Abstract
The present study examined the association of specific virus infections with acute respiratory tract conditions among hospitalized and outpatient children in a subtropical country. A total of 2,295 virus infections were detected in 6,986 patients between 1997 and 1999, including infections caused by respiratory syncytial virus (RSV) (1.7%), parainfluenza virus (2.0%), influenza B virus (2.6%), adenovirus (4.0%), herpes simplex virus type 1 (4.4%), influenza A virus (5.5%), and enterovirus (12.7%). There were 61 mixed infections, and no consistent seasonal variation was found. One or more viruses were detected among 24.8% of hospitalized patients and 35.0% of outpatients. The frequencies and profiles of detection of various viruses among in- and outpatients were different. The occurrence of enterovirus infections exceeded that of other viral infections detected in 1998 and 1999 due to outbreaks of enterovirus 71 and coxsackievirus A10. RSV was the most prevalent virus detected among hospitalized children, whereas influenza virus was the most frequently isolated virus in the outpatient group. Most respiratory viral infections (39.3%) occurred in children between 1 and 3 years old. RSV (P < 0.025) and influenza A virus (P < 0.05) infections were dominant in the male inpatient group. In addition, most pneumonia and bronchiolitis (48.4%) was caused by RSV among hospitalized children less than 6 months old. Adenovirus was the most common agent associated with pharyngitis and tonsilitis (45.5%). These data expand our understanding of the etiology of acute respiratory tract viral infections among in- and outpatients in a subtropical country and may contribute to the prevention and control of viral respiratory tract infections.
Viruses, including respiratory syncytial virus (RSV), parainfluenza viruses, influenza viruses, adenoviruses, rhinoviruses, and enteroviruses, are a frequent cause of respiratory tract infections in children (2, 6, 11). In hospitalized children, RSV infections occur at greater frequency than other viral infections of the lower respiratory tract (1, 7, 15, 23, 28). Symptoms of RSV infections include coughing, wheezing, hypoxia, bronchiolitis, and pneumonia (18, 21). Recently, several strategies for prophylaxis and treatment of RSV infection have been developed (8, 20, 21). Passive immunization using RSV immunoglobulin and monoclonal antibodies for prevention of RSV disease in premature infants have provided effective forms of prophylactic intervention for high-risk groups (8, 20, 21). A combination of RSV immunoglobulin and the antiviral agent ribavirin given to bone marrow transplant recipients infected with RSV has also been shown to result in higher survival compared to untreated patients (31). Influenza virus is the most frequent cause of acute respiratory illness, which results in local, regional or worldwide epidemics with various degrees of severity each year (16). Although the new antiviral drug zanamivir for influenza A and B and live attenuated intranasal influenza vaccine were shown to be highly effective in clinical trials, continuous surveillance of influenza viruses is still required in order to properly select the vaccine strains (4). Worldwide, enteroviruses appear to account for 2 to 15% of upper respiratory tract viral infections (3). In addition, enteroviruses have been associated with a number of disease manifestations, such as common cold, herpangina, hand-foot-and-mouth disease, exanthema, conjunctivitis, myocarditis, generalized infections of newborns, and involvement of the central nervous system ranging from mild meningeal symptoms to fatal cases of encephalitis and paralysis (3).
The relationship between clinical symptoms and respiratory infections has been frequently discussed in the literature, but there are still conflicting results with influenza virus and RSV (9). Viral detection provides more specific information on the correlation of clinical symptoms with specific infections. The epidemiological data and clinical symptoms observed in infected patients will also help clinicians and researchers involved in prevention and control of respiratory tract viral infections (19). Causes of respiratory tract infections in infants and children are diverse. Most of the reports are based on studies of severely ill hospitalized children (2, 11, 25, 32, 34) or acute respiratory infections in community surveillance studies (6, 10, 16, 27). The present study was designed to examine comprehensively within a defined population the role of respiratory virus infections in an acute respiratory condition. In order to investigate the etiology of viral infections, specimens from hospitalized patients and outpatients with respiratory symptoms were collected and viral isolations were performed. Viral epidemiological data and seasonal variations were analyzed. In addition, the correlation of viruses and clinical syndromes was investigated. These data may provide information on the etiology and management of respiratory tract viral infection.
MATERIALS AND METHODS
Cases. (i) Inpatients.
National Cheng Kung University Hospital (NCKUH) and seven related hospitals located in southern Taiwan were included in this study. A total of 2,077 pediatric patients (≤12 years old) who were hospitalized for respiratory tract infections during the study period from January 1997 to December 1999 were investigated. On admission, specimens for viral culture were usually drawn by pediatric residents. On the following day the study physician reviewed all data relating to patient hospitalizations (i.e., disease onset, signs and symptoms, and risk factors) and collected the remaining clinical specimens for virological examination.
(ii) Outpatients.
Outpatients were from the outpatient department at NCKUH and 10 other local clinics in southern Taiwan. A total of 4,909 pediatric patients were included in the study. During the outpatient visit, information on clinical presentations, symptoms and signs, time of onset of symptoms, and patient demographics was collected and specimens for virological examination were obtained.
Definition of respiratory infections.
The respiratory tract infections are divided into upper and lower respiratory tract infections. The symptoms of upper respiratory tract infections include cough, sore throat, tonsillitis, pharyngitis, and herpangina. Pneumonia and bronchiolitis were considered lower respiratory tract infections. Infected patients with one or more of these syndromes were included in this study.
Collection and transport of specimens.
