Abstract
A means for distinguishing between clinical isolates of Renibacterium salmoninarum that is based on the PCR amplification of length polymorphisms in the tRNA intergenic spacer regions (tDNA-ILPs) was investigated. The method used primers specific to nucleotide sequences of R. salmoninarum tRNA genes and tRNA intergenic spacer regions that had been generated by using consensus tRNA gene primers. Twenty-one PCR products were sequenced from five isolates of R. salmoninarum from the United States, England, and Scotland, and four complete tRNA genes and spacer regions were identified. Sixteen specific PCR primers were designed and tested singly and in all possible pairwise combinations for their potential to discriminate between isolates from recent clinical outbreaks of bacterial kidney disease (BKD) in the United Kingdom. Fourteen of the isolates were cultured from kidney samples taken from fish displaying clinical signs of BKD on five farms, and some of the isolates came from the same farm and at the same time. The tDNA-ILP profiles separated 22 clinical isolates into nine groups and highlighted that some farms may have had more than one source of infection. The grouping of isolates improved on the discriminatory power of previously reported typing methods based on randomly amplified polymorphic DNA analysis and restriction fragment length profiles developed using insertion sequence IS994. Our method enabled us to make divisions between closely related clinical isolates of R. salmoninarum that have identical exact tandem repeat (ETR-A) loci, rRNA intergenic spacer sequences, and IS994 profiles.
Renibacterium salmoninarum is an obligate pathogen and the causative agent of bacterial kidney disease (BKD), a chronic systemic infection of salmonid fish (15). The pathogen is a gram-positive bacterium that represents a genospecies placed within the high-G+C subgroup of the actinomycetes (4, 24, 27, 36). R. salmoninarum survives intracellularly and can be transmitted both vertically inside the ova and horizontally between cohabiting fish. Although BKD is geographically widespread and is responsible for significant losses in farmed and wild salmonids, knowledge of the epizootiology of the disease has been hampered due to a remarkable degree of uniformity among isolates of the pathogen (5, 19, 37).
We have examined the rRNA genes of R. salmoninarum for evidence of variation and have shown that the bacterium possesses two copies of the rRNA operon, which are identical or nearly identical and which are highly conserved among a wide variety of isolates (20). The spacer regions between the rRNA genes often vary in size and nucleotide sequence and can be useful for typing bacterial species (10, 18), but this is not the case in R. salmoninarum. Analysis of the 16S-23S rRNA intergenic spacer (ITS1) of R. salmoninarum has shown that the spacers of all isolates are identical in length, and, although four sequence variants (SV) have been described, most isolates from a wide variety of sources belong to a single sequevar, SV1 (21, 22). An exact tandem repeat locus, ETR-A, has been identified and has been shown to be a specific marker for SV1 isolates (21). The three remaining ITS1 sequence variants, which have from 1 to 3 base substitutions, are confined to isolates with temporal and spatial origins that set them apart from the mainstream of salmonid fisheries. Variation in the 23S-5S rRNA intergenic spacer (ITS2) was less obvious, and two sequence variants, SV21 and SV22, were identified (20).
Examining variation throughout the whole R. salmoninarum genome using randomly amplified polymorphic DNA (RAPD) and recently characterized insertion element IS994 has provided up to 21 arbitrary groupings on the basis of banding patterns (21, 22, 34). Nevertheless, many isolates from obviously unrelated sources are still indistinguishable, and there is a need to identify more specific markers of variation that will facilitate a better understanding of the relationships between isolates that share the same spatial and temporal origins.
Use of length polymorphisms of the spacer regions that separate tRNA genes is a PCR-based strategy for exploring the degree of relatedness between bacteria. The arrangement of tRNA gene clusters in multiple tandem repeating units on the bacterial genome (23, 40) allows the amplification of intergenic length polymorphisms (tDNA-ILPs) by PCR employing consensus primers that are annealed at low stringency. Welsh and McClelland developed four “universal” tRNA gene primers designed to face outwards from the end of the tRNA genes, which have been shown to amplify a tDNA-ILP fingerprint that is determined by the arrangement of tRNA genes on the bacterial genome (42, 43). The order and arrangement of tRNA genes are highly conserved, and the fingerprints generated by the consensus primers are often characteristic of a particular species (12, 30), although in some cases consensus tRNA gene primers have been used to generate divisions below species level (7, 9, 35). Furthermore, specific tRNA gene primers can be developed from the DNA sequences of PCR products generated using consensus primers, and this approach was used to distinguish between streptococci on the basis of tRNA gene spacer length polymorphisms (31).
The tRNA genes and their flanking regions in a wide range of bacteria have been reported to be prone to disruption by mobile genetic elements including insertion sequences, tandem repeats, pathogenicity islands, prophage, and plasmids (6, 8, 11, 17, 32). An area of the genome prone to such a high degree of genetic change may have the potential to differentiate between isolates in a highly conserved organism such as R. salmoninarum. Here we report the development of a tool for epizootiological studies of R. salmoninarum based on tDNA-ILPs which uses PCR primers specific to tRNA genes and spacer sequences. We show that the method can discriminate between isolates of R. salmoninarum from the United Kingdom that possess identical ITS1 and ITS2 nucleotide sequences, IS994 patterns, and ETR-A profiles.
MATERIALS AND METHODS
Bacterial isolates and DNA extraction.
A total of 67 isolates of R. salmoninarum were used in this study, and their designation codes, countries of origin, and sources of isolation are listed in Tables 1 and 2. The isolates were cultured for 6 to 8 weeks in selective kidney disease medium supplemented with 5% spent culture broth at 15°C (3, 14) and then freeze-dried and checked for purity. The names and addresses of colleagues who have kindly provided isolates, derived from confirmed clinical outbreaks of BKD, have been previously published (22, 34). The isolates are maintained in a culture collection at the University of Plymouth, in collaboration with the Centre for Environmental, Fisheries and Aquacultural Sciences, Weymouth, United Kingdom.
TABLE 1.
