple) after exposure to S. aureus biofilm-conditioned medium (left-hand side) and S. aureus planktonic culture-conditioned medium (right-hand side). Modified from Torres et al. (383).
Once bacteria are on a surface or located nearby, hydrodynamic interactions can trigger bacterial movement in the direction opposite to the flow: this behavior is observed in flagellated bacteria with an elongated body, swimming very close to a surface, and in bacteria moving on the surface thanks to type IV pili, the so-called “twitching motility.” Upstream swimming has been studied in E. coli (290) and is a faster mechanism for surface colonization than colony spread by growth (291), with important implications for spreading infections in catheters and medical devices. Upstream twitching has been observed in P. aeruginosa (292), Xylella fastidiosa (293), and Mycoplasma mobile (294). Despite being 100-fold slower than upstream swimming, twitching still confers P. aeruginosa advantages in the colonization of flow networks (295) and in the competition over its natural competitor Proteus mirabilis (296). Hydrodynamic interactions not only increase the surface exploration capabilities of E. coli and P. aeruginosa but also increase their residence time on surfaces (297) by activating, for example, in the case of E. coli specific catch-bonds between proteins present on type I pili and mannose adsorbed on the surface (298). Similar shear-enhanced adhesion mechanisms have been observed in S. epidermis (299) and S. aureus (300).
Surface colonization is the starting point for biofilm formation. Surface adhesion triggers virulence (301) and starts biofilm development in P. aeruginosa due to to hydrodynamic force-sensing mechanisms allowing bacteria to tune their adhesion strength (302). Mechanosensing has been recently recognized as an essential resource that bacteria have developed to optimize biofilm formation and pathogenicity, as discussed in a recent review (303). Once biofilm development has started, local flow can influence biofilm architecture, for example, determining the formation of a bacterial monolayer under high-flow conditions or multilayer structure under low flows (304, 305), affecting cell membrane permeability (306), determining the shape of the colony (307), or driving the formation of biofilm streamers when obstacles and constrictions are present (308–311).
Biofilm streamers, which are filamentous bacterial aggregates suspended in the middle of the flow, can have a more significant impact on the flow itself compared to classic biofilms, for example, by causing rapid clogging of artificial and natural conduits, porous media, and medical devices (Fig. 3B) (312, 313). An in vitro experiment conducted using a bare-metal stent has shown that streamers rapidly develop and span the gaps between metal wires (312). Given their potential impact on the clogging of medical devices, it is worth investigating their formation in in vivo scenarios. If biofilms are formed by several bacterial species, flow can affect population dynamics and biofilm morphology by segregating more-adhesive and less-adhesive cells (314). This interplay could also influence the evolution of biofilm multispecies infection in a medical context.
EPS is mainly constituted from polysaccharides, proteins, nucleic acids, and lipids (21, 316). Its composition can vary greatly depending on the microorganisms, nutrient availability, and environmental conditions, including the presence of fluid flow and the temperature (317–320). In biofilm formed in complex host environments, such as those in medical devices, extra-microbial-host-derived components may be incorporated in the biofilm. Examples of incorporation include fibronectin, a glycoprotein found in the matrix of eukaryotic cells, which plays a crucial role in the formation of S. aureus biofilm on devices, and calcium phosphate and magnesium phosphate crystals formed in urinary catheters due to urinary pH and incorporated in biofilms, resulting in a crystalline structure. However, despite the differences in EPS composition, its functions are universal: EPS forms the scaffold of the biofilm structure, is responsible for adhesion to surfaces and internal cohesion, and protects the microbial community from chemical and mechanical insults (21). In a medical context, surface adhesion is also influenced by the conditioning film formed by host-derived components attached to the surface and potentially interacting with the EPS. In addition, the viscoelastic nature of EPS confers biofilm mechanical resistance: when a force is applied, biofilms instantaneously undergo an elastic deformation as solids and then slowly flow as viscous fluids, further spreading on surfaces, while maintaining their structural integrity (321). The viscoelastic behavior increases the surface spreading and allows the formation of streamers (308, 309).
The number of studies focusing on the influence of local flow on biofilm growth in medical devices is still limited, despite the benefits of a better understanding of the role of fluid dynamics. Biliary stents are a primary example of devices subject to severe occlusion, requiring their removal and replacement. Studies have been conducted on removed stents (Fig. 3C) to shed light on the parameters affecting the clogging dynamics (89, 322, 323). The diameter has been shown to play a primary role since stents with a smaller diameter are more prone to occlusions, whereas stents with a larger lumen allow greater bile flow velocity (but lower shear rate) and, in turn, they are less prone to bile salt precipitation and protein accumulation (89). For this reason, metal stents, having a larger diameter, seem preferable to plastic stents (324) in this respect. Bile viscosity is another factor influencing the flow-pressure relation and consequently biofilm growth, since a higher viscosity implies a lower flow rate and consequently increases the probability of occlusion (28). When considering EPS composition, the flow has been shown to influence the ability to produce specific molecules in S. epidermidis strains isolated from high-shear and low-shear environments in the human body (325) and, in turn, the structure of the biofilm, as shown in an in vitro study (326).
Computational fluid dynamic simulations have been used to characterize the flow field within different configurations of an occluded and stented ureter, and it has been observed that bacterial attachment occurred preferentially near a ureteric occlusion (327). In this case, it has been recently recognized that UTIs should be studied in the context of the bladder’s physical environment, which includes flow (328). For example, orthodontic appliances bonded to the tooth surface are exposed to salivary flow with a self-cleaning action. However, the shape of the appliances affects the flow pattern, potentially favoring the creation of vortexes and a resulting bacterial accumulation, as investigated using computational fluid dynamic simulations (329). In vivo studies focused primarily on the effect of the material, showing that plastic and stainless steel (330) and ceramic and metallic (331) brackets do not influence the composition of the bacterial population.
Surface Properties
Surface properties represent another critical factor that influences biofilm accumulation and the occlusion of implants. In blood-contacting medical devices, proteins contained in the blood, upon contact with the surface of the device, adhere to the surface, followed by platelets, and activate an immune response, resulting in the formation of a fibrin matrix that traps red blood cells and clogs the devices. Biofilm growth can contribute to speeding up this process. Central venous catheters are blood-contacting medical devices, subject to biofouling. In vitro experiments conducted with S. epidermidis using glass and polymer catheters have shown that these materials do not affect biofilm formation or morphology (332). However, very specific surface treatments can reduce biofouling: coating with superhydrophobic titania nanoflowers was shown to increase hemocompatibility and reduce protein deposition and consequently biofilm growth in an in vitro study (Fig. 3D) (333); similar results were obtained with a covalently attached layer of perfluorocarbon (334).
Studies on the influence of the implant material on biofilm formation have been conducted for biliary stents made of polyethylene and hydrophilic polymer-coated polyurethane (335). Inspection of the biofilms using SEM and confocal scanning microscopy (CSM) did not highlight any difference either in the type of biofilm or in the clogging dynamic. Although the type of material does not influence the fouling process, pilot studies have shown that gold coatings reduce the biofouling of different types of catheters (336–338). Furthermore, treatment of surface plasma with direct thrombin inhibitors has been shown to reduce staphylococcal binding, revealing the potential of these agents for implant surface coatings to prevent device-related infections (339). In vitro experiments have shown that biofilm formation on the surface of dental implant materials, such as titanium and zirconium, is comparable to that on hydroxyapatite, a typical tooth surface (340). These results have been obtained with six different bacterial species typically found in the human mouth. Not only were biofilms, imaged using CSM, structurally similar, but the composition of the bacterial community, analyzed by qPCR, also did not show appreciable differences. In another study of dental plaque formation in a flow chamber under anaerobic conditions (341), zirconia surfaces showed a statistically significant reduction in bacterial adhesion after 3 days of incubation compared to titanium surfaces. Nevertheless, differences between zirconia and titanium surfaces with regard to biofilm formation are still under debate.
Surface roughness is an important parameter when considering bacterial surface colonization. Roughness is a characterizing parameter of breast implants, in which the introduction of texture to the outer shell was aimed at increasing tissue incorporation. Based on their texture, breast implants can be divided into smooth (i.e., having a rugosity of <10 μm), microtextured (with rugosity ranging between 10 and 50 μm), and macrotextured (with a rugosity of >50 μm) (342). Several studies confirm that bacterial colonization on rough textures is generally favored (343), while the exact trend depends on the model of the implant and the bacterial species considered (343, 344). Differences in colonization have been recently attributed to different surface areas: rough textures offer a better surface area to which the bacteria can adhere. Plate counting, the methodology used to quantify surface colonization, normalized for the surface area did not show significant differences between textures and bacterial species (343). Similar results were obtained on typical surfaces of dental implants (345) and on biotic surfaces in general (346).
Material properties are essential parameters in controlling the colonization of soft contact lenses. Contact lenses are characterized by a different water content, which changes their comfort and long-term usability (347). A high water content (between 58 and 64%) results in softer lenses that are better suited for long-term use and also reduces biofilm formation (217, 225). Surface wettability is another parameter influencing bacterial adhesion on contact lenses: in vitro studies have shown that P. aeruginosa, S. aureus, or S. epidermidis adhere in greater numbers to the hydrophobic silicone hydrogel lenses compared to hydrophilic hydrogel lenses (217, 348). The same study revealed that a higher surface roughness increased the colonization by S. epidermidis, whereas no differences were reported for P. aeruginosa (217). When fungal biofilms are considered, they have been shown to have different structures on different types of lenses, but the differences have not been systematically analyzed in terms of surface properties (349). Finally, bacteria also respond to the mechanical properties of a surface, such as stiffness, which is positively correlated with the adhesion of different bacterial species, such as S. epidermidis and E. coli, independently of other surface properties (350). Conversely, another study showed that, when exposed to high-shear conditions, E. coli cells exhibit stronger adhesion properties on soft silicone substrates than on stiff ones, a difference that decreased when native silicone surfaces were coated with extracellular matrix molecules (351).
IMMUNOLOGICAL ASPECTS
Although the interplay between host immune response and biofilms has been investigated in the last decade (352, 353), very little is known about their crosstalk associated with medical devices. Most of the literature has focused on the immune response to biofilms formed by P. aeruginosa under specific pathological conditions, such as, for example, in cystic fibrosis patients (354, 355). P. aeruginosa biofilms stimulate the host immune response via extracellular polysaccharides (356), eDNA (357), outer membrane vesicles (OMVs) containing extracellular proteins (358), and small molecules, such as 3-oxo-C12-HSL (359, 360) or pyocyanin (361, 362). EPS components can impair complement activation (363), inhibit macrophage killing (364), and escape the action of neutrophil extracellular traps (NETs) (365). In general, it is known that some components of biofilms formed by Salmonella enterica serovar Typhi, Burkholderia cepacia, or other probiotic bacteria have displayed anti-inflammatory properties (366–368), whereas those formed by other bacteria, such as Thermus aquaticus and some Lactobacillus strains, seemed to be proinflammatory (369, 370). An overview of devices associated with biofilms and their induced host response mechanisms—which can result in either an immune response activation or its suppression—is provided in Table 4.
TABLE 4.
Biofilms and human immune response
| Biofilm component | Antigen | Organism involved | Effect | Immune response | Reference(s) |
|---|---|---|---|---|---|
| Exoproteins | β-Lactamase, lipoprotein, lipase, autolysin, and an ABC transporter lipoprotein | S. aureus | Induce antibody recognition and immune system activation | Activation | 422 |
| Leukocidin, hemolysin, PG, LTA, Hla | S. aureus | Induce cell death in macrophages or in neutrophils via the secretion of leukocidins PVL and HlgAB | Evasion | 375, 383 | |
| Nuc1 | S. aureus | Protects against NET formation by secreting LukAB and Hla | Evasion | 382 | |
| EPS (exopolysaccharides) | Capsular EPS | Streptococcus spp. | Release of IL-1, IL-6, IL-8, and CCL2 | Activation | 423 |
| PIA/PNAG | S. aureus, S. epidermidis | Evasion from neutrophils killing NETs and the bacteria acquired the ability to resist to NET-mediated killing | Evasion | 373, 376–379, 424 | |
| Psl and alginate | P. aeruginosa | Elicit neutrophil aggressive response | Activation | 356 | |
| Alginate | P. aeruginosa | Inhibition of complement activation and macrophage killing | Evasion | 363, 364 | |
| Extracellular DNA | EPS-eDNA | P. aeruginosa | Release of IL-8, IL-1β and Net induction | Activation | 357, 425 |
| Outer membrane vesicles (OMVs) | OMVs containing EPS proteins | P. aeruginosa | Have multiple PAMPs and are highly immunogenic | Activation | 358 |
| Small molecules | 3-Oxo-C12-HSL | P. aeruginosa | Stimulation of neutrophils and IL-8 release | Activation | 359, 360, 426–429 |
| Pyocyanin | P. aeruginosa | Induces ROS and NET production | Activation | 361, 362, 430, 431 | |
| High level of pyocyanin | P. aeruginosa | Create excessive level of NETosis that lead to host tissue damage and chronic infections | Evasion | 432 – 434 |
Bacteria belonging to the Staphylococcus genus, such as S. aureus and S. epidermidis, have been found to form biofilms on implanted medical devices. The immune system is less able to recognize bacteria belonging to the Staphylococcus genus when grown as biofilms, especially mature biofilms, compared to their planktonic growth (371, 372); in fact, biofilm bacteria can resist phagocytosis, the release of toxic granule components, and NET-mediated killing (373–375). One of the components of Staphylococcus biofilms, polysaccharide intercellular adhesin [also known as poly-N-acetyl-β-(1-6)-glucosamine (PIA/PNAG)], can protect against phagocytosis by neutrophils and macrophages (373, 376, 377), whereas S. aureus extracellular nuclease can destroy NETs (378, 379). In S. aureus biofilm, several proteins and toxins are highly expressed and are associated with immune response evasion, even at the early stage of biofilm formation (371, 380, 381). The release of Nuc1 protects against NET formation by neutrophils (382), the secretion of LukAB and Hla induces cell death in macrophages (Fig. 3E) (383), and the release of the leukocidins PVL and HlgAB allows the evasion of neutrophil-mediated killing (375).
Results from S. aureus biofilm secretome have demonstrated the presence of proteins with an immunogenic ability (384, 385); however, their presence is not sufficient to inhibit biofilm formation. In vivo studies on murine S. aureus infections and adaptive immune response found that inflammatory Th1/Th17 responses result in a link with biofilm formation; in contrast, protective Th2/Treg responses are associated with spontaneous clearance of infection without adjunctive therapy (386, 387). Also, the site of infection has an impact on the interaction between S. aureus and the host immune system (388); therefore, the insertion of a device should consider whether the site chosen for the surgery can be reached by the pathogens directly, such as through the blood flow, or indirectly, such as only after passing through the epithelial barrier and draining lymph nodes. Furthermore, the promotion of an anti-inflammatory and profibrotic environment derived from staphylococcal biofilm could occur via alternative macrophage activation (374) or via a distortion of the macrophage response to promote bacterial persistence (389). Between host mechanisms able to clear biofilm, both staphylococcal and that for P. aeruginosa, there is opsonization enhanced by ROS production (390) and the presence of polymorphonuclear neutrophils (PMN) at the site of infection, which have shown in vitro the ability to phagocytose bacteria (391, 392). Probiotic biofilms participate in the formation or destruction of pathological biofilm, depending on their anti-inflammatory ability (368, 369). For example, L. plantarum can help polymorphonuclear leukocytes (PMNs) destroy P. aeruginosa in vitro (393), whereas L. rhamnosus, when incubated with S. aureus, decreases ROS production and phagocytosis, helping S. aureus evasion to the host immune system (394).
In the study of biofilms associated with medical devices, the topography and the implant surface material must be considered as part of the crosstalk with the host immune system. Implants, also built with biodegradable biomaterials, generally induce a host reaction (395, 396) that can be recapitulated in four phases: (i) implantation, (ii) blood-biomaterial interaction, (iii) inflammation, and (iv) tissue remodeling (397). The foreign body reaction could be responsible for many aseptic device failures (398); for all the implants in contact with blood, it is necessary to consider the hemocompatibility of biomaterials (333). Indeed, to prevent thrombosis, an in vitro study using superhydrophobic titania nanoflowers recently improved hemocompatibility, as well as reduced bacterial adhesion compared to both nontextured and unmodified surfaces. A recent study by Doloff et al. demonstrated that breast implants with a roughness of ∼4 μm can induce a greater immune response compared less-rough implants (399).
A better understanding of the interplay between implants and the immune system may allow treatment of the surfaces of medical devices to trigger the host immune system to avoid biofilm formation or implant failure. As a proof of concept, Hanke et al. (400) targeted MΦ proinflammatory activity to reduce biofilm formation in a mouse model of MRSA catheter-associated infection, thus showing the possibility of using the host’s endogenous innate immune cells to control device-associated biofilm infections. The role of IL-1β, in a mouse model of postarthroplasty S. aureus joint infection, has also been demonstrated (401) in controlling bacterial adhesion and biofilm formation through neutrophil recruitment to the site of infection. Based on this finding, Bernthal et al. speculate on the possibility of enhancing the early protective IL-1β response while minimizing any sustained inflammation as a therapeutic strategy to help prevent postarthroplasty infections.
CONCLUDING REMARKS
The recalcitrance to conventional antibiotic therapies of biofilms grown on medical devices usually requires revision operations and replacement of the implant, with increased morbidity, mortality, and a devastating impact on the patient’s quality of life. In addition, the spread of multidrug-resistant bacteria is constantly growing, increasing public concern about the health risks for hospitalized patients due to device-associated microbial infections. Although much has been done to elucidate the mechanisms by which biofilm-forming microbes adhere to indwelling devices and interact with the host immune system, this has been primarily limited to selected pathogens and to a small number of medical implants. Efforts are needed to advance our knowledge of the different microbiota compositions that correlate with a particular device and with the presence of mechanical forces. Furthermore, studies on in vivo biofilms or under more physiological and clinically relevant conditions—such as, for example, in microfluidic systems—should be pursued to shed more light on the complex interplay between surface-attached bacteria and the peri-prosthetic microenvironment.
