Abstract
Purpose
Medulloblastoma is known to be associated with multiple cancer-predisposition syndromes. In this article, we explore a possible association among a patient's Aarskog-Scott syndrome, development of medulloblastoma, and subsequent brainstem radiation necrosis.
Case Presentation
A 5-year-old male with Aarskog-Scott syndrome initially presented to his pediatrician with morning emesis, gait instability, and truncal weakness. He was ultimately found to have a posterior fossa tumor with pathology consistent with group 3 medulloblastoma. After receiving a gross total resection and standard proton beam radiation therapy with concurrent vincristine, he was noted to develop brainstem radiation necrosis, for which he underwent therapy with high-dose dexamethasone, bevacizumab, and hyperbaric oxygen therapy with radiographic improvement and clinical stabilization.
Conclusion
Based on several possible pathologic correlates in the FDG1 pathway, there exists a potential association between this patient's Aarskog-Scott syndrome and medulloblastoma, which needs to be investigated further. In patients with underlying, rare genetic syndromes, further caution should be taken when evaluating chemotherapy and radiation dosimetry planning.
Keywords: brainstem radiation necrosis, medulloblastoma, Aarskog-Scott syndrome, hyperbaric oxygen therapy
Introduction
Medulloblastoma is the most common pediatric central nervous system malignancy [1]. The mainstay of treatment is surgical resection, followed by radiation therapy and adjuvant chemotherapy [2, 3]. The emergence of proton beam radiation has been critical for medulloblastoma therapy because of its ability to spare damage to healthy tissue, resulting in a reduction in both short-term and long-term side effects [3, 4]. Brainstem radiation necrosis, however, has occurred in the settings of both photon and proton radiotherapy [5].
Medulloblastoma is associated with several cancer predisposition syndromes, including Li-Fraumeni syndrome, nevoid basal cell carcinoma syndrome, and familial adenomatous polyposis [6]. Newer sequencing technologies allow identification of underlying cancer predispositions or other syndromes that would have been missed historically [7, 8]. In this case report, we present a patient with Aarskog-Scott syndrome who developed medulloblastoma with subsequent brainstem radiation necrosis.
Case Presentation
A 5-year-old male with Aarskog-Scott syndrome, gastroesophageal reflux, and beta thalassemia minor initially presented with a 2-month history of morning emesis and decreased oral intake. He had subsequent difficulties with balance, truncal and extremity weakness, and a wide-based gait. His medical history, as part of his Aarskog-Scott syndrome, was significant for small stature and decreased weight, tracking under the first percentile for his age. His family history was significant for a father with history of childhood seizures and a younger brother who also has Aarskog-Scott syndrome.
Initial exam was significant for 4/5 strength in all extremities, truncal ataxia with a wide-based gait, and dysmetria. Magnetic resonance imaging (MRI) showed a 4.1 × 5.7 × 6.3-cm, heterogeneously enhancing tumor in the fourth ventricle, with obstructive hydrocephalus. An MRI of the spine and a lumbar puncture showed no signs of distant disease. The patient underwent gross total resection of the tumor. Pathology showed medulloblastoma, classic variant, World Health Organization grade IV, MYC/MYCN non-amplified, with loss of heterozygosity secondary to copy number gains of chromosome band 1q, 7, 9, and 14, as well as a copy number loss of chromosome band 16q, overlapping with the group 3 subtype [9].