R. salmoninarum isolates which were used for the PCR amplification of tRNA genes and intergenic spacer regions using consensus tRNA primers
| Isolate | Geographic origin and yr | Biological sourcea |
|---|---|---|
| 980036-150 | Wales, 1998 | Rainbow trout (f) |
| 980036-87 | Wales, 1998 | Rainbow trout (f) |
| 970083-88 | England, 1997 | Rainbow trout (f) |
| 970083-102 | England, 1997 | Rainbow trout (f) |
| 980106#1.1.5 | England, 1998 | Rainbow trout (f) |
| 970419-1.2.3 | England, 1997 | Atlantic salmon (w) |
| 970153-19 | England, 1997 | Grayling (w) |
| A6 | England, 1998 | Rainbow trout (f) |
| A80 | England, 1998 | Rainbow trout (f) |
| MT409 | Scotland, 1987 | Unknown |
| MT417 | Scotland, 1988 | Atlantic salmon (f) |
| MT239 | Scotland, 1986 | Atlantic salmon (f) |
| MT426 | Scotland, 1988 | Unknown |
| NCIMB1111 | Scotland, 1962 | Atlantic salmon (w)b |
| NCIMB1112 | Scotland, 1962 | Atlantic salmon (w)b |
| NCIMB1113 | Scotland, 1962 | Atlantic salmon (w)b |
| NCIMB1114 | Scotland, 1962 | Atlantic salmon (w) |
| NCIMB1115 | Scotland, 1962 | Atlantic salmon (w) |
| NCIMB1116 | Scotland, 1962 | Atlantic salmon (w) |
| MT420 | Scotland, 1988 | Atlantic salmon (f) |
| MT452 | Scotland, 1988 | Rainbow trout (f) |
| MT1363 | Scotland, 1993 | Rainbow trout (f) |
| MT410 | Scotland, 1987 | Unknown |
| FT-10 | Scotland | Atlantic salmon |
| DR143 | Alberta, Canada, 1972 | Brook trout (f) |
| DR384 | British Columbia, Canada, 1979 | Coho salmon (f) |
| 960023 | British Columbia, Canada, 1996 | Coho salmon (f) |
| 960046 | British Columbia, Canada, 1996 | Coho salmon (f) |
| 980002 | British Columbia, Canada, 1998 | Chinook salmon (f) |
| RS-TSA | Nova Scotia, Canada | Atlantic salmon (f) |
| AcF6-1 | Northwest Territories, Canada, 1985 | Arctic char (w) |
| F-120-87(P-2) | Iceland, 1987 | Atlantic salmon (f) |
| F-130-87(P-4) | Iceland, 1987 | Rainbow trout (f) |
| F-138-87(O-78) | Iceland, 1987 | Atlantic salmon (f) |
| F-273-87(P-19) | Iceland, 1987 | Atlantic salmon (f) |
| F-283-87(P-10) | Iceland, 1987 | Atlantic salmon (f) |
| F-358-87(P-13) | Iceland, 1987 | Atlantic salmon (w) |
| S-182-90(P-7) | Iceland, 1990 | Atlantic salmon (f) |
| Siletz | Oregon, 1976 | Coho salmon (f) |
| Marion Forks | Oregon, 1987 | Chinook salmon (f) |
| Little Goose | Washington, 1994 | Chinook salmon (f) |
| CCM6205 | Washington, 1975 | Coho salmon (f) |
| 84-019-OC | Washington, 1984 | Chinook salmon (w) |
| SS-ChS-94-1 | Oregon, 1994 | Chinook salmon |
| Cow ChS94 P22 | Washington, 1994 | Chinook salmon (f) |
| Idaho 91-126 | Idaho, 1993 | Sockeye salmon (f) |
| RFL-643.94#1 | Washington, 1994 | Sockeye salmon (f) |
| CCM6206 | Oregon, 1978 | Chinook salmon (f) |
| Round Butte | Oregon, 1973 | Chinook salmon (f) |
| NCIMB2235 | Oregon, 1981 | Chinook salmon (f) |
| BY1996 | Alaska, 1998 | Chinook salmon (f) |
| Rs 9 | Sweden, 1985 | Rainbow trout |
| Rs 19 | Sweden, 1987 | Atlantic salmon |
| Rs 61 | Sweden, 1989 | Arctic char |
| Rs 116 | Sweden, 1993 | Grayling |
| Rs 122 | Sweden, 1994 | Rainbow trout (f) |
| Rs 125 | Sweden | Rainbow trout |
| Rs 126 | Sweden | Rainbow trout |
| 3015-86 | Norway | Atlantic salmon |
| 4451-86 | Norway | Atlantic salmon |
Isolates were obtained from wild fish (w) or farm-raised fish (f). The full histories of some isolates are not known.
The host species is uncertain but probably Atlantic salmon.
TABLE 2.
Isolates of R. salmoninarum which were used for the PCR amplification of tDNA-ILPs with specific tRNA gene and intergenic spacer primers
| No. | Isolate | Geographic origin | Biological sourceb | Details of isolation (date) |
|---|---|---|---|---|
| 1 | 970083-88c | England | Rainbow trout (f) | Farm A, tank A (February 1997) |
| 2 | 970083-102cd | England | Rainbow trout (f) | Farm A, tank A (February 1997) |
| 3f | 980106#1.1.5 | England | Rainbow trout (f) | Farm B, raceway (March 1998) |
| 4f | 980036-150c | Wales | Rainbow trout (f) | Farm C, pond (February 1998) |
| 5f | 980036-87cd | Wales | Rainbow trout (f) | Farm C, raceway (February 1998) |
| 6 | 970419-1.2.3d | England | Atlantic salmon (w) | Unknown (1997) |
| 7 | 970153-19 | England | Grayling (w) | Unknown (1997) |
| 8 | A6cd | England | Rainbow trout (f) | Farm D (March 1998) |
| 9 | A80cd | England | Rainbow trout (f) | Farm D (March 1998) |
| 10 | 980297#97cde | England | Rainbow trout (f) | Hatchery E, raceway (1998) |
| 11g | F95cd | England | Rainbow trout (f) | Farm A, tank B (1998) |
| 12g | F85cd | England | Rainbow trout (f) | Farm A, tank B (1998) |
| 13g | F82cde | England | Rainbow trout (f) | Farm A, tank C (1998) |
| 14g | F60cd | England | Rainbow trout (f) | Farm A, tank D (1998) |
| 15g | F47cd | England | Rainbow trout (f) | Farm A, tank E (1998) |
| 16g | F3cd | England | Rainbow trout (f) | Farm A, raceway (1998) |
| 17 | NCIMB1111cd | Scotland | Atlantic salmon (w)a | River Dee (1962) |
| 18 | NCIMB1112cd | Scotland | Atlantic salmon (w)a | River Dee (1962) |
| 19 | NCIMB1113cd | Scotland | Atlantic salmon (w)a | River Dee (1962) |
| 20 | NCIMB1114 | Scotland | Atlantic salmon (w) | River Dee (1962) |
| 21 | NCIMB1115cd | Scotland | Atlantic salmon (w) | River Dee (1962) |
| 22 | NCIMB1116d | Scotland | Atlantic salmon (w) | River Dee (1962) |
The host species is uncertain but probably Atlantic salmon.
Isolates were obtained from wild fish (w) or farm-raised fish (f). The full histories of some isolates are not known.
The ponds and raceways contained fish from the same hatchery but not from hatchery E.
The fish came from various unlisted origins, probably not the same as that of no. 3 to 5.
DNA extraction and analysis.
Genomic DNA was extracted and analyzed as described previously (22) using the Puregene D-6000 DNA isolation kit according to the manufacturer's instructions (Gentra Systems Inc.). The DNA concentration was determined for each isolate by Kodak digital imaging following agarose gel electrophoresis, and the identity of R. salmoninarum DNA was confirmed for all isolates by PCR using six sets of primers specific for R. salmoninarum genes msa, hly, and rsh as previously described (22).
Amplification of tRNA genes and intergenic spacer regions using consensus primers.
