Graphical abstract
Keywords: Thermochemical conversion, Chemical recycling, Characterization, Steam cracking, Contaminants, Upgrading
Abbreviations: AAS, Atomic absorption spectroscopy; ABS, Acrylonitrile butadiene styrene; AED, Atomic emission detector; ASTM, American Society for Testing and Materials; ASR, Automotive shredder residue; ATR, Attenuated total reflection; CHNS/O, Carbon, hydrogen, nitrogen, sulfur / oxygen elemental analyzer; CIC, Combustion Ion Chromatography; CSTR, Continuous stirred tank reactor; ECD, Electron capture detector; EDXRF, Energy dispersive X-ray fluorescent spectroscopy; EPA, Environmental protection agency (US); EU, European Union; FBP, Final boiling point; FCC, Fluid catalytic cracking; FIA, Fluorescent indicator adsorption; FID, Flame ionization detector; FTIR, Fourier-transformed infrared; GC, Gas chromatography; GC × GC, Two-dimensional gas chromatography; HDPE, High-density polyethylene; HIPS, High impact polystyrene; HPLC, High performance liquid chromatography; IBP, Initial boiling point; ICP, Inductively coupled plasma; Incl., Including; JIS, Japanese industrial standards; LC, Liquid chromatography; (L)LDPE, (linear) low-density polyethylene; LOD, Limit of detection; LOQ, Limit of quantification; MAPD, Methyl acetylene and propadiene; MPO, Mixed polyolefins; MS, Mass spectrometry; NCD, Nitrogen chemiluminescence detector; ND, Not detected; NMR, Nuclear Magnetic Resonance; OES, Optical emission spectrometry; PA, Polyamide; PAH, Polyaromatic hydrocarbons; PET, Polyethylene terephthalate; PFO, Pyrolysis Fuel Oil; PIONA, Paraffins, (iso-) paraffins, olefins, naphthenes, aromatics; PMMA, Polymethylmethacrylate; PP, Polypropylene; ppb, Parts per billion; ppm, Parts per million; PS, Polystyrene; PTFE, Polytetrafluoroethylene; PUR, Polyurethane; PVC, Polyvinylchloride; PVDC, Polyvinylidene chloride; SCD, Sulfur chemiluminescence detector; STR, Stirred tank reactor; TCD, Thermal conductivity detector; TGA, Thermogravimetric analysis; ToF, Time-of-flight; WEEE, Waste electrical and electronic equipment
Highlights
-
•
Contaminants determine the chemical recycling potential of pyrolysis oils.
-
•
Pyrolysis oils contain more and different contaminants than fossil feedstocks.
-
•
Contaminants cause corrosion, process fouling and downstream catalyst poisoning.
-
•
The main contaminants are nitrogen, oxygen, chlorine, iron, lead and calcium.
-
•
Advanced analytical techniques and standardization are crucial.
Abstract
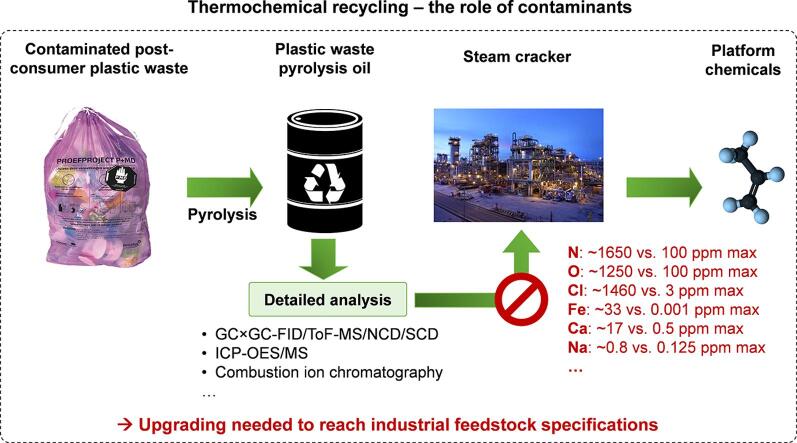
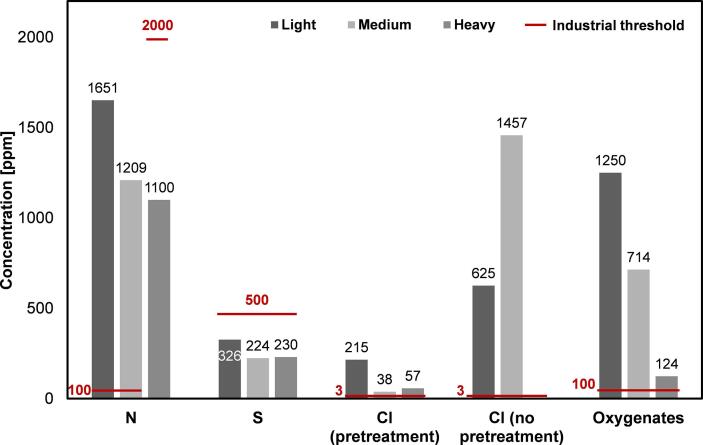
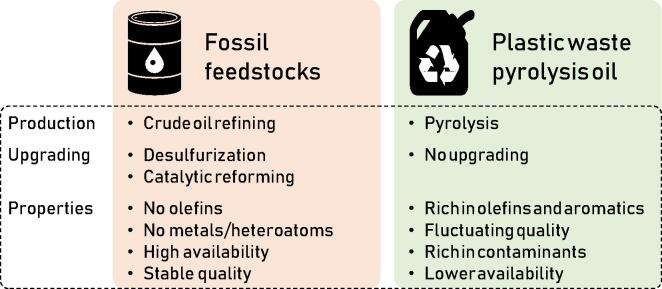
Thermochemical recycling of plastic waste to base chemicals via pyrolysis followed by a minimal amount of upgrading and steam cracking is expected to be the dominant chemical recycling technology in the coming decade. However, there are substantial safety and operational risks when using plastic waste pyrolysis oils instead of conventional fossil-based feedstocks. This is due to the fact that plastic waste pyrolysis oils contain a vast amount of contaminants which are the main drivers for corrosion, fouling and downstream catalyst poisoning in industrial steam cracking plants. Contaminants are therefore crucial to evaluate the steam cracking feasibility of these alternative feedstocks.
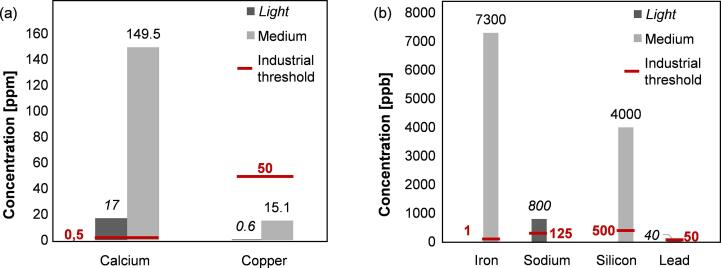
Indeed, current plastic waste pyrolysis oils exceed typical feedstock specifications for numerous known contaminants, e.g. nitrogen (∼1650 vs. 100 ppm max.), oxygen (∼1250 vs. 100 ppm max.), chlorine (∼1460 vs. 3 ppm max.), iron (∼33 vs. 0.001 ppm max.), sodium (∼0.8 vs. 0.125 ppm max.) and calcium (∼17 vs. 0.5 ppm max.). Pyrolysis oils produced from post-consumer plastic waste can only meet the current specifications set for industrial steam cracker feedstocks if they are upgraded, with hydrogen based technologies being the most effective, in combination with an effective pre-treatment of the plastic waste such as dehalogenation.
Moreover, steam crackers are reliant on a stable and predictable feedstock quality and quantity representing a challenge with plastic waste being largely influenced by consumer behavior, seasonal changes and local sorting efficiencies. Nevertheless, with standardization of sorting plants this is expected to become less problematic in the coming decade.
1. Introduction
During recent years, energy and carbon efficiency became increasingly important in the transition from a linear to a circular economy. In this scenario, recycling of plastics plays a key role due to their energy and material intensive production, their abundant use and their long lifetime as a pollutant (Meys et al., 2021).
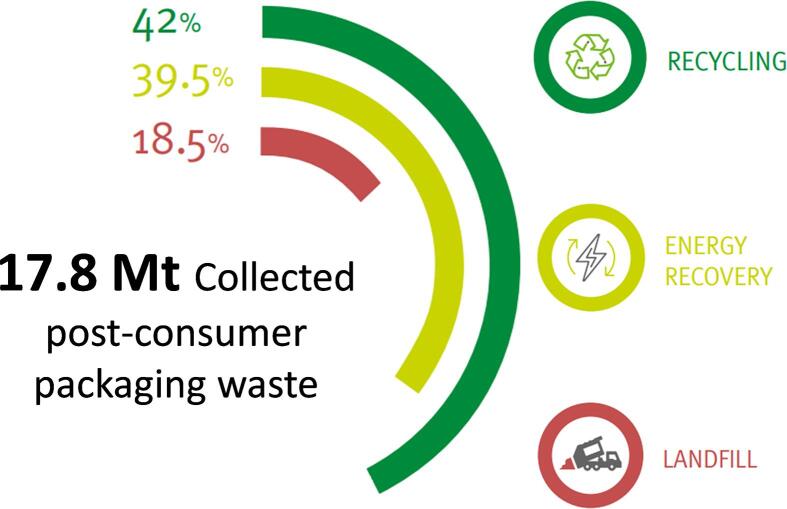
Plastics production in Europe (EU28 plus Norway and Switzerland) reached almost 62 million tons in 2018 with plastic packaging making up almost 40 % of the produced plastics. Of the total plastic packaging waste (∼17.8 million tons), approximately 42 % are collected for recycling purposes with another 39.5 % being used for energy recovery in waste incineration plants (see Fig. 1). The remaining 18.5 % of plastic packaging material is disposed of in landfills (Plastics Europe, 2020).
Fig. 1.
Post-consumer plastic packaging waste treatment distribution in Europe in 2018 (adapted from (Plastics Europe, 2020)).
Consequently, the demand increases for plastic waste recycling technologies capable of creating valuable products with similar properties as their original fossil-derived counterparts.
Post-consumer plastic waste is a mixture of several different polymers such as polyethylene (PE), polypropylene (PP), polystyrene (PS), polyvinylchloride (PVC) and polyethylene terephthalate (PET) with traces of polyamide (PA), polyurethane (PUR), poly(methyl methacrylate) (PMMA) and others which further contains numerous additives and auxiliary materials (Geyer et al., 2017, Ügdüler et al., 2020).
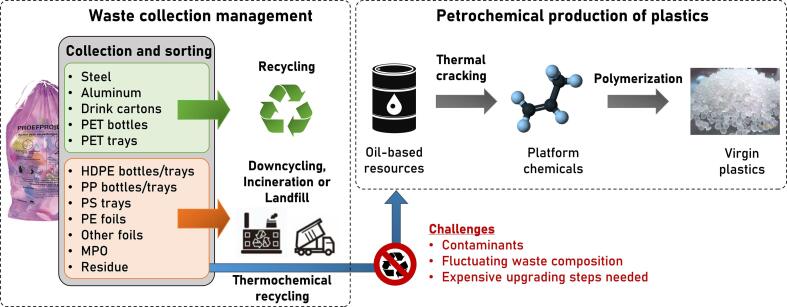
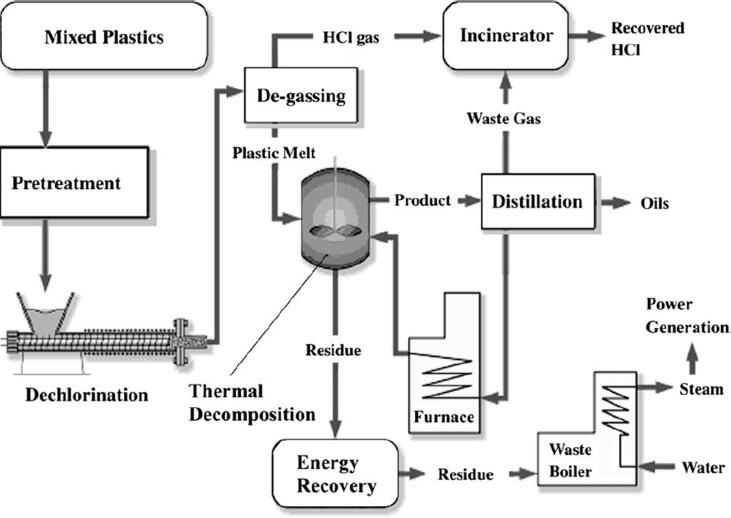
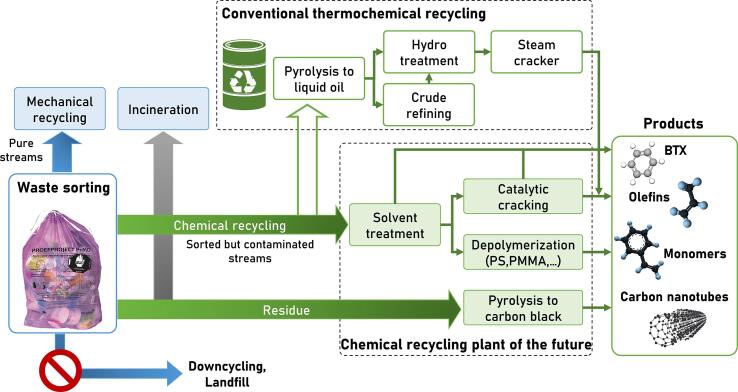
Only for a limited number of plastic waste streams efficient mechanical recycling schemes exist (e.g. for PET bottles and trays). The remaining sorted but contaminated waste is currently either downcycled, landfilled or incinerated. The primary route to recycle the major part of polyolefinic (i.e. PE and PP) plastic packaging waste in the coming decade will be chemical recycling (Solis and Silveira, 2020, Dogu et al., 2021). In particular, pyrolysis towards liquid feedstocks for producing olefins and aromatics in petrochemical process chains gains momentum with numerous demonstration projects in Europe (Fitzsimons, 2020, Bailey, 2020, Pilkington, 2020, Young, 2021). In this concept, the material loop can be closed since new virgin plastics are produced from the base chemicals ethylene, propylene, 1,3-butadiene and benzene (Ragaert et al., 2017). However, there are still challenges in place such as fluctuating waste compositions, feedstock contaminants and the need for expensive upgrading steps which lower the economic prospect. Fig. 2 depicts a scheme of the state-of-the-art plastics waste management and a potential integration of thermochemical recycling into the petrochemical production of plastics.
Fig. 2.
Schematic overview of the state-of-the-art domestic waste recycling schemes and a potential integration of thermochemical recycling.
In a pyrolysis reactor, solid plastics are thermally decomposed in an inert atmosphere with or without catalyst, providing a distribution of gaseous, liquid and solid products as function of temperature, pressure and residence time (Lopez et al., 2017, Al-Salem et al., 2017, Kannan et al., 2014). A distinction can be made between polymers such as PMMA, polytetrafluorethylene (PTFE) or PS, which decompose primarily into their corresponding monomers (Miandad et al., 2017, Garforth et al., 2004). On the other hand, PE and PP rich streams, which are based on volume the most widely available plastic packaging waste feedstocks, decompose in a random fragmentation reaction (Plastics Plastics Europe, 2020, Ragaert et al., 2017, Dogu et al., 2021).
The main pyrolysis product is a liquid containing a complex mixture of hydrocarbons with a wide carbon number distribution which may be utilized as petrochemical feedstock (Al-Salem et al., 2010). Contaminants present in the original plastic waste distribute over the entire product range and are known to diminish the liquid product quality making further treatment necessary to obtain a high-quality petrochemical feedstock such as fossil naphtha (Al-Salem et al., 2017). The total content of inorganics, or ash, in post-consumer mixed polyolefin (MPO) streams after sorting and washing (with cold water) can still be appreciable (2.8 wt%) (Eschenbacher et al., 2022).
Many different (petro-) chemical treatment options for plastic waste pyrolysis products have been researched in the past including the conversion to transportation fuels (Nanda and Berruti, 2021, Papari et al., 2021), or to feedstocks for fluid catalytic cracking (Rodríguez et al., 2019a, Lee, 2009, Rodríguez et al., 2019b), hydrocrackers (Joo and Guin, 1997, Li et al., 2016) or coker units (Palos et al., 2021). Obviously, the conversion of plastics to fuel results in a low value product which makes it economically very challenging to compete with, for example olefin production, not even considering the circularity of fuels. In Europe, the projections are that the utilization of refineries will decrease drastically by 2040 due to a more aggressive transition towards electrified transportation (Bousso and Sanicola, 2020). Given the enormous scale of operation for commercial steam crackers and the fact that economic penalties such as carbon taxes are being introduced, there is a huge potential for using pyrolysis oils as feedstocks for already existing steam cracker units. Furthermore, since steam cracking is the main process for the production of base chemicals (e.g. ethylene and propylene) of which the majority is converted to virgin plastics, it would provide the highest degree of circularity if plastic waste is converted to steam cracker feedstocks. However, one of the key reasons why this post-treatment pathway has not yet taken off industrially is the uncertainty that is associated with the contaminants and impurities found in plastic waste pyrolysis oils.
In steam cracking, a substantial amount of energy is used in order to decompose large hydrocarbon molecules into light olefins (Amghizar et al., 2017). Steam cracking of plastic waste pyrolysis oils requires a liquid feedstock that is compatible with industrial steam cracking units in terms of physical properties (e.g. viscosity, boiling point), molecular composition (e.g. paraffinic, iso-paraffinic, olefinic, naphthenic and aromatic content: PIONA) and potential contaminant limits (Sundaram and Stancato, 2018). Furthermore, sufficient amounts of high quality feedstock are needed to match the scale of operation of industrial steam crackers. Typically, one naphtha cracking furnace requires feedstock in the order of >100,000 tons per year (Oliveira and Van Dril, 2021). Therefore, it is likely that plastic waste pyrolysis products will be blended with fossil feedstocks rather than entirely replacing them (Amghizar et al., 2017).
Feedstock contaminants play a crucial role for the economics of a steam cracking furnace due to their accelerating effect on coke formation, fouling, corrosion and downstream catalyst poisoning leading to reduced run-length and/or off-spec products (Sundaram and Stancato, 2018). Contaminant levels found in plastic waste pyrolysis oils will therefore be the determining factor for a successful integration of a thermochemical recycling process into a petrochemical complex. The most important contaminants are heteroatomic compounds (nitrogen, sulfur, oxygen and halogens) as well as metals. High amounts of aromatics and olefins are also known to amplify coke formation and fouling. This has been known as far back as thirty years (Kopinke et al., 1993a, Kopinke et al., 1993b). In order to assess the steam cracking feasibility of plastic waste pyrolysis oils in terms of contaminants, molecular characterization techniques play a key role. Therefore, as a starting point of this review the most important analytical techniques for the analysis of plastic waste pyrolysis oils are discussed with special emphasis on the analysis of trace amounts of highly important contaminants such as nitrogen, sulfur, oxygen and halogens as well as metals.
In scientific literature, little systematic information is available on contaminants in pyrolysis oil and their potential consequences in the closed loop recycling chain towards steam cracking. In this review, contaminant levels in real post-consumer plastic waste derived feedstocks are listed and compared to the maximum allowable concentrations in industrial steam cracker feedstocks, giving an indication of how challenging it is to use plastic waste pyrolysis oils in a steam cracker and what potential risks are. Thus, a guideline is provided for steam cracker operators when attempting to implement plastic waste pyrolysis oils into their existing petrochemical infrastructure.
The presented study is a strong foundation for benchmarking plastic waste pyrolysis oils to conventional fossil feedstocks in order to assess the potential effects from plastic waste contaminants on chemical recycling and for potential upgrading technologies.
2. State-of-the-art molecular characterization techniques
Plastic waste pyrolysis oils as steam cracking feedstocks will be evaluated based on their chemical composition especially in light of potentially harmful compounds with the ability to put stable operation of industrial crackers at risk. Determining and quantifying of elements in trace amounts down to ppb ranges is not straight forward and requires sophisticated analytical techniques. A crucial aspect regarding plastic waste pyrolysis oils as steam cracking feedstocks is the setting of quality standards which are based on the most accurate detection techniques.
The most flexible and powerful analytical tool is gas chromatography (GC) coupled to various detectors. In essence, all GC techniques make use of the chromatographic separation of compounds prior to analysis on a detector targeted to a certain compound group. Thus, considering the entire range of compatible detectors, GC techniques are suitable to analyze almost the entire chemical composition of a plastic waste pyrolysis oil.
2.1. Capillary gas chromatography coupled to various detectors for the characterization of the hydrocarbon matrix and crucial contaminants
The most frequently used GC method is one-dimensional (1D) GC where the sample is separated in a column based on a specific mechanism (e.g. boiling point or polarity). However, the high complexity of plastic waste pyrolysis oils can often not be fully represented by 1D-GC due to the limited peak capacity leading to an insufficient chromatographic separation of the vast amount of compounds over the various compound families. Although the separation may be improved by increasing the length of the column or by choosing a more selective stationary phase, experience shows that 1D-GC is limited to a certain feedstock complexity (Djokic et al., 2018, Mondello et al., 2008). Also, the quantification of heteroatomic compounds containing nitrogen, sulfur or oxygen is difficult due to the complex dominant hydrocarbon matrix (Toraman et al., 2014).