ACKNOWLEDGMENTS
M.C. was supported by grants awarded to N. Marmiroli and E. Maestri (SITEIA.UNIPR-University of Parma). E.S. was supported by the SNSF PRIMA grant 179834. R.R. was supported by the Italian Ministry of Health–Fondi 5x1000 Ricerca Sanitaria.
Biographies

Marina Caldara, Ph.D., is currently a senior researcher at the University of Parma and collaborates with SITEIA.PARMA. She studied Industrial Biotechnology in Milan-Bicocca and received her doctoral degree in Bioscience Engineering in 2007 at the VU Brussel. From 2007 to 2008 she worked as a postdoctoral fellow with Professor Kevin Verstrepen studying tandem repeats regions of yeast DNA at the FAS Center for Systems Biology at Harvard University and from 2008 to 2011 with Professor Katharina Ribbeck in the Department of Biological Engineering at the Massachusetts Institute of Technology, working on the properties of P. aeruginosa biofilm within a reconstituted mucus layer. From 2011 to 2012, she was a FWO (Flanders Research Foundation) fellow at KU Leuven (Belgium). Since 2014, she has worked at the University of Parma. Her current research focuses on drug repositioning and on understanding the properties of biofilms grown in nature and possibly their exploitation.

Cristina Belgiovine, Ph.D., is a postdoctoral researcher at Humanitas Research Hospital and a student in the Postgraduate School of Microbiology and Virology at Università degli Studi di Pavia. She graduated in Applied Biology and earned a Ph.D. in Genetics and Molecular Biology from the Università degli studi di Pavia. In 2011, she moved to the laboratory of Cellular Immunology of Professor Paola Allavena at the Humanitas Clinical and Research Institute in Milan. She has worked on strategies for targeting tumor-associated macrophages (TAMs), investigating the mechanism of action of drugs used for chemotherapy on TME. She has obtained an AIRC fellowship (2013-2015) and a Veronesi fellowship (2017-2018). More recently, she is investigating the role of soluble proteins produced by macrophages in tumors and in periprosthetic fluids of hip and knee prosthesis and breast implants, focusing on the interplay between immune system, bacterial biofilms, and surface topography.

Eleonora Secchi, Ph.D., is the Principal Investigator of the bioMatter Microfluidics Group in the Institute of Environmental Engineering at ETH Zurich. She earned a B.A. in Physical Engineering, a M.S. in Nuclear Engineering, and a Ph.D. in Chemical Engineering and Industrial Chemistry from the Polytechnic University of Milan. From 2014 to 2016, she has been a postdoctoral fellow in the Laboratoire de Physique Statistique directed by Professor Lyderic Bocquet at Ecole Normale Supérieure. From 2016 to 2018, she was awarded an ETH Postdoctoral Fellowship to work in the laboratory of Professor Roman Stocker in the Department of Civil, Environmental, and Geomatic Engineering at ETH Zurich. Her current research focuses on understanding the physical mechanisms influencing bacterial surface colonization and biofilm formation in fluids and their implications in environmental processes. Her experimental approach relies on an innovative combination of experimental techniques, mainly based on microfluidics and advanced optical visualization techniques.

Roberto Rusconi, Ph.D., is Associate Professor of Applied Physics in the Department of Biomedical Sciences at Humanitas University and Principal Investigator at Humanitas Research Hospital. He earned an M.S. in Nuclear Engineering and a Ph.D. in Radiation Science and Technology from the Polytechnic University of Milan. His graduate research work investigated out-of-equilibrium effects in colloidal dispersions. From 2007 to 2010, he has been a postdoctoral fellow in the group of Professor Howard Stone in the School of Engineering and Applied Sciences at Harvard University and from 2010 to 2015 in the group of Professor Roman Stocker in the Department of Civil and Environmental Engineering at MIT. From 2016 to 2017, he was a Research Scientist at ETH Zurich. By combining microfluidics and mathematical modeling, his research aims to identify fundamental aspects of bacterial transport and biofilm formation in response to the environment, including fluid mechanical forces and chemical gradients.
Contributor Information
Marina Caldara, Email: marina.caldara@unipr.it.
Cristina Belgiovine, Email: Cristina.Belgiovine@humanitasresearch.it.
Eleonora Secchi, Email: secchi@ifu.baug.ethz.ch.
Roberto Rusconi, Email: roberto.rusconi@hunimed.eu.
REFERENCES
- 1.Guerra F, Brambatti M, Matassini MV, Capucci A. 2017. Current therapeutic options for heart failure in elderly patients. Biomed Res Int 2017:1483873. 10.1155/2017/1483873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andries E, Gilles A, Topsakal V, Vanderveken OM, Van de Heyning P, Van Rompaey V, Mertens G. 2020. Systematic review of quality of life assessments after cochlear implantation in older adults. Audiol Neurootol 26:61–75. 10.1159/000508433. [DOI] [PubMed] [Google Scholar]
- 3.Hake ME, Davis ME, Perdue AM, Goulet JA. 2019. Modern implant options for the treatment of distal femur fractures. J Am Acad Orthop Surg 27:e867–e875. 10.5435/JAAOS-D-17-00706. [DOI] [PubMed] [Google Scholar]
- 4.Sandhu FA, Dowlati E, Garica R. 2020. Lumbar arthroplasty: past, present, and future. Neurosurgery 86:155–169. 10.1093/neuros/nyz439. [DOI] [PubMed] [Google Scholar]
- 5.Global Market Insights. 2018. Implantable medical devices market. Global Market Insights, Selbyville, DE. [Google Scholar]
- 6.Darouiche RO. 2004. Treatment of infections associated with surgical implants. New Engl J Med 350:1422–1429. 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 7.Percival SL, Suleman L, Vuotto C, Donelli G. 2015. Healthcare-associated infections, medical devices and biofilms: risk, tolerance, and control. J Med Microbiol 64:323–334. 10.1099/jmm.0.000032. [DOI] [PubMed] [Google Scholar]
- 8.Arciola CR, Campoccia D, Montanaro L. 2018. Implant infections: adhesion, biofilm formation and immune evasion. Nat Rev Microbiol 16:397–409. 10.1038/s41579-018-0019-y. [DOI] [PubMed] [Google Scholar]
- 9.Adlhart C, Verran J, Azevedo NF, Olmez H, Keinänen-Toivola MM, Gouveia I, Melo LF, Crijns F. 2018. Surface modifications for antimicrobial effects in the healthcare setting: a critical overview. J Hosp Infect 99:239–249. 10.1016/j.jhin.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Stewart PS, Bjarnsholt T. 2020. Risk factors for chronic biofilm-related infection associated with implanted medical devices. Clin Microbiol Infect 26:1034–1038. 10.1016/j.cmi.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 11.Plachouras D, Kärki T, Hansen S, Hopkins S, Lyytikäinen O, Moro ML, Reilly J, Zarb P, Zingg W, Kinross P, Weist K, Monnet DL, Suetens C. 2018. Antimicrobial use in European acute care hospitals: results from the second point prevalence survey (PPS) of healthcare-associated infections and antimicrobial use, 2016 to 2017. Euro Surveill 23:1800393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russo PL, Stewardson AJ, Cheng AC, Bucknall T, Mitchell BG. 2019. The prevalence of healthcare associated infections among adult inpatients at nineteen large Australian acute-care public hospitals: a point prevalence survey. Antimicrob Resist Infect Control 8:114. 10.1186/s13756-019-0570-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labi A-K, Obeng-Nkrumah N, Owusu E, Bjerrum S, Bediako-Bowan A, Sunkwa-Mills G, Akufo C, Fenny AP, Opintan JA, Enweronu-Laryea C, Debrah S, Damale N, Bannerman C, Newman MJ. 2019. Multi-centre point-prevalence survey of hospital-acquired infections in Ghana. J Hosp Infect 101:60–68. 10.1016/j.jhin.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Fortaleza CMCB, Padoveze MC, Kiffer CRV, Barth AL, Carneiro IC, Giamberardino HIG, Rodrigues JLN, Santos Filho L, de Mello MJG, Pereira MS, Gontijo Filho PP, Rocha M, Servolo de Medeiros EA, Pignatari ACC. 2017. Multi-state survey of healthcare-associated infections in acute care hospitals in Brazil. J Hosp Infect 96:139–144. 10.1016/j.jhin.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 15.De la Fuente-Núñez C, Reffuveille F, Fernández L, Hancock REW. 2013. Bacterial biofilm development as a multicellular adaptation: antibiotic resistance and new therapeutic strategies. Curr Opin Microbiol 16:580–589. 10.1016/j.mib.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Mah T, Pitts B, Pellock B, Walker GC, Stewart PS, O’Toole GA. 2003. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426:306–310. 10.1038/nature02122. [DOI] [PubMed] [Google Scholar]
- 17.Figueiredo AMS, Ferreira FA, Beltrame CO, Côrtes MF. 2017. The role of biofilms in persistent infections and factors involved in Ica-independent biofilm development and gene regulation in Staphylococcus aureus. Crit Rev Microbiol 43:602–620. 10.1080/1040841X.2017.1282941. [DOI] [PubMed] [Google Scholar]
- 18.Koo H, Allan RN, Howlin RP, Stoodley P, Hall-Stoodley L. 2017. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat Rev Microbiol 15:740–755. 10.1038/nrmicro.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flemming H-CC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. 2016. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14:563–575. 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 20.Flemming HC, Wuertz S. 2019. Bacteria and archaea on Earth and their abundance in biofilms. Nat Rev Microbiol 17:247–260. 10.1038/s41579-019-0158-9. [DOI] [PubMed] [Google Scholar]
- 21.Flemming H-C, Wingender J. 2010. The biofilm matrix. Nat Rev Microbiol 8:623–633. 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Toyofuku M, Sakai R, Nomura N. 2017. Influence of the alginate production on cell-to-cell communication in Pseudomonas aeruginosa PAO1. Environ Microbiol Rep 9:239–249. 10.1111/1758-2229.12521. [DOI] [PubMed] [Google Scholar]
- 23.Águila-Arcos S, Álvarez-Rodríguez I, Garaiyurrebaso O, Garbisu C, Grohmann E, Alkorta I. 2017. Biofilm-forming clinical staphylococcus isolates harbor horizontal transfer and antibiotic resistance genes. Front Microbiol 8:1–12. 10.3389/fmicb.2017.02018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y, Cai P, Jing X, Niu X, Ji D, Ashry NM, Gao C, Huang Q. 2019. Soil biofilm formation enhances microbial community diversity and metabolic activity. Environ Int 132:105116. 10.1016/j.envint.2019.105116. [DOI] [PubMed] [Google Scholar]
- 25.Sun S, Noorian P, McDougald D. 2018. Dual role of mechanisms involved in resistance to predation by protozoa and virulence to humans. Front Microbiol 9:1–12. 10.3389/fmicb.2018.01017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu D, Jia R, Li Y, Gu T. 2017. Advances in the treatment of problematic industrial biofilms. World J Microbiol Biotechnol 33:97. 10.1007/s11274-016-2203-4. [DOI] [PubMed] [Google Scholar]
- 27.Le KY, Park MD, Otto M. 2018. Immune evasion mechanisms of Staphylococcus epidermidis biofilm infection. Front Microbiol 9:359. 10.3389/fmicb.2018.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campoccia D, Mirzaei R, Montanaro L, Arciola CR. 2019. Hijacking of immune defences by biofilms: a multifront strategy. Biofouling 35:1055–1074. 10.1080/08927014.2019.1689964. [DOI] [PubMed] [Google Scholar]
- 29.Costerton JW, Stewart PS. 2014. Biofilms and device-related infections, p 423–439. In Persistent bacterial infections. Wiley, Hoboken, NJ. [Google Scholar]
- 30.Hall CW, Mah T-F. 2017. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol Rev 41:276–301. 10.1093/femsre/fux010. [DOI] [PubMed] [Google Scholar]
- 31.Schlafer S, Meyer RL. 2017. Confocal microscopy imaging of the biofilm matrix. J Microbiol Methods 138:50–59. 10.1016/j.mimet.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Rodríguez-Suárez JM, Butler CS, Gershenson A, Lau BLTT. 2020. Heterogeneous diffusion of polystyrene nanoparticles through an alginate matrix: the role of cross-linking and particle size. Environ Sci Technol 54:5159–5166. 10.1021/acs.est.9b06113. [DOI] [PubMed] [Google Scholar]
- 33.Anderl JN, Franklin MJ, Stewart PS. 2000. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother 44:1818–1824. 10.1128/AAC.44.7.1818-1824.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh R, Ray P, Das A, Sharma M. 2010. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Antimicrob Chemother 65:1955–1958. 10.1093/jac/dkq257. [DOI] [PubMed] [Google Scholar]
- 35.Tseng BS, Zhang W, Harrison JJ, Quach TP, Song JL, Penterman J, Singh PK, Chopp DL, Packman AI, Parsek MR. 2013. The extracellular matrix protects Pseudomonas aeruginosa biofilms by limiting the penetration of tobramycin. Environ Microbiol 15:2865–2878. 10.1111/1462-2920.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinclair P, Carballo-Pacheco M, Allen RJ. 2019. Growth-dependent drug susceptibility can prevent or enhance spatial expansion of a bacterial population. Phys Biol 16. 10.1088/1478-3975/ab131e. [DOI] [PubMed] [Google Scholar]
- 37.Fisher RA, Gollan B, Helaine S. 2017. Persistent bacterial infections and persister cells. Nat Rev Microbiol 15:453–464. 10.1038/nrmicro.2017.42. [DOI] [PubMed] [Google Scholar]
- 38.Dengler Haunreiter V, Boumasmoud M, Häffner N, Wipfli D, Leimer N, Rachmühl C, Kühnert D, Achermann Y, Zbinden R, Benussi S, Vulin C, Zinkernagel AS. 2019. In-host evolution of Staphylococcus epidermidis in a pacemaker-associated endocarditis resulting in increased antibiotic tolerance. Nat Commun 10:1–14. 10.1038/s41467-019-09053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan J, Bassler BL. 2019. Surviving as a community: antibiotic tolerance and persistence in bacterial biofilms. Cell Host Microbe 26:15–21. 10.1016/j.chom.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stewart PS, Franklin MJ. 2008. Physiological heterogeneity in biofilms. Nat Rev Microbiol 6:199–210. 10.1038/nrmicro1838. [DOI] [PubMed] [Google Scholar]
- 41.Karampatzakis A, Sankaran J, Kandaswamy K, Rice SA, Cohen Y, Wohland T. 2017. Measurement of oxygen concentrations in bacterial biofilms using transient state monitoring by single plane illumination microscopy. Biomed Physics Eng Express 3:e035020. 10.1088/2057-1976/aa6db7. [DOI] [Google Scholar]
- 42.Arciola CR, Montanaro L, Costerton JW. 2011. New trends in diagnosis and control strategies for implant infections. Int J Artif Organs 34:727–736. 10.5301/IJAO.2011.8784. [DOI] [PubMed] [Google Scholar]
- 43.Prinz V, Bayerl S, Renz N, Trampuz A, Vajkoczy P, Finger T. 2019. Sonication improves pathogen detection in ventriculoperitoneal shunt-associated infections. Clin Neurosurg 85:516–523. 10.1093/neuros/nyy383. [DOI] [PubMed] [Google Scholar]
- 44.Esteban J, Gomez-Barrena E, Cordero J, Martin-de-Hijas NZ, Kinnari TJ, Fernandez-Roblas R. 2008. Evaluation of quantitative analysis of cultures from sonicated retrieved orthopedic implants in diagnosis of orthopedic infection. J Clin Microbiol 46:488–492. 10.1128/JCM.01762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carlson BC, Hines JT, Robinson WA, Sebastian AS, Greenwood-Quaintance KE, Patel R, Huddleston PM. 2020. Implant sonication versus tissue culture for the diagnosis of spinal implant infection. Spine 45:E525–E532. 10.1097/BRS.0000000000003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Langbach O, Kristoffersen AK, Abesha-Belay E, Enersen M, Røkke O, Olsen I. 2016. Oral, intestinal, and skin bacteria in ventral hernia mesh implants. J Oral Microbiol 8:31854. 10.3402/jom.v8.31854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al-Ahmad A, Muzafferiy F, Anderson AC, Wölber JP, Ratka-Krüger P, Fretwurst T, Nelson K, Vach K, Hellwig E. 2018. Shift of microbial composition of peri-implantitis-associated oral biofilm as revealed by 16s rRNA gene cloning. J Med Microbiol 67:332–340. 10.1099/jmm.0.000682. [DOI] [PubMed] [Google Scholar]
- 48.Fritz B, Stavnsbjerg C, Markvart M, Damgaard PDB, Nielsen SH, Bjørndal L, Qvortrup K, Bjarnsholt T. 2019. Shotgun sequencing of clinical biofilm following scanning electron microscopy identifies bacterial community composition. Pathog Dis 77:ftz013. 10.1093/femspd/ftz013. [DOI] [PubMed] [Google Scholar]
