Abstract
Background
Autism spectrum disorders (ASDs) are a wide range of behavioural disabilities for which there are no definite interventional modalities available. Remedial therapies remain the only option but with varying outcomes. We have evaluated the Childhood Autism Rating Scale (CARS) and alpha-synuclein levels in this parallel-group, multiple-arm pilot clinical study after supplementation with a biological response modifier beta-glucan food supplement (Nichi Glucan).
Methods
Six subjects with ASD (n=6) Gr. 1 underwent conventional treatment comprising remedial behavioural therapies and L-carnosine 500 mg per day, and 12 subjects (n=12) Gr. 2 underwent supplementation with the Nichi Glucan 0.5 g two times per day along with the conventional treatment.
Results
There was a significant decrease in the CARS score in all of the children of the Nichi Glucan Gr.2 compared with the control (p=0.034517). Plasma levels of alpha-synuclein were significantly higher in Gr. 2 (Nichi Glucan) than in the control group Gr. 1 (p=0.091701).
Conclusion
Improvement of the behavioural pattern CARS score and a correlating alpha-synuclein level, followed by a safe beta-glucan food supplement, warrants further research on other parameters, such as gut-microbiota evaluation, and relevant neuronal biomarkers which is likely to cast light on novel solutions.
Keywords: autism, neural networks, behavioural disorder, paediatric neurology
Introduction
Autism spectrum disorders (ASDs) are a group of developmental disabilities that can cause significant impairment in social, emotional and communication skills (cdc.gov). Several causes and underlying mechanisms have been postulated for the pathogenesis of ASD, including genetic, environmental, immune dysregulation, neuroinflammation and oxidative stress. Neuronal synaptic imbalance and mutation in synaptic proteins and receptors have also been reported to be associated with ASD.1 Synucleins are small soluble proteins that are present in presynaptic terminals, and they regulate synaptic plasticity and neurotransmitter release. Synucleins are important in the context of brains and neurons.1 2 Alpha-synuclein as a presynaptic neurotransmitter plays a key role in the synaptic functions of neurons by regulating calcium dependent secretion activator 2 (CADPS2) messenger RNA (mRNA) expression.3–5 Alpha-synuclein has already been reported to be associated with several neurodegenerative disorders, collectively called synucleinopathies such as Alzheimer’s disease (AD), Parkinson’s disease (PD), dementia with Lewy bodies and multiple system atrophy.1 The levels of alpha-synuclein have been variedly reported between ASD and PD wherein in ASD, levels lower than age-matched controls has been reported3–6 while in PD, some have reported lower than normal levels and others higher. At present, there is no definitive cure for ASD. Interventions involve speech and behavioural therapies to improve the symptoms. According to the research, the microbiota-gut-brain axis is significant because dysbiosis has been observed in gut-related diseases and other generalised disorders, especially of the nervous system, such as AD, multiple sclerosis, PD and ASD.7 Therefore, nutritional supplements are considered potential interventions in alleviating gastrointestinal and behavioural symptoms in ASD.8 Beta-glucans, especially yeast-derived ones, have shown a considerable positive outcome as food supplements in modulating gut microbiota.9 Nichi Glucan is a black yeast-derived AFO-202 beta-glucan that has been in consumption for the past two decades10 and has been shown to have potential as a nutritional supplement to balance metabolic levels of glucose, lipids and immunomodulators.11–13 Studies on children with ASD have indicated there is an underlying neuroinfilammatory process occurring in different regions of the brain involved in microglial activation, thus resulting in a loss of connections or underconnectivity of neurons and leading to behavioural manifestations.14 monocyte chemoattractant protein-1 (MCP-1), interleukin 6 (IL-6), interleukin 10 (IL-10) and tumor necrosis factor-alpha (TNF-α) have been shown to be expressed in higher levels in children with autism.15 Beta-glucan has been proven to reduce the expression of inflammatory and proinflammatory markers, including Il-6 and TNF-α,16 apart from having a neuroprotective effect by attenuating inflammatory cytokine production through microglia.17 In another study, beta-glucan reduced induced microglia activation and its phagocytosis of synaptic puncta and upregulation of proinflammatory cytokine (TNF-α, IL-1β and IL-6) mRNA expression apart from promoting Tau signalling and improving cognition and brain function via the gut-brain axis.18
This study was undertaken to investigate the effects of Nichi Glucan as a food supplement in children with ASD, especially with relevance to the Childhood Autism Rating Scale (CARS) score and alpha-synuclein levels.