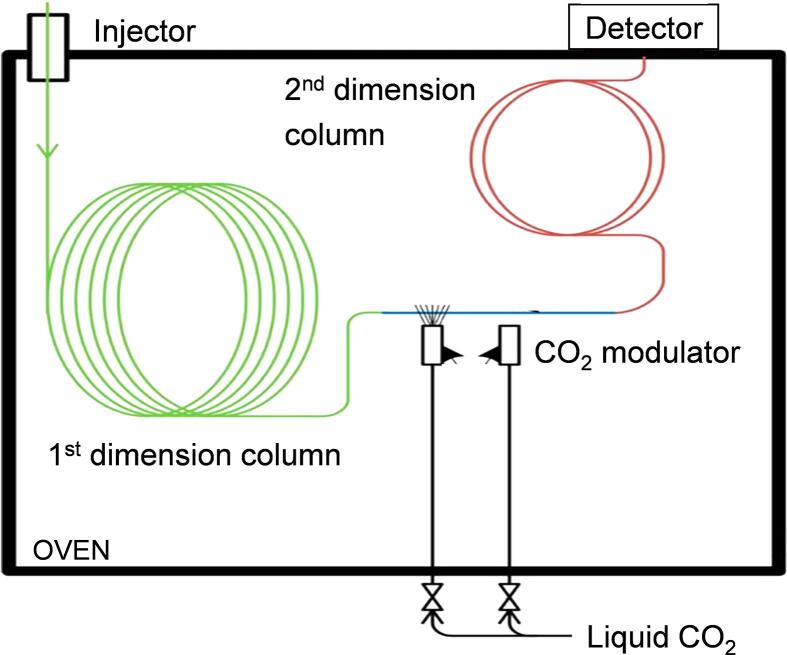
In order to obtain comprehensive data of highly complex component mixtures, hyphenated methods such as comprehensive two-dimensional gas chromatography (GC × GC) coupled to different detectors can be used due to the significantly higher resolution and signal-to-noise ratio (Djokic et al., 2018, Djokic et al., 2012, Dijkmans et al., 2014). Comprehensive GC × GC separates the entire effluent spectrum using two columns with two different, statistically independent separation mechanisms. In this way, no information gained during the first column separation is lost in the cause of the second column separation (Dallüge et al., 2003). The first column separation is typically based on a non-polar stationary phase whereas the shorter and narrower second column separates the effluent compounds based on polarity. Both columns may be placed in a single heated oven (see Fig. 3). For the comprehensive analysis of plastic waste pyrolysis oils which often contain high amounts of large molecular weight hydrocarbons, the temperature resistance of the used columns is highly important. High temperature chromatographic columns are available which are produced from fused silica, aluminum or stainless steel that can withstand temperatures up to 440 °C (Philp et al., 1995). This way it is possible to separate the entire carbon number range of the feedstock.
Fig. 3.
Schematic overview of a GC × GC setup. Re-drawn from (Dijkmans et al., 2014).
Between both columns, there is a modulator which can be a valve modulator (Sinha et al., 2003), thermal modulator (Libardoni et al., 2010, Phillips and Xu, 1995, Beens et al., 1998a, Phillips et al., 1999, Beens et al., 1998b, Gaines et al., 1999) or cryogenic modulator (Phillips and Beens, 1999, Kinghorn et al., 1998, Pursch et al., 2003) functioning as an interface. The main purpose of the modulator is to trap adjacent fractions of the effluent, to refocus and rapidly send them to the second dimension column (Adahchour et al., 2008, Beens et al., 2001, Adahchour et al., 2006). The resulting GC × GC chromatograms are structured along two axes based on the respective separation mechanisms (boiling point, polarity), leading to an improved resolution compared to 1D-GC (Pyl et al., 2011, Dallüge et al., 2003, Mondello et al., 2004, Mondello et al., 2008, Van Geem et al., 2010).
GC × GC is especially interesting for samples consisting of both well-known compound families such as paraffins, olefins, naphthenes or aromatics and a high number of isomers and homologues (Dallüge et al., 2003). In contrast to 1D-GC, GC × GC is not an ASTM standard technique. Comprehensive GC × GC was introduced 30 years ago and since then became the major analytical tool used as a platform for a broad range of detectors (see Table 1). For the latest state-of-the-art of GC × GC the reader is referred to a recently published review article by (Zanella et al., 2021). An overview of detectors compatible with GC or GC × GC is given in Table 1. For a detailed review of ionization-based detectors for gas chromatography, the reader is referred to (Poole, 2015).
Table 1.
Overview of analytical detectors compatible with GC or GC × GC.
| Detector | Properties | Reference |
|---|---|---|
| FID |
|
(Djokic et al., 2012, Marsman et al., 2007, De Saint Laumer et al., 2010, Tranchida and Mondello, 2019, Biedermann and Grob, 2009, Luong et al., 2019) |
| ToF-MS |
|
(Van Geem et al., 2010, Dijkmans et al., 2015, Toraman et al., 2014, Djokic et al., 2018, Tranchida and Mondello, 2019) |
| TCD |
|
(Budiman and Nuryatini & Zuas, 2015, Miskolczi, 2013, Park et al., 2020) |
| SCD/NCD |
|
(Yan, 1999, Yan, 2002, Yan, 2006, Tranchida and Mondello, 2019) |
| ECD |
|
(Eguchi et al., 2021, Hall and Williams, 2006, Bhaskar et al., 2007, Pellizzari, 1974, Muscalu et al., 2011, Booij et al., 1998) |
| AED |
|
(Ross et al., 2001, Tranchida and Mondello, 2019, Bartle et al., 2009, Van Stee et al., 2002, Van Stee et al., 2003, Lorentz et al., 2017) |
| ICP-OES/MS |
|
(Foppiano et al., 2020, Linge, 2008, Lienemann et al., 2007, Rahmi et al., 2007, Haraguchi, 2004, Navarro et al., 2002, Clases et al., 2021) |
Numerous studies exist reporting the analysis of plastic waste pyrolysis oils using 1D-GC coupled to a thermal conductivity detector (TCD) (Miskolczi, 2013, Park et al., 2020), flame ionization detector (FID) (Demirbas, 2004, Park et al., 2003, Park et al., 2020) or mass spectrometer (MS) (Anuar Sharuddin et al., 2017, Lee, 2007, Seo et al., 2003, Lee et al., 2003, Miranda et al., 2001a, Miskolczi and Ateş, 2016, Park et al., 2003, Park et al., 2020, Tsuge et al., 2011). Obviously, for the analysis of plastic waste pyrolysis oils, the more sophisticated GC × GC is still not frequently used.
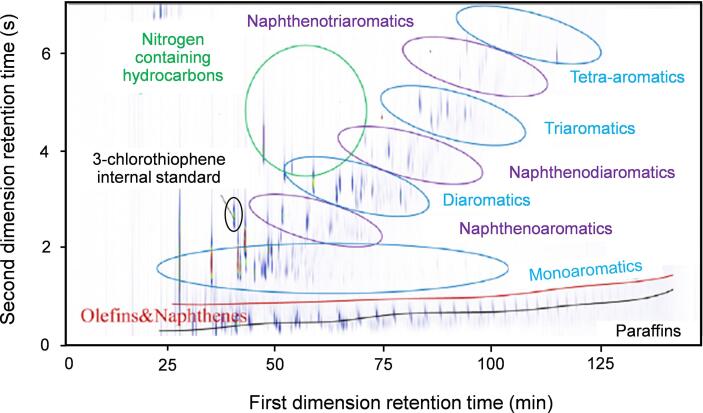
The principle of a TCD is based on the difference in thermal conductivity of the analyte and the carrier gas. Regarding their applicability, TCD can be compared to FID, however, with a lower sensitivity and linearity range making FID more suitable for highly complex mixtures such as plastic waste pyrolysis oils (Budiman et al., 2015). 1D-GC-TCD complies with the ASTM D7833 – 20 standard. For conventional hydrocarbon feedstocks, FID is the most widely applied detector combined with GC (× GC) due to its universal applicability for organic compounds (Tranchida and Mondello, 2019). 1D-GC-FID complies with the ASTM D8028 – 17 standard. The basic principle of the FID is the measurement of variations in the ionization current in a hydrogen–oxygen flame caused by the presence of analytes. The FID response of a specific compound is proportional to the sum of the carbon atoms capable of hydrogenation (Sevcik, 2011, Schofield, 2008). FID has a wide detector linearity range and high flexibility regarding response factors which can be predicted or calculated (Djokic et al., 2012, Marsman et al., 2007, De Saint Laumer et al., 2010, Scanlon and Willis, 1985). Fig. 4 depicts a GC × GC-FID color plot of plastic waste pyrolysis oil showing the benefit of the second dimension separation.
Fig. 4.
GC × GC-FID chromatogram of a plastic waste pyrolysis oil. Re-drawn from (Toraman et al., 2014).
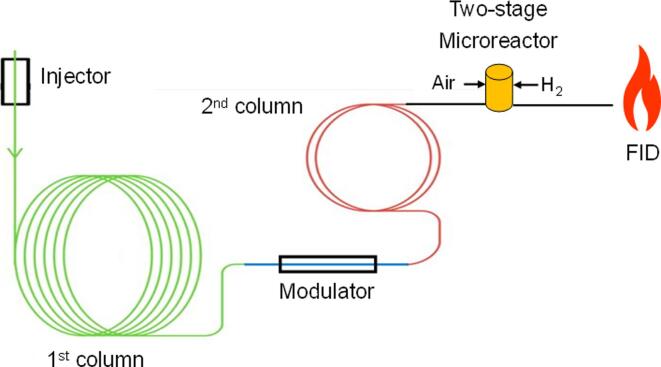
However, FID shows no response to non-combustible compounds such as H2O, CO2, SO2 or NOx. Furthermore, functional groups such as alcohol or acidic groups as well as halogenic functionalities show little or no response (Tranchida and Mondello, 2019). In a recent publication (Luong et al., 2019), GC × GC separation was combined with post-column reaction in a Polyarc reactor prior to detection on an FID (see Fig. 5). In the reactor, all organic molecules are converted to methane, thus eliminating the need for multilevel calibration. The authors observed improved FID uniformity and detector sensitivity (Luong et al., 2019). Furthermore, (Ferraz-Almeida et al., 2020) were able to analyze CO2 in soil samples using post-column methanization.
Fig. 5.
Two-dimensional gas chromatography combined with a Polyarc reactor. ().
Adapted from Luong et al., 2019
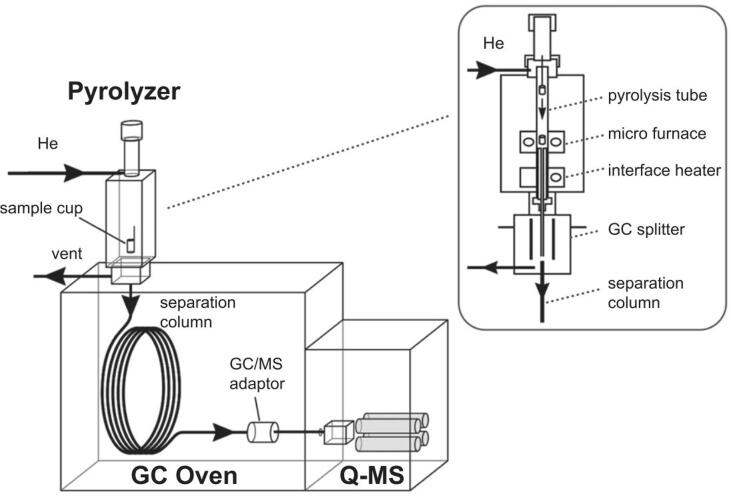
In MS, compounds are ionized by an ion source and consequently analyzed in the mass analyzer. The most widely used MS devices are low-resolution time-of-flight (LR-ToF) for GC × GC–MS applications and quadrupole mass spectrometry (qMS) for GC–MS application (Frysinger and Gaines, 1999, Van Deursen et al., 2000). ToF detectors separate ions based on different velocities in a flight tube. Lighter ions reach the detector at the end of the flight tube faster than heavier ions (Tranchida and Mondello, 2019, De Hoffmann, 2000, Tranchida et al., 2015, Zushi et al., 2014). 1D-GC–MS is the most suitable technique for fast screening, for instance, in a micro-pyrolyzer setup (see Fig. 6) (Tsuge et al., 2011), however, data obtained is primarily qualitative unless laborious absolute calibration is performed (Djokic et al., 2018). To combine qualitative and quantitative analysis using one GC × GC setup, the second-dimension column effluent may be split onto two second-dimension columns for the simultaneous analysis via FID and time-of-flight (ToF)-MS (Tranchida and Mondello, 2019, Sgorbini et al., 2015, Nicolotti et al., 2014). Several ASTM standard methods exist for 1D-GC–MS analyses of different hydrocarbon samples which are also applicable for plastic waste pyrolysis oils (i.e. ASTM D4128-18, ASTM D6420-18, ASTM D8276-19). For a detailed overview of recent developments in mass spectrometry, the reader is referred to a recent review by (Rankin-Turner and Heaney, 2021).
Fig. 6.
Schematic overview of the micro-pyrolysis setup mounted to a 1D-GC–MS. Re-used with permission (Tsuge et al., 2011).
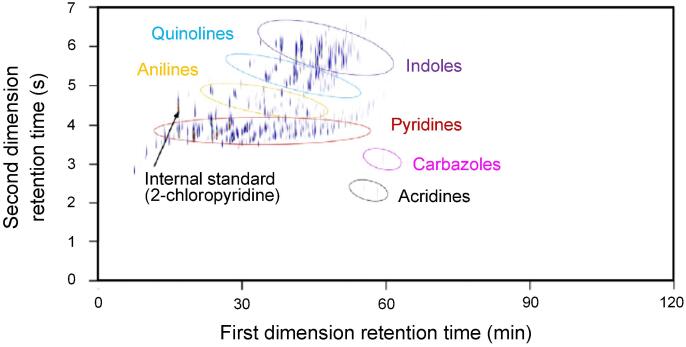
Element-selective detection techniques are essential since metals or heteroatomic compounds may exist in extremely low concentrations dispersed in complex hydrocarbon matrices, making detection with unselective methods such as FID or MS extremely difficult. For the specific analysis of nitrogen or sulfur compounds, detectors utilizing ozone-induced chemiluminescence have been introduced. Combined with GC × GC, a powerful technique for the analysis of nitrogen (GC × GC-NCD) and sulfur (GC × GC-SCD) compounds is provided (Yan, 1999, Yan, 2002). The mechanisms of NCD and SCD are very similar beginning with the conversion of nitrogen/sulfur containing analytes into their chemiluminescent species. In a second step, the chemiluminescence emitting from the reactions of these species with ozone is detected (Yan, 1999). Non-sulfur/nitrogen compounds are converted into non-chemiluminescent CO2 and H2O in the oxidative combustion step (Yan, 2002). GC × GC coupled to SCD and NCD have been applied for complex hydrocarbon samples in various publications and have proven to be valuable techniques for the analysis of trace amounts of sulfur (Dijkmans et al., 2015, Djokic et al., 2017, Ruiz-Guerrero et al., 2006, Hua et al., 2003, Blomberg et al., 2004) and nitrogen (Adam et al., 2009, Adam et al., 2007, Lissitsyna et al., 2013, Ristic et al., 2016, Dijkmans et al., 2015, Wang et al., 2004, Toraman et al., 2016, Dao Thi et al., 2021), respectively (see Fig. 7). Methods applied for complex fossil-based samples are applicable for plastic waste pyrolysis oils as well. In order to improve the separation of heteroatomic compounds such as sulfur and nitrogen containing compounds as well as oxygenates from the dominant complex hydrocarbon matrix it is possible to use reversed-phase GC × GC using the polar column first, followed by the non-polar column. This specific mechanism was used by (Dao Thi et al., 2021) and (Toraman et al., 2014) who reported improved accuracy for the characterization of plastic waste pyrolysis oils.
Fig. 7.
GC × GC-NCD chromatogram of a shale oil sample with individually marked compound groups. Re-used with permission (Dijkmans et al., 2015).
For the oxygen-specific analysis of complex hydrocarbon matrices, only few detection techniques exist. Using an O-FID analyzer, it is possible to detect oxygenates via conversion to CO and subsequent hydrogenation to methane (Juntarachat et al., 2013). However, O-FID is limited to lighter and “cleaner” samples. Therefore, GC coupled to an atomic emission detector (GC-AED or GC × GC-AED) is a viable option (Lorentz et al., 2017). An AED is a multi-element detector technique which, coupled to a GC, has the widest element range of all detectors (up to 23 elements including halogens and metals such as Fe, Pb, Ni and Si) (Ross et al., 2001, Tranchida and Mondello, 2019). The GC (× GC)-effluent is directed into a He plasma chamber where all elements are atomized and excited. When the atoms transition from the excited to the relaxed state, radiation at a characteristic wavelength for every element is released and detected (Tranchida and Mondello, 2019, Bartle et al., 2009).
A prominent detector for the analysis of halogens is the electron capture detector (ECD) which, combined with GC, has been used extensively for the analysis of halogens in plastic waste (Eguchi et al., 2021, Hall and Williams, 2006, Bhaskar et al., 2007). The ECD makes use of the electron absorption by highly electronegative atoms and molecules such as halogens but also some forms of nitrogen, sulfur and oxygen such as NO2/3, O2, CO2, SO2 among others. The reduced current between a collector anode and a cathode caused by the absorption of electrons by the sample eluting from a GC is proportional to the analyte concentration (Pellizzari, 1974). The high electronegativity makes this technique highly suitable for halogen containing plastic waste pyrolysis oils since it enables the identification of individual halogenated compounds such as HBr, bromophenols and bromobenzenes as reported by (Hall and Williams, 2006b) who analyzed the pyrolysis oil of brominated HIPS using GC-ECD. With this technique, the authors were able to detect >110 peaks indicating a large variety of brominated products. (Bhaskar et al., 2007) analyzed the pyrolysis oils of a PE/PP/PS/HIPS mix using GC-ECD and identified several brominated hydrocarbons. For the analysis of halogenated compounds in highly complex hydrocarbon matrices such as plastic waste pyrolysis oils, ECD can be coupled to GC × GC as shown by (Muscalu et al., 2011), who analyzed soil and sediment samples by GC × GC-μECD with enhanced selectivity and sensitivity without necessary prior fractionation of the samples. A disadvantage of the ECD for the analysis of complex matrices is the potential co-elution of compounds with low electron-capture affinity which can interfere with the electron capture of the target compounds causing a change in the relationship between the compound concentration and the response intensity (Booij et al., 1998). Combination with a higher resolution separation technique such as GC × GC is therefore advised for complex samples such as plastic waste pyrolysis oils.
Due to the fact that post-consumer plastic waste is largely contaminated with metals stemming mostly from performance-enhancing additives, trace metal analysis is essential when considering plastic waste pyrolysis oils as steam cracking feedstocks. An important role in this is held by inductively coupled plasma spectrometry combined with optical emission spectroscopy (ICP-OES) or mass spectrometry (ICP-MS) since it is capable of detecting metals in ultra-trace (ppb) concentrations (Al-Momani, 2003, Gazulla et al., 2017, Navarro et al., 2002, Rahmi et al., 2007, Haraguchi, 2004, Wysocka, 2021). In ICP, a sample is ionized in a plasma. Excited atoms will emit electromagnetic radiation at a wavelength which is characteristic to the respective element (Nelms, 2005, Dean, 2005). Next to stand-alone use, it is also possible to combine ICP-OES/MS with GC or even GC × GC (Bouyssiere et al., 2003, Clases et al., 2021). ICP-MS has lower detection limits than ICP-OES making it especially attractive for the analysis of trace elements in the ppb range. However, ICP-MS is less robust than ICP-OES. This is, for instance, due to carbon deposits derived from organic matrices. A recent review on ICP-MS analysis for trace metal concentrations was published by (Wysocka, 2021).
2.2. Other frequently used analytical techniques
Next to the analytical techniques involving gas-chromatographic separation prior to analysis, there are several characterization techniques that have been used extensively for the qualitative and quantitative characterization of plastic waste pyrolysis oils, in particular for the heavy tail that consists of compounds with a large molecular weight. These compounds are typically unwanted in steam cracking due to high coke formation tendencies in the convection sections of commercial steam crackers (Sundaram and Stancato, 2018). Several of these methods are ASTM standardized which aids the comparability of analytical data produced at different laboratories. The most important methods including the respective ASTM standards are listed in Table 2.
Table 2.
Overview of detection techniques used for the analysis of plastic waste pyrolysis oils.
High performance liquid chromatography (HPLC) is a technique applicable for the identification of the hydrocarbon matrix including large molecular weight compounds, which includes a column-based separation mechanism prior to detection of the time-resolved elution of individual compounds on separate detectors such as UV (Carné Sánchez and Collinson, 2011, Uzumkesici et al., 1999, Kicinski et al., 1989, Oña-Ruales et al., 2016) or MS (Pivnenko et al., 2017, Schlummer et al., 2005, Thiäner et al., 2019). Gel permeation chromatography (GPC) is a type of size exclusion chromatography (SEC) which makes use of the different elution velocity of the respective analytes in a gel prior to detection on separate detectors. It has been frequently used for the analysis of pyrolysis oils and other high molecular weight hydrocarbons (Choi et al., 2014, Mercader et al., 2010, Miskolczi et al., 2008, Çit et al., 2010, Arabiourrutia et al., 2012, Hoekstra et al., 2011, Miskolczi et al., 2009, Williams and Williams, 1997b, Apicella et al., 2006, Gargiulo et al., 2015, Altgelt and Hirsch, 1970, Panda et al., 2019).
