Abstract
Eating palatable foods reduces behavioral and hypothalamic-pituitary-adrenocortical (HPA) axis responses to stress – an idea referred to by the colloquial term “comfort” food. To study the underlying stress-relieving mechanisms of palatable foods, we previously developed a paradigm of limited sucrose feeding in which male rats are given twice-daily access to a small amount of sucrose drink and subsequently have reduced stress responses. Prior research in humans and rodents implicates high dietary sugars/carbohydrates with reduced stress responsivity. However, it is not clear whether the stress relieving effects the limited sucrose paradigm depend upon its macronutrient content. To test this idea, the current work measures stress responses in male rats following the limited intermittent intake of cheese – a highly palatable food that is low in sugar and other carbohydrates. The data show that a history of limited cheese intake (LCI) reduced HPA axis responses to acute psychological (restraint) and physiological (hypoxia) stressors. LCI also reduced behavioral struggling during restraint, increased sociability during a social interaction test, and increased open arm activity in the elevated plus-maze test. Z-score analyses evaluated the extent to which these behavioral effects extended within and across assays, and indicated that there was an overall reduction in stress-related behaviors following LCI. Finally, LCI increased immunolabeling for FosB/deltaFosB (a protein associated with repeated or chronic neuronal activation) in the nucleus accumbens. These results indicate that palatable foods can provide stress blunting regardless of their sugar/carbohydrate composition, and support the idea that food reward per se contributes to stress relief.
Keywords: Cheese, palatable food, corticosterone, anxiety-related behavior, deltaFosB
1. Introduction
Individuals respond to real or perceived threats to homeostasis or well-being (i.e., stressors), with a combination of physiological, emotional, and behavioral responses. The physiological responses to stress include activation of the hypothalamic-pituitary-adrenocortical (HPA) axis, resulting in elevated levels of circulating glucocorticoid hormones (Ulrich-Lai and Herman 2009). The emotional and behavioral responses include irritability, reduced sociability, diminished mood, and increased threat avoidance (or “anxiety”) (Packard et al. 2016). Importantly, the extent which these various stress responses occur is modifiable, with diet and nutritional state playing an important role in establishing stress responsivity.
Approximately 35–70% of people increase their total caloric intake during times of stress (Epel et al. 2004, Kandiah et al. 2006, Oliver and Wardle 1999, Weinstein et al. 1997, Zellner et al. 2006). Stress also promotes the consumption of highly-palatable, calorically-dense foods relative to less palatable, more nutritious alternatives (Barrington et al. 2014, Cartwright et al. 2003, Epel et al. 2001, Groesz et al. 2012, Kandiah et al. 2006, Laugero et al. 2011, Oliver and Wardle 1999, Wardle et al. 2000), even among many people who do not increase their total caloric intake during stress (Oliver and Wardle 1999). When individuals are asked why they eat these foods during stress, the majority reply that the primary reason is because these foods make them feel better (i.e., more relaxed or comforted) (Zellner et al. 2006). This supports the colloquial idea of “comfort” eating, in which many individuals alter their dietary patterns as a means of stress relief. Indeed, the consumption of palatable comfort foods is associated with improved emotional states and blunted HPA axis stress reactivity in people (Dube et al. 2005, Finch and Tomiyama 2015, Macht and Mueller 2007, Markus et al. 2000, Tomiyama et al. 2011, Tryon et al. 2013, Tryon et al. 2015).
To study the underlying stress-relieving mechanisms for this “comfort” feeding effect, we developed a paradigm of limited sucrose feeding in rats that is intended to mimic typical human “snacking” patterns (Ulrich-Lai et al. 2007). In this paradigm, adult rats with free access to normal chow and water are offered additional brief access to a small amount (up to 4 ml) of 30% sucrose drink twice-daily for upwards of a week, with controls instead receiving a second bottle with water. The rats typically begin to drink the full amount of offered sucrose (i.e., 8 ml/d, or ~9 kcal/d) within the first few days of access, and reduce their normal chow intake isocalorically, thereby maintaining body weight and body composition at the same levels as water controls (Christiansen et al. 2011, Egan et al. 2019, Egan and Ulrich-Lai 2015, Ulrich-Lai et al. 2010, Ulrich-Lai et al. 2011, Ulrich-Lai et al. 2007). Notably, the history of limited sucrose intake (LSI) attenuates HPA axis and behavioral responses to stress (Christiansen et al. 2011, Egan et al. 2019, Egan et al. 2018, Ulrich-Lai et al. 2010, Ulrich-Lai et al. 2011, Ulrich-Lai et al. 2007), suggesting that the paradigm is ideal for studying the mechanisms by which palatable foods confer stress protection, while limiting the potential confounding effect of palatable food-induced obesity.
An important unresolved question regarding the limited sucrose paradigm is the extent to which its stress relieving effects depend upon its nutritional content. On the one hand, there is a large amount of evidence indicating that high dietary carbohydrates and/or sugars reduce physiological and/or emotional responses to stress in people and rodents (Anderson et al. 1987, Bell et al. 2002, Markus et al. 2000, Strack et al. 1997, Suchecki et al. 2003, Tryon et al. 2015, Utter et al. 1999). But on the other hand, our prior work indicates that the stress-blunting effects of LSI are replicated by the consumption of a non-caloric artificial sweetener, and prevented by when the sucrose is placed directly into the stomach via gavage (Ulrich-Lai et al. 2010, Ulrich-Lai et al. 2007). These observations suggest that the pleasurable and rewarding properties of sucrose, as opposed to its carbohydrate composition, provide the stress relief. To test this idea, the current work measures stress responses in rats following the limited intermittent intake of a highly palatable, low-carbohydrate food – cheese. We hypothesized the limited cheese intake (LCI) will effectively reduce HPA axis activity and stress-related behaviors (i.e., reducing threat avoidance, or increasing threat appraisal and sociability) similar to the effects we have previously reported for LSI. Moreover, as immunolabeling for deltaFosB (a protein associated with repeated or chronic neuronal activation (Nestler et al. 1999)) is increased in the nucleus accumbens (NAc) core by repeated reward experiences, including drugs of abuse and limited sucrose feeding (Christiansen et al. 2011, Nestler et al. 1999, Wallace et al. 2008), we evaluated the extent that LCI similarly impacts FosB/deltaFosB protein expression.
Finally, there is general agreement that to assess a particular emotional state (e.g., threat appraisal/avoidance), multiple behavioral assays should be utilized, many of which include several relevant behavioral end points. However, this raises the key question of how to evaluate the extent to which treatment effects extend across multiple behavioral endpoints within an assay, as well as across different assays. Z-score analyses have been proposed as a means to normalize, compare and combine behavioral end points that otherwise have distinct units, thereby allowing identification of treatment effects that generalize (Guilloux et al. 2011, Page and Coutellier 2018, Shepard et al. 2016). We therefore used z-score analyses to evaluate the extent to which the behavioral effects of LCI extend within and across assays and compare the results to a more traditional approach where each end point is analyzed independently.
2. Materials and Methods
2.1. Animals
Adult male Long-Evans rats (250–275 g body weight) were purchased from Envigo (Indianapolis, IN). Rats were single-housed (to facilitate accurate monitoring of food and drink intake) in a temperature- and humidity-controlled housing facility on a 12/12-hour light cycle (lights on at 06:00 h, lights off at 18:00 h). Housing facilities were accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All procedures were approved by the University of Cincinnati Institutional Animal Care and Use Committee and are consistent with the United States‟ National Institutes of Health “Guide for the Care and Use of Laboratory Animals”.
2.2. Experimental overview
Figure 1 illustrates the time course of the experimental procedures. On experimental day 0, rats were weighed, and body composition was measured via nuclear magnetic resonance (NMR, EchoMRI, Houston, TX). Rats were then divided into two treatment groups (n=12 cheese, n=12 chow) that were matched for initial body weight and percent body fat to minimize the potential confound of differences in starting body weight and composition. The limited LCI paradigm began on the following day (d1) and continued through d28. HPA axis responsivity to an acute stressor was determined using two different types of stress – restraint and hypoxia – on d14 and d22, respectively. The use of two different types of HPA axis stressors establishes the generalizability of any treatment effects, and the use of restraint permits the analysis of stress-related struggling behavior concordant with the HPA axis assessment. Sociability was evaluated in a social interaction test on d18–19. Threat appraisal (i.e., exploratory behaviors evaluating the possibility of and degree of threat) vs. avoidance (i.e., behaviors that minimize exposure to and interaction with potential threats) was assessed in the novel object interaction test on d11 and in the elevated plus-maze test on d25. The experiment concluded on d29 with the collection of the brains in a non-stressed state for measurement of FosB/deltaFosB immunolabeling in the nucleus accumbens core. Each procedure is described in greater detail below.
Figure 1.
Experimental timeline. Schematic representation of the timing of each assessment relative to the onset of the limited cheese intake (LCI) paradigm on experimental day (d) 1.
2.3. Limited cheese intake (LCI) paradigm
Rats were maintained with free access to normal chow (LM-485, product #7012, Teklad Diets, Madison, WI) and water. Twice-daily, at 10:00 h and 16:30 h, rats were offered an additional piece of cheese (Colby Jack, Meijer store brand, Grand Rapids, MI) that was pre-weighed to contain ~4.5 kcal. This cheese amount was calorically matched to that provided by the twice-daily limited sucrose paradigm, and contained ~33% fat, 24% protein and 5% carbohydrate by weight (based on the food package labeling). The feeding session began with dropping the cheese onto the floor of the cage, and after 30 min, any remaining cheese was removed and weighed to determine the amount of „snack‟ consumed. Control rats instead received a small piece of normal chow that was pre-weighed to contain the same number of calories (4.5 kcal). This piece of chow was dyed with a small amount of food coloring (McCormick and Company, Inc., Hunt Valley, MD) to permit the identification, removal and weighing of its remains after 30 min (i.e., to distinguish it from any other pieces of chow that the rat had pulled from its hopper). To evaluate the effects of LCI on energy balance, body weight and non-snack food intake (i.e., ad libitum chow) were monitored throughout experiment, and body composition was re-assessed by NMR on d28.