Throat swabs or nasopharyngeal aspirates were obtained from hospitalized patients and outpatients with symptoms of respiratory infections. Throat swabs were collected into transport medium containing 2 ml of Eagle's minimum essential medium (EMEM) (pH 7.2) with gelatin (5 mg/liter), penicillin (400 U/liter), streptomycin (400 μg/liter), gentamicin (50 μg/liter) and amphotericin B (Fungizone) (1.25 μg/liter). Specimens were placed on ice and transported to the NCKUH virology laboratory within 24 h after collection.
Virus isolation and identification.
Respiratory specimens were inoculated onto appropriate tissue cultures (Madin-Darby canine kidney [MDCK], Vero, A549, rabdomyosarcoma [RD], and green monkey kidney [GMK] cells) to isolate influenza virus, parainfluenza virus, adenovirus, RSV, enterovirus, and herpesvirus. Cells were cultured in EMEM (supplemented with 10% fetal bovine serum, penicillin [100 U/ml], streptomycin [100 μg/ml], and amphotericin B [0.25 μg/ml]) and incubated at 35°C with 5% CO2. Each culture tube was inoculated with 0.2 ml of clinical specimen and incubated for 1 h to allow for adsorption, and then viral growth medium was added. The viral growth medium for Vero, A549, RD, and GMK cells was EMEM containing 2% fetal bovine serum and antibiotics; that for MDCK cells was serum-free EMEM with 2 μg of bovine pancreatic crystalline trypsin per ml to promote growth of influenza virus. These culture tubes were incubated at 35°C and examined for cytopathic effect daily for 10 to 14 days. When typical virus-induced cytopathic effect (for adenovirus, enterovirus, herpesvirus, or RSV) was observed, viral identification was done by immunofluorescent staining with virus-specific monoclonal antibody (Chemicon International Inc.). Hemadsorption and hemagglutination were done on MDCK cell cultures to detect hemagglutinin-containing viruses, such as influenza virus and parainfluenza virus, and final identification was performed by using a screening kit for respiratory viruses (Chemicon International Inc.). Influenza virus isolates were also subtyped by hemagglutination inhibition assay using the World Health Organization influenza reagent kit, kindly provided by Centers for Disease Control and Prevention, Atlanta, Ga. Enterovirus strains were typed antigenically by neutralization tests using Lim and Benyesh-Melnick pools and type-specific antiserum or immunofluorescence tests using available monoclonal antibodies (Chemicon International Inc.) (13).
Statistical analysis.
The data in this study were analyzed by the chi-square test; a P value of <0.05 was significant.
RESULTS
Association of virus infections among in- and outpatients.
A total of 6,986 pediatric patients were investigated over the time period studied; 4,909 (70%) were from outpatient clinics (Table 1). Detection of viral infections was carried out by cell culture and immunofluorescent staining. Viral infections were detected in 2,234 of the 6,986 patients analyzed (32.0%), with a higher proportion in the outpatient group (35.0%; 1,719 of 4,909) than in the inpatient group (24.8%; 515 of 2077). A total of 2,295 viruses were isolated, including 384 influenza A virus isolates, 181 influenza B virus isolates, 53 parainfluenza virus 1 isolates, 22 parainfluenza virus 2 isolates, 62 parainfluenza virus 3 isolates, 277 adenovirus isolates, 889 enterovirus isolates, 120 RSV isolates, and 307 herpes simplex virus type 1 (HSV-1) isolates. Sixty-one patients (0.9%) had more than one virus infection detected. The most frequent combinations were adenovirus or HSV-1 plus enterovirus (21 cases; 34.4%) and enterovirus or HSV-1 plus influenza virus (13 cases; 21.3%). Infections with enterovirus (213; 10.3%), RSV (92; 4.4%), adenovirus (69; 3.3%), and HSV-1 (63; 3.0%) were the most commonly detected in the hospitalized children, comprising about 83.3% of all virus infections. In contrast, enterovirus (676; 13.8%), influenza A virus (334; 6.8%), HSV-1 (244; 5.0%), and adenovirus (208; 4.2%) accounted for the majority of viral infections (87.8%) in the outpatient group. In 1997, RSV infection (6.8%) was predominant in the hospitalized patient group, while influenza A and B viruses (16.4%) were more prevalent in the outpatient group (Table 1). In contrast, during 1998 and 1999, the majority of infections in both groups were caused by enteroviruses, due to an epidemic of enterovirus 71 and coxsackievirus A10.
TABLE 1.