Spectroscopic techniques are also frequently used to analyze (heavy) petroleum samples. Fourier-transform infrared spectroscopy (FTIR) is a technique in which the compounds are analyzed and categorized based on the type of chemical bond (Williams and Williams, 1999a, Jin et al., 2016, Singh et al., 2012, Qin et al., 2018, Wu et al., 2014, Miskolczi and Bartha, 2008, Camacho and Karlsson, 2001, Ioakeimidis et al., 2016, Aguado et al., 2002, Sogancioglu et al., 2017, Liu et al., 2017, Arnold et al., 2010). Several other spectroscopic techniques can be used, such as atomic absorption spectroscopy (AAS) (Okuwaki et al., 2006, Vasile et al., 2007, Hall and Williams, 2006, Kaminsky et al., 1996), nuclear magnetic resonance (NMR) (Siddiqui and Redhwi, 2009, Doğan and Kayacan, 2008, Das and Tiwari, 2018, Kiran et al., 2000, Edwards, 2011, Mattsson et al., 2016) or energy dispersive X-ray fluorescent spectroscopy (EDXRF) (Miskolczi et al., 2004, Srakeaw et al., 2014). EDXRF is also a suitable technique to analyze halogens (Ziegler et al., 2008, Pereira et al., 2015, Krishna et al., 2012). Another potent technique for the analysis of the total amount of halogens is combustion ion chromatography (CIC) as used by (Hall and Williams, 2008, Wongkhorsub and Chindaprasert, 2013, Takeshita et al., 2004, Bhaskar et al., 2007, Kakuta et al., 2008, Hall and Williams, 2006a, Österlund et al., 2009, Miskolczi et al., 2011, Roosen et al., 2020). UV–vis spectroscopy is an analytical method that makes use of the absorption of electromagnetic waves in the ultraviolet (UV) and visible (vis) light spectrum. It has been frequently used for the analysis of pyrolysis oils (Li et al., 2017, Chen et al., 2021). For a comprehensive overview of further analytical techniques for the characterization of complex pyrolysis (bio) oils including solvent fractionation techniques and volumetric methods, the reader is referred to the recent review of (Staš et al., 2020).
3. Composition of post-consumer plastic waste pyrolysis products
In the following section an overview is given on the composition of (polyolefinic) post-consumer plastic waste pyrolysis oils reported in open literature. Special emphasis is put on the hydrocarbon matrix (i.e. PIONA composition), heteroatoms and metals. Plastic waste streams which predominantly depolymerize into monomers such as PS or PMMA are not included since the pyrolysis products of these polymers can be used for the production of new polymers without the need for steam cracking towards light olefins.
Furthermore, a distinction has to be made between catalytic and thermal pyrolysis since the use of catalysts has a substantial influence on the composition of the pyrolysis products (Murata et al., 2009, Anene et al., 2018). However, contaminants present in post-consumer plastic waste may lead to prompt catalyst deactivation, hence, in the majority of studies involving catalytic decomposition, virgin or pre-treated plastic streams were used. Therefore, it is uncertain if contaminated mixed plastic waste is tolerable for commercial pyrolysis catalysts. The focus of this review thus lies on plastic waste pyrolysis oils obtained by thermal pyrolysis.
3.1. Hydrocarbon composition
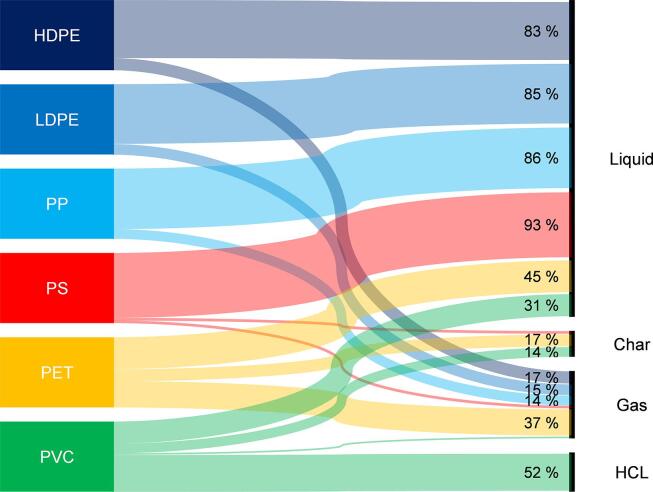
In a pyrolysis reactor, solid plastics are thermally decomposed in an inert, oxygen-free atmosphere yielding a distribution of gaseous, liquid and solid products depending on process conditions and on the pyrolyzed polymer type (Buekens, 2006, Lopez et al., 2017, Donaj et al., 2012). It has been reported already more than two decades ago that pyrolysis of virgin HDPE in a fixed bed batch reactor at 700 °C yields roughly 17 % gaseous and 80 % liquid product, while LDPE and PP yield between 14 and 15 % gaseous and roughly 84 % liquid products with no measurable char production. The mass balances were not normalized and closed between 96.5 and 99.3 % (Williams and Williams, 1999b). Heteroatom containing polymers (PVC and PET) yielded far less liquid product (<50 %, respectively) with significant amounts of char of roughly 15 %. Furthermore, PVC pyrolysis yields about 50 wt% HCl (Williams and Williams, 1999b) (see Fig. 8). The liquid pyrolysis product of pure PET consists exclusively of aromatics and aromatic acids such as benzoic acid (Çit et al., 2010).
Fig. 8.
Sankey chart depicting the pyrolysis mass balances of the respective pure virgin polymers pyrolyzed in a fixed-bed batch reactor at 700 °C, based on (Williams and Williams, 1997b). For the sake of comparability, the reported mass balances by were normalized to 100 %.
The pyrolysis product distribution strongly depends on the chosen pyrolysis conditions. At lower pyrolysis temperatures in the range 400 – 450 °C, heavier liquid products with a waxy quality are formed comparable with atmospheric residue from crude oil. At higher temperatures, a lighter oil is formed which is more suitable for the application as fuel or steam cracking feedstock. However, more non-condensable gaseous products are formed at higher temperatures (Williams and Williams, 1997a, Predel and Kaminsky, 2000, Al-Salem et al., 2017). It has further been shown that longer reaction times increase the formation of gas and coke with a corresponding decrease in liquid product yield (Murata et al., 2004, Murata et al., 2002, Al-Salem et al., 2017). Regarding the pressure effect on the decomposition, it was found that with higher reactor pressure the carbon number distribution of the liquid pyrolysis product of PE pyrolysis is shifted towards lower molecular weight products which can be related to the increased residence time in the reactor (Murata et al., 2004, Al-Salem et al., 2017).
Gaseous products of polyolefin waste pyrolysis contain mostly methane, ethane, ethylene, propane, propylene, butane and butylene with small amounts of pentane and pentene (Williams and Williams, 1997b, Williams and Williams, 1999a, Onwudili et al., 2009). The composition of the gaseous product is rather independent of the process temperature and residence time (Miskolczi et al., 2004). However, the gaseous product composition differs in terms of the pyrolyzed material. Gaseous products from HDPE pyrolysis consist of higher concentrations of ethane and ethylene, while product gas of PP pyrolysis contains higher amounts of propane and propylene (Miskolczi et al., 2009), which relates to their molecular structure.
Table 3 provides an overview of the hydrocarbon (PIONA) compositions of liquid pyrolysis products from different post-consumer plastic waste streams. For the sake of comparability between pyrolysis oils and steam cracking feedstocks such as naphtha or gas oil, the carbon number and if reported the boiling point ranges are given as well.
Table 3.
Overview of PIONA data of liquid products from pyrolysis of different plastic waste streams. Concentrations as specified in the respective reference.
| Raw material composition | Pyrolysis conditions, reactor type, analytical technique | PIONA | Carbon range, IBP-FBP | Reference |
|---|---|---|---|---|
| 28.9 % LDPE 51.7 % HDPE 11.2 % PP 2.5 % PVC 5.5 % Others |
500 °C Atmospheric pressure Stirred batch reactor GC–MS |
33 % Paraffins 50 % Olefins 4 % Naphthenes 4 % Oxygenates 9 % Others |
C9 – C34 30 °C – 324 °C |
(Miskolczi and Ateş, 2016) |
| 31 % LDPE 26 % HDPE 8 % PP 16 % PET 4 % PVC 1% PS |
500 °C Atmospheric pressure Fixed bed reactor GC–MS(a) |
3 % Paraffins 28 % Iso-paraffins 9 % Olefins 4 % Naphthenes 28 % Aromatics 29 % Oxygenates |
C5 – C17 | (Anuar Sharuddin et al., 2017) |
| Pure waste HDPE | 430 °C Atmospheric pressure Stirred semi-batch reactor GC–MS/FID |
40 % Paraffins 40 % Olefins 15 % Naphthenes 5 % Others |
C5 – C28 35 °C – 431 °C(b) |
(Lee et al., 2003) |
| Waste HDPE | 450 °C Atmospheric pressure Batch reactor GC–MS |
41 % Paraffins 40 % Olefins 1 % Aromatics 18 % Naphthenes |
C6 – C33 40 °C – 560 °C |
(Seo et al., 2003) |
| Waste HDPE | 450 °C Atmospheric pressure Batch reactor GC-TCD |
45 % Paraffins 49 % Olefins 3 % Aromatics 2 % Others |
C5 – C26 24 °C – 280 °C |
(Miskolczi, 2013) |
| Pure waste PE | 400 °C Steel tube GC-FID |
34 % Paraffins(c) 41 % Olefins 6 % Aromatics 19 % Naphthenes 1 % Others |
C7 – C20 | (Demirbas, 2004) |
| Pure waste PE | 450 °C Steel tube GC-FID |
40 % Paraffins 39 % Olefins 2 % Aromatics 19 % Naphthenes 1 % Others |
C7 – C20 | (Demirbas, 2004) |
| Pure waste PE | 525 °C Steel tube GC-FID |
44 % Paraffins 35 % Olefins 4 % Aromatics 18 % Naphthenes |
C7 – C20 | (Demirbas, 2004) |
| Pure waste PE | 600 °C Steel tube GC-FID |
44 % Paraffins 33 % Olefins 6 % Aromatics 17 % Naphthenes |
C7 – C20 | (Demirbas, 2004) |
| Pure waste LDPE | 400 °C Atmospheric pressure Stirred semi-batch reactor GC–MS |
36 % Paraffins 42 % Olefins 22 % Naphthenes |
C6 – C26 | (Lee, 2007) |
| Pure waste HDPE | 400 °C Atmospheric pressure Stirred semi-batch reactor GC–MS |
52 % Paraffins 33 % Olefins 16 % Naphthenes |
C6 – C26 | (Lee, 2007) |
| Pure waste PP | 400 °C Atmospheric pressure Stirred semi-batch reactor GC–MS |
5 % Paraffins 72 % Olefins 22 % Naphthenes |
C6 – C26 | (Lee, 2007) |
| Pure waste PP | 400 °C Steel tube GC-FID |
30 % Paraffins 45 % Olefins 1 % Aromatics 22 % Naphthenes 2 % Others |
C7 – C20 | (Demirbas, 2004) |
| Pure waste PP | 450 °C Steel tube GC-FID |
28 % Paraffins 42 % Olefins 5 % Aromatics 23 % Naphthenes 2 % Others |
C7 – C20 | (Demirbas, 2004) |
| Pure waste PP | 525 °C Steel tube GC-FID |
25 % Paraffins 40 % Olefins 9 % Aromatics 24 % Naphthenes 3 % Others |
C7 – C20 | (Demirbas, 2004) |
| Pure waste PP | 600 °C Steel tube GC-FID |
30 % Paraffins 36 % Olefins 10 % Aromatics 24 % Naphthenes 1 % Others |
C7 – C20 | (Demirbas, 2004) |
| Municipal plastic waste (mixture of PE, PP and PS) | 400 °C Steel tube GC-FID |
33 % Paraffins 37 % Olefins 8 % Aromatics 21 % Naphthenes 2 % Others |
C7 – C20 | (Demirbas, 2004) |
| Municipal plastic waste (mixture of PE, PP and PS) | 450 °C Steel tube GC-FID |
31 % Paraffins(d) 33 % Olefins 13 % Aromatics 21 % Naphthenes 2 % Others |
C7 – C20 | (Demirbas, 2004) |
| Municipal plastic waste (mixture of PE, PP and PS) | 525 °C Steel tube GC-FID |
31 % Paraffins 32 % Olefins 13 % Aromatics 23 % Naphthenes 1 % Others |
C7 – C20 | (Demirbas, 2004) |
| Municipal plastic waste (mixture of PE, PP and PS) | 600 °C Steel tube GC-FID |
33 % Paraffins 32 % Olefins 12 % Aromatics 23 % Naphthenes 1 % Others |
C7 – C20 | (Demirbas, 2004) |
| Plastic solid waste consisting of PE, PP, PS, PA and trace amounts of food residuals. | 430 °C Atmospheric pressure GC × GC-FID |
5 % Paraffins 8 % Iso-paraffins 12 % Olefins and naphthenes 67 % Aromatics 2 % Oxygenates 5 % Nitrogen containing compounds |
C5 – C44 | (Toraman et al., 2014) |
| 32 % PE 13 % PP 18 % PS 8 % PVC 15 % PET 14 % Others |
420 °C CSTR Niigata waste plastics liquefaction process |
18 % Paraffins and naphthenes(e) 26 % Olefins(f) 56 % Aromatics |
Light fraction 40 °C – 250 °C | (Okuwaki et al., 2006) |
| 32 % PE 13 % PP 18 % PS 8 % PVC 15 % PET 14 % Others |
420 °C CSTR Niigata waste plastics liquefaction process |
41 % Paraffins and naphthenes(e) 27 % Olefins(f) 32 % Aromatics |
Medium fraction 110 °C – 500 °C |
(Okuwaki et al., 2006) |
| 38 % PE 16 % PP 16 % PS 3 % PVC 12 % PET 15 % Others |
400 °C Rotary kiln reactor Sapporo waste plastics liquefaction process |
9 % Paraffins and naphthenes(e) 20 % Olefins(f) 71 % Aromatics |
Light fraction C4 – C12 55 °C – 180 °C |
(Okuwaki et al., 2006) |
| 38 % PE 16 % PP 16 % PS 3 % PVC 12 % PET 15 % Others |
400 °C Rotary kiln reactor Sapporo waste plastics liquefaction process |
21 % Paraffins and naphthenes(e) 52 % Olefins(f) 27 % Aromatics |
Medium fraction C7 – C20 195 °C – 320 °C |
(Okuwaki et al., 2006) |
| 35 % LDPE 32 % HDPE 24 % PP 4 % PVC 5 % Others |
550 – 560 °C Horizontal tubular reactor GC-FID/TCD |
26 % Paraffins 28 % Olefins 44 % Branched hydrocarbons 2 % Aromatics |
C5 – C35 | (Fekhar et al., 2019) |
| Municipal plastic waste (unspecified) | 400 °C CSTR Mikasa waste plastics liquefaction plant |
31 % Paraffins and naphthenes(e) 18 % Olefins(f) 51 % Aromatics |
Light fraction 40 °C – 240 °C |
(Okuwaki et al., 2006) |
| Municipal plastic waste (unspecified) | 400 °C CSTR Mikasa waste plastics liquefaction plant |
55 % Paraffins 23 % Olefins 22 % Aromatics |
Heavy fraction 110 °C – >360 °C |
(Okuwaki et al., 2006) |
| Mixed plastic packaging waste | CSTR GC × GC-FID |
15 % Paraffins 2 % Iso-paraffins 35 % Olefins 9 % Iso-olefins 4 % Diolefins 26 % Naphthenes 9 % Aromatics |
Light fraction C7 – C22 |
(Dao Thi et al., 2021) |
| Mixed plastic packaging waste | CSTR GC × GC-FID |
28 % Paraffins 4 % Iso-paraffins 36 % Olefins 9 % Iso-olefins 4 % Diolefins 17 % Naphthenes 2 % Aromatics |
Heavy fraction C5 – C11 |
(Dao Thi et al., 2021) |
(a)Composition based on GC–MS peak areas reported.
(b)Carbon number range and boiling points based on molar weight distribution of product reported.
(c)PIONA data normalized due to a reported mass balance of 105 %.
(d)PIONA data normalized due to a reported mass balance of 102 %.
(e)Given as “saturated compounds”
(f)Given as “unsaturated compounds”
From Table 3 it can be seen that the reported hydrocarbon compositions of the liquid pyrolysis products differ substantially. This has several reasons, namely the feedstock material, the pyrolysis conditions, the reactor design, the analyzed product fraction and the analytical methods used.
3.1.1. Influence of the plastic composition
The composition of the liquid pyrolysis product is largely dependent on the type of polymer which was pyrolyzed and can, therefore, be anticipated to a certain extent. An important publication was provided by (Tsuge et al., 2011), who performed qualitative (1D-)GC–MS analyses for a vast number of pure, virgin plastics which have been pyrolyzed in a pyrolysis reactor directly mounted to a 1D-GC–MS setup (see Fig. 6). Other important experimental studies have been provided by (Soják et al., 2007, Sojak et al., 2006). Apart from experimental studies, several theoretical studies have been conducted on modeling the decomposition kinetics of individual polymers as well as common plastic mixtures (De Witt and Broadbelt, 2000, Wong and Broadbelt, 2001, Vinu and Broadbelt, 2012, Dogu et al., 2021).
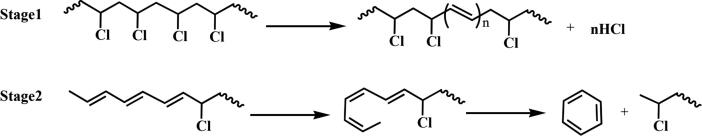
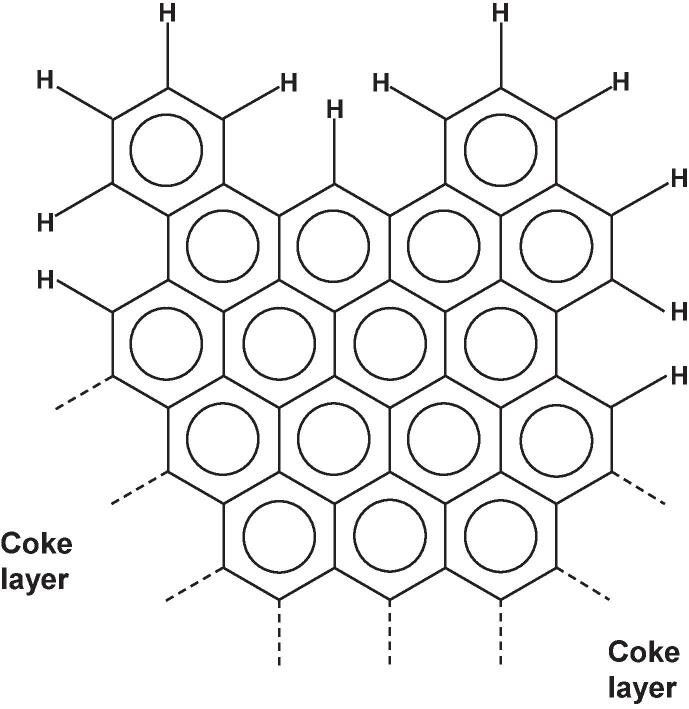
Regarding plastic packaging waste which mostly consists of PE and PP, the different branching tendencies of PE and PP are an important aspect. A high PP content shifts the liquid product composition towards higher concentrations of (branched) olefins (Tsuge et al., 2011, Soják et al., 2007, Sojak et al., 2006). This can be explained by the methyl side groups occurring in the PP chains which are mostly isotactic in commercial PPs (Anderson-Wile et al., 2012). In fact, it has been observed that the fingerprint products of PP pyrolysis are indeed oligomers of propylene: 2-methylpent-1-ene (dimer), 2,4-dimethylhept-1-ene (trimer), 2,4,6-trimethylnon-1-ene (tetramer) and 2,4,6,8-tetramethylundec-1-ene (pentamer) (Ballice and Reimert, 2002, Kusch, P., 2017, De Amorim et al., 1982, Predel and Kaminsky, 2000, Sojak et al., 2006). It can therefore be stated that the molecular structure of the PP polymer chain has a high influence on the decomposition chemistry. In the recent review paper by (Dogu et al., 2021), it is explained that due to the side groups of PP, multiple types of secondary radicals are formed during pyrolysis while in PE pyrolysis only primary radicals are formed. A high PE content, therefore, leads to higher paraffin concentrations. Pyrolysis of PS favors aromatic products (Pinto et al., 1999, Al-Salem et al., 2017). Furthermore, PS has an accelerating effect on the pyrolysis of polyolefins due to the formation of free radicals (Wong and Broadbelt, 2001). PVC decomposes in a two-step mechanism releasing HCl gas before the polymer backbone is decomposed (Miranda et al., 2001b, López et al., 2011a) (see Fig. 9). It can also be seen that in stage 2 of the PVC decomposition, chlorinated hydrocarbons are forming which are considered crucial contaminants in plastic waste pyrolysis oils.
Fig. 9.
Thermal decomposition mechanism of PVC (Ye et al., 2019).
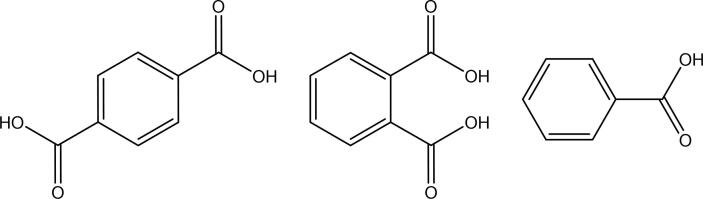
PET pyrolysis yields oxygenated compounds which may form organic acids as shown in Fig. 10. (Williams and Williams, 1997b).
Fig. 10.
Structural formulas of terephthalic acid, phthalic acid and benzoic acid (from left to right).
The findings show that it is highly important to monitor the composition of the post-consumer plastic waste since 100 % pure polymer streams are difficult to achieve in most modern waste sorting plants (Kleinhans et al., 2021).