- 49.Ghensi P, Manghi P, Zolfo M, Armanini F, Pasolli E, Bolzan M, Bertelle A, Dell’Acqua F, Dellasega E, Waldner R, Tessarolo F, Tomasi C, Segata N. 2020. Strong oral plaque microbiome signatures for dental implant diseases identified by strain-resolution metagenomics. NPJ Biofilms Microbiomes 6:47. 10.1038/s41522-020-00155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baldan R, Sendi P. 2020. Precision medicine in the diagnosis and management of orthopedic biofilm infections. Front Med 7:580671. 10.3389/fmed.2020.580671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dempsey KE, Riggio MP, Lennon A, Hannah VE, Ramage G, Allan D, Bagg J. 2007. Identification of bacteria on the surface of clinically infected and noninfected prosthetic hip joints removed during revision arthroplasties by 16S rRNA gene sequencing and by microbiological culture. Arthritis Res Ther 9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martellacci L, Quaranta G, Patini R, Isola G, Gallenzi P, Masucci L. 2019. A literature review of metagenomics and culturomics of the peri-implant microbiome: current evidence and future perspectives. Materials 12:3010. 10.3390/ma12183010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su C, Lei L, Duan Y, Zhang K-Q, Yang J. 2012. Culture-independent methods for studying environmental microorganisms: methods, application, and perspective. Appl Microbiol Biotechnol 93:993–1003. 10.1007/s00253-011-3800-7. [DOI] [PubMed] [Google Scholar]
- 54.Green SJ, Leigh MB, Neufeld JD. 2010. Denaturing gradient gel electrophoresis (DGGE) for microbial community analysis, p 4137–4158. In Timmis KN (ed), Handbook of hydrocarbon and lipid microbiology. Springer, Berlin, Germany. 10.1007/978-3-540-77587-4_323. [DOI] [Google Scholar]
- 55.Ercolini D. 2004. PCR-DGGE fingerprinting: novel strategies for detection of microbes in food. J Microbiol Methods 56:297–314. 10.1016/j.mimet.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 56.Fedi S, Tremaroli V, Scala D, Perez-Jimenez JR, Fava F, Young L, Zannoni D. 2005. T-RFLP analysis of bacterial communities in cyclodextrin-amended bioreactors developed for biodegradation of polychlorinated biphenyls. Res Microbiol 156:201–210. 10.1016/j.resmic.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 57.Amann R, Fuchs BM. 2008. Single-cell identification in microbial communities by improved fluorescence in situ hybridization techniques. Nat Rev Microbiol 6:339–348. 10.1038/nrmicro1888. [DOI] [PubMed] [Google Scholar]
- 58.Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT, Pavri BB, Kurtz SM. 2011. 16-Year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States. J Am College Cardiol 58:1001–1006. 10.1016/j.jacc.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 59.Mela T, McGovern BA, Garan H, Vlahakes GJ, Torchiana DF, Ruskin J, Galvin JM. 2001. Long-term infection rates associated with the pectoral versus abdominal approach to cardioverter- defibrillator implants. Am J Cardiol 88:750–753. 10.1016/s0002-9149(01)01845-8. [DOI] [PubMed] [Google Scholar]
- 60.Maisel WH, Moynahan M, Zuckerman BD, Gross TP, Tovar OH, Tillman D-B, Schultz DB. 2006. Pacemaker and ICD generator malfunctions: analysis of Food and Drug Administration annual reports. JAMA 295:1901. 10.1001/jama.295.16.1901. [DOI] [PubMed] [Google Scholar]
- 61.Tarakji KG, Chan EJ, Cantillon DJ, Doonan AL, Hu T, Schmitt S, Fraser TG, Kim A, Gordon SM, Wilkoff BL. 2010. Cardiac implantable electronic device infections: presentation, management, and patient outcomes. Heart Rhythm 7:1043–1047. 10.1016/j.hrthm.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 62.Kirkfeldt RE, Johansen JB, Nohr EA, Jorgensen OD, Nielsen JC. 2014. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J 35:1186–1194. 10.1093/eurheartj/eht511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Habib A, Le KY, Baddour LM, Friedman PA, Hayes DL, Lohse CM, Wilson WR, Steckelberg JM, Sohail MR. 2013. Predictors of mortality in patients with cardiovascular implantable electronic device infections. Am J Cardiol 111:874–879. 10.1016/j.amjcard.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 64.Sohail MR, Uslan DZ, Khan AH, Friedman PA, Hayes DL, Wilson WR, Steckelberg JM, Stoner S, Baddour LM. 2007. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am College Cardiol 49:1851–1859. 10.1016/j.jacc.2007.01.072. [DOI] [PubMed] [Google Scholar]
- 65.Kusumoto FM, Schoenfeld MH, Wilkoff BL, Berul CI, Birgersdotter-Green UM, Carrillo R, Cha Y-M, Clancy J, Deharo J-C, Ellenbogen KA, Exner D, Hussein AA, Kennergren C, Krahn A, Lee R, Love CJ, Madden RA, Mazzetti HA, Moore JC, Parsonnet J, Patton KK, Rozner MA, Selzman KA, Shoda M, Srivathsan K, Strathmore NF, Swerdlow CD, Tompkins C, Wazni O. 2017. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 14:e503–e551. 10.1016/j.hrthm.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 66.Döring M, Richter S, Hindricks G. 2018. The diagnosis and treatment of pacemaker-associated infection. Deutsches Aerzteblatt Online 10.3238/arztebl.2018.0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ortega-Loubon C, Muñoz-Moreno MF, García IA, Álvarez FJ, Gómez-Sánchez E, Bustamante-Munguira J, Lorenzo-López M, Tamayo-Velasco Á, Jorge-Monjas P, Resino S, Tamayo E, Heredia-Rodríguez M. 2019. Nosocomial versus community-acquired infective endocarditis in Spain: location, trends, clinical presentation, etiology, and survival in the 21st century. J Clin Med 10.3390/jcm8101755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Talha KM, Desimone DC, Sohail MR, Baddour LM. 2020. Pathogen influence on epidemiology, diagnostic evaluation, and management of infective endocarditis. Heart 106:1878–1882. 10.1136/heartjnl-2020-317034. [DOI] [PubMed] [Google Scholar]
- 69.Mamtani SS, Aljanabi NM, Gupta Rauniyar RP, Acharya A, Malik BH. 2020. Candida endocarditis: a review of the pathogenesis, morphology, risk factors, and management of an emerging and serious condition. Cureus 11:e6345. 10.7759/cureus.6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nagpal A, Baddour LM, Sohail MR. 2012. Microbiology and pathogenesis of cardiovascular implantable electronic device infections. Circ Arrhythm Electrophysiol 5:433–441. 10.1161/CIRCEP.111.962753. [DOI] [PubMed] [Google Scholar]
- 71.Murdoch DR. 2009. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century. Arch Intern Med 169:463–473. 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta J-P, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska-Gosciniak E, Price S, Roos-Hesselink J, Snygg-Martin U, Thuny F, Tornos Mas P, Vilacosta I, Zamorano JL. 2015. 2015 ESC guidelines for the management of infective endocarditis. Eur Heart J 36:3075–3128. 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 73.Ali S, Kanjwal Y, Bruhl SR, Alo M, Taleb M, Ali SS, Kabour A, Khawaja O. 2017. A meta-analysis of antibacterial envelope use in prevention of cardiovascular implantable electronic device infection. Ther Adv Infect Dis 4:75–82. 10.1177/2049936117702317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seymour RA, Lowry R, Whitworth JM, Martin MV. 2000. Infective endocarditis, dentistry, and antibiotic prophylaxis: time for a rethink? Br Dent J 189:610–616. 10.1038/sj.bdj.4800845. [DOI] [PubMed] [Google Scholar]
- 75.Nakano K, Inaba H, Nomura R, Nemoto H, Takeda M, Yoshioka H, Matsue H, Takahashi T, Taniguchi K, Amano A, Ooshima T. 2006. Detection of cariogenic Streptococcus mutans in extirpated heart valve and atheromatous plaque specimens. J Clin Microbiol 44:3313–3317. 10.1128/JCM.00377-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li X, Kolltveit KM, Tronstad L, Olsen I. 2000. Systemic diseases caused by oral infection. Clin Microbiol Rev 13:547–558. 10.1128/CMR.13.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bensing BA, Li L, Yakovenko O, Wong M, Barnard KN, Iverson TM, Lebrilla CB, Parrish CR, Thomas WE, Xiong Y, Sullam PM. 2019. Recognition of specific sialoglycan structures by oral streptococci impacts the severity of endocardial infection. PLoS Pathog 15:e1007896. 10.1371/journal.ppat.1007896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abranches J, Zeng L, Kajfasz JK, Palmer SR, Chakraborty B, Wen ZT, Richards VP, Brady LJ, Lemos JA. 2018. Biology of oral streptococci. Microbiol Spectrum 6:GPP3-0042-2018. 10.1128/microbiolspec.GPP3-0042-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park SY, Kim SH, Kang SH, Yoon CH, Lee HJ, Yun PY, Youn TJ, Chae IH. 2019. Improved oral hygiene care attenuates the cardiovascular risk of oral health disease: a population-based study from Korea. Eur Heart J 40:1138–1145. 10.1093/eurheartj/ehy836. [DOI] [PubMed] [Google Scholar]
- 80.Pierce D, Calkins BC, Thornton K. 2012. Infectious endocarditis: diagnosis and treatment. Am Fam Physician 85:981–986. [PubMed] [Google Scholar]
- 81.Okuda KI, Nagahori R, Yamada S, Sugimoto S, Sato C, Sato M, Iwase T, Hashimoto K, Mizunoe Y. 2018. The composition and structure of biofilms developed by Propionibacterium acnes isolated from cardiac pacemaker devices. Front Microbiol 9:182. 10.3389/fmicb.2018.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.DeLegge RL, DeLegge MH. 2005. Percutaneous endoscopic gastrostomy evaluation of device materials: are we “failsafe”? Nutr Clin Pract 20:613–617. 10.1177/0115426505020006613. [DOI] [PubMed] [Google Scholar]
- 83.Le MN-T, Kayama S, Yoshikawa M, Hara T, Kashiyama S, Hisatsune J, Tsuruda K, Onodera M, Ohge H, Tsuga K, Sugai M. 2020. Oral colonization by antimicrobial-resistant Gram-negative bacteria among long-term care facility residents: prevalence, risk factors, and molecular epidemiology. Antimicrob Resist Infect Control 9:45. 10.1186/s13756-020-0705-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dautle MP, Wilkinson TR, Gauderer MWL. 2003. Isolation and identification of biofilm microorganisms from silicone gastrostomy devices. J Pediatr Surg 38:216–220. 10.1053/jpsu.2003.50046. [DOI] [PubMed] [Google Scholar]
- 85.Trevisani L, Sartori S, Rossi MR, Bovolenta R, Scoponi M, Gullini S, Abbasciano V. 2005. Degradation of polyurethane gastrostomy devices: what is the role of fungal colonization? Dig Dis Sci 50:463–469. 10.1007/s10620-005-2459-2. [DOI] [PubMed] [Google Scholar]
- 86.Boulay BR, Parepally M. 2014. Managing malignant biliary obstruction in pancreas cancer: choosing the appropriate strategy. World J Gastroenterol 20:9345–9353. 10.3748/wjg.v20.i28.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kruse EJ. 2010. Palliation in pancreatic cancer. Surg Clinics North Am 90:355–364. 10.1016/j.suc.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 88.Guaglianone E, Cardines R, Vuotto C, di Rosa R, Babini V, Mastrantonio P, Donelli G. 2010. Microbial biofilms associated with biliary stent clogging. FEMS Immunol Med Microbiol 59:410–420. 10.1111/j.1574-695X.2010.00686.x. [DOI] [PubMed] [Google Scholar]
- 89.Vaishnavi C, Samanta J, Kochhar R. 2018. Characterization of biofilms in biliary stents and potential factors involved in occlusion. World J Gastroenterol 24:112–123. 10.3748/wjg.v24.i1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weber A, Schneider J, Wagenpfeil S, Winkle P, Riedel J, Wantia N, Feihl S, Römmler F, Baur DM, Schmid RM, Algül H, Huber W. 2013. Spectrum of pathogens in acute cholangitis in patients with and without biliary endoprosthesis. J Infect 67:111–121. 10.1016/j.jinf.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 91.Scheufele F, Aichinger L, Jäger C, Demir IE, Schorn S, Sargut M, Erkan M, Kleeff J, Friess H, Ceyhan GO. 2017. Effect of preoperative biliary drainage on bacterial flora in bile of patients with periampullary cancer. Br J Surg 104:e182–e188. 10.1002/bjs.10450. [DOI] [PubMed] [Google Scholar]
- 92.Lübbert C, Wendt K, Feisthammel J, Moter A, Lippmann N, Busch T, Mössner J, Hoffmeister A, Rodloff AC. 2016. Epidemiology and resistance patterns of bacterial and fungal colonization of biliary plastic stents: a prospective cohort study. PLoS One 11:e0155479. 10.1371/journal.pone.0155479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. 2008. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res 466:1710–1715. 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nodzo SR, Boyle KK, Spiro S, Nocon AA, Miller AO, Westrich GH. 2017. Success rates, characteristics, and costs of articulating antibiotic spacers for total knee periprosthetic joint infection. Knee 24:1175–1181. 10.1016/j.knee.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 95.Triffault-Fillit C, Ferry T, Laurent F, Pradat P, Dupieux C, Conrad A, Becker A, Lustig S, Fessy MH, Chidiac C, Valour F, Ferry T, Valour F, Perpoint T, Boibieux A, Biron F, Miailhes P, Ader F, Becker A, Roux S, Triffault-Fillit C, Daoud F, Lippman J, et al. 2019. Microbiologic epidemiology depending on time to occurrence of prosthetic joint infection: a prospective cohort study. Clin Microbiol Infect 25:353–358. 10.1016/j.cmi.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 96.Lourtet-Hascoët J, Bicart-See A, Félicé MP, Giordano G, Bonnet E. 2016. Staphylococcus lugdunensis, a serious pathogen in periprosthetic joint infections: comparison to Staphylococcus aureus and Staphylococcus epidermidis. Int J Infect Dis 51:56–61. 10.1016/j.ijid.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 97.Lee J, Zilm PS, Kidd SP. 2020. Novel research models for Staphylococcus aureus small colony variants (SCV) development: co-pathogenesis and growth rate. Front Microbiol 11:1–8. 10.3389/fmicb.2020.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.de Mesy Bentley KL, Trombetta R, Nishitani K, Bello-Irizarry SN, Ninomiya M, Zhang L, Chung HL, McGrath JL, Daiss JL, Awad HA, Kates SL, Schwarz EM. 2017. Evidence of Staphylococcus aureus deformation, proliferation, and migration in canaliculi of live cortical bone in murine models of osteomyelitis. J Bone Miner Res 32:985–990. 10.1002/jbmr.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paharik AE, Horswill AR. 2016. The staphylococcal biofilm: adhesins, regulation, and host response. Microbiol Spectr 4. 10.1128/microbiolspec.VMBF-0022-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stoodley P, Nistico L, Johnson S, Lasko LA, Baratz M, Gahlot V, Ehrlich GD, Kathju S. 2008. Direct demonstration of viable Staphylococcus aureus biofilms in an infected total joint arthroplasty: a case report. J Bone Joint Surg Ser A 90:1751–1758. 10.2106/JBJS.G.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dastgheyb SS, Hammoud S, Ketonis C, Liu AY, Fitzgerald K, Parvizi J, Purtill J, Ciccotti M, Shapiro IM, Otto M, Hickok NJ. 2015. Staphylococcal persistence due to biofilm formation in synovial fluid containing prophylactic cefazolin. Antimicrob Agents Chemother 59:2122–2128. 10.1128/AAC.04579-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lew DP, Waldvogel FA. 2004. Osteomyelitis. Lancet 364. 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 103.Kelly-Quintos C, Cavacini LA, Posner MR, Goldmann D, Pier GB. 2006. Characterization of the opsonic and protective activity against Staphylococcus aureus of fully human monoclonal antibodies specific for the bacterial surface polysaccharide poly-N-acetylglucosamine. Infect Immun 74:2742–2750. 10.1128/IAI.74.5.2742-2750.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maira-Litrán T, Kropec A, Goldmann DA, Pier GB. 2005. Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated staphylococcal poly-n-acetyl-β-(1-6)-glucosamine. Infect Immun 73:6752–6762. 10.1128/IAI.73.10.6752-6762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Levack AE, Cyphert EL, Bostrom MP, Hernandez CJ, von Recum HA, Carli AV. 2018. Current options and emerging biomaterials for periprosthetic joint infection. Curr Rheumatol Rep 20:33. 10.1007/s11926-018-0742-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zajonz D, Birke U, Ghanem M, Prietzel T, Josten C, Roth A, Fakler JKM. 2017. Silver-coated modular Megaendoprostheses in salvage revision arthroplasty after periimplant infection with extensive bone loss: a pilot study of 34 patients. BMC Musculoskelet Disord 18:383. 10.1186/s12891-017-1742-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wafa H, Grimer RJ, Reddy K, Jeys L, Abudu A, Carter SR, Tillman RM. 2015. Retrospective evaluation of the incidence of early periprosthetic infection with silver-treated endoprostheses in high-risk patients. Bone Joint J 97-B. 10.1302/0301-620X.97B2.34554. [DOI] [PubMed] [Google Scholar]
- 108.Mijnendonckx K, Leys N, Mahillon J, Silver S, van Houdt R. 2013. Antimicrobial silver: uses, toxicity, and potential for resistance. Biometals 26:609–621. 10.1007/s10534-013-9645-z. [DOI] [PubMed] [Google Scholar]
- 109.Sussman EM, Casey BJ, Dutta D, Dair BJ. 2015. Different cytotoxicity responses to antimicrobial nanosilver coatings when comparing extract-based and direct-contact assays. J Appl Toxicol 35. 10.1002/jat.3104. [DOI] [PubMed] [Google Scholar]