PCRs were performed on DNA extracted from 60 isolates of R. salmoninarum (Table 1) using consensus tRNA gene primers T5A (5′-AGTCCGGTGCTCTAACCAACTGAG-3′), T5B (5′-AATGCTCTACCAACTGAACT-3′), T3A (5′-GGGGGTTCGAATTCCCGCCGGCCCCA-3′), and T3B (5′-AGGTCGCGGGTTCGAATCC-3′) in each of the six possible paired combinations in accordance with the published protocols (42, 43). The 50-μl reaction mixtures consisted of 1 U of Taq polymerase and reaction buffer containing 1.5 mM MgCl2 (Roche), 24 pmol of each primer (Genosys), 0.2 mM deoxynucleoside triphosphates, and 10 ng of bacterial DNA. PCR amplification was performed in a DNA thermal cycler (Perkin-Elmer). The reaction mixture was overlaid with mineral oil (Sigma), incubated at 94°C for 2 min, and then subjected to 44 cycles of 94°C for 30 s, 45°C for 30 s, and 72°C for 90 s and a final cycle of 94°C for 30 s, 45°C for 30 s, and 72°C for 10 min. The amplification products were visualized after electrophoresis at 80 V in 1.2% agarose gels.
Cloning of tRNA genes and intergenic spacers from R. salmoninarum.
Products which were generated in PCRs using consensus primers were purified using the Prep-a-Gene DNA purification kit (Bio-Rad), and were blunted, kinased, and ligated into pUC18 using the Sureclone kit (Amersham Pharmacia Biotech) according to the manufacturer's instructions. Host Escherichia coli DH5α cells were transformed with the ligation mixture, and recombinants were recovered on Luria-Bertani agar containing 50 μg of ampicillin/ml with a 5-ml overlay containing 240 μg of IPTG (isopropyl-β-d-thiogalactopyranoside/ml and 250 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside/ml.
DNA sequencing and analysis of cloned products.
Plasmid DNA containing cloned inserts was isolated using the Quantum Prep plasmid miniprep kit (Bio-Rad) according to the manufacturer's instructions. Both strands of the cloned insert DNA were sequenced by MWG-Biotech Ltd. (Milton Keynes, United Kingdom). The DNA sequences obtained in this way were compared with sequences from other organisms obtained from the GenBank database (1). Pairwise and multiple alignments were performed using the BLAST 2 sequence program (38) available at GenBank and the GeneBee multiple-alignment program available at http://www.genebee.msu.su/services/malign_reduced.html. The tRNA genes were identified using the tRNA-scan program available at http://www.genetics.wustl.edu/eddy/tRNAscan-SE/ (16, 29).
Amplification of tRNA intergenic spacer regions using specific primers.
The isolates that were used for tDNA-ILP analysis are listed in Table 2 and represent 22 isolates of R. salmoninarum from England, Wales, and Scotland, 14 of which were cultured from the kidneys of farmed fish displaying clinical symptoms of BKD and 8 of which were from wild fish. Sixteen PCR primers (Table 3) were designed using Amplify software (13) on the basis of the nucleotide sequences of amplicons derived from PCRs using consensus tRNA primers. The primers were tested singly and in all possible pairwise combinations in PCRs for their ability to amplify reproducible tDNA-ILPs. The PCR mixtures were prepared as described for amplification using consensus primers. Each reaction mixture was incubated at 96°C for 2 min and then subjected to 40 cycles consisting of 96°C for 30 s, 50°C for 30 s, and 72°C for 90 s.
TABLE 3.
PCR primers specific to R. salmoninarum DNA sequences that were used to amplify tRNA intergenic spacer regions
| Designation | Sequence (5′–3′) | Sourcea |
|---|---|---|
| A35K+754 | TTGGTAAGAACGAGGTCACCGGAT | tRNA gene Thr (GGT) |
| A35K−760 | TTACCAAGAACGCGCTCTACCACT | tRNA gene Thr (GGT) |
| T17C+80 | GACAGGATTCGAACCTGTGACCAT | tRNA gene Arg (TCT) |
| T17C−135 | TCAGTTGGATAGAGCATCCGCCTT | tRNA gene Arg (TCT) |
| T25D+115 | CCTTAGTTCGTAGCCAAGTGCTCT | tRNA gene Arg (ACG) |
| T25D−120 | TGGATAGAGCACTTGGCTACGAAC | tRNA gene Arg (ACG) |
| T35E+128 | ACCAACTGCGCTACAGGGCCTTGC | tRNA gene Asp (GTC) |
| T35E−94 | GTTCAAGTCCCGTCAGGGTCGCTA | tRNA gene Asp (GTC) |
| A7A+46 | GCGTGAAAGATCTTAACCGGTGAG | tRNA ITS region |
| A7A−309 | ATGATCGACGTCAGCTCCATCAAG | tRNA ITS region |
| A25A+4 | CCGGTGCTCTAACCAACTGAGCTA | tRNA ITS region |
| A25A−247 | GGTTGTTGCGTTTAGCTCAAGACG | tRNA ITS region |
| T3C+42 | GGCTCGTGTCAAGACGGTTTTTGA | tRNA ITS region |
| T3C−123 | TGCTCTAACCAACTGAGCTACACC | tRNA ITS region |
| T25E+19 | AACTGAGCTAAGCGCCCTTGAGAA | tRNA ITS region |
| T25E−128 | CGCCATTTTCTAGATCCCTTGTGC | tRNA ITS region |
tRNA gene anticodons are in parentheses.
RAPD analysis of isolates from the same outbreak of BKD.
RAPD analysis was carried out on isolates 980297#97, F3, F47, F60, F82, F85, and F95, which were isolated from the kidneys of rainbow trout showing clinical signs of BKD, and six of these isolates were obtained at the same time from a single farm in England (Table 2). Two methods of RAPD analysis were used as previously described (22). The first method employed Ready-to-go RAPD analysis beads (Amersham Pharmacia Biotech), which were used according to the manufacturer's instructions. Briefly, six distinct random 10-mer primers, namely, P1 (GGTGCGGGAA), P2 (GTTTCGCTCC), P3 (GTAGACCCGT), P4 (AAGAGCCCGT), P5 (AACGCGCAAC), and P6 (CCCGTCAGCA), were used at a concentration of 25 pmol with 10 ng of template DNA in a 25-μl volume. The PCR conditions consisted of 1 cycle of 95°C for 4 min and then 45 cycles of 95°C for 1 min, 36°C for 1 min, and 72°C for 2 min.
For the second method, described by Atienzar et al. (2), we used primers OPA9 (GGGTAACGCC) and OPB1 (GTTTCGCTCC) (Operon Technologies Inc.). The 25-μl reaction mixtures contained 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 5.11 mM MgCl2, 0.1% Triton X-100, 0.1% gelatin, each deoxynucleoside triphosphate at a concentration of 0.33 mM, 2 μM primer, 2.5 μg of bovine serum albumin, 2.8 U of Taq DNA polymerase (Immunogen International), and 10 ng of bacterial DNA. The PCR conditions consisted of 1 cycle of 95°C for 4 min, 39 cycles of 95°C for 1 min, 50°C for 1 min, and 74°C for 1 min, and a final cycle of 95°C for 1 min, 50°C for 1 min, and 74°C for 10 min.
ITS1 sequence analysis.
The analysis of ITS1 was performed in accordance with the published protocol (22). Briefly, PCR mixtures were prepared using primers RS+1002 and ML−1329, which span ITS1 and which have previously been shown to amplify a single unambiguous 751-bp product of known sequence from R. salmoninarum DNA (22). Each 50-μl reaction mixture was prepared as described for tRNA gene and intergenic spacer amplification, incubated at 96°C for 2 min, and then subjected to 24 cycles of 96°C for 30 s, 65°C for 30 s, and 72°C for 90 s and a final cycle of 96°C for 30 s, 65°C for 30 s, and 72°C for 5 min. The reaction products were analyzed in 1.0% agarose gels.