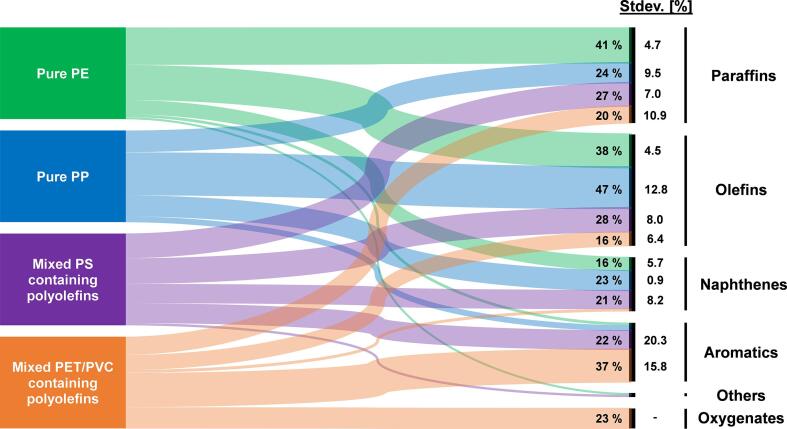
Fig. 11 presents a Sankey chart with an overview of the respective hydrocarbon families in the pyrolysis products depending on the feedstock material. For the diagram, liquid product compositions for similar post-consumer plastic waste feedstock compositions were averaged based on Table 3.
Fig. 11.
Sankey chart depicting the influence of the feedstock material on the hydrocarbon composition. Feedstock data is based on averaged values from Table 3 (numbers in wt%).
Pure PE waste yields equal amounts of paraffins and olefins with only minor amounts of naphthenes and aromatics. Pure PP waste yields higher amounts olefins and smaller amounts of paraffins, naphthenes and aromatics. This effect can be seen well by means of the data provided by (Demirbas, 2004) who pyrolyzed pure PE and PP in separate experiments using the same reactor and pyrolysis conditions. For the pyrolysis oil of pure PE waste the authors reported 40 % and 39 % of paraffins and olefins, respectively. For pure PP waste the authors detected 28 % of paraffins and 42 % of olefins in the pyrolysis oil. An even more drastic trend was reported by (Lee, 2007) who reported 52 % of paraffins and 33 % of olefins in waste HDPE pyrolysis oil and 5 % of paraffins and 72 % of olefins in pyrolysis oil from waste PP (Demirbas, 2004, Lee, 2007).
For mixed plastic waste samples, the liquid product composition is highly dependent on the PS, PVC and PET concentration, respectively. PS containing mixed polyolefin waste yields high amounts of aromatics. In case PET is present in the mixed plastic waste, high amounts of oxygenates are formed.
Importantly, it can be seen that inaccurately sorted waste plastics may lead to high amounts of aromatics or oxygenates in the pyrolysis oil deriving from minor amounts of PS, PET or PVC. However, considering the given standard deviations it becomes clear that there are large discrepancies between the respective reported concentrations. Thus, it is highlighted that, on the one hand, standardized analytic techniques are needed in order to reliably compare data produced at different laboratories and that, on the other hand, standardized waste sorting and separation procedures are needed.
3.1.2. Influence of the pyrolysis conditions
The main influencing factor of the pyrolysis product composition is the pyrolysis temperature which decides when chemical decomposition occurs. The different decomposition temperatures of various polymers can be assessed by thermogravimetric analysis (TGA), where a polymer is thermally degraded at gradually increasing temperatures to assess the weight loss of the polymer according to temperature and time (Wilkie, 1999). However, in a continuous process, another highly important factor is the residence time which is influenced by the reactor design and by the reactor pressure. At low pressure, for instance, the reactants have a shorter residence time in the reactor which leads to less severe cracking of the polymer chains compared to high pressure conditions which lead to the formation of lighter products (Murata et al., 2004, Schubert et al., 2019). Both residence time and temperature are an important driver for secondary reactions which may cause the formation of heavy (aromatic) products due to Diels-Alder reactions and polymerization reactions (Dogu et al., 2021, Westmoreland et al., 1989, Kislov et al., 2013). (López et al., 2011b) pyrolyzed a PE/PP mixture in a semi-batch reactor with reaction times of up to 120 min and reported that the olefin concentrations of the pyrolysis product decreased from 22 wt% to < 1 wt% with a corresponding substantial increase of polyaromatic hydrocarbons as the temperature increased from 460 to 600 °C. The decreasing effect of the pyrolysis temperature on the olefin concentration can also be seen, however to a lesser extent, by means of the data provided by (Demirbas, 2004) who reported a gradually decreasing olefin concentration when testing pyrolysis temperatures between 400 °C and 600 °C in a steel tube reactor. This observation was made both with pure PE waste, pure PP waste as well as mixed municipal plastic waste.
3.1.3. Influence of the reactor design
Obviously, there is an effect of the reactor design on the pyrolysis chemistry due to crucial aspects such as mass and heat distribution as well as residence times. In order to provide the highest efficiency regarding use of energy and reactor size, optimal heat and mass transfer in the reactor are of the highest importance. In the recently published review paper by (Dogu et al., 2021), several reactor types were assessed. In the past, numerous reactor types have been used industrially and in research such as fixed bed reactors, fluidized bed reactors, semi-batch and batch reactors or more specific solutions such as microwave-assisted reactors (Dogu et al., 2021). The different reactor technologies that have been used on an industrial scale in the past were reviewed by (Sasse and Emig, 1998) and (Butler et al., 2011).
The most commonly applied reactors for the pyrolysis of plastics are stirred tank reactors (STRs) which use a stirrer to improve heat and mass transfer as well as to scrape of coke from the reactor walls. Advantages of STRs are the rather simple design and the high conversion. However, due to the large volumes, heat gradients exist and the poor temperature control leads to secondary reactions and thus higher amounts of large molecular weight compounds in the pyrolysis oils. Furthermore, STRs typically require frequent maintenance (Butler et al., 2011). It was reported that the fluidized bed reactor shows the best heat transfer compared to concepts such as rotary kilns and tubular reactors. Therefore, in a larger scale, heat transfer media such as quartz sand at different grain sizes have been used (Sasse and Emig, 1998). Fluidized bed reactors were extensively tested in the pioneering work by Kaminsky and co-workers in the so-called Hamburg Process with highly promising results (Kaminsky, 1995, Simon et al., 1996, Predel and Kaminsky, 2000, Kaminsky et al., 2000, Kaminsky et al., 2004, Kaminsky et al., 1996). As described above, the use of a fluidized bed reactor significantly increased the light product fraction and reduces the formation of large molecular weight compounds. Furthermore, innovative solutions such as the dual-fluidized bed system for steam cracking of plastic waste developed at Chalmers University in Sweden provide a more integrated approach for the direct valorization of plastic waste to light olefins avoiding the two-step process of pyrolysis and subsequent steam cracking (Thunman and Seemann., 2010, Thunman et al., 2019). The use of supercritical fluids is another potential technology for the decomposition of plastic waste (Goto, 2009).
PIONA values shown in Table 3 were quite similar for different reactor types used at similar conditions in terms of feed material, pyrolysis temperature and analytical method. This was shown in pyrolysis experiments with pure waste PE at 430 – 450 °C in a stirred semi batch reactor (Lee et al., 2003), a batch reactor (Seo et al., 2003) and a steel tube vessel (Demirbas, 2004). Paraffin and olefin yields were in a similar range at around 40 %, followed by naphthenes in the range of 15 – 18 % and aromatics in the range of 1 – 2 %. These observations indicate that the influence of different reaction vessels on the hydrocarbon composition of the liquid pyrolysis products might indeed be minor and that the process conditions potentially have a far higher impact on the composition of the pyrolysis oils. However, when comparing the final boiling points (FBP) (or reported carbon number ranges), it becomes obvious that pyrolysis products from batch (FBP 560 °C, (Seo et al., 2003)) and semi-batch reactors (FBP 431 °C, (Lee et al., 2003)) have a much higher share of large molecular weight hydrocarbons compared to the pyrolysis products of a steel tube reactor (C7 – C20, (Demirbas, 2004)). This high discrepancy is clearly related to the different residence times of the respective reactor types and becomes even more obvious by means of the results reported by Kaminsky et al. (Kaminsky, 1995, Simon et al., 1996, Predel and Kaminsky, 2000, Kaminsky et al., 2000, Kaminsky et al., 2004, Kaminsky et al., 1996) who used a fluidized bed reactor and reported a high yield of gaseous and light liquid products
3.1.4. Influence of the analytical technique
The influence of the analytical techniques used by the respective researchers should not be neglected. This is especially evident when dealing with a great amount of isomers and homologues which are difficult to separate using 1D-GC. The fact that several authors did not report naphthenes in their products indicates that the paraffinic and olefinic concentrations might be overestimated due to lumping and misidentification. The difficulty of identifying the entire hydrocarbon matrix of plastic waste pyrolysis oils is also affected by the pyrolyzed polymers. The high degree of branching as found in PP pyrolysis oil as reported by (Tsuge et al., 2011, Soják et al., 2007, Sojak et al., 2006) complicates an accurate compound identification making alternative techniques such as GC × GC-FID necessary. Furthermore, the choice of columns for the first and second dimension separation in GC × GC has a substantial impact on the analytical accuracy. Different column combinations including reverse-phase (polar/non-polar instead of non-polar/polar) have been tested, making the identification of highly complex hydrocarbon matrices possible (Dao Thi et al., 2021, Toraman et al., 2014). This way it is also possible to distinguish between α-olefins, branched olefins and diolefins present in the pyrolysis oils (Dao Thi et al., 2021). Furthermore, in order to assess plastic waste pyrolysis oils as a steam cracking feedstock from an industry point-of-view, standardized methods are of highest importance to increase and guarantee comparability of compositional data. In light of trace amounts of highly harmful contaminants present in plastic waste pyrolysis oils, this becomes even more obvious
3.1.5. Influence of the boiling point range of the pyrolysis oils
In the liquid pyrolysis product, the hydrocarbon composition (i.e. distribution of PIONA), often varies with the carbon number and boiling point range. Since steam crackers are designed for a certain feedstock boiling point range, assessment of individual fractions of plastic waste pyrolysis oils is crucial in order to compare the composition with conventional feedstocks such as fossil naphtha. Data from the Niigata (Japan) plastic waste liquefaction plant, reported by (Okuwaki et al., 2006), shows that the light fraction (IBP 40 °C, FBP 250 °C) of the plastic waste pyrolysis product contained 18 % paraffins, 26 % olefins and 56 % aromatics. The medium fraction (IBP 110 °C, FBP 500 °C) contained 41 % paraffins, 28 % olefins and 41 % aromatics. The processed mixed plastic waste contained substantial amounts of PS (18 %) and PET (15 %). The values indicate that the formed aromatics more likely end up in the lighter product fractions. This can be explained by the presence of PS in the waste since its pyrolysis yields mostly (C8) mono-aromatics as confirmed elsewhere (Toraman et al., 2014). In the recently published study by (Dao Thi et al., 2021), two different fractions of plastic waste pyrolysis oils were thoroughly analyzed using GC × GC coupled to various detectors including the assessment of different column combinations. The authors reported around 10 wt% of aromatics in the light fraction (C5 – C11) while the heavy fraction (C7 – C23) only contained very small amounts (<1 wt%) of aromatics. This is in agreement with the abovementioned observations by (Okuwaki et al., 2006).
Next to the aromatic concentration which is likely to accumulate in the light fractions, similar amounts of linear and branched olefins as well as diolefins were found in the analyzed light fraction and heavy fraction indicating that olefins distribute more evenly throughout the entire boiling range of the pyrolysis oils, again agreeing with the abovementioned observations made by (Okuwaki et al., 2006). Finally, according to (Dao Thi et al., 2021), the paraffin content of the light fractions is substantially lower than in the heavier fractions which is again in agreement with (Okuwaki et al., 2006). However, it needs to be noted that (Okuwaki et al., 2006) reported lumped concentrations of paraffins and napthenes as “saturated” compounds.
Furthermore, it has been shown that PP pyrolysis oil contains predominantly branched olefins while PE pyrolysis oils contains more linear hydrocarbons (Tsuge et al., 2011, Soják et al., 2007, Sojak et al., 2006). An important implication can be drawn from this observation due to the fact that branched (acyclic) compounds are known to have lower boiling points than their linear counterparts which can be explained by an increased compactness reducing their intermolecular interactions (Wessel and Jurs, 1995, Monteiro and Firme, 2014, Santak and Conduit, 2019, Prahlada Rao and Sunkada, 2007). Therefore, there is a discrepancy between the carbon number range of the pyrolysis oil and the boiling point range depending on the concentration of branched and linear compounds and hence depending on the share of PP and PE in the original plastic waste sample. A fixed boiling point range distillation cut of PP pyrolysis oil will thus contain a larger carbon number range compared to the same boiling point range distillation cut of a PE pyrolysis oil.
3.2. Heteroatom levels in liquid plastic waste pyrolysis products
In the following section it is explained in which product fractions the majority of contaminants and heteroatoms will end up based on data reported in open literature. Table 4 provides an overview of heteroatom levels in liquid pyrolysis products. The data is distributed according to the carbon number range and, if reported, the boiling point range of the analyzed product. In this way, the contamination can be traced throughout the respective product fractions defined by their carbon number or boiling point range which is crucial for further (petro-) chemical processing. However, as mentioned above, the boiling point and carbon number ranges are not necessarily equivalent due to the different boiling points of branched hydrocarbon compounds compared to their linear counterparts. If reported, the concentration of the respective contaminant in the original plastic waste material is given as well. Due to the fact that oxygen is largely present in form of organic acids (see Fig. 10), the concentrations of terephthalic acid, phthalic acid and benzoic acid are listed separately.
Table 4.
Overview of heteroatom levels found in plastic pyrolysis products based on literature data.
| Composition in original material | Product fraction | Concentration | Detection technique | LOD | Reference | |
|---|---|---|---|---|---|---|
| N | 49 ppm | C5 – C26 Solid residue |
< 10 ppm 73 ppm |
ASTM D6366-99 | 0.05 ppm | (Miskolczi, 2013) |
| n.a. | C5 – C20 | 1700 ppm | ASTM D-5291 | n.a. | (Anuar Sharuddin et al., 2017) | |
| 6.1 ppm | C5 – C15 64 °C – 268 °C |
16.5 ppm 16.2 ppm |
ASTM D6366-99 | 0.05 ppm | (Angyal et al., 2007) | |
| 4 % PA/PUR | 35 °C – 204 °C 189 °C – 307 °C |
1459 ppm 1142 ppm |
ASTM D6366-99 | 0.05 ppm | (Miskolczi et al., 2004) | |
| 775 ppm | C5 – C32 | 100 ppm | CHN-600 LECO | n.a. | (Miranda et al., 2001a) | |
| n.a. | 70 °C – 500 °C | 1400 ppm | CHN LECO | n.a. | (Lee, 2009) | |
| n.a. | C5 – C11 | 1.1 wt% | GC × GC-NCD | n.a. | (Toraman et al., 2014) | |
| n.a. | 40 °C – 250 °C 110 °C – 500 °C 190 °C – 600 °C Solid residue |
850 ppm 1200 ppm 1100 ppm 2000 ppm |
Elemental analysis | n.a. | (Okuwaki et al., 2006) | |
| n.a. | 55 °C – 180 °C 195 °C – 320 °C 250 °C – 500 °C Solid residue |
440 ppm 1910 ppm 800 ppm 3700 ppm |
JIS K 2609 TCD | n.a. | (Okuwaki et al., 2006) | |
| n.a. | 40 °C – 240 °C 110 °C – >360 °C Solid residue |
820 ppm 800 ppm 3600 ppm |
Elemental analysis | n.a. | (Okuwaki et al., 2006) | |
| n.a. | C6 – C23 | 2100 ppm | Leco CHN600 | n.a. | (Baena-González et al., 2020) | |
| 10 % ABS | C5 – C22 | 1214 ppm | GC-AED | n.a. | (Brebu et al., 2005) | |
| n.a. | C5 – C18 | 180 ppm | Chemiluminescence detection | n.a. | (Borsodi et al., 2011) | |
| n.a. | C5 – C11 | 30 ppm | GC × GC-NCD | n.a. | (Dao Thi et al., 2021) | |
| S | 58 ppm | C5 – C26 Solid residue |
36 ppm 52 ppm |
ASTM D6366-99 | 0.05 ppm | (Miskolczi, 2013) |
| n.a. | C5 – C20 | 100 ppm | ASTM D-5291 | n.a. | (Anuar Sharuddin et al., 2017) | |
| 14.8 ppm | C5 – C15 64 °C – 268 °C |
17 ppm 15 ppm |
ASTM D 6428 | 0.05 ppm | (Angyal et al., 2007) | |
| 2 % PUR rubber | 35 °C – 204 °C 189 °C – 307 °C |
52 ppm 5 ppm |
ASTM D 6428 | 0.05 ppm | (Miskolczi et al., 2004) | |
| 0.25 wt% | C5 – C32 | 200 ppm | CHN-600 LECO | n.a. | (Miranda et al., 2001a) | |
| n.a. | C5 – C11 C12 – C16 |
1016 ppm 684 ppm |
GC × GC-SCD | n.a. | (Toraman et al., 2014) | |
| n.a. | 40 °C – 250 °C 110 °C – 500 °C 190 °C – 600 °C Solid residue |
12 ppm 43 ppm 60 ppm 430 ppm |
Elemental analysis | n.a. | (Okuwaki et al., 2006) | |
| n.a. | 55 °C – 180 °C 195 °C – 320 °C 250 °C – 500 °C Solid residue |
<100 ppm <100 ppm 400 ppm 800 ppm |
JIS K 2541 | 3 ppm | (Okuwaki et al., 2006) | |
| n.a. | 40 °C – 240 °C 110 °C – >360 °C Solid residue |
540 ppm 1510 ppm <100 ppm |
Elemental analysis | n.a. | (Okuwaki et al., 2006) | |
| n.a. | C6 – C23 | 300 ppm | LECO SC 132 | n.a. | (Baena-González et al., 2020) | |
| n.a. | 30 °C – 324 °C | 71 ppm | EDXRF | n.a. | (Miskolczi and Ateş, 2016) | |
| 135 ppm | 118 °C – 376 °C | 51 ppm | EDXRF | n.a. | (Miskolczi et al., 2013) | |
| n.a. | C5 – C11 C7 – C22 |
211 ppm 7 ppm |
GC × GC-SCD | n.a. | (Dao Thi et al., 2021) | |
| n.a. | C5 – C18 | 300 ppm | Oxidative combustion microcoulometry | n.a. | (Borsodi et al., 2011) | |
| O | n.a. | C5 – C44 | 1 wt% | ASTM D 5622 | n.a. | (Toraman et al., 2014) |
| n.a. | C5 – C20 | 3.83 wt% | By difference | n.a. | (Anuar Sharuddin et al., 2017) | |
| 5.78 % PET (Niigata, Japan) | 40 °C – 250 °C 110 °C – 500 °C 190 °C – 600 °C Solid residue |
<0.1 wt% 0.2 wt% <0.1 wt% 5 wt% |
n.a. | n.a. | (Okuwaki et al., 2006) | |
| n.a. | C6 – C23 | 2.30 wt% | By difference | n.a. | (Baena-González et al., 2020) | |
| 5.1 wt% | C4 – C20 | 0.04 wt% (oxygenated compounds) | GC–MS | n.a. | (Cho et al., 2010) | |
| n.a. | C5 – C11 C7 – C22 |
1400 ppm 100 ppm |
GC × GC-FID/MS | n.a. | (Dao Thi et al., 2021) | |
| Terephthalic acid | 5.78 % PET (Niigata, Japan) | 40 °C – 250 °C 110 °C – 500 °C 190 °C – 600 °C Solid residue |
<10 ppm 100 ppm 50 ppm 23000 ppm |
n.a. | n.a. | (Okuwaki et al., 2006) |
| 12.2 % PET Sapporo, Japan) | 195 °C – 320 °C 250 °C – 500 °C Solid residue |
<10 ppm 300 ppm 490 ppm |
GC–MS HPLC |
n.a. | (Okuwaki et al., 2006) | |
| Phthalic acid | 5.78 % PET (Niigata, Japan) | 40 °C – 250 °C 110 °C – 500 °C 190 °C – 600 °C Solid residue |
<10 ppm 110 ppm 70 ppm <10 ppm |
n.a. | n.a. | (Okuwaki et al., 2006) |
| Benzoic acid | 5.78 % PET (Niigata, Japan) | 40 °C – 250 °C | 1100 ppm | n.a. | n.a. | (Okuwaki et al., 2006) |
| 12.2 % PET Sapporo, Japan) | 195 °C – 320 °C 250 °C – 500 °C Solid residue |
42 ppm 100 ppm 210 ppm |
GC–MS HPLC |
n.a. | (Okuwaki et al., 2006) | |
| Cl | 46 ppm | C5 – C26 Solid residue |
< 10 ppm 45 ppm |
EDXRF | Low ppm | (Miskolczi, 2013) |
| 0.6 wt% | C5 – C14 | 15 ppm | n.a. | n.a. | (Kaminsky and Kim, 1999) | |
| 1.1 wt% | C6 – C20 | 2000 ppm | EPA 5050 | n.a. | (López et al., 2011c) | |
| 7.9 % PVC | C5 – C32 | 12 ppm | ASTM D4208-8 | n.a. | (Miranda et al., 2001a) | |
| 3.1 % PVC 1.4 % PVDC (Sapporo, Japan) |
55 °C – 180 °C 195 °C – 320 °C 250 °C – 500 °C Full range + ash Solid residue |
90 ppm 47 ppm 71 ppm 3800 ppm 14000 ppm |
TS Z 0025 | n.a. | (Okuwaki et al., 2006) | |
| 5.23 % PVC (Niigata, Japan) | 40 °C – 250 °C 110 °C – 500 °C 190 °C – 600 °C Solid residue |
550 ppm 45 ppm 43 ppm 1 ppm |
TS Z 0025 | n.a. | (Okuwaki et al., 2006) | |
| n.a. | 40 °C – 240 °C 110 °C – >360 °C Solid residue |
5 ppm 23 ppm 13800 ppm |
Elemental analysis | n.a. | (Okuwaki et al., 2006) | |
| 1.13 wt% | C4 – C20 Without additive With CaO |
358 – 506 ppm 50–74 ppm |
Trace chlorine analyzer (TCL-100 | n.a. | (Cho et al., 2010) | |
| n.a. | C6 – C23 | 400 ppm | ASTM2361 m | n.a. | (Baena-González et al., 2020) | |
| n.a. | C9 – C34 (30 °C – 324 °C) |
1285 ppm | EDXRF | n.a. | (Miskolczi and Ateş, 2016) | |
| 0.63 wt% | bp. < 210 °C Dist. residue |
4 ppm 1.66 wt% |
AAS | n.a. | (Kaminsky et al., 1996) | |
| 10 % PVC | C5 – C22 | 4972 ppm | GC-AED | n.a. | (Brebu et al., 2005) | |
| 2689 ppm | 118 °C – 376 °C | 618 ppm | EDXRF | n.a. | (Miskolczi et al., 2013) | |
| n.a. | C5 – C18 | 600 ppm | Oxidative combustion microcoulometry | n.a. | (Borsodi et al., 2011) | |
| Br | <5 ppm | C5 – C26 | < 10 ppm | EDXRF | Low ppm | (Miskolczi, 2013) |
| 10 % brominated ABS | C5 – C22 | 1924 ppm | GC-AED | n.a. | (Brebu et al., 2005) | |
| n.a. | 30 °C – 324 °C | 1533 ppm | EDXRF | n.a. | (Miskolczi and Ateş, 2016) | |
| 2316 ppm | 118 °C – 376 °C | 253 ppm | EDXRF | n.a. | (Miskolczi et al., 2013) | |
| P | n.a. | 30 °C – 324 °C | 498 ppm | EDXRF | n.a. | (Miskolczi and Ateş, 2016) |
Table 4 indicates large differences in the concentrations of the respective elements throughout all studies. This can be explained by different pre-treatment steps of the waste streams prior to pyrolysis such as increased sorting, extensive washing or the specialized removal of PVC. Since nitrogen stems from dirt and food residuals, extensive washing may result in lower concentrations in the pyrolysis oil. Next to organic residues (i.e. amino acids), a potential source of nitrogen are other nitrogen containing compounds such as detergents which were in contact with the plastic material due to its original use as detergent bottles (Roosen et al., 2020). It can further be seen that the “rest” fraction of the plastic waste is highly relevant for the heteroatomic content. In their study, (Miskolczi et al., 2004) pyrolyzed plastic waste containing 4 % PA/PUR which both contain nitrogen in their polymer structure. The resulting pyrolysis oil consequently contained >1000 ppm of nitrogen in both the lighter (IBP 35 °C, FBP 204 °C) and in the medium fraction (IBP 189 °C, FBP 307 °C). Depending on the nitrogen source, different chemical decomposition pathways might occur during pyrolysis which, in turn, have an influence on the nitrogen compounds found in the pyrolysis oils: While amino acids mostly decompose to ammonia which subsequently decomposes to N2 and H2, the chemical decomposition products of PA and PUR are compounds such as nitriles and amines (Weiss et al., 2018, Schaberg et al., 2018, Blazso, 2006, Takamoto and Petrich, 1994, Herrera et al., 2001b, Herrera et al., 2001a). This again underlines the importance of advanced waste separation and sorting. However, it has been shown that plastic waste pyrolysis oils may contain substantial amounts of nitrogen which is highly problematic for further petrochemical processing steps.