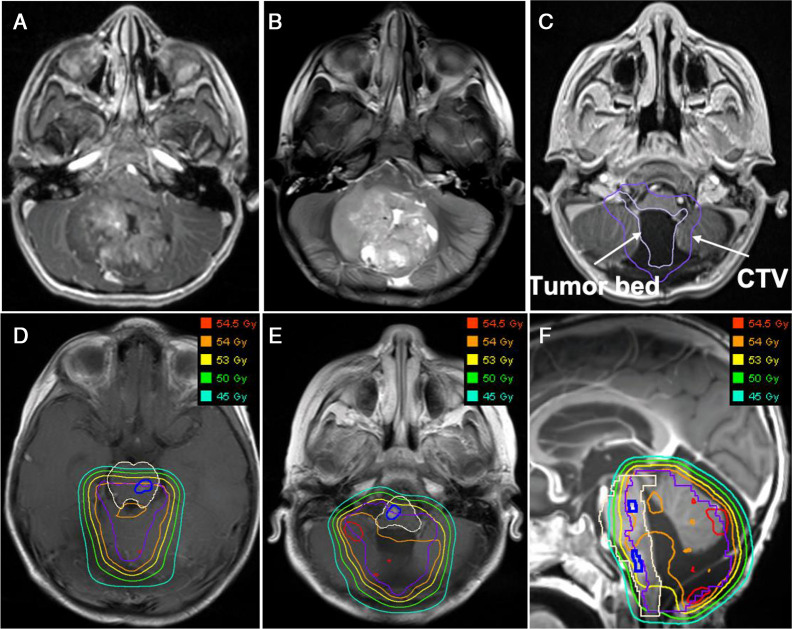
Four days after the surgery, the patient was diagnosed with severe posterior fossa syndrome with poor global muscle tone, ataxia, mutism, and dysphagia. Subclinical seizures were confirmed by video electroencephalogram. A literature search revealed no contraindication or increased sensitivity to radiotherapy associated with Aarskog-Scott syndrome. His radiotherapy consisted of 23.4 GyRBE to the craniospinal axis (Figure 1) with an additional 30.6 GyRBE to the tumor bed, for a total dose of 54 GyRBE (Figure 2) with weekly vincristine at 1.5 mg/m2. Maximum and D50 (median) doses to the patient's brainstem were 54.4 GyRBE and 53.7 GyRBE, respectively, well within published guidelines for healthy brainstem tolerance [10–13]. Modest improvements were made in strength, mobility, and mutism with intensive rehabilitation.
Figure 1.
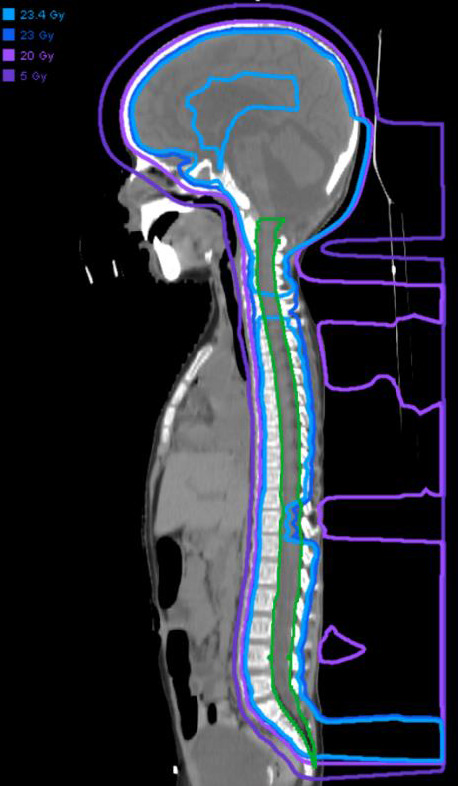
Craniospinal irradiation phase dosimetry. The radiation target is the whole brain and spinal canal (dark green line) inclusive of the thecal sac. The doses received by these regions are shown by a series of isodose lines for the 5 Gy (dark purple), 20 Gy (light purple), 23 Gy (dark blue), and 23.4 Gy (light blue) dose levels. Note the lack of low-dose radiation received by structures anterior to the spinal canal when using a proton beam that enters from the posterior surface.
Figure 2.
Boost phase dosimetry. (A–C) Representative magnetic resonance imaging (MRI) contrast-enhanced T1 (A) and T2 (B) images of the tumor in the preoperative setting and tumor bed in the postoperative setting (C, light purple). Note the extensive contact between the tumor and brainstem in the axial plane accompanied by brainstem displacement and compression. A margin was added to the known at-risk tissue volume to account for microscopic disease spread, also known as a clinical target volume (CTV; dark purple). (D–F) The doses received by the brainstem (cream) and neighboring tissues at the level of the superior and inferior lesions (dark blue) are shown by a series of isodose lines in transverse (D and E) and sagittal (F) views for the 45 Gy (aqua), 50 Gy (green), 53 Gy (yellow), 54 Gy (orange), and 54.5 Gy (red) dose levels.