2.4. Novel object interaction test
The novel object interaction test balances the motivation to explore a novel object with the avoidance of the center of an open field arena (Abuhamdah et al. 2012, Ennaceur et al. 2005, Haba et al. 2012). This test was performed on d11 between 11:30–16:00 h (i.e., between that day‟s snack sessions). Rats were individually placed for 5 minutes (min) into a plastic open field arena (~1 m x ~1 m) that contained a novel object at its center. The novel object was comprised of plastic toy blocks (a pyramid shape affixed to the top of a cube), and was taped to the bottom of the arena to prevent its movement by the rats. Rat behavior was video recorded for later analysis by two observers that were unaware of treatment group assignments. Both observers scored the number and duration of interaction events (defined as touching or sniffing the object) and the average of the scores was taken as the value for each rat. The observers‟ scores were typically within 10% of each other; if larger discrepancies occurred, then that rat‟s behavior was rescored to reach consensus.
2.5. HPA axis and behavioral struggling responses to an acute restraint stressor
On the morning of d14, rats were given a 20-min restraint stress as described previously (Christiansen et al. 2011, Egan et al. 2018, Ulrich-Lai et al. 2010, Ulrich-Lai et al. 2011, Ulrich-Lai et al. 2007). Prior to testing, rats were left undisturbed in their home cages in their normal housing room to prevent any stress-associated HPA axis activity, and hence did not receive the cheese/chow snack paradigm that day. The home cage for each rat was then individually carried to an adjacent procedure room, where each rat was placed into a well-ventilated plastic restraint tube. A tail-clip blood sample (200 μl) was rapidly collected into ice-cold EDTA-coated tubes. This first blood collection (i.e., at 0 min) was completed within 3 min of first touching/moving each rat‟s home cage to ensure assessment of basal, pre-stress plasma corticosterone levels (Vahl et al. 2005). Post-stress blood samples were collected at 20, 40 and 60 min after stress onset, and were all completed within 3 min. Blood samples were centrifuged (3000 x g, 15 min, 4°C) and plasma was stored at −80°C until measurement of plasma corticosterone by radioimmunoassay (product #07120103, MP Biomedicals, Solon, OH). The minimum detection of this assay is 7.7 ng/ml, and the intra-assay and inter-assay coefficients of variance are both 7%, as indicated by the manufacturer.
Rat behavior during the restraint was also video recorded for later analysis by two observers that were unaware of treatment group assignments. Struggling behaviors were defined as any attempt to make or find a way out of the restraint tube, including biting, chewing, scratching or pushing at the walls of the restrainer, or attempting to turn or spin around in the restrainer (Egan et al. 2019, Grissom et al. 2008, Weinberg et al. 2010). Each rat‟s value was taken as the mean of the two observers‟ scores, and inter-observer consensus was reached as described above. Since we have previously observed that struggling behavior can vary across the duration of the restraint (Egan et al. 2019), data were grouped into consecutive 5-min segments for analysis.
2.6. Social interaction test
Sociability testing occurred over 2 days (d18–19), with half of the rats in each treatment group being tested on each day, so that testing could be completed between the morning and afternoon snack sessions. The social interaction apparatus was comprised of 3 chambers; the center chamber was connected to the left and right chambers by an opening in the wall, allowing rats to freely move across the 3 chambers. Rats were first placed into the chamber for 5 min to habituate to the testing apparatus; during habituation, the left and right chambers each contained an empty rat enclosure. These enclosures were upright plastic cylinders with solid bottoms and tops, and walls that were vertical bars to limit physical contact between the test rat and its social target. The test phase occurred immediately following habituation. During the test phase, a novel male conspecific was placed into one of the enclosures (as a social target), while the other enclosure remained empty (as a non-social target). The test rats were allowed to freely explore the apparatus for 10 min while their behavior was video recorded for later analysis by two observers that were unaware of treatment group assignments. Both observers scored the number of entries and time spent in the social vs. non-social chambers, as well as the number and duration of sniffing events for the social and non-social targets. Sniffing events were defined as the test rat placing its nose against the enclosure and/or between its vertical bars in an attempt to smell the enclosure and/or its contents. Each rat‟s value was taken as the mean of the two observers‟ scores, and inter-observer consensus was reached as described above. The preference for a social target was calculated by expressing the time spent sniffing the social target as a percentage of the total time sniffing both the social and non-social targets (Lo et al. 2016).
2.7. HPA axis response to an acute hypoxia stressor
On the morning of d22, rats were given a 20-min hypoxia stress. Acute hypoxia was used because it is a physiological stressor that has been shown to produce transient HPA axis activation of a similar magnitude and duration as restraint stress (Jones et al. 2011, Ostrander et al. 2006). As for the restraint stressor, rats were left undisturbed prior to hypoxia stress testing and did not receive the cheese/chow snack paradigm that day. The home cage for each rat was individually carried to an adjacent procedure room, where the rats were placed into a chamber containing 8% oxygen/92% nitrogen for 20 min. Immediately after the hypoxia stressor (i.e., at 20 min after stress onset), a tail-clip blood sample (200 μl) was rapidly collected into ice-cold EDTA-coated tubes. Additional post-stress blood samples were collected at 40 and 60 min after stress onset. All blood sample collections were completed within 3 min. Plasma corticosterone levels were measured as described above. Research staff noted that there were technical difficulties with one of the hypoxia chambers on the day of this experiment that prevented it from becoming fully hypoxic for 3 rats/group; the data from these rats were therefore removed from the plasma corticosterone analysis.
2.8. Elevated plus-maze test
An elevated plus-maze test was performed on d25 (between the morning and afternoon snack sessions) as an assay of threat appraisal vs. avoidance (or “anxiety-related”) behavior (Cruz et al. 1994, File et al. 2004, Pellow et al. 1985). Rats were placed into an open arm of an elevated plus-maze apparatus (immediately adjacent to and facing the center of the maze) and were allowed to explore for 5 min. The apparatus had an approximate arm size of 4-inches wide X 40-inches long, with 14-inch high black walls on the closed arms. Rat behavior was video recorded for later analysis by two observers that were unaware of treatment group assignments. Observers scored the number of open and closed arm entries, the time spent in the open arms, and the time spent performing head dipping behavior. Head dipping was defined as the rat moving its head beyond the edge of the apparatus (e.g., to look over the edge of the open arm). Each rat‟s value was taken as the mean of the two observers‟ scores, and inter-observer consensus was reached as described above. Since we (Egan et al. 2019) and others (Arabo et al. 2014, Bertoglio and Carobrez 2002, Carobrez and Bertoglio 2005, Casarrubea et al. 2013) have previously observed that elevated plus-maze behavioral patterns vary across the duration of the test, data were grouped into consecutive 1-min segments for analysis.
2.9. Brain collection and immunolabeling for FosB/deltaFosB
On the morning of d29, rats were given a pentobarbital (ip) anesthesia overdose prior to transcardiac perfusion with 0.9% saline and 3.7% paraformaldehyde. The rats were undisturbed (i.e., not stressed or handled) prior to receiving the pentobarbital and did not receive the cheese/chow snack paradigm that day. Brains were then removed and post-fixed overnight in 3.7% paraformaldehyde at room temperature, after which they were stored in a solution of 30% sucrose in phosphate-buffered saline (PBS) at 4°C. Brains were sectioned (25 μm) in a 1-in-12 series on a freezing-stage microtome (Leica Biosystems, Wetzler, Germany). Brain slices were stored at −20°C in cryoprotectant solution (PBS with 1% polyvinylpyrrolidone (PVP-40, Sigma Chemical, Perth, WA), 30% ethylene glycol (Fisher Scientific, Pittsburgh, PA) and 30% sucrose (Amaresco, Solon, OH)) until undergoing immunolabeling for FosB/deltaFosB protein.
Immunolabeling for total FosB (FosB/deltaFosB) was performed as described previously (Christiansen et al. 2011, Egan et al. 2018), using rabbit primary antisera H-75 (1:300, sc-7203, Santa Cruz, Dallas, TX), biotinylated goat anti-rabbit secondary antibody (1:250, BA1000, Vector Laboratories, Burlingame, CA), avidin-biotin-peroxidase (Vectastain ABC solution, Vector Laboratories), and 3,3‟-diaminobenzidine (Sigma Chemical). This primary antibody immunoreacts with both FosB and deltaFos B (a truncated splice variant of the full length FosB) (Marttila et al. 2006). FosB is expressed transiently after neuronal activation, whereas deltaFosB is a stable, long-lasting protein that accumulates following repeated or chronic neuronal activation (Nestler et al. 1999, Perrotti et al. 2004). Available antibodies cannot distinguish deltaFosB from FosB, so we cannot definitively identify which isoform is being detected in the present study. However, the brain tissue was collected ~18 h after the last exposure to cheese, well after the transiently-induced FosB would have returned to baseline (Nestler et al. 1999, Perrotti et al. 2004, Wallace et al. 2008), suggesting that the immunolabeling primarily represents the more long-lasting deltaFosB protein. Consistent with this idea, continuous long-term access to 10% sucrose drink increases FosB/deltaFosB immunolabeling in the nucleus accumbens, and western blot analysis has attributed this effect to the deltaFosB (35–37 kDa) isoform (Wallace et al. 2008).
The FosB/deltaFosB immunolabeling was visualized using an Axio Imager.M2 microscope with an AxioCam camera and Zen 2012 software (Carl Zeiss Microscopy, Jena, Germany), and analyzed using ImageJ analysis software (W. Rasband, National Institutes of Health, USA, http://imagej.nih.gov/ij). The analysis was performed by lab personnel that were unaware of treatment group assignments. The density of FosB/deltaFos-positive cells was measured bilaterally in the core of the NAc in coronal tissue sections that were 1.2 mm anterior to bregma, as defined by a standard rat brain atlas (Paxinos and Watson 1998).
Prior work has established that a single acute stress exposure does not significantly increase deltaFosB expression in the NAc (Perrotti et al. 2004), whereas it is increased by chronic repeated stress paradigms, including 5–10 days of daily restraint, 10 days of chronic unpredictable stress, and 10 days of chronic social defeat (Perrotti et al. 2004, Vialou et al. 2010). This suggests that cumulatively, the five stress/test exposures that occurred in this experiment (novel object interaction test, restraint, social interaction test, hypoxia, and elevated plus-maze test) could have contributed to the deltaFosB expression assessed on experiment day 29. However, stress-induced elevations in NAc deltaFosB expression are also markedly reduced (by ~50%) after 7 days recovery from chronic stress exposure (Perrotti et al. 2004), suggesting that the recovery days that were scheduled between each test (Figure 1) likely mitigated any stress effects. Moreover, as stress-induced deltaFosB expression should occur to the same extent in the Cheese and Chow groups, any differences in deltaFosB expression can be attributed primarily to the effects of feeding history.