Again, the analytical techniques play a crucial role when discussing the reported values. (Toraman et al., 2014) analyzed plastic waste pyrolysis oil using comprehensive GC × GC-SCD/NCD and reported that the N containing compounds present are pyridines, nitriles, quinolines and indoles which are mostly in the range between C7 and C11. Sulfur in plastic waste stems partly from organic residues and on the other hand from sulfur containing additives. The most important S compounds are thiophenes, thiols, benzothiophenes, naphthenobenzothiophenes and dibenzothiophenes (Toraman et al., 2014). Due to the extreme fluctuations in the respective heteroatom concentrations, the validity of individual data points and especially averaged values is rather qualitative than quantitative.
The chlorine content in the pyrolysis oil is directly related to the PVC removal efficiency of the waste sorting plant but also to potential dehalogenation steps during the thermochemical recycling process as applied in the Niigata plastics waste liquefaction plant in Japan (see Fig. 12).
Fig. 12.
Schematic overview of the Niigata plastic waste liquefaction plant. Re-used with permission (Okuwaki, 2004).
Obviously, industrial and lab-scale processes differ significantly regarding their priorities (production of valuable products vs. generation of knowledge). Industrial-scale processes typically employ measures to reduce the negative impact of impurities such as PVC. This becomes evident when comparing Cl concentrations in liquid products from pyrolysis with and without implementation of dehalogenation steps. For instance, industrially produced PVC/PVDC containing plastic waste pyrolysis oil had a reported Cl content of 47 ppm in a boiling point range of 195 °C – 320 °C (Okuwaki et al., 2006). Pyrolysis oils of a similar carbon number range from plastic waste with a similar PVC/PVDC content processed without dehalogenation step had a reported Cl concentration of 2000 ppm (López et al., 2011c). Furthermore, (Cho et al., 2010) have pyrolyzed plastic waste with an original Cl content of 1.13 wt% with and without addition of CaO and reported a substantial reduction of a factor 5–10 of the Cl content of the corresponding pyrolysis oils (C4 – C20).
Bromine is a prominent flame retardant which predominantly occurs in waste electrical and electronic equipment WEEE (Yang et al., 2013, Ma et al., 2016). Separate take-back schemes for WEEE exist, as reported by (De Meester et al., 2019) and (Kawecki et al., 2018), and WEEE may require processing in a separate (thermochemical) recycling system. Pyrolysis of WEEE for the production of fuels and chemical feedstocks requires several specialized upgrading steps such as dehalogenation prior, during or after pyrolysis in order to produce a chemical feedstock suitable for further petrochemical processing (Yang et al., 2013, Ma et al., 2016). Potential debromination steps include solvent extraction, supercritical fluid technology and others, as reviewed in the recent article of (Charitopoulou et al., 2020). Next to WEEE, brominated flame retardants are also used in numerous other plastics which was shown by (Pivnenko et al., 2017) with HIPS as well as ABS containing the by far highest amounts of bromine. Low concentrations of brominated compounds in the range of the detection limit (3–20 ppb) were found in polyolefinic waste fractions, and may be a result of sorting inefficiencies, e.g. from WEEE debris. In the study of (Roosen et al., 2020), chlorine and fluorine concentrations in the high ppm range were reported in plastic waste samples representative for European waste. However, bromine was below the detection limit of the used analytical method (CIC).
(Brebu et al., 2005), (Miskolczi et al., 2013) and (Miskolczi and Ateş, 2016) showed that the pyrolysis oils of plastic waste mixtures containing small fractions of brominated polymers indeed contain small amounts of Br. (Brebu et al., 2005) assessed the removal of N, Br and Cl from mixtures of virgin polyolefins containing 10 % of brominated ABS as well as 10 % of PVC. The authors tested different catalysts next to purely thermal pyrolysis and found that the pyrolysis oil from thermal pyrolysis still contained 1900 ppm of Br and 5000 ppm of Cl along with 1200 ppm of N. In the work by (Miskolczi et al., 2013), mixed plastic waste with a Br content of 2316 ppm and a Cl content of 11196 ppm was pyrolyzed. The authors reported a concentration of 253 ppm of Br and 618 ppm of Cl in the liquid pyrolysis product. In another study by (Miskolczi and Ateş, 2016), Br and Cl concentrations of 1533 ppm and 1285 ppm, respectively, were reported in the pyrolysis oils of mixed plastic waste. Hence, it can be stated that indeed substantial amounts of Br may end up in the liquid pyrolysis product comparable with Cl. However, the cause of Br contamination is clearly related to certain additives which predominantly occur in WEEE as well as in certain polymers such as flame retarded HIPS and ABS. Flame retardants act as radical scavengers during combustion reactions thus slowing down the exothermic combustion reaction (Maier and Calafut, 1998). All halogen flame retardants have this effect, however, Br is more potent than Cl and F. Iodine, being the most effective radical scavenger, is not used in flame retardants due to lower thermal stability (Maier and Calafut, 1998). The radical scavenging activity plays a role during pyrolysis. It has been reported by (Grause et al., 2008) and (Barontini et al., 2004) that the radical scavenging activity of Br leads to the formation of brominated hydrocarbons next to HBr.
However, it can be expected that the Br-rich waste fractions will be reduced significantly in modern waste sorting and separation plants, thus reducing the risk of Br in pyrolysis oils from polyolefinic plastic packaging wastes (De Meester et al., 2019). Of course, an uncertainty remains in the detection techniques applied by the respective research groups which need to be highly accurate to detect trace amounts of Br and F.
Few studies exist reporting on the oxygen content of plastic waste pyrolysis oils. However, it can be stated that one of the major sources for oxygen in the pyrolysis oils is PET impurity in the (polyolefinic) plastic waste while oxygen contamination stemming from organic residues contributes to a lesser extent. In industrial processes, measures are applied to minimize the impact of oxygen compounds present in the feedstock as for instance addition of Ca salts to bind organic acids within the pyrolysis process minimizing blockage and corrosion issues (Okuwaki et al., 2006). This also explains why most of the acids ended up in the residue fractions (see Table 4).
3.3. Metal contaminant levels in liquid plastic waste pyrolysis products
It has been reported that the solid residue (i.e. char) from pyrolysis contains the highest number of contaminants (Miskolczi, 2013, Kaminsky and Kim, 1999) with levels below the (unspecified) detection limits in the liquid pyrolysis products. However, it is highly important to trace metal contaminants throughout the pyrolysis product fractions which are most likely used for steam cracking. Table 5 contains an overview of metal contaminant concentrations as reported in open literature.
Table 5.
Overview of metal contaminant levels found in plastic pyrolysis products based on literature data.
| Original concentration | Liquid fraction | Concentration | Detection technique | LOD | Reference | |
|---|---|---|---|---|---|---|
| Al | n.a. | Residue | 16400 ppm | n.a. | n.a. | (Okuwaki et al., 2006) |
| n.a. | 55 °C – 180 °C 195 °C – 320 °C 250 °C – 500 °C Full range + ash Solid residue |
<0.2 ppm ND 17 ppm 150 ppm 15000 ppm |
JIS K 0102 58.4 | n.a. | (Okuwaki et al., 2006) | |
| n.a. | Solid residue 1 Solid residue 2 |
<400 ppm 2500 ppm |
n.a. | n.a. | (Okuwaki et al., 2006) | |
| n.a. | C4 – C20 Dist. residue |
2 ppm 2 – 2.7 ppm |
ICP-OES | n.a. | (Cho et al., 2010) | |
| 8320 ppm | C8 – C28 | <200 ppm | ICP-OES | n.a. | (Velghe et al., 2011) | |
| As | n.a. | Solid residue | <0.1 ppm | JIS K 0102 61.3 | n.a. | (Okuwaki et al., 2006) |
| n.a. | Solid residue | <1 ppm | n.a. | n.a. | (Okuwaki et al., 2006) | |
| Ba | n.a. | C4 – C20 Dist. residue |
1 ppm 3.2 – 21 ppm |
ICP-OES | n.a. | (Cho et al., 2010) |
| Ca | n.a. | 55 °C – 180 °C 195 °C – 320 °C 250 °C – 500 °C Full range + ash Solid residue |
17 ppm 2 ppm 82 ppm 8200 ppm 10 wt% |
JIS K 0102 50.3 | n.a. | (Okuwaki et al., 2006) |
| n.a. | Solid residue 1 Solid residue 2 |
1000 ppm 2500 ppm |
n.a. | n.a. | (Okuwaki et al., 2006) | |
| 189 ppm | >C30 | 368 ppm | EDXRF | 1 ppm | (Miskolczi et al., 2004) | |
| 1504 ppm | 118 °C – 376 °C | 297 ppm | EDXRF | n.a. | (Miskolczi et al., 2013) | |
| 7700 ppm | C8 – C28 | <30 ppm | ICP-OES | n.a. | (Velghe et al., 2011) | |
| Cd | n.a. | Solid residue | 3.3 ppm | JIS K 0102 55.3 | n.a. | (Okuwaki et al., 2006) |
| n.a. | Solid residue | 13 ppm | n.a. | n.a. | (Okuwaki et al., 2006) | |
| Cr | n.a. | Solid residue | 3 ppm | n.a. | n.a. | (Kaminsky and Kim, 1999) |
| n.a. | Solid residue | 24 ppm | n.a. | n.a. | (Okuwaki et al., 2006) | |
| n.a. | 55 °C – 180 °C 195 °C – 320 °C 250 °C – 500 °C Full range + ash Solid residue |
<0.2 ppm ND 8.2 ppm 37 ppm 200 ppm |
JIS K 0102 65.1.4 | n.a. | (Okuwaki et al., 2006) | |
| n.a. | Solid residue | 28 ppm | JIS K 0102 65.1.4 | n.a. | (Okuwaki et al., 2006) | |
| n.a. | C4 – C20 Distillation residue |
1 ppm 1–4 ppm |
ICP-OES | n.a. | (Cho et al., 2010) | |
| Cu | n.a. | Solid residue | 270 ppm | n.a. | n.a. | (Okuwaki et al., 2006) |
| n.a. | 55 °C – 180 °C 195 °C – 320 °C 250 °C – 500 °C Full range + ash |
0.6 ppm 0.1 ppm 2.3 ppm 84 ppm |
JIS K 0102 52.4 | n.a. | (Okuwaki et al., 2006) | |
| 109 ppm | C8 – C28 | 6–30 ppm | ICP-OES | n.a. | (Velghe et al., 2011) | |
| Fe | n.a. | 55 °C – 180 °C 195 °C – 320 °C 250 °C – 500 °C Full range + ash Solid residue |
<0.2 ppm 7.3 ppm 33 ppm 99 ppm 3200 ppm |
JIS K 0102 57.4 | n.a. | (Okuwaki et al., 2006) |
| K | n.a. | Solid residue | 1300 ppm | n.a. | n.a. | (Okuwaki et al., 2006) |
| 1200 ppm | C8 – C28 | 20–200 ppm | ICP-OES | n.a. | (Velghe et al., 2011) | |
| Mn | n.a. | Solid residue | 160 ppm | JIS K 0102 56.4 | n.a. | (Okuwaki et al., 2006) |
| Na | n.a. | Solid residue | 3700 ppm | n.a. | n.a. | (Okuwaki et al., 2006) |
| n.a. | 55 °C – 180 °C 195 °C – 320 °C 250 °C – 500 °C Full range + ash Solid residue |
0.8 ppm ND 2.8 ppm 200 ppm 3300 ppm |
AAS | n.a. | (Okuwaki et al., 2006) | |
| Ni | n.a. | Solid residue | 32 ppm | JIS K 0102 59.3 | n.a. | (Okuwaki et al., 2006) |
| Pb | n.a. | Solid residue | 4 ppm | n.a. | n.a. | (Kaminsky and Kim, 1999) |
| n.a. | Solid residue | 410 ppm | n.a. | n.a. | (Okuwaki et al., 2006) | |
| n.a. | 70 °C – 460 °C | 37 ppm | JIS 0102 54.3 | n.a. | (Okuwaki et al., 2006) | |
| n.a. | Solid residue | 33 ppm | JIS K 0102 55.4 | n.a. | (Okuwaki et al., 2006) | |
| n.a. | C4 – C20 Distillation residue |
0.02 – 0.04 ppm 4.4 – 21.5 ppm |
ICP-OES | n.a. | (Cho et al., 2010) | |
| Sb | 749 ppm | 118 °C – 376 °C | 105 ppm | EDXRF | n.a. | (Miskolczi et al., 2013) |
| n.a. | C9 – C34 (30 °C – 324 °C) |
189 ppm | EDXRF | n.a. | (Miskolczi and Ateş, 2016) | |
| Si | n.a. | 55 °C – 180 °C 195 °C – 320 °C 250 °C – 500 °C Full range + ash Solid residue |
<100 ppm 4 ppm <50 ppm 2000 ppm 45000 ppm |
ICP-OES | n.a. | (Okuwaki et al., 2006) |
| Ti | 284 ppm | >C30 | 456 ppm | EDXRF | 1 ppm | (Miskolczi et al., 2004) |
| Zn | n.a. | Solid residue | 100 ppm | n.a. | n.a. | (Kaminsky and Kim, 1999) |
| 145 ppm | >C30 | 254 ppm | EDXRF | 1 ppm | (Miskolczi et al., 2004) | |
| n.a. | Solid residue | 300 ppm | n.a. | n.a. | (Okuwaki et al., 2006) | |
| n.a. | 55 °C – 180 °C 195 °C – 320 °C 250 °C – 500 °C Full range + ash Solid residue |
0.6 ppm ND 2.1 ppm 18 ppm 350 ppm |
JIS K 0102 53.3 | n.a. | (Okuwaki et al., 2006) | |
| 1310 ppm | 118 °C – 376 °C | 124 ppm | EDXRF | n.a. | (Miskolczi et al., 2013) | |
| 472 ppm | C8 – C28 | <8 ppm | ICP-OES | n.a. | (Velghe et al., 2011) | |
| n.a. | C4 – C20 Distillation residue |
0.03 – 0.18 ppm 5 – 24.3 ppm |
ICP-OES | n.a. | (Cho et al., 2010) |
Reported values for trace metals depend not only on the starting material and the product fraction, but also largely on the analytical technique used and their respective lower limits of detection and potential memory effects. Therefore, it is likely that, on the one hand, the actual metal contamination is higher than listed in Table 5 and that, on the other hand, trace metals were not detected at all. Furthermore, metal contaminant data reported only for solid residue was not reported due to the fact that the data is not relevant in terms of steam cracking of the liquid pyrolysis oils. Since trace metal analysis in plastic waste pyrolysis oils is still a field largely unexplored, it is difficult to provide a representative overview. Importantly, the presented data underlines the importance of accurate analytical methods such as ICP-OES/MS for the analysis of trace metals.
By average, the metal concentrations that could be clearly allocated to the respective product fractions, end up to almost 99 % in the pyrolysis residue fraction. Only traces of the metals reach the liquid product in increasing order with increasing “heaviness” of the product fractions (0.01 % in the light, 0.36 % in the medium and 0.75 % in the heavy fraction). The higher concentration in the heavy fraction can be explained by metal contaminants bound in form of organometallic complexes such as metalloporphyrins (see Fig. 13) which are mostly present in asphaltenes in heavier fractions ranging around C27 – C33 (Mello et al., 2012, Ali and Abbas, 2006). Furthermore, it has been reported that fine solid particles can be entrained by the volatile pyrolysis products thus ending up in the condensed liquid fractions. To prevent this, hot-gas filters might be applied downstream of the pyrolysis reactor (Velghe et al., 2011).
Fig. 13.
Structural formula of a metalloporphyrin.
Thus, it can be assumed that, while most of the metals end up in the solid pyrolysis residue, there is indeed a dependency on the respective boiling range visible. The findings indicate that, although there is a substantial metal contamination in the liquid pyrolysis product, the most interesting carbon number range for steam cracking, which is between C5 up to ∼ C30 shows indeed quite low metal contaminant levels. Therefore, distillation to remove the heavy fractions of the crude pyrolysis oils is a viable first upgrading technique. However, the severe impact of some metal contaminants in steam cracking requires ultra-low concentrations which are still exceeded in the lighter fractions of plastic waste pyrolysis oils by orders of magnitude requiring further upgrading steps or low drop-in concentrations in order to reach the strict specifications.
4. Contaminants as bottleneck in steam cracking
In this section, a link between pyrolysis oil contaminants and feedstock specifications for industrial steam crackers is established to shed light on the steam cracking feasibility of plastic waste pyrolysis oils. Furthermore, potential harmful effects of the contaminants are discussed. According to the thresholds and the found contaminant levels, upgrading pathways can be designed to meet the described feedstock quality. Of course, blending plastic waste pyrolysis oils with conventional petrochemical feedstocks to lower the contaminant levels according to the mixing ratio is always an option.
Steam cracking is a large-scale industrial process which is sensitive to certain contaminants and feedstock impurities. As steam crackers are designed for a specific feedstock range, different thresholds for contaminants and impurities apply in order to guarantee stable operation of the steam cracking unit and to avoid off-spec products (Sundaram and Stancato, 2018). Thresholds can vary between steam crackers depending on the exact design of the furnace and the separation section. Steam crackers designed for heavier feedstocks such as gasoil are typically more resistant to heteroatom contaminations as can be seen from a survey among industrial plant operators (Baumgartner et al., 2004).
4.1. Conventional fossil-based steam cracker feedstocks
As a good benchmark for plastic waste pyrolysis oils, fossil naphtha can be taken into account. Naphtha is a product of crude oil distillation and a mixture of a large number of hydrocarbon compounds (Gary et al., 2007). The initial boiling point (IBP) of naphtha lies around 35 °C and the final boiling point (FBP) at around 200 °C. Naphtha can be distilled into a light fraction which has a carbon number range of C4 to C7 (FBP 145 °C) and a heavy fraction with a carbon number range of up to C12 (FBP 200 °C) (Robinson, 2011, Speight, 2015). Since naphtha feedstocks are produced from crude oil, there are hundreds of variations depending on the origin of the crude oil source. Naphthas typically consist mostly of paraffins and naphthenes with lower amounts of aromatics and contain, in contrast to plastic waste pyrolysis oils, no olefins (Pandey et al., 2004). Gas oils are heavier but less favored steam cracker feedstocks due to higher amounts of sulfur compounds and aromatics. Steam cracking of these feedstocks leads to lower ethylene yields and more heavy products (C10+) which reduce the run length of a steam cracker due to their higher fouling potential. Heavy fossil feedstocks such as petroleum or shale oils can contain significant amounts of heteroatomic compounds which may substantially exceed the specifications set for petrochemical feedstocks (Djokic et al., 2017). To mitigate the fouling potential, these feedstocks can be upgraded using hydrotreatment to reduce the aromatic and sulfur content prior to cracking (Matar and Hatch, 2001). When comparing fossil feedstocks with plastic waste pyrolysis oils it has to be noted that certain upgrading steps such as desulfurization and catalytic reforming are performed prior to steam cracking (Speight, 2015).