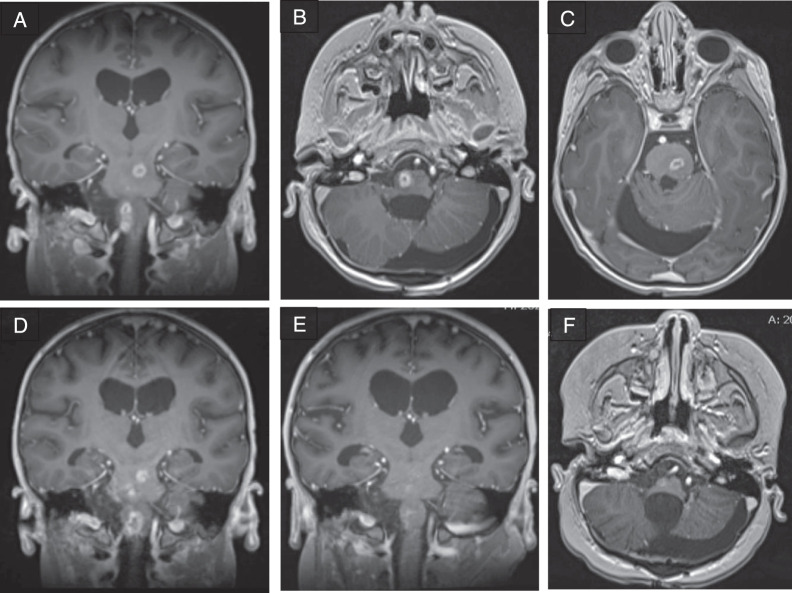
He received his first 6-week cycle of maintenance chemotherapy with cisplatin, lomustine, and vincristine per ACNS0331 [14], cycle A prior to being discharged to home. Adjuvant chemotherapy continued with another A cycle, and then a 4-week B cycle comprising cyclophosphamide with Mesna and vincristine. Routine MRI, at 21 weeks after radiotherapy completion, revealed new, rim-enhancing lesions with central necrosis and surrounding edema in the left upper, middle, and lower pons, as well as in the right medulla and cervicomedullary junction, measuring up to 1 cm (Figure 3A–3C). All lesions were within the tumor-bed boost portion of the radiotherapy field and were judged to be consistent with radiation injury, although the patient had not manifested any neurologic deterioration. Further chemotherapy was held, and the patient was started on dexamethasone.
Figure 3.
Development of rim enhancing lesions within the brainstem 5 months after radiation therapy. (A–C) Postgadolinium T1-weighted imaging approximately 5 months after radiation. (D) Postgadolinium T1-weighted imaging 6 weeks after initial imaging. (E and F) Postgadolinium T1-weighted imaging after completion of bevacizumab therapy.
An MRI performed 6 weeks later showed lesion progression (Figure 3D). The patient developed worsening hemiparesis in the following days. Dexamethasone was continued, and bevacizumab was initiated at 10 mg/kg every 2 weeks for 6 doses [15, 16]. Mild improvement in symptoms occurred, and radiographic stability was observed on the following MRI 2 months later. Radiographic improvement continued after completion of bevacizumab therapy, with enhancing lesions subsequently subsiding (Figure 3E and 3F); however, his neurologic deficits persisted.
Daily hyperbaric oxygen therapy was initiated 6 weeks later [17–20]. During this treatment, mild clinical improvement was reported. To date, the patient continues to have slow clinical improvement.
Methods and Results
Genomic DNA of the patient, his father, mother, and younger brother was isolated from blood, and whole-exome sequencing was performed, as previously described [21]. Exon capture was performed using the IDT xGen Exome capture kit (Integrated DNA Technologies, Coralville, Iowa), followed by 101 base paired–end sequencing on the HiSeq 4000 platform (Illumina, San Diego, California). Sequence reads were aligned to the human reference genome GRCh37/hg19 using the BWA-MEM software and further processed to call variants, following the GATK best practices workflow [22, 23]. Variants annotated with ANNOVAR and MetaSVM software were used to predict the deleteriousness of nonsynonymous variants (herein, referred to as D-mis) [24, 25]. All variants covered by independent-aligned sequencing reads with a depth of 8× or greater were visualized in silico to eliminate false-positives.