- 110.Ciobanu G, Ilisei S, Luca C. 2014. Hydroxyapatite-silver nanoparticles coatings on porous polyurethane scaffold. Mater Sci Eng C Mater Biol Appl 35:36–42. 10.1016/j.msec.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 111.Indelli PF, Ghirardelli S, Iannotti F, Indelli AM, Pipino G. 2021. Nanotechnology as an anti-infection strategy in periprosthetic joint infections (PJI). Trop Med Infect Dis 6. 10.3390/tropicalmed6020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ricciardi BF, Muthukrishnan G, Masters EA, Kaplan N, Daiss JL, Schwarz EM. 2020. New developments and future challenges in prevention, diagnosis, and treatment of prosthetic joint infection. J Orthop Res 38:1423–1435. 10.1002/jor.24595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lindfors N, Geurts J, Drago L, Arts JJ, Juutilainen V, Hyvönen P, Suda AJ, Domenico A, Artiaco S, Alizadeh C, Brychcy A, Bialecki J, Romanò CL. 2017. Antibacterial bioactive glass, S53P4, for chronic bone infections - a multinational study. Adv Exp Med Biol 971:81–92. 10.1007/5584_2016_156. [DOI] [PubMed] [Google Scholar]
- 114.Franceschini M, Sandiford NA, Cerbone V, de Araujo LCT, Kendoff D. 2020. Defensive antibacterial coating in revision total hip arthroplasty: new concept and early experience. HIP Int 30. 10.1177/1120700020917125. [DOI] [PubMed] [Google Scholar]
- 115.Gabrilovich DI, Nagaraj S. 2009. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9:162–174. 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sica A, Bronte V. 2007. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest 117:1155–1166. 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brudecki L, Ferguson DA, McCall CE, El Gazzar M. 2012. Myeloid-derived suppressor cells evolve during sepsis and can enhance or attenuate the systemic inflammatory response. Infect Immun 80:2026–2034. 10.1128/IAI.00239-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Youn J-I, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI. 2012. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol 91:167–181. 10.1189/jlb.0311177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Heim CE, Vidlak D, Kielian T. 2015. Interleukin-10 production by myeloid-derived suppressor cells contributes to bacterial persistence during Staphylococcus aureus orthopedic biofilm infection. J Leukoc Biol 98:1003–1013. 10.1189/jlb.4VMA0315-125RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Niska JA, Shahbazian JH, Ramos RI, Francis KP, Bernthal NM, Miller LS. 2013. Vancomycin-rifampin combination therapy has enhanced efficacy against an experimental Staphylococcus aureus prosthetic joint infection. Antimicrob Agents Chemother 57:5080–5086. 10.1128/AAC.00702-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thrane JF, Sunde NA, Bergholt B, Rosendal F. 2014. Increasing infection rate in multiple implanted pulse generator changes in movement disorder patients treated with deep brain stimulation. Stereotact Funct Neurosurg 92:360–364. 10.1159/000365576. [DOI] [PubMed] [Google Scholar]
- 122.Bjerknes S, Skogseid IM, Saehle T, Dietrichs E, Toft M. 2014. Surgical site infections after deep brain stimulation surgery: frequency, characteristics and management in a 10-year period. PLoS One 9:e105288. 10.1371/journal.pone.0105288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Martin RM, Zimmermann LL, Huynh M, Polage CR. 2018. Diagnostic approach to health care- and device-associated central nervous system infections. J Clin Microbiol 56:e00861-18. 10.1128/JCM.00861-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vinchon M, Dhellemmes P. 2006. Cerebrospinal fluid shunt infection: risk factors and long-term follow-up. Childs Nerv Syst 22:692–697. 10.1007/s00381-005-0037-8. [DOI] [PubMed] [Google Scholar]
- 125.Di Rienzo A, Colasanti R, Gladi M, Pompucci A, Della Costanza M, Paracino R, Esposito D, Iacoangeli M. 2020. Sinking flap syndrome revisited: the who, when and why. Neurosurg Rev 43:323–335. 10.1007/s10143-019-01148-7. [DOI] [PubMed] [Google Scholar]
- 126.Zarrouk V, Vassor I, Bert F, Bouccara D, Kalamarides M, Bendersky N, Redondo A, Sterkers O, Fantin B. 2007. Evaluation of the management of postoperative aseptic meningitis. Clin Infect Dis 44:1555–1559. 10.1086/518169. [DOI] [PubMed] [Google Scholar]
- 127.Conen A, Fux CA, Vajkoczy P, Trampuz A. 2017. Management of infections associated with neurosurgical implanted devices. Expert Rev Anti Infect Ther 15:241–255. 10.1080/14787210.2017.1267563. [DOI] [PubMed] [Google Scholar]
- 128.Zimmerli W, Trampuz A, Ochsner PE. 2004. Current concepts: prosthetic-joint infections. N Engl J Med 351:1645–1654. 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 129.Arocho-Quinones EV, Huang C-C, Ward BD, Pahapill PA. 2019. Care bundle approach to minimizing infection rates after neurosurgical implants for neuromodulation: a single-surgeon experience. World Neurosurg 128:e87–e97. 10.1016/j.wneu.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gilbert DN, Chambers HF, Eliopoulos GM, Saag MS, Pavia AT (ed). 2019. The Sanford guide to antimicrobial therapy 2019—50 years: 1969-2019. Antimicrobial Therapy, Sperryville, VA. [Google Scholar]
- 131.Chen Y, Zhang L, Qin T, Wang Z, Li Y, Gu B. 2019. Evaluation of neurosurgical implant infection rates and associated pathogens: evidence from 1118 postoperative infections. Neurosurg Focus 47:E6. 10.3171/2019.5.FOCUS18582. [DOI] [PubMed] [Google Scholar]
- 132.Strahm C, Albrich WC, Zdravkovic V, Schöbi B, Hildebrandt G, Schlegel M. 2018. Infection rate after cranial neurosurgical procedures: a prospective single-center study. World Neurosurg 111:e277–e285. 10.1016/j.wneu.2017.12.062. [DOI] [PubMed] [Google Scholar]
- 133.Sabih A, Leslie SW. 2021. Complicated urinary tract infections. In StatPearls, StatPearls Publishing, Treasure Island, FL. [PubMed] [Google Scholar]
- 134.Paick SH, Park HK, Oh SJ, Kim HH. 2003. Characteristics of bacterial colonization and urinary tract infection after indwelling of double-J ureteral stent. Urology 62:214–217. 10.1016/s0090-4295(03)00325-x. [DOI] [PubMed] [Google Scholar]
- 135.Riedl CR, Plas E, Hubner WA, Zimmerl H, Ulrich W, Pfluger H. 1999. Bacterial colonization of ureteral stents. Eur Urol 36:53–59. 10.1159/000019927. [DOI] [PubMed] [Google Scholar]
- 136.Kehinde EO, Rotimi VO, Al-awadi KA, Aabdul-Halim H, Boland F, Al-Hunayan A, Pazhoor A. 2002. Factors predisposing to urinary tract infection after J ureteral stent insertion. J Urol 167:1334–1337. 10.1016/S0022-5347(05)65294-9. [DOI] [PubMed] [Google Scholar]
- 137.Tenke P, Köves B, Nagy K, Hultgren SJ, Mendling W, Wullt B, Grabe M, Wagenlehner FME, Cek M, Pickard R, Botto H, Naber KG, Johansen TEB. 2012. Update on biofilm infections in the urinary tract. World J Urol 30:51–57. 10.1007/s00345-011-0689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Scotland KB, Lo J, Grgic T, Lange D. 2019. Ureteral stent-associated infection and sepsis: pathogenesis and prevention: a review. Biofouling 35:117–127. 10.1080/08927014.2018.1562549. [DOI] [PubMed] [Google Scholar]
- 139.Wollin TA, Tieszer C, Riddell JV, Denstedt JD, Reid G. 1998. Bacterial biofilm formation, encrustation, and antibiotic adsorption to ureteral stents indwelling in humans. J Endourol 12:101–111. 10.1089/end.1998.12.101. [DOI] [PubMed] [Google Scholar]
- 140.Tomer N, Garden E, Small A, Palese M. 2021. Ureteral stent encrustation: epidemiology, pathophysiology, management, and current technology. J Urol 201:68–67. [DOI] [PubMed] [Google Scholar]
- 141.Chew BH, Lange D. 2009. Ureteral stent symptoms and associated infections: a biomaterials perspective. Nat Rev Urol 6:440–448. 10.1038/nrurol.2009.124. [DOI] [PubMed] [Google Scholar]
- 142.Holá V, Ruzicka F, Horka M. 2010. Microbial diversity in biofilm infections of the urinary tract with the use of sonication techniques. FEMS Immunol Med Microbiol 59:525–528. 10.1111/j.1574-695X.2010.00703.x. [DOI] [PubMed] [Google Scholar]
- 143.Tenke P, Kovacs B, Jäckel M, Nagy E. 2006. The role of biofilm infection in urology. World J Urol 24:13–20. 10.1007/s00345-005-0050-2. [DOI] [PubMed] [Google Scholar]
- 144.Gao G, Lange D, Hilpert K, Kindrachuk J, Zou Y, Cheng JTJ, Kazemzadeh-Narbat M, Yu K, Wang R, Straus SK, Brooks DE, Chew BH, Hancock REW, Kizhakkedathu JN. 2011. The biocompatibility and biofilm resistance of implant coatings based on hydrophilic polymer brushes conjugated with antimicrobial peptides. Biomaterials 32:3899–3909. 10.1016/j.biomaterials.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 145.Lo J, Lange D, Chew BH. 2014. Ureteral stents and Foley catheters-associated urinary tract infections: the role of coatings and materials in infection prevention. Antibiotics 3:87–97. 10.3390/antibiotics3010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Andersen MJ, Flores-Mireles AL. 2019. Urinary catheter coating modifications: the race against catheter-associated infections. Coatings 10. 10.3390/coatings10010023. [DOI] [Google Scholar]
- 147.Singha P, Locklin J, Handa H. 2017. A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater 50. 10.1016/j.actbio.2016.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Al-Qahtani M, Safan A, Jassim G, Abadla S. 2019. Efficacy of anti-microbial catheters in preventing catheter associated urinary tract infections in hospitalized patients: a review on recent updates. J Infect Public Health 12:760–766. 10.1016/j.jiph.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 149.Gayani B, Dilhari A, Kottegoda N, Ratnaweera DR, Weerasekera MM. 2021. Reduced crystalline biofilm formation on superhydrophobic silicone urinary catheter materials. ACS Omega 6. 10.1021/acsomega.1c00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mosayyebi A, Lange D, Yann Yue Q, Somani BK, Zhang X, Manes C, Carugo D. 2019. Reducing deposition of encrustation in ureteric stents by changing the stent architecture: a microfluidic-based investigation. Biomicrofluidics 13:1–15. 10.1063/1.5059370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Isguven S, Chung PH, Machado P, Delaney LJ, Chen AF, Forsberg F, Hickok NJ. 2020. Minimizing penile prosthesis implant infection: what can we learn from orthopedic surgery? Urology 146:6–14. 10.1016/j.urology.2020.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dawn LE, Henry GD, Tan GK, Wilson SK. 2017. Biofilm and infectious agents present at the time of penile prosthesis revision surgery: times are a changing. Sex Med Rev 5:236–243. 10.1016/j.sxmr.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 153.Henry GD, Wilson SK, Delk JR, Carson CC, Silverstein AD, Cleves MA, Donatucci CF. 2004. Penile prosthesis cultures during revision surgery: a multicenter study. J Urol 172:153–156. 10.1097/01.ju.0000132141.48587.f1. [DOI] [PubMed] [Google Scholar]
- 154.Silverstein AD, Henry GD, Evans B, Pasmore M, Simmons CJ, Donatucci CF. 2006. Biofilm formation on clinically noninfected penile prostheses. J Urol 176:1008–1011. 10.1016/j.juro.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 155.O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, Raad II, Randolph AG, Rupp ME, Saint S, Healthcare Infection Control Practices Advisory Committee (HICPAC) . 2011. Guidelines for the prevention of intravascular catheter-related infections Clin Infect Dis 52. 10.1093/cid/cir257. [DOI] [Google Scholar]
- 156.Satou K, Kusanagi R, Nishizawa A, Hori S. 2018. Scrubbing technique for needleless connectors to minimize contamination risk. J Hosp Infect 100:e200–e203. 10.1016/j.jhin.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 157.Moureau N. 2015. Disinfection of needleless connector hubs: clinical evidence systematic review. J Assoc Vasc Access 20:266. 10.1016/j.java.2015.10.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chernecky C, Waller J. 2011. Comparative evaluation of five needleless intravenous connectors. J Adv Nursing 67:1601–1613. 10.1111/j.1365-2648.2010.05598.x. [DOI] [PubMed] [Google Scholar]
- 159.Schulze A, Mitterer F, Pombo JP, Schild S. 2021. Biofilms by bacterial human pathogens: clinical relevance—development, composition and regulation—therapeutic strategies. Microbial Cell 8:28–56. 10.15698/mic2021.02.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Scoppettuolo G, Donato C, De Carolis E, Vella A, Vaccaro L, La Greca A, Fantoni M. 2014. Candida utilis catheter-related bloodstream infection. Med Mycol Case Rep 6:70–72. 10.1016/j.mmcr.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wisplinghoff H, Ebbers J, Geurtz L, Stefanik D, Major Y, Edmond MB, Wenzel RP, Seifert H. 2014. Nosocomial bloodstream infections due to Candida spp. in the USA: species distribution, clinical features, and antifungal susceptibilities. Int J Antimicrob Agents 43:78–81. 10.1016/j.ijantimicag.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 162.Maki DG, Kluger DM, Crnich CJ. 2006. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clinic Proc 81:1159–1171. 10.4065/81.9.1159. [DOI] [PubMed] [Google Scholar]
- 163.Albert O, Bonnet E, Cassard B, Chambrier C, Charmillon A, Diamantis S, Gachot B, Lafaurie M, Lebeaux D, Lucas N, Strady C, Toubiana J. 2021. Antibiotic lock therapy for the conservative treatment of long-term intravenous catheter-related infections in adults and children: when and how to proceed? Guidelines for clinical practice 2020. Infect Dis Now 51:236–246. 10.1016/j.idnow.2021.02.004. [DOI] [PubMed] [Google Scholar]
- 164.Messing B, Peitra-Cohen S, Debure A, Beliah M, Bernier JJ. 1988. Antibiotic-lock technique: a new approach to optimal therapy for catheter-related sepsis in home-parenteral nutrition patients. J Parenteral Enteral Nutr 12:185–189. 10.1177/0148607188012002185. [DOI] [PubMed] [Google Scholar]
- 165.Norris LAB, Kablaoui F, Brilhart MK, Bookstaver PB. 2017. Systematic review of antimicrobial lock therapy for prevention of central-line-associated bloodstream infections in adult and pediatric cancer patients. Int J Antimicrob Agents 10.1016/j.ijantimicag.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 166.Bookstaver B, Justo JA. 2014. Antibiotic lock therapy: review of technique and logistical challenges. Infect Drug Resist 7:343. 10.2147/IDR.S51388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Moon DJ, Deva AK. 2021. Adverse events associated with breast implants: the role of bacterial infection and biofilm. Clin Plastic Surg 48:101–108. 10.1016/j.cps.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 168.Gabriel SE, Woods JE, O’Fallon WM, Beard CM, Kurland LT, Melton LJ. 1997. Complications leading to surgery after breast implantation. New Engl J Med 336:677–682. 10.1056/NEJM199703063361001. [DOI] [PubMed] [Google Scholar]
- 169.Camirand A, Doucet J. 2000. Breast augmentation: teaching our patients how compression can help prevent capsular contracture. Aesthetic Plast Surg 24:221–226. 10.1007/s002660010037. [DOI] [PubMed] [Google Scholar]
- 170.Wiener TC. 2008. Relationship of incision choice to capsular contracture. Aesthetic Plast Surg 32:303–306. 10.1007/s00266-007-9061-2. [DOI] [PubMed] [Google Scholar]
- 171.Namnoum JD, Largent J, Kaplan HM, Oefelein MG, Brown MH. 2013. Primary breast augmentation clinical trial outcomes stratified by surgical incision, anatomical placement and implant device type. J Plast Reconstruct Aesthetic Surg 66:1165–1172. 10.1016/j.bjps.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 172.Montemurro P, Demir IA, Cheema M, Hedén P. 2018. Exploring the genetic role of capsular contracture in three family generations with a case report and a literature review. Aesthetic Surg J 38:6–9. 10.1093/asj/sjx176. [DOI] [PubMed] [Google Scholar]
- 173.Stevens WG, Nahabedian MY, Calobrace MB, Harrington JL, Capizzi PJ, Cohen R, D’incelli RC, Beckstrand M. 2013. Risk factor analysis for capsular contracture: a 5-year sientra study analysis using round, smooth, and textured implants for breast augmentation. Plast Reconstruct Surg 132:1115–1123. 10.1097/01.prs.0000435317.76381.68. [DOI] [PubMed] [Google Scholar]
- 174.Pajkos A, Deva AK, Vickery K, Cope C, Chang L, Cossart YE. 2003. Detection of subclinical infection in significant breast implant capsules. Plast Reconstruct Surg 111:1605–1611. 10.1097/01.PRS.0000054768.14922.44. [DOI] [PubMed] [Google Scholar]
- 175.Schrem S, Heine N, Eisenmann-Klein M, Prantl L. 2007. Bacterial colonization is of major relevance for high-grade capsular contracture after augmentation mammoplasty. Ann Plast Surg 59:126–130. 10.1097/01.sap.0000252714.72161.4a. [DOI] [PubMed] [Google Scholar]
- 176.Ahn CY, Ko CY, Wagar EA, Wong RS, Shaw WW. 1996. Microbial evaluation: 139 implants removed from symptomatic patients. Plast Reconstruct Surg 98:1225–1229. 10.1097/00006534-199612000-00016. [DOI] [PubMed] [Google Scholar]
- 177.Washer LL, Gutowski K. 2012. Breast Implant Infections. Infect Dis Clinics North Am 26:111–125. 10.1016/j.idc.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 178.Ajdic D, Zoghbi Y, Gerth D, Panthaki ZJ, Thaller S. 2016. The relationship of bacterial biofilms and capsular contracture in breast implants. Aesthetic Surg J 36:297–309. 10.1093/asj/sjv177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jacombs A, Tahir S, Hu H, Deva AK, Almatroudi A, Wessels WLF, Bradshaw DA, Vickery K. 2014. In vitro and in vivo investigation of the influence of implant surface on the formation of bacterial biofilm in mammary implants. Plast Reconstruct Surg 133:471–480. [DOI] [PubMed] [Google Scholar]