4.1.1. Effects of the hydrocarbon composition
In general, olefins inhibit the cracking of paraffins while paraffins accelerate cracking of olefins. This effect was observed already decades ago for paraffin cracking in the presence of propylene and with higher olefins (Albright et al., 1983). Furthermore, olefins are subject to polymerization reactions (i.e. “chemical reaction fouling”) (Ibrahim et al., 2005). Due to the fact that the walls of the heat transfer surface are typically at higher temperatures than the bulk flow, chemical reactions might be catalyzed by the wall material yielding insoluble deposits at the inner walls (Müller-Steinhagen et al., 2011, Müller-Steinhagen, 2010). Consequently, deposits cause a decrease of heat transfer through the externally fired reactor tubes inside the steam cracking furnace. Ultimately, blockages of the tubes might occur (Asomaning, 1990, Müller-Steinhagen et al., 2011). An important fouling reaction is the oxidation of unsaturated hydrocarbons. Olefins are more prone to oxidation reactions than paraffins due to their lower stability (Ibrahim, 2012). At more severe conditions as found in steam crackers, autoxidation is the major mechanism causing fouling (Asomaning, 1990, Epstein, 1983). It has been further reported that high olefin concentrations in liquid feedstocks result in increased radiant coke formation and transfer line exchanger fouling due to secondary reactions (Van Geem et al., 2009). This effect becomes dominant at an olefin concentration of > 5 % as found by (Kopinke et al., 1993a, Kopinke et al., 1993b) decades ago. According to industrial plant operators, the maximum allowable olefin concentration in naphtha range feedstocks is 2 wt% (Baumgartner et al., 2004).
Typically, naphthenes are slightly more stable than straight chain paraffins due to a presumably slower initiation step (H* elimination) of the thermal decomposition. Furthermore, naphthenes pose a higher coking tendency (by ∼ 50 %) than paraffins due to dehydrogenation reaction towards aromatic products. This has been known for decades (Albright et al., 1983, Kopinke et al., 1993a) and is especially valid for olefinic naphthenes such as cyclopentadiene which are prominent coke precursors (Guisnet and Magnoux, 2001).
However, it has been reported decades ago that the coking potential of a feedstock is essentially dependent on the aromatic content (Kopinke et al., 1993a, Kopinke et al., 1993b, Letzsch and Ashton, 1993). Aromatics have a higher coking tendency than paraffins due to combination reactions with other aromatics forming heavier species which may condense to highly stable polyaromatic hydrocarbons (PAHs) (Richter and Howard, 2000, Wauters and Marin, 2002) (see Fig. 14).
Fig. 14.
Coke layer represented by a polyaromatic structure. Re-drawn from (Wauters and Marin, 2002).
PAHs may result in coke deposition in the reactor and the transfer line which ultimately leads to severe blockages (Towfighi et al., 2002, Ristic et al., 2018).
4.1.2. Steam cracking feasibility of pyrolysis oils in terms of PIONA
Table 6 lists the concentrations of hydrocarbon families found in plastic waste pyrolysis oils distributed over the light, medium and heavy product fractions. The fractions were defined according to the reported ranges of the respective literature data. As light fraction, fractions with carbon number ranges below C20 and final boiling points below 250 °C were considered. Fractions were considered medium fractions, when the reported carbon number range and boiling point range did not exceed C30 and 450 °C, respectively. The values are averaged values of all the PIONA values listed in Table 3, thus providing a rough estimate of the average compositions to be expected from mixed plastic waste pyrolysis oils. Potential uncertainties lie, of course, in the accuracy of the reported literature data regarding lumping of compound groups or unrepresentative plastic waste samples. Hydrocarbon compositions which could not be clearly allocated to a certain product fraction are not included. Furthermore, impure polyolefinic plastic waste has a huge impact which can be seen by means of the high aromatic content of some reported hydrocarbon compositions, largely caused by PS impurities. However, by average, the impact will, of course, be reduced when considering polyolefinic waste with only minor PS contents.
Table 6.
Distribution of paraffins, olefins, naphthenes and aromatics over the light, medium and heavy product fractions of all post-consumer plastic waste pyrolysis oils (averaged values taken from Table 3).
| Light fraction(a) [wt%] | Medium fraction(b) [wt%] | Heavy fraction(c) [wt%] | |
|---|---|---|---|
| Paraffins | 28 | 29 | 44 |
| Olefins | 32 | 40 | 29 |
| Naphthenes | 18 | 14 | 17 |
| Aromatics | 19 | 12 | 11 |
| Others | 4 | 5 | 0 |
(a) defined as carbon number up to C20 and boiling point up to 250 °C.
(b) defined as carbon number up to C30 and boiling point up to 450 °C
(c) defined as carbon number > C30 and FBP > 450 °C
Table 6 shows that the lighter fractions are most likely off-spec in terms of their steam cracking feasibility due to the high aromatic concentration. In this case, the aromatic content must be recovered prior to steam cracking using, for instance, extractive-azeotropic distillation (Gaile et al., 2004). It is also recommended to remove PS prior to pyrolysis to recover styrene in a separate process instead of removing the aromatics from the pyrolysis oil prior to steam cracking.
The olefin concentrations listed in Table 6 and Table 3 exceed the olefin threshold value of 2 wt% substantially (Baumgartner et al., 2004) which indicates a high coking and fouling potential when steam cracking these untreated products. Tuning the olefin concentration to reach industrial limits may be performed by hydrotreatment. Another option to reach the proposed olefin limits would be co-feeding with fossil feedstocks thus tuning down the olefin concentration according to the mixing ratio to an acceptable value. For the light product fractions this would mean a blending ratio of ∼ 6.3 wt% with olefin-free fossil naphtha. The medium fraction would require a blending ratio of ∼ 5 wt% in order to reach the specifications.
4.2. Steam cracking feasibility of pyrolysis oils in terms of heteroatoms
Heteroatoms in feedstocks can have a severe impact on the steam cracker in terms of fouling potential, coke formation, corrosion and downstream catalyst poisoning. Therefore, strict specifications exist which will be the basis for a feasibility assessment of plastic waste pyrolysis oils as steam cracking feedstocks (see Table 7).
Table 7.
Overview of typical heteroatom specifications for fossil-based steam cracker feedstocks.
| Contaminant | Industrial limit | Reference |
|---|---|---|
| Nitrogen | Naphtha: 100 ppm Gas oils: 2000 ppm |
(Baumgartner et al., 2004) |
| Sulfur | 500 ppm | (Baumgartner et al., 2004) |
| Oxygen | 100 ppm | (Baumgartner et al., 2004) |
| Chlorine | 3 ppm | (Baumgartner et al., 2004, Sundaram and Stancato, 2018, Alanazi et al., 2017) |
| Phosphorus | 0.5 ppm | (Baumgartner et al., 2004) |
An overview of the heteroatom concentrations found in the respective light, medium and heavy fractions of plastic waste pyrolysis oils compared to the corresponding contaminant limits for industrial steam crackers is given in Fig. 15. The heteroatom values are averaged data from Table 4 which are subject to large fluctuations. Therefore, the data should be considered qualitative rather than quantitative. However, the data gives an indication of the challenges for plastic waste pyrolysis oils in terms of heteroatoms.
Fig. 15.
Overview of the heteroatom content in the light, medium and heavy fractions compared with the threshold value for industrial steam crackers. Values taken from Table 4 and (Baumgartner et al., 2004).
In the following sub-sections, the steam cracking feasibility of plastic waste pyrolysis oils is discussed based on their heteroatom concentration. Potential upgrading techniques in order to reach industrial feedstock specifications are suggested.
4.2.1. Nitrogen
The average nitrogen values found in the light and medium fractions are substantially higher than the allowable nitrogen concentrations in liquid feedstocks for industrial crackers (100 ppm). The average N concentration in the heavy fraction of plastic waste pyrolysis oils lies below the industrial threshold for gas oils of 2000 ppm (Baumgartner et al., 2004). However, the data presented by (Toraman et al., 2014) who detected a nitrogen concentration of 1 wt% using GC × GC-NCD shows the high impact of nitrogen containing polymers such as PA/PUR.
Nitrogen compounds are responsible for catalyst deactivation in hydrodesulfurization units, hydrocrackers and reforming processes (Adam et al., 2009). Furthermore, they may cause explosive gum formation in the cold-box of the downstream section of a steam cracker plant, which has been known for decades (Charlesworth, 1986, Dinneen and Bickel, 1951). Thermal decomposition of nitrogen compounds leads to the formation of NOx species which cause air pollution and acid rain (Dijkmans et al., 2015). Amines and other nitrogen containing compounds have a negative impact on the process since they decompose to ammonia and nitriles which are harmful for downstream catalysts, causing the steam cracking products to be off-spec (Letzsch and Ashton, 1993) (see Fig. 16).
Fig. 16.
Decrease of performance of commercial downstream catalysts as a function of the feedstock nitrogen concentration. Re-drawn from (Letzsch and Ashton, 1993).
Furthermore, nitrogen gas may contaminate the hydrogen product (Baumgartner et al., 2004). Therefore, efficient and accurate nitrogen monitoring for plastic pyrolysis oils is highly important. An example for a removal technique for nitrogen compounds is hydrodenitrogenation (Prado et al., 2017) according to the reaction equation below.
| (1) |
Blending of plastic waste pyrolysis products with fossil naphtha in order to tune the nitrogen level down to an acceptable concentration would require a minimum dilution factor of 12–17.
4.2.2. Sulfur
The detected sulfur concentrations in the respective light, medium and heavy fractions of plastic waste pyrolysis oil do not exceed the maximum allowable sulfur concentration of 500 ppm (Baumgartner et al., 2004). However, due to the fluctuations in the composition of plastic waste, the sulfur contents of the pyrolysis products need to be monitored carefully and sulfur removal processes such as hydrodesulfurization or selective oxidation might need to be applied prior to petrochemical processing (Khan and Al-Sayed, 2007). Furthermore, due to inaccurate measurements it is possible that the actual sulfur concentrations present in plastic waste pyrolysis oils are indeed higher. This has been shown by (Toraman et al., 2014) who measured >1000 ppm of sulfur using GC × GC-SCD. Considering a value of 1000 ppm, a dilution factor of 2 would be required to reach industrial feedstock specifications.
The influence of sulfur in steam cracking may be beneficial or harmful depending on the amount. On the one hand, sulfur has a beneficial effect in terms of controlling the formation of CO and CO2. The effect is based on the coverage of active nickel sites on the metal surface leading to a reduction of steam reforming reactions (Reyniers and Froment, 1995, Jakobi and Karduck, 2018, Sarris et al., 2017). However, the role of sulfur in terms of coke formation is controversial (Symoens et al., 2018, Patil et al., 2020). On the other hand, sulfur containing compounds may lead to the formation of hazardous substances, pose threats to the reactor material and have detrimental effects on the final product (Dhuyvetter et al., 2001, Depeyre et al., 1985). Thermal decomposition of sulfur compounds leads to the formation of hydrogen sulfide, carbon disulfide and SOx which pose a threat to the environment and are therefore subject to strict regulations (Jafarinejad, 2016). Moreover, the Ni-Cr-Fe alloys often used for the steam cracking coil may suffer from corrosive attacks by hydrogen sulfide (Reyniers and Froment, 1995). Therefore, it is crucial to control and monitor the elemental sulfur content of liquid hydrocarbon feedstocks.
4.2.3. Halogens
Fig. 15 indicates that the industrial threshold value of 3 ppm (Baumgartner et al., 2004, Alanazi et al., 2017) is exceeded substantially both by plastic waste pyrolysis oils including dechlorination pre-treatment (215 ppm in the light fraction, 38 ppm in the medium fraction) as well as without pre-treatment (615 ppm in the light fraction, 1457 ppm in the medium fraction). Thus, it can be concluded that upgrading steps of the pyrolysis products or improved dechlorination steps prior to pyrolysis are of paramount importance. A potential process to remove chlorine from pyrolysis products prior to further petrochemical processing is catalytic hydrotreatment (Lingaiah et al., 2001). The hydrodechlorination of chlorobenzene as a popular chlorinated hydrocarbon compound is shown in the reaction equation below.
| (2) |
Organic and inorganic chlorides cause corrosion of equipment such as metal surfaces, heat exchangers and downstream facilities (Gutzeit, 2000, Alanazi et al., 2017). Inorganic chlorides such as sodium chloride may originate from entrained salt water and in the case of plastic waste from the adsorption of salt which was in the packaged product. Sodium chloride is especially known for corrosion of austenic steel above temperatures of 500 °C (Baumgartner et al., 2004). Organic chlorides such as chloroform (CHCl3) and dichloromethane (CH2Cl2) are more difficult to detect and separate compared to inorganics due to their higher solubility in liquid hydrocarbons (Arjang et al., 2018, Alanazi et al., 2017). The main thermal decomposition product of organic chlorides is hydrogen chloride which may lead to severe corrosion in downstream facilities. Moreover, hydrogen chloride can form ammonium chloride with nitrogen which can cause blockages (Li et al., 2018). Finally, chloride contamination in products can lead to environmental concerns (Arjang et al., 2018). The extremely low threshold for chlorine indicates that essentially no PVC or salts are allowed in the plastic waste fractions. Therefore, both efficient PVC separation and washing techniques must be applied in modern waste sorting plants and dehalogenation techniques need to be integrated in plastic waste pyrolysis units. Furthermore, standardized high-quality analytic techniques such as GC × GC-AED are needed in order to establish pyrolysis oils from a chlorine point-of-view.
Next to the mentioned harmful effects of chlorine, bromine and fluorine may have similar negative effects regarding corrosion of heat exchanger surfaces and the formation of unwanted halogenated hydrocarbon products as mentioned by (Elliott et al., 1985). However, no threshold exists for industrial steam crackers regarding the Br concentration in the feedstocks. Nevertheless, the reported Br concentration of almost 2000 ppm in a medium sample (C5 – C22) as reported by (Brebu et al., 2005), indicates that Br is indeed a problematic contaminant which needs to be subject of further research. Several debromination techniques have been reviewed by (Shen et al., 2016). For fluorides, a maximum allowable value of < 2 ppm was reported by (Sundaram and Stancato, 2018), however, no fluorine was reported in plastic waste pyrolysis oils.
4.2.4. Oxygen
The oxygen concentration found in (PET-containing) plastic waste pyrolysis oils exceeds the threshold value for industrial steam crackers (100 ppm (Baumgartner et al., 2004)) by at least ten times. Therefore, a detailed characterization of the plastic waste pyrolysis oils prior to petrochemical processing with close monitoring of the oxygenate concentration is advised. Considering the inevitable PET content in polyolefinic plastic waste streams, the corresponding pyrolysis oils are most likely not feasible for industrial crackers regarding the oxygen content. When considering to tune the oxygen levels by diluting the plastic waste pyrolysis products with fossil naphtha, a minimum dilution factor of 7–13 would be necessary.
Oxygenates can diminish the value of steam cracking feedstocks due to the potential formation of methanol, formaldehyde and CO next to the formation of corrosive acids (Reid and Nowowiejski, 2003). Furthermore, organic acids formed in quench water systems or elemental oxygen which reacts with butadiene species to promote gum formation are strong drivers for process fouling and corrosion issues (Ristic et al., 2017, Li et al., 2007). Oxygen and its decomposition products (especially CO) can also be a poison to a number of catalysts downstream of the steam cracker. Furthermore, off-spec propylene can occur due to contamination with formed methanol. In ethylene and propylene product specifications, the elemental oxygen content is typically limited to 5 ppm (Reid and Nowowiejski, 2003). Therefore, removal of oxygen through catalytic processes such as hydrodeoxygenation is necessary (Zacher et al., 2014). Hydrodeoxygenation of the hydroxyl group of an oxygenated compound is schematically shown below.
| (3) |
Other oxygen removal technologies are presented elsewhere (Chuang et al., 2008).
4.2.5. Phosphorus
P is a contaminant of high concern in industrial crackers (Baumgartner et al., 2004). Phosphine (PH3) may poison hydrogenation catalysts, while elemental phosphorus leads to corrosion of surfaces (Sundaram and Stancato, 2018). However, it has been reported that phosphine in steam cracking feedstocks may lead to a reduction of coke formation by 35 % when aluminum containing alloys were used (Patil et al., 2019). For industrial steam crackers, a maximum phosphorous concentration of 500 ppb was reported (Baumgartner et al., 2004). This value was exceeded substantially by the P concentration of 498 ppm in a medium fraction as reported by (Miskolczi and Ateş, 2016).
4.3. Steam cracking feasibility of pyrolysis oils in terms of metals and inorganics
Metal contamination in steam cracking feedstocks may cause issues such as accumulation and blockages in process equipment, corrosion of metal surfaces as well as irreversible poisoning of pre-treatment or downstream catalysts. Furthermore, coke formation and fouling can be increased due to catalytic effects (López et al., 2010). An overview of contaminant levels found in plastic waste pyrolysis oils as well as the corresponding contaminant limits for industrial steam crackers are shown in Table 8. The listed metal concentrations are the ones found in the liquid fractions of the pyrolysis oils. Metals only found in distillation or pyrolysis residues are not listed.
Table 8.
Comparison of metal contamination levels in plastic pyrolysis oil and known contaminant limits for industrial crackers (values taken from Table 5).
(a) defined as carbon number up to C20 and boiling point up to 250 °C.
(b) defined as carbon number up to C30 and boiling point up to 450 °C.
(c) defined as carbon number > C30 and FBP > 450 °C.
It has to be noted that for some metals, which are typically not present in fossil-based feedstocks such as aluminum, chromium or titanium, no specifications exist. Since these metals may occur in plastic waste pyrolysis oils stemming from numerous additives and auxiliary materials used in plastic products, there is a knowledge gap still to be filled. Importantly, it is implied that the absence of a maximum specification does not mean that the contaminant is harmless. It indeed indicates that further research is needed targeted at metal contaminants predominantly occurring in plastic waste pyrolysis oils.
Furthermore, it is more likely that certain metals remained undetected by the respective research groups rather than that they are indeed absent. This is especially valid for metals such as arsenic, nickel and vanadium which are harmful already in ppb levels. Due to the extremely difficult detection of trace metals, it is therefore likely that the actually occurring metal contamination is substantially higher than reported. Again, the need for highly accurate analytic techniques such as ICP-MS is highlighted.
Fig. 17 compares the metal contaminants in the light and medium product fractions which are most interesting for steam cracking with the known feedstock specifications for industrial steam crackers. Metals for which no maximum allowable concentration is known are not included.
Fig. 17.
Concentrations of (a) Ca and Cu (in ppm) and (b) Fe, Na, Si and Pb (in ppb) in light and medium fractions of plastic waste pyrolysis oils vs. the thresholds for industrial steam crackers.
In the following sub-sections, the steam cracking feasibility of plastic waste pyrolysis oils is discussed based on the respective metal contaminants.
4.3.1. Aluminum
Aluminum contamination in plastic waste stems from foils and cans which were not separated from the polyolefinic plastic waste (Roosen et al., 2020). Since Al is not typically present in fossil feedstocks, no specification exists for industrial crackers. However, Al contamination in steam cracking has been related with furnace problems (Baumgartner et al., 2004). Aluminum contamination will be an issue in plastic waste pyrolysis oils which underlines the need for additional investigation in Al’s impact in steam cracking. Potential accumulation and catalytic effects must be monitored carefully.
4.3.2. Antimony
Sb is a metal which is frequently used as a synergist in form of antimony trioxide for brominated flame retardants (Montezin et al., 1997, Zhang and Horrocks, 2003). Substantial amounts of Sb between 105 and 189 ppm in medium fractions of plastic waste pyrolysis oils were reported by (Miskolczi et al., 2013) and (Miskolczi and Ateş, 2016). In both mentioned studies, Br was also found in the pyrolysis oils which shows that, indeed, Sb and Br are often associated with each other as additives and that both elements partly end up in the pyrolysis oils. Furthermore, in one of the studies, (Miskolczi et al., 2013) report an initial Sb concentration in the pyrolyzed waste of 749 ppm. The fact that with 105 ppm a large portion of this initial concentration ended up in the pyrolysis oil shows that Sb is a metal which cannot be neglected when considering pyrolysis oils of mixed plastic waste as steam cracker feedstocks. No limit value for the Sb concentration in steam cracker feedstock has been reported which can likely be explained by the fact that Sb seldom occurs in feedstocks of fossil origin. Furthermore, in the book chapter by (Kunzru, 2000) it was reported that antimony has a coke inhibiting effect in ethane cracking reactions. Addition of 100 ppm of an antimony salt lead to a coke reduction of 63 % (Kunzru, 2000). This finding indicates that Sb is a contaminant of lower concern for commercial steam crackers. However, it also shows that additional research is needed in light of the fact that Sb is likely to become increasingly important due to its presence in plastic waste.
4.3.3. Barium
Ba is another metal that is often used as additive, namely as a filler substance in form of BaSO4 among others and is added to a broad range of plastic products including PP and PE-based food packaging (Turner and Filella, 2020). (Cho et al., 2010) is the only group of authors who reported low amounts of Ba (1 ppm) in plastic waste pyrolysis oils using ICP-OES analysis. No industrial threshold value exists for Ba contamination in steam cracker feedstocks. However, the fact that it was detected in various consumer plastic products underlines that additional research is needed regarding potential harmful effects in steam cracking.
