Abstract
We present an unusual case of takotsubo cardiomyopathy (TTC) following administration of the second dose of the DNA ChadOX1 nCOV-19 (AZD122) vaccination. This woman in her early 50s presented to the emergency department 8 days following her vaccine with central chest pain. Initial investigations revealed a raised troponin and evolving T wave inversion on ECG. Acute coronary syndrome management was commenced. Further investigations revealed non-obstructive coronary arteries on coronary angiography and imaging revealed hypokinesia of the anterior and anterior-septal walls in the apex and midcavity level, myocardial oedema and no infarction, all in keeping with TTC. Given the large-scale roll out of vaccinations during the COVID-19 pandemic better understanding of potential adverse events is essential. This is the first case report of TTC following a second dose of the DNA ChadOX1 nCOV-19 (AZD122) vaccination.
Keywords: cardiovascular medicine, COVID-19, healthcare improvement and patient safety
Background
Takotsubo cardiomyopathy (TTC) is an acute transient heart failure syndrome often triggered by an acute physical or emotional stressor. It characteristically presents with features that mimic acute myocardial infarction (AMI).1
This condition predominatly affects women (90%) and accounts for approximately 5%–6% of female patients presenting with suspected ST elevation myocardial infarction (STEMI).2 Two-thirds of TTC cases have an identifiable trigger, which can be emotional (depression, death of a family member, divorce) or physical (stroke, cancer, sepsis). Up to one quarter of cases do not have an identifiable trigger.3 4
Patients typically present with chest pain and/or dyspnoea. Acute abnormal ECG changes are common and resemble those of AMI. The most common patterns seen are ST elevation, T-wave inversion, ST depression and left bundle branch block.5 Serum troponin and other cardiac biomarkers are invariably elevated, although typically to a lesser degree than seen in STEMI.6
The diagnosis of TTC is generally established at coronary angiography, which shows non-obstructed coronary arteries with left ventriculography demonstrating one of the typical patterns of dyskinesia, known as ballooning.
Cardiac MRI (CMR) allows for multiparametric quantification of myocardial tissue characteristics with the absence of late gadolinium enhancement and evidence of myocardial oedema on T2-STIR sequencing being typical.
Pathophysiology of this condition has not been fully elucidated; however, studies have suggested potential mechanisms inclusive of coronary vasospasm, activation of central autonomic neurons expressing oestrogen receptors, metabolic dysfunction and increased sensitivity to a surge in circulating catecholamines. Catecholamine levels have been shown by some to be greater in TTC in comparison to STEMI.7 8
The incidence of TTC has increased during the COVID-19 pandemic from 1.5% to 7.8%.9 COVID-19 infection has been shown to cause hyperinflammatory states through a ‘cytokine storm’ such as interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-a), which is already implicated as a mechanistic pathway in the course of TTC.10 11 Where individuals have presented without COVID-19 infection or other notable risk factors, the psychosocial burden of living in a pandemic may have been the trigger for TTC. Individuals with psychological illness prior to the pandemic are more likely to have a heightened stress response and increased risk of developing TTC.9 12–14
In the literature to date, there have been three cases of TTC following the first dose of the mRNA-1273 (Moderna) vaccination,15–17 one case following the first dose of the ChadOX1 nCOV-19 (AZD122) Astra Zeneca vaccination18 and one case following the Pfizer-BioNTech vaccination in a patient on maintenance haemodialysis.19
In this report, we discuss a case of TTC 8 days following administration of a second dose of the ChadOX1 nCOV-19 (AZD122) vaccination, in absence of any other identifiable trigger.
Case presentation
A woman in her early 50s with a history of chronic obstructive pulmonary disease (COPD) was admitted to the emergency department with intermittent sharp central chest pain radiating between her scapulae. This pain began at rest with associated symptoms of diaphoresis, dyspnoea and vomiting.
A week prior to her presentation, she had received her second dose of the COVID-19 vaccination and developed pain around the injection site and ecchymosis on her left leg. She acknowledged increased levels of stress at this time.