Comparison of positive viral isolations in inpatients and outpatients
| Virus | No. (%) of positive isolations
|
Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Inpatients
|
Outpatients
|
||||||||
| 1997 | 1998 | 1999 | Total | 1997 | 1998 | 1999 | Total | ||
| Influenza A virus | 3 (0.9) | 1 (0.1) | 46 (4.8) | 50 (2.4) | 88 (9.7) | 73 (4.3) | 173 (7.5) | 334 (6.8) | 384 (5.5) |
| Influenza B virus | 0 | 3 (0.4) | 21 (2.2) | 24 (1.2) | 61 (6.7) | 6 (0.4) | 90 (3.9) | 157 (3.2) | 181 (2.6) |
| Parainfluenza virus 1 | 2 (0.6) | 0 | 1 (0.1) | 3 (0.1) | 14 (1.5) | 15 (0.9) | 21 (0.9) | 50 (1.0) | 53 (0.8) |
| Parainfluenza virus 2 | 0 | 3 (0.4) | 0 | 3 (0.1) | 0 | 16 (0.9) | 3 (0.1) | 19 (0.4) | 22 (0.3) |
| Parainfluenza virus 3 | 2 (0.6) | 2 (0.3) | 2 (0.2) | 6 (0.3) | 10 (1.1) | 22 (1.3) | 24 (1.0) | 56 (1.1) | 62 (0.9) |
| Adenovirus | 14 (4.1) | 17 (2.2) | 38 (4.0) | 69 (3.3) | 61 (6.7) | 64 (3.8) | 83 (3.6) | 208 (4.2) | 277 (4.0) |
| RSV | 23 (6.8) | 36 (4.6) | 33 (3.5) | 92 (4.4) | 2 (0.2) | 15 (0.9) | 11 (0.5) | 28 (0.6) | 120 (1.7) |
| Enterovirus | 9 (2.6) | 99 (12.6) | 105 (11.0) | 213 (10.3) | 44 (4.8) | 154 (9.1) | 478 (20.8) | 676 (13.8) | 889 (12.7) |
| HSV-1 | 4 (1.2) | 30 (3.8) | 29 (3.0) | 63 (3.0) | 17 (1.9) | 110 (6.5) | 117 (5.1) | 244 (5.0) | 307 (4.4) |
| Total no. positive/ no. studied (% positive) | 56/340 (16.8) | 188/786 (23.9) | 271/951 (28.4) | 515/2,077 (24.8) | 287/910 (31.5) | 460/1,699 (27.1) | 972/2,300 (42.3) | 1,719/4,909 (35.0) | 2,234a/6,986 (32.0) |
Among 2,234 patients, 61 patients (8 inpatients and 53 outpatients) had multiple viral infections.
Seasonal distribution of viral infections.
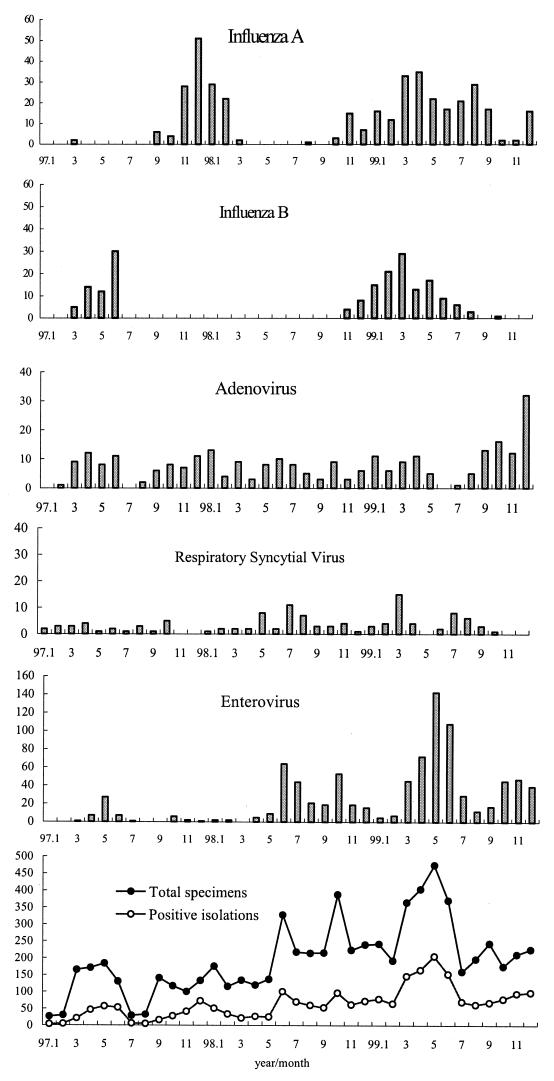
The monthly distribution of respiratory tract viruses is shown in Fig. 1. In Taiwan, the four seasons were generally recognized as spring (March to May), summer (June to August), fall (September to November), and winter (December to February). The average temperatures during spring, summer, fall, and winter in the studied area from 1997 to 1999 were 25, 32, 28, and 20°C, respectively. Respiratory viral agents usually have characteristic seasonal patterns in temperate and tropical climates. Although infections with influenza A and B viruses predominate in winter seasons in temperate climates, influenza A viruses were observed in the spring, summer, and early fall of 1999 in Taiwan. Similarly, influenza B viruses were also seen in the spring and early summer of 1997 and 1999 in this study. During the 3-year study period, there were two outbreaks of influenza A virus (H3N2); one (123 cases) occurred from November 1997 to February 1998, and the other (222 cases) was from November 1998 to September 1999 (11 months). Interestingly, a switch to influenza A virus subtype H1N1 (123 cases) occurred from December 1999 to February 2000, with peak activity in January 2000 (data not shown). There were also two peaks of influenza B virus outbreaks observed in this period; one occurred in 1997 from March to June (B/Beijing/184/93-like subtype, 40%; B/Guangdong/8/93 like subtype, 48%), and the other was from November 1998 to August 1999 (B/Beijing/184/93-like subtype, 97.2%). Adenovirus was detected throughout the year; however, an outbreak was identified in the winter of 1999. Although RSV infections occur predominantly in the winter in temperate climates and are associated with rainfall in tropical climates, RSV was recovered in Taiwan in all four seasons without significant seasonality over the period of analysis. Enterovirus infections were dominant in the summer, with two peaks observed each year of the study; the first peak was in May to June, and the second peak was between October and November. Coxsackievirus A9 represented 20.8% of positive enterovirus isolates in 1997. A dramatic increase in enterovirus isolations was due to an epidemic of enteroviruses 71 in 1998 and an outbreak of coxsackieviruses A10 in 1999. Two peaks of enterovirus 71 infections were seen in Taiwan, one in June and the other in October of 1998 (12, 17, 29, 33). Coxsackieviruses A10 with herpangina represented 23.9% of enterovirus infections in 1999, and the major peak was in May. HSV-1 was found year round without a particular seasonality (data not shown). Parainfluenza viruses 1, 2, and 3 were detected sporadically in small numbers during the entire study period (data not shown).