2.10. Z-score analysis of behavioral data
Z-score analysis was used to evaluate the extent to which the behavioral effects of LCI generalize within and across assays (Guilloux et al. 2011, Page and Coutellier 2018, Shepard et al. 2016). In this process, each data value was converted to a z-score that indicated how many standard deviations the value was below or above the population mean, thereby allowing data on different scales or from different tests to be compared and/or combined. For the novel object interaction test, the number object interactions and the total time spent in object interactions were each converted to a z-score; these z-scores were then averaged to obtain a net ZNOI score, where a higher ZNOI score indicates overall increased exploratory/appraisal behavior. For struggling behavior during the restraint stress, the total number of struggling events and the total time spent struggling were each converted to a z-score; these z-scores were then averaged and their direction reversed to yield a net ZRest score where a higher value denotes overall reduced struggling behavior. For the social interaction test, the number and duration of social chamber visits, the number of duration of sniffing bouts, and the social preference were each converted to z-scores and averaged to obtain ZSI, where a higher value denotes increased social/approach behaviors in this test. For the elevated plus-maze test, the total number and duration of open arm visits and the duration of head dipping behavior were each converted to z-scores and averaged to obtain ZEPM, where a higher value indicates greater exploratory/appraisal behavior in this test. ZNOI, ZRest, ZSI, and ZEPM were then averaged to obtain ZTotal. Note that as LSI has previously been shown to decrease struggling during restraint (Egan et al. 2019) and increase threat appraisal behaviors in the social interaction and elevated plus-maze tests (Egan et al. 2019, Ulrich-Lai et al. 2010), the direction of the ZRest score was reversed, thereby allowing it to be averaged with the ZNOI, ZSI and ZEPM to reflect the overall effect of LCI on stress-related behavior across all assays.
2.11. Statistical analyses
Data are shown as mean +/− SEM. When comparing two treatment groups, data were analyzed by two-tailed t-test (for parametric data with homogenous variance), and by MannWhitney U (MWU) test (for parametric data with non-homogenous variance, and for nonparametric data). For multiple group comparisons, data were analyzed by ANOVA, with repeated measures when appropriate, followed by a protected Newman-Keuls post-hoc analysis. For ANOVA, if the variance between treatment groups was not homogenous, then analyses were performed following square root transformation. Potential outliers were evaluated using two standard outlier tests an d were removed only if they failed both tests, as described previously (Christiansen et al. 2011, Egan et al. 2019, Egan et al. 2018, Egan and Ulrich-Lai 2015, Ulrich-Lai et al. 2010, Ulrich-Lai et al. 2011, Ulrich-Lai et al. 2007). Statistical significance was taken as p < 0.05.
When statistical differences were observed, effect size estimates were performed to indicate the strength of the treatment effects. For 2-tailed t-tests, Hedges‟ g was utilized due to the relatively small group sizes (< 20 samples/group) and was determined using the website: https://www.socscistatistics.com/effectsize/default3.aspx. For Mann Whitney U tests, the effect size r was determined using the formula: . For two-way and three-way ANOVAs, eta squared (η2, the proportion of total variation attributed to each main or interactive effect) was determined using the formula: . In addition, as eta squared does not allow comparisons of effect sizes across experiments, partial eta squared, (ηp2, the proportion of variation attributed to each main or interactive effect after excluding the variance explained by the other effects) was determined using the formula: .
3. Results
3.1. Effects of LCI on feeding behavior, body weight and body composition
Rats that were offered twice-daily access to a limited amount of cheese began to eat all the cheese that was offered (e.g., ~4.5 kcal/snack, or ~9 kcal/d) within the first 2 days of access, suggesting that cheese is high palatable to rats (Figure 2A). In contrast, controls that were offered an isocaloric piece of normal chow ate very little (averaging ~1.7 kcal/day over the course of the experiment), as they were maintained with ad libitum access to chow, and thus had little incentive to eat more. Statistical analysis of snack intake indicated main effects of FOOD (F1,22=535.82, p<0.001, η2 = 0.80, ηp2 = 0.96) and TIME (F25,550=4.65, p<0.001, η2 = 0.02, ηp2 = 0.17), and a FOOD x TIME interaction (F25,550=7.66, p<0.001, η2 = 0.04, ηp2 = 0.26). Posthoc analysis indicated greater intake of cheese (relative to chow controls) on every day of snack exposure.
Figure 2.
The effects of limited cheese intake (LCI) on feeding behavior, body weight and body composition. (A) Snack consumption when rats were offered brief (up to 30 min), twice-daily access to a limited amount (~4.5 kcal/snack, or ~9 kcal/d) of cheese vs. an isocaloric piece of chow (control). Not shown on panel (A) – all cheese are greater (p < 0.05) than their respective chow control for every day of limited cheese exposure. (B) Non-snack consumption of ad libitum chow. (C) Total caloric intake from all (snack and non-snack) sources. (D) Body weight, (E) body fat mass, and (F) body lean mass prior to the onset (Day 0) and at the completion (Day 28) of the snacking paradigm. n = 12 rats/group. *p < 0.05 vs. Chow controls, and #p < 0.05 vs. Day 0 by post-hoc analysis.
Non-snack food intake (i.e., ad libitum chow) was reduced in rats offered limited cheese beginning on day 7 of exposure, from ~71 kcal/d in chow controls to ~ 66 kcal/d in cheese-fed rats, suggesting the possibility that they reduced their chow intake to compensate for the additional calories provided by the cheese (Figure 2B). Statistical analysis of non-snack food intake showed main effects of FOOD (F1,22=8.01, p=0.010, η2 = 0.17, ηp2 = 0.27) and TIME (F7,154=3.36, p=0.002, η2 = 0.04, ηp2 = 0.13), and a FOOD x TIME interaction (F7,154=2.95, p=0.006, η2 = 0.04, ηp2 = 0.12). Post-hoc analysis indicated reduced ad libitum chow intake by cheese-fed rats (relative to chow controls) on days 7–28 of snack exposure.
Total caloric intake from all sources (snack and non-snack) was increased on days 4–6 of snack exposure in cheese-fed rats, and normalized thereafter, consistent with the idea that cheese-fed rats began to decrease their non-snack food intake on day 7 to compensate for the excess calories provided by the cheese (Figure 2C). Statistical analysis of total caloric intake showed a main effect of TIME (F25,550=4.70, p<0.001, η2 = 0.07, ηp2 = 0.18) and a FOOD x TIME interaction (F25,550=1.69, p=0.021, η2 = 0.03, ηp2 = 0.07), without a main effect of FOOD (F1,22=2.87, p=0.104). Post-hoc analysis indicated increased total caloric intake by cheese-fed rats (relative to chow controls) on days 4–6 of snack exposure.
Body weight increased over the duration of the experiment and was not affected by cheese intake (Figure 2D; main effect of TIME (F9,198=968.20, p<0.001, η2 = 0.85, ηp2 = 0.98), with no main (F1,22=0.46, p=0.506) or interactive (F9,198=0.88, p=0.545) effects of FOOD. Likewise, both body fat mass (Figure 2E) and lean mass (Figure 2F) increased over time and were not affected by cheese intake (fat mass: main effect of TIME (F1,22=190.76, p<0.001, η2 = 0.62, ηp2 = 0.90), with no main (F1,22=0.06, p=0.813) or interactive (F1,22=0.64, p=0.434) effects of FOOD; lean mass: main effect of TIME (F1,22=1882.59, p<0.001, η2 = 0.95, ηp2 = 0.99), with no main (F1,22=0.05, p=0.824) or interactive (F1,22=0.003, p=0.955) effects of FOOD). Collectively, these data suggest that the LCI paradigm did not affect body weight or body composition.
3.2. Effects of LCI on behavior in the novel object interaction test
Limited intermittent cheese intake did not alter behavior in the novel object interaction test. The number of object interactions (Figure 3A; U=56, p=0.321), and the time spent in object interactions (Figure 3B; U=56.5, p=0.339), were unaffected by cheese consumption, suggesting that cheese did not alter threat appraisal (or anxiety-related) behavior in this test. Cheese intake also did not alter total locomotion in the novel object interaction test, as the total number of lines crossed did not vary with food type (Figure 3C; T22=0.04, p=0.972).
Figure 3.
A history of limited cheese intake (LCI) did not alter behavior in the novel object interaction test. (A) Number of object interactions, (B) time spent in object interactions, and (C) the total number of lines crossed. n = 12 rats/group.
3.3. Effects of LCI on HPA axis and behavioral responses to an acute restraint stressor
The time course of the plasma corticosterone response to an acute restraint stress was attenuated by prior cheese intake (Figure 4A; main effects of FOOD (F1,22=7.62, p=0.011, η2 = 0.01, ηp2 = 0.26) and TIME (F3,66=469.36, p<0.001, η2 = 0.92, ηp2 = 0.96), with no FOOD x TIME interaction (F3,66=0.75, p=0.524)). Post-hoc analysis indicated that the reduced plasma corticosterone was most pronounced at the 40-min time point after stress onset. Analysis of the integrated plasma corticosterone response to restraint showed a similar effect, with cheese intake reducing the total corticosterone response by ~15% relative to chow controls (Figure 4B; T22=2.59, p=0.017, g=1.06). Cheese intake did not affect pre-stress plasma corticosterone values at the 0-min time point (Chow = 56.4 ± 15.4 ng/ml, Cheese = 28.1 ± 2.9 ng/ml, U=66, p=0.75).
Figure 4.
Limited cheese intake (LCI) reduced HPA axis and behavioral responses to an acute restraint stress. (A) Time course and (B) integrated (area-under-the curve) plasma corticosterone response. (C) Time course and (D) total time spent struggling. (E) Time course and (F) total number of struggling events. n=11–12 rats/group. *p < 0.05 vs. Chow controls by post-hoc analysis (A, C, E) or 2-tailed t-test or MWU (B, D, F).
