Abstract
COVID-19 has been declared a global pandemic which has brought the world economy and the society to a standstill. The current emphasis of testing is on detection of genetic material of SARS-CoV-2. Such tests are useful for assessing the current state of a subject: Infected or not infected. In addition to such tests, antibody testing is necessary to stratify the population into three groups: never exposed, infected, and immune. Such a stratification is necessary for safely reopening the society and remobilizing the economy. The aim of this review article is to inform the audience of the current diagnostic and surveillance technologies that are being employed for the detection of SARS-CoV-2 antibodies along with their shortcomings, and to highlight microfluidic sensors and devices that show promise of being commercialized for detection and quantification of SARS-CoV-2 antibodies in low-resource and Point-of-Care (POC) settings.
Keywords: Antibody, biosensors, chemical and biological sensors, enzyme linked immunosorbent assay (ELISA), protein
I. Introduction
COVID-19 is an infectious disease caused by SARS-CoV-2 virus; a virus closely related to the SARS virus. The disease surfaced in late 2019 in the city of Wuhan, capital of Hubei province in mainland China. According to the most recent statistics, as of October 2020, coronavirus has spread across the world, by infecting more than 38 million people and has claimed about 1,089,000 lives [1]. In the United States alone, almost 8 million cases of coronavirus have been reported and the fatalities have amounted to approximately 214,000 and these numbers keep on increasing with each passing day. In addition to claiming precious human lives, coronavirus has also negatively impacted the world economy. Europe is on track to lose about 18.4 million tourism-related jobs and $1 trillion in GDP in 2020 because of Covid-19 related cancellations. [2]. Moreover, the US Department of labor statistics reported that in the month of April 2020, the US economy lost 20.5 million jobs and the unemployment rate soared to staggering 14.7 percent [3]. The economic fallout of coronavirus can have far-reaching consequences that can wipe up to US$ 2.7 trillion from the global economy [4]. With no proven vaccine or treatment available till date, monitoring and containment is the only strategy available for stopping the spread of the virus.
A recent report [5] lays out a plan for remobilizing the US economy through the massive up-scale of testing, paired with contact tracing and supported isolation. According to the report, 5 million tests per day were required by early June for a safe social opening and 20 million tests by mid-summer to fully re-mobilize the US economy. Currently, the major emphasis of testing is on detecting the genetic material of the virus. Though necessary, these tests provide an incomplete picture. While they may indicate if a person is currently infected with the virus or not, they do not provide information if the person had been previously exposed to the virus and has recovered.
The human body produces Immunoglobulin M (IgM) antibodies as a first line defense against viral pathogens while Immunoglobulin G (IgG) antibodies are produced later and are responsible for long-term immunity [6]. Fig. 1 shows the probability of detection for various types of diagnostic tests after the subject is exposed to the virus. Polymerase Chain Reaction (PCR) test can likely test positive only for a specific time frame. Antibodies are developed at a later stage and can be monitored to ascertain the severity of the disease and recovery progress. A recent study has pointed out that the antibodies against SARS-CoV-2 virus are detectable even after 5 to 7 months following infection [7]. Furthermore, the infection rate and the number of people infected can be estimated by antibody testing. Testing for antibodies specific to the virus along with the current infection status, therefore, provides a full picture and statistics derived from these types of multiplexed tests such as mortality rate, infection rate and recovery rate etc. are less fallible [8].
Fig. 1.
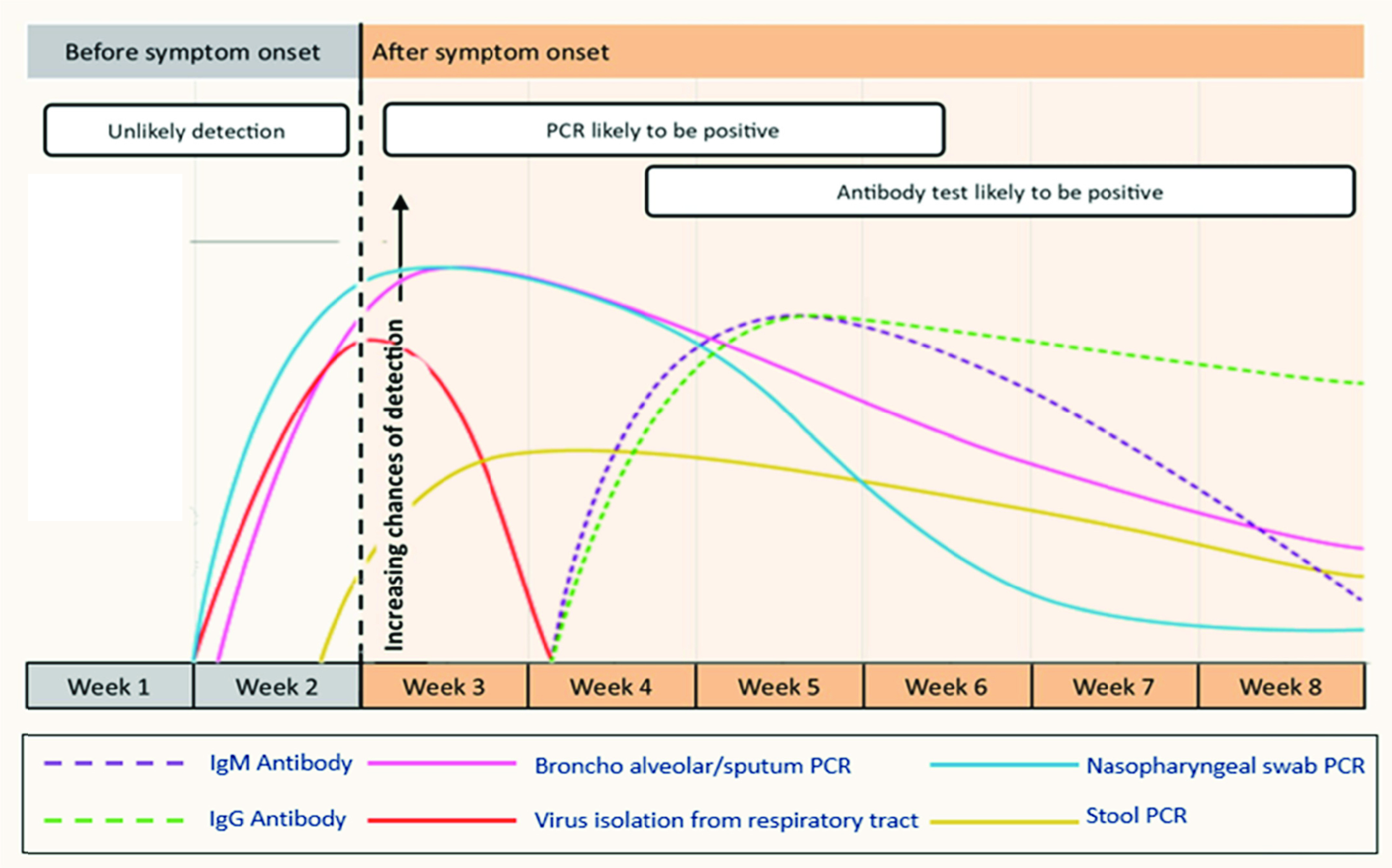
A chart qualitatively showing the probability of detection of various diagnostic techniques (on average) vs. time after a subject is exposed to SARS-CoV-2. IgM levels fall quickly after a few weeks making it difficult to detect whereas IgG levels remain almost constant. Adapted from [6].
This paper is exclusively focused on microfluidic devices having the potential for detecting COVID-19 specific antibodies at Point-of-Care (POC). Devices and technologies for detecting SARS-CoV-2 virus and its envelope proteins are not covered and are out of the scope of this review. The review is divided into two parts. In the first part, we list the antibody detection tests approved for use in at least one country and their shortcomings, whereas, in the second part, we have highlighted a number of microfluidic devices and discussed their potential to be used for large-scale antibody detection at Point-of-Care (POC).
II. Commercially Available Tests
Serological and Immunological assays have been approved by multiple countries including the United States, Brazil, Australia, Germany, Belgium, and South Korea. These assays come in various flavors: Enzyme Linked Immunosorbent Assay (ELISA), Lateral Flow Immunoassays, Neutralization Assays, Luminescent immunoassay, Rapid Antigen Testing, and electrical biosensing. Some of these techniques are highlighted below, along with countries that have either approved or are currently deploying them to detect SARS-CoV-2 antibodies.
A. Lateral Flow Immunoassays
Lateral flow immunoassays typically provide results in a 10–20 minute time window, can test either plasma/serum or even whole blood, check for the presence of both IgG as well as IgM antibodies and do not require highly specialized equipment, making them popular for field use. These lateral flow COVID-19 IgG/IgM tests contain two components, an IgG component, and an IgM component. Anti-human IgG is coated in the IgG test line region whereas anti-human IgM is coated in the IgM test line region (see Fig. 2(a)). During testing, the antibodies in the sample (whole blood/serum/plasma) react with the SARS-CoV-2 antigen-coated particles in the test cassette. These particles then move upwards in the device chromatically by capillary action to react with anti-human IgG and anti-human IgM in their respective testing regions to form visible colored lines indicating the presence of antibodies in the sample. In addition to the IgG and IgM lines, a control line is also usually present to check whether proper sample volume was used, and membrane wicking had occurred [9].