Methods
The caregiver of all the subjects gave their informed consent for inclusion before participation in the study. The study was conducted in accordance with the Declaration of Helsinki.
Study design
The subjects enrolled in the study had received a diagnosis of ASD by a developmental paediatrician and were verified by a psychologist using a clinical interview for a behavioural pattern that incorporated CARS.
Eighteen subjects (n=18) with ASD in total were enrolled in this prospective, open-label, pilot clinical trial comprising of two arms. The Consolidated Standards of Reporting Trials (CONSORT) flow diagram is presented as figure 1.
Figure 1.
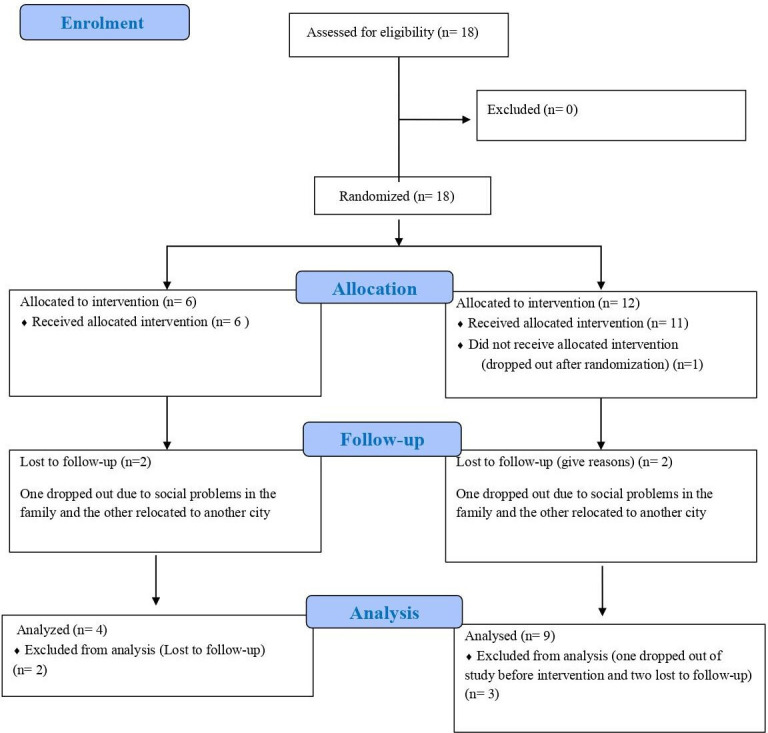
Consolidated Standards of Reporting Trials (CONSORT) flow diagram for description of the clinical trial.
Arm 1 or Gr. 1: Control: Six subjects with ASD (n=6) underwent conventional treatment comprising remedial behavioural therapies and L-Carnosine 500 mg per day.
Arm 2 or Gr. 2: Treatment arm: 12 subjects (n=12) underwent supplementation with Nichi Glucan food supplement along with conventional treatment (remedial behavioural therapies and L-Carnosine 500 mg per day). Each subject consumed two sachets (0.5 g each) of Nichi Glucan daily—one sachet with a meal twice daily—for a period of 90 days.
Inclusion criteria
Age: 3–18 years.
Gender: Both male and female.
ASD criteria as per CARS score.
Parents/caretakers willing to provide consent for their children to actively participate in the study.
Exclusion criteria
Subjects aged more than 18 years old.
Any child with acute general illness or who has been on any antibiotic, anti-inflammatory, or antioxidant treatment in the 2 weeks prior to enrolment in the study.
Hyperallergic to any of the investigational products.