FIG. 1.
Seasonal distribution of viral infections.
Age and sex distributions of virus-infected children.
The age distribution of patients at the time of hospital admission is presented in Table 2. The highest incidence of viral infections was in children between 1 and 3 years old. Influenza virus infections were detected in 384 persons distributed among all age groups. Children between 1 and 6 years of age (53.6%) were the most common age group affected by influenza A virus among the inpatient (62%) and outpatient (52.4%) groups. Influenza B virus was mostly detected in children between 6 and 10 years old among both the inpatient (37.5%) and outpatient (36.3%) groups. Parainfluenza virus infections accounted for 137 infections in both groups. More than 56% of the parainfluenza virus infections of children younger than 3 years were type 3. Adenovirus infections were more common in 1- to 3-year-olds in both groups. Of 92 RSV infections in hospitalized children, 45 (48.9%) of the children were less than 6 months of age. However, RSV infections were dominant in 1- to 3-year-olds in the outpatient group. Enteroviruses and herpesviruses were detected in all age distributions, but mainly in 1- to 3-year-olds in both the in- and outpatient groups. The overall male-to-female ratio for patients with viral infection was 1.4 to 1. Males with RSV and influenza A virus infections were dominant in the inpatient group (RSV, P < 0.025; influenza A virus, P < 0.05).
TABLE 2.
Age and sex distributions of virus-infected children
| Virus | Inpatients
|
Outpatients
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) of isolations from patients of age:
|
Male/female ratioa | No. (%) of isolations from patients of age:
|
Male/female ratiob | |||||||||||||||
| <6 mo | 6 mo–1 yr | 1–3 yr | 3–6 yr | 6–10 yr | >10 yr | Unknown | Total | <6 mo | 6 mo–1 yr | 1–3 yr | 3–6 yr | 6–10 yr | >10 yr | Unknown | Total | |||
| Influenza A virus | 3 (6.0) | 9 (18.0) | 21 (42.0) | 10 (20.0) | 4 (8.0) | 1 (2.0) | 2 | 50 | 2.7c | 13 (3.9) | 20 (6.0) | 81 (24.3) | 94 (28.1) | 50 (14.9) | 28 (8.3) | 48 | 334 | 1.2 |
| Influenza B virus | 0 | 0 | 7 (29.2) | 8 (33.3) | 9 (37.5) | 0 | 0 | 24 | 1.4 | 0 | 1 (0.6) | 16 (10.2) | 42 (26.8) | 57 (36.3) | 13 (8.3) | 28 | 157 | 1.4 |
| Parainfluenza virus 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 3 | 3.0 | 4 (8.0) | 1 (2.0) | 21 (40.3) | 21 (40.3) | 3 (6.0) | 0 | 0 | 50 | 1.6 |
| Parainfluenza virus 2 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 3 | 2 | 1 | 1 | 7 | 7 | 1 | 0 | 2 | 19 | 0.4 |
| Parainfluenza virus 3 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 6 | 1 | 2 (3.6) | 10 (17.9) | 30 (53.6) | 10 (17.9) | 1 (1.8) | 0 | 3 | 56 | 1.4 |
| Adenovirus | 4 (5.8) | 6 (8.7) | 28 (40.6) | 20 (29.0) | 4 (5.8) | 2 (2.9) | 5 | 69 | 2.1 | 4 (1.9) | 20 (9.6) | 77 (37.0) | 60 (28.8) | 25 (12.0) | 3 (1.4) | 19 | 208 | 1.5 |
| RSV | 45 (48.9) | 22 (23.9) | 21 (22.8) | 4 (4.3) | 0 | 0 | 0 | 92 | 2.8d | 2 (8.3) | 3 (10.7) | 15 (53.6) | 4 (14.3) | 3 (10.7) | 0 | 1 | 28 | 1 |
| Enterovirus | 45 (21.1) | 31 (14.6) | 81 (38.0) | 36 (16.9) | 13 (6.1) | 2 (0.9) | 5 | 213 | 1.4 | 14 (2.0) | 56 (8.3) | 322 (47.6) | 178 (26.3) | 52 (7.7) | 15 (2.2) | 39 | 676 | 1.5 |
| HSV-1 | 1 (1.6) | 2 (3.2) | 35 (55.6) | 9 (14.3) | 8 (12.7) | 5 (7.9) | 5 | 63 | 1.1 | 2 (0.8) | 16 (6.6) | 135 (55.3) | 45 (18.4) | 27 (11.7) | 7 (2.9) | 12 | 244 | 1.1 |
| No. (%) positive | 98 (18.7) | 72 (13.8) | 197 (37.7) | 90 (17.2) | 38 (7.3) | 10 (1.9) | 18 (3.4) | 523 | 42 (2.4) | 128 (7.2) | 704 (39.7) | 461 (26.0) | 219 (12.4) | 66 (3.7) | 152 (8.6) | 1,772 | ||
Mean, 1.77.
Mean, 1.3.
P < 0.05.
P < 0.025.
Clinical symptoms associated with viral agents.