Fig. 2.
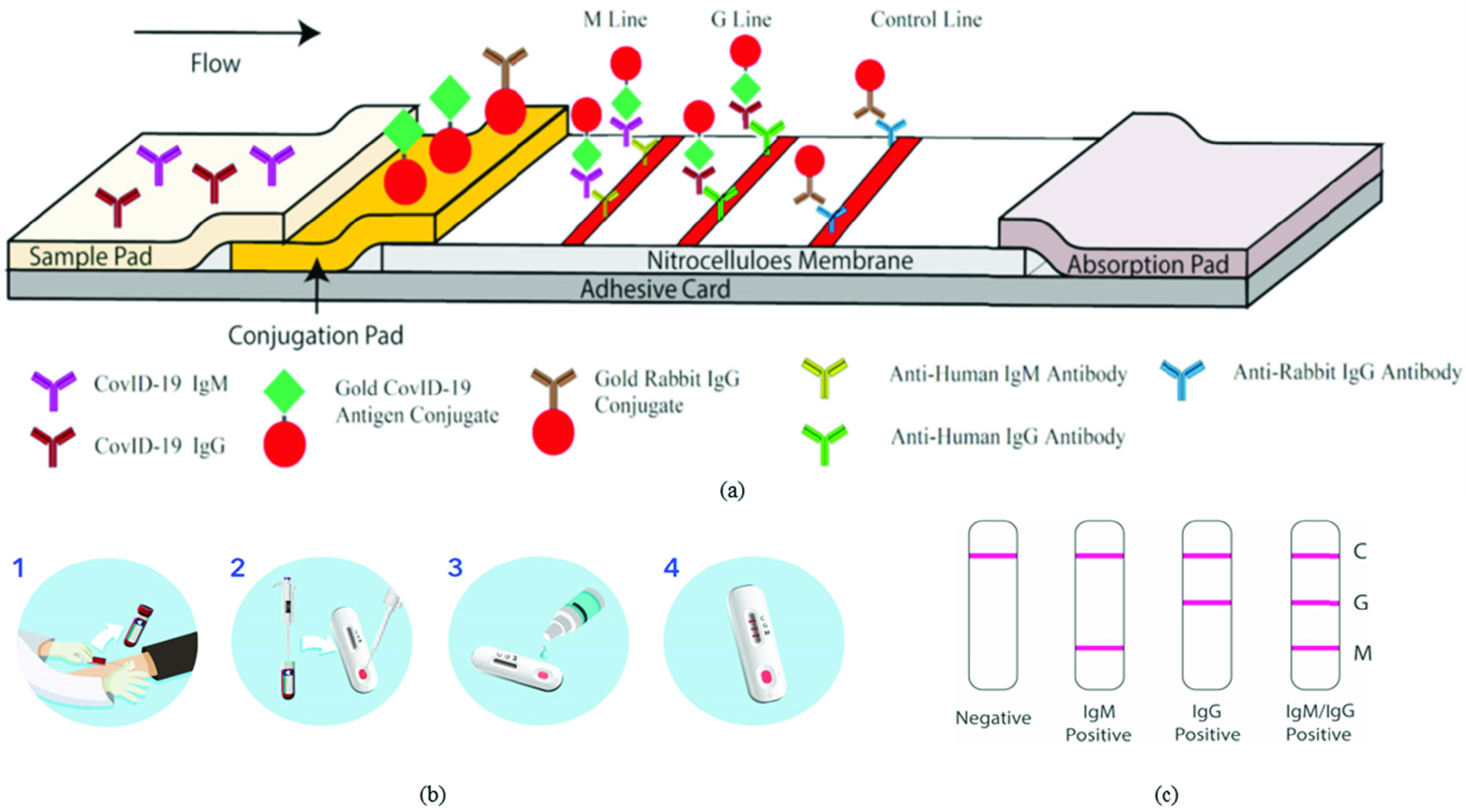
(a) Process flow diagram of a lateral flow immunoassay. Adapted from [9]. (b) Steps for antibody detection using BioMedomics lateral flow device, Courtesy of BioMedomics. (c) Demonstration of results for a lateral flow Immunoassay device, Courtesy of BioMedomics.
These lateral flow assays have been approved for usage in at least one country. Rapid lateral flow tests developed independently by BioMedomics (Fig. 2 (b), (c)) [10] and Cellex Inc. [11] are in use in the United States. Other lateral flow assays that have been approved for usage include the ones developed by Advagen Biotech [12], Chembio Diagnostics [13] and Celer Biotecnologia (Brazil) [14], CTK Biotech Inc. (developed in the USA, in use in Australia) [15], a cassette based test by Hangzhou Alltest Biotech Co. Ltd (in use in Australia, not approved in the US) [16], PharmACT (Germany) [17] and a test developed by SD Biosensor (South Korea) [18], Artron Lab simultaneous detection and differentiation of IgM & IgG antibodies in blood sample (Canada) [19], Acro Biotech 15 min test for detection of patient-generated IgG and IgM antibodies against SARS-CoV-2 (USA) [20], and a test developed by Hardy Diagnostics (Autobio) in the USA for the detection and differentiation of IgG and IgM antibodies to SARS-CoV-2 in plasma [21]. Furthermore, a magnetic particle-based chemiluminescence immunoassay developed by Bioscience (Chongqing) Diagnostic Technology Co., Ltd. (only for IgG) is in use in China [22]. Lassaunière et al. ranked the overall performance of six of these POC tests in which AutoBio is the foremost and Hangzhou Alltest Biotech is the last in the rank order [23]. Though these tests can be used for reliable detection of SARS-CoV-2 antibodies at POC, they can only be employed for qualitative testing and their inability to quantify the level of antibodies in test subjects is a major drawback that needs to be addressed.
B. ELISA Tests
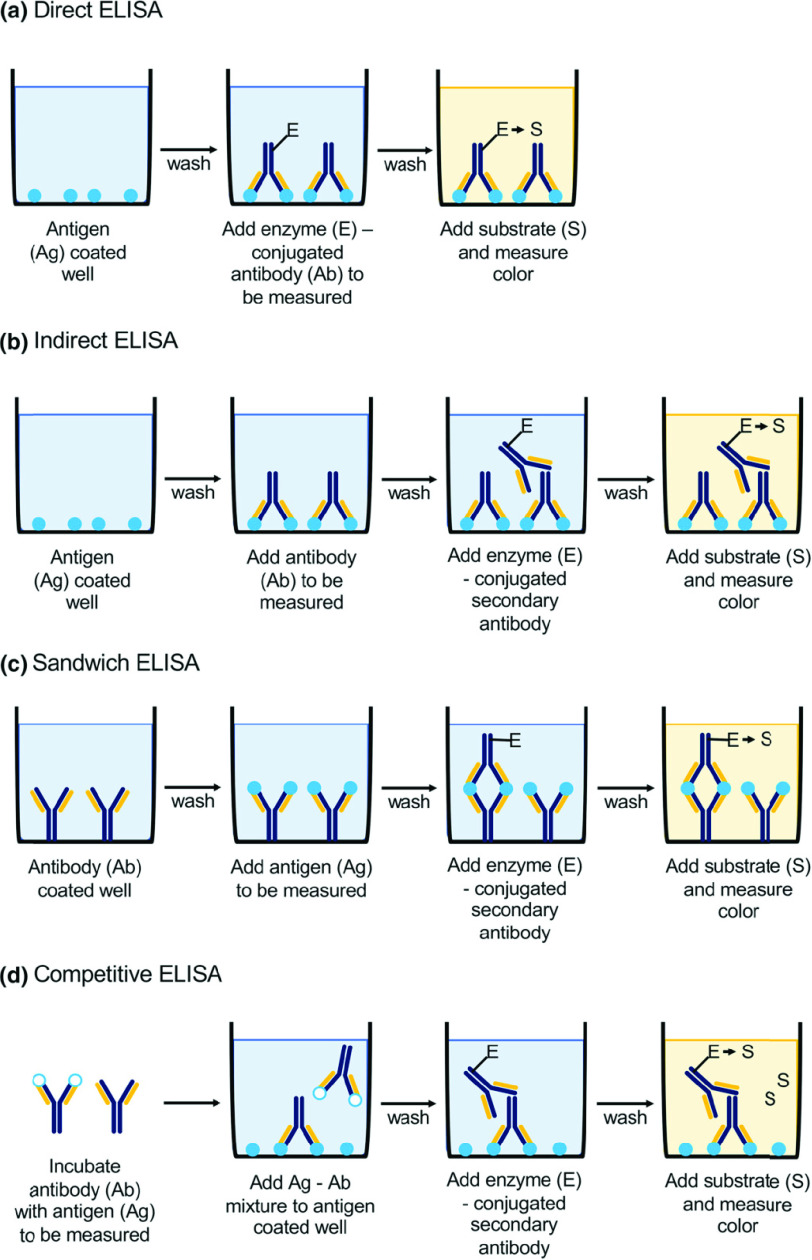
Enzyme Linked Immunosorbent Assay (ELISA) tests have seen high usage rates during the present pandemic. The ELISA technique itself has four main flavors: Direct, Indirect, Sandwich and Competitive ELISA [24]. The different types of ELISA can be seen in Fig. 3 [25]. In Direct ELISA, the enzyme-linked antibodies bind directly to the antigens that are immobilized on a surface to produce a detectable signal. Indirect ELISA involves the binding of a primary antibody with the antigen first, followed by the binding of a secondary antibody which is linked to the enzyme. In Sandwiched ELISA it is not the antigen, but the antibody that is immobilized to a surface (unlike direct and indirect ELISA), called the capture antibody. The antigen binds to this capture body. Next, the enzyme labeled antigen binds to the “captured” antigen, and an enzyme-substrate reaction follows to produce a signal. Finally, the above three techniques can be adapted into a fourth “competitive” ELISA protocol where in addition to the target antigen, there is a reference antigen that competes to bind with the antibodies: therefore the weaker the signal, the greater is the concentration of the target antigen [26].