LCI reduced the time spent struggling during acute restraint. The time course of struggling duration (Figure 4C) showed a main effect of FOOD (F1,22=6.54, p=0.018, η2 = 0.12, ηp2 = 0.23) and a FOOD x TIME interaction (F2,44=3.23, p=0.049, η2 = 0.05, ηp2 = 0.13), without a main effect of TIME (F2,44=2.86, p=0.068). Post-hoc analysis indicated that cheese-fed rats spent less time struggling during minutes 6–15 of restraint. Cheese intake also reduced the total time spent struggling by ~55% relative to chow controls (Figure 4D; U=33, p=0.024, r= −0.46).
LCI had a more modest effect on the number of struggling events. For the time course of struggling events (Figure 4E), statistical analysis indicated a FOOD x TIME interaction (F2,44=3.80, p=0.030, η2 = 0.06, ηp2 = 0.15), without main effects of FOOD (F1,22=0.760, p=0.393) or TIME (F2,44=3.16, p=0.052). Post-hoc analysis revealed that cheese-fed rats had fewer struggling events specifically during minutes 6–10 of restraint. Consistent with this observation, cheese intake did not alter the total number of struggling events during the entire restraint (Figure 4F; U=61, p=0.758).
3.4. Effects of LCI on behavior in the social interaction task
LCI increased the amount of time spent in the chamber containing a social target (i.e., social chamber) and reduced time spent in the chamber containing an empty target (i.e., non-social chamber) (Figure 5A). Statistical analysis indicated a main effect of CHAMBER (F1,44=457.74, p<0.001, η2 = 0.89, ηp2 = 0.91) and a CHAMBER x FOOD interaction (F1,44=14.52, p<0.001, η2 = 0.03, ηp2 = 0.25), with no main effect of FOOD (F1,44=0.04, p=0.847). Post-hoc analysis indicated that while both cheese-fed and chow control rats spent more time in the social vs. non-social chamber, cheese further increased social chamber duration, and reduced non-social chamber duration, relative to chow controls. Time spent in the central, connecting chamber was low and unaffected by cheese intake (Chow = 71.0 ± 8.7 sec, Cheese = 64.0 ± 7.0 sec, T22=0.62, p=0.541). Moreover, rats made more entries into the social vs. non-social chamber (Figure 5B; main effect of CHAMBER (F1,43=10.78, p=0.002, η2 = 0.19, ηp2 = 0.20), without main (F1,43=1.02, p=0.319) or interactive (F1,43=0.53, p=0.470) effects of FOOD), and the post-hoc analysis indicates that this effect was most pronounced for the cheese-fed rats. The total number of chamber entries (social + non-social) (Figure 5C; U=60.5, p=0.506) was unaffected by food type, suggesting that cheese intake did not alter general locomotor activity during this test.
Figure 5.
Limited cheese intake (LCI) increased social interaction. (A) Time spent and (B) number of entries for the chamber containing a social target vs. an empty, non-social target. (C) Total number of entries into the social and non-social chambers. (D) Time spent sniffing and (E) number of sniffing events for the social vs. non-social targets. (F) Preference for the social target, defined as sniff time for the social target expressed as a percentage of the total time spent sniffing the social and non-social targets. n = 12 rats/group. *p < 0.05 vs. Chow controls, and #p < 0.05 vs. Social by post-hoc analysis (A, B, D, E) or 2-tailed t-test or MWU (C, F).
LCI also increased the amount of time spent sniffing the social target and reduced time spent sniffing the empty, non-social target (Figure 5D). Statistical analysis of sniffing duration revealed a main effect of CHAMBER (F1,44=863.00, p<0.001, η2 = 0.93, ηp2 = 0.95) and a CHAMBER x FOOD interaction (F1,44=20.61, p<0.001, η2 = 0.02, ηp2 = 0.32), with no main effect of FOOD (F1,44=0.53, p=0.472). Post-hoc analysis indicated that while both cheese-fed and chow control rats spent more time sniffing the social vs. non-social target, cheese further increased social sniffing duration, and reduced non-social sniffing duration, relative to chow controls. In addition, statistical analysis of the number of sniffing events (Figure 5E) revealed a main effect of CHAMBER (F1,44=430.41, p<0.001, η2 = 0.89, ηp2 = 0.91) and a CHAMBER x FOOD interaction (F1,44=7.27, p=0.100, η2 = 0.02, ηp2 = 0.14), with no main effect of FOOD (F1,44=0.35, p=0.560). Post-hoc analysis indicated that both cheese-fed and chow control rats had more sniffing events directed towards the social vs. non-social target, and that cheese further reduced the non-social sniffing events relative to chow controls. Cheese also increased preference for the social target (Figure 5F; T22=4.18, p<0.001, g=1.71).
3.5. Effects of LCI on the HPA axis response to an acute hypoxia stressor
LCI reduced the plasma corticosterone response to hypoxia. Statistical analysis of the time course of the plasma corticosterone response (Figure 6A) indicated a FOOD x TIME interaction (F2,32=3.34, p=0.048, η2 = 0.03, ηp2 = 0.17) and a main effect of TIME (F2,32=62.81, p<0.001, η2 = 0.58, ηp2 = 0.80), without a main effect of FOOD (F1,16=2.99, p=0.103). Post-hoc analysis identified that cheese-fed rats had lower plasma corticosterone levels at 40 min after hypoxia onset. The integrated plasma corticosterone response to hypoxia was not affected by prior cheese intake (Figure 6B; T16=2.06, p=0.056). However, we cannot exclude the possibility that reduced statistical power for the hypoxia test (due to a technical error for 3 rats/group; see Materials and Methods for further details) may have impacted these results.
Figure 6.
Limited cheese intake (LCI) reduced the HPA axis response to an acute hypoxia stress. (A) Time course and (B) integrated (area-under-the curve) plasma corticosterone response. n=9 rats/group. *p < 0.05 vs. Chow controls by post-hoc analysis.
3.6. Effects of LCI on behavior in the elevated plus-maze test
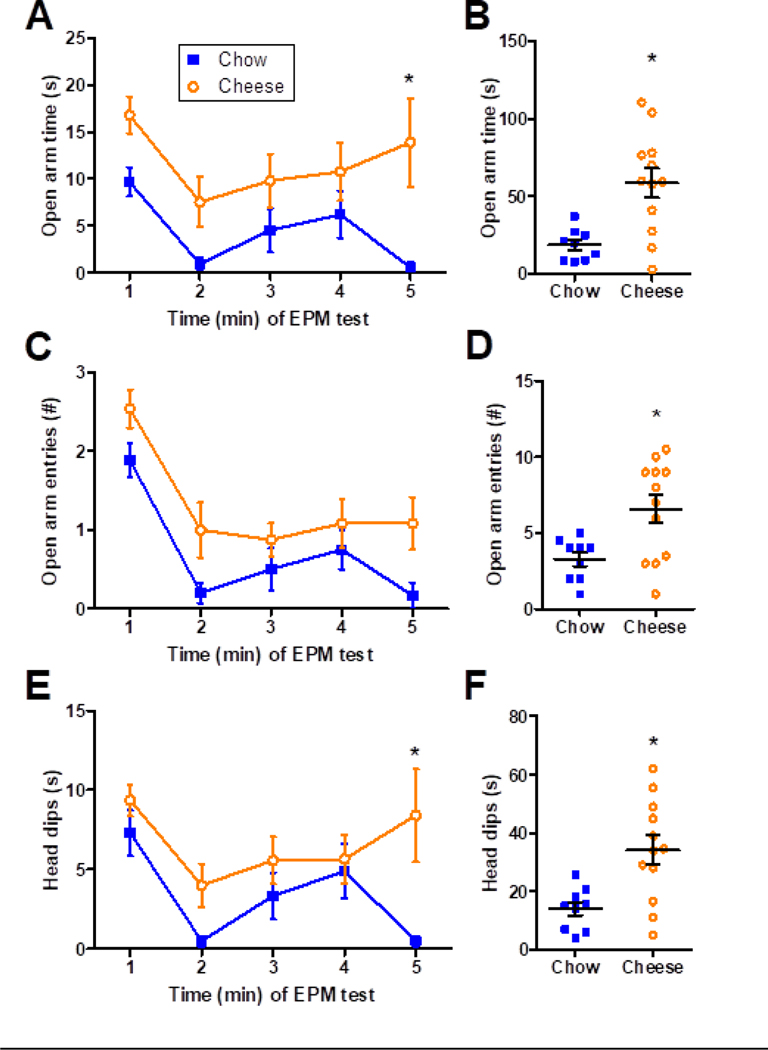
LCI increased open arm behavior in the elevated plus-maze test. Statistical analysis of the amount of time spent in the open arm (Figure 7A) showed main effects of FOOD (F1,20=11.14, p=0.003, η2 = 0.14, ηp2 = 0.36) and TIME (F4,80=3.49, p=0.011, η2 = 0.09, ηp2 = 0.15) without a FOOD x TIME interaction (F4,80=0.98, p=0.423). Post-hoc analysis identified that cheese increased open arm time predominantly at the 5-min time point. While over the entire test, cheese increased open arm time by ~3-fold over chow controls (Figure 7B; U=15, p=0.006). Statistical analysis of the number of open arm entries (Figure 7C) indicated main effects of FOOD (F1,20=6.95, p=0.016) and TIME (F4,80=17.47, p<0.001) without a FOOD x TIME interaction (F4,80=0.61, p=0.655). Post-hoc analysis did not identify any particular time points that were affected by cheese, and cheese increased the total number of open arm entries over the entire test by ~2-fold (Figure 7D; U=24.5, p=0.035, r= −0.61).
Figure 7.
Limited cheese intake (LCI) increased open arm behavior in the elevated plus-maze test. (A) Time course and (B) total amount of time spent in the open arm. (C) Time course and (D) total number of open arm entries. (E) Time course and (F) total amount of time spent engaging in head dips. n=9–12 rats/group. *p < 0.05 vs. Chow controls by post-hoc analysis (A, C, E) or 2-tailed MWU test (B, D, F).