- 180.Yeo IS, Kim HY, Lim KS, Han JS. 2012. Implant surface factors and bacterial adhesion: a review of the literature. Int J Artif Organs 35:762–772. 10.5301/ijao.5000154. [DOI] [PubMed] [Google Scholar]
- 181.Wong CH, Samuel M, Tan BK, Song C. 2006. Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: a systematic review. Plast Reconstruct Surg 118:1224–1236. 10.1097/01.prs.0000237013.50283.d2. [DOI] [PubMed] [Google Scholar]
- 182.Liu X, Zhou L, Pan F, Gao Y, Yuan X, Fan D. 2015. Comparison of the postoperative incidence rate of capsular contracture among different breast implants: a cumulative meta-analysis. PLoS One 10:e0116071. 10.1371/journal.pone.0116071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Keech JA, Creech BJ. 1997. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstruct Surg 100:554–555. [DOI] [PubMed] [Google Scholar]
- 184.Hu H, Johani K, Almatroudi A, Vickery K, Van Natta B, Kadin ME, Brody G, Clemens M, Cheah CY, Lade S, Joshi PA, Prince HM, Deva AK. 2016. Bacterial biofilm infection detected in breast implant-associated anaplastic large-cell lymphoma. Plast Reconstruct Surg 137:1659–1669. 10.1097/PRS.0000000000002010. [DOI] [PubMed] [Google Scholar]
- 185.Cordeiro PG, Ghione P, Ni A, Hu Q, Ganesan N, Galasso N, Dogan A, Horwitz SM. 2020. Risk of breast implant associated anaplastic large cell lymphoma (BIA-ALCL) in a cohort of 3546 women prospectively followed long term after reconstruction with textured breast implants. J Plast Reconstruct Aesthetic Surg 73:841–846. 10.1016/j.bjps.2019.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wiener TC. 2012. Minimizing capsular contracture in a “clean-contaminated site.” Aesthetic Surg J 32. 10.1177/1090820X11433819. [DOI] [PubMed] [Google Scholar]
- 187.Wixtrom RN, Stutman RL, Burke RM, Mahoney AK, Codner MA. 2012. Risk of breast implant bacterial contamination from endogenous breast flora, prevention with nipple shields, and implications for biofilm formation. Aesthetic Surg J 32. 10.1177/1090820X12456841. [DOI] [PubMed] [Google Scholar]
- 188.Awad AN, Heiman AJ, Patel A. 2021. Implants and breast pocket irrigation: outcomes of antibiotic, antiseptic, and saline irrigation. Aesthetic Surg J 10.1093/asj/sjab181. [DOI] [PubMed] [Google Scholar]
- 189.Barker AS, Law J, Nicholson M, Collett D, Deva AK. 2020. The reversed glove sleeve: a readily available and cost-effective way to achieve “no touch” breast implant insertion. Plast Reconstr Surg - Glob Open 8:e2650. 10.1097/GOX.0000000000002650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Huttenhower C, Gevers D, Knight R, Abubucker S, Badger JH, Chinwalla AT, Creasy HH, Earl AM, Fitzgerald MG, Fulton RS, Giglio MG, Hallsworth-Pepin K, Lobos EA, Madupu R, Magrini V, Martin JC, Mitreva M, Muzny DM, Sodergren EJ, Versalovic J, Wollam AM, et al. 2012. Structure, function, and diversity of the healthy human microbiome. Nature 486:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Awano S, Ansai T, Takata Y, Soh I, Akifusa S, Hamasaki T, Yoshida A, Sonoki K, Fujisawa K, Takehara T. 2008. Oral health and mortality risk from pneumonia in the elderly. J Dent Res 87:334–339. 10.1177/154405910808700418. [DOI] [PubMed] [Google Scholar]
- 192.Beck JD, Offenbacher S. 2005. Systemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular disease. J Periodontol 76:2089–2100. 10.1902/jop.2005.76.11-S.2089. [DOI] [PubMed] [Google Scholar]
- 193.Genco RJ, Grossi SG, Ho A, Nishimura F, Murayama Y. 2005. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J Periodontol 76:2075–2084. 10.1902/jop.2005.76.11-S.2075. [DOI] [PubMed] [Google Scholar]
- 194.Joshipura KJ, Rimm EB, Douglass CW, Trichopoulos D, Ascherio A, Willett WC. 1996. Poor oral health and coronary heart disease. J Dent Res 75:1631–1636. 10.1177/00220345960750090301. [DOI] [PubMed] [Google Scholar]
- 195.Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. 2007. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect 13:3–10. 10.1111/j.1469-0691.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- 196.Grössner-Schreiber B, Teichmann J, Hannig M, Dörfer C, Wenderoth DF, Ott SJ. 2009. Modified implant surfaces show different biofilm compositions under in vivo conditions. Clinical Oral Implants Res 20:817–826. 10.1111/j.1600-0501.2009.01729.x. [DOI] [PubMed] [Google Scholar]
- 197.Renvert S, Roos-Jansåker A-M, Claffey N. 2008. Non-surgical treatment of peri-implant mucositis and peri-implantitis: a literature review. J Clin Periodontol 35:305–315. 10.1111/j.1600-051X.2008.01276.x. [DOI] [PubMed] [Google Scholar]
- 198.Cenci MS, Lund RG, Pereira CL, de Carvalho RM, Demarco FF. 2006. In vivo and in vitro evaluation of class II composite resin restorations with different matrix systems. J Adhesive Dent 8:127–32. [PubMed] [Google Scholar]
- 199.Beyth N, Bahir R, Matalon S, Domb AJ, Weiss EI. 2008. Streptococcus mutans biofilm changes surface-topography of resin composites. Dent Mater 24:732–736. 10.1016/j.dental.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 200.Busscher HJ, Rinastiti M, Siswomihardjo W, van der Mei HC. 2010. Biofilm formation on dental restorative and implant materials. J Dent Res 89:657–665. 10.1177/0022034510368644. [DOI] [PubMed] [Google Scholar]
- 201.Papaioannou W, Gizani S, Nassika M, Kontou E, Nakou M. 2007. Adhesion of Streptococcus mutans to different types of brackets. Angle Orthod 77:1090–1095. 10.2319/091706-375.1. [DOI] [PubMed] [Google Scholar]
- 202.Lee A, Wang HLH-L. 2010. Biofilm related to dental implants. Implant Dent 19:387–393. 10.1097/ID.0b013e3181effa53. [DOI] [PubMed] [Google Scholar]
- 203.Larsen T, Fiehn N-E. 2017. Dental biofilm infections: an update. APMIS 125:376–384. 10.1111/apm.12688. [DOI] [PubMed] [Google Scholar]
- 204.Marsh PD. 2005. Dental plaque: biological significance of a biofilm and community life-style. J Clin Periodontol 32:7–15. 10.1111/j.1600-051X.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 205.Fürst MM, Salvi GE, Lang NP, Persson GR. 2007. Bacterial colonization immediately after installation on oral titanium implants. Clin Oral Implants Res 18:501–508. 10.1111/j.1600-0501.2007.01381.x. [DOI] [PubMed] [Google Scholar]
- 206.Quirynen M, Vogels R, Peeters W, van Steenberghe D, Naert I, Haffajee A. 2006. Dynamics of initial subgingival colonization of ‘pristine’ peri-implant pockets. Clin Oral Implants Res 17:25–37. 10.1111/j.1600-0501.2005.01194.x. [DOI] [PubMed] [Google Scholar]
- 207.Botero JE, González AM, Mercado RA, Olave G, Contreras A. 2005. Subgingival microbiota in peri-implant mucosa lesions and adjacent teeth in partially edentulous patients. J Periodontol 76:1490–1495. 10.1902/jop.2005.76.9.1490. [DOI] [PubMed] [Google Scholar]
- 208.Tabanella G, Nowzari H, Slots J. 2009. Clinical and microbiological determinants of ailing dental implants. Clin Implant Dent Relat Res 11:24–36. 10.1111/j.1708-8208.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- 209.Jain A, Rai A, Singh A, Taneja S. 2020. Efficacy of preoperative antibiotics in prevention of dental implant failure: a meta-analysis of randomized controlled trials. Oral Maxillofacial Surg 24:469–475. 10.1007/s10006-020-00872-5. [DOI] [PubMed] [Google Scholar]
- 210.Alghamdi HS, Jansen JA. 2020. The development and future of dental implants. Dent Mater J 39:167–172. 10.4012/dmj.2019-140. [DOI] [PubMed] [Google Scholar]
- 211.Jiang X, Yao Y, Tang W, Han D, Zhang L, Zhao K, Wang S, Meng Y. 2020. Design of dental implants at materials level: an overview. J Biomed Mater Res Part A 108:1634–1661. 10.1002/jbm.a.36931. [DOI] [PubMed] [Google Scholar]
- 212.Page MA, Mathers WD. 2013. Acanthamoeba keratitis: a 12-year experience covering a wide spectrum of presentations, diagnoses, and outcomes. J Ophthalmol 2013:670242. 10.1155/2013/670242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Khalil MA, Sonbol FI. 2014. Investigation of biofilm formation on contact eye lenses caused by methicillin resistant Staphylococcus aureus. Niger J Clin Pract 17:776–784. 10.4103/1119-3077.144398. [DOI] [PubMed] [Google Scholar]
- 214.Willcox MDP, Harmis N, Cowell BA, Williams T, Holden BA. 2001. Bacterial interactions with contact lenses; effects of lens material, lens wear, and microbial physiology. Biomaterials 22:3235–3247. 10.1016/S0142-9612(01)00161-2. [DOI] [PubMed] [Google Scholar]
- 215.Thomas PA, Kaliamurthy J. 2013. Mycotic keratitis: epidemiology, diagnosis, and management. Clin Microbiol Infect 19:210–220. 10.1111/1469-0691.12126. [DOI] [PubMed] [Google Scholar]
- 216.Bispo P, Haas W, Gilmore M. 2015. Biofilms in infections of the eye. Pathogens 4:111–136. 10.3390/pathogens4010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Dutta D, Cole N, Willcox M. 2012. Factors influencing bacterial adhesion to contact lenses. Mol Vision 18:14–21. [PMC free article] [PubMed] [Google Scholar]
- 218.Read ML, Navascues-Cornago M, Keir N, Maldonado-Codina C, Morgan PB. 2020. The impact of contact lens wear on ocular surface mucins using a novel clinical fluorescence imaging system. Contact Lens Anterior Eye 43:378–388. 10.1016/j.clae.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 219.Ruiz-Alcocer J, Monsálvez-Romín D, García-Lázaro S, Albarrán-Diego C, Hernández-Verdejo JL, Madrid-Costa D. 2018. Impact of contact lens material and design on the ocular surface. Clin Exp Optometry 101:188–192. 10.1111/cxo.12622. [DOI] [PubMed] [Google Scholar]
- 220.Morgan PB, Murphy PJ, Gifford KL, Gifford P, Golebiowski B, Johnson L, Makrynioti D, Moezzi AM, Moody K, Navascues-Cornago M, Schweizer H, Swiderska K, Young G, Willcox M. 2021. CLEAR: effect of contact lens materials and designs on the anatomy and physiology of the eye. Contact Lens Anterior Eye 44:192–219. 10.1016/j.clae.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 221.Stapleton F, Tan J. 2017. Impact of contact lens material, design, and fitting on discomfort. Eye Contact Lens Sci Clin Pract 43:32–39. 10.1097/ICL.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 222.Robertson DM, Parks QM, Young RL, Kret J, Poch KR, Malcolm KC, Nichols DP, Nichols M, Zhu M, Cavanagh HD, Nick JA. 2011. Disruption of contact lens-associated Pseudomonas aeruginosa biofilms formed in the presence of neutrophils. Invest Ophthalmol Vis Sci 52:2844–2850. 10.1167/iovs.10-6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cho P, Boost MV. 2019. Evaluation of prevention and disruption of biofilm in contact lens cases. Ophthalmic Physiol Opt 39:337–349. 10.1111/opo.12635. [DOI] [PubMed] [Google Scholar]
- 224.Cope JR, Collier SA, Nethercut H, Jones JM, Yates K, Yoder JS. 2017. Risk behaviors for contact lens-related eye infections among adults and adolescents—United States, 2016. MMWR Morb Mortal Wkly Rep 66:841–845. 10.15585/mmwr.mm6632a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Dosler S, Hacioglu M, Yilmaz FN, Oyardi O. 2020. Biofilm modelling on the contact lenses and comparison of the in vitro activities of multipurpose lens solutions and antibiotics. PeerJ 8:e9419. 10.7717/peerj.9419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Efron N, Morgan PB. 2017. Rethinking contact lens aftercare. Clin Exp Optometry 100. 10.1111/cxo.12588. [DOI] [PubMed] [Google Scholar]
- 227.di Onofrio V, Gesuele R, Maione A, Liguori G, Liguori R, Guida M, Nigro R, Galdiero E. 2019. Prevention of Pseudomonas aeruginosa biofilm formation on soft contact lenses by allium sativum fermented extract (BGE) and cannabinol oil extract (CBD). Antibiotics 8:258. 10.3390/antibiotics8040258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ádám A, Pál Z, Terhes G, Szucs M, Gabay ID, Urbán E. 2018. Culture- and PCR-based detection of BV associated microbiological profile of the removed IUDs and correlation with the time period of IUD in place and the presence of the symptoms of genital tract infection. Ann Clin Microbiol Antimicrob 17:40. 10.1186/s12941-018-0293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Madden T, Grentzer JM, Secura GM, Allsworth JE, Peipert JF. 2012. Risk of bacterial vaginosis in users of the intrauterine device: a longitudinal study. Sex Transm Dis 39:217–222. 10.1097/OLQ.0b013e31823e68fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Pál Z, Urbán E, Dósa E, Pál A, Nagy E. 2005. Biofilm formation on intrauterine devices in relation to duration of use. J Med Microbiol 54:1199–1203. 10.1099/jmm.0.46197-0. [DOI] [PubMed] [Google Scholar]
- 231.Taylor BD, Darville T, Haggerty CL. 2013. Does bacterial vaginosis cause pelvic inflammatory disease? Sex Transmit Dis 40:117–122. 10.1097/OLQ.0b013e31827c5a5b. [DOI] [PubMed] [Google Scholar]
- 232.Lazarevic V, Gaïa N, Emonet S, Girard M, Renzi G, Despres L, Wozniak H, Yugueros Marcos J, Veyrieras J-B, Chatellier S, van Belkum A, Pugin J, Schrenzel J. 2014. Challenges in the culture-independent analysis of oral and respiratory samples from intubated patients. Front Cell Infect Microbiol 4:65. 10.3389/fcimb.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gibbs K, Holzman IR. 2012. Endotracheal tube: friend or foe? Bacteria, the endotracheal tube, and the impact of colonization and infection. Semin Perinatol 36:454–461. 10.1053/j.semperi.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 234.Craven DE, Hjalmarson KI. 2010. Ventilator-associated tracheobronchitis and pneumonia: thinking outside the box. Clin Infect Dis 51:S59–S66. 10.1086/653051. [DOI] [PubMed] [Google Scholar]
- 235.Antonelli PJ, Lee JC, Burne RA. 2004. Bacterial biofilms may contribute to persistent cochlear implant infection. Otol Neurotol 25:953–957. 10.1097/00129492-200411000-00015. [DOI] [PubMed] [Google Scholar]
- 236.Cristobal R, Edmiston CE, Runge-Samuelson CL, Owen HA, Firszt JB, Wackym PA. 2004. Fungal biofilm formation on cochlear implant hardware after antibiotic-induced fungal overgrowth within the middle ear. Pediatr Infect Dis J 23:774–778. 10.1097/01.inf.0000134315.24413.92. [DOI] [PubMed] [Google Scholar]
- 237.Pawlowski KS, Wawro D, Roland PS. 2005. Bacterial biofilm formation on a human cochlear implant. Otol Neurotol 26:972–975. 10.1097/01.mao.0000169047.38759.8b. [DOI] [PubMed] [Google Scholar]
- 238.Ruellan K, Frijns JHM, Bloemberg GV, Hautefort C, Van Den Abbeele T, Lamers GEM, Herman P, Ba Huy PT, Kania RE. 2010. Detection of bacterial biofilm on cochlear implants removed because of device failure, without evidence of infection. Otol Neurotol 31:1320–1324. 10.1097/MAO.0b013e3181e3d36b. [DOI] [PubMed] [Google Scholar]
- 239.Gaddy JA, Actis LA. 2009. Regulation of Acinetobacter baumannii biofilm formation. Future Microbiol 4:273–278. 10.2217/fmb.09.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582. 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wilks M, Wilson A, Warwick S, Price E, Kennedy D, Ely A, Millar MR. 2006. Control of an outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus colonization and infection in an intensive care unit (ICU) without closing the ICU or placing patients in isolation. Infect Control Hosp Epidemiol 27:654–658. 10.1086/507011. [DOI] [PubMed] [Google Scholar]
- 242.Tomaras AP, Dorsey CW, Edelmann RE, Actis LA. 2003. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: involvement of a novel chaperone-usher pili assembly system. Microbiology 149:3473–3484. 10.1099/mic.0.26541-0. [DOI] [PubMed] [Google Scholar]
- 243.Maragakis LL, Perl TM. 2008. Antimicrobial resistance: Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis 46:1254–1263. 10.1086/529198. [DOI] [PubMed] [Google Scholar]
- 244.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner ACR, Yu W-H, Lakshmanan A, Wade WG. 2010. The human oral microbiome. J Bacteriol 192:5002–5017. 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Romanos GE, Biltucci MT, Kokaras A, Paster BJ. 2016. Bacterial composition at the implant-abutment connection under loading in vivo. Clin Implant Dent Relat Res 18:138–145. 10.1111/cid.12270. [DOI] [PubMed] [Google Scholar]
- 246.Persson GR, Renvert S. 2014. Cluster of bacteria associated with peri-implantitis. Clin Implant Dent Relat Res 16:783–793. 10.1111/cid.12052. [DOI] [PubMed] [Google Scholar]
- 247.Kojic EM, Darouiche RO. 2004. Candida infections of medical devices. Clin Microbiol Rev 17:255–267. 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Heilmann C. 2011. Adhesion mechanisms of staphylococci. Adv Exp Med Biol 715:105–123. 10.1007/978-94-007-0940-9_7. [DOI] [PubMed] [Google Scholar]