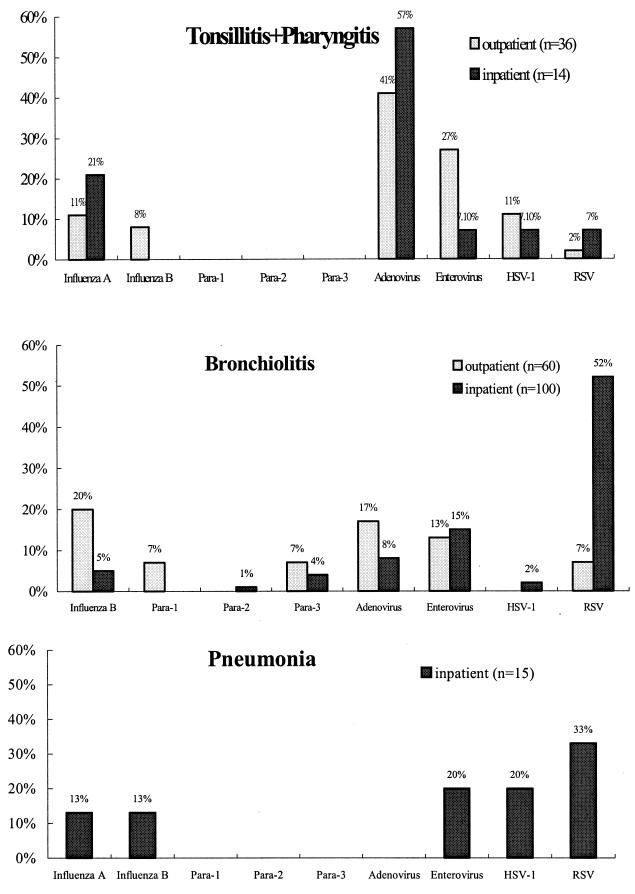
The association of tonsillitis-pharyngitis, bronchiolitis, and pneumonia with individual viral agents is shown in Fig. 2. RSV was the most common agent associated with bronchiolitis and pneumonia in the low respiratory tract infections, whereas pharyngitis and tonsillitis of the upper respiratory tract were caused predominately by adenovirus infections. In the inpatient group, pneumonia was mostly caused by RSV infection. However, only a few cases of pneumonia were found within the outpatient group (data not shown).
FIG. 2.
Clinical symptoms associated with viral agents. Para-1, parainfluenza virus 1.
DISCUSSION
This study analyzed the occurrence of various viral respiratory infections among hospitalized patients and outpatients. The frequency of virus detection was higher for the outpatient group than the inpatient group, suggesting that more outpatients were in the acute phase when the specimens were taken. The agents most frequently detected in the hospitalized children were enterovirus (10.3%), RSV (4.4%), adenovirus (3.3%), and HSV-1 (3.0%). In contrast, enterovirus (13.8%), influenza A virus (6.8%), HSV-1 (5.0%), and adenovirus (4.2%) were identified mostly in the outpatient group, indicating that the profile of virus detection is different among in- and outpatient groups.
Table 3 summarizes the recent studies of respiratory infections in different countries. Viruses were detected in 32.9% of our clinical cases of acute respiratory infection in children under 12 years old. This rate is likely to be underestimated, since only culture results were employed; however, our detection rate was within the range of 22.0 to 51.9% found by other workers (Table 3). In most studies, RSV is the leading cause of lower respiratory tract infection in children (7, 15, 28). This study may have underestimated the rate of detection of RSV infection, since only the culture method was used and the rate would increase if detection of antigen was included. It was less frequently isolated in the outpatient population during this study period, but in hospitalized patients, it was the major cause of bronchopneumonia and was also the most frequently isolated agent except during the enterovirus outbreaks in 1998 and 1999.
TABLE 3.
Studies of respiratory infections in various countries
| % of respiratory infections caused bya:
|
Total detection rate (no.) | Mixed infection rate | Total no. of patients or specimens (% in- or outpatients) | Age (yr) | Symptomsb | Study site | Method usedc | Study period | Reference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inf-A | Inf-B | Para-1 | Para-2 | Para-3 | ADV | RSV | Rhino | Corona | EV | HSV | Others | |||||||||
| 14.5d | 3.7e | 5.0 | 3.3 | 11.1 | 4.7 | 3.3 | 2.6 | 6.3 | 49.8 (148) | 5.1 | 297 (100% outpatients) | <14 | LRI | United States | Culture | 1975–1976 | 10 | |||
| 5.2 | 2.1 | 5.3f | 5.2 | 4.5 | 3.0 | 0.6 | 2.2 | 9.2 | 37.4 (2,051) | 5.5 | 5,474 (100% outpatients) | 0–>25 | ARI | Czechoslavakia | Ab, Culture | 1976–1986 | 27 | |||
| 15.9 | 5.8 | 4.2 | 1.0 | 3.6 | 3.9 | 7.1 | 5.1 | 1.6 | 22.7 | 51.9 (198) | 312 (58.0% outpatients) | <5 | LRI | Philippines | Ag, Ab, Culture | 1984 | 22 | |||
| 2.0d | 5.8f | 3.6 | 16.7 | 5.7 | 2.6 | 35 (386) | 1,052 (100% outpatients) | <5 | ARI | Brazil | Ag, Culture | 1984–1986 | 5 | |||||||
| 2.8 | 1.3 | 3.9 | 9.0 | 6.7 | 20.3 | 4.3 | 45 (268) | 3.6 | 596 (52.8% outpatients) | <5 | LRI | Thailand | Ag, Ab, Culture | 1986–1987 | 26 | |||||
| 4.0 | 2.0 | 2.0 | 3.0 | 3.0 | 5.0 | 3.0 | 22.0 (164) | 736 (both groups) | <5 | ARI | India | Ag, Culture | 1986–1989 | 14 | ||||||
| 0.6 | 0.4 | 38 | 0.8 | 41.3 (292) | 0.8 | 707 (39.1% outpatients) | <5 | ARI | Brazil | Ag, Culture | 1987–1989 | 25 | ||||||||
| 3.9 | 1.4 | 1.7 | 0.5 | 7.8 | 3.9 | 27.2 | 1.0 | 45.9 (369) | 1.5 | 804 (20% outpatients) | <12 (most) | LRI | Korea | Ag, Culture | 1990–1994 | 34 | ||||
| 2.7 | 0.7 | 1.0 | 0.2 | 2.1 | 1.6 | 22.8 | 0.1 | 0.2 | 29.0 (3,904) | 12,354 (29% outpatients) | 0–≥20 | RI | Singapore | Ag, Ab, Culture | 1990–1994 | 2 | ||||
| 7.2 | 0.1 | 1.9 | 2.4 | 3.2 | 1.1 | 10.3 | 4.2 | 2.5 | 1.7 | 4.0 | 0.07 | 35.6 (366) | 1,029 (100% inpatients) | 0–≥65 | ARI | United States | Ab, Culture | 1991–1995 | 11 | |
| 6.6 | 4.9 | 0.3f | 2.3 | 11.2 | 3.6 | 6.7 | 0.5 | 36.1 (348) | 1.7 | 962 (100% outpatients) | 0–65 | Flu-like | France | Ag, Culture | 1994–1995 | 16 | ||||
| 5.5 | 2.6 | 0.8 | 0.3 | 0.9 | 4.0 | 1.7 | 12.7 | 4.4 | 32.9 (2,295) | 0.9 | 6,986 (70.3% outpatients) | <12 | ARI | Taiwan | Culture | 1997–1999 | This study | |||
Inf-A, influenza A virus; Inf-B, influenza B virus; Para-1, parainfluenza virus 1, Para-2, parainfluenza virus 2; Para-3, parainfluenza virus 3; ADV, adenovirus; Rhino, rhinovirus; Corona, coronavirus; EV, enterovirus; others, mixed infections with viruses or bacteria.
LRI, lower respiratory tract infection; ARI, acute respiratory tract infection; RI, respiratory tract infection.
Ab, detection of antibody; Ag, detection of antigen.