Head dip behavior was likewise increased by cheese intake (Figure 7E), with main effects of FOOD (F1,20=7.43, p=0.013, η2 = 0.08, ηp2 = 0.27) and TIME (F4,80=4.34, p=0.003, η2 = 0.11, ηp2 = 0.18), but no FOOD x TIME interaction (F4,80=1.71, p=0.156). Post-hoc analysis identified that cheese increased head dip time predominantly at the 5-min time point. Over the entire test, cheese increased the amount of time spent in head dips by ~2.5-fold over chow controls (Figure 7F; U=17, p=0.009, r= −0.57).
The total number of arm entries (open + closed) was not affected by cheese intake (Chow = 11.4 ± 1.4 entries, Cheese = 14.7 ± 1.4 entries, T20=1.59, p=0.127), suggesting a lack of effects on general locomotor activity in the elevated plus-maze test. Likewise, the total number of closed arm entries was not altered by cheese intake (Chow = 6.7 ± 0.9 entries, Cheese = 8.1 ± 0.7 entries, T19=1.28, p=0.215).
3.7. Use of z-score analyses to evaluate the generalizability of behavioral effects within and across assays
Z-score analyses were used to examine the extent to which the behavioral effects of LCI generalized within and across the behavioral assays. For the novel object interaction test, Z-score analysis combining the total number and duration of object interactions was not altered by cheese intake (Table 1; U=55, p=0.294). The restraint z-score, combining the total number and duration of struggling bouts, was also not affected by cheese intake (Table 1; U=37, p=0.074). However, the social interaction z-score, which combined the number and duration of social chamber visits, the number and duration of sniffing bouts, and the social preference, was increased by cheese intake relative to chow controls (Table 1; U=27, p=0.016, r= −0.50). Likewise, the elevated plus-maze z-score, comprised of the number and duration of open arm visits and the duration of head dips, was increased by cheese intake (Table 1; U=17, p=0.009, r= −0.57). Finally, LCI increased the total z-score across all behavioral assays (Table 1; U=11, p<0.001, r= −0.72), suggesting an overall reduction in stress-related behaviors across assays.
Table 1.
Z-score analyses for behavioral assays.
| Behavioral test | Behavioral measures in cluded in Z-score | Chow | Cheese |
|---|---|---|---|
| Novel object interaction (Z-scoreNOI) |
Number and duration of object interactions (Fig. 3A, B) | 0.00 ± 0.27 | 1.29 ± 0.68 |
| Restraint (Z-scoreRest) |
Number and duration of struggling bouts (Fig. 4D, F) | 0.00 ± 0.28 | 0.65 ± 0.10 |
| Social interaction (Z-scoreSI) |
Number and duration of social chamber visits and sniffing bouts, and social preference (Fig. 5A, B, D, E, F) | 0.01 ± 0.18 | 0.63 ± 0.10* |
| Elevated plus-maze (Z-scoreEPM) |
Number and duration of open arm visits and duration of head dips (Fig. 7B, D, F) | 0.00 ± 0.32 | 3.10 ± 0.76* |
| Total (Z-scoreTotal) |
Mean of z-scores for each assay | −0.01 ± 0.13 | 1.50 ± 0.30* |
p<0.05 vs. Chow.
3.8. Effects of LCI on the number of FosB/deltaFosB-positive neurons in the nucleus accumbens (NAc)
Immunolabeling was used to evaluate whether LCI increased the number of FosB/deltaFosB-positive neurons in the NAc core like other types of natural and pharmacological rewards. In fact, the density of FosB/deltaFosB-positive cells (Figure 8A, B) was increased by ~33 % in rats with a history of cheese intake relative to chow controls (Figure 8C; T20=3.86, p=0.001, g=1.65).
Figure 8.
Limited cheese intake (LCI) increased FosB/deltaFosB immunolabeling in the nucleus accumbens. Representative images of FosB/deltaFosB-positive cells in the nucleus accumbens core following (A) chow control and (B) limited cheese feeding. The dotted lines indicate the outer bounds of the region that was analyzed. Scale bar = 200 μm. ac = anterior commissure, LV = lateral ventricle. (C) The density of FosB/deltaFosB-positive cells in the nucleus accumbens core. n=12 rats/group. *p < 0.05 vs. Chow controls by 2-tailed t-test.
4. Discussion
4.1. Effects of the limited cheese vs. sucrose feeding paradigms on energy balance
Ad libitum chow-fed rats that were given additional twice-daily access to a small cheese “snack” (4.5 kcal/session, or 9 kcal/d) began to eat the full amount of cheese that was offered by days 2–3 of exposure. They also ate more during the “snack” session than controls that were instead offered an isocaloric piece of normal chow, beginning on the first day of exposure, suggesting that cheese is a highly palatable, preferred food for rats. Cheese-fed rats maintained their normal (non-snack) chow intake through day 7 of LCI, and when taken together with the calories provided by the cheese, this resulted in an elevated total caloric intake on days 4–6 of LCI. Cheese-fed rats then reduced their chow intake after day 7 (perhaps to compensate for the calories provided by the cheese) such that their total caloric intake returned to control levels thereafter. As a result, body weight and body composition were not affected by the LCI paradigm.
The metabolic effects of the limited cheese “snack” paradigm are similar to those produced by LSI. When rats with free access to chow and water are given additional twice daily access to 4 ml of 30% sucrose solution, they typically drink a larger volume relative to control rats that are instead offered a second bottle of water by day 1 of exposure, and begin to drink the full amount of sucrose by days 4–5 of exposure (Egan and Ulrich-Lai 2015, Packard et al. 2017, Ulrich-Lai et al. 2010, Ulrich-Lai et al. 2011, Ulrich-Lai et al. 2007) consistent with the known high preference of rats for sucrose solutions (reviewed in (Smith and Sclafani 2002)). Sucrose fed rats consistently reduce their chow intake during the first week of sucrose exposure, thereby maintaining total caloric intake, body weight and body composition at control levels throughout the entire LSI paradigm (Christiansen et al. 2011, Egan and Ulrich-Lai 2015, Ulrich-Lai et al. 2010, Ulrich-Lai et al. 2011).
Ultimately, both LCI and LSI rats decrease chow intake in an apparent attempt to compensate for the ~10% of daily calories provided by the palatable food. This replicates prior work showing that when satiated rats that are maintained on a bland, standard chow diet are also given intermittent access to limited amounts of palatable foods, they effectively reduce their standard chow intake to compensate for the calories provided by the palatable food (Corwin et al. 1998, Dailey et al. 2012, Hume et al. 2016, Merkestein et al. 2012). However, there were notable differences in the timing of this effect between the LSI and LCI paradigms. LSI rats consistently reduce their chow intake as early as the first week of sucrose exposure, whereas LCI rats did not begin to reduce their chow intake until after day 7, producing an early, transient increase in total caloric intake. The reasons for the delayed decrease in chow intake in LCI vs. LSI rats are not clear. However, this may be related to the observation that it often takes several days for sucrose-fed rats to begin consuming the full amount of sucrose they are offered, whereas cheese-fed rats consumed the full amount of cheese by the second day. It is possible that the more tempered onset rate for sucrose taking provides a larger window of time to engage compensatory behavioral mechanisms to decrease chow intake, thereby avoiding increases to total caloric intake. Though it should be noted that occasionally a particular cohort of LSI rats will begin to consume the maximal amount of sucrose sooner (as early as day 2 of the feeding paradigm), and in these instances chow intake is still reduced during the first week (Egan and Ulrich-Lai 2015), casting some doubt on this possible explanation.
Alternatively, the delayed decrease in chow intake in LCI vs. LSI rats may be related to inherent differences in the macronutrient composition of the palatable foods. It is possible that compensatory behavioral mechanisms occur more rapidly during the consumption of high sugar vs. high fat/protein palatable foods as these dietary nutrients are sensed differently. Dietary sugars are detected by type 1 taste receptors (e.g., T1R2-T1R3 heterodimers) that are expressed in the tongue and gastrointestinal tract, and by glucose-sensing neurons in brain (reviewed in (Efeyan et al. 2015, Levin et al. 2011)). In contrast, fatty acids are detected by the fatty acid translocase CD36 and several G protein-coupled receptors (e.g., GPR40, GPR120) that are expressed in the tongue and/or gastrointestinal tract, as well as by fatty acid-sensing neurons in brain (reviewed in (Efeyan et al. 2015, Lam et al. 2005, Levin et al. 2011, Sundaresan and Abumrad 2015)). Thus, there are distinct mechanisms for detecting the presence of ingested sugars vs. fats that may contribute to a more rapid decrease in chow intake during the LSI vs. LCI paradigms. Though it should be noted that when standard chow-fed rats are given daily 2-h access to a palatable fat (hydrogenated vegetable shortening), they consume ~33% of their total daily calories as shortening, and compensatory decreases in standard chow intake occur quickly, preventing an increase in total caloric intake (Corwin et al. 1998). This suggests that the amount of palatable food consumed may also be a contributing factor, with larger amounts promoting more rapid decreases in chow intake. Importantly, the observed differences in total caloric intake between the LCI and LSI paradigms are transient, and neither alter body weight and body composition, suggesting that both paradigms are useful for assessing the effects of palatable food in the absence of potential confounds due to the development of obesity.
4.2. Effects of limited cheese vs. sucrose intake on stress responses
The present data indicate that the LCI paradigm blunts multiple types of stress responses. Reduced HPA axis (plasma corticosterone) responsivity was observed following two different types of stressors – restraint and hypoxia – and the reduced HPA axis response to restraint was accompanied by less behavioral struggling during this stressor. LCI increased sociability in the social interaction test, and increased threat appraisal (or decreased threat avoidance) in the elevated plus maze test. Collectively, these results suggest a generalized stress-blunting by LCI across multiple types of stressors, and across multiple types of stress responses. Though it should be noted that LCI did not impact threat appraisal behavior in the novel object interaction test. This may indicate that LCI stress relief is not universal, or as the novel object test on day 11 was the first test performed, it may indicate that a greater duration of LCI is needed to observe effects.