ITS2 sequence analysis.
The analysis of ITS2 was performed by PCR amplification using primers R5+118 (5′-CTGACCGGTACTAATAGGCCAACA-3′) and R5-433 (5′-GTCTTAGCTTCCGGGTTCGAGATG-3′) under the same reaction conditions used for ITS1 analysis. Primers R5+118 and R5−433 are specific to sequences in the 3′ end of the 23S rRNA gene and the 5′ end of the 5S rRNA gene, respectively, thereby flanking the ITS2 region of the rRNA operon of R. salmoninarum. The primers amplify a single 318-bp reaction product with a nucleotide sequence which corresponds to the ITS2 regions of a variety of isolates of R. salmoninarum from many sources (20).
ETR-A locus profile of R. salmoninarum isolates.
The analysis of the ETR-A locus was performed using a previously described protocol (21). PCR amplification was carried out using primers 17D+95 and 17D−344, which previously have been shown to amplify a single fragment spanning the ETR-A locus of R. salmoninarum (21). The PCR conditions were the same as those described for the amplification of ITS1 and ITS2. The identities of the PCR products were confirmed by nucleotide sequencing for isolates 980297#97 and F82.
DNA sequencing and sequence analysis of PCR products.
Both strands of all PCR products were sequenced using a cycle sequencing method by MWG-Biotech Ltd. Pairwise and multiple alignments of the nucleotide sequences were performed using the BLAST 2 sequence program (38) and the GeneBee multiple-alignment program, respectively.
Nucleotide sequence accession numbers.
The nucleotide sequences reported here have been deposited in GenBank with accession no. AF239179 to AF239195 (ITS1 regions), AF239890 to AF239906 (ITS2 regions), AF242882 to AF242889, AF245384 to AF245386 (tRNA genes and tRNA intergenic spacer regions), and AF242882 and AF242883 (ETR-A locus).
RESULTS
After confirmation of culture purity and DNA extraction, the identity of the template DNA was confirmed using six sets of primers that are believed to be specific for R. salmoninarum DNA. Each of the PCRs produced a single distinct band of the expected size (22).
The ITS1 sequences and the ITS2 sequences are conserved among recent clinical isolates of R. salmoninarum.
ITS1 and ITS2 were amplified by PCR from the genomic DNA of 17 isolates of R. salmoninarum including recent clinical isolates from five fish farms in England and Wales. Many of the isolates had been cultured from the kidneys of fish showing clinical signs of BKD which were held on the same farm and sampled at the same time (Table 2). The ITS1 and ITS2 sequences of the remaining five isolates listed in Table 2 have been previously published (21, 22). All of the isolates examined in this way were found to have ITS1 regions of identical size and nucleotide sequence that matched that of previously designated sequevar SV1 (22). In addition, the analysis of ITS2 sequences from these isolates (Table 2) showed that all isolates had regions identical in size and nucleotide sequence, which exactly matched that of previously described ITS2 sequevar SV21 (20).
Among recent isolates from the same farm the ETR-A locus has two copies of a 51-bp tandem repeat.
The ETR-A loci of seven isolates were amplified by PCR using primers specific to sequences that are known to flank this region of the R. salmoninarum genome. The seven isolates, 980297#97, F3, F47, F60, F82, F85, and F95, were isolated from the kidneys of rainbow trout showing clinical signs of BKD, and six of these were obtained at the same time from a single farm in England (Table 2). The ETR-A loci of the remaining 15 isolates listed in Table 2 have been previously examined (21). All of the isolates were found to possess a single amplicon of 301 bp, which corresponds to the expected size of a PCR product containing two copies of the 51-bp tandem repeat unit at ETR-A. This was confirmed by the nucleotide sequence analysis of the PCR products from two selected isolates, 980297#97 and F82.
The RAPD profiles of recent isolates from the same farm are almost identical.
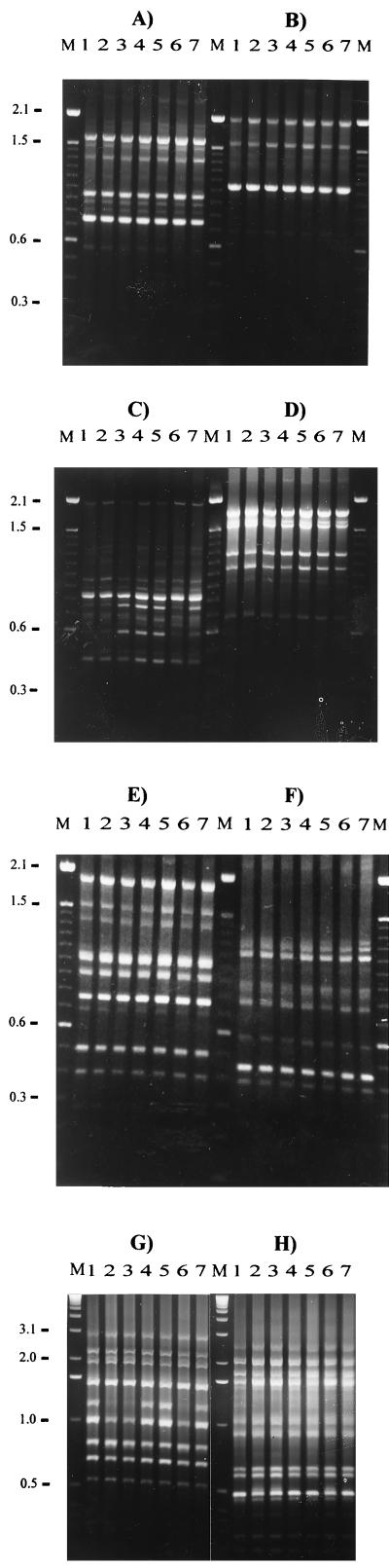
RAPD analysis was carried out on 7 isolates, 980297#97, F3, F47, F60, F82, F85, and F95, which are listed in Table 2. The remaining 15 isolates listed in Table 2 have been previously characterized by RAPD analysis (21). RAPD analysis revealed that all seven isolates produced identical RAPD profiles using primers P1, P2, P4, P5, and OPA9 (Fig. 1). However, the RAPD patterns obtained using primers P3, P6, and OPB1 did reveal differences in the intensities of some bands between some of the isolates. When primer P3 was used, isolates F3, F60, F82, and F85 possessed stronger bands of 600 and 750 bp than isolates 980297#97, F47, and F95 (Fig. 1C). Primer P6 generated a strong band of 1.25 kb in isolate F3; this band was weak or absent in the other isolates (Fig. 1F). Furthermore, the RAPD patterns generated using primer OPB1 showed that a 1.15-kb band, which was strongly present in isolates 980297#97, F3, F60, and F82, was indistinct in isolates F47, F85, and F95 (Fig. 1G).
FIG. 1.
The RAPD patterns of seven isolates of R. salmoninarum. The patterns were generated using primers P1 (A), P2 (B), P3 (C), P4 (D), P5 (E), P6 (F), OPB1 (G), and OPA9 (H). Lanes 1 to 7 correspond to isolates 980297#97, F95, F85, F82, F60, F47, and F3, respectively. Lanes M, 100-bp (A to F) or 1-kb (G and H) DNA ladder (Gibco BRL).