Fig. 3.
Schematic presentation of basic types of ELISA (enzyme-linked immunosorbent assay): (a) direct, (b) indirect, (c) sandwich, (d) competitive; Ag antigen, Ab antibody, E enzyme, S substrate. Reprinted with permission from [25].
ELISA tests typically require separation of plasma/serum and require much longer time windows to complete (~several hours). Some ELISA tests approved and in use in the United States include the KT-1033 EDI Novel Coronavirus COVID-19 ELISA kit (Epitope Diagnostics) [27], VITROS Anti-SARS-CoV-2 (Ortho-Clinical Diagnostics) [28], EuroImmun (IgG detection in Serum or Plasma) [29], and the DEIASL019/020 SARS-CoV-2 IgG ELISA kit, part of a suite of ELISA based test kits (Creative Diagnostics) [30].
Moreover, in a research in the Netherlands, samples from patients were screened for antibodies using a SARS-CoV-2 total antibody ELISA (Wantai Biological Pharmacy Enterprise Co. China) [31]. Specificity and sensitivity of three ELISA tests were compared using serum samples from positive cases of COVID-19 and control serum samples in [23]. Results indicated that specificities are 100%, 96%, and 93% while sensitivities are 90%, 65%, and 90% respectively for Wantai SARS-CoV-2 Total Antibody test, Euroimmun IgG, and IgM tests. Though these tests provide accurate, reliable, and quantifiable results, the requirement for trained technicians and a proper lab setting make their usage difficult for POC testing of SARS-COV-2 antibodies.
C. Other Techniques
Other less common tests have also been successfully used for antibody-based detection of SARS-CoV-2. These include but are not limited to MAGLUMI IgG/IgM de 2019-nCoV chemiluminescence immunoassay developed by Snibe Diagnostic (in use in Brazil) [32], and LIAISON proposed by Diasorin (Italy) [33]. Furthermore, Wadsworth test which was based on the extensive antigenic similarity between SARS-CoV-l and SARS-CoV-2, can simultaneously detect IgA, IgG and IgM antibodies [34]. Elecsys developed by Cobos in the USA, an anti-SARS-CoV-2 electrochemiluminescence test, utilizes a recombinant protein which represents nucleocapsid (N) antigen for antibodies determination against SARS-CoV-2 [35]. Abbott laboratories have developed a chemi-luminescent microparticle immunoassay for qualitative detection of IgG antibodies against SARS-CoV-2 [36]. Furthermore, Siemens Healthineers SARS-CoV-2 Total (COV2T) Assay, is another chemi-luminescent microparticle immunoassay for total antibody detection against RBD of SARS-CoV-2 S1 antigen [37]. Though these tests are capable of providing accurate and reliable results and have been approved for usage, the requirement of trained technicians and bulky benchtop instruments to perform these tests pose serious challenges towards their usage at POC settings.
III. Microfluidic Devices Having Potential for Commercialization as Point-of-Care Sensors
Microfluidic devices can be used to quantify antibodies and protein biomarkers with precision and relative ease as compared to traditional benchtop methods. These devices have the potential to be commercialized for easy, reliable, and cost-effective SARS-CoV-2 antibody detection. Researchers have developed POC devices relying on different detection techniques. Some of these are discussed below.
A. Impedance-Based Sensors
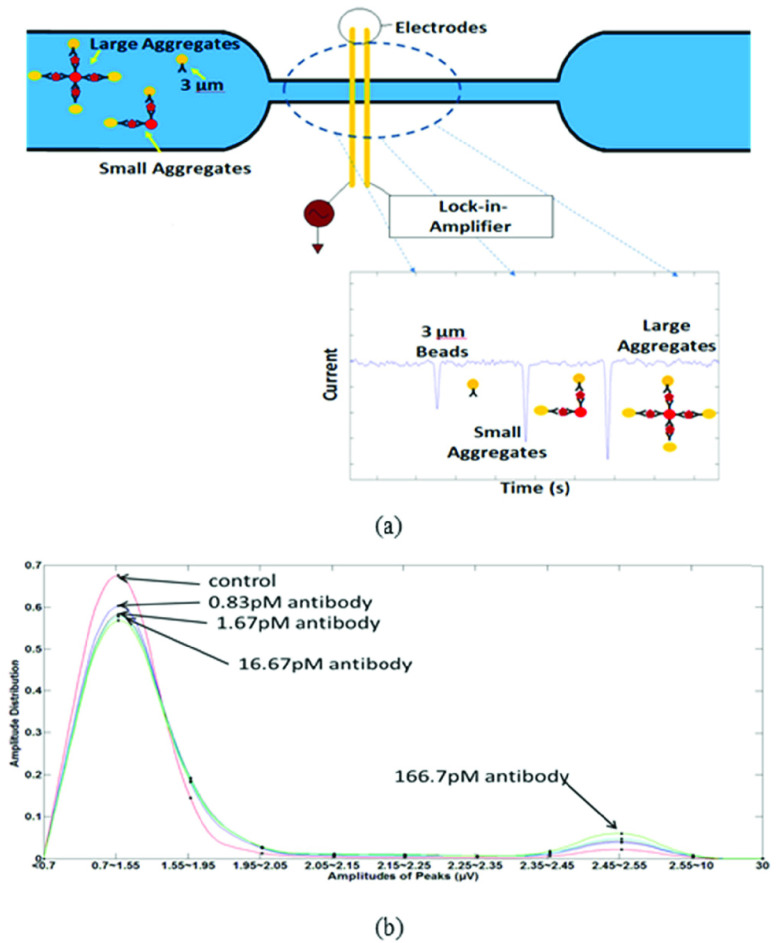
Significant progress has been made in recent years on electrical detection of protein biomarkers and antibodies using microfluidic devices. Zheng [38] demonstrated the use of a silicon nanowire array field-effect antibody functionalized sensor to detect femtomolar level concentrations of prostate specific antigen (PSA). However, such sensors require very low salt concentrations for their operation making them incompatible with physiological conditions in clinical samples such as blood. Devices employing impedance change as a detection mechanism using Lock-in Amplification are another example for the electrical detection of proteins. Javanmard [39] developed a label-free impedance-based protein biomarker sensor using bioactivated microchannels and antibody functionalized microspheres. Researchers demonstrated the use of their system for the detection of anti-hCG antibodies at concentrations as low as 1 ng/ml with an assay time of 1 hour. The beads were coated with the primary receptors whereas antibodies were immobilized on the sensing region. After the functionalized beads were allowed to interact with the sample, they were introduced in the sensing region. The resistance therefore increased with the introduction of the polystyrene beads which was measured by a commercial Impedance Spectroscope utilizing Lock-in Amplification (Zurich Instruments, HF2IS). Although, anti-hCG antibody quantification was chosen for the study, the technique can be modified by modifying the bioactivated microchannels with specific antibodies for other protein biomarkers. Valera [40] used a pillar-based capture chamber to measure protein concentration in human plasma. The limit of detection achieved was reported as 127 pg/ml. Though measurement took 5 mins, a long incubation time was required for the beads to react with the sample (plasma) before they could be passed through the device and the impedance could be measured. Mok [41] developed a two-chamber system that separated the reaction/capture and the detection steps of bead-based assays to allow independent optimization of both. The detection of Interleukin-6 for concentrations as low as 50 pM was reported. IgG detection using bead aggregation was demonstrated by Rodrigeuz-Trujillo [42] for rat IgG diluted in PBS at 14 ng/ml concentration whereas Lin [43] utilized the same detection principle to demonstrate the quantification of mouse IgG down to the picomolar level. The biosensor (Fig. 4 (a)) detected change in impedance using gold-electrodes in a PDMS microchannel. Polystyrene and magnetic beads coated with anti-mouse IgG were used. During the presence of the specific antibody, bead aggregates were formed. The varying sizes of the bead aggregates resulted in change in impedance and produced electrical signals of varying amplitudes. The protein concentration is therefore related to the amplitude distribution i.e. a higher protein concentration yields larger clusters which in turn produces a greater impedance change which in turn results in a larger amplitude (see Fig. 4 (b)). The sensitivity of the device was limited by non-specific binding and theoretical calculations indicated the detection limit to be as low as 100 fM level in the case of 0.01% non-specific binding. The impedance-based sensors provide good detection limits for antibody or protein quantification. However, automation for serum/plasma separation poses a challenge for use in a low-resource setting. Furthermore, the researchers used commercial Lock-in-Amplifiers (LIA’s) which should be replaced with a CMOS device to reduce the cost and the footprint of the systems.