Subjects with long-standing infections.
Outcome measures
Primary endpoints
1. An improvement on the CARS by at least 4.5 points.19
The CARS was monitored at baseline and after 90 days between the Gr.1 (control) and Gr.2 (Nichi Glucan). The CARS score was calculated based on a cumulative score obtained on the CARS scale, wherein a score below 30 indicates absence of sufficient signs and symptoms indicative of autism, a score between 30 and 36 indicates mild-to-moderately severe autism, and a score from 37 to 60 is correlated with severe autism.19 The psychologist who performed the assessment and the parents were blind to the participant’s treatment status.
2. Atleast 30% increase in plasma alpha-synuclein levels from baseline.
Human alpha-synuclein (α-syn) levels in plasma were measured in peripheral blood at baseline and after the study’s completion at 90 days. The measurement was performed using the human α-synuclein (α-syn) ELISA kit (KINESISDx, USA) as per the manufacturer’s instructions. Normal range of values were 0.50–100 ng/dL.
Secondary endpoints
Safety Monitoring: Adverse events frequency and severity; clinically abnormal safety parameters.
Target population for analysis
Intention-to-treat (ITT) analysis included all the subjects who were enrolled in the study. Per-protocol analysis (PPS) was performed on the subjects who completed the entire study without dropping out.
Method of analysis
All data were analysed using Excel software statistics package analysis software (Microsoft Office Excel); Student’s paired t-tests were also calculated using this package; and values of p<0.05 were considered significant.
Results
ITT and PPS subjects
Eighteen patients who fulfilled all the selection criteria and none of the exclusion criteria were selected to start the study. These 18 patients were included in the ITT analysis.
During enrolment, in the treatment group (Gr. 2), one of them dropped out before the start of the study.
Figure 1 shows the CONSORT diagram of the trial.
During the study, four subjects were lost to follow-up: two in Gr. 1 (one dropped out due to social problems in the family, and the other relocated to another city) and two in Gr. 2 (one dropped out due to social problems in the family, and the other relocated to another city).
After excluding these four subjects, 13 subjects were included in the PPS.
ITT analysis
Primary end points
CARS score
Among the children in the control group (Gr.1), all four were in the category of severe autism, and their score at baseline ranged from 37 to 52 (mean=42.75 ± 5.76). Among the nine children in Gr.2, two were in the mild-to-moderate category of autism (mean=33.5±2.5), whereas the remaining seven were in the category of severe autism (mean=43.71±4.80).
ITT analysis
Gr.1 Control: None of the participants achieved the endpoint of improvement in CARS score by 4.5 points.
Gr.2 Nichi Glucan: Only 25% of the subjects achieved the endpoint of improvement in CARS score by 4.5 points. Proportion of subjects is not greater than 50% after intervention.
PPS analysis
Gr.1 Control: None of the participants achieved the endpoint of improvement in CARS score by 4.5 points.
Gr.2 Nichi Glucan: Only 33% of the subjects achieved the endpoint of improvement in CARS score by 4.5 points. Proportion of subjects is not greater than 50% after intervention.
Though the proportion of subjects who have achieved the end point is not greater than 50% in both the groups, Nichi Glucan is significantly better than Control in terms of proportion of subjects who have achieved the end point (p=0.034517).
Absolute values
After the intervention, the mean CARS score in the four children of the control group was 42.5±5.4, while in Gr.2 (Nichi Glucan), the mean of the CARS score in the two children with mild-to-moderate autism was 32.5±0.5. In the remaining seven children, the CARS score after Nichi Glucan intervention had a mean of 40.1±5.96. There was an average of three points in the improvement of autism’s signs and symptoms in the Nichi Glucan group Gr.2, whereas the improvement was very mild or nil in Gr.1 (figure 2). Among the various parameters assessed on the CARS, there was visible subjective improvement in the emotional response, including reduction in irritability and anger (88%), sleep improvement (88%), speech characteristics with improvement in finger pointing and monosyllables in 77%, and improved responses to the caregiver in 77% of the children in Nichi Glucan Gr. 2, but these improvements were very mild or nil in Gr.1.