Notably, the effects of LCI closely resemble those seen previously with the limited sucrose feeding paradigm. LSI consistently reduces the plasma corticosterone response to restraint (Christiansen et al. 2011, Packard et al. 2017, Ulrich-Lai et al. 2010, Ulrich-Lai et al. 2011, Ulrich-Lai et al. 2007), and also attenuates the plasma corticosterone response to hypoxia (unpublished observation). Behaviorally, LSI increases the time spent engaging in social behaviors (e.g., sniffing, touching) in a direct exploration social test, in which each rat is placed into a neutral cage with an unfamiliar, body weight-matched conspecific (i.e., interactor rat) for 10 min (Ulrich-Lai et al. 2010). LSI also increases open arm exploration, particularly at the far end of the open arm, and head dipping behavior in the elevated plus-maze test (Ulrich-Lai et al. 2010), and reduces struggling behavior during a restraint stressor (unpublished observations). Taken together, these data suggest that LCI and LSI similarly reduce HPA axis and behavioral responses to stress.
4.3. Stress dampening by palatable foods: the role of macronutrients vs. reward
When offered as a choice, the voluntary intake of palatable foods reduces physiological and psychological response to stress. Much of this prior work has focused on the ability of dietary sugars and other carbohydrates to reduce stress responses. In humans, eating a high carbohydrate diet is associated with reduced resting and stress-evoked cortisol levels (Anderson et al. 1987, Deuster et al. 1992, Markus et al. 2000, Utter et al. 1999), and the consumption of high calorie sweet foods can improve emotional states (Dube et al. 2005, Macht and Mueller 2007). A similar effect is observed in rodents, as rats that are offered unlimited access to sucrose drink in addition to standard chow consume ~30–45% of their daily calories as sucrose and have blunted HPA axis responses to stress (Bell et al. 2002, Strack et al. 1997, Suchecki et al. 2003). However, little is known about how this effect occurs, including the relative contribution of the foods‟ macronutrient and caloric properties, as opposed to its hedonic and rewarding properties.
A primary role for the metabolic properties of dietary carbohydrates in stress dampening has been supported by comparisons between the stress effects of sucrose vs. noncaloric artificial sweeteners, particularly when feeding paradigms are used that provide large and/or unlimited amounts of dietary carbohydrates. Unlimited access to sucrose drink prevents the stimulatory effect of adrenalectomy on the HPA axis of rats, and this effect does not occur with the artificial sweetener saccharin, suggesting that the metabolic consequences of sucrose contribute to HPA axis down-regulation (Bell et al. 2000, Bhatnagar et al. 2000, Laugero et al. 2001). Similarly, women who drink a sucrose-sweetened beverage three times daily for two weeks (corresponding to ~25% of their energy requirement) have a reduced salivary cortisol response to a laboratory stressor, and this effect does not occur in women who drink an artificially (aspartame) sweetened beverage, attributing the HPA effect to the metabolic effects of sucrose (Tryon et al. 2015). However, it should be noted that this interpretation is complicated by the fact that sucrose and artificial sweeteners may not be equivalently rewarding. People generally prefer the taste of sucrose over that of artificial sweeteners like aspartame (Sprowl and Ehrcke 1984, Tan et al. 2019), and post-ingestive factors like macronutrients or calories can also increase the rewarding properties of foods (Sclafani 2004). This leaves open the possibility that food reward contributes to physiological and psychological stress dampening by palatable foods. In support of this idea, unlimited access to either sucrose or saccharin drink reduces the plasma ACTH and corticosterone responses to paradoxical sleep deprivation in rats (Suchecki et al. 2003), and people report greater mood improvements after eating palatable chocolate relative to unpalatable chocolate (Macht and Mueller 2007).
Conceptually, a scheduled limited feeding paradigm may be ideally suited for exploring stress relief by food reward per se. Offering palatable food as a choice, in a limited amount, and on an intermittent schedule likely maximizes its rewarding properties, while minimizing its metabolic impact. In support of this idea, the blunted HPA axis reactivity that occurs following LSI is replicated by limited intermittent intake of saccharin (Ulrich-Lai et al. 2010, Ulrich-Lai et al. 2007), and prevented if the pleasurable taste of the sucrose is minimized by direct gavage into the stomach (Ulrich-Lai et al. 2010), suggesting that the impact of LSI is mediated by reward rather than calories. The present data extend this by showing that the limited intake of cheese – a palatable food that is low in sugars and carbohydrates – provides a similar stress blunting in this limited feeding paradigm. LCI also increases the expression of FosB/deltaFosB (a protein associated with repeated or chronic neuronal activation) in the NAc core similar to LSI and other types of natural and pharmacological rewards (Christiansen et al. 2011, Nestler et al. 1999, Wallace et al. 2008), suggesting that LCI activates brain reward pathways. Moreover, limited sucrose and cheese feeding both reduce HPA axis reactivity in a manner analogous to that provided by another type of natural reward (sexual activity) (Ulrich-Lai et al. 2010). Collectively, this evidence strongly supports the idea that food reward can contribute to the stress relief provided by palatable foods, and that rodent limited feeding paradigms are ideal for studying the mechanisms by which food reward meditates these effects.
4.4. Behavioral analysis: the use of z-scores
The present work utilized several different tests of stress-related behavior to increase overall rigor and reproducibility, and analyzed them with a traditional approach where each end point was assessed independently. However, this leads to the important question of how to evaluate the extent to which the behavioral effects of LCI extend within and across these assays. Z-score analyses were therefore performed to normalize and combine different end points within each behavioral test, as well as across all tests, thereby allowing identification of generalized LCI effects.
Traditional and z-score analyses identified similar behavioral effects for LCI in most, but not all, of the assays utilized. In the novel object interaction test, LCI did not alter either the time spent or number of object interactions, and this was reflected in a ZNOI that was not impacted by LCI. In the social interaction test, LCI increased social chamber time, social sniffing time and social preference. When these variables were combined with the number of social chamber visits and sniffing bouts to determine the ZSI, this was also increased by LCI, suggesting that LCI generally increased social behavior in this assay. In the elevated plus-maze test, LCI increased time spent in the open arm, the number of open arm entries, and time spent in head dips. When all of these variables were combined to calculate ZEPM, this was increased by LCI, indicating an effect that extended across multiple end points in this assay. However, the traditional and z-score analyses identified key differences for the effect of LCI on restraint stress behavior. While traditional analysis identified that LCI reduced overall struggling time, as well as the number of struggling bouts during minutes 6–10 of this test, when total struggling time and bouts were combined, it did not result in a significantly increased ZRES. This discrepancy is likely because only two variables (struggling time and number of bouts) were used to calculate ZRES, and the LCI effect for struggling events only occurred at a specific time point and did not alter the total number during the entire restraint. Thus, while the traditional analysis suggests that LCI clearly impacted key aspects of struggling behavior, the ZRES suggests that this effect did not generalize across all indices in this test. Finally, when the z-scores for each assay were combined to determine a ZTOTAL, it was significantly increased by LCI. This suggests that LCI reduced overall stress-related behavior across all tests, despite a lack of effects on ZNOI and ZRES. This is likely because both ZNOI and ZRES tended (non-significantly) to be increased by LSI, so when combined with the several other assays that had significant z-scores, it resulted in a significant increase in ZTOTAL by LCI.
The present findings indicate that there are clear strengths and limitations to the z-score analysis approach that should be considered prior to its use. Importantly, z-score analysis provides a way to evaluate the extent that behavioral effects are consistent and generalizable across multiple end points within and across behavioral assays, thereby supporting overall scientific rigor and reproducibility. This is critical for behavioral phenotypes like threat appraisal or avoidance that are not readily evaluated by any single behavioral end point or assay. However, it is also important to note that the z-score analysis weights each behavioral component equally when they are averaged to obtain the overall z-score for a behavioral test. This assumes that each of the factors included in the overall z-score is similarly regulated and equally important. However, this may not be the case. It is possible that experimental interventions that target specific neural systems could impact some, but not all, components of an overall z-score, and this effect could go undetected if z-scores were the sole method of analysis. For example, in the forced swim test for coping behavior, antidepressants that increase serotonin signaling are primarily associated with altered swimming behavior, whereas those that increase catecholaminergic signaling are primarily linked with altered climbing behavior (Cryan et al. 2005, Detke et al. 1995). Relying solely on a z-score that combines multiple behavioral indices could compromise the ability to identify behavioral subtypes that are differentially regulated by the brain.
The equal weighting of each behavioral component within the z-score calculation also has implications for the selection of which and how many factors to include in the calculation. Limiting the analysis to just the most critical behavioral end points seems more likely to give a positive result than if a large number of less informative variables are included that could potentially “dilute” the overall effect. Going forward, it may be useful to perform modeling on large historical datasets to establish the optimal number and type of behavioral end points that should be included for each type of assay, as well as whether various end points should be differentially weighted in the overall z-score.
Finally, while z-score analyses are a useful way to perform intra-animal comparisons across behavioral assays, this approach necessarily requires that each animal receive multiple behavioral tests. This then raises the possibility of carryover effects whereby the stress associated with an earlier behavioral assay may impact performance on later tests. While we mitigated this concern by incorporating recovery days between each assay, it is important to acknowledge that this is a potential limitation that is inherent to this type of experimental design.
4.5. Conclusions
The present study demonstrates that when ad libitum chow-fed rats are offered additional twice-daily access to a small cheese “snack”, they quickly begin to eat the entire amount of cheese, and ultimately reduce their chow intake to maintain normal body weight. Rats with a history of this LCI paradigm have overall reduced behavioral and HPA axis responses to acute stressors, as well as increased FosB/deltaFosB immunolabeling in the nucleus accumbens – effects that mirror those that occur following a history of LSI. Collectively, these data suggest that limited intake paradigms are particularly well-suited for exploring the mechanisms by which food reward impacts stress responsivity regardless of specific macronutrient compositions. In addition, the observation that rats decrease their chow intake as an apparent means to compensate for the calories provided by the limited palatable foods to maintain body weight suggests that this type of paradigm can be used to isolate the stress relieving mechanisms of palatable foods from their obesogenic effects. Finally, the present work supports the idea that z-score analysis may be a useful approach to identify behavioral effects that are consistent within and across behavioral assays.
Highlights.
Limited intermittent intake of cheese blunts HPA and behavioral responses to stress
Z-score analyses indicate that the behavioral effects extend across multiple assays
Limited cheese intake increases FosB/deltaFosB expression in the nucleus accumbens
The collective effects of limited cheese intake resemble those produced by sucrose
Stress relief by limited palatable “comfort” feeding is not macronutrient specific
Acknowledgements
This work was supported by the National Institutes of Health [grant numbers R01 DK118292 (YU-L), R01 MH119814 (YU-L), and R56 DK118292 (YU-L)]. The funding source played no role in study design, in data collection, analysis and interpretation, in manuscript preparation, or in the decision to submit the work for publication. The authors thank Abaegail Czop for her assistance with the behavioral analyses.