PCR amplification using consensus tRNA gene primers.
A series of PCRs were carried out using four consensus tRNA gene primers in each of the six possible paired combinations. The primers are located within the tRNA consensus sequence and are designed to amplify the intergenic spacer region from the 5′ and 3′ ends of tRNA genes (31, 42). PCR amplification of DNA templates from 60 R. salmoninarum isolates (Table 1) resulted in profiles that were identical in appearance for many of the isolates regardless of their origin (Fig. 2). For example, with consensus primer set T3B-T5A all of the isolates showed identical tDNA PCR profiles consisting of a major band of 230 bp (Fig. 2A). Similarly, the use of primer set T3B-T5B showed that a fragment of 230 bp was present in all of the isolates examined (data not shown). However, using other primer combinations revealed that some isolates, particularly Marion Forks, 970153-19, and AcF6-1, consistently showed distinct differences. In the majority of isolates primer set T5A-T5B produced two major bands of approximately 1.1 and 2.4 kb, but 970153-19 and Marion Forks showed only single main bands of 490 and 300 bp, respectively (Fig. 2B). The patterns produced by primer set T3A-T3B were identical for most of the isolates and consisted of three main bands of approximately 120, 790, and 840 bp. Three isolates differed from this pattern. Cow ChS94 P22 showed an additional band of 180 bp, 970153-19 possessed two additional bands of 620 bp and 1.2 kb, and AcF6-1 possessed an additional band of 900 bp (Fig. 2C). The use of primer set T3A-T5A (Fig. 2D to F) or T3A-T5B (data not shown) produced the same fingerprints, which consisted of a faint band of 290 bp and a major band of 830 bp in all except three isolates. The three isolates which differed from this pattern were 970153-19 (major band of 200 bp), Marion Forks (major band of 230 bp), and AcF6-1 (major band of 870 bp).
FIG. 2.
The tDNA PCR profiles of 60 isolates of R. salmoninarum. The patterns were generated using consensus primer sets T3B-T5A (A), T5A-T5B (B), T3A-T3B (C), and T3A-T5A (D to F). (A and F) Lanes 1 to 20, isolates 960046, F-120-87(P-2), F-130-87(P-4), F-138-87(O-78), F-273-87(P-19), F-283-87(P-10), F-358-87(P-13), S-182-90(P-7), Rs 9, Rs 19, Rs 61, Rs 116, Rs 122, Rs 125, Rs 126, 3015-86, 4451-86, RS-TSA, FT10, and BY1996, respectively. (C and E) Lanes 1 to 20, isolates MT452, MT1363, MT410, Siletz, Marion Forks, Little Goose, CCM6205, 84-019-OC, SS-ChS-94-1, Cow ChS94 P22, Idaho 91-126, RFL-643.94#1, CCM6206, Round Butte, NCIMB2235, AcF6-1, DR143, DR384, 980002, and 960023, respectively. (B and D) Lanes 1 to 20, isolates 970083-88, 970083-102, 980106#1.1.5, 980036-150, 980036-87, 970419-1.2.3, 970153-19, A6, A80, MT409, MT417, MT239, MT426, NCIMB1111, NCIMB1112, NCIMB1113, NCIMB1114, NCIMB1115, NCIMB1116, and MT420, respectively. Lanes M, 100-bp DNA ladder (Gibco BRL).
Sequencing R. salmoninarum tRNA genes and intergenic spacers.
A variety of products that had been amplified in tDNA PCRs using consensus tRNA gene primers were purified and cloned into pUC18. The products were chosen from R. salmoninarum isolates NCIMB2235, Marion Forks, 970153-19, 980106#1.1.5, and NCIMB1114, which represented some of the variations that were found in the tRNA intergenic spacer regions. Sequencing 21 clones revealed that many of the products spanned part of the tRNAIle gene and all of the tRNAAsp gene, anticodon GTC. In a single clone, the tRNAThr gene, anticodon GGT, preceded a partial tRNAMet gene sequence. Two distinct and complete tRNAArg genes, anticodons TCT and ACG, were identified, and these were flanked by partial tRNAAla and tRNAVal sequences, respectively. Other partial tRNA gene sequences were found in the primer sites but could not be fully identified.
PCR amplification using primers specific to R. salmoninarum tRNA genes and intergenic spacer sequences.
Sixteen specific primers were designed based on the sequences of the tRNA genes and intergenic spacer regions that had been obtained from amplicons generated using consensus tRNA gene primers (Table 3). The primers were specific for tRNA gene sequences encoding threonine, aspartic acid, and two forms of arginine and for intergenic spacer sequences. The primers were tested singly and in paired combinations in a series of PCRs in order to determine their potential to discriminate between 22 R. salmoninarum isolates from sources in England, Wales, and Scotland (Table 2). The results of this initial screening showed that most of the isolates possessed identical banding patterns (Fig. 3). For example, primers A35K+754 and T25D−120 span the intergenic region between the tRNAThr and tRNAArg (ACG) genes and amplified two bands of 210 and 650 bp that were present in all of the 22 isolates examined (Fig. 3A). Similarly, PCRs using primers T17C+80 and T25E−128 amplified a single band of 780 bp that was present in each isolate (Fig. 3B).
FIG. 3.
The tDNA PCR profiles of 22 isolates of R. salmoninarum generated when using specific primers A35K+754 and T25D−120 (A), T17C+80 and T25E−128 (B), A35K+754 and T17C−135 (C), T3C+42 and T25E−128 (D), and T25E−128 (E). Lanes 1 to 22 correspond to the isolate numbers in Table 2. Lanes 23, negative control; lanes M, 100-bp DNA ladder (Gibco BRL).
In contrast, some primers did reveal differences. Primers A35K+754 and T17C−135 amplify the spacer region between the tRNAThr and tRNAArg (TCT) genes. In these reactions all isolates showed two bands of 110 and 680 bp but isolate 970153-19 also possessed an additional band of 1.2 kb (Fig. 3C). Furthermore, the profiles produced using primers T3C+42 and T25E−128 showed two major bands of 200 and 870 bp in all of the isolates, while an additional 300-bp fragment was found to be present in 970419-1.2.3, 970153-19, A6, 980297#97, F3, F47, F60, F82, and F95 (Fig. 3D). Using primer T25E-128 alone produced a banding pattern of up to six amplicons that separated the isolates into nine groups (Fig. 3E). The groupings of the isolates in relation to the observed band sizes are shown in Table 4. Some isolates from the same source were placed into separate groups, for example, isolates A6 and A80 from farm D were placed into groups 2 and 4, respectively, and isolates from farm E were placed into groups 2, 5, 8, and 9. Group 1 contained five isolates from farms A, B, and C in England and Wales and four isolates from the River Dee, Scotland. Three of these isolates were obtained in 1998 from two farms, one in England and one in Wales, and are known to have a common hatchery source.
TABLE 4.