Figure 2.
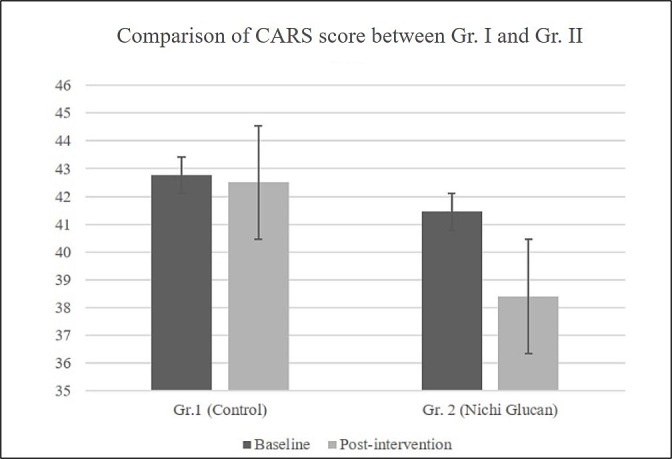
Comparison between Gr.1 and Gr.2 showed a significant decrease in the CARS score, indicating improvement in autism’s signs and symptoms in Gr.2 (Nichi Glucan) compared with Gr.1 (control) postintervention (p=0.034). CARS, Childhood Autism Rating Scale.
Table 1 shows the values of CARS score in all the study subjects of the PPS analysis.
Table 1.
The score on the CARS in Gr.1 (control) and Gr.2 (Treatment—Nichi Glucan) at baseline and postintervention
| Patient | Baseline | Postintervention |
| Gr.1 Control | ||
| I | 52 | 50 |
| II | 43 | 45 |
| III | 39 | 39 |
| IV | 37 | 36 |
| Gr.2 Treatment | ||
| I | 38 | 32 |
| II | 42 | 37 |
| III | 31 | 32 |
| IV | 37 | 35 |
| V | 45 | 39 |
| VI | 46 | 45 |
| VII | 36 | 33 |
| VIII | 46 | 42 |
| IX | 52 | 51 |
CARS, Childhood Autism Rating Scale.
Plasma levels of alpha-synuclein
ITT analysis
Gr.1 Control: Only 25% of the participants achieved the endpoint of at least 30% improvement in plasma levels of alpha-synuclein. Proportion of subjects is not greater than 50% after intervention.
Gr.2 Nichi Glucan: Only 31.25% of the participants achieved the endpoint of at least 30% improvement in plasma levels of alpha-synuclein. Proportion of subjects is not greater than 50% after intervention.
PPS analysis
Gr.1 Control: 50% of the participants achieved the endpoint of at least 30% improvement in plasma levels of alpha-synuclein. Proportion of subjects is greater than 50% after intervention.
Gr.2 Nichi Glucan: 55% of the subjects achieved the endpoint of at least 30% improvement in plasma levels of alpha-synuclein. Proportion of subjects is greater than 50% after intervention.
Nichi Glucan (Gr. 2) is better than Control (Gr. 1) in terms of proportion of subjects who have achieved the end point (p=0.091701),
Absolute values
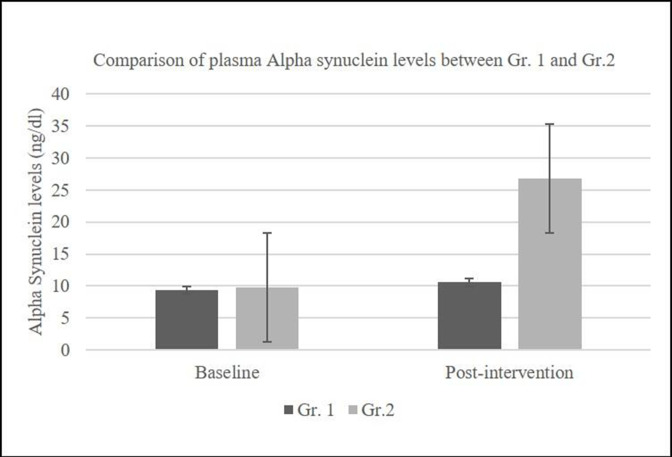
Plasma levels of alpha-synuclein ranged between 0.12 and 20.41 ng/dL (mean=9.73 ng/dL) in the control group and between 0.45 and 41.12 ng/dL (mean=9.39 ng/dL) in the treatment group at baseline. After the intervention, plasma levels of alpha-synuclein increased, with a mean increase in levels of 26.72 ng/dL in the treatment (Nichi Glucan) Gr.2 group compared with the control group Gr. 1 (mean increase=10.56 ng/dL) (figure 3).