Fig. 4.
(a) Schematic of device. Presence of target protein results in bead aggregation. The ratio of bead aggregates to the total bead count is used to quantify antibody. (b) Normalized amplitude distribution for various concentrations of IgG ranging from 166 pM to 0 pM. The first peak represents unaggregated beads whereas the second peak represents aggregated peaks. Increasing concentration makes the second peak larger in size while decreases the first peak size. Adapted from [43].
B. Electrochemical-Based Sensors
These microfluidic devices rely on sandwich-type electrochemistry for detecting and quantifying the antibodies present in biological samples by recording the electrical response of a chemical reaction. This response is related to the concentration of the target molecules which need to be detected. Researchers in [44] developed a screen-printed microfluidic device which can be used for the quantification of IgG antibodies in serum samples. Anti-mouse IgG was covalently attached to the electrode surface via electropolymerized polypyrrole propylic acid (PPA) film. This device has many steps for introducing reagents. After undergoing the protocol, the device electrochemically measures the reduction of p-aminophenyl phosphate (PAPP) to p-aminophenol (PAP) because of alkaline phosphatase conjugated secondary antibodies present in the chip. The rate of increase of the PAP concentration (the slope of I vs. t) is proportional to the concentration of target analyte. The limit of detection of this device is about 10ng/mL and the dynamic range is 100ng/ml to 10ug/ml. No human serum samples were tested with this device. Furthermore, the need for multiple washing steps and long incubation times make this device difficult for use at Point-of-Care. In [45], researchers presented a microfluidic device for the quantification of IgG anti-Trypanosoma cruzi antibodies in serum of patients that suffer from Chagas disease. For the detection of mentioned antibodies, the microfluidic system relied on a screen-printed carbon electrode (SPCE) which was modified by electrodeposition of gold nanoparticles (AuNPs) functionalized with Trypanosoma cruzi proteins. Serum sample was then introduced in the microfluidic channel and IgG anti-T. cruzi antibodies present in the serum were allowed to react with the Trypanosoma cruzi proteins present on the SPCE surface. Horseradish peroxidase (HRP) labelled secondary antibodies specific to human IgG were then introduced in the channel using 4-tert-butylcatechol (4-TBC) as enzymatic mediator. HRP in the presence of hydrogen peroxide (H2O2) catalyzes the oxidation of 4-TBC which was detected as a change in the electrode current. This change in the electrode current is directly proportional to the IgG concentrations in the range of 11 to 205 ng/ml. The calculated detection limit for electrochemical detection was 3.065 ng/ml and the total assay time is about 26 minutes. Similarly, researchers in [46], [47] used antigen-coated beads for the quantification of human serum IgG anti-Helicobacter pylori and IgG anti-gliadin antibodies respectively. The assay time in both these devices was about 29 minutes and the limit of detection was about 0.37 U/mL and 2.72 U/mL* respectively. Even though we can quickly get sensitive and precise results using biochips relying on electrochemical detection, the need for multiple washing and incubation steps poses challenges in the commercialization of the chip.
C. Optical Detection
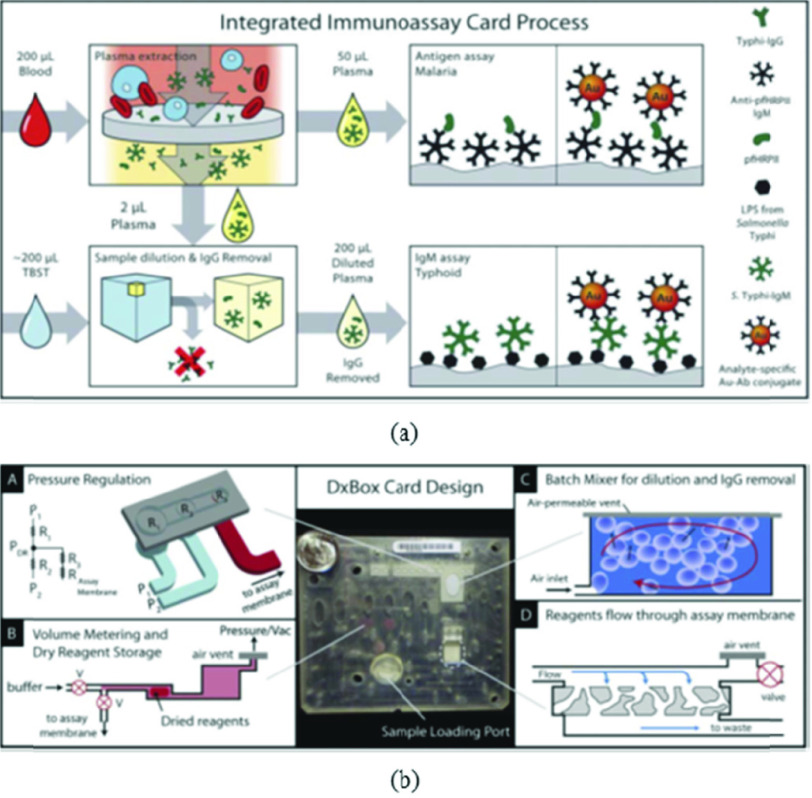
Sensors utilizing optical means of detection have been used conventionally for detecting various biomarkers via use of expensive benchtop instruments such as spectrophotometers. Here, we present a few microfluidic sensors which use rather inexpensive equipment to achieve detection using similar principles. To illustrate, researchers in [48] developed a miniaturized ELISA microfluidic chip which they named mChip (mobile microfluidic chip for immunoassay on protein biomarkers) and demonstrated its use in low-resource settings in countries such as Rwanda using small quantity (< 1 uL) of unprocessed whole blood. Optical detection can be employed using LED’s and photodetectors. The different reagents used in ELISA were injected into the channel sequentially using metered plugs which were actuated using a syringe on the other side. The chip achieved signal amplification using the reduction of silver ions onto gold antibody conjugated nanoparticles. This signal was further amplified using meandering channels which reduced the need for bulky and expensive optics. The commercialization of such a system is particularly promising due to the cost reduction achieved by using injection molding as the fabrication technique ($0.10 per chip). Interestingly, authors in another study [49] developed a disposable multiplexed microfluidic system (Fig. 5) based on immunoassay that detected disease-specific antigens or IgM antibodies from blood. They carried out detection of malaria antigen and IgM to Salmonella Typhi LPS. The microfluidic chip was based on flow through the membrane immunoassay on porous nitrocellulose. After blood was introduced to the system, the cells were removed by passing the sample onto a plasma extraction membrane. The separated plasma was further divided into 2 parts/samples. One sample was intended for antigen detection and another for IgM detection. A Limit of Detection of 20 ng/ml was achieved in 30 minutes which is comparable to benchtop ELISA tests. A large body of work is also focused on using Surface Plasmon Resonance (SPR) and Localized Surface Plasmon Resonance (LSPR) for the detection of target molecules. Researchers in [50] employed SPR phase imaging for IgG detection. They used microheaters and temperature sensors since SPR is sensitive to temperature. A monochromatic light source is provided by a He-Ne laser and the reflected light is measured using a CCD device. Although both SPR and LSPR devices are sensitive and specific, implementation of a low-cost portable reader for this sensing modality is a challenge that is yet to be solved.
Fig. 5.
(a) Process flow diagram of the DxBox integrated immunoassay cards. Unprocessed blood was added to the card and drawn through a plasma extraction membrane to remove blood cells. All processes from sample to result were carried out on card. All reagents were contained on the cards in dry form; only sample and buffer needed to be supplied to run the assays. (b) Image of the immunoassay card and illustrations of processes designed for fluid manipulation by pneumatic control. Adapted from [49] with the permission of Royal Society of Chemistry.