- 249.Becker K, Heilmann C, Peters G. 2014. Coagulase-negative staphylococci. Clin Microbiol Rev 27:870–926. 10.1128/CMR.00109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Galar A, Weil AA, Dudzinski DM, Muñoz P, Siedner MJ. 2019. Methicillin-resistant staphylococcus aureus prosthetic valve endocarditis: pathophysiology, epidemiology, clinical presentation, diagnosis, and management. Clin Microbiol Rev 32. 10.1128/CMR.00041-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Haddad Kashani H, Schmelcher M, Sabzalipoor H, Seyed Hosseini E, Moniri R. 2017. Recombinant endolysins as potential therapeutics against antibiotic-resistant Staphylococcus aureus: current status of research and novel delivery strategies. Clin Microbiol Rev 31. 10.1128/CMR.00071-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Mosolygó T, Kincses A, Csonka A, Tönki ÁS, Witek K, Sanmartín C, Marć MA, Handzlik J, Kieć-Kononowicz K, Domínguez-Álvarez E, Spengler G. 2019. Selenocompounds as novel antibacterial agents and bacterial efflux pump inhibitors. Molecules 24:1487. 10.3390/molecules24081487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lakhundi S, Zhang K. 2018. Methicillin-resistant Staphylococcus aureus: molecular characterization, evolution, and epidemiology. Clin Microbiol Rev 31. 10.1128/CMR.00020-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kranjec C, Morales Angeles D, Torrissen Mårli M, Fernández L, García P, Kjos M, Diep DB. 10.3390/antibiotics10020131. [DOI] [PMC free article] [PubMed]
- 255.Nguyen HTT, Nguyen TH, Otto M. 2020. The staphylococcal exopolysaccharide PIA: biosynthesis and role in biofilm formation, colonization, and infection. Comput Struct Biotechnol J 18:3324–3334. 10.1016/j.csbj.2020.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.de Oliveira DMP, Forde BM, Kidd TJ, Harris PNA, Schembri MA, Beatson SA, Paterson DL, Walker MJ. 2020. Antimicrobial resistance in ESKAPE pathogens. Clin Microbiol Rev 33:e00181-19. 10.1128/CMR.00181-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. 2017. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev 30:409–447. 10.1128/CMR.00058-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Mea HJ, Yong PVC, Wong EH. 2021. An overview of Acinetobacter baumannii pathogenesis: motility, adherence, and biofilm formation. Microbiol Res 247:126722. 10.1016/j.micres.2021.126722. [DOI] [PubMed] [Google Scholar]
- 259.Colquhoun JM, Rather PN. 2020. Insights into mechanisms of biofilm formation in Acinetobacter baumannii and implications for uropathogenesis. Front Cell Infect Microbiol 10. 10.3389/fcimb.2020.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. 2012. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 25:682–707. 10.1128/CMR.05035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Davin-Regli A, Lavigne J-P, Pagès J-M. 2019. Enterobacter spp.: update on taxonomy, clinical aspects, and emerging antimicrobial resistance. Clin Microbiol Rev 32:e00002-19. 10.1128/CMR.00002-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Wang G, Zhao G, Chao X, Xie L, Wang H. 2020. The characteristic of virulence, biofilm, and antibiotic resistance of Klebsiella pneumoniae. Int J Environ Res Public Health 17:1–17. 10.3390/ijerph17176278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ageorges V, Monteiro R, Leroy S, Burgess CM, Pizza M, Chaucheyras-Durand F, Desvaux M. 2020. Molecular determinants of surface colonization in diarrhoeagenic Escherichia coli (DEC): from bacterial adhesion to biofilm formation. FEMS Microbiol Rev 44:314–350. 10.1093/femsre/fuaa008. [DOI] [PubMed] [Google Scholar]
- 264.Horcajada JP, Montero M, Oliver A, Sorlí L, Luque S, Gómez-Zorrilla S, Benito N, Grau S. 2019. Epidemiology and treatment of multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa infections. Clin Microbiol Rev 32:e00031-19. 10.1128/CMR.00031-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Scales BS, Dickson RP, LiPuma JJ, Huffnagle GB. 2014. Microbiology, genomics, and clinical significance of the Pseudomonas fluorescens species complex, an unappreciated colonizer of humans. Clin Microbiol Rev 27:927–948. 10.1128/CMR.00044-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Behzadi P, Baráth Z, Gajdács M. 2021. It’s not easy being green: a narrative review on the microbiology, virulence and therapeutic prospects of multidrug-resistant Pseudomonas aeruginosa. Antibiotics 10:42. 10.3390/antibiotics10010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Urwin L, Okurowska K, Crowther G, Roy S, Garg P, Karunakaran E, MacNeil S, Partridge LJ, Green LR, Monk PN. 2020. Corneal infection models: tools to investigate the role of biofilms in bacterial keratitis. Cells 9:2450. 10.3390/cells9112450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.García-Solache M, Rice LB. 2019. The Enterococcus: a model of adaptability to its environment. Clin Microbiol Rev 32:1–28. 10.1128/CMR.00058-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Gipson KS, Nickerson KP, Drenkard E, Llanos-Chea A, Dogiparthi SK, Lanter BB, Hibbler RM, Yonker LM, Hurley BP, Faherty CS. 2020. The great ESKAPE: exploring the crossroads of bile and antibiotic resistance in bacterial pathogens. Infect Immun 88:e00865-19. 10.1128/IAI.00865-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Dréno B, Pécastaings S, Corvec S, Veraldi S, Khammari A, Roques C. 2018. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: a brief look at the latest updates. J Eur Acad Dermatol Venereol 32:5–14. 10.1111/jdv.15043. [DOI] [PubMed] [Google Scholar]
- 271.Mayslich C, Grange PA, Dupin N. 2021. Cutibacterium acnes as an opportunistic pathogen: an update of its virulence-associated factors. Microorganisms 9:303. 10.3390/microorganisms9020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Foster AL, Cutbush K, Ezure Y, Schuetz MA, Crawford R, Paterson DL. 2020. Cutibacterium (Proprionibacterium) acnes in shoulder surgery: a scoping review of strategies for prevention, diagnosis and treatment. J Shoulder Elbow Surg 10.1016/j.jse.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 273.Corvec S. 2018. Clinical and biological features of Cutibacterium (formerly Propionibacterium) avidum, an underrecognized microorganism. Clin Microbiol Rev 31:1–42. 10.1128/CMR.00064-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Ravaioli S, Campoccia D, Speziale P, Pietrocola G, Zatorska B, Maso A, Presterl E, Montanaro L, Arciola CR. 2020. Various biofilm matrices of the emerging pathogen Staphylococcus lugdunensis: exopolysaccharides, proteins, eDNA, and their correlation with biofilm mass. Biofouling 36:86–100. 10.1080/08927014.2020.1716217. [DOI] [PubMed] [Google Scholar]
- 275.Heilbronner S, Foster TJ. 2021. Staphylococcus lugdunensis: a skin commensal with invasive pathogenic potential. Clin Microbiol Rev 34:e00205-20. 10.1128/CMR.00205-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Douedi S, Upadhyaya VD, Obagi A, Hossain M. 2020. Aggressive Staphylococcus lugdunensis endocarditis in a young healthy patient: a case report. Cardiol Res 11:192–195. 10.14740/cr1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Yamazaki K, Minakata K, Sakamoto K, Sakai J, Ide Y, Kawatou M, Kanemitsu H, Ikeda T, Minatoya K, Sakata R. 2020. A case of aggressive aortic prosthetic valve endocarditis aggressive caused by Staphylococcus lugdunensis. Surg Case Rep 6:280. 10.1186/s40792-020-01062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Isabel R, Monica M. 2019. Cutibacterium avidum: a rare but expected agent of breast implant infection. IDCases 17:e00546. 10.1016/j.idcr.2019.e00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Maurer SM, Kursawe L, Rahm S, Prinz J, Zinkernagel AS, Moter A, Kuster SP, Zbinden R, Zingg PO, Achermann Y. 2021. Cutibacterium avidum resists surgical skin antisepsis in the groin: a potential risk factor for periprosthetic joint infection: a quality control study. Antimicrobial Resist Infect Control 10:27. 10.1186/s13756-021-00883-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Hughes S, Troise O, Donaldson H, Mughal N, Moore LSP. 2020. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect 26:1395–1399. 10.1016/j.cmi.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Montoya-Hinojosa E, Bocanegra-Ibarias P, Garza-González E, Alonso-Ambriz ÓM, Salazar-Mata GA, Villarreal-Treviño L, Pérez-Alba E, Camacho-Ortiz A, Morfín-Otero R, Rodríguez-Noriega E, Flores-Treviño S. 2020. Discrimination of biofilm-producing Stenotrophomonas maltophilia clinical strains by matrix-assisted laser desorption ionization–time of flight. PLoS One 15:e0244751. 10.1371/journal.pone.0244751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Pompilio A, Ranalli M, Piccirilli A, Perilli M, Vukovic D, Savic B, Krutova M, Drevinek P, Jonas D, Fiscarelli EV., Tuccio Guarna Assanti V, Tavío MM, Artiles F, Di Bonaventura G. 2020. Biofilm formation among Stenotrophomonas maltophilia isolates has clinical relevance: the ANSELM prospective multicenter study. Microorganisms 9:49. 10.3390/microorganisms9010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Ramos JN, Souza C, Faria YV, da Silva EC, Veras JFC, Baio PVP, Seabra SH, de Oliveira Moreira L, Hirata Júnior R, Mattos-Guaraldi AL, Vieira VV. 2019. Bloodstream and catheter-related infections due to different clones of multidrug-resistant and biofilm producer Corynebacterium striatum. BMC Infect Dis 19:672. 10.1186/s12879-019-4294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kalt F, Schulthess B, Sidler F, Herren S, Fucentese SF, Zingg PO, Berli M, Zinkernagel AS, Zbinden R, Achermann Y. 2018. Corynebacterium species rarely cause orthopedic infections. J Clin Microbiol 56:e01200-18. 10.1128/JCM.01200-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.De Souza C, Mota HF, Faria YV, Cabral FDO, De Oliveira DR, Sant’Anna L de O, Nagao PE, Santos CDS, Moreira LO, Mattos-Guaraldi AL. 2020. Resistance to antiseptics and disinfectants of planktonic and biofilm-associated forms of Corynebacterium striatum. Microb Drug Resist 26:1546–1558. 10.1089/mdr.2019.0124. [DOI] [PubMed] [Google Scholar]
- 286.Tong JCK, Sparrow EM, Abraham JP. 2007. Numerical simulation of the urine flow in a stented ureter. J Biomech Eng 129:187–192. 10.1115/1.2472381. [DOI] [PubMed] [Google Scholar]
- 287.Wheeler JD, Secchi E, Rusconi R, Stocker R. 2019. Not just going with the flow: the effects of fluid flow on bacteria and plankton. Annu Rev Cell Dev Biol 35:213–237. 10.1146/annurev-cellbio-100818-125119. [DOI] [PubMed] [Google Scholar]
- 288.Rusconi R, Guasto JS, Stocker R. 2014. Bacterial transport suppressed by fluid shear. Nat Physics 10:212–217. 10.1038/nphys2883. [DOI] [Google Scholar]
- 289.Secchi E, Vitale A, Miño GL, Kantsler V, Eberl L, Rusconi R, Stocker R. 2020. The effect of flow on swimming bacteria controls the initial colonization of curved surfaces. Nat Commun 11:2851. 10.1038/s41467-020-16620-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Kaya T, Koser H. 2012. Direct upstream motility in Escherichia coli. Biophys J 102:1514–23. 10.1016/j.bpj.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Hill J, Kalkanci O, McMurry JL, Koser H. 2007. Hydrodynamic surface interactions enable Escherichia coli to seek efficient routes to swim upstream. Phys Rev Lett 98:e068101. 10.1103/PhysRevLett.98.068101. [DOI] [PubMed] [Google Scholar]
- 292.Shen Y, Siryaporn A, Lecuyer S, Gitai Z, Stone HA. 2012. Flow directs surface-attached bacteria to twitch upstream. Biophys J 103:146–151. 10.1016/j.bpj.2012.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Chatelet DS, Matthews MA, Rost TL. 2006. Xylem structure and connectivity in grapevine (Vitis vinifera) shoots provides a passive mechanism for the spread of bacteria in grape plants. Ann Bot 98:483–494. 10.1093/aob/mcl124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Miyata M, Ryu WSS, Berg HCC. 2002. Force and velocity of Mycoplasma mobile gliding. J Bacteriol 184:1827–1831. 10.1128/JB.184.7.1827-1831.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Kannan A, Yang Z, Kim MK, Stone HA, Siryaporn A. 2018. Dynamic switching enables efficient bacterial colonization in flow. Proc Natl Acad Sci USA 115:5438–5443. 10.1073/pnas.1718813115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Siryaporn A, Kim MK, Shen Y, Stone HA, Gitai Z. 2015. Colonization, competition, and dispersal of pathogens in fluid flow networks. Curr Biol 25:1201–1207. 10.1016/j.cub.2015.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Lecuyer S, Rusconi R, Shen Y, Forsyth A, Vlamakis H, Kolter R, Stone HA. 2011. Shear stress increases the residence time of adhesion of Pseudomonas aeruginosa. Biophys J 100:341–350. 10.1016/j.bpj.2010.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Thomas WE, Vogel V, Sokurenko E. 2008. Biophysics of catch bonds. Annu Rev Biophys 37:399–416. 10.1146/annurev.biophys.37.032807.125804. [DOI] [PubMed] [Google Scholar]
- 299.Weaver WM, Dharmaraja S, Milisavljevic V, Di Carlo D. 2011. The effects of shear stress on isolated receptor-ligand interactions of Staphylococcus epidermidis and human plasma fibrinogen using molecularly patterned microfluidics. Lab Chip 11:883–889. 10.1039/c0lc00414f. [DOI] [PubMed] [Google Scholar]
- 300.Pappelbaum KI, Gorzelanny C, Grässle S, Suckau J, Laschke MW, Bischoff M, Bauer C, Schorpp-Kistner M, Weidenmaier C, Schneppenheim R, Obser T, Sinha B, Schneider SW. 2013. Ultralarge von willebrand factor fibers mediate luminal Staphylococcus aureus adhesion to an intact endothelial cell layer under shear stress. Circulation 128:50–59. 10.1161/CIRCULATIONAHA.113.002008. [DOI] [PubMed] [Google Scholar]
- 301.Siryaporn A, Kuchma SL, O’Toole GA, Gitai Z. 2014. Surface attachment induces Pseudomonas aeruginosa virulence. Proc Natl Acad Sci USA 111:16860–16865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Rodesney CA, Roman B, Dhamani N, Cooley BJ, Touhami A, Gordon VD. 2017. Mechanosensing of shear by Pseudomonas aeruginosa leads to increased levels of the cyclic-di-GMP signal initiating biofilm development. Proc Natl Acad Sci USA 114:5906–5911. 10.1073/pnas.1703255114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Dufrêne YF, Persat A. 2020. Mechanomicrobiology: how bacteria sense and respond to forces. Nat Rev Microbiol 18:227–240. 10.1038/s41579-019-0314-2. [DOI] [PubMed] [Google Scholar]
- 304.Paul E, Ochoa JC, Pechaud Y, Liu Y, Liné A. 2012. Effect of shear stress and growth conditions on detachment and physical properties of biofilms. Water Res 46:5499–5508. 10.1016/j.watres.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 305.Stoodley P, Dodds I, Boyle JDD, Lappin-Scott HMM. 1998. Influence of hydrodynamics and nutrients on biofilm structure. J Appl Microbiol 85:19S–28S. 10.1111/j.1365-2672.1998.tb05279.x. [DOI] [PubMed] [Google Scholar]
- 306.Wang L, Keatch R, Zhao Q, Wright JA, Bryant CE, Redmann AL, Terentjev EM. 2018. Influence of type I fimbriae and fluid shear stress on bacterial behavior and multicellular architecture of early Escherichia coli biofilms at single-cell resolution. Appl Environ Microbiol 84:1–13. 10.1128/AEM.02343-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Pearce P, Song B, Skinner DJ, Mok R, Hartmann R, Singh PK, Jeckel H, Oishi JS, Drescher K, Dunkel J. 2019. Flow-induced symmetry breaking in growing bacterial biofilms. Phys Rev Lett 123:258101. 10.1103/PhysRevLett.123.258101. [DOI] [PubMed] [Google Scholar]
- 308.Rusconi R, Lecuyer S, Guglielmini L, Stone HA. 2010. Laminar flow around corners triggers the formation of biofilm streamers. J R Soc Interface 7:1293–1299. 10.1098/rsif.2010.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Rusconi R, Lecuyer S, Autrusson N, Guglielmini L, Stone HA. 2011. Secondary flow as a mechanism for the formation of biofilm streamers. Biophys J 100:1392–1399. 10.1016/j.bpj.2011.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Guglielmini L, Rusconi R, Lecuyer S, Stone HA. 2011. Three-dimensional features in low-Reynolds-number confined corner flows. J Fluid Mech 668:33–57. 10.1017/S0022112010004519. [DOI] [Google Scholar]
- 311.Autrusson N, Guglielmini L, Lecuyer S, Rusconi R, Stone Ha. 2011. The shape of an elastic filament in a two-dimensional corner flow. Physics Fluids 23:e063602. 10.1063/1.3601446. [DOI] [Google Scholar]
- 312.Drescher K, Shen Y, Bassler BL, Stone Ha. 2013. Biofilm streamers cause catastrophic disruption of flow with consequences for environmental and medical systems. Proc Natl Acad Sci USA 110:4345–4350. 10.1073/pnas.1300321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Kevin Kim M, Drescher K, Shun Pak O, Bassler BL, Stone HA. 2014. Filaments in curved streamlines: rapid formation of Staphylococcus aureus biofilm streamers. New J Physics 16. 10.1088/1367-2630/16/6/065024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Nadell CD, Ricaurte D, Yan J, Drescher K, Bassler BL. 2017. Flow environment and matrix structure interact to determine spatial competition in Pseudomonas aeruginosa biofilms. eLife 6:21855. 10.7554/eLife.21855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Reference deleted.