Value applies to both influenza A virus and influenza B virus.
Value applies to parainfluenza viruses 1 and 2.
Value applies to parainfluenza viruses 1, 2, and 3.
In contrast to other reports (6, 10), influenza A virus infections accounted for only 2.4% of respiratory tract infections among the inpatient group, and influenza viruses were not a predominant cause of pneumonia among hospitalized patients in this study. However, the rate of detection of influenza A virus (6.8%) is second only to that of enterovirus in the outpatient group. Two outbreaks of each influenza A and B were observed in this 3-year study. Most influenza A viruses isolated (98.2%) were of the H3N2 subtype between 1997 and September 1999. A change of serotype prevalence to H1N1 occurred from December 1999 to February 2000. An outbreak of mixed infections of influenza B virus (B/Beijing/184/93-like subtype, 40%; B/Guangdong/8/93 like subtype, 48%) was seen from March to June 1997; in contrast, most isolates (97.2%) from the other outbreak from November 1998 to August 1999 were of the B/Beijing/184/93-like subtype. It was unexpected that influenza A virus infections continued throughout the 11-month study period. This was considerably longer than the 4 to 6 weeks usually seen in temperate climates, suggesting a prolonged circulation of influenza A virus in the area for which seasonal change is not evident. Although the influenza epidemic did not caused a high hospitalization rate during the study period, continuous observation of outpatient epidemics will provide the right choice of prevention and control of the outbreak of diseases.
The incidence of enterovirus in this study is much higher than those in other studies. Although some studies have recorded rates ranging from 0 to 5.7% (Table 3), these rates are still much lower than our findings (12.7%). In 1997, the dominant enterovirus was coxsackievirus A9; however, in 1998, due to an outbreak, enterovirus 71 infections resulted in 46% of enterovirus-positive cases in inpatients (29). Many of these cases were associated with symptoms of respiratory tract infections, with some having complications involving the central nervous system. In 1999, an outbreak of coxsackievirus A10 also contributed to a large number of enteroviruses being isolated. The characteristics of the enteroviruses obtained during the current study period suggest a significant type-specific variability in enterovirus outbreaks from year to year. Isolation of HSV-1 is somewhat higher than isolation of other viruses; however, the significance is not known. Adenovirus accounted for 4.0% of respiratory infections, and a similar rate was found in other reports. Parainfluenza virus was responsible for only 2.0% of cases, and the proportion of type 3 was higher than that of type 1. No rhinovirus was recovered; the likely explanation is that the culture conditions were not appropriate for rhinovirus because human diploid fibroblast cells, which are known to be more sensitive for rhinoviruses, were not used in the study period.
Respiratory tract infections are common in young children, decrease in frequency with age, and predominate in boys (1, 6, 34). This male predominance is reflected in the present study, which demonstrates a higher incidence of respiratory tract infection in boys. The results of this study are similar to the report of Denny et al. (6) in terms of the sex distribution of respiratory tract infection but not in terms of the age distribution, in which the influenza viruses occurred equally in all age group. The age distribution of RSV in developing countries appears to be similar to that in developed countries (30). Sixty percent of RSV infections were in patients under 1 year of age in this study. This report also described the seasonal variation of respiratory viral infections in Taiwan. The relative frequency of each viral agent and the pattern of occurrence in this study are not similar to those described in previous reports in countries with temperate and tropical climates (22, 26). In temperate climates, the majority of isolations of influenza virus and RSV are in the winter and enterovirus infections occur more frequently during the summer and autumn. In contrast, this study showed less clear-cut seasonality of influenza virus, RSV, and enterovirus infections. The possible reason for this is that Taiwan is situated on an island with a subtropical climate, where the difference in average temperature between two seasons is only about 8°C and the effect of rainfall is not so evident. Outbreaks of influenza virus and enterovirus lasted as long as 11 months in this study. Neither temperature nor rainfall appears to be the main determinant of the timing of those outbreaks. RSV appears in distinct outbreaks in temperate climates, and the seasonal pattern of RSV in our study is also different from those in the reports from Singapore and Hong Kong, which showed that in tropical climates, the incidence of RSV infections appears to be associated with seasonal rainfall rather than cold weather (2, 24, 30).