Figure 3.
Plasma alpha-synuclein levels in Nichi Glucan group GR. 2 showing a significant increase compared with Gr.1 (p=0.091).
Table 2 shows the values of plasma levels of alpha-synuclein in all the study subjects of the PPS analysis.
Table 2.
Plasma alpha-synuclein levels in Gr. 1 (control) and Gr.2 (treatment—Nichi Glucan) at baseline and postintervention
| Patient | Baseline (ng/dL) | Postintervention (ng/dL) | Fold increase |
| Gr.1 Control | |||
| I | 1.06 | 1.75 | 1.66 |
| II | 0.12 | 1.8 | 15 |
| III | 15.99 | 20.1 | 1.25 |
| IV | 20.41 | 18.6 | 0.91 |
| Gr.2 Treatment | |||
| I | 41.12 | 56.1 | 1.36 |
| II | 0.9 | 26.74 | 29.72 |
| III | 3.67 | 24.1 | 6.57 |
| IV | 0.44 | 11.12 | 25.27 |
| V | 11.38 | 9.6 | 0.84 |
| VI | 24.99 | 28.5 | 1.14 |
| VII | 0.45 | 70 | 155.5 |
| VIII | 2.46 | 11.98 | 4.87 |
| IX | 2.18 | 2.4 | 1.1 |
Secondary endpoint
Adverse effects
Only one child exhibited possible mild adverse effects related to increased bowel movements in Gr. 2 for 1 week after supplementation with Nichi Glucan, which settled on its own. No adverse effects were found in any of the other children.
Discussion
In this study of 13 subjects, the behavioural pattern evaluated by the CARS score improved in all nine subjects of Gr.2 (Nichi Glucan) (figure 1), especially on the emotional aspects and sleep-related parameters, and the alpha-synuclein levels increased significantly in these nine subjects compared with the control (figure 2). The improvement in behaviour can be attributed to the improvement in sleep parameters20 as consumption of Nichi Glucan for 90 days has been able to improve the sleep quality and sleep pattern by a correlating increase in serum melatonin levels as well.21 In a correlating hypothesis of the plasma alpha-synuclein level, between autism and neurodegenerative diseases, it has been proposed that alpha-synuclein aggregation in the neural synapse may lead to lower plasma levels.5 Whether the increase in alpha-synuclein levels in plasma in the ASD patients after Nichi Glucan supplementation is due to regulation/prevention of alpha-synuclein’s aggregation in the neural synapse must be investigated because an earlier study on beta-glucan from yeast showed reduction in alpha-synuclein expression on the brain substantia nigra in Parkinson’s rat model.22 Another postulated mechanism is association of the gut microbiota. Gram-negative enteric bacteria such as the Enterobacter and Escherichia coli secrete the amyloid curli which causes misfolding23 and accumulation of the neuronal protein alpha-synuclein in the form of insoluble amyloid aggregations, which has also been shown to propagate in a prion-like fashion from the gut to the brain via the vagus nerve and/or spinal cord, thus culminating in the neurological disorders such as ASD and PD.24 In a follow-up analysis of this study, we have reported a significant decrease in Enterobacter and E. coli25 which logically will result in lesser production of alpha-synuclein. In spite of such decreased production, the increase in plasma alpha-synuclein levels can be probably due to the disintegration of the amyloid deposits by natural killer cells leading to these alpha-synuclein entering the blood stream.26 27 These postulated mechanisms while need further in-depth research, no single intervention, or therapy has proven its ability to regulate alpha-synuclein levels, especially in children with ASD. This study, thus, gains significance as the first of its kind which demonstrates a significant increase in the plasma alpha-synuclein levels after Nichi Glucan supplementation and the levels being in line with those that were reported for children without ASD,28 probably implies a regulation of the alpha-synuclein but this requires further validation.