Investigations
On admission to the emergency department, vital signs were measured and were within normal limits. A 12-lead ECG revealed sinus rhythm. Arterial blood sampling was normal, excluding an acute exacerbation of COPD, and a chest radiograph showed absence of consolidation or pleural effusion, pointing towards a cardiac cause of her symptoms. Pulmonary embolism was not suspected as the patient was not tachycardic, tachypnoeic or hypoxic.
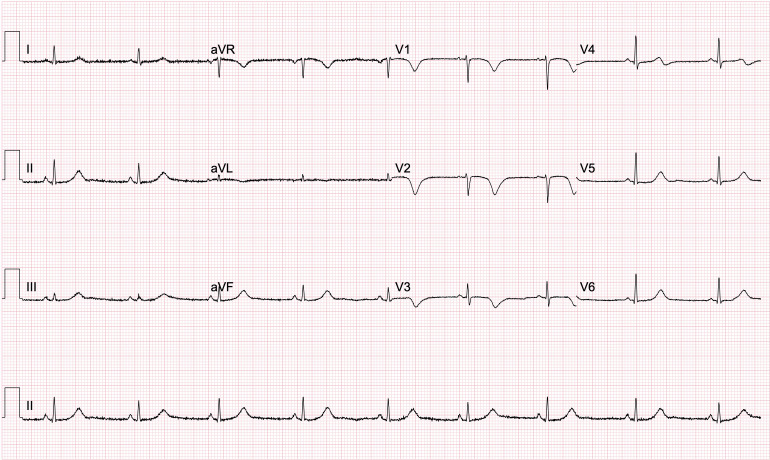
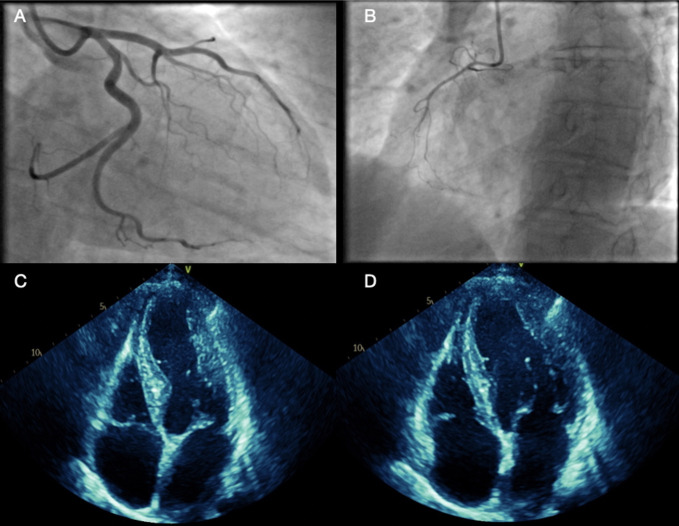
Biochemistry showed an elevated peak troponin of 1662 ng/L with a normal C-reactive protein. The full blood count was normal (platelets 225×109/L and haemoglobin 140 g/L) as was the coagulation screen. Repeat ECG highlighted anterior T wave inversion with a corrected QT interval of 480 ms, which evolved over 48 hours (figure 1). Since blood results and ECG findings were suggestive of acute coronary syndrome (ACS), coronary angiography was performed revealing left ventricular hypokinesia of the mid-cavity anterior wall with no significant coronary artery disease present and left dominant coronary arteries (figure 2A, B). STEMI and non-STEMI (NSTEMI) were, therefore, excluded and TTC was likely. Transthoracic echocardiography (TTE) was performed 48 hours after admission and further showed hypokinesia of the mid-cavity anteroseptum and the apical septum with overall mildly impaired left ventricular systolic contraction. No significant valvular heart disease, normal dimensions of the atria and ventricles and good right ventricular systolic contraction were noted (figure 2C, D).
Figure 1.