4.3.4. Calcium
Calcium is another metal which will be increasingly relevant in terms of plastic waste pyrolysis, since CaCO3 is a popular performance-enhancing additive (Ügdüler et al., 2020). Another possible source for Ca is the addition of Ca salts in order to bind organic acids forming when PET is thermally decomposed (Okuwaki et al., 2006). Calcium has been found to cause fouling and corrosion leading to a reported concentration limit in liquid steam cracker feedstocks of 0.5 ppm (Sundaram and Stancato, 2018). As can be seen in Fig. 17, the Ca concentration in the light and medium fractions of plastic waste pyrolysis oils exceed the maximum allowable concentration substantially. In order to reach an acceptable concentration, the light fraction of plastic waste pyrolysis oil needs to be diluted with naphtha by a factor of ∼ 34. It has been further shown that the Ca concentration in the light fraction is substantially lower compared to the medium fraction which indicated that higher upgrading effort or dilution is needed for heavier fractions. Considering the strong possibility that the reported Ca concentration is underestimated, steam cracking of these feedstocks is not feasible regarding the Ca concentration. Potential Ca removal techniques include aqueous solution using a calcium removal agent (Yoon and Jung, 2016).
4.3.5. Chromium
Chromium is a very important compound of the reactor coil metallurgy since a chromium oxide layer works as a protector against carbon diffusion and catalytic coking (Sundaram and Stancato, 2018). It was further stated that chromium is able to absorb unwanted sulfur oxides aiding to maintain the sulfur oxide concentration in the regeneration zone effluent gas stream (Tatterson and Vasalos, 1981). Therefore, it can be anticipated that trace amounts of chromium will not harm the process to a great extent. However, chromium is a potential component of pigments and dyes used in plastic products. Therefore, it needs to be monitored carefully since it will likely play a more important role in the future if steam cracking of plastic waste pyrolysis oil is established.
4.3.6. Copper
Copper has catalytic activity promoting dehydrogenation reactions of hydrocarbons which can substantially influence the character of the cracking reactions ultimately causing higher coking rates. A maximum allowable copper concentration of 50 ppm has been reported (Tatterson and Vasalos, 1981). With respect to Fig. 17 it can be stated that the reported Cu concentration in the light (0.6 ppm) and medium fraction (15.1 ppm) of plastic waste pyrolysis oil are well below the given threshold. However, considering the likely underestimation due to insufficiently accurate detection techniques and the harmful impact of Cu careful monitoring is advised.
4.3.7. Iron
The catalytic effect of iron attached to the reactor wall surface may induce rapid coke formation causing a reduction of the furnace run length. It has been reported that the run length of an industrial liquid feedstock cracker was halved within days when exposed to iron contamination (Orriss, 1996). Iron further causes corrosion and (pre-treatment) catalyst poisoning issues. Especially iron oxide has been listed as a high concern contaminant due to plugging issues (Baumgartner et al., 2004). The maximum tolerable concentration in steam cracking feedstocks has been reported as < 1 ppb, underlining the importance of highly accurate measurements (Sundaram and Stancato, 2018). It can be seen from Fig. 17 that while no iron was reported in the light fraction of the pyrolysis oil, the concentration in the medium fractions exceeds the industrial threshold by orders of magnitude. To comply with the industrial threshold value, the most accurate metal detection technique ICP-MS is needed. This can be seen by means of the limits of quantification of other techniques which are already substantially higher than 1 ppb, indicating that the actual iron concentration in plastic waste pyrolysis oils is always higher than the threshold value. Considering the large iron concentration in the medium fraction, blending of the pyrolysis oils with iron-free naphtha will not be feasible due to the extremely high dilution necessary to reach the given specification. Instead, metal removal techniques are needed such as hydrodemetallization, solvent extraction (Ali and Abbas, 2006) or membrane filtration (Ates and Uzal, 2018).
4.3.8. Potassium
Potassium can be used as an additive in plastic composites (Yu et al., 2000, Tjong and Meng, 1998a, Tjong and Meng, 1998b). Potassium contamination was reported by (Velghe et al., 2011), who detected a K concentration of 20–200 ppm in a C8 – C28 fraction of a plastic waste pyrolysis oil. The original K content of the pyrolyzed waste was 1200 ppm indicating that indeed a considerable amount of the original potassium content may end up in the plastic waste pyrolysis oil. However, the fact that only one group of authors reported K as a contaminant does not allow a final judgement of the importance of this particular element. Nevertheless, potassium has been associated with radiant coil corrosion and fouling in steam cracking, leading to a maximum acceptable concentration of < 0.5 ppmw (Sundaram and Stancato, 2018) which is substantially exceeded by the value of 20–200 ppm as reported by (Velghe et al., 2011). Therefore, careful monitoring of the K concentration in both plastic waste as well as in the pyrolysis oil is advised.
4.3.9. Sodium
Sodium contamination may occur in liquid feeds of fossil origin following desalting of crudes or from certain storage modes, for instance in salt caverns. In plastic waste it occurs due to absorption of salts present in the packaged goods. Na can cause severe corrosion issues which is called sodium attack (Orriss, 1996). Na reacts with Cr in the steam cracking coil, forming compounds such as sodium chromite (NaCrO2) which has a melting point of 762 °C. By this mechanism, the chromium layer of the coil will gradually be depleted, permanently damaging the radiant coil (Sundaram and Stancato, 2018). Furthermore, Na is a strong catalyst poison (Letzsch and Ashton, 1993). This can pose severe problems to potential pre-treatment catalysts. Moreover, Na is a strong coke promoter (Brayden et al., 2006). Due to the listed issues, Na is among the top five problematic contaminants for liquid crackers with a maximum tolerable concentration of 1 ppm. Even lower concentrations have been reported by other authors, namely 125 ppb (Sundaram and Stancato, 2018). A Na concentration of 800 ppb was reported in the light fraction of plastic waste pyrolysis oil. This implies that a minimum blending ratio of ∼ 15 wt% in naphtha can be sufficient to reach the specifications. However, due to the very low threshold, data must be treated with care and additional analyses are advised.
4.3.10. Lead
Pb containing compounds in steam cracker feedstocks are not desirable due to their thermally induced decomposition into elemental Pb which deposits irreversibly on surfaces of potential pre-treatment catalysts (Audeh, 1985, Griffing et al., 1957). Additionally, Pb induced corrosion problems have been reported (Sundaram and Stancato, 2018). Several operators of liquid feedstock crackers named values below 100 ppb as maximum tolerable Pb level in the feedstock (Baumgartner et al., 2004). Other authors reported values < 50 ppb (Sundaram and Stancato, 2018). (Okuwaki et al., 2006) reported a Pb concentration of 37 ppm in the heavy fraction and concentrations below the limit of detection in the medium and light fraction of the plastic waste pyrolysis oil. (Cho et al., 2010), reported a Pb concentration in a light pyrolysis oil fraction (C4 – C20) of 40 ppb which shows that the concentrations in the light fractions are substantially lower than in the heavy fractions. This observation is promising regarding distillation of the crude pyrolysis oils as a first treatment step, as it was reported by (Cho et al., 2010) that the distillation residue contained up to 21.5 ppm of Pb. Since the maximum Pb threshold is in the low ppb range, it can, nevertheless, be assumed that traces of Pb in the lighter fractions were not detected by some authors due to limited detector capacities.
4.3.11. Silicon
Silicon is another relevant contaminant in terms of plastic waste due its use in additives and coatings for plastic products (Ügdüler et al., 2020). Silicon has been known to poison catalysts irreversibly (Gazulla et al., 2017). Furthermore, silicon dioxide is known to cause fouling (Reid and Nowowiejski, 2003). The maximum tolerable concentration in liquid feedstocks has been reported to be between 0.5 and 1 ppm (Sundaram and Stancato, 2018, Baumgartner et al., 2004). (Okuwaki et al., 2006) reported a Si concentration of 4 ppm in the medium fraction which exceeds the threshold by far. Furthermore, the reported value for the light fraction is < 100 ppm which indicates that the accuracy of the used analytical method is insufficient. However, based on the found concentration a dilution factor of 4–8 would be sufficient.
4.3.12. Titanium
In the light and medium product fractions of pyrolysis oils, no titanium was reported. However, (Miskolczi et al., 2004) reported a Ti concentration of 456 ppm in the heavy fraction which indicates that indeed a substantial amount of titanium might be present. Since Ti is a prominent component of pigments and dyes, this particular metal might become more relevant in the future (Ügdüler et al., 2020). Titanium has been used as matrix material for cracking catalysts and therefore poses catalytic activity which may influence the cracking reactions.
4.3.13. Zinc
Zinc is an element frequently used in plastic additives such as stabilizers, flame retardants, lubricants and slip agents (Ügdüler et al., 2020). In the light pyrolysis oil fractions, an average Zn concentration of 0.4 ppm was reported (Cho et al., 2010, Okuwaki et al., 2006). In the middle fraction, 124 ppm were reported by (Miskolczi et al., 2013) and in the heavy fraction, a concentration of 254 ppm was reported (Miskolczi et al., 2004) which indicates that there is indeed a substantial amount of Zinc present in plastic waste pyrolysis products. Again, the discrepancy between the respective light, medium and heavy fractions indicates that distillation as a first upgrading step is suitable to already remove certain metals. (Baumgartner et al., 2004) listed Zn as catalyst poison of limited concern. Zinc has also been used as a passivating agent for the treatment of metal contaminants in fluidized catalytic cracking (FCC) feedstock diminishing the negative impact of metals such as nickel, copper, vanadium and iron (Hirschberg et al., 1982). No further negative impacts caused by zinc contamination in petrochemical feedstocks have been reported.
4.3.14. Effect of metal contaminants not reported in pyrolysis oils
Next to the metals found in plastic waste pyrolysis oils, other metals are known to have partly severe impacts on steam crackers. In the following, the most important metals are addressed briefly.
Traces of As in the ppb range may cause irreversible poisoning of downstream or pre-treatment catalysts (Brandão et al., 2006, Powers et al., 1959). AsH3 (Arsine) is extremely poisonous and might stem from dry gas from an FCC unit or from the feedstock itself. Arsine is a strong poison for methylacetylene/propadiene (MAPD) catalysts (Baumgartner et al., 2004). The maximum tolerable concentration of As has been reported as 5 ppb (Sundaram and Stancato, 2018). Due to the fact that As was indeed reported in the pyrolysis residue (Okuwaki et al., 2006), potential entrainment of solid particles into the condensable pyrolysis product must be avoided.
Mercury may be present in crude oil in several different species depending on the sample source. A prominent suspended species is, for instance, mercuric sulfide (HgS), while the dissolved species are mostly elemental mercury or ionic halides (Wilhelm and Bloom, 2000). Furthermore, it can be present in form of organometallic complexes such as methyl mercury (Baumgartner et al., 2004). It has been reported that Hg is highly aggressive against aluminum heat exchangers causing a catastrophic shutdown at the Skikda facility in Algeria which was destroyed due to lowest amounts of Hg in natural gas (Boschee, 2013, Wilhelm, 2009) (see Fig. 18).
Fig. 18.
Skikda explosion, January 19, 2004 (Mashyanov, 2021).
Obviously, the release of toxic elements such as As, Hg and Pb into the environment is a further concern (Jai Kumar and Gangadharan, 1999). Due to the high toxicity of Hg, strict regulations regarding the emission are in place which is a significant concern regarding furnace maintenance. Mercury distributes among all product fractions with the highest presence in the propylene and butane/butylene product. It is further known to deposit on and deactivate MAPD catalysts. Maximum levels reported by several operators ranged below 10 ppb of Hg in the liquid steam cracker feedstock (Baumgartner et al., 2004). Other authors reported an even lower threshold of 5 ppb for liquid feedstocks (Sundaram and Stancato, 2018).
Nickel is one of the most prominent contaminants in fossil feedstocks predominantly found in the heavy fractions of crude oil, specifically in asphaltenes. Ni is known as a severe poison for downstream or pre-treatment catalysts (Tatterson and Vasalos, 1981, Groenenboom, 1989). Furthermore, Ni is known as an active dehydrogenation catalyst which can influence the cracking reaction to a certain extent, ultimately leading to higher coke formation. It has been reported that processing of a feedstock is not economically feasible if it contains >100 ppm of Ni (Letzsch and Ashton, 1993). Again, entrainment of solid pyrolysis residue which may contain traces of Ni as reported by (Okuwaki et al., 2006) must be avoided during pyrolysis.
Vanadium is a strong poison for downstream or pre-treatment catalysts. The catalyst deactivation tendency of V is known to be higher than the one of Ni (Letzsch and Ashton, 1993). The maximum tolerable concentration of V in naphtha feedstocks is as low as 50 ppb (Baumgartner et al., 2004). Experience from the pilot steam cracker at Ghent University in Belgium shows that V can deposit on the reactor wall and enhance the gasification of coke and hydrocarbons towards CO, CO2 and H2 (K.M. Van Geem, personal communication, February 11, 2021).
5. Implications for plastic waste pyrolysis oils as steam cracking feedstocks
It has been shown in the previous sections that (untreated) plastic waste pyrolysis oils are indeed not feasible to steam crack regarding several contaminants (see Table 9). However, it needs to be noted that the largest steam crackers require roughly 100,000 tons of feedstock per furnace with multiple furnaces in parallel operation. On the contrary, the largest waste sorting plants in Europe process 100,000 tons of collected waste per year of which only a fraction will be available for thermochemical recycling (PlasticsEurope, 2021). Therefore, using plastic waste pyrolysis oils as a drop-in feedstock in the range of 5–20 % is the most likely scenario.
Table 9.
Feasibility matrix of plastic waste pyrolysis oils as steam cracking feedstocks.
| Category | Recommended analytical method | Critical contaminant | Minimum dilution factor | Further treatment necessary? |
|---|---|---|---|---|
| Hydrocarbon composition | GC × GC-FID/ToF-MS | Olefins | 15–20 | |
| Aromatics | ∼2 | × | ||
| Heteroatoms | GC × GC-NCD | Nitrogen | 12–16 | |
| GC × GC-SCD | Sulfur | – | × | |
| GC × GC-AED | Oxygen | 7–13 | ||
| Chlorine | >12–485 | |||
| Metals | ICP-MS | Calcium | >34 | |
| Iron | >7000 | |||
| Sodium | >7 | × | ||
| Lead | 1->350 | |||
| Silicon | >4 | × |
The only contaminants which most likely do not require additional treatment are sulfur, sodium and silicon due to the fact that the dilution factor is sufficiently small. All other contaminants such as nitrogen, chlorine, calcium, iron and lead are exceeding the feedstock specifications for steam crackers to such an extent that additional upgrading technologies such as hydrotreatment are needed, which, of course, challenge the economic potential of plastic waste pyrolysis oil as steam cracking feedstock. Table 9 is a rough estimation due to the fact that it is based on partly inaccurate measurements with detection limits above the maximal allowable concentration. Therefore, the actual upgrading effort will likely be higher. However, it clearly shows which contaminants should have the highest priority regarding upgrading. In order to reach the feedstock specifications, catalytic hydrotreatment is the most promising technology capable of significantly reducing the concentrations of most contaminants. Typical catalysts contain Co-Mo, Ni-Mo as active metals on a Al2O3 support and hydrogen pressures of up to 200 bar and temperatures up to 450 °C are used. It has been shown in several studies that full removal of oxygen and nitrogen from liquid hydrocarbon samples is possible (Khan and Al-Sayed, 2007, Murena and Gioia, 1998, Zacher et al., 2014). Furthermore, Miller et al. (2006) reported a reduction of the chlorine content of a plastic waste pyrolysis oil using hydroprocessing with a HZSM-5 catalyst from an initial Cl concentration of 50–70 ppm to a final concentration of 2–8 ppm showing that hydrotreatment is a viable technique to reduce the chlorine content in the order of magnitude needed to comply with the mentioned Cl threshold of 3 ppm (Baumgartner et al., 2004). However, a challenge in hydrotreatment is the co-existence of a large set of contaminants that may inhibit the catalytic activity (Gioia and Murena, 1998, Murena and Gioia, 1998). Since hydrotreatment is a catalytic process, the issue of catalyst poisoning by metal contaminants present in the pyrolysis oils must be taken into account. For these issues, it is possible to install guard beds with mostly cheap aluminum oxide catalysts removing certain metal contaminants prior to hydrotreatment in order to avoid swift catalyst deactivation (Ali and Abbas, 2006, Van Dongen et al., 1980). It has been reported that a substantial metal removal is possible reducing the concentrations of V, Ni, and Fe from an initial concentration of 80 ppm down to ∼ 8 ppm, however, still exceeding the threshold values for these metals which are in the ppb range (Baumgartner et al., 2004, Ali and Abbas, 2006).
Next to the removal of metals via hydroprocessing, other demetallization processes such as solvent extraction (Ali and Abbas, 2006), membrane filtration (Ates and Uzal, 2018) as well as acidic demetallization (Ali and Abbas, 2006, Blytas, 1978, Kukes and Battiste, 1985), have been performed and showed great potential for the full removal of metals. However, all mentioned processes introduce additional cost drivers, challenging the economic potential of plastic waste pyrolysis oils compared to conventional feedstocks. Therefore, dilution with fossil feedstocks will be needed to a certain extent in order to comply with all mentioned feedstock specifications. As already mentioned, it is likely that the metal contaminants can be removed to a certain extent by distillation which will be the first treatment step of plastic waste pyrolysis oils when being integrated into a petrochemical cluster.
Obviously, upgrading processes are part of every petrochemical cluster guaranteeing a stable feedstock quality (Matar and Hatch, 2001). Therefore, a comparison between pyrolysis oils and fossil feedstocks is not entirely fair (see Fig. 19).
Fig. 19.
Comparison of fossil feedstocks and plastic waste pyrolysis oils.
In a future integrated chemical recycling scheme (see Fig. 20), alternative technologies such as solvent treatment of contaminated plastic waste streams may be employed. Subsequent catalytic cracking and depolymerization steps may be used to produce a broad range of important chemicals. This way, energy intensive conversion to platform chemicals via conventional petrochemical routes can be avoided. Furthermore, pyrolysis of the waste residue can be used for the production of carbon nanotubes. Finally, downcycling and disposal in landfills can be avoided entirely while energy recovery via incineration can be reduced to a minimum.
Fig. 20.
Plastic waste recycling scheme of the future.
6. Conclusion and outlook
Plastic waste pyrolysis and subsequent steam cracking of the pyrolysis oil has the potential to be an economically attractive and sustainable technique for the recycling of plastic waste. However, the composition of plastic waste pyrolysis oil significantly differs from liquid fossil-based steam cracking feedstocks. Contaminant levels exceed established feedstock quality specifications by one or more orders of magnitude such as for nitrogen, chlorine and iron. All these contaminants are known to cause corrosion issues, increase coke formation, destroy expensive reactor tubes or deactivate catalysts in the separation sections of a steam cracker. Even the typical amounts of olefins, oxygenates and aromatics found in plastic waste pyrolysis oils are substantially off-spec. In a nutshell, today the quality of crude plastic waste pyrolysis oils is unacceptable as feedstocks for industrial steam crackers. In order to close the quality gap between conventional fossil feedstocks and plastic waste pyrolysis oils, thorough upgrading is needed using, for instance, hydrotreatment.
However, due to the enormous scale of operation of industrial steam crackers which exceeds the availability of sorted plastic waste by far, a steam cracker will unlikely run on 100 % plastic waste feedstock but rather use blends with fossil feedstocks, thereby reducing the contaminant levels. Furthermore, improved automation and standardization in waste sorting plants will help to systematically purify the polyolefinic waste from other polymers thereby reducing a main source of contaminants such as nitrogen, chlorine and oxygen.
A key aspect in establishing plastic waste pyrolysis oils as steam cracking feedstocks is detailed characterization together with a better understanding of the impact of contaminants on process fouling and coke formation. Quality standards which are based on standardized methods to assess important feedstock properties such as chlorine or nitrogen concentrations and the most important metals are needed. Removing the uncertainty for plant operators and thus the associated risk of using these alternative synthetic feedstocks instead of conventional fossil-based ones is needed in order to overcome the hurdles of thermochemical plastic waste recycling.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge financial support by the Catalisti ICON project MATTER (Mechanical and Thermochemical Recycling of mixed plastic waste), the Catalisti SBO project WATCH (Plastic Waste to Chemicals), the Fund for Scientific Research Flanders (FWO) project WASTE and the Catalisti Moonshot project PREFER (The Plastics Refinery: No More Waste). Furthermore, Kevin M. Van Geem is holder of the ERC Grant OPTIMA (Process Intensification and Innovation in Olefin Production by Multiscale Analysis and Design) with the grant agreement ID 818607.
References
- Adahchour, M., Beens, J. & Brinkman, U. a. T. 2008. Recent developments in the application of comprehensive two-dimensional gas chromatography. J. Chromatogr A, 1186(1), 67–108, https://doi.org/10.1016/j.chroma.2008.01.002. [DOI] [PubMed]
- Adahchour, M., Beens, J., Vreuls, R. J. J. & Brinkman, U. a. T. 2006. Recent developments in comprehensive two-dimensional gas chromatography (GC×GC): I. Introduction and instrumental set-up. TrAC, Trends Anal Chem, 25(5), 438-454, https://doi.org/10.1016/j.trac.2006.03.002.