The mechanism by which the beta-glucan promoted behavioural improvement in this study and correlated with the regulation of alpha-synuclein levels needs further in-depth research, not only for ASD but also for neurodegenerative diseases such as AD, PD, and so on, especially with regard to its effects on the gut-microbial ecosystem. The evolving data on the gut-brain axis and gut microbiota indicate there are two major approaches to balancing gut microbiota: probiotic and prebiotic. Probiotic approaches, such as nutritional probiotics, faecal transplantation, and so on, involve direct administration of the beneficial microorganisms that have to colonise the gut.23 However, the gut environment must be conducive for such probiotic supplementation. This is where prebiotic approaches come in, such as Nichi Glucan, which help in regulating the gut-microbial ecosystem and preventing chronic inflammatory status28; this must be validated by future studies in terms of the effects of Nichi Glucan and gut microbiota in their relevance to ASD.
The limitation of the study is the limited number of participants, the unequal distribution of genders, and the number of participants between the groups. Also, the drop-out rate from the study is very high. Further, a recent study has suggested that clinicians on an average would like to see a 4.5-point improvement to conclude to a meaningful improvement after an intervention in the context of ASD.19 In this study, only a three-point improvement was observed after Nichi-Glucan supplementation. However, this is only a pilot study, and larger randomised, multi-centric clinical trials of longer duration for validation to attain a clinical meaningful response and to overcome the above-mentioned limitations are warranted. Nevertheless, the study is significant as it has identified a simple nutritional supplemental intervention based on a naturally derived beta-glucan, the Nichi Glucan, which could stimulate endogenous alpha-synuclein secretion, promote better synaptic regulation and improve the behaviour symptoms of children with autism. However, the results suggest that the benefits will be considerable when evaluated in terms of social and emotional well-being and alleviation of caregiver stress, which is extremely significant.
Conclusion
Patients with ASD showed improvement in behavioural symptoms and improved levels of plasma alpha-synuclein; thus, this pilot clinical study of nutritional supplementation with an AFO-202 strain of black yeast Aureobasidium pullulans produced the biological response modifier beta-glucan (Nichi Glucan). Evaluation as per the CARS score has also shown significant beneficial effects. Although further validations need to be performed, the study definitely confirms the potential of Nichi Glucan as a simple but effective food supplement to be considered as a routine in children with ASD. Further research on the mechanisms of its action in improving alpha-synuclein levels and balancing the immune system in the context of managing chronic inflammation and gut-microbiota regulation as a prebiotic is likely to improve understanding of other neurodegenerative diseases such as PD and AD.
Acknowledgments
The authors thank: Mr. Mohan Ponnusamy & staff of Kenmax for their assistance during the clinical study and data collection of the manuscript; Mr. Takashi Onaka, Mr. Yasunori Ikeue, Mr. Mitsuru Nagataki (Sophy Inc, Kochi, Japan), for necessary technical clarifications; Dr. Ramesh Shankar Kandaswamy, Consultant Psychiatrist & Clinical Director, Lincolnshire Partnership NHS Foundation Trust, United Kingdom for his critical inputs during the revision of the manuscript; Loyola-ICAM College of Engineering and Technology (LICET) for their support to our research work.
Footnotes
Contributors: KR, NI and SJKA contributed to conception and design of the study. KR and NI helped in data collection and analysis. SJKA and SP drafted the manuscript. VDD, TS and MI performed critical revision of the manuscript. All the authors read, and approved the submitted version.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: SJKA is a shareholder in GN Corporation, Japan which in turn is a shareholder in the manufacturing company of the AFO 202 Beta Glucan.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
Ethics approval
The study was approved by the Institutional Ethics Committee (IEC) of Saravana Multispeciality Hospital, Madurai, India on 24th August, 2019 (Ref ID: GLU/2020/01).