Infections with respiratory viruses are a common cause of morbidity and mortality around the world. A better understanding of the epidemiology of respiratory viral infections may be used for timely, specific antiviral therapy, prophylaxis, and vaccination. This study described the epidemiology and clinical presentations of specific respiratory viral infections in hospitalized patients and outpatients in southern Taiwan from 1997 to 1999. With the development of effective antiviral therapy for many respiratory viruses, this study may provide valuable information for better management of patients suffering from respiratory tract infections in the subtropical climate.
ACKNOWLEDGMENTS
The study was supported by the grants NHRI-CN-CR8801 from the National Health Research Institutes and DOH89-TD-1134C from the Department of Health, Taiwan.
We thank David Kiang for critically reviewing the manuscript.
REFERENCES
- 1.Åkerlind B, Norrby E. Occurrence of respiratory syncytial virus subtypes A and B strains in Sweden. J Med Virol. 1986;19:241–247. doi: 10.1002/jmv.1890190306. [DOI] [PubMed] [Google Scholar]
- 2.Chew F T, Doraisingham S, Ling A E, Kumarasinghe G, Lee B W. Seasonal trend of viral respiratory tract infections in the tropics. Epidemiol Infect. 1998;121:121–128. doi: 10.1017/s0950268898008905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chonmaitree T, Mann L. Respiratory infections. In: Rotbart H A, editor. Human enterovirus infections. Washington, D.C.: American Society for Microbiology; 1995. pp. 255–270. [Google Scholar]
- 4.Cox N J, Subbarao K. Influenza. Lancet. 1999;354:1277–1282. doi: 10.1016/S0140-6736(99)01241-6. [DOI] [PubMed] [Google Scholar]
- 5.de Arruda E, N, Hayden F G, McAuliffe J F, Auxiliadora. de Sousa M, Mota S B, McAauliffe M I, Geist F C, Carvalho E P, Fernandes M C, Guerrant R L, Gwaltney J M., Jr Acute respiratory viral infections in ambulatory children of urban northeast Brazil. J Infect Dis. 1991;164:252–258. doi: 10.1093/infdis/164.2.252. [DOI] [PubMed] [Google Scholar]
- 6.Denny F W, Clyde W A., Jr Acute lower respiratory tract infections in nonhospitalized children. J Pediatr. 1986;108:635–646. doi: 10.1016/s0022-3476(86)81034-4. [DOI] [PubMed] [Google Scholar]
- 7.De Sliva L M, Hanlon M G. Respiratory syncytial viruses: a report of a 5-year study at a children's hospital. J Med Virol. 1986;19:299–305. doi: 10.1002/jmv.1890190402. [DOI] [PubMed] [Google Scholar]
- 8.Englund J A. Prevention strategies for respiratory syncytial virus: passive and active immunization. J Pediatr. 1999;135:S38–S44. [PubMed] [Google Scholar]
- 9.Fleming D M, Cross K W. Respiratory syncytial or influenza virus? Lancet. 1993;342:1507–1510. doi: 10.1016/s0140-6736(05)80082-0. [DOI] [PubMed] [Google Scholar]
- 10.Glezen W P, Paredes A, Taber L H. Influenza in children. JAMA. 1980;243:1345–1349. doi: 10.1001/jama.243.13.1345. [DOI] [PubMed] [Google Scholar]
- 11.Glezen W P, Greenberg S B, Atmar R L, Piedra P A, Couch R B. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA. 2000;283:499–505. doi: 10.1001/jama.283.4.499. [DOI] [PubMed] [Google Scholar]
- 12.Ho M, Chen E R, Hsu K H, Twu S J, Chen K T, Tsai S F, Wang J R, Shih S R. An epidemic of enterovirus 71 infection in Taiwan. N Engl J Med. 1999;341:929–935. doi: 10.1056/NEJM199909233411301. [DOI] [PubMed] [Google Scholar]
- 13.Hsiung G D. Picornaviridae. In: Hsiung G D, Fong C K Y, Landry M L, editors. Hsiung's diagnostic virology. 4th ed. New Haven, Conn: Yale University Press; 1994. pp. 119–140. [Google Scholar]
- 14.Jain A, Pande A, Misra P K, Mathur A, Chatuvedi U C. An Indian hospital study of viral causes of acute respiratory infection in children. J Med Microbiol. 1991;35:219–223. doi: 10.1099/00222615-35-4-219. [DOI] [PubMed] [Google Scholar]
- 15.Krasinski K. Severe respiratory syncytial virus infection: clinical features, nosocomial acquisition and outcome. Pediatr Infect Dis J. 1985;4:250–257. doi: 10.1097/00006454-198505000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Lina B, Valette M, Foray S, Luciani J, Stagnara J, See D M, Aymard M. Surveillance of community-acquired viral infections due to respiratory viruses in Rhone-Alpes (France) during winter 1994 to 1995. J Clin Microbiol. 1996;34:3007–3011. doi: 10.1128/jcm.34.12.3007-3011.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu C C, Tseng H W, Wang S M, Wang J R, Su I J. An outbreak of enterovirus 71 in Taiwan, 1998: epidemiology and clinical manifestations. J Clin Virol. 2000;17:23–30. doi: 10.1016/s1386-6532(00)00068-8. [DOI] [PubMed] [Google Scholar]