Groupings of 22 isolates of R. salmoninarum from United Kingdom sources based on band sizes generated using primer T25E−128
| Group | Bands sizes (bp) | Isolatesa |
|---|---|---|
| 1 | 690, 1,250, 1,600 | 970083-88, 970083-102, 980106#1.1.5, 980036-150, 980036-87, NCIMB1111, NCIMB1112, NCIMB1115, NCIMB1116 |
| 2 | 320, 690, 1,250, 1,600 | 970419-1.2.3, A6, F47 |
| 3 | 320, 480, 690, 1,250, 1,600 | 970153-19 |
| 4 | 550, 690, 1,250, 1,600 | A80 |
| 5 | 690, 790, 850, 1,250, 1,600 | 980297#97, F3, F60, F82 |
| 6 | 690, 780, 1,250, 1,600 | NCIMB1113 |
| 7 | 690, 850, 1,250, 1,600 | NCIMB1114 |
| 8 | 350, 690, 1,250, 1,600 | F95 |
| 9 | 480, 690, 790, 850, 1,250, 1,600 | F85 |
Isolates 980106#1.1.5, 980036-150, and 980036-87 were derived from fish sampled from ponds and raceways that contained fish from the same hatchery.
DISCUSSION
In this study tDNA PCR profiling was used to separate 22 isolates of R. salmoninarum from various sources in the United Kingdom into nine groups on the basis of the sizes of the amplicons which were generated using primers specific to tRNA genes and intergenic spacer regions. Many of these isolates were cultured from samples which had been gathered from the same farm and at the same time in England and Wales and from wild fish sampled in 1962 from the River Dee. Using other methods of analysis we have shown that 20 of the isolates possessed identical ITS1 and ITS2 nucleotide sequences, SV1 and SV21, respectively, and two copies of a 51-bp repeat unit at the ETR-A locus. We have previously shown that two of the isolates used here, NCIMB1114 and NCIMB1116, from the River Dee possess a different ITS1 nucleotide sequence, SV4, and have a single copy of a 51-bp sequence at ETR-A (21). In addition, many of the isolates have been shown to be identical using IS994 restriction fragment length polymorphism (RFLP) and RAPD analysis (21, 34).
The ITS1 and ITS2 sequence analyses support the findings of our previous work showing that all R. salmoninarum isolates from England and Wales which have been examined in this way are SV1 (ITS1) and SV21 (ITS2). These are the most commonly occurring sequence variants among R. salmoninarum isolates, particularly in regions of the world with a history of intensive salmonid aquaculture. In the United Kingdom, only isolates which have been gathered from Scotland show any variation in the ITS1 region, and these date back to 1962, before the substantial development of salmonid culture in that region (21). We have previously found that SV1 isolates are distinguished by the possession of two copies of a 51-bp tandem repeat at ETR-A, an exact tandem repeat locus on the R. salmoninarum genome (21). The present work supports this finding.
Consensus tRNA primers were used in each of the possible paired combinations to generate tDNA PCR profiles of 60 isolates of R. salmoninarum. Welsh and McClelland speculated that because of the highly conserved nature of tRNA genes and their arrangement on the genome tDNA-ILPs are often characteristic of a species or even a genus, and this has been apparent in a number of other studies (42, 43). However, our work has shown that isolates 970153-19, Marion Forks, Cow CHs94 P22, and AcF6-1 produced different tDNA-ILPs when consensus primers T3A-T5B, T3A-T5A, T3A-T3B and T5A-T5B were used. We previously showed that isolates 970153-19, Marion Forks, and AcF6-1 differed in their RAPD profiles (21). Furthermore, isolate AcF6-1 has unique ITS1 and ITS2 sequences, SV3 and SV22, respectively, a single copy of the 51-bp repeat unit at the ETR-A locus, and a unique IS994 RFLP pattern (20, 21, 22, 34). Interestingly, while RAPDistance analysis grouped Cow Chs94 P22 together with another 28 isolates of R. salmoninarum from different geographical origins (21), the IS994 RFLP profile of this isolate was unique (34).
We have shown that several sets of consensus primers were capable of amplifying more than one major PCR product for each isolate. This probably occurred because the products are derived from multiple tRNA genes from the same tRNA gene cluster or from different clusters (25). Sequence analysis of a selection of PCR products in our study provided evidence that this was indeed the case. It is also possible that some of the amplicons that were generated using consensus primers were not from tRNA genes but rather represented fragments produced by the arbitrary amplification of unrelated loci. However, the lack of tRNA or tRNA-like genes in the sequenced amplicons occurred in only two cases.
Primers with perfect homology to specific tRNA genes were designed in order to discriminate, and assist in determining the epizootiological relationships, among a selection of United Kingdom isolates of R. salmoninarum. Twenty-two isolates were examined; of these 7 isolates had been cultured from a single outbreak of BKD at a fish farm in England, while a further 7 isolates were derived from single outbreaks on four separate farms. The remaining eight isolates were cultured from wild fish including six isolates from the River Dee in 1962. Most of the PCRs using specific primers showed that there were no differences between these isolates. However, the use of primers A35K+754 and T17C−135 to amplify the spacer region between the tRNAThr and tRNAArg genes showed that most isolates produced two bands of 110 and 630 bp but that 970153-19 possessed an additional band. This suggests there is some fundamental difference either in the sequence or the order of tRNA genes and the associated intergenic spacer regions in this isolate. Generating two or more products from primers that correspond to specific tRNA genes is possible because bacterial genomes can contain multiple copies of tRNA genes and because the organization of the genes within a cluster is often the same (23, 25, 35). The tDNA PCR profiles produced using primers T3C+42 and T25E−128 divided the 22 isolates into two groups. Interestingly, one of these groups contained six clinical isolates from fish held on the same farm but three other isolates with which they were grouped were from different host species and from different areas of the United Kingdom. These three isolates have previously been grouped together by RAPDistance analysis, suggesting that they may be derived from the same clone (21). When T25E-128 was used as a single primer, banding patterns of up to six amplicons, which enabled the differentiation of the 22 isolates into nine groups, were produced. Of particular interest was the finding that some of the isolates that had been cultured from fish held on the same farm and sampled at the same time possessed different tDNA PCR fingerprints. We can only speculate as to whether the groups emerged on the farm over the course of time from an initial isolate, in which case the past use of antibiotic and chemical treatments may have played some role in the selection of genetic variants. Alternatively, there may have been successive introductions from external sources which are related to differences in the hatchery supply of eggs, the cohorts of fish held on the farm, and the identity of the brood stock. Interestingly, 980106#1.1.5, 980036-87, and 980036-150 were grouped together and, although they were isolated from fish held on two farms, one in Wales and one in England, they had a common hatchery source. While it is possible to draw links between some isolates, it is unfortunate that the full details of the precise origins of all of the fish stocks from which R. salmoninarum was isolated are unavailable.