References
- 1.Al-Mazidi S, Al-Ayadhi LY. Plasma levels of alpha and gamma synucleins in autism spectrum disorder: an indicator of severity. Med Princ Pract 2021;30:160–7. 10.1159/000513935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vargas KJ, Schrod N, Davis T, et al. Synucleins have multiple effects on presynaptic architecture. Cell Rep 2017;18:161–73. 10.1016/j.celrep.2016.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Obergasteiger J, Überbacher C, Pramstaller PP, et al. CADPS2 gene expression is oppositely regulated by LRRK2 and alpha-synuclein. Biochem Biophys Res Commun 2017;490:876–81. 10.1016/j.bbrc.2017.06.134 [DOI] [PubMed] [Google Scholar]
- 4.Siddique A, Khan HF, Ali S, et al. Estimation of alpha-synuclein monomer and oligomer levels in the saliva of the children with autism spectrum disorder: a possibility for an early diagnosis. Cureus 2020;12:e9936. 10.7759/cureus.9936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sriwimol W, Limprasert P. Significant changes in plasma alpha-synuclein and beta-synuclein levels in male children with autism spectrum disorder. Biomed Res Int 2018;2018:1–7. 10.1155/2018/4503871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadak MT, Cetin I, Tarakçıoğlu MC, et al. Low serum level α-synuclein and tau protein in autism spectrum disorder compared to controls. Neuropediatrics 2015;46:410–5. 10.1055/s-0035-1565273 [DOI] [PubMed] [Google Scholar]
- 7.Srikantha P, Mohajeri MH. The possible role of the Microbiota-Gut-Brain-Axis in autism spectrum disorder. Int J Mol Sci 2019;20:2115. 10.3390/ijms20092115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karhu E, Zukerman R, Eshraghi RS, et al. Nutritional interventions for autism spectrum disorder. Nutr Rev 2020;78:515–31. 10.1093/nutrit/nuz092 [DOI] [PubMed] [Google Scholar]
- 9.Xu M, Mo X, Huang H, et al. Yeast β-glucan alleviates cognitive deficit by regulating gut microbiota and metabolites in Aβ1-42-induced AD-like mice. Int J Biol Macromol 2020;161:258–70. 10.1016/j.ijbiomac.2020.05.180 [DOI] [PubMed] [Google Scholar]
- 10.Ikewaki N, Fujii N, Onaka T, et al. Immunological actions of Sophy beta-glucan (beta-1,3-1,6 glucan), currently available commercially as a health food supplement. Microbiol Immunol 2007;51:861–73. 10.1111/j.1348-0421.2007.tb03982.x [DOI] [PubMed] [Google Scholar]
- 11.Dedeepiya VD, Sivaraman G, Venkatesh AP, et al. Potential effects of nichi glucan as a food supplement for diabetes mellitus and hyperlipidemia: preliminary findings from the study on three patients from India. Case Rep Med 2012;2012:1–5. 10.1155/2012/895370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganesh JS, Rao YY, Ravikumar R, et al. Beneficial effects of black yeast derived 1-3, 1-6 Beta Glucan-Nichi Glucan in a dyslipidemic individual of Indian origin--a case report. J Diet Suppl 2014;11:1–6. 10.3109/19390211.2013.859211 [DOI] [PubMed] [Google Scholar]
- 13.Ikewaki N, Iwasaki M, Kurosawa G, et al. β-glucans: wide-spectrum immune-balancing food-supplement-based enteric (β-WIFE) vaccine adjuvant approach to COVID-19. Hum Vaccin Immunother 2021;17:2808–13. 10.1080/21645515.2021.1880210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez JI, Kern JK. Evidence of microglial activation in autism and its possible role in brain underconnectivity. Neuron Glia Biol 2011;7:205–13. 10.1017/S1740925X12000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez-Fernandez A, de la Torre-Aguilar MJ, Gil-Campos M, et al. Children with autism spectrum disorder with regression exhibit a different profile in plasma cytokines and adhesion molecules compared to children without such regression. Front Pediatr 2018;6:264. 