- 316.Flemming H-C, Neu T, Wingender J. 2015. The perfect slime. IWA Publishing, London, United Kingdom. [Google Scholar]
- 317.Stoodley P, Dodds I, Boyle JD, Lappin-Scott HMM. 1999. Influence of hydrodynamics and nutrients on biofilm structure. J Appl Microbiol 85:19–28. [DOI] [PubMed] [Google Scholar]
- 318.Conrad JC, Poling-Skutvik R. 2018. Confined flow: consequences and implications for bacteria and biofilm. Annu Rev Chem Biomol Eng 9:175–200. 10.1146/annurev-chembioeng-060817-084006. [DOI] [PubMed] [Google Scholar]
- 319.Pavlovsky L, Sturtevant RA, Younger JG, Solomon MJ. 2015. Effects of temperature on the morphological, polymeric, and mechanical properties of Staphylococcus epidermidis bacterial biofilms. Langmuir 31:2036–2042. 10.1021/la5044156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Emge P, Moeller J, Jang H, Rusconi R, Yawata Y, Stocker R, Vogel V. 2016. Resilience of bacterial quorum sensing against fluid flow. Sci Rep 6:33115. 10.1038/srep33115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Shaw T, Winston M, Rupp CJ, Klapper I, Stoodley P. 2004. Commonality of elastic relaxation times in biofilms. Phys Rev Lett 93:e98102. 10.1103/PhysRevLett.93.098102. [DOI] [PubMed] [Google Scholar]
- 322.Ikenberry SO, Sherman S, Hawes RH, Smith M, Lehman GA. 1994. The occlusion rate of pancreatic stents. Gastrointest Endoscopy 40:611–613. 10.1016/s0016-5107(94)70264-0. [DOI] [PubMed] [Google Scholar]
- 323.Donelli G, Guaglianone E, Di Rosa R, Fiocca F, Basoli A. 2007. Plastic biliary stent occlusion: factors involved and possible preventive approaches. Clin Med Res 5:53–60. 10.3121/cmr.2007.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Soderlund C, Linder S. 2006. Covered metal versus plastic stents for malignant common bile duct stenosis: a prospective, randomized, controlled trial. Gastrointest Endosc 63:986–995. 10.1016/j.gie.2005.11.052. [DOI] [PubMed] [Google Scholar]
- 325.Schaeffer CR, Hoang T-MN, Sudbeck CM, Alawi M, Tolo IE, Robinson DA, Horswill AR, Rohde H, Fey PD. 2016. Versatility of biofilm matrix molecules in Staphylococcus epidermidis clinical isolates and importance of polysaccharide intercellular adhesin expression during high shear stress. mSphere 1:e00165-16. 10.1128/mSphere.00165-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Weaver WM, Milisavlijevic V, Di Carlo D. 2012. Fluid flow induces biofilm formation in Staphylococcus epidermidis polysaccharide intracellular adhesin-positive clinical isolates. Appl Environ Microbiol 78:5890–5896. 10.1128/AEM.01139-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.De Grazia A, LuTheryn G, Meghdadi A, Mosayyebi A, Espinosa-Ortiz E, Gerlach R, Carugo D. 2020. A microfluidic-based investigation of bacterial attachment in ureteral stents. Micromachines 11:408. 10.3390/mi11040408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Murray BO, Flores C, Williams C, Flusberg DA, Marr EE, Kwiatkowska KM, Charest JL, Isenberg BC, Rohn JL. 2021. Recurrent urinary tract infection: a mystery in search of better model systems. Front Cell Infect Microbiol 11:1–29. 10.3389/fcimb.2021.691210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Xue J, Zhu P, He Y, Li Q, Xu Y. 2020. Application of computational fluid dynamics models for the evaluation of salivary flow patterns and related bacterial accumulation around orthodontic brackets. Orthod Craniofac Res 23:291–299. 10.1111/ocr.12369. [DOI] [PubMed] [Google Scholar]
- 330.Jurela A, Repic D, Pejda S, Juric H, Vidakovic R, Matic I, Bosnjak A. 2013. The effect of two different bracket types on the salivary levels of S. mutans and S. sobrinus in the early phase of orthodontic treatment. Angle Orthod 83:140–145. 10.2319/030612-187.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Anhoury P, Nathanson D, Hughes CV, Socransky S, Feres M, Chou LL. 2002. Microbial profile on metallic and ceramic bracket materials. Angle Orthod 72:338–343. [DOI] [PubMed] [Google Scholar]
- 332.Van kerckhoven M, Hotterbeekx A, Lanckacker E, Moons P, Lammens C, Kerstens M, Ieven M, Delputte P, Jorens PG, Malhotra-Kumar S, Goossens H, Maes L, Cos P. 2016. Characterizing the in vitro biofilm phenotype of Staphylococcus epidermidis isolates from central venous catheters. J Microbiol Methods 127:95–101. 10.1016/j.mimet.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 333.Montgomerie Z, Popat KC. 2021. Improved hemocompatibility and reduced bacterial adhesion on superhydrophobic titania nanoflower surfaces. Mater Sci Eng C 119:111503. 10.1016/j.msec.2020.111503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Leslie DC, Waterhouse A, Berthet JB, Valentin TM, Watters AL, Jain A, Kim P, Hatton BD, Nedder A, Donovan K, Super EH, Howell C, Johnson CP, Vu TL, Bolgen DE, Rifai S, Hansen AR, Aizenberg M, Super M, Aizenberg J, Ingber DE. 2014. A bioinspired omniphobic surface coating on medical devices prevents thrombosis and biofouling. Nat Biotechnol 32:1134–1140. 10.1038/nbt.3020. [DOI] [PubMed] [Google Scholar]
- 335.van Berkel AM, van Marle J, Groen AK, Bruno MJ. 2005. Mechanisms of biliary stent clogging: confocal laser scanning and scanning electron microscopy. Endoscopy 37:729–734. 10.1055/s-2005-870131. [DOI] [PubMed] [Google Scholar]
- 336.Rocca DM, Aiassa V, Zoppi A, Silvero Compagnucci J, Becerra MC. 2020. Nanostructured gold coating for prevention of biofilm development in medical devices. J Endourol 34:345–351. 10.1089/end.2019.0686. [DOI] [PubMed] [Google Scholar]
- 337.Björling G, Johansson D, Bergström L, Strekalovsky A, Sanchez J, Frostell C, Kalman S. 2018. Evaluation of central venous catheters coated with a noble metal alloy-A randomized clinical pilot study of coating durability, performance, and tolerability. J Biomed Mater Res B Appl Biomater 106:2337–2344. 10.1002/jbm.b.34041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Stenzelius K, Persson S, Olsson U-B, Stjärneblad M. 2011. Noble metal alloy-coated latex versus silicone Foley catheter in short-term catheterization: a randomized controlled study. Scand J Urol Nephrol 45:258–264. 10.3109/00365599.2011.560007. [DOI] [PubMed] [Google Scholar]
- 339.Hogan S, Kasotakis E, Maher S, Cavanagh B, O’Gara JP, Pandit A, Keyes TE, Devocelle M, O’Neill E. 2019. A novel medical device coating prevents Staphylococcus aureus biofilm formation on medical device surfaces. FEMS Microbiol Lett 366:fnz107. 10.1093/femsle/fnz107. [DOI] [PubMed] [Google Scholar]
- 340.Sánchez MC, Llama-Palacios A, Fernández E, Figuero E, Marín MJ, León R, Blanc V, Herrera D, Sanz M. 2014. An in vitro biofilm model associated with dental implants: structural and quantitative analysis of in vitro biofilm formation on different dental implant surfaces. Dent Mater 30:1161–1171. 10.1016/j.dental.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 341.Roehling S, Astasov-Frauenhoffer M, Hauser-Gerspach I, Braissant O, Woelfler H, Waltimo T, Kniha H, Gahlert M. 2017. In vitro biofilm formation on titanium and zirconia implant surfaces. J Periodontol 88:298–307. 10.1902/jop.2016.160245. [DOI] [PubMed] [Google Scholar]
- 342.Atlan M, Kinney BM, Perry TA. 2020. Intra- and inter-shell roughness variability of breast implant surfaces. Aesthetic Surg J 40:NP324–NP326. 10.1093/asj/sjz369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.James GA, Boegli L, Hancock J, Bowersock L, Parker A, Kinney BM. 2019. Bacterial adhesion and biofilm formation on textured breast implant shell materials. Aesthetic Plast Surg 43:490–497. 10.1007/s00266-018-1234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Jones P, Mempin M, Hu H, Chowdhury D, Foley M, Cooter R, Adams WP, Jr, Vickery K, Deva AK. 2018. The functional influence of breast implant outer shell morphology on bacterial attachment and growth. Plast Reconstruct Surg 142:837–849. 10.1097/PRS.0000000000004801. [DOI] [PubMed] [Google Scholar]
- 345.Lang NP, Berglundh T. 2011. Periimplant diseases: where are we now? Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol 38:178–181. 10.1111/j.1600-051X.2010.01674.x. [DOI] [PubMed] [Google Scholar]
- 346.De-la-Pinta I, Cobos M, Ibarretxe J, Montoya E, Eraso E, Guraya T, Quindós G. 2019. Effect of biomaterials hydrophobicity and roughness on biofilm development. J Mater Sci Mater Med 30:1–11. 10.1007/s10856-019-6281-3. [DOI] [PubMed] [Google Scholar]
- 347.Jiri M, Duskova R, Hobzova M, Pradny M. 2010. Hydrogel contact lenses. In Ottenbrite R, Park K, Okano T (ed), Biomedical applications of hydrogels handbook. Springer, New York, NY. [Google Scholar]
- 348.Bruinsma GM, van der Mei HC, Busscher HJ. 2001. Bacterial adhesion to surface hydrophilic and hydrophobic contact lenses. Biomaterials 22:3217–3224. 10.1016/s0142-9612(01)00159-4. [DOI] [PubMed] [Google Scholar]
- 349.Imamura Y, Chandra J, Mukherjee PK, Lattif AA, Szczotka-Flynn LB, Pearlman E, Lass JH, O’Donnell K, Ghannoum MA. 2008. Fusarium and Candida albicans biofilms on soft contact lenses: model development, influence of lens type, and susceptibility to lens care solutions. Antimicrob Agents Chemother 52:171–182. 10.1128/AAC.00387-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Lichter JA, Thompson MT, Delgadillo M, Nishikawa T, Rubner MF, Van Vliet KJ. 2008. Substrata mechanical stiffness can regulate adhesion of viable bacteria. Biomacromolecules 9:1571–1578. 10.1021/bm701430y. [DOI] [PubMed] [Google Scholar]
- 351.Siddiqui S, Chandrasekaran A, Lin N, Tufenkji N, Moraes C. 2019. Microfluidic shear assay to distinguish between bacterial adhesion and attachment strength on stiffness-tunable silicone substrates. Langmuir 35:8840–8849. 10.1021/acs.langmuir.9b00803. [DOI] [PubMed] [Google Scholar]
- 352.Fazli M, Almblad H, Rybtke ML, Givskov M, Eberl L, Tolker-Nielsen T. 2014. Regulation of biofilm formation in Pseudomonas and Burkholderia species. Environ Microbiol 16:1961–1981. 10.1111/1462-2920.12448. [DOI] [PubMed] [Google Scholar]
- 353.Moser C, Pedersen HT, Lerche CJ, Kolpen M, Line L, Thomsen K, Høiby N, Jensen PØ. 2017. Biofilms and host response: helpful or harmful. APMIS 125:320–338. 10.1111/apm.12674. [DOI] [PubMed] [Google Scholar]
- 354.Moser C, Jensen PØ, Thomsen K, Kolpen M, Rybtke M, Lauland AS, Trøstrup H, Tolker-Nielsen T. 2021. Immune responses to Pseudomonas aeruginosa biofilm infections. Front Immunol 12:625597. 10.3389/fimmu.2021.625597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Turkina MV., Vikström E. 2019. Bacterium-host crosstalk: sensing of the quorum in the context of Pseudomonas aeruginosa infections. J Innate Immun 11:263–279. 10.1159/000494069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Rybtke M, Jensen PØ, Nielsen CH, Tolker-Nielsen T. 2020. The extracellular polysaccharide matrix of Pseudomonas aeruginosa biofilms is a determinant of polymorphonuclear leukocyte responses. Infect Immun 89:e00631-20. 10.1128/IAI.00631-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Fuxman Bass JI, Russo DM, Gabelloni ML, Geffner JR, Giordano M, Catalano M, Zorreguieta Á, Trevani AS, Zorreguieta A, Trevani AS. 2010. Extracellular DNA: a major proinflammatory component of Pseudomonas aeruginosa biofilms. J Immunol 184:6386–6395. 10.4049/jimmunol.0901640. [DOI] [PubMed] [Google Scholar]
- 358.Toyofuku M, Roschitzki B, Riedel K, Eberl L. 2012. Identification of proteins associated with the Pseudomonas aeruginosa biofilm extracellular matrix. J Proteome Res 11:4906–4915. 10.1021/pr300395j. [DOI] [PubMed] [Google Scholar]
- 359.Holban AM, Bleotu C, Chifiriuc MC, Bezirtzoglou E, Lazar V. 2014. Role of Pseudomonas aeruginosa quorum sensing (QS) molecules on the viability and cytokine profile of human mesenchymal stem cells. Virulence 5:303–310. 10.4161/viru.27571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Schwarzer C, Wong S, Shi J, Matthes E, Illek B, Ianowski JP, Arant RJ, Isacoff E, Vais H, Foskett JK, Maiellaro I, Hofer AM, Machen TE. 2010. Pseudomonas aeruginosa homoserine lactone activates store-operated cAMP and cystic fibrosis transmembrane regulator-dependent Cl− secretion by human airway epithelia. J Biol Chem 285:34850–34863. 10.1074/jbc.M110.167668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Managò A, Becker KA, Carpinteiro A, Wilker B, Soddemann M, Seitz AP, Edwards MJ, Grassmé H, Szabò I, Gulbins E. 2015. Pseudomonas aeruginosa pyocyanin induces neutrophil death via mitochondrial reactive oxygen species and mitochondrial acid sphingomyelinase. Antioxidants Redox Signal 22:1097–1110. 10.1089/ars.2014.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Rada B, Jendrysik MA, Pang L, Hayes CP, Yoo DG, Park JJ, Moskowitz SM, Malech HL, Leto TL. 2013. Pyocyanin-enhanced neutrophil extracellular trap formation requires the NADPH oxidase. PLoS One 8:e54205. 10.1371/journal.pone.0054205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Pier GB, Coleman F, Grout M, Franklin M, Ohman DE. 2001. Role of alginate O acetylation in resistance of mucoid Pseudomonas aeruginosa to opsonic phagocytosis. Infect Immun 69:1895–1901. 10.1128/IAI.69.3.1895-1901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Leid JG, Willson CJ, Shirtliff ME, Hassett DJ, Parsek MR, Jeffers AK. 2005. The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-γ-mediated macrophage killing. J Immunol 175:7512–7518. 10.4049/jimmunol.175.11.7512. [DOI] [PubMed] [Google Scholar]
- 365.Ríos-López AL, González GM, Hernández-Bello R, Sánchez-González A. 2021. Avoiding the trap: mechanisms developed by pathogens to escape neutrophil extracellular traps. Microbiol Res 243:126644. 10.1016/j.micres.2020.126644. [DOI] [PubMed] [Google Scholar]
- 366.Raffatellu M, Chessa D, Wilson RP, Dusold R, Rubino S, Bäumler AJ. 2005. The Vi capsular antigen of Salmonella enterica serotype Typhi reduces Toll-like receptor-dependent interleukin-8 expression in the intestinal mucosa. Infect Immun 73:3367–3374. 10.1128/IAI.73.6.3367-3374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Bylund J, Burgess L-A, Cescutti P, Ernst RK, Speert DP. 2006. Exopolysaccharides from Burkholderia cenocepacia inhibit neutrophil chemotaxis and scavenge reactive oxygen species. J Biol Chem 281:P2526–P2532. 10.1074/jbc.M510692200. [DOI] [PubMed] [Google Scholar]
- 368.Rieu A, Aoudia N, Jego G, Chluba J, Yousfi N, Briandet R, Deschamps J, Gasquet B, Monedero V, Garrido C, Guzzo J. 2014. The biofilm mode of life boosts the anti-inflammatory properties of Lactobacillus. Cell Microbiol 16:1836–1853. 10.1111/cmi.12331. [DOI] [PubMed] [Google Scholar]
- 369.Jones SE, Versalovic J. 2009. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol 9:35. 10.1186/1471-2180-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Lin M-H, Yang Y-L, Chen Y-P, Hua K-F, Lu C-P, Sheu F, Lin G-H, Tsay S-S, Liang S-M, Wu S-H. 2011. A novel exopolysaccharide from the biofilm of Thermus aquaticus YT-1 induces the immune response through Toll-like receptor 2. J Biol Chem 286:17736–17745. 10.1074/jbc.M110.200113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.de Vor L, Rooijakkers SHM, van Strijp JAG. 2020. Staphylococci evade the innate immune response by disarming neutrophils and forming biofilms. FEBS Lett 594:2556–2569. 10.1002/1873-3468.13767. [DOI] [PubMed] [Google Scholar]
- 372.Günther F, Wabnitz GH, Stroh P, Prior B, Obst U, Samstag Y, Wagner C, Hänsch GM. 2009. Host defence against Staphylococcus aureus biofilm infection: phagocytosis of biofilms by polymorphonuclear neutrophils (PMN). Mol Immunol 46:1805–1813. 10.1016/j.molimm.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 373.Kristian SA, Birkenstock TA, Sauder U, Mack D, Götz F, Landmann R. 2008. Biofilm formation induces C3a release and protects Staphylococcus epidermidis from IgG and complement deposition and from neutrophil-dependent killing. J Infect Dis 197:1028–1035. 10.1086/528992. [DOI] [PubMed] [Google Scholar]