D. Fluorescence-Based Detection
Multiple fluorescence-based microfluidic biochips have been reported for the quantification of antibodies of interest. In [51], researchers reported a magnetic force-based biochip for the quantification of rabbit IgG and mouse IgG antibodies with detection limits of 244 pg/mL and 15.6 ng/mL, respectively. YG fluorescent beads were immobilized with goat anti-mouse IgG whereas the red fluorescent beads were immobilized with goat anti-rabbit IgG. The superparamagnetic antibody complex was separated on the basis of the magnetic field which was detected using optical instruments (CCD). The velocity of the bead correlates to the concentration and is limited by the surface area of the microbead available thereby providing an upper limit of concentration after which saturation occurs (1 ug/ml). The lowest concentration that was detected is 244 pg/ml (1.5 pM). In another study, a digital microfluidic (DMF) device for the quantification of human IgG antibodies in BSA was presented in [52]. The requirement of manual sample preparation and bulky optical instruments for data acquisition hinders the usage of these sensors at POC. The biosensor presented in [53] quantifies human serum IgG antibodies to Helicobacter pylori by using antigen-coated beads, an enzyme-conjugated secondary antibody specific to human IgG, and 4-methylumbelliferyl phosphate (4-MUP), as an enzymatic substrate. A detection limit of 0.17 U/ml* was reported along with an assay time of 30 mins. This sensor requires serum dilution before usage which is a drawback as it adds to the manual processing steps. Researchers in [54] presented the design and working of an integrated microfluidic sensor for the detection of dengue virus IgG and IgM antibodies in human serum. Anti-human IgG-FITC antibodies and anti-human IgM-R-PE antibodies were used as fluorescent markers. The detection limit is reported to be 21 pg and the total assay time is about 30 mins. One of the major advantages of using this biochip is that it can be used for the simultaneous detection of IgG and IgM in human serum. Even though the setup involves multiple washing and incubation steps, all of them have been automated and are done automatically on-chip, which increases the likelihood of the sensor’s application at POC.
E. Chemiluminescent-Based Sensors
Chemiluminescent techniques used in conjunction with microfluidic devices make for a rapid and easy-to-use means for detection of antibodies and proteins. In such tests, the concentration of antibodies is generally directly proportional to the chemiluminescence produced. Previous work [55] depicts the use of this technique for RNA aptamer-based SARS coronavirus nucleocapsid protein detection. In [56], researchers were able to quantify anti-IgG using protein microarrays with microfluidic pumps and valves for actuation in a chip with 6 reaction chambers down to 66 pM under optimized conditions. Researchers in [57] miniaturized the Luciferase Immunoprecipitation System (LIPS) to a microfluidic format for the antibody detection in serum. The researchers demonstrated their operation by detecting HSV-2 antibodies with an assay time of 10 mins. A recent study [58] reported the use of a digital microfluidic (DMF) device using unprocessed whole blood for quantification of anti-measles and anti-rubella IgG antibodies at a field setting in Kenya. Their device has a Limit-of-Detection (LOD) of 0.15 IU/ml** for rubella IgG and 0.15 mIU/ml** for measles IgG with an assay time of 35 mins. Chemiluminescence based sensors require many sample handling steps such as washing, incubating etc. but researchers in [58] have developed a controlling unit which they have named Measles-Rubella (MR) box (Fig. 6) for automating most of these steps thereby making it ideal for use in low-resource settings.
Fig. 6.
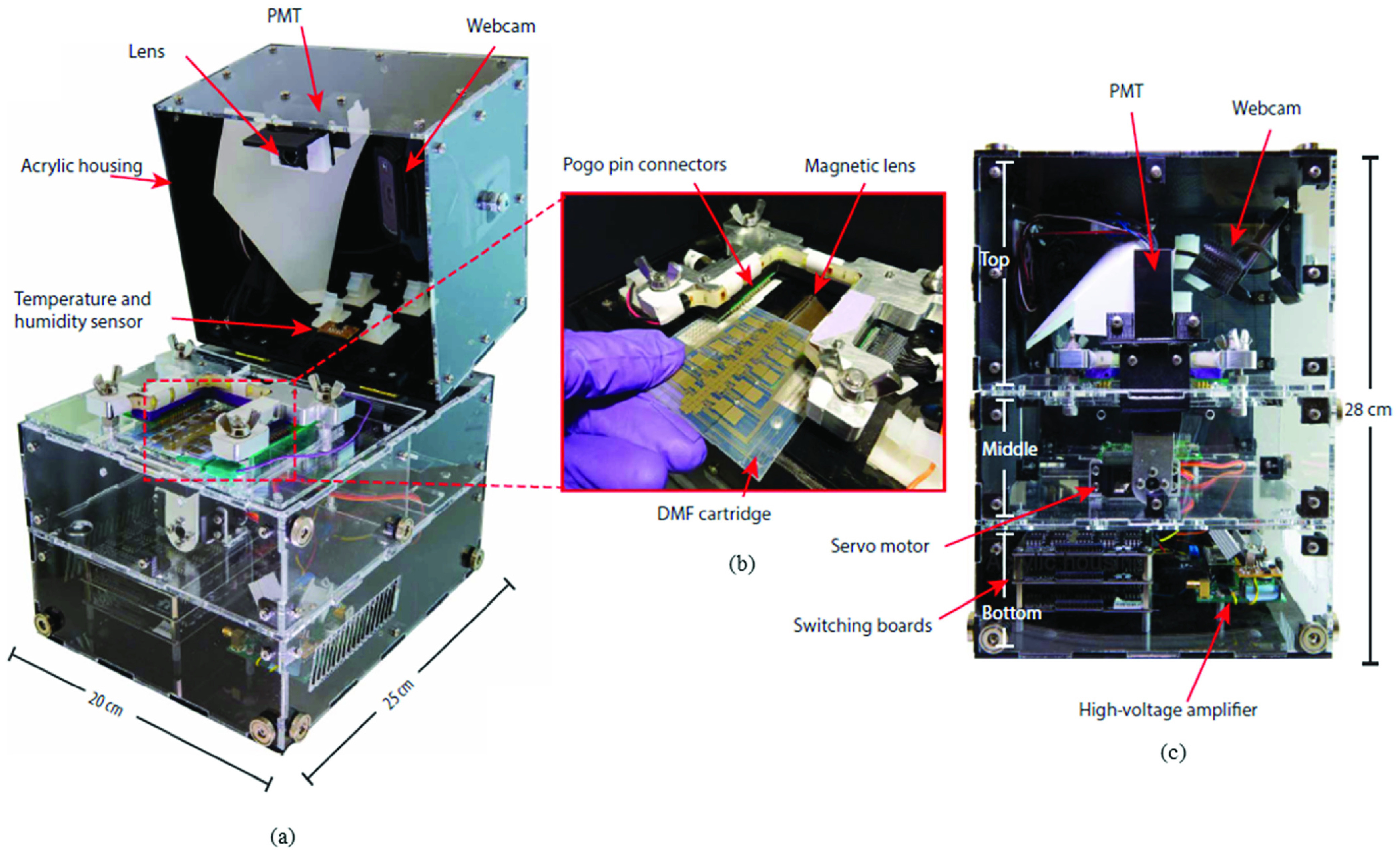
(a) Photograph of the MR Box with transparent sides showing the photomultiplier tube (PMT), lens, webcam, and temperature and humidity sensors (b) Photograph showing detail of a DMF device being inserted into the MR Box interface. The DMF device sits atop a motorized magnetic lens and interfaces with the control system via pogo pin connectors. (c) Photograph (front view; transparent panels) of the MR Box showing the PMT, webcam, servo motor, switching boards, and high-voltage amplifier. With the lid closed, the MR Box measures 28 cm tall. Adapted from [58] with permission from The American Association for the Advancement of Science.