To identify potential causal mutations for the disease phenotypes in the affected siblings, we filtered for rare, damaging mutations shared by both individuals. A non–frameshift deletion (c.2729_2746del, p.910_916del) in FGD1 was identified in both siblings, which was transmitted from their unaffected mother. FGD1 encodes “FYVE, RhoGEF and PH domain containing 1.” X-linked recessive variants in FGD1 have been associated with Aarskog-Scott syndrome (OMIM: 305400) [26], which is consistent with the phenotype and the inheritance model observed in this family. Variants in this domain have previously been associated with Aarskog-Scott syndrome [27–29].
Discussion
Despite radiotherapy dosimetry being well within published brainstem constraints for healthy tissue, this patient suffered radiation-related injury causing neurologic decline [10–13]. Dose heterogeneity in a radiation plan is unavoidable, but in this case, there were very few and mild hot spots.
Importantly, the pediatric radiation oncology community is increasingly recognizing that brainstem tolerance may be less with proton radiotherapy than it is with photon radiotherapy. The current Children's Oncology Group ependymoma study (ACNS0831) [30] uses lower brainstem dose constraints for proton radiotherapy compared with photon radiotherapy, a topic recently addressed at a workshop sponsored by the National Cancer Institute [10]. Nonetheless, this patient was treated with doses meeting those newer and lower brainstem dose constraints and still suffered symptomatic brainstem injury.
Integral to this discussion is consideration of the linear energy transfer (LET) and relative biologic effectiveness (RBE)–weighted dose distributions. The LET distribution here is typical for patients treated with this technique. The RBE depends heavily on the a/b ratio, which was 3 in this case (Figure 4). This RBE model attempts to account for the biological effective dose. Although it appears that the injury occurred in regions of higher RBE, it does not occur in the region of highest LET, and previous reports have been inconclusive. However, the LET differences across the proton-dose distribution may partially account for these unexpected findings. Better models for LET or RBE are needed to truly understand the potential effect on the patient. Newer radiation-planning systems are testing the incorporation of LET into the model, which could help diminish the apparent increased biological effective dose seen in certain parts of the dose distribution.
Figure 4.
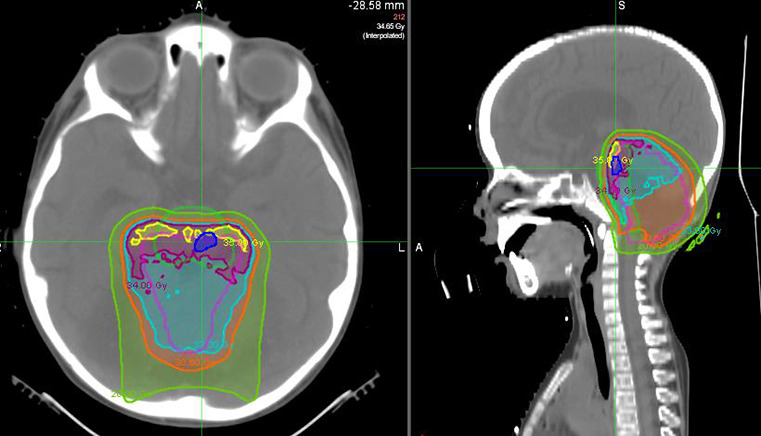
Relative biologic effectiveness (RBE) modeling overlay using a/b = 3. The brainstem (lime green) and superior contrast-enhancing brainstem lesion (royal blue) are outlined. The RBE contours are shown for 20 Gy (lime green), 30.6 (orange), 33 (aqua), 34 (magenta), and 35 (yellow). The area of brainstem injury partially overlaps the modeled area of highest RBE that, when added to the craniospinal irradiation (CSI) dose, equates to 57.4–58.4 Gy.
Many patients are treated with similar RBE/LET dose distributions and do not develop injuries. Thus, factors contributing to host radiosensitivity are also likely contributory. It behooves us to consider those factors that may have predisposed this patient to radiation sensitivity or injury and to consider those factors moving forward as we treat patients with genetic syndromes, blood dyscrasias, nutritional deficiencies, or other comorbidities that may impair growth and healing [31].