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abuhamdah RM, van Rensburg R, Lethbridge NL, Ennaceur A Chazot PL (2012). “Effects of methimepip and jnj-5207852 in wistar rats exposed to an open-field with and without object and in balb/c mice exposed to a radial-arm maze.” Front Syst Neurosci 6: 54. DOI: 10.3389/fnsys.2012.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KE, Rosner W, Khan MS, New MI, Pang SY, Wissel PS Kappas A. (1987). “Diet-hormone interactions: Protein/carbohydrate ratio alters reciprocally the plasma levels of testosterone and cortisol and their respective binding globulins in man.” Life Sci 40(18): 1761–1768. [DOI] [PubMed] [Google Scholar]
- Arabo A, Potier C, Ollivier G, Lorivel T Roy V. (2014). “Temporal analysis of free exploration of an elevated plus-maze in mice.” J Exp Psychol Anim Learn Cogn 40(4): 457–466. DOI: 10.1037/xan0000031 [DOI] [PubMed] [Google Scholar]
- Barrington WE, Beresford SA, McGregor BA White E. (2014). “Perceived stress and eating behaviors by sex, obesity status, and stress vulnerability: Findings from the vitamins and lifestyle (vital) study.” J Acad Nutr Diet 114(11): 1791–1799. DOI: 10.1016/j.jand.2014.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ME, Bhargava A, Soriano L, Laugero K, Akana SF Dallman MF (2002). “Sucrose intake and corticosterone interact with cold to modulate ingestive behaviour, energy balance, autonomic outflow and neuroendocrine responses during chronic stress.” J Neuroendocrinol 14(4): 330–342. [DOI] [PubMed] [Google Scholar]
- Bell ME, Bhatnagar S, Liang J, Soriano L, Nagy TR Dallman MF (2000). “Voluntary sucrose ingestion, like corticosterone replacement, prevents the metabolic deficits of adrenalectomy.” J Neuroendocrinol 12(5): 461–470. [DOI] [PubMed] [Google Scholar]
- Bertoglio LJ Carobrez AP (2002). “Behavioral profile of rats submitted to session 1-session 2 in the elevated plus-maze during diurnal/nocturnal phases and under different illumination conditions.” Behav Brain Res 132(2): 135–143. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Bell ME, Liang J, Soriano L, Nagy TR Dallman MF (2000). “Corticosterone facilitates saccharin intake in adrenalectomized rats: Does corticosterone increase stimulus salience?” J Neuroendocrinol 12(5): 453–460. [DOI] [PubMed] [Google Scholar]
- Carobrez AP Bertoglio LJ (2005). “Ethological and temporal analyses of anxiety-like behavior: The elevated plus-maze model 20 years on.” Neurosci Biobehav Rev 29(8): 1193–1205. DOI: 10.1016/j.neubiorev.2005.04.017 [DOI] [PubMed] [Google Scholar]
- Cartwright M, Wardle J, Steggles N, Simon AE, Croker H Jarvis MJ (2003). “Stress and dietary practices in adolescents.” Health Psychol 22(4): 362–369. [DOI] [PubMed] [Google Scholar]
- Casarrubea M, Roy V, Sorbera F, Magnusson MS, Santangelo A, Arabo A. Crescimanno G. (2013). “Temporal structure of the rat’s behavior in elevated plus maze test.” Behav Brain Res 237: 290–299. DOI: 10.1016/j.bbr.2012.09.049 [DOI] [PubMed] [Google Scholar]
- Christiansen AM, Dekloet AD, Ulrich-Lai YM Herman JP (2011). ““Snacking” causes long term attenuation of hpa axis stress responses and enhancement of brain fosb/deltafosb expression in rats.” Physiol Behav 103(1): 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin RL, Wojnicki FH, Fisher JO, Dimitriou SG, Rice HB Young MA (1998). “Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats.” Physiol Behav 65(3): 545–553. DOI: 10.1016/s0031-9384(98)00201-7 [DOI] [PubMed] [Google Scholar]
- Cruz AP, Frei F Graeff FG (1994). “Ethopharmacological analysis of rat behavior on the elevated plus-maze.” Pharmacol Biochem Behav 49(1): 171–176. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ Lucki I. (2005). “Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test.” Neurosci Biobehav Rev 29(4–5): 547–569. DOI: 10.1016/j.neubiorev.2005.03.008 [DOI] [PubMed] [Google Scholar]
- Dailey MJ, Stingl KC Moran TH (2012). “Disassociation between preprandial gut peptide release and food-anticipatory activity.” Endocrinology 153(1): 132–142. DOI: 10.1210/en.2011-1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detke MJ, Rickels M Lucki I. (1995). “Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants.” Psychopharmacology (Berl) 121(1): 66–72. DOI: 10.1007/BF02245592 [DOI] [PubMed] [Google Scholar]
- Deuster P, Singh A, Hofmann A, Moses F Chrousos G. (1992). “Hormonal responses to ingesting water or a carbohydrate beverage during a 2 h run.” Med Sci Sports Exerc 24(1): 72–79. [PubMed] [Google Scholar]
- Dube L, LeBel JL Lu J. (2005). “Affect asymmetry and comfort food consumption.” Physiol Behav 86(4): 559–567. [DOI] [PubMed] [Google Scholar]
- Efeyan A, Comb WC Sabatini DM (2015). “Nutrient-sensing mechanisms and pathways.” Nature 517(7534): 302–310. DOI: 10.1038/nature14190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan AE, Seemiller LR, Packard AEB, Solomon MB Ulrich-Lai YM (2019). “Palatable food reduces anxiety-like behaviors and hpa axis responses to stress in female rats in an estrous-cycle specific manner.” Horm Behav 115: 104557. DOI: 10.1016/j.yhbeh.2019.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan AE, Thompson AMK, Buesing D, Fourman SM, Packard AEB, Terefe T, Li D, Wang X, Song S, Solomon MB Ulrich-Lai YM (2018). “Palatable food affects hpa axis responsivity and forebrain neurocircuitry in an estrous cycle-specific manner in female rats.” Neuroscience 384: 224–240. DOI: 10.1016/j.neuroscience.2018.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan AE Ulrich-Lai YM (2015). “Activation of physiological stress responses by a natural reward: Novel vs. Repeated sucrose intake.” Physiol Behav 150: 43–52. DOI: 10.1016/j.physbeh.2015.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Michalikova S, Bradford A. Ahmed S. (2005). “Detailed analysis of the behavior of lister and wistar rats in anxiety, object recognition and object location tasks.” Behav Brain Res 159(2): 247–266. DOI: 10.1016/j.bbr.2004.11.006 [DOI] [PubMed] [Google Scholar]
- Epel E, Jimenez S, Brownell K, Stroud L, Stoney C. Niaura R. (2004). “Are stress eaters at risk for the metabolic syndrome?” Ann N Y Acad Sci 1032: 208–210. DOI: 10.1196/annals.1314.022 [DOI] [PubMed] [Google Scholar]
- Epel E, Lapidus R, McEwen B. Brownell K(2001). “Stress may add bite to appetite in women: A laboratory study of stress-induced cortisol and eating behavior.” Psychoneuroendocrinology 26(1): 37–49. [DOI] [PubMed] [Google Scholar]
- File SE, Lippa AS, Beer B. Lippa MT (2004). “Animal tests of anxiety.” Curr Protoc Neurosci Chapter 8: Unit 8 3. DOI: 10.1002/0471142301.ns0803s26 [DOI] [PubMed] [Google Scholar]
- Finch LE Tomiyama AJ (2015). “Comfort eating, psychological stress, and depressive symptoms in young adult women.” Appetite 95: 239–244. DOI: 10.1016/j.appet.2015.07.017 [DOI] [PubMed] [Google Scholar]
- Grissom N, Kerr W Bhatnagar S. (2008). “Struggling behavior during restraint is regulated by stress experience.” Behav Brain Res 191(2): 219–226. DOI: 10.1016/j.bbr.2008.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groesz LM, McCoy S, Carl J, Saslow L, Stewart J, Adler N, Laraia B. Epel E(2012). “What is eating you? Stress and the drive to eat.” Appetite 58(2): 717–721. DOI: 10.1016/j.appet.2011.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloux JP, Seney M, Edgar N Sibille E. (2011). “Integrated behavioral z-scoring increases the sensitivity and reliability of behavioral phenotyping in mice: Relevance to emotionality and sex.” J Neurosci Methods 197(1): 21–31. DOI: 10.1016/j.jneumeth.2011.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haba R, Shintani N, Onaka Y, Wang H, Takenaga R, Hayata A, Baba A. Hashimoto H. (2012). “Lipopolysaccharide affects exploratory behaviors toward novel objects by impairing cognition and/or motivation in mice: Possible role of activation of the central amygdala.” Behav Brain Res 228(2): 423–431. DOI: 10.1016/j.bbr.2011.12.027 [DOI] [PubMed] [Google Scholar]
- Hume C, Jachs B Menzies J. (2016). “Homeostatic responses to palatable food consumption in satiated rats.” Obesity (Silver Spring) 24(10): 2126–2132. DOI: 10.1002/oby.21606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KR, Myers B Herman JP(2011). “Stimulation of the prelimbic cortex differentially modulates neuroendocrine responses to psychogenic and systemic stressors.” Physiol Behav 104(2): 266–271. DOI: 10.1016/j.physbeh.2011.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandiah J, Yake M, Jones J Meyer M. (2006). “Stress influences appetite and comfort food preferences in college women.” Nutr Res 26: 118–123. [Google Scholar]
- Lam TK, Schwartz GJ Rossetti L. (2005). “Hypothalamic sensing of fatty acids.” Nat Neurosci 8(5): 579–584. DOI: 10.1038/nn1456 [DOI] [PubMed] [Google Scholar]
- Laugero KD, Bell ME, Bhatnagar S, Soriano L Dallman MF (2001). “Sucrose ingestion normalizes central expression of corticotropin-releasing-factor messenger ribonucleic acid and energy balance in adrenalectomized rats: A glucocorticoid-metabolic-brain axis?” Endocrinology 142(7): 2796–2804. [DOI] [PubMed] [Google Scholar]