F. Paper/Colorimetric Detection
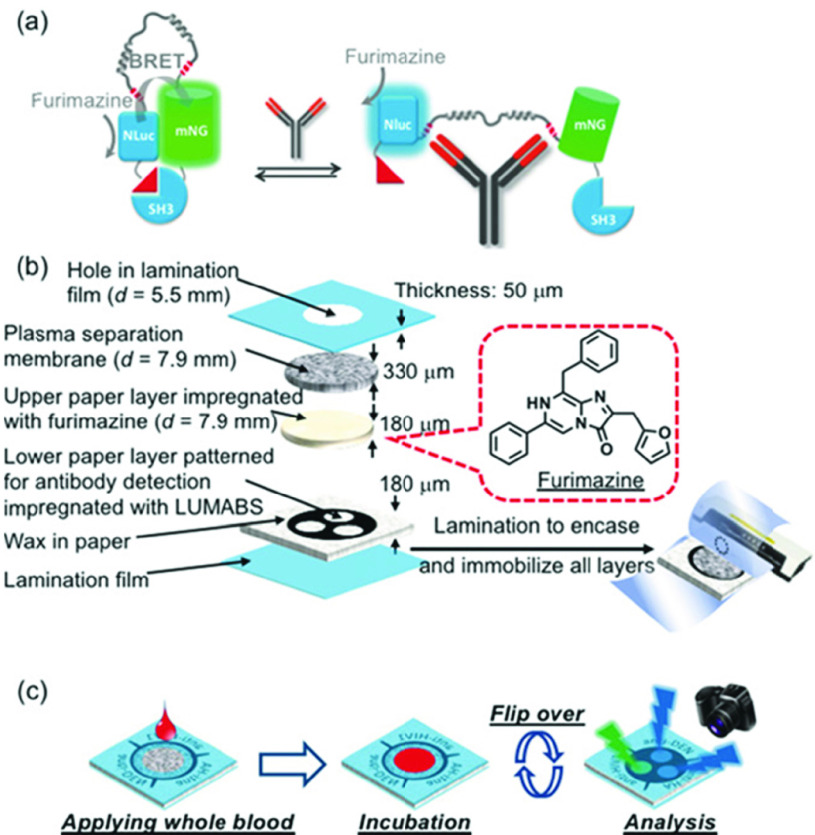
A paper/polymer-based low-cost microfluidic device for antibody quantification was presented in [59]. Immunoglobulin G (IgG) and Hepatitis B surface Antigen (HBsAg) were quantified using the microfluidic device. For the quantification of the IgG and HBsAg, alkaline phosphatase (ALP) conjugated secondary antibody and BCIP/NBT was used. Assay time for this microfluidic device is around 1 hour and the limits of detection of 1.6ng/mL for IgG and 1.3ng/mL for HBsAg were achieved. The limitations of this study include the assay time and the fact that IgG was not quantified in human serum samples, instead known concentrations of IgG spiked PBS samples were tested. Another paper-based immunoassay is presented in [60] which relied on the phenomenon of bioluminescence resonance energy transfer (BRET) for the detection of antibodies in blood serum integrating antibody binding and signal generation in a single protein switch referred to as LUMABS (Fig. 7). The device is simple to use as the user only has to apply a drop of whole blood and take a picture of the sensor 20 minutes after sample introduction. Image processing will then be done for the detection of the target analyte. The device can detect three antibodies in whole blood/plasma simultaneously, thereby, making it ideal for use at POC for SARS-CoV-2 antibody detection.
Fig. 7.
(a) Schematic of the LUMABS working principle with the “closed form” green light-emitting and the “open form” blue light emitting protein sensor in the absence and presence of target antibody, respectively (NLuc = NanoLuc luciferase; mNG = mNeonGreen fluorescent protein) (b) Schematic of a multi-layer 3D-mPAD. All layers are kept together through lamination. (c) Schematic of the use of a 3D-mPAD for simultaneous detection of three different antibodies. Reprinted from [60] with permission of IEEE.
IV. Discussion and Conclusion
In Table I we have listed the salient features of each of the potential microfluidic technologies discussed in this paper. These features include the sensors’ multiplexing capability, sample type, assay time, and the effort required to commercialize them for quantification of Covid-19 specific antibodies at POC.
TABLE I. Salient Features of the Discussed Microfluidic Devices.
| Reference No. | Sensing Methodology | Multiplexing Capability | Sample type | Assay time | Effort required for commercialization to a Point-of-Care setting |
|---|---|---|---|---|---|
| 38 | Impedance | Yes | Serum | <30 min | Substantial |
| 39 | Impedance | No | Buffer | 60 min | Substantial |
| 40 | Impedance | No | Serum | >2 hrs | Substantial |
| 41 | Impedance | No | Serum | >1 hr | Substantial |
| 42 | Impedance | No | Buffer | >20 min | Substantial |
| 43 | Impedance | No | Buffer | >3 hrs | Substantial |
| 44 | Electrochemical | No | Buffer | >4 hrs | Substantial |
| 45 | Electrochemical | No | Serum | <30 min | Moderate |
| 46 | Electrochemical | No | Serum | <30 min | Moderate |
| 47 | Electrochemical | No | Serum | <30 min | Moderate |
| 48 | Optical | Yes | Blood | 20 min | Minimal |
| 49 | Optical | Yes | Blood | 30 min | Minimal |
| 50 | Optical | No | Buffer | >4 hrs | Substantial |
| 51 | Fluorescence | Yes | Buffer | >30 min | Substantial |
| 52 | Fluorescence | No | Buffer | 2.5 hrs | Substantial |
| 53 | Fluorescence | No | Serum | 30 min | Moderate |
| 54 | Fluorescence | Yes | Serum | 30 min | Moderate |
| 56 | Chemiluminescent | Yes | Serum | 60 min | Substantial |
| 57 | Chemiluminescent | No | Plasma | <10 min | Substantial |
| 58 | Chemiluminescent | Yes | Blood | 35 min | Minimal |
| 59 | Colorimetric | Yes | Serum | 1 hour | Moderate |
| 60 | Colorimetric | Yes | Blood | 20 min | Minimal |
All the impedance based microfluidic sensors discussed in this paper can be used for quantitative detection of antibodies with great precision. These sensors, however, are not without their own drawbacks and limitations. As listed in Table I, almost all these sensors require serum samples for the detection of antibodies and none of them can work directly with human blood samples. Moreover, most of these sensors use bead-based sandwich type immune assay which requires long incubation times. Lastly, for quantification of electrical signals, they depend on bulky lock in amplifiers which cannot be used at POC. These problems can however be addressed, using a plasma separation unit can make these sensors capable of working with human blood samples [61], replacing the commercial lock-in amplifier with a portable one [62], and on chip incubation of beads with serum samples can be performed to minimize manual processing. This will require considerable effort and time.
Microfluidic sensors employing electrochemical detection discussed in this paper are all quantitative in nature. When compared to impedance-based sensors, these sensors are more mature and can efficiently quantify antibodies in human serum in under 30 minutes but have some limitations of their own. Just like impedance-based sensors, they also cannot work directly with human blood samples, cannot do multiplexed detection, and require bulky instrumentation for the quantification of electrical signals. Moreover, most of these sensors also require multiple washing and incubation steps which need to be automated before usage at POC. The inclusion of a plasma separation stage, and the automation of the multiple washing and incubation steps can address these limitations. This will however require time and effort.
The optical microfluidic devices discussed in [48], [49] in this paper are both capable of quantitative multiplexed detection of antibodies in human blood samples in under 30 minutes. The immunoassay card based microfluidic sensor [49] needs some minute modifications for the quantification of antibodies at POC but the effort required to do so should be minimal. The microfluidic device discussed in [48] specifically, has already been field tested in Rwanda to quantify antibodies against HIV and Treponema pallidum (the causative agent of syphilis) from needle-prick sample volumes of blood samples with great accuracy. Therefore, in our opinion, it can be quickly deployed for the quantification of COVID-19 specific antibodies in human blood samples by making minute changes.
Some fluorescence based microfluidic devices are also capable of quantitative multiplexed detection of antibodies. Similar to impedance and electrochemical devices, these devices are as of now not capable of working with whole blood and require multiple washing and incubation steps which need to be automated. Traditionally, the requirement of bulky fluorescent microscopes, or photomultiplier tubes for detection and quantification of the fluorescent signals has posed challenges towards large scale adoption of these fluorescent devices. However, the usage of these devices in conjunction with portable smartphone-based fluorescent microscopes such as the ones discussed in [63] and [64] may be used to overcome this challenge.
Chemiluminescence based microfluidic devices reviewed in this paper rely on quantifying antibodies using enzyme-linked immunosorbent assay (ELISA) and Luciferase Immunoprecipitation Systems (LIPS) technology in a microfluidic format. Both these techniques require multiple washing and incubation steps in addition to the requirement of bulky and expensive chemiluminescent signal detectors. Authors in [58] automated all these steps and miniaturized the detection platform as well for multiplexed quantification of antibodies in whole blood in about 35 minutes. They also field tested their platform in North western Kenya for the quantification anti-measles IgG and anti-rubella IgG in children with great accuracy. Therefore, in our opinion, their microfluidic platform can also be urgently deployed for the quantification of Covid-19 antibodies in humans at POC with minimal changes.
The colorimetric microfluidic devices discussed rely on the principles of ELISA and bioluminescence resonance energy transfer (BRET) switches for analyte recognition and signal generation. Both ELISA and BRET based devices can be used for multiplexed quantitative detection of antibodies, however, the ELISA based device discussed in [59] is limited to working with serum samples only and has an assay time of about 1 hour which makes its usage difficult at POC. On the other hand, BRET based device [60] is capable of working with blood samples and can simultaneously be used for the quantification of up to 3 target analytes simultaneously in just 20 minutes which makes it ideal for usage at POC applications. Furthermore, the working of the device is also a lot simpler compared to the devices that were pointed out earlier [48], [58] for usage at POC. One small drawback though is the fact that this device has not been field tested before like [48], [58].
Funding Statement
This work was supported by the National Science Foundation under Grant 1711165.
Contributor Information
Muhammad Tayyab, Email: muhammad.tayyab@rutgers.edu.
Muhammad Ahsan Sami, Email: ahsan.sami@rutgers.edu.