10.3389/fped.2018.00264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah VB, Williams DL, Keshvara L. Beta-Glucan attenuates TLR2- and TLR4-mediated cytokine production by microglia. Neurosci Lett 2009;458:111–5. 10.1016/j.neulet.2009.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raghavan K, Kandaswamy RS, Ikewaki N, et al. Potentials to alleviate coagulopathy and enhance microglial function of beta (β)- glucans, making them worth a clinical study for COVID-19’s neurological sequalae. J Neurol Sci 2021;427:117554. 10.1016/j.jns.2021.117554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi H, Yu Y, Lin D, et al. β-glucan attenuates cognitive impairment via the gut-brain axis in diet-induced obese mice. Microbiome 2020;8:143. 10.1186/s40168-020-00920-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jurek L, Baltazar M, Gulati S, et al. Response (minimum clinically relevant change) in ASD symptoms after an intervention according to CARS-2: consensus from an expert elicitation procedure. Eur Child Adolesc Psychiatry 2021;55:1–10. 10.1007/s00787-021-01772-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen S, Conduit R, Lockley SW, et al. The relationship between sleep and behavior in autism spectrum disorder (ASD): a review. J Neurodev Disord 2014;6:44. 10.1186/1866-1955-6-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raghavan K, Dedeepiya VD, Kandaswamy R. Improvement of sleep patterns and serum melatonin levels in children with autism spectrum disorders after consumption of beta-1,3/1,6-glucan in a pilot clinical study. Research Square 2021. 10.21203/rs.3.rs-701988/v1 [DOI] [Google Scholar]
- 22.Rahayu M, Nandar Kurniawan S, Husna M, et al. The effect of beta glucan of saccharomyces cerevisae on the decrease of alpha synuclein expression in the brain substantia nigra of parkinson’s wistar strain rats (rattus novergicus) model induced with rotenone. MNJ 2016;2:01. 10.21776/ub.mnj.2016.002.01.2 [DOI] [Google Scholar]
- 23.Peng M, Tabashsum Z, Anderson M, et al. Effectiveness of probiotics, prebiotics, and prebiotic-like components in common functional foods. Compr Rev Food Sci Food Saf 2020;19:1908–33. 10.1111/1541-4337.12565 [DOI] [PubMed] [Google Scholar]
- 24.Miller AL, Bessho S, Grando K, et al. Microbiome or infections: Amyloid-Containing biofilms as a trigger for complex human diseases. Front Immunol 2021;12:638867. 10.3389/fimmu.2021.638867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raghavan K, Dedeepiya VD, Yamamoto N. Beneficial reconstitution of gut microbiota and control of alpha-synuclein and curli-amyloids-producing enterobacteria, by beta 1,3-1,6 glucans in a clinical pilot study of autism and potentials in neurodegenerative diseases. medRxiv 2021. 10.1101/2021.10.26.21265505 [DOI] [Google Scholar]
- 26.Earls RH, Menees KB, Chung J, et al. Nk cells clear α-synuclein and the depletion of NK cells exacerbates synuclein pathology in a mouse model of α-synucleinopathy. Proc Natl Acad Sci U S A 2020;117:1762–71. 10.1073/pnas.1909110117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raghavan K, Kandaswamy RS, Ikewaki N, et al. Potentials to alleviate coagulopathy and enhance microglial function of beta (β)- glucans, making them worth a clinical study for COVID-19's neurological sequalae. J Neurol Sci 2021;427:117554. 10.1016/j.jns.2021.117554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramaekers VT, Sequeira JM, DiDuca M, et al. Improving outcome in infantile autism with folate receptor autoimmunity and nutritional derangements: a self-controlled trial. Autism Res Treat 2019;2019:1–12. 10.1155/2019/7486431 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.