- 18.McBride J T. Pulmonary function changes in children after respiratory syncytial virus infection in infancy. J Pediatr. 1999;135:S28–S32. [PubMed] [Google Scholar]
- 19.McIntosh K, Halonen P, Ruuskanen O. Report of a workshop on respiratory viral infections: epidemiology, diagnosis, treatment, and prevention. Clin Infect Dis. 1993;16:151–164. doi: 10.1093/clinids/16.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prober C G, Sullender W M. Advances in prevention of respiratory syncytial virus infections. J Pediatr. 1999;135:546–558. doi: 10.1016/s0022-3476(99)70051-x. [DOI] [PubMed] [Google Scholar]
- 21.Robinson R F, Nahata M C. Respiratory syncytial virus (RSV) immune globulin and palivizumab for prevention of RSV infection. Am J Health Syst Pharm. 2000;57:259–266. doi: 10.1093/ajhp/57.3.259. [DOI] [PubMed] [Google Scholar]
- 22.Ruutu P, Halonen P, Meurman O, Torres C, Paladin F, Yamaoka K, Tupasi T E. Viral lower respiratory tract infections in Filipino children. J Infect Dis. 1990;161:175–179. doi: 10.1093/infdis/161.2.175. [DOI] [PubMed] [Google Scholar]
- 23.Shay D K, Holman R C, Newman R D, Liu L L, Stout J W, Anderson L J. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 24.Sung R Y T, Murray H G S, Chan R C K, Davies D P, French G L. Seasonal patterns of respiratory syncytial virus infection in Hong Kong: a preliminary report. J Infect Dis. 1987;156:527–528. doi: 10.1093/infdis/156.3.527. [DOI] [PubMed] [Google Scholar]
- 25.Sutomöller F, Ferro Z P A, Asensi M D, Ferreira V, Mazzei I S, Cunha B L. Etiology of acute respiratory tract infections among in a combined community and hospital study in Rio de Janeiro. Clin Infect Dis. 1995;20:854–860. doi: 10.1093/clinids/20.4.854. [DOI] [PubMed] [Google Scholar]
- 26.Suwanjutha S, Chantarojanasiri T, Watthana-kasetr S, Sirinavin S, Ruangkanchanasetr S, Hotrakitya S, Wasi C, Puthavathana P. A study of nonbacterial agents of acute lower respiratory tract infection in Thai children. Rev Infect Dis. 1990;12:S923–S928. doi: 10.1093/clinids/12.supplement_8.s923. [DOI] [PubMed] [Google Scholar]
- 27.Tumova B, Heinz F, Syrucek L, Bruckova M, Fedova D, Kunzova L, Strizova V. Occurrence and aetiology of acute respiratory diseases: results of a longterm surveillance programme. Acta Virol. 1988;33:50–62. [PubMed] [Google Scholar]
- 28.Uduman S A, Ijaz M K, Kochiyil J, Mathew T, Hossam M K. Respiratory syncytial virus infection among hospitalized young children with acute lower respiratory illness in A1 AIN, UAE. J Commun Dis. 1996;28:245–252. [PubMed] [Google Scholar]
- 29.Wang J R, Tsai H P, Chen P F, Lai Y J, Yan J J, Kiang D, Lin K H, Liu C C, Su I J. An outbreak of enterovirus 71 in Taiwan, 1998: laboratory diagnosis and genetic analysis. J Clin Virol. 2000;17:91–100. doi: 10.1016/s1386-6532(00)00079-2. [DOI] [PubMed] [Google Scholar]
- 30.Weber M W, Mulholland E K, Greenwood B M. Respiratory syncytial virus infection in tropical and developing countries. Trop Med Int Health. 1998;3:268–280. doi: 10.1046/j.1365-3156.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- 31.Whimbey E, Champlin R E, Englund J A, Mirza N Q, Piedra P A, Goodrich J M, Przepiorka D, Luna M A, Morice R C, Neumann J L, Elting L S, Bodey G P. Combination therapy with aerosolized ribavirin and intravenous immunoglobulin for respiratory syncytial virus disease in adult bone marrow transplant recipients. Bone Marrow Transplant. 1995;16:393–399. [PubMed] [Google Scholar]
- 32.Winter G F, Hallam N F, Hargreaves F D, Molyneaux P J, Burns S M, Inglis J M. Respiratory viruses in a hospitalized pediatric population in Edinburgh 1985–1994. J Infect. 1996;33:207–211. doi: 10.1016/s0163-4453(96)92297-5. [DOI] [PubMed] [Google Scholar]
- 33.Yan J J, Wang J R, Liu C C, Yang H B, Su I J. An outbreak of enterovirus 71 in Taiwan, 1998: a comprehensive pathologic, virologic and molecular study on a case of fulminant encephalitis. J Clin Virol. 2000;17:13–22. doi: 10.1016/s1386-6532(00)00067-6. [DOI] [PubMed] [Google Scholar]
- 34.Yun B-Y, Kim M-R, Park J-Y, Choi E-H, Lee H-J, Yun C-K. Viral etiology and epidemiology of acute lower respiratory tract infections in Korean children. Pediatr Infect Dis J. 1995;14:1054–1059. doi: 10.1097/00006454-199512000-00005. [DOI] [PubMed] [Google Scholar]