Hassan Raji, Email: hassan.raji@rutgers.edu.
Srinivas Mushnoori, Email: scm177@scarletmail.rutgers.edu.
Mehdi Javanmard, Email: mehid.javanmard@rutgers.edu.
References
- [1].(2020). WHO Coronavirus Disease (COVID-19) Dashboard. Covid19.who.int. Accessed: Oct. 15, 2020. [Online]. Available: https://covid19.who.int/
- [2].(2020). Europe Hopes Summer Tourism Will Boost Economy Amid COVID-19. Time. Accessed: Aug. 8, 2020. [Online]. Available: https://time.com/5859217/europe-tourism-coronavirus/
- [3].(2020). U.S. Unemployment Rate Soars to 14.7 Percent, the Worst Since the Depression Era. Accessed: Jun. 4, 2020. [Online]. Available: https://www.washingtonpost.com/business/2020/05/08/april-2020-jobs-report/
- [4].(2020). Coronavirus Could Cost the Global Economy 2.7 Trillion. Here’s How. Bloomberg.com. Accessed: Jun. 4, 2020. [Online]. Available: https://www.bloomberg.com/graphics/2020-coronavirus-pandemic-global-economic-risk/
- [5].(2020). Ethics.Harvard.Edu. Accessed: Jun. 4, 2020. [Online]. Available: https://ethics.harvard.edu/files/center-for-ethics/files/roadmaptopandemicresilience_updated_4.20.20.pdf
- [6].Sethuraman N., Jeremiah S. S., and Ryo A., “Interpreting diagnostic tests for SARS-CoV-2,” Jama, vol. 323, no. 22, p. 2249, Jun. 2020. Accessed: Jun. 4, 2020, doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- [7].Ripperger T. J.et al. , “Orthogonal SARS-CoV-2 serological assays enable surveillance of low-prevalence communities and reveal durable humoral immunity,” Immunity, Oct. 2020, doi: 10.1016/j.immuni.2020.10.004. [DOI] [PMC free article] [PubMed]
- [8].Wu X., Fu B., Chen L., and Feng Y., “Serological tests facilitate identification of asymptomatic SARS-CoV-2 infection in Wuhan, China,” J. Med. Virol., vol. 92, no. 10, pp. 1795–1796, 2020, doi: 10.1002/jmv.25904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li Z.et al. , “Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis,” J. Med. Virol., vol. 92, no. 9, pp. 1518–1524, 2020, doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].(2020). COVID-19 IgM/IgG Rapid Test–BioMedomics Inc. Accessed: Jun. 4, 2020. [Online]. Available: https://www.biomedomics.com/products/infectious-disease/covid-19-rt/
- [11].(2020). COVID-19, lgG/lgM Rapid Test, Antibody Detection, Blood Test, Serological Test, Coronavirus Disease, Lateral Flow Immunoassay. Accessed: Jun. 4, 2020. [Online]. Available: https://cellexcovid.com/
- [12].(2020). AdvaGen Biotech. Accessed: Jun. 4, 2020. [Online]. Available: http://advagen.tecnologia.ws/produtos/detalhado/413/COVID-19-IgG-IgM-LF
- [13].(2020). Chembio |DPP COVID-19 IgM/IgG System. Accessed: Jun. 4, 2020. [Online]. Available: http://chembio.com/dpp-covid-19-igm-igg-system/
- [14].(2020). Celer Biotecnologia S.A. Accessed: Jun. 4, 2020. [Online]. Available: https://celer.ind.br/
- [15].(2020). New! COVID-19 IgG/IgM Rapid Test–CTK Biotech. Accessed: Jun. 4, 2020. [Online]. Available: https://ctkbiotech.com/2020/04/15/new-covid-19-igg-igm-rapid-test/
- [16].(2020). SELL SHEET COVID-19 IgG/IgM Rapid Test-Hangzhou Biotest Biotech Co., LTD. Accessed: Jun. 4, 2020. [Online]. Available: http://en.biotests.com.cn/newsitem/278470281
- [17].(2020). Cov-2 RAPID TEST–Test Kit for SARS-CoV-2-Detects IgM/IgG |PharmACT. Accessed: Jun. 4, 2020. [Online]. Available: https://pharmact-health.com/en/sars-cov-2-rapid-test/
- [18].(2020). Products–STANDARD Q COVID-19 IgM/IgG Duo. Accessed: Jun. 4, 2020. [Online]. Available: http://sdbiosensor.com/xe/product/7662
- [19].(2020). Artron |Diagnostics Made Simple. Accessed: Jun. 4, 2020. [Online]. Available: http://www.artronlab.com/home.html
- [20].(2020). COVID-19 15 min RAPID POC Test. Accessed: Jun. 4, 2020. [Online]. Available: https://www.assaygenie.com/covid-19-15-min-rapid-poc-test
- [21].Anti-SARS-CoV-2 Rapid Test. Accessed: Jun. 4, 2020. [Online]. Available: https://www.fda.gov/media/137367/download
- [22].(2020). Bioscience (Tianjin) Diagnostic Technology Co. Ltd. Accessed: Jun. 4, 2020. [Online]. Available: http://www.bioscience-tj.com/en/newn.php?id=1000077
- [23].Lassaunière R.et al. , “Evaluation of nine commercial SARS-CoV-2 immunoassays,” medRxiv, 2020, Art. no. 2020.04.09.20056325, doi: 10.1101/2020.04.09.20056325. [DOI] [Google Scholar]
- [24].Sciences L., Biology P., Center P., Library P., Methods P., and Elisa O.. (2020). Overview of ELISA | Thermo Fisher Scientific–UK. Accessed: Jul. 13, 2020. [Online]. Available: https://www.thermofisher.com/us/en/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/overview-elisa.html [Google Scholar]
- [25].Boguszewska K., Szewczuk M., Urbaniak S., and Karwowski B. T., “Review: Immunoassays in DNA damage and instability detection,” Cellular Mol. Life Sci., vol. 76, no. 23, pp. 4689–4704, Dec. 2019. Accessed: Oct. 18, 2020, doi: 10.1007/s00018-019-03239-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kong W.-H.et al. , “SARS-CoV-2 detection in patients with influenza-like illness,” Nature Microbiol., vol. 5, no. 5, pp. 675–678, May 2020, doi: 10.1038/s41564-020-0713-1. [DOI] [PubMed] [Google Scholar]
- [27].(2020). ELISA for Novel Coronavirus (2019-nCoV, SARS-Cov-2) Causing an Outbreak of Pneumonia (COVID-19)–Epitope Diagnostics, Inc. Accessed: Jun. 4, 2020. [Online]. Available: http://www.epitopediagnostics.com/covid-19-elisa
- [28].(2020). Ortho’s COVID-19 Answer. Accessed: Jun. 4, 2020. [Online]. Available: https://www.orthoclinicaldiagnostics.com/en-us/home/ortho-covid-19-answer
- [29].(2020). Anti-SARS-CoV-2 ELISA (IgG). Accessed: Jun. 4, 2020. [Online]. Available: https://www.fda.gov/media/137609/download
- [30].(2020). SARS-CoV-2 Immunoassay Kits - Creative Diagnostics. Accessed: Jun. 4, 2020. [Online]. Available: https://www.creative-diagnostics.com/news-sars-cov-2-immunoassay-kits-83.htm
- [31].(2020). COVID-19 Products. Accessed: Jun. 4, 2020. [Online]. Available: https://www.sanbio.nl/resources/news/covid-19/
- [32].(2020). Snibe Co., Ltd. Accessed: Jun. 4, 2020. [Online]. Available: http://www.snibe.com/zh_en/en_newsView.aspx?id=576
- [33].(2020). LIAISON SARS-CoV-2 S1/S2 IgG. Accessed: Jun. 4, 2020. [Online]. Available: https://www.fda.gov/media/137359/download
- [34].(2020). Accelerated Emergency Use Authorization (EUA) Summary New York SARS-COV Microsphere Immunoassay for Antibody Detection. Accessed: Jun. 4, 2020. [Online]. Available: https://www.fda.gov/media/137541/download
- [35].(2020). Elecsys Anti-SARS-CoV-2. Accessed: Jun. 4, 2020. [Online]. Available: https://www.fda.gov/media/137605/download
- [36].(2020). SARS-CoV-2 Immunoassay |Abbott Core Laboratory. Accessed: Oct. 18, 2020. [Online]. Available: https://www.corelaboratory.abbott/us/en/offerings/segments/infectious-disease/sars-cov-2
- [37].(2020). SARS-CoV-2 Total Assay. Accessed: Oct. 18, 2020. [Online]. Available: https://www.siemens-healthineers.com/en-us/laboratory-diagnostics/assays-by-diseases-conditions/infectious-disease-assays/cov2t-assay
- [38].Zheng G., Patolsky F., Cui Y., Wang W. U., and Lieber C. M., “Multiplexed electrical detection of cancer markers with nanowire sensor arrays,” Nature Biotechnol., vol. 23, no. 10, pp. 1294–1301, Oct. 2005, doi: 10.1038/nbt1138. [DOI] [PubMed] [Google Scholar]