In some cases, the groups based on tDNA-ILPs corresponded with previous divisions that had been created using either IS994 or RAPDistance analyses (21, 34). However, some United Kingdom isolates that were grouped together by either IS994 RFLP or RAPDistance analysis were found to be different using tDNA-ILP profiling and vice versa. For example, it was possible to differentiate between isolates NCIMB1114 and NCIMB1116 by using tDNA-ILPs even though the isolates were identical using RAPDistance analysis (21). On the other hand, isolates 970419-1.2.3 and A6 were found to be different using RAPDistance software but were identical using tDNA PCR. The methods measure molecular variation in different ways; RAPD and IS994 analyses measure variation throughout the genome, while tDNA-ILP analyses are restricted to variation within the tRNA intergenic spacer regions, and so this outcome is not surprising. Our previous studies have shown that there is molecular variation in the R. salmoninarum genome that cannot be attributed to variation within the rRNA operon (20). Recent work by Rhodes et al. (34) has shown that the pattern of insertion of putative IS3 family insertion element IS994 throughout the genome helps to explain some of this variation. We propose that polymorphisms in both the tRNA genes and the tRNA intergenic spacer regions may also help to explain some of this variation. tRNA genes have consistently been reported to be sites for the integration of pathogenicity islands, bacteriophage, repeat elements, transposons, and plasmid DNA. Due to their highly conserved and repeated nature tRNA genes are prone to the insertion of mobile genetic elements and indeed may play a key role in the evolution of microbial pathogens. The variations that exist between and within tRNA genes on the R. salmoninarum genome may also be a reflection of this process.
One of the major shortcomings of the objective analysis of RAPD profiles is that the binary matrix that is used to input data into RAPDistance software necessitates that bands be scored as either present or absent. Consequently, there is no account taken of variation in the brightness of bands. For example, for primer P6, compare the brightness of the 1.25-kb band in isolate F3 with that of the faint bands in the same position in some of the other isolates (Fig. 1F). RAPDistance analysis necessitates that either the bands be determined to be the same, that is, present no matter how faint or bright, or else the band at that position is not used in the analysis. In addition, it is often necessary to reject the use of some bands because of ambiguities between the isolates, for example, bands that are amplified in RAPD reactions to which a present or absent score cannot be definitively made for every isolate, either because of their proximity to other bands, e.g., multiple bands, or because of background or smeared amplification. In such cases there is a loss of discriminatory power, and these are the main reasons why 28 isolates of R. salmoninarum were grouped into a single cluster in our previous work using RAPDistance analysis (21).
When comparing tDNA PCR with other methods for determining the relationships between isolates of R. salmoninarum it would be advisable to use a multifactorial approach. Each of the techniques developed so far for this purpose seems to complement the information that can be gained from the use of just a single technique. Therefore, it would be better to use a number of techniques in order to attach more confidence to the outcome. In conclusion, tDNA PCR profiling provides another method for discriminating between clinical isolates of R. salmoninarum which show identical rRNA ITS sequences, ETR-A loci, and IS994 patterns. The tDNA PCR profiling was in good agreement with previous divisions made using RAPD profiles and in some cases improved on the discriminatory power of RAPD. When used in combination with other molecular typing methods, tDNA-ILPs will be a useful tool for epizootiological studies of BKD outbreaks in populations of both wild and farmed fish.
ACKNOWLEDGMENTS
This work was funded by the Ministry for Agriculture, Fisheries and Food UK, project codes FC1103 and OC9613.
We are indebted to the individuals who provided bacterial isolates and to Michele Stone, CEFAS, Weymouth, UK, for help with farm infection documentation.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipmann D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atienzar F, Child P, Evenden A, Jha A, Savva D, Walker C, Depledge M. Application of the arbitrarily primed polymerase chain reaction for the detection of DNA damage. Mar Environ Res. 1998;46:331–335. [Google Scholar]
- 3.Austin B, Embley T M, Goodfellow M. Selective isolation of Renibacterium salmoninarum. FEMS Microbiol Lett. 1983;17:111–114. [Google Scholar]
- 4.Banner C R, Rohovec J S, Fryer J L. A new value for mol percent guanine + cytosine of DNA for the salmonid fish pathogen Renibacterium salmoninarum. FEMS Microbiol Lett. 1991;63:57–59. doi: 10.1016/0378-1097(91)90527-h. [DOI] [PubMed] [Google Scholar]
- 5.Bruno D W, Munro A L S. Uniformity in the biochemical properties of Renibacterium salmoninarum isolates obtained from several sources. FEMS Microbiol Lett. 1986;33:247–250. [Google Scholar]
- 6.Campbell A M. Chromosomal insertion sites from phages and plasmids. J Bacteriol. 1992;174:7495–7499. doi: 10.1128/jb.174.23.7495-7499.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claros M C, Haase G, Schonian G, Citron D M, Gerardo S H, Goldstein E J C, Schumacher U, Rodloff A C. Differentiation of clinical Bacteroides ovatus isolates using RNA gene size polymorphisms. Rev Med Microbiol. 1997;8(Suppl. 1):S103–S104. [Google Scholar]
- 8.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Conner R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail M A, Rajandream M A, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston J E, Taylor K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature (London) 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 9.Daffonchio D, Borin S, Frova G, Manachini L, Sorlini C. PCR fingerprinting of whole genomes: the spacers between the 16S and 23S rRNA genes and of intergenic tRNA gene regions reveal a different intraspecific genomic variability of Bacillus cereus and Bacillus licheniformis. Int J Syst Bacteriol. 1998;48:107–116. doi: 10.1099/00207713-48-1-107. [DOI] [PubMed] [Google Scholar]
- 10.Dolzani L, Tonin E, Lagatolla C, Monti-Bragadin C. Typing of Staphylococcus aureus by amplification of the 16S–23S rRNA intergenic spacer sequences. FEMS Microbiol Lett. 1994;119:167–174. doi: 10.1111/j.1574-6968.1994.tb06884.x. [DOI] [PubMed] [Google Scholar]
- 11.Dundon W G, Marshall D G, Morain C A, Smyth C J. A novel tRNA-associated locus (trl) from Helicobacter pylori is co-transcribed with tRNAgly and reveals genetic diversity. Microbiology. 1999;145:1289–1298. doi: 10.1099/13500872-145-6-1289. [DOI] [PubMed] [Google Scholar]
- 12.Ehrenstein B, Bernards A T, Dijkshoorn L, Gerner-Smidt P, Towner K J, Bouvet P J M, Daschner F D, Grundmann H. Actinetobacter species identification by using tRNA spacer fingerprinting. J Clin Microbiol. 1996;34:2414–2420. doi: 10.1128/jcm.34.10.2414-2420.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engels W R. Contributing software to the Internet: the Amplify program. Trends Biochem Sci. 1993;18:448–450. doi: 10.1016/0968-0004(93)90148-g. [DOI] [PubMed] [Google Scholar]