In this case, it is unclear what genetic components of Aarskog-Scott may have had a role in sensitivity. The FGD1 gene implicated in Aarskog-Scott syndrome codes for a protein that activates Cdc42. This GTPase is important in cell signaling and is involved with many functions, including remodeling of the extracellular matrix and regulation of cell growth [32, 33]. The upregulation of Cdc42 is also associated with increased invasion of medulloblastoma, potentially making these tumors more aggressive [34, 35]. Review of the literature did not reveal any cases of patients with Aarskog-Scott who received proton or photon radiation.
This patient's relatively poor radiotherapy tolerance could also be related to phenotypic manifestations of his underlying syndrome, namely, his nutritional status and growth delay. He was < 1% of expected weight and height for his age, and patients with an impaired nutritional status may be predisposed to injury and have more difficulty repairing any sustained injury [36].
Conclusion
It is likely that multiple factors, including an underlying syndrome and poor nutritional and growth status, contributed to this patient's high toxicity to treatment. When treating a child with a genetic syndrome, failure to thrive, and/or nutritional deficiencies, additional care must be taken in developing the radiotherapy plan. If radiation-related injury develops during adjuvant chemotherapeutic treatment, we recommend that treatment be halted immediately with early initiation of anti-inflammatory agents to avoid neurologic decline, regardless of symptoms at the time. Chemotherapy administration can cause further deterioration. Only when imaging findings of brainstem radiation necrosis resolve and there is no further clinical deterioration should chemotherapy be reinstated, if at all. Prolonged steroid and bevacizumab use is typically needed when clinically significant injury is present. Interestingly, hyperbaric oxygen therapy appears to have had some benefit for this patient and merits consideration going forward.
ADDITIONAL INFORMATION AND DECLARATIONS
CRediT: Vidya Puthenpura: conceptualization, data curation, investigation, methodology, resources, validation, visualization, writing – original draft, writing- review and editing; Nicholas DeNunzio: data curation, investigation, methodology, resources, validation, visualization, writing – original draft, writing – review and editing; Xue Zeng: data curation, investigation, methodology, validation, writing – original draft: Drosoula Giantsoudi: data curation, investigation, methodology, validation, writing – review and editing; Mariam Aboian: investigation, validation, writing – review and editing; David Ebb: validation, writing – review and editing; Kristopher T. Kahle: data curation, methodology, resources, validation, writing – review and editing; Torunn I. Yock: conceptualization, data curation, investigation, methodology, resources, validation, visualization, writing – original draft, writing – review and editing; Asher M. Marks: conceptualization, data curation, investigation, methodology, resources, validation, visualization, writing – original draft, writing – review and editing.
Conflicts of Interest: The authors have no relevant conflicts of interest to disclose.
Funding: The authors have no funding to disclose.
Ethical Approval: Institutional review board approval was not required by our institution to report this single case.
References
- 1.Smoll NR, Drummond KJ. The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J Clin Neurosci . 2012;19:1541–4. doi: 10.1016/j.jocn.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Kline CN, Packer RJ, Hwang EI, Raleigh DR, Braunstein S, Raffel C, Bandopadhayay P, Solomon DA, Aboian M, Cha S, Mueller S. Case-based review: pediatric medulloblastoma. Neurooncol Pract . 2017;4:138–50. doi: 10.1093/nop/npx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devine CA, Liu KX, Ioakeim-Ioannidou M, Susko M, Poussaint TY, Huisman T, Aboian M, Brown D, Zaslowe-Dude C, Rao AD, Orlina LT, Rawal B, Mueller S, Marcus KJ, Terezakis SA, Braunstein SE, Haas-Kogan DA. Brainstem injury in pediatric patients receiving posterior fossa photon radiation. Int J Radiat Oncol Biol Phys . 2019;105:1034–42. doi: 10.1016/j.ijrobp.2019.08.039. [DOI] [PubMed] [Google Scholar]