An ECG taken 48 hours after admission showing prominent T wave inversion in the anterior leads V1-3 and a QT interval of 480 ms.
Figure 2.
(A, B) Coronary angiography showing left dominant system with non-obstructive coronaries, (C, D) transthoracic echocardiography showing hypokinesia of the mid-apical and anterior septum with mildly impaired left ventricular systolic contraction.
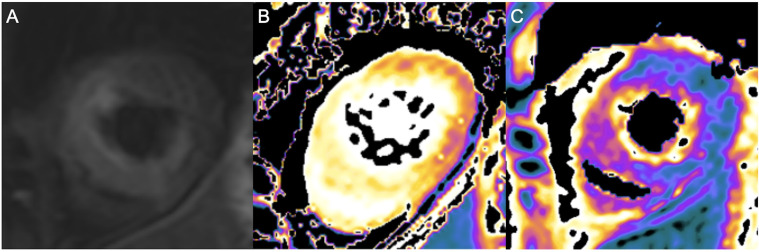
TTC was diagnosed 14 days post-acute event by CMR, which showed acute, predominantly apical, TTC in the intermediate stage of resolution. There was hypokinesia of the anterior and septal walls in the apex and less so in the mid-cavity level. On T2-STIR-FS images, increased signal intensity was evident in the base, mid-cavity and apex (figure 3A) with increased T1/T2 mapping values consistent with myocardial oedema (figure 3B, C). No evidence of myocardial infarction was seen on late gadolinium enhancement.
Figure 3.
(A) CMR T2-STIR sequence carried out 14 days post-acute event showing increased signal in the apex, most pronounced in the septal, anterior and inferior walls, (B) native T1 mapping of apex showing increased signalling, most pronounced in the anterior and septal walls, (C) T2 mapping of apex showing increased signalling, most pronounced in the anterior and septal walls. CMR, cardiac MRI; STEMI, ST elevation myocardial infarction.
Differential diagnosis
The differential diagnoses of STEMI and NSTEMI were excluded at angiography by non-obstructive coronary arteries and the absence of late gadolinium enhancement on CMR. CMR further excluded myocardial infarction with non-obstructive coronary arteries and the absence of typical wall mortion abnormalities.
Spontaneous coronary artery dissection was excluded at angiography.
Myocarditis was excluded by the absence of typical subendothelial late gadolinimun enhancement on CMR and the absence of preceding viral illness.
Treatment
The patient was initially managed with the ACS protocol of dual antiplatelet therapy. Following diagnosis of TTC, her ACS protocol medications were stopped.
Outcome and follow-up
The patient improved clinically and had no evidence of high-risk features on 12-lead ECG or TTE and was discharged home 5 days post-event. The patient was followed up after 3 months. Her follow-up CMR showed resolving myocardial oedema in the apex and septum. Repeat TTE showed normal left ventricular (LV) systolic function and resolution of previously noted wall motion abnormalities.