- 14.Evelyn T P T, Prosperi-Porta L, Ketcheson J E. Two new techniques for obtaining consistent results when growing Renibacterium salmoninarum on KDM2 culture medium. Dis Aquat Org. 1990;9:209–212. [Google Scholar]
- 15.Evenden A J, Grayson T H, Gilpin M L, Munn C B. Renibacterium salmoninarum and bacterial kidney disease—the unfinished jigsaw. Annu Rev Fish Dis. 1993;3:87–104. [Google Scholar]
- 16.Fichant G A, Burks C. Identifying potential tRNA genes in genomic DNA sequences. J Mol Biol. 1991;220:659–671. doi: 10.1016/0022-2836(91)90108-i. [DOI] [PubMed] [Google Scholar]
- 17.Folkesson A, Advani A, Sukupolvi S, Pfeifer J, Normark S, Lofdahl S. Multiple insertions of Salmonella serovars responsible for human disease. Mol Microbiol. 1999;33:612–622. doi: 10.1046/j.1365-2958.1999.01508.x. [DOI] [PubMed] [Google Scholar]
- 18.Forsman P, Tilsala-Timisjarvi A, Alatossava T. Identification of staphylococcal and streptococcal causes of bovine mastitis using 16S–23S rRNA spacer regions. Microbiology. 1997;143:3491–3500. doi: 10.1099/00221287-143-11-3491. [DOI] [PubMed] [Google Scholar]
- 19.Goodfellow M, Embley T M, Austin B. Numerical taxonomy and emended description of Renibacterium salmoninarum. J Gen Microbiol. 1985;131:2739–2752. [Google Scholar]
- 20.Grayson T H, Alexander S M, Cooper L F, Gilpin M L. Renibacterium salmoninarum isolates from different sources possess two highly conserved copies of the rRNA operon. Antonie Leeuwenhoek. 2000;78:51–61. doi: 10.1023/a:1002745129625. [DOI] [PubMed] [Google Scholar]
- 21.Grayson T H, Atienzar F A, Alexander S M, Cooper L F, Gilpin M L. Molecular diversity of Renibacterium salmoninarum isolates determined by randomly amplified polymorphic DNA. Appl Environ Microbiol. 2000;66:435–438. doi: 10.1128/aem.66.1.435-438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grayson T H, Cooper L F, Atienzar F A, Knowles M R, Gilpin M L. Molecular differentiation of Renibacterium salmoninarum isolates from world-wide locations. Appl Environ Microbiol. 1999;65:961–968. doi: 10.1128/aem.65.3.961-968.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green C J, Vold B. Staphylococcus aureus has clustered tRNA genes. J Bacteriol. 1993;175:5091–5096. doi: 10.1128/jb.175.16.5091-5096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutenberger S K, Giovannoni S J, Field K G, Fryer J L, Rohovec J S. A phylogenetic comparison of the 16S rRNA sequence of the fish pathogen, Renibacterium salmoninarum, to gram-positive bacteria. FEMS Microbiol Lett. 1991;77:151–156. doi: 10.1016/0378-1097(91)90543-j. [DOI] [PubMed] [Google Scholar]
- 25.Honeycutt R J, Sobral B W, McClelland M. tRNA intergenic spacers reveal polymorphisms diagnostic for Xanthomonas albilineas. Microbiology. 1995;141:3229–3239. doi: 10.1099/13500872-141-12-3229. [DOI] [PubMed] [Google Scholar]
- 26.Kersulyte D, Woods J P, Keath E J, Goldmann W E, Berg D E. Diversity among clinical isolates of Histoplasma capsulatum detected by polymerase chain reaction with arbitrary primers. J Bacteriol. 1992;174:7075–7095. doi: 10.1128/jb.174.22.7075-7079.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koch C F, Rainey F A, Stackebrandt E. 16S rDNA studies on members of Arthrobacter and Micrococcus: and aid for their future taxonomic restructuring. FEMS Microbiol Lett. 1994;123:167–172. [Google Scholar]
- 28.Kunze Z M, Wall S, Appelberg R, Silva M T, Portaels F, McFadden J J. IS901, a new member of a widespread class of atypical insertion sequences, is associated with pathogenicity in Mycobacterium avium. Mol Microbiol. 1991;5:2265–2272. doi: 10.1111/j.1365-2958.1991.tb02157.x. [DOI] [PubMed] [Google Scholar]
- 29.Lowe T M, Eddy S R. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maes N, Degheldre Y. Rapid and accurate identification of Staphylococcus species by tRNA intergenic spacer length polymorphism analysis. J Clin Microbiol. 1997;35:2477–2481. doi: 10.1128/jcm.35.10.2477-2481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McClelland M, Petersen C, Welsh J. Length polymorphisms in tRNA intergenic spacers detected by using the polymerase chain reaction can distinguish streptococcal strains and species. J Clin Microbiol. 1992;30:1499–1504. doi: 10.1128/jcm.30.6.1499-1504.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moss F E, Cardozo T J, Zychlinsky A, Groisman E A. The selC-associated SHI-2 pathogenicity isolate of Shigella flexneri. Mol Microbiol. 1999;33:74–83. doi: 10.1046/j.1365-2958.1999.01449.x. [DOI] [PubMed] [Google Scholar]
- 33.Norton R, Roberts B, Freeman M, Wilson M, Ashurst-Smith C, Lock W, Brooks D, La Brooy J. Characterisation and molecular typing of Burkholderia pseudomallei: are disease presentations of melioidosis clonally related? FEMS Immunol Med Microbiol. 1998;20:37–44. doi: 10.1111/j.1574-695X.1998.tb01109.x. [DOI] [PubMed] [Google Scholar]
- 34.Rhodes L D, Grayson T H, Alexander S M, Strom M S. Description and characterisation of IS994, a putative IS3 family insertion sequence from the salmon pathogen, Renibacterium salmoninarum. Gene. 2000;244:97–107. doi: 10.1016/s0378-1119(99)00573-9. [DOI] [PubMed] [Google Scholar]
- 35.Seal S E, Jackson L A, Daniels M J. Use of tRNA consensus primers to indicate subgroups of Pseudomonas solanacearum by polymerase chain reaction amplification. Appl Environ Microbiol. 1992;58:3759–3761. doi: 10.1128/aem.58.11.3759-3761.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stackebrandt E, Wehmeyer U, Nader H, Fiedler F. Phylogenetic relationship of the fish pathogen Renibacterium salmoninarum to Arthrobacter, Micrococcus and related taxa. FEMS Microbiol Lett. 1988;50:117–120. [Google Scholar]
- 37.Starliper C E. Genetic diversity of North American isolates of Renibacterium salmoninarum. Dis Aquat Org. 1996;27:207–213. [Google Scholar]
- 38.Tatusova T A, Madden T L. BLAST 2 sequences, a new tool for comparing protein and nucleotide sequences. FEMS Microbiol Lett. 1999;174:247–250. doi: 10.1111/j.1574-6968.1999.tb13575.x. [DOI] [PubMed] [Google Scholar]
- 39.van der Zee A, Mooi F, van Emben J, Musser J. Molecular evolution and host adaptation of Bordetella sp.: phylogenetic analysis using multilocus enzyme electrophoresis and typing with three insertion sequences. J Bacteriol. 1997;179:6609–6617. doi: 10.1128/jb.179.21.6609-6617.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vold B. Structure and organization of genes for transfer ribonucleic acid in Bacillus subtilis. Microbiol Rev. 1985;49:71–80. doi: 10.1128/mr.49.1.71-80.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang G, van Dam A P, Spanjaard L, Dankert J. Molecular typing of Borrelia burgdorferi sensu lato by randomly amplified polymorphic DNA fingerprinting analysis. J Clin Microbiol. 1998;36:768–776. doi: 10.1128/jcm.36.3.768-776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welsh J, McClelland M. Genomic fingerprints produced by PCR with consensus tRNA gene primers. Nucleic Acids Res. 1991;19:861–866. doi: 10.1093/nar/19.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welsh J, McClelland M. PCR-amplified length polymorphisms in tRNA intergenic spacers for categorizing Staphylococci. Mol Microbiol. 1992;6:1673–1680. doi: 10.1111/j.1365-2958.1992.tb00892.x. [DOI] [PubMed] [Google Scholar]