- 4.Ho ESQ, Barrett SA, Mullaney LM. A review of dosimetric and toxicity modeling of proton versus photon craniospinal irradiation for pediatrics medulloblastoma. Acta Oncol . 2017;56:1031–42. doi: 10.1080/0284186X.2017.1324207. [DOI] [PubMed] [Google Scholar]
- 5.Davanzo J, Greiner RJ, Barbour M, Rizk E. Radiation necrosis following proton beam therapy in the pediatric population: a case series. Cureus . 2017;9:e1785. doi: 10.7759/cureus.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waszak SM, Northcott PA, Buchhalter I, Robinson GW, Sutter C, Groebner S, Grund KB, Brugieres L, Jones DTW, Pajtler KW, Morrissy AS, Kool M, Sturm D, Chavez L, Ernst A, Brabetz S, Hain M, Zichner T, Segura-Wang M, Weischenfeldt J, Rausch T, Mardin BR, Zhou X, Baciu C, Lawerenz C, Chan JA, Varlet P, Guerrini-Rousseau L, Fults DW, Grajkowska W, Hauser P, Jabado N, Ra YS, Zitterbart K, Shringarpure SS, De La Vega FM, Bustamante CD, Ng HK, Perry A, MacDonald TJ, Hernaiz Driever P, Bendel AE, Bowers DC, McCowage G, Chintagumpala MM, Cohn R, Hassall T, Fleischhack G, Eggen T, Wesenberg F, Feychting M, Lannering B, Schuz J, Johansen C, Andersen TV, Roosli M, Kuehni CE, Grotzer M, Kjaerheim K, Monoranu CM, Archer TC, Duke E, Pomeroy SL, Shelagh R, Frank S, Sumerauer D, Scheurlen W, Ryzhova MV, Milde T, Kratz CP, Samuel D, Zhang J, Solomon DA, Marra M, Eils R, Bartram CR, von Hoff K, Rutkowski S, Ramaswamy V, Gilbertson RJ, Korshunov A, Taylor MD, Lichter P, Malkin D, Gajjar A, Korbel JO, Pfister SM. Spectrum and prevalence of genetic predisposition in medulloblastoma: a retrospective genetic study and prospective validation in a clinical trial cohort. Lancet Oncol . 2018;19:785–98. doi: 10.1016/S1470-2045(18)30242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kline CN, Joseph NM, Grenert JP, van Ziffle J, Talevich E, Onodera C, Aboian M, Cha S, Raleigh DR, Braunstein S, Torkildson J, Samuel D, Bloomer M, Campomanes AGA, Banerjee A, Butowski N, Raffel C, Tihan T, Bollen AW, Phillips JJ, Korn WM, Yeh I, Bastian BC, Gupta N, Mueller S, Perry A, Nicolaides T, Solomon DA. Targeted next-generation sequencing of pediatric neuro-oncology patients improves diagnosis, identifies pathogenic germline mutations, and directs targeted therapy. Neuro Oncol . 2017;19:699–709. doi: 10.1093/neuonc/now254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garber JE, Offit K. Hereditary cancer predisposition syndromes. J Clin Oncol . 2005;23:276–92. doi: 10.1200/JCO.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 9.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S, Gajjar A, Ellison DW, Lichter P, Gilbertson RJ, Pomeroy SL, Kool M, Pfister SM. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol . 2012;123:465–72. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haas-Kogan D, Indelicato D, Paganetti H, Esiashvili N, Mahajan A, Yock T, Flampouri S, MacDonald S, Fouladi M, Stephen K, Kalapurakal J, Terezakis S, Kooy H, Grosshans D, Makrigiorgos M, Mishra K, Poussaint TY, Cohen K, Fitzgerald T, Gondi V, Liu A, Michalski J, Mirkovic D, Mohan R, Perkins S, Wong K, Vikram B, Buchsbaum J, Kun L. National Cancer Institute workshop on proton therapy for children: considerations regarding brainstem injury. Int J Radiat Oncol Biol Phys . 2018;101:152–68. doi: 10.1016/j.ijrobp.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gentile MS, Yeap BY, Paganetti H, Goebel CP, Gaudet DE, Gallotto SL, Weyman EA, Morgan ML, MacDonald SM, Giantsoudi D, Adams J, Tarbell NJ, Kooy H, Yock TI. Brainstem injury in pediatric patients with posterior fossa tumors treated with proton beam therapy and associated dosimetric factors. Int J Radiat Oncol Biol Phys . 2018;100:719–29. doi: 10.1016/j.ijrobp.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 12.Indelicato DJ, Flampouri S, Rotondo RL, Bradley JA, Morris CG, Aldana PR, Sandler E, Mendenhall NP. Incidence and dosimetric parameters of pediatric brainstem toxicity following proton therapy. Acta Oncol . 2014;53:1298–1304. doi: 10.3109/0284186X.2014.957414. [DOI] [PubMed] [Google Scholar]