Discussion
The COVID-19 pandemic has seen the largest global vaccination programme in history with over 7.9 billion vaccination doses administered from December 2020 to 6 December 2021.20 With increasing populations being vaccinated daily, we are seeing an increase in reports of associated side effects. The most common side effects reported are pain, swelling and redness around the injection site. Systemic effects such as fever, fatigue, myalgia and headache can also occur in around one in four. Higher prevalence of these systemic effects has been noted in women, individuals aged 55 years or under, those who have had past SARS-CoV-2 infection and after the second vaccination dose.21–24 Moreover, severe side effects have been reported and are under current monitoring: thrombocytopenic thrombosis, anaphylaxis, Bell’s palsy and capillary leak syndrome.25 The Medicines and Healthcare products Regulatory Agency has recently warned of rare reports of pericarditis and myocarditis with revisions being made to the Moderna and Pfizer/BioNTech vaccine product information. International reports have shown the highest incidence of myocarditis in men aged under 40 years within 2 weeks of receiving their second vaccine dose. Symptoms are mostly mild with rest and supportive treatment required.26
This case demonstrates an extremely rare adverse event of the ChadOX1 nCOV-19 (AZD122) second dose vaccination, which has only been reported once in the literature in a 72-year old man. No physical or emotional triggers were identified and it was hypothesised that 4 days of an exaggerated systemic inflammatory response to the first dose of the vaccine was sufficient to trigger a catecholamine surge causing TTC.18 Cases of TTC after the mRNA-1273 vaccination15–17 were documented in postmenopausal women presenting with symptoms earlier, between 17 hours and 2 days. Similarly, no acute stressors were identified making vaccination the most likely cause. In the case reported by Fearon et al,17 ‘diffuse regional microvascular dysfunction’ caused by neurohormonal imbalance was thought to have increased TTC susceptibility after vaccination. They also noted significant anticipatory vaccination stress in the patient suggesting that psychological factors may have played a role. The final case report of TTC was presented with symptoms of fatigue and poor appetite before being admitted with hypotension during maintenance haemodialysis 4 days after the Pfizer-BioNTech vaccination.19 They hypothesised a similar mechanism of action, sympathovagal imbalance, to Jani et al15 and Fearon et al.17
In contrast to Boscolo et al,16 the classical pattern of apical ballooning was not noted in our case; however, it is well recognised that not all cases of TTC follow this classical pattern. CMR imaging was done 14 days post-index event, during which time LV systolic function is likely to have started to resolve. However, similar to Boscolo et al,16 the key features of increased T1 and T2 mapping and increased signal on T2-STIR imaging suggestive of myocardial oedema were noted.
In our case and those mentioned above, the majority of patients were postmenopausal women who had multiple comorbidities including ischaemic heart disease, asthma, bronchiectasis, COPD, hypertension, diabetes, hyperlipidaemia, ulcerative colitis and chronic kidney disease. No cause is immediately obvious; however, an immune substrate could be responsible, as TTC has been seen clustered with other autoimmune conditions.27 Furthermore, in all cases documented, it is difficult to determine whether the vaccine was exclusively the causative factor or whether a stress response to the vaccine side effects was sufficient to induce TTC. It is evident, however, that since the cases of TTC occurred in close time proximity to vaccinations with no obvious acute trigger identified, vaccination is the most likely cause.
In addition to COVID-19 vaccinations, there are limited reports of other vaccinations causing TTC; only two case reports have been published describing uncommon adverse event following the influenza vaccination. One report suggested that the immediate cardiac sympathetic discharge with reduced heart rate variability and a myocardium already sensitive to a surge in catecholamines could be attributable mechanisms following vaccination.28 Similarly, the second report suggested a role of a catecholamine surge along with adrenergic predominance stimulating the sympathetic nervous system.29
TTC is a relatively novel disease entity whose pathophysiology is yet to be fully elucidated. While once thought to be a transient and self-limiting condition, evidence is building to suggest that these patients can have persistent symptoms as part of a heart failure with preserved ejection fraction phenotype.30 Therefore, understanding potential triggers and the clinical course of this under-reported condition is vital. While there have been several reports of TTC following varying types of COVID-19 vaccinations, it is important to highlight the rarity of this adverse event, which should not stop or alter the current recommended vaccination strategies.
Patient’s perspective.
After I received my first COVID-19 vaccination, I had a large bruise on my left knee. This returned 1 week after my second dose and my son was concerned that it was a blood clot, so I visited the doctor for tests. I did not feel particularly anxious about this but in the back of my mind, I probably was stressed. Luckily, tests excluded a clot. The next day while sitting on my sofa, I felt a squeezing pain in the centre of my chest, which spread to my back in between my shoulder blades. I also vomited and struggled to breathe. I was admitted to A&E by ambulance and at the time I was worried I was having a heart attack. Being a patient in hospital during the pandemic was very lonely as I was unable to have visitors and staff seemed very busy. I did not fully understand my diagnosis at the time apart from it being caused by stress and that it was also called ‘broken heart syndrome’. I have had a lot of stress over the past 5 years after my mother passed away, which is when I started noticing my physical health deteriorating. Now, I am in recovery, I am doing well and have not had any chest pain. I have been trying my best to relax as much as possible by going for walks with my dog, watching movies, seeing friends and attending emotional support sessions. However, I am worried that it can happen again and there are stresses in my life that I am unable to control. Hopefully, my follow-up scans will show improvement and I can relax a little more and try to get on with life.