- [39].Javanmard M., Talasaz A. H., Nemat-Gorgani M., Pease F., Ronaghi M., and Davis R. W., “Electrical detection of protein biomarkers using bioactivated microfluidic channels,” Lab a Chip, vol. 9, no. 10, p. 1429, 2009, doi: 10.1039/b818872f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Valera E.et al. , “A microfluidic biochip platform for electrical quantification of proteins,” Lab a Chip, vol. 18, no. 10, pp. 1461–1470, 2018, doi: 10.1039/c8lc00033f. [DOI] [PubMed] [Google Scholar]
- [41].Mok J., Mindrinos M. N., Davis R. W., and Javanmard M., “Digital microfluidic assay for protein detection,” Proc. Nat. Acad. Sci. USA, vol. 111, no. 6, pp. 2110–2115, Feb. 2014, doi: 10.1073/pnas.1323998111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rodriguez-Trujillo R.et al. , “Label-free protein detection using a microfluidic coulter-counter device,” Sens. Actuators B, Chem., vol. 190, pp. 922–927, Jan. 2014, doi: 10.1016/j.snb.2013.09.038. [DOI] [Google Scholar]
- [43].Lin Z., Cao X., Xie P., Liu M., and Javanmard M., “PicoMolar level detection of protein biomarkers based on electronic sizing of bead aggregates: Theoretical and experimental considerations,” Biomed. Microdevices, vol. 17, no. 6, p. 119, Dec. 2015, doi: 10.1007/s10544-015-0022-2. [DOI] [PubMed] [Google Scholar]
- [44].Dong H., Li C.-M., Zhang Y.-F., Cao X.-D., and Gan Y., “Screen-printed microfluidic device for electrochemical immunoassay,” Lab a Chip, vol. 7, no. 12, p. 1752, 2007, doi: 10.1039/b712394a. [DOI] [PubMed] [Google Scholar]
- [45].Pereira S. V.et al. , “A microfluidic device based on a screen-printed carbon electrode with electrodeposited gold nanoparticles for the detection of IgG anti-trypanosoma cruzi antibodies,” Analyst, vol. 136, no. 22, p. 4745, 2011, doi: 10.1039/c1an15569e. [DOI] [PubMed] [Google Scholar]
- [46].Pereira S. V., Messina G. A., and Raba J., “Integrated microfluidic magnetic immunosensor for quantification of human serum IgG antibodies to helicobacter pylori,” J. Chromatography B, vol. 878, no. 2, pp. 253–257, Jan. 2010, doi: 10.1016/j.jchromb.2009.05.007. [DOI] [PubMed] [Google Scholar]
- [47].Pereira S. V., Raba J., and Messina G. A., “IgG anti-gliadin determination with an immunological microfluidic system applied to the automated diagnostic of the celiac disease,” Anal. Bioanal. Chem., vol. 396, no. 8, pp. 2921–2927, Apr. 2010, doi: 10.1007/s00216-010-3589-8. [DOI] [PubMed] [Google Scholar]
- [48].Chin C. D.et al. , “Microfluidics-based diagnostics of infectious diseases in the developing world,” Nature Med., vol. 17, no. 8, pp. 1015–1019, Aug. 2011, doi: 10.1038/nm.2408. [DOI] [PubMed] [Google Scholar]
- [49].Lafleur L.et al. , “Progress toward multiplexed sample-to-result detection in low resource settings using microfluidic immunoassay cards,” Lab a Chip, vol. 12, no. 6, p. 1119, 2012, doi: 10.1039/c2lc20751f. [DOI] [PubMed] [Google Scholar]
- [50].Lee K.-H., Su Y.-D., Chen S.-J., Tseng F.-G., and Lee G.-B., “Microfluidic systems integrated with two-dimensional surface plasmon resonance phase imaging systems for microarray immunoassay,” Biosensors Bioelectron., vol. 23, no. 4, pp. 466–472, Nov. 2007, doi: 10.1016/j.bios.2007.05.007. [DOI] [PubMed] [Google Scholar]
- [51].Sung Kim K. and Park J.-K., “Magnetic force-based multiplexed immunoassay using superparamagnetic nanoparticles in microfluidic channel,” Lab a Chip, vol. 5, no. 6, p. 657, 2005, doi: 10.1039/b502225h. [DOI] [PubMed] [Google Scholar]
- [52].Miller E. M., Ng A. H. C., Uddayasankar U., and Wheeler A. R., “A digital microfluidic approach to heterogeneous immunoassays,” Anal. Bioanal. Chem., vol. 399, no. 1, pp. 337–345, Jan. 2011, doi: 10.1007/s00216-010-4368-2. [DOI] [PubMed] [Google Scholar]
- [53].Seia M. A., Pereira S. V., Fontán C. A., De Vito I. E., Messina G. A., and Raba J., “Laser-induced fluorescence integrated in a microfluidic immunosensor for quantification of human serum IgG antibodies to helicobacter pylori,” Sens. Actuators B, Chem., vol. 168, pp. 297–302, Jun. 2012, doi: 10.1016/j.snb.2012.04.026. [DOI] [Google Scholar]
- [54].Lee Y.-F., Lien K.-Y., Lei H.-Y., and Lee G.-B., “An integrated microfluidic system for rapid diagnosis of dengue virus infection,” Biosensors Bioelectron., vol. 25, no. 4, pp. 745–752, Dec. 2009, doi: 10.1016/j.bios.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ahn D.-G.et al. , “RNA aptamer-based sensitive detection of SARS coronavirus nucleocapsid protein,” Analyst, vol. 134, no. 9, p. 1896, 2009, doi: 10.1039/b906788d. [DOI] [PubMed] [Google Scholar]
- [56].Heyries K. A., Loughran M. G., Hoffmann D., Homsy A., Blum L. J., and Marquette C. A., “Microfluidic biochip for chemiluminescent detection of allergen-specific antibodies,” Biosensors Bioelectron., vol. 23, no. 12, pp. 1812–1818, Jul. 2008, doi: 10.1016/j.bios.2008.02.025. [DOI] [PubMed] [Google Scholar]
- [57].Zubair A., Burbelo P. D., Vincent L. G., Iadarola M. J., Smith P. D., and Morgan N. Y., “Microfluidic LIPS for serum antibody detection: Demonstration of a rapid test for HSV-2 infection,” Biomed. Microdevices, vol. 13, no. 6, pp. 1053–1062, Dec. 2011, doi: 10.1007/s10544-011-9575-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ng A. H. C.et al. , “A digital microfluidic system for serological immunoassays in remote settings,” Sci. Transl. Med., vol. 10, no. 438, Apr. 2018, Art. no. eaar6076, doi: 10.1126/scitranslmed.aar6076. [DOI] [PubMed] [Google Scholar]
- [59].Sanjay S. T., Dou M., Sun J., and Li X., “A paper/polymer hybrid microfluidic microplate for rapid quantitative detection of multiple disease biomarkers,” Sci. Rep., vol. 6, no. 1, Sep. 2016, Art. no.030474, doi: 10.1038/srep30474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Tenda K., van Gerven B., Arts R., Hiruta Y., Merkx M., and Citterio D., “Paper-based antibody detection devices using bioluminescent BRET-switching sensor proteins,” Angew. Chem., vol. 130, no. 47, pp. 15595–15599, 2018, doi: 10.1002/ange.201808070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Xue X., Patel M. K., Kersaudy-Kerhoas M., Desmulliez M. P. Y., Bailey C., and Topham D., “Analysis of fluid separation in microfluidic T-channels,” Appl. Math. Model., vol. 36, no. 2, pp. 743–755, Feb. 2012, doi: 10.1016/j.apm.2011.07.009. [DOI] [Google Scholar]
- [62].Furniturewalla A., Chan M., Sui J., Ahuja K., and Javanmard M., “Fully integrated wearable impedance cytometry platform on flexible circuit board with online smartphone readout,” Microsyst. Nanoeng., vol. 4, no. 1, p. 20, Dec. 2018, doi: 10.1038/s41378-018-0019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sami M. A., Wagner K., Parikh P., and Hassan U., “Smartphone based microfluidic biosensor for leukocyte quantification at the Point-of-Care,” in Proc. IEEE Healthcare Innov. Point Care Technol. (HI-POCT), Bethesda, MD, USA, Nov. 2019, pp. 119–122, doi: 10.1109/HI-POCT45284.2019.8962697. [DOI] [Google Scholar]
- [64].Ghonge T.et al. , “Smartphone-imaged microfluidic biochip for measuring CD64 expression from whole blood,” Analyst, vol. 144, no. 13, pp. 3925–3935, Jun. 2019. Accessed: Oct. 15, 2020, doi: 10.1039/c9an00532c. [DOI] [PubMed] [Google Scholar]