- 13.Yock TI, Constine LS, Mahajan A. Protons, the brainstem, and toxicity: ingredients for an emerging dialectic. Acta Oncol . 2014;53:1279–82. doi: 10.3109/0284186X.2014.957415. [DOI] [PubMed] [Google Scholar]
- 14.Michalski J. A study evaluating limited target volume boost irradiation and reduced dose craniospinal radiotherapy (18 Gy) and chemotherapy in children with newly diagnosed standard risk medulloblastoma a phase III double randomized trial. Children's Oncology Group ID: ACNS0331; Published June 24, 2013. Updated June 28, 2013. Accessed May 12, 2021. https://childrensoncologygroup.org/acns0331. [Google Scholar]
- 15.Wong ET, Huberman M, Lu XQ, Mahadevan A. Bevacizumab reverses cerebral radiation necrosis. J Clin Oncol . 2008;26:5649–50. doi: 10.1200/JCO.2008.19.1866. [DOI] [PubMed] [Google Scholar]
- 16.Liu AK, Macy ME, Foreman NK. Bevacizumab as therapy for radiation necrosis in four children with pontine gliomas. Int J Radiat Oncol Biol Phys . 2009;75:1148–54. doi: 10.1016/j.ijrobp.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aghajan Y, Grover I, Gorsi H, Tumblin M, Crawford JR. Use of hyperbaric oxygen therapy in pediatric neuro-oncology: a single institutional experience. J Neurooncol . 2019;141:151–8. doi: 10.1007/s11060-018-03021-x. [DOI] [PubMed] [Google Scholar]
- 18.Co J, De Moraes MV, Katznelson R, Evans AW, Shultz D, Laperriere N, Millar BA, Berlin A, Kongkham P, Tsang DS. Hyperbaric oxygen for radiation necrosis of the brain. Can J Neurol Sci . 2019;47:92–9. doi: 10.1017/cjn.2019.290. [DOI] [PubMed] [Google Scholar]
- 19.Chuba PJ, Aronin P, Bhambhani K, Eichenhorn M, Zamarano L, Cianci P, Muhlbauer M, Porter AT, Fontanesi J. Hyperbaric oxygen therapy for radiation-induced brain injury in children. Cancer . 1997;80:2005–12. doi: 10.1002/(sici)1097-0142(19971115)80:10<2005::aid-cncr19>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Ashamalla HL, Thom SR, Goldwein JW. Hyperbaric oxygen therapy for the treatment of radiation-induced sequelae in children: the University of Pennsylvania experience. Cancer . 1996;77:2407–12. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2407::AID-CNCR33>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 21.Duran D, Zeng X, Jin SC, Choi J, Nelson-Williams C, Yatsula B, Gaillard J, Furey CG, Lu Q, Timberlake AT, Dong W, Sorscher MA, Loring E, Klein J, Allocco A, Hunt A, Conine S, Karimy JK, Youngblood MW, Zhang J, DiLuna ML, Matouk CC, Mane S, Tikhonova IR, Castaldi C, Lopez-Giraldez F, Knight J, Haider S, Soban M, Alper SL, Komiyama M, Ducruet F, Zabramski JM, Dardik A, Walcott BP, Stapleton CJ, Aagaard-Kienitz B, Rodesch G, Jackson E, Smith ER, Orbach DB, Berenstein A, Bilguvar K, Vikkula M, Gunel M, Lifton RP, Kahle KT. Mutations in chromatin modifier and ephrin signaling genes in vein of Galen malformation. Neuron . 2019;101:429–43.e424. doi: 10.1016/j.neuron.2018.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, Freudenberg J. Characterizing regions in the human genome unmappable by next-generation-sequencing at the read length of 1000 bases. Comput Biol Chem . 2014;53(pt A):108–17. doi: 10.1016/j.compbiolchem.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 23.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res . 2010;20:1297–303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res . 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong C, Wei P, Jian X, Gibbs R, Boerwinkle E, Wang K, Liu X. Comparison and integration of deleteriousness prediction methods for nonsynonymous SNVs in whole exome sequencing studies. Hum Mol Genet . 2015;24:2125–37. doi: 10.1093/hmg/ddu733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University Schoool of Medicine. Online Mendelian Inheritance in Man: Aarskog-Scott Syndrome; AAS. OMIM: 305400. Published June 4, 1986. Updated September 22, 2011. Accessed March 23, 2021. https://www.omim.org/entry/305400 .