- Laugero KD, Falcon LM Tucker KL (2011). “Relationship between perceived stress and dietary and activity patterns in older adults participating in the boston puerto rican health study.” Appetite 56(1): 194–204. DOI: 10.1016/j.appet.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE, Magnan C, Dunn-Meynell A Le Foll C. (2011). “Metabolic sensing and the brain: Who, what, where, and how?” Endocrinology 152(7): 2552–2557. DOI: 10.1210/en.2011-0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S-C, Scearce-Levie K Sheng M. (2016). “Characterization of social behaviors in caspase3 deficient mice.” Scientific Reports 6: 18335. DOI: 10.1038/srep18335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macht M Mueller J. (2007). “Immediate effects of chocolate on experimentally induced mood states.” Appetite 49(3): 667–674. DOI: 10.1016/j.appet.2007.05.004 [DOI] [PubMed] [Google Scholar]
- Markus R, Panhuysen G, Tuiten A Koppeschaar H. (2000). “Effects of food on cortisol and mood in vulnerable subjects under controllable and uncontrollable stress.” Physiol Behav 70(3–4): 333–342. [DOI] [PubMed] [Google Scholar]
- Marttila K, Raattamaa H Ahtee L. (2006). “Effects of chronic nicotine administration and its withdrawal on striatal fosb/deltafosb and c-fos expression in rats and mice.” Neuropharmacology 51(1): 44–51. DOI: 10.1016/j.neuropharm.2006.02.014 [DOI] [PubMed] [Google Scholar]
- Merkestein M, Brans MA, Luijendijk MC, de Jong JW, Egecioglu E, Dickson SL Adan RA (2012). “Ghrelin mediates anticipation to a palatable meal in rats.” Obesity (Silver Spring) 20(5): 963–971. DOI: 10.1038/oby.2011.389 [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Kelz MB Chen J. (1999). “Deltafosb: A molecular mediator of long-term neural and behavioral plasticity.” Brain Res 835(1): 10–17. [DOI] [PubMed] [Google Scholar]
- Oliver G Wardle J. (1999). “Perceived effects of stress on food choice.” Physiol Behav 66(3): 511–515. [DOI] [PubMed] [Google Scholar]
- Ostrander MM, Ulrich-Lai YM, Choi DC, Richtand NM Herman JP (2006). “Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress.” Endocrinology 147(4): 2008–2017. DOI: 10.1210/en.2005-1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard AE, Egan AE Ulrich-Lai YM (2016). “Hpa axis interactions with behavioral systems.” Compr Physiol 6(4): 1897–1934. DOI: 10.1002/cphy.c150042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard AEB, Di S, Egan AE, Fourman SM, Tasker JG Ulrich-Lai YM(2017). “Sucrose-induced plasticity in the basolateral amygdala in a ‘comfort’ feeding paradigm.” Brain Struct Funct. DOI: 10.1007/s00429-017-1454-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page CE Coutellier L(2018). “Adolescent stress disrupts the maturation of anxiety-related behaviors and alters the developmental trajectory of the prefrontal cortex in a sex- and age-specific manner.” Neuroscience 390: 265–277. DOI: 10.1016/j.neuroscience.2018.08.030 [DOI] [PubMed] [Google Scholar]
- Paxinos G Watson C. (1998). The rat brain in stereotaxic coordinates. New York, Academic Press. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE Briley M. (1985). “Validation of open:Closed arm entries in an elevated plus-maze as a measure of anxiety in the rat.” J Neurosci Methods 14(3): 149–167. [DOI] [PubMed] [Google Scholar]
- Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS Nestler EJ (2004). “Induction of deltafosb in reward-related brain structures after chronic stress.” J Neurosci 24(47): 10594–10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A. (2004). “Oral and postoral determinants of food reward.” Physiol Behav 81(5): 773–779. [DOI] [PubMed] [Google Scholar]
- Shepard R, Page CE Coutellier L. (2016). “Sensitivity of the prefrontal gabaergic system to chronic stress in male and female mice: Relevance for sex differences in stress-related disorders.” Neuroscience 332: 1–12. DOI: 10.1016/j.neuroscience.2016.06.038 [DOI] [PubMed] [Google Scholar]
- Smith JC Sclafani A. (2002). “Saccharin as a sugar surrogate revisited.” Appetite 38(2): 155–160. [DOI] [PubMed] [Google Scholar]
- Sprowl DJ Ehrcke LA (1984). “Sweeteners: Consumer acceptance in tea.” J Am Diet Assoc 84(9): 1020–1022. [PubMed] [Google Scholar]
- Strack AM, Akana SF, Horsley CJ Dallman MF (1997). “A hypercaloric load induces thermogenesis but inhibits stress responses in the sns and hpa system.” Am J Physiol 272(3 Pt 2): R840–848. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Antunes J Tufik S. (2003). “Palatable solutions during paradoxical sleep deprivation: Reduction of hypothalamic-pituitary-adrenal axis activity and lack of effect on energy imbalance.” J Neuroendocrinol 15(9): 815–821. [DOI] [PubMed] [Google Scholar]
- Sundaresan S Abumrad NA (2015). “Dietary lipids inform the gut and brain about meal arrival via cd36-mediated signal transduction.” J Nutr 145(10): 2195–2200. DOI: 10.3945/jn.115.215483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan VWK, Wee MSM, Tomic O Forde CG (2019). “Temporal sweetness and side tastes profiles of 16 sweeteners using temporal check-all-that-apply (tcata).” Food Res Int 121: 39–47. DOI: 10.1016/j.foodres.2019.03.019 [DOI] [PubMed] [Google Scholar]
- Tomiyama AJ, Dallman MF Epel ES (2011). “Comfort food is comforting to those most stressed: Evidence of the chronic stress response network in high stress women.” Psychoneuroendocrinology 36(10): 1513–1519. DOI: 10.1016/j.psyneuen.2011.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryon MS, DeCant R Laugero KD (2013). “Having your cake and eating it too: A habit of comfort food may link chronic social stress exposure and acute stress-induced cortisol hyporesponsiveness.” Physiol Behav 114-115: 32–37. DOI: 10.1016/j.physbeh.2013.02.018 [DOI] [PubMed] [Google Scholar]
- Tryon MS, Stanhope KL, Epel ES, Mason AE, Brown R, Medici V, Havel PJ Laugero KD (2015). “Excessive sugar consumption may be a difficult habit to break: A view from the brain and body.” J Clin Endocrinol Metab 100(6): 2239–2247. DOI: 10.1210/jc.2014-4353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Christiansen AM, Ostrander MM, Jones AA, Jones KR, Choi DC, Krause EG, Evanson NK, Furay AR, Davis JF, Solomon MB, de Kloet AD, Tamashiro KL, Sakai RR, Seeley RJ, Woods SC Herman JP (2010). “Pleasurable behaviors reduce stress via brain reward pathways.” Proc Natl Acad Sci U S A 107(47): 20529–20534. DOI: 10.1073/pnas.1007740107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM Herman JP (2009). “Neural regulation of endocrine and autonomic stress responses.” Nat Rev Neurosci 10(6): 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Ostrander MM Herman JP (2011). “HPA axis dampening by limited sucrose intake: Reward frequency vs. Caloric consumption.” Physiol Behav 103(1): 104–110. DOI: 10.1016/j.physbeh.2010.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Ostrander MM, Thomas IM, Packard BA, Furay AR, Dolgas CM, Van Hooren DC, Figueiredo HF, Mueller NK, Choi DC Herman JP (2007). “Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses.” Endocrinology 148(4): 1823–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utter AC, Kang J, Nieman DC, Williams F, Robertson RJ, Henson DA, Davis JM Butterworth DE (1999). “Effect of carbohydrate ingestion and hormonal responses on ratings of perceived exertion during prolonged cycling and running.” Eur J Appl Physiol Occup Physiol 80(2): 92–99. [DOI] [PubMed] [Google Scholar]
- Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D’Alessio DA Herman JP (2005). “Comparative analysis of acth and corticosterone sampling methods in rats.” Am J Physiol Endocrinol Metab 289(5): E823–828. DOI: 10.1152/ajpendo.00122.2005 [DOI] [PubMed] [Google Scholar]
- Vialou V, Maze I, Renthal W, LaPlant QC, Watts EL, Mouzon E, Ghose S, Tamminga CA Nestler EJ (2010). “Serum response factor promotes resilience to chronic social stress through the induction of deltafosb.” J Neurosci 30(43): 14585–14592. DOI: 10.1523/JNEUROSCI.2496-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DL, Vialou V, Rios L, Carle-Florence TL, Chakravarty S, Kumar A, Graham DL, Green TA, Kirk A, Iniguez SD, Perrotti LI, Barrot M, DiLeone RJ, Nestler EJ Bolanos-Guzman CA (2008). “The influence of deltafosb in the nucleus accumbens on natural reward-related behavior.” J Neurosci 28(41): 10272–10277. DOI: 10.1523/JNEUROSCI.1531-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle J, Steptoe A, Oliver G Lipsey Z. (2000). “Stress, dietary restraint and food intake.” J Psychosom Res 48(2): 195–202. [DOI] [PubMed] [Google Scholar]
- Weinberg MS, Grissom N, Paul E, Bhatnagar S, Maier SF Spencer RL (2010). “Inescapable but not escapable stress leads to increased struggling behavior and basolateral amygdala c-fos gene expression in response to subsequent novel stress challenge.” Neuroscience 170(1): 138–148. DOI: 10.1016/j.neuroscience.2010.06.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein SE, Shide DJ Rolls BJ (1997). “Changes in food intake in response to stress in men and women: Psychological factors.” Appetite 28(1): 7–18. DOI: 10.1006/appe.1996.0056 [DOI] [PubMed] [Google Scholar]
- Zellner DA, Loaiza S, Gonzalez Z, Pita J, Morales J, Pecora D Wolf A. (2006). “Food selection changes under stress.” Physiol Behav 87(4): 789–793. DOI: 10.1016/j.physbeh.2006.01.014 [DOI] [PubMed] [Google Scholar]