- 374.Scherr TD, Heim CE, Morrison JM, Kielian T. 2014. Hiding in plain sight: interplay between staphylococcal biofilms and host immunity. Front Immunol 5:37. 10.3389/fimmu.2014.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Bhattacharya M, Berends ETM, Chan R, Schwab E, Roy S, Sen CK, Torres VJ, Wozniak DJ. 2018. Staphylococcus aureus biofilms release leukocidins to elicit extracellular trap formation and evade neutrophil-mediated killing. Proc Natl Acad Sci USA 115:7416–7421. 10.1073/pnas.1721949115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, DeLeo FR, Otto M. 2004. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol 6:269–275. 10.1046/j.1462-5822.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 377.Schommer NN, Christner M, Hentschke M, Ruckdeschel K, Aepfelbacher M, Rohde H. 2011. Staphylococcus epidermidis uses distinct mechanisms of biofilm formation to interfere with phagocytosis and activation of mouse macrophage-like cells 774A.1. Infect Immun 79:2267–2276. 10.1128/IAI.01142-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Thammavongsa V, Missiakas DM, Schneewind O. 2013. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science 342:863–866. 10.1126/science.1242255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Berends ETM, Horswill AR, Haste NM, Monestier M, Nizet V, von Köckritz-Blickwede M. 2010. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J Innate Immun 2:576–586. 10.1159/000319909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Sultan AR, Swierstra JW, den Toom NAL, Snijders Sv., Maňásková SH, Verbon A, van Wamel WJB. 2018. Production of staphylococcal complement inhibitor (SCIN) and other immune modulators during the early stages of Staphylococcus aureus biofilm formation in a mammalian cell culture medium. Infect Immun 86:e00352-18. 10.1128/IAI.00352-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Bosch ME, Bertrand BP, Heim CE, Alqarzaee AA, Chaudhari SS, Aldrich AL, Fey PD, Thomas VC, Kielian T. 2020. Staphylococcus aureus ATP synthase promotes biofilm persistence by influencing innate immunity. mBio 11:e01581-20. 10.1128/mBio.01581-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Sultan AR, Hoppenbrouwers T, Lemmens-Den Toom NA, Snijders Sv., van Neck JW, Verbon A, de Maat MPM, van Wamel WJB. 2019. During the early stages of staphylococcus aureus biofilm formation, induced neutrophil extracellular traps are degraded by autologous thermonuclease. Infect Immun 87:e00605-19. 10.1128/IAI.00605-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Torres VJ, Scherr TD, Hanke ML, Huang O, James DBA, Horswill AR, Bayles KW, Fey PD, Torres VJ, Kielian T. 2015. Staphylococcus aureus biofilms induce macrophage dysfunction through leukocidin AB and alpha-toxin mBio 6:25–27. 10.1128/mBio.01021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Sadowska B, Więckowska-Szakiel M, Paszkiewicz M, Różalska B. 2013. The immunomodulatory activity of staphylococcus aureus products derived from biofilm and planktonic cultures. Arch Immunol Ther Exp 61:413–420. 10.1007/s00005-013-0240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Gil C, Solano C, Burgui S, Latasa C, García B, Toledo-Arana A, Lasa I, Valle J. 2014. Biofilm matrix exoproteins induce a protective immune response against Staphylococcus aureus biofilm infection. Infect Immun 82:1017–1029. 10.1128/IAI.01419-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Prabhakara R, Harro JM, Leid JG, Harris M, Shirtliff ME. 2011. Murine immune response to a chronic Staphylococcus aureus biofilm infection. Infect Immun 79:1789–1796. 10.1128/IAI.01386-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Prabhakara R, Harro JM, Leid JG, Keegan AD, Prior ML, Shirtliff ME. 2011. Suppression of the inflammatory immune response prevents the development of chronic biofilm infection due to methicillin-resistant Staphylococcus aureus. Infect Immun 79:5010–5018. 10.1128/IAI.05571-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Nippe N, Varga G, Holzinger D, Löffler B, Medina E, Becker K, Roth J, Ehrchen JM, Sunderkötter C. 2011. Subcutaneous infection with S. aureus in mice reveals association of resistance with influx of neutrophils and Th2 response. J Invest Dermatol 131:125–132. 10.1038/jid.2010.282. [DOI] [PubMed] [Google Scholar]
- 389.Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH, Engebretsen IL, Bayles KW, Horswill AR, Kielian T. 2011. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J Immunol 186:6585–6596. 10.4049/jimmunol.1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Stroh P, Günther F, Meyle E, Prior B, Wagner C, Hänsch GM. 2011. Host defence against Staphylococcus aureus biofilms by polymorphonuclear neutrophils: oxygen radical production but not phagocytosis depends on opsonization with immunoglobulin G. Immunobiology 216:351–357. 10.1016/j.imbio.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 391.Wagner C, Kaksa A, Müller W, Denefleh B, Heppert V, Wentzensen A, Hänsch GM. 2004. Polymorphonuclear neutrophils in posttraumatic osteomyelitis: cells recovered from the inflamed site lack chemotactic activity but generate superoxides. Shock 22:108–115. 10.1097/01.shk.0000132488.71875.15. [DOI] [PubMed] [Google Scholar]
- 392.Scherr TD, Roux CM, Hanke ML, Angle A, Dunman PM, Kielian T. 2013. Global transcriptome analysis of Staphylococcus aureus biofilms in response to innate immune cells. Infect Immun 81:4363–4376. 10.1128/IAI.00819-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Ramos AN, Gobbato N, Rachid M, González L, Yantorno O, Valdez JC. 2010. Effect of Lactobacillus plantarum and Pseudomonas aeruginosa culture supernatants on polymorphonuclear damage and inflammatory response. Int Immunopharmacol 10:247–251. 10.1016/j.intimp.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 394.Vong L, Lorentz RJ, Assa A, Glogauer M, Sherman PM. 2014. Probiotic Lactobacillus rhamnosus inhibits the formation of neutrophil extracellular traps. J Immunol 192:1870–1877. 10.4049/jimmunol.1302286. [DOI] [PubMed] [Google Scholar]
- 395.Anderson JM, Rodriguez A, Chang DT. 2008. Foreign body reaction to biomaterials. Semin Immunol 20:86–100. 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Sheikh Z, Brooks PJ, Barzilay O, Fine N, Glogauer M. 2015. Macrophages, foreign body giant cells, and their response to implantable biomaterials. Materials 8:5671–5701. 10.3390/ma8095269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Frazão LP, Vieira de Castro J, Neves NM. 2020. In vivo evaluation of the biocompatibility of biomaterial device. Adv Exp Med Biol 1250:109–124. 10.1007/978-981-15-3262-7_8. [DOI] [PubMed] [Google Scholar]
- 398.Saleh LS, Bryant SJ. 2017. In vitro and in vivo models for assessing the host response to biomaterials. Drug Discov Today Dis Models 24:13–21. 10.1016/j.ddmod.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Doloff JC, Veiseh O, de Mezerville R, Sforza M, Perry TA, Haupt J, Jamiel M, Chambers C, Nash A, Aghlara-Fotovat S, Stelzel JL, Bauer SJ, Neshat SY, Hancock J, Romero NA, Hidalgo YE, Leiva IM, Munhoz AM, Bayat A, Kinney BM, Hodges HC, Miranda RN, Clemens MW, Langer R. 2021. The surface topography of silicone breast implants mediates the foreign body response in mice, rabbits and humans. Nat Biomed Eng 10.1038/s41551-021-00739-4. [DOI] [PubMed] [Google Scholar]
- 400.Hanke ML, Heim CE, Angle A, Sanderson SD, Kielian T. 2013. Targeting macrophage activation for the prevention and treatment of Staphylococcus aureus biofilm infections. J Immunol 190:2159–2168. 10.4049/jimmunol.1202348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Bernthal NM, Pribaz JR, Stavrakis AI, Billi F, Cho JS, Ramos RI, Francis KP, Iwakura Y, Miller LS. 2011. Protective role of IL-1β against post-arthroplasty Staphylococcus aureus infection. J Orthop Res 29:1621–1626. 10.1002/jor.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Mendiratta P, Tilford JM, Prodhan P, Curseen K, Azhar G, Wei JY. 2012. Trends in percutaneous endoscopic gastrostomy placement in the elderly from 1993 to 2003. Am J Alzheimers Dis Other Dementias 27:609–613. 10.1177/1533317512460563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Darouiche RO, Bella AJ, Boone TB, Brock G, Broderick GA, Burnett AL, Carrion R, Carson C, Christine B, Dhabuwala CB, Hakim LS, Henry G, Jones LA, Khera M, Montague DK, Nehra A. 2013. North American consensus document on infection of penile prostheses. Urology 82:937–942. 10.1016/j.urology.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 404.Ambrosioni J, Martinez-Garcia C, Llopis J, Garcia-de-la-Maria C, Hernández-Meneses M, Tellez A, Falces C, Almela M, Vidal B, Sandoval E, Fuster D, Quintana E, Tolosana JM, Marco F, Moreno A, Miró JM. 2018. HACEK infective endocarditis: epidemiology, clinical features, and outcome: a case–control study. Int J Infect Dis 76:120–125. 10.1016/j.ijid.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 405.Oliva A, Mascellino M, Nguyen B, De Angelis M, Cipolla A, Di Berardino A, Ciccaglioni A, Mastroianni CM, Vullo V. 2018. Detection of biofilm-associated implant pathogens in cardiac device infections: high sensitivity of sonication fluid culture even in the presence of antimicrobials. J Global Infect Dis 10:74–79. 10.4103/jgid.jgid_31_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Sendi P, Wustmann K, Büchi AE, Noti F, Klaeser B, Sonderegger B, Der Maur CA, Mercier T, Schwerzmann M, Ruppen C. 2019. Cardiac implantable electronic device-related infection due to granulicatella adiacens. Open Forum Infect Dis 6. 10.1093/ofid/ofz130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Maille B, Koutbi L, Resseguier N, Lemoine C, Thuny F, Peyrol M, Hourdain J, Deharo JC, Franceschi F. 2019. Seasonal variations in cardiac implantable electronic device infections. Heart Vessels 34:824–831. 10.1007/s00380-018-1292-4. [DOI] [PubMed] [Google Scholar]
- 408.Zhu J, Yang Q, Pan J, Shi H, Jin B, Chen Q. 2019. Cardiac resynchronization therapy-defibrillator pocket infection caused by Mycobacterium fortuitum: a case report and review of the literature. BMC Cardiovasc Disord 19:53. 10.1186/s12872-019-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Del Pozo JL, Tran NV, Petty PM, Johnson CH, Walsh MF, Bite U, Clay RP, Mandrekar JN, Piper KE, Steckelberg JM, Patel R. 2009. Pilot study of association of bacteria on breast implants with capsular contracture. J Clin Microbiol 47:1333–1337. 10.1128/JCM.00096-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Macadam SA, Mehling BM, Fanning A, Dufton JA, Kowalewska-Grochowska KT, Lennox P, Anzarut A, Rodrigues M. 2007. Nontuberculous mycobacterial breast implant infections. Plast Reconstruct Surg 119:337–344. 10.1097/01.prs.0000244924.61968.d2. [DOI] [PubMed] [Google Scholar]
- 411.Chang DC, Grant GB, O’Donnell K, Wannemuehler KA, Noble-Wang J, Rao CY, Jacobson LM, Crowell CS, Sneed RS, Lewis FMT, Schaffzin JK, Kainer MA, Genese CA, Alfonso EC, Jones DB, Srinivasan A, Fridkin SK, Park BJ, Fusarium Keratitis Investigation Team . 2006. Multistate outbreak of fusarium keratitis associated with use of a contact lens solution. JAMA 296:953. 10.1001/jama.296.8.953. [DOI] [PubMed] [Google Scholar]
- 412.Keay L, Stapleton F, Schein O. 2007. Epidemiology of contact lens-related inflammation and microbial keratitis: a 20-year perspective. Eye Contact Lens 33:346–53 (Discussion 33: 362–363.) 10.1097/ICL.0b013e318157c49d. [DOI] [PubMed] [Google Scholar]
- 413.Jin Q, Zhang X, Li X, Wang J. 2010. Dynamics analysis of bladder-urethra system based on CFD. Front Mech Eng China 5:336–340. 10.1007/s11465-010-0027-8. [DOI] [Google Scholar]
- 414.Vahidi B, Fatouraee N, Imanparast A, Moghadam AN. 2010. A mathematical simulation of the ureter: effects of the model parameters on ureteral pressure/flow relations. J Biomech Eng 133:e031004. 10.1115/1.4003316. [DOI] [PubMed] [Google Scholar]
- 415.Sebastian B, Dittrich PS. 2018. Microfluidics to mimic blood flow in health and disease. Annu Rev Fluid Mech 50:483–504. 10.1146/annurev-fluid-010816-060246. [DOI] [Google Scholar]
- 416.Secomb TW. 2017. Blood flow in the microcirculation. Annu Rev Fluid Mech 49:443–461. 10.1146/annurev-fluid-010816-060302. [DOI] [Google Scholar]
- 417.Dawes C. 2008. Salivary flow patterns and the health of hard and soft oral tissues. J Am Dent Assoc 139:18S–24S. 10.14219/jada.archive.2008.0351. [DOI] [PubMed] [Google Scholar]
- 418.Ooi RC, Luo XY, Chin SB, Johnson AG, Bird NC. 2004. The flow of bile in the human cystic duct. J Biomech 37:1913–1922. 10.1016/j.jbiomech.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 419.Li WG, Luo XY, Chin SB, Hill NA, Johnson AG, Bird NC. 2008. Non-Newtonian bile flow in elastic cystic duct: one- and three-dimensional modeling. Ann Biomed Eng 36:1893–1908. 10.1007/s10439-008-9563-3. [DOI] [PubMed] [Google Scholar]
- 420.Matsui H, Randell SH, Peretti SW, Davis CW, Boucher RC. 1998. Coordinated clearance of periciliary liquid and mucus from airway surfaces. J Clin Invest 102:1125–1131. 10.1172/JCI2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Janott M, Gayler S, Gessler A, Javaux M, Klier C, Priesack E, Brodersen CR, Choat B, Chatelet DS, Shackel K, Matthews M, McElrone AJ, The Mendeley Support Team, Feil H, Purcell AH, Biology I, Lieth JH, Meyer MM, Yeo K, Kirkpatrick BC, Meng Y, Li Y, Galvani CD, Hao G, Turner N, Burr TJ, Hoch HC, Turner JN, Pérez-Donoso AG, Greve LC, Walton JH, Labavitch JM, Sun Q, Roper MC, Wistrom CM, Rost TL, Feil WS. 2011. Upstream migration of Xylella fastidiosa via pilus-driven twitching motility. Plant Physiol 108:1748–1759. [Google Scholar]
- 422.Brady RA, Leid JG, Camper AK, Costerton JW, Shirtliff ME. 2006. Identification of Staphylococcus aureus proteins recognized by the antibody-mediated immune response to a biofilm infection. Infect Immun 74:3415–3426. 10.1128/IAI.00392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Graveline R, Segura M, Radzioch D, Gottschalk M. 2007. TLR2-dependent recognition of Streptococcus suis is modulated by the presence of capsular polysaccharide which modifies macrophage responsiveness. Int Immunol 19:375–389. 10.1093/intimm/dxm003. [DOI] [PubMed] [Google Scholar]
- 424.Cerca N, Jefferson KK, Oliveira R, Pier GB, Azeredo J. 2006. Comparative antibody-mediated phagocytosis of Staphylococcus epidermidis cells grown in a biofilm or in the planktonic state. Infect Immun 74:4849–4855. 10.1128/IAI.00230-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Mulcahy H, Charron-Mazenod L, Lewenza S. 2008. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog 4:e1000213. 10.1371/journal.ppat.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Smith RS, Fedyk ER, Springer TA, Mukaida N, Iglewski BH, Phipps RP. 2001. IL-8 production in human lung fibroblasts and epithelial cells activated by the Pseudomonas autoinducer N-3-oxododecanoyl homoserine lactone is transcriptionally regulated by NF-κB and activator protein-2. J Immunol 167:366–374. 10.4049/jimmunol.167.1.366. [DOI] [PubMed] [Google Scholar]
- 427.Tateda K, Ishii Y, Horikawa M, Matsumoto T, Miyairi S, Pechere JC, Standiford TJ, Ishiguro M, Yamaguchi K. 2003. The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect Immun 71:5785–5793. 10.1128/IAI.71.10.5785-5793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Shiner EK, Terentyev D, Bryan A, Sennoune S, Martinez-zaguilan R, Li G, Gyorke S, Williams SC, Rumbaugh KP. 2006. Pseudomonas aeruginosa autoinducer modulates host cell responses through calcium signaling. Cell Microbiol 8:1601–1610. 10.1111/j.1462-5822.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 429.Li H, Wang L, Ye L, Mao Y, Xie X, Xia C, Chen J, Lu Z, Song J. 2009. Influence of Pseudomonas aeruginosa quorum sensing signal molecule N-(3-oxododecanoyl) homoserine lactone on mast cells. Med Microbiol Immunol 198:113–121. 10.1007/s00430-009-0111-z. [DOI] [PubMed] [Google Scholar]
- 430.Das T, Manefield M. 2012. Pyocyanin promotes extracellular DNA release in Pseudomonas aeruginosa. PLoS One 7:e46718. 10.1371/journal.pone.0046718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Das T, Kutty SK, Kumar N, Manefield M. 2013. Pyocyanin facilitates extracellular DNA binding to Pseudomonas aeruginosa influencing cell surface properties and aggregation. PLoS One 8:e58299. 10.1371/journal.pone.0058299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Lau GW, Hassett DJ, Ran H, Kong F. 2004. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol Med 10:599–606. 10.1016/j.molmed.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 433.Fothergill JL, Panagea S, Hart CA, Walshaw MJ, Pitt TL, Winstanley C. 2007. Widespread pyocyanin over-production among isolates of a cystic fibrosis epidemic strain. BMC Microbiology 7:1–10. 10.1186/1471-2180-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Lögters T, Margraf S, Altrichter J, Cinatl J, Mitzner S, Windolf J, Scholz M. 2009. The clinical value of neutrophil extracellular traps. Med Microbiol Immunol 198:211–219. [DOI] [PubMed] [Google Scholar]