- 27.Orrico A, Galli L, Falciani M, Bracci M, Cavaliere ML, Rinaldi MM, Musacchio A, Sorrentino V. A mutation in the pleckstrin homology (PH) domain of the FGD1 gene in an Italian family with faciogenital dysplasia (Aarskog-Scott syndrome) FEBS Lett . 2000;478:216–20. doi: 10.1016/s0014-5793(00)01857-3. [DOI] [PubMed] [Google Scholar]
- 28.Blomberg N, Baraldi E, Nilges M, Saraste M. The PH superfold: a structural scaffold for multiple functions. Trends Biochem Sci . 1999;24:441–5. doi: 10.1016/s0968-0004(99)01472-3. [DOI] [PubMed] [Google Scholar]
- 29.Fruman DA, Rameh LE, Cantley LC. Phosphoinositide binding domains: embracing 3-phosphate. Cell . 1999;97:817–20. doi: 10.1016/s0092-8674(00)80792-8. [DOI] [PubMed] [Google Scholar]
- 30.Smith A, Madden J, Hoeppner C. Phase III Randomized Trial of Post-Radiation Chemotherapy in Patients with Newly Diagnosed Ependymoma Ages 1 to 21 years Children's Oncology Group ID: ACNS0831. Published March 29, 2010. Updated January 30, 2018. Accessed May 15, 2021. https://childrensoncologygroup.org/acns0831 .
- 31.Murphy ES, Merchant TE, Wu S, Xiong X, Lukose R, Wright KD, Qaddoumi I, Armstrong GT, Broniscer A, Gajjar A. Necrosis after craniospinal irradiation: results from a prospective series of children with central nervous system embryonal tumors. Int J Radiat Oncol Biol Phys . 2012;83:e655–60. doi: 10.1016/j.ijrobp.2012.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavone P, Marino S, Maniaci A, Cocuzza S. Aarskog-Scott syndrome: clinical and molecular characterisation of a family with the coexistence of a novel FGD1 mutation and 16p13.11-p12.3 microduplication. BMJ Case Rep . 2020;13:e235183. doi: 10.1136/bcr-2020-235183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozawa PM, Ariza CB, Ishibashi CM, Fujita TC, Banin-Hirata BK, Oda JM, Watanabe MA. Role of CXCL12 and CXCR4 in normal cerebellar development and medulloblastoma. Int J Cancer . 2016;138:10–3. doi: 10.1002/ijc.29333. [DOI] [PubMed] [Google Scholar]
- 34.Lau KM, Chan QK, Pang JC, Li KK, Yeung WW, Chung NY, Lui PC, Tam YS, Li HM, Zhou L, Wang Y, Mao Y, Ng HK. Minichromosome maintenance proteins 2, 3 and 7 in medulloblastoma: overexpression and involvement in regulation of cell migration and invasion. Oncogene . 2010;29:5475–89. doi: 10.1038/onc.2010.287. [DOI] [PubMed] [Google Scholar]
- 35.Bhoopathi P, Gondi CS, Gujrati M, Dinh DH, Lakka SS. SPARC mediates Src-induced disruption of actin cytoskeleton via inactivation of small GTPases Rho-Rac-Cdc42. Cell Signal . 2011;23:1978–87. doi: 10.1016/j.cellsig.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koom WS, Ahn SD, Song SY, Lee CG, Moon SH, Chie EK, Jang HS, Oh YT, Lee HS, Keum KC. Nutritional status of patients treated with radiotherapy as determined by subjective global assessment. Radiat Oncol J . 2012;30:132–9. doi: 10.3857/roj.2012.30.3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]