Learning points.
Incidence of takotsubo cardiomyopathy (TTC) has increased during the COVID-19 pandemic.
Cases of TTC have been reported following varying types of COVID-19 vaccinations.
Multimodal imaging is required to diagnose and risk stratify this condition.
Acknowledgments
Janaki Srinivasan for preparing the echocardiography images used in this report.
Footnotes
Contributors: CS was involved with the preparation of the first draft of this paper and subsequent review. DG was involved in the clinical care of this patient, preparation of the images, first draft of this paper and subsequent review. DD had the idea of this case report, was involved in the clinical care of this patient, review of the first draft of this paper and subsequent review.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
References
- 1.Dawson DK. Acute stress-induced (takotsubo) cardiomyopathy. Heart 2018;104:96–102. 10.1136/heartjnl-2017-311579 [DOI] [PubMed] [Google Scholar]
- 2.Ghadri J-R, Wittstein IS, Prasad A, et al. International expert consensus document on takotsubo syndrome (Part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J 2018;39:2032–46. 10.1093/eurheartj/ehy076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zvonarev V. Takotsubo Cardiomyopathy: Medical and Psychiatric Aspects. Role of Psychotropic Medications in the Treatment of Adults with "Broken Heart" Syndrome. Cureus 2019;11:e5177. 10.7759/cureus.5177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato K, Lyon AR, Ghadri J-R, et al. Takotsubo syndrome: aetiology, presentation and treatment. Heart 2017;103:1461–9. 10.1136/heartjnl-2016-309783 [DOI] [PubMed] [Google Scholar]
- 5.Ghadri J-R, Wittstein IS, Prasad A, et al. International expert consensus document on takotsubo syndrome (Part II): diagnostic workup, outcome, and management. Eur Heart J 2018;39:2047–62. 10.1093/eurheartj/ehy077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dagrenat C, Von Hunolstein JJ, Matsushita K, et al. Value of cardiac biomarkers in the early diagnosis of takotsubo syndrome. J Clin Med 2020;9:2985. 10.3390/jcm9092985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med 2015;373:929–38. 10.1056/NEJMoa1406761 [DOI] [PubMed] [Google Scholar]
- 8.Ono R, Falcão LM. Takotsubo cardiomyopathy systematic review: pathophysiologic process, clinical presentation and diagnostic approach to takotsubo cardiomyopathy. Int J Cardiol 2016;209:196–205. 10.1016/j.ijcard.2016.02.012 [DOI] [PubMed] [Google Scholar]
- 9.Shah RM, Shah M, Shah S, et al. Takotsubo syndrome and COVID-19: associations and implications. Curr Probl Cardiol 2021;46:100763. 10.1016/j.cpcardiol.2020.100763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh S, Desai R, Gandhi Z, et al. Takotsubo syndrome in patients with COVID-19: a systematic review of published cases. SN Compr Clin Med 2020;2:2102–8. 10.1007/s42399-020-00557-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scally C, Abbas H, Ahearn T, et al. Myocardial and systemic inflammation in acute stress-induced (takotsubo) cardiomyopathy. Circulation 2019;139:1581–92. 10.1161/CIRCULATIONAHA.118.037975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jabri A, Kalra A, Kumar A, et al. Incidence of stress cardiomyopathy during the coronavirus disease 2019 pandemic. JAMA Netw Open 2020;3:e2014780. 10.1001/jamanetworkopen.2020.14780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbieri L, Galli F, Conconi B, et al. Takotsubo syndrome in COVID-19 era: is psychological distress the key? J Psychosom Res 2021;140:110297. 10.1016/j.jpsychores.2020.110297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivers J, Ihle JF. COVID-19 social isolation-induced takotsubo cardiomyopathy. Med J Aust 2020;213:336–336.e1. 10.5694/mja2.50770 [DOI] [PubMed] [Google Scholar]
- 15.Jani C, Leavitt J, Al Omari O, et al. COVID-19 vaccine-associated takotsubo cardiomyopathy. Am J Ther 2021;28:361–4. 10.1097/MJT.0000000000001379 [DOI] [PubMed] [Google Scholar]
- 16.Boscolo Berto M, Spano G, Wagner B, et al. Takotsubo cardiomyopathy after mRNA COVID-19 vaccination. Heart Lung Circ 2021;30:e119–20. 10.1016/j.hlc.2021.06.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fearon C, Parwani P, Gow-Lee B, et al. Takotsubo syndrome after receiving the COVID-19 vaccine. J Cardiol Cases 2021;24:223–6. 10.1016/j.jccase.2021.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crane P, Wong C, Mehta N, et al. Takotsubo (stress) cardiomyopathy after ChAdOx1 nCoV-19 vaccination. BMJ Case Rep 2021;14:e246580. 10.1136/bcr-2021-246580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toida R, Uezono S, Komatsu H, et al. Takotsubo cardiomyopathy after vaccination for coronavirus disease 2019 in a patient on maintenance hemodialysis. CEN Case Reports 2021;98. 10.1007/s13730-021-00657-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organisation . WHO coronavirus (COVID-19). Available: https://covid19.who.int [Accessed 06 Aug 2021].
- 21.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med Overseas Ed 2020;383:2603–15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinde V, Bhikha S, Hoosain Z, et al. Efficacy of NVX-CoV2373 Covid-19 vaccine against the B.1.351 variant. N Engl J Med 2021;384:1899–909. 10.1056/NEJMoa2103055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu L, McPhee R, Huang W, et al. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine 2021;39:2791–9. 10.1016/j.vaccine.2021.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menni C, Klaser K, May A, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID symptom study APP in the UK: a prospective observational study. Lancet Infect Dis 2021;21:939–49. 10.1016/S1473-3099(21)00224-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medicines & Healthcare products Regulatory Agency . Coronavirus vaccine - weekly summary of Yellow Card reporting. Available: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting [Accessed 12 May 2021].
- 26.Medicines & Healthcare products Regulatory Agency . COVID-19 vaccines: updates for July 2021. Available: https://www.gov.uk/drug-safety-update/covid-19-vaccines-updates-for-july-2021#review-of-extremely-rare-reports-of-myocarditis-and-pericarditis [Accessed 16 July 2021].
- 27.Gupta S, Goyal P, Idrees S, et al. Association of endocrine conditions with takotsubo cardiomyopathy: a comprehensive review. J Am Heart Assoc 2018;7:e009003. 10.1161/JAHA.118.009003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh K, Marinelli T, Horowitz JD. Takotsubo cardiomyopathy after anti-influenza vaccination: catecholaminergic effects of immune system. Am J Emerg Med 2013;31:1627.e1–1627.e4. 10.1016/j.ajem.2013.06.039 [DOI] [PubMed] [Google Scholar]
- 29.Santoro F, Ieva R, Ferraretti A, et al. Tako-Tsubo cardiomyopathy after influenza vaccination. Int J Cardiol 2013;167:e51–2. 10.1016/j.ijcard.2013.03.147 [DOI] [PubMed] [Google Scholar]
- 30.Scally C, Rudd A, Mezincescu A, et al. Persistent long-term structural, functional, and metabolic changes after stress-induced (takotsubo) cardiomyopathy. Circulation 2018;137:1039–48. 10.1161/CIRCULATIONAHA.117.031841 [DOI] [PMC free article] [PubMed] [Google Scholar]