- Adam F., Bertoncini F., Brodusch N., Durand E., Thiébaut D., Espinat D., Hennion M.-C. New benchmark for basic and neutral nitrogen compounds speciation in middle distillates using comprehensive two-dimensional gas chromatography. J. Chromatogr. A. 2007;1148(1):55–64. doi: 10.1016/j.chroma.2007.01.142. [DOI] [PubMed] [Google Scholar]
- Adam F., Bertoncini F., Dartiguelongue C., Marchand K., Thiébaut D., Hennion M.-C. Comprehensive two-dimensional gas chromatography for basic and neutral nitrogen speciation in middle distillates. Fuel. 2009;88(5):938–946. doi: 10.1016/j.fuel.2008.11.032. [DOI] [Google Scholar]
- Aguado R., Olazar M., San José M.J., Gaisán B., Bilbao J. Wax Formation in the Pyrolysis of Polyolefins in a Conical Spouted Bed Reactor. Energy Fuels. 2002;16(6):1429–1437. doi: 10.1021/ef020043w. [DOI] [Google Scholar]
- Al-Momani I.F. Trace elements in atmospheric precipitation at Northern Jordan measured by ICP-MS: acidity and possible sources. Atmos. Environ. 2003;37(32):4507–4515. doi: 10.1016/S1352-2310(03)00562-4. [DOI] [Google Scholar]
- Al-Salem S.M., Antelava A., Constantinou A., Manos G., Dutta A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW) J. Environ. Manage. 2017;197:177–198. doi: 10.1016/j.jenvman.2017.03.084. [DOI] [PubMed] [Google Scholar]
- Al-Salem S.M., Lettieri P., Baeyens J. The valorization of plastic solid waste (PSW) by primary to quaternary routes: From re-use to energy and chemicals. Prog. Energy Combust. Sci. 2010;36(1):103–129. doi: 10.1016/j.pecs.2009.09.001. [DOI] [Google Scholar]
- Alanazi, N., Adam, F. & Nagu, M. 2017. Organochloride Contamination in a Refinery Naphtha Hydrotreater Unit. Mater Perform, 56(10), 1-5, https://www.researchgate.net/profile/Nayef_Alanazi2/publication/325545109_Organochloride_Contamination_in_a_Refinery_Naphtha_Hydrotreater_Unit/links/5da59eb5a6fdcc8fc3533d68/Organochloride-Contamination-in-a-Refinery-Naphtha-Hydrotreater-Unit.pdf.
- Albright L.F., Crynes B.L., Corcoran W.H. Academic Press Inc; New York: 1983. Pyrolysis: Theory and Industrial Practice. [Google Scholar]
- Ali M.F., Abbas S. A review of methods for the demetallization of residual fuel oils. Fuel Process. Technol. 2006;87(7):573–584. doi: 10.1016/j.fuproc.2006.03.001. [DOI] [Google Scholar]
- Altgelt K.H., Hirsch E. GPC Separation and Integrated Structural Analysis of Petroleum Heavy Ends. Sep. Sci. 1970;5(6):855–862. doi: 10.1080/00372367008055545. [DOI] [Google Scholar]
- Amghizar I., Vandewalle L.A., Van Geem K.M., Marin G.B. New Trends in Olefin Production. Engineering. 2017;3(2):171–178. doi: 10.1016/J.ENG.2017.02.006. [DOI] [Google Scholar]
- Anderson-Wile A.M., Edson J.B., Coates G.W. In: Polymer Science: A Comprehensive Reference. Matyjaszewski K., Möller M., editors. Elsevier; Amsterdam: 2012. 3.23 - Living Transition Metal-Catalyzed Alkene Polymerization: Polyolefin Synthesis and New Polymer Architectures; pp. 739–778. [Google Scholar]
- Anene A.F., Fredriksen S.B., Sætre K.A., Tokheim L.-A. Experimental Study of Thermal and Catalytic Pyrolysis of Plastic Waste Components. Sustainability. 2018;10(11):3979. doi: 10.3390/su10113979. [DOI] [Google Scholar]
- Angyal A., Miskolczi N., Bartha L. Petrochemical feedstock by thermal cracking of plastic waste. J. Anal. Appl. Pyrolysis. 2007;79(1):409–414. doi: 10.1016/j.jaap.2006.12.031. [DOI] [Google Scholar]
- Anuar Sharuddin S.D., Abnisa F., Wan Daud W.M.A., Aroua M.K. Energy recovery from pyrolysis of plastic waste: Study on non-recycled plastics (NRP) data as the real measure of plastic waste. Energy Convers. Manage. 2017;148:925–934. doi: 10.1016/j.enconman.2017.06.046. [DOI] [Google Scholar]
- Apicella B., Millan M., Herod A.A., Carpentieri A., Pucci P., Ciajolo A. Separation and measurement of flame-formed high molecular weight polycyclic aromatic hydrocarbons by size-exclusion chromatography and laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2006;20(7):1104–1108. doi: 10.1002/rcm.2419. [DOI] [PubMed] [Google Scholar]
- Arabiourrutia M., Elordi G., Lopez G., Borsella E., Bilbao J., Olazar M. Characterization of the waxes obtained by the pyrolysis of polyolefin plastics in a conical spouted bed reactor. J. Anal. Appl. Pyrolysis. 2012;94:230–237. doi: 10.1016/j.jaap.2011.12.012. [DOI] [Google Scholar]
- Arjang S., Motahari K., Saidi M. Experimental and Modeling Study of Organic Chloride Compounds Removal from Naphtha Fraction of Contaminated Crude Oil Using Sintered γ-Al2O3 Nanoparticles: Equilibrium, Kinetic, and Thermodynamic Analysis. Energy Fuels. 2018;32(3):4025–4039. doi: 10.1021/acs.energyfuels.7b03845. [DOI] [Google Scholar]
- Arnold J.C., Watson T., Alston S., Carnie M., Glover C. The use of FTIR mapping to assess phase distribution in mixed and recycled WEEE plastics. Polym. Test. 2010;29(4):459–470. doi: 10.1016/j.polymertesting.2010.02.006. [DOI] [Google Scholar]
- Asomaning S. The University of British Columbia; Master of Applied Sciences: 1990. The Role of Olefins in Fouling of Heat Exchangers. [Google Scholar]
- Ates N., Uzal N. Removal of heavy metals from aluminum anodic oxidation wastewaters by membrane filtration. Environ. Sci. Pollut. Res. 2018;25(22):22259–22272. doi: 10.1007/s11356-018-2345-z. [DOI] [PubMed] [Google Scholar]
- Audeh C.A. Removal of lead alkyl contaminants from naphtha reformer feed. Ind. Eng. Chem. Prod. Res. Dev. 1985;24(4):666–668. doi: 10.1021/i300020a031. [DOI] [Google Scholar]
- Baena-González J., Santamaria-Echart A., Aguirre J.L., González S. Chemical recycling of plastic waste: Bitumen, solvents, and polystyrene from pyrolysis oil. Waste Manage. 2020;118:139–149. doi: 10.1016/j.wasman.2020.08.035. [DOI] [PubMed] [Google Scholar]
- Bailey, M. P. 2020. Neste successfully processes liquefied plastic waste at industrial scale [Online]. Available: https://www.chemengonline.com/neste-successfully-processes-liquefied-plastic-waste-at-industrial-scale/ [Accessed 04/01/2021].
- Ballice L., Reimert R. Classification of volatile products from the temperature-programmed pyrolysis of polypropylene (PP), atactic-polypropylene (APP) and thermogravimetrically derived kinetics of pyrolysis. Chem. Eng. Process. 2002;41(4):289–296. doi: 10.1016/S0255-2701(01)00144-1. [DOI] [Google Scholar]
- Barontini F., Marsanich K., Petarca L., Cozzani V. The Thermal Degradation Process of Tetrabromobisphenol A. Ind. Eng. Chem. Res. 2004;43(9):1952–1961. doi: 10.1021/ie034017c. [DOI] [Google Scholar]
- Bartle K.D., Hall S.R., Holden K., Mitchell S.C., Ross A.B. Analysis of oxygen-containing polycyclic aromatic compounds by gas chromatography with atomic emission detection. Fuel. 2009;88(2):348–353. doi: 10.1016/j.fuel.2008.08.014. [DOI] [Google Scholar]
- Baumgartner, A. J., Blaschke, M. W., Coleman, S. T., Kohler, R. & Paxson, T. E. Feedstock Contaminants in Ethylene Plants - an Update. AIChE Spring National Meeting, Ethylene Producers Conference, April 25-29 2004 New Orleans, Louisiana.
- Beens, J., Adahchour, M., Vreuls, R. J. J., Van Altena, K. & Th. Brinkman, U. A. 2001. Simple, non-moving modulation interface for comprehensive two-dimensional gas chromatography. J Chromatogr A, 919(1), 127-132, https://doi.org/10.1016/S0021-9673(01)00785-3. [DOI] [PubMed]
- Beens J., Boelens H., Tijssen R., Blomberg J. Quantitative Aspects of Comprehensive Two-Dimensional Gas Chromatography (GC×GC) J. High Resolut. Chromatogr. 1998;21(1):47–54. doi: 10.1002/(sici)1521-4168(19980101)21:1<47::Aid-jhrc47>3.0.Co;2-5. [DOI] [Google Scholar]
- Beens J., Tijssen R., Blomberg J. Prediction of comprehensive two-dimensional gas chromatographic separations: A theoretical and practical exercise. J. Chromatogr. A. 1998;822(2):233–251. doi: 10.1016/S0021-9673(98)00649-9. [DOI] [Google Scholar]
- Bhaskar T., Hall W.J., Mitan N.M.M., Muto A., Williams P.T., Sakata Y. Controlled pyrolysis of polyethylene/polypropylene/polystyrene mixed plastics with high impact polystyrene containing flame retardant: Effect of decabromo diphenylethane (DDE) Polym. Degrad. Stab. 2007;92(2):211–221. doi: 10.1016/j.polymdegradstab.2006.11.011. [DOI] [Google Scholar]
- Biedermann M., Grob K. Comprehensive two-dimensional GC after HPLC preseparation for the characterization of aromatic hydrocarbons of mineral oil origin in contaminated sunflower oil. J. Sep. Sci. 2009;32(21):3726–3737. doi: 10.1002/jssc.200900366. [DOI] [PubMed] [Google Scholar]
- Blazso M. In: Feedstock Recycling and Pyrolysis of Waste Plastics. Scheirs J., Kaminsky W., editors. John Wiley & Sons Ltd; 2006. Composition of Liquid Fuels Derived from the Pyrolysis of Plastics; pp. 314–344. [Google Scholar]
- Blomberg J., Riemersma T., Zuijlen M.V., Chaabani H. Comprehensive two-dimensional gas chromatography coupled with fast sulphur-chemiluminescence detection: implications of detector electronics. J. Chromatogr. A. 2004;1050(1):77–84. doi: 10.1016/j.chroma.2004.07.105. [DOI] [PubMed] [Google Scholar]
- Booij, K., Theo J. Hillebrand, M. & M. Van Weerlee, E. 1998. Calibration of the electron-capture detector for the determination of polychlorinated biphenyls. Analyst, 123(3), 415-420, https://doi.org/10.1039/A706157I.
- Borsodi, N., Miskolczi, N., Angyal, A., Bartha, L., Kohán, J. & Lengyel, A. Hydrocarbons obtained by pyrolysis of contaminated waste plastics. 45th International Petroleum Conference, Bratislava, Slovak Republic, 2011.
- Blytas G.C. Process for demetallizing of heavy hydrocarbons. US Patent. 1978;US 4116820 [Google Scholar]
- Boschee P. Advancements in the removal of Mercury from crude oil. J Oil & Gas Facilities. 2013;2(02), 12–17:2224–4514. [Google Scholar]
- Bousso, R. & Sanicola, L. 2020. Facing wave of closures, oil refiners turn to biofuels [Online]. Reuters. Available: https://www.reuters.com/article/uk-europe-refining-idUKKBN2741B3 [Accessed 07/10/2021].
- Bouyssiere B., Szpunar J., Lespes G., Lobinski R. Gas Chromatography with Inductively Coupled Plasma Mass Spectrometric Detection (GC-ICP-MS) Adv. Chromatogr. 2003;42:107–137. doi: 10.1201/9780203911266.ch3. [DOI] [PubMed] [Google Scholar]
- Brandão G.P., De Campos R.C., Luna A.S., De Castro E.V.R., De Jesus H.C. Determination of arsenic in diesel, gasoline and naphtha by graphite furnace atomic absorption spectrometry using microemulsion medium for sample stabilization. Anal. Bioanal. Chem. 2006;385(8):1562. doi: 10.1007/s00216-006-0585-0. [DOI] [PubMed] [Google Scholar]
- Brayden, M., Wines, T. H. & Del Giudice, K. Improve Steam Cracking Furnace Productivity and Emissions Control through Filtration and Coalescence. AIChE Spring National Meeting, Ethylene Producers Conference, April 23 – 27 2006 Orlando, Florida.
- Brebu M., Bhaskar T., Murai K., Muto A., Sakata Y., Uddin M.A. Removal of nitrogen, bromine, and chlorine from PP/PE/PS/PVC/ABS–Br pyrolysis liquid products using Fe- and Ca-based catalysts. Polym. Degrad. Stab. 2005;87(2):225–230. doi: 10.1016/j.polymdegradstab.2004.08.008. [DOI] [Google Scholar]
- Budiman, H., Nuryatini & Zuas, O. 2015. Comparison between GC-TCD and GC-FID for the determination of propane in gas mixture. Procedia Chem, 16, 465-472, https://doi.org/10.1016/j.proche.2015.12.080.
- Buekens A.G. In: Feedstock Recycling and Pyrolysis of Waste Plastics. Scheirs J., Kaminsky W., editors. John Wiley & Sons Ltd; 2006. Introduction to Feedstock Recycling of Plastics; pp. 1–41. [Google Scholar]
- Butler E., Devlin G., Mcdonnell K. Waste Polyolefins to Liquid Fuels via Pyrolysis: Review of Commercial State-of-the-Art and Recent Laboratory Research. Waste Biomass Valorization. 2011;2(3):227–255. doi: 10.1007/s12649-011-9067-5. [DOI] [Google Scholar]
- Camacho W., Karlsson S. NIR, DSC, and FTIR as quantitative methods for compositional analysis of blends of polymers obtained from recycled mixed plastic waste. Polym. Eng. Sci. 2001;41(9):1626–1635. doi: 10.1002/pen.10860. [DOI] [Google Scholar]
- Carné Sánchez A., Collinson S.R. The selective recycling of mixed plastic waste of polylactic acid and polyethylene terephthalate by control of process conditions. Eur. Polym. J. 2011;47(10):1970–1976. doi: 10.1016/j.eurpolymj.2011.07.013. [DOI] [Google Scholar]
- Charitopoulou M.A., Kalogiannis K.G., Lappas A.A., Achilias D.S. Novel trends in the thermo-chemical recycling of plastics from WEEE containing brominated flame retardants. Environ. Sci. Pollut. Res. 2020 doi: 10.1007/s11356-020-09932-5. [DOI] [PubMed] [Google Scholar]
- Charlesworth J.M. Monitoring the products and kinetics of oil shale pyrolysis using simultaneous nitrogen specific and flame ionization detection. Fuel. 1986;65(7):979–986. doi: 10.1016/0016-2361(86)90208-5. [DOI] [Google Scholar]
- Chen Z., Zhang X., Yang F., Peng H., Zhang X., Zhu S., Che L. Deactivation of a Y-zeolite based catalyst with coke evolution during the catalytic pyrolysis of polyethylene for fuel oil. Appl Catal A Gen. 2021;609 doi: 10.1016/j.apcata.2020.117873. [DOI] [Google Scholar]
- Cho M.-H., Jung S.-H., Kim J.-S. Pyrolysis of Mixed Plastic Wastes for the Recovery of Benzene, Toluene, and Xylene (BTX) Aromatics in a Fluidized Bed and Chlorine Removal by Applying Various Additives. Energy Fuels. 2010;24(2):1389–1395. doi: 10.1021/ef901127v. [DOI] [Google Scholar]
- Choi Y.S., Johnston P.A., Brown R.C., Shanks B.H., Lee K.-H. Detailed characterization of red oak-derived pyrolysis oil: Integrated use of GC, HPLC, IC, GPC and Karl-Fischer. J. Anal. Appl. Pyrol. 2014;110:147–154. doi: 10.1016/j.jaap.2014.08.016. [DOI] [Google Scholar]
- Chuang S.-Y., Nanda R., Rizopoulos J. Contaminants key to refinery offgas treatment unit design. Oil Gas J. 2008;106(35), 68–72:0030–1388. [Google Scholar]
- Çit İ., Sınağ A., Yumak T., Uçar S., Mısırlıoğlu Z., Canel M. Comparative pyrolysis of polyolefins (PP and LDPE) and PET. Polym. Bull. 2010;64(8):817–834. doi: 10.1007/s00289-009-0225-x. [DOI] [Google Scholar]
- Clases D., Ueland M., Gonzalez De Vega R., Doble P., Pröfrock D. Quantitative speciation of volatile sulphur compounds from human cadavers by GC-ICP-MS. Talanta. 2021;221 doi: 10.1016/j.talanta.2020.121424. [DOI] [PubMed] [Google Scholar]
- Dallüge, J., Beens, J. & Brinkman, U. a. T. 2003. Comprehensive two-dimensional gas chromatography: a powerful and versatile analytical tool. J Chromatogr A, 1000(1), 69-108, https://doi.org/10.1016/S0021-9673(03)00242-5. [DOI] [PubMed]
- Dao Thi H., Djokic M.R., Van Geem K.M. Detailed Group-Type Characterization of Plastic-Waste Pyrolysis Oils: By Comprehensive Two-Dimensional Gas Chromatography Including Linear, Branched, and Di-Olefins. Separations. 2021;8(7) doi: 10.3390/separations8070103. [DOI] [Google Scholar]
- Das P., Tiwari P. Valorization of packaging plastic waste by slow pyrolysis. Resour. Conserv. Recycl. 2018;128:69–77. doi: 10.1016/j.resconrec.2017.09.025. [DOI] [Google Scholar]
- De Amorim M.T.S.P., Comel C., Vermande P. Pyrolysis of polypropylene: I. Identification of compounds and degradation reactions. J. Anal. Appl. Pyrol. 1982;4(1):73–81. doi: 10.1016/0165-2370(82)80028-4. [DOI] [Google Scholar]
- De Hoffmann E. John Wiley & Sons; Kirk-Othmer Encyclopedia of Chemical Technology: 2000. Mass Spectrometry. [Google Scholar]
- De Meester S., Nachtergaele P., Debaveye S., Vos P., Dewulf J. Using material flow analysis and life cycle assessment in decision support: A case study on WEEE valorization in Belgium. Resour. Conserv. Recycl. 2019;142:1–9. doi: 10.1016/j.resconrec.2018.10.015. [DOI] [Google Scholar]
- De Saint Laumer J.-Y., Cicchetti E., Merle P., Egger J., Chaintreau A. Quantification in Gas Chromatography: Prediction of Flame Ionization Detector Response Factors from Combustion Enthalpies and Molecular Structures. Anal. Chem. 2010;82(15):6457–6462. doi: 10.1021/ac1006574. [DOI] [PubMed] [Google Scholar]
- De Witt M.J., Broadbelt L.J. Binary Interactions between High-Density Polyethylene and 4-(1-Naphthylmethyl)bibenzyl during Low-Pressure Pyrolysis. Energy Fuels. 2000;14(2):448–458. doi: 10.1021/ef990172l. [DOI] [Google Scholar]
- Dean J.R. Wiley; 2005. Practical Inductively Coupled Plasma Spectroscopy. [Google Scholar]
- Demirbas A. Pyrolysis of municipal plastic wastes for recovery of gasoline-range hydrocarbons. J. Anal. Appl. Pyrol. 2004;72(1):97–102. doi: 10.1016/j.jaap.2004.03.001. [DOI] [Google Scholar]
- Depeyre D., Flicoteaux C., Ossebi J.G. Pure n-nonane steam cracking and the influence of sulfur compounds. Ind. Eng. Chem. Process Des. Dev. 1985;24(4):920–924. doi: 10.1021/i200031a005. [DOI] [Google Scholar]
- Dhuyvetter I., Reyniers M.-F., Froment G.F., Marin G.B., Viennet D. The Influence of Dimethyl Disulfide on Naphtha Steam Cracking. Ind. Eng. Chem. Res. 2001;40(20):4353–4362. doi: 10.1021/ie001131b. [DOI] [Google Scholar]
- Dijkmans T., Djokic M.R., Van Geem K.M., Marin G.B. Comprehensive compositional analysis of sulfur and nitrogen containing compounds in shale oil using GC×GC – FID/SCD/NCD/TOF-MS. Fuel. 2015;140(1):398–406. doi: 10.1016/j.fuel.2014.09.055. [DOI] [Google Scholar]
- Dijkmans T., Van Geem K.M., Djokic M.R., Marin G.B. Combined Comprehensive Two-Dimensional Gas Chromatography Analysis of Polyaromatic Hydrocarbons/Polyaromatic Sulfur-Containing Hydrocarbons (PAH/PASH) in Complex Matrices. Ind. Eng. Chem. Res. 2014;53(40):15436–15446. doi: 10.1021/ie5000888. [DOI] [Google Scholar]
- Dinneen G.U., Bickel W.D. Gum Formation in Shale-Oil Naphtha. Ind. Eng. Chem. 1951;43(7):1604–1607. doi: 10.1021/ie50499a039. [DOI] [Google Scholar]
- Djokic M.R., Dijkmans T., Yildiz G., Prins W., Van Geem K.M. Quantitative analysis of crude and stabilized bio-oils by comprehensive two-dimensional gas-chromatography. J. Chromatogr. A. 2012;1257(1):131–140. doi: 10.1016/j.chroma.2012.07.035. [DOI] [PubMed] [Google Scholar]
- Djokic M.R., Muller H., Ristic N.D., Akhras A.R., Symoens S.H., Marin G.B., Van Geem K.M. Combined characterization using HT-GC × GC-FID and FT-ICR MS: A pyrolysis fuel oil case study. Fuel Process. Technol. 2018;182(1):15–25. doi: 10.1016/j.fuproc.2018.10.007. [DOI] [Google Scholar]
- Djokic M.R., Ristic N.D., Olahova N., Marin G.B., Van Geem K.M. Quantitative on-line analysis of sulfur compounds in complex hydrocarbon matrices. J. Chromatogr. A. 2017;1509(1):102–113. doi: 10.1016/j.chroma.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Doğan Ö.M., Kayacan İ. Pyrolysis of Low and High Density Polyethylene. Part II: Analysis of Liquid Products Using FTIR and NMR Spectroscopy. ENERG Part A. 2008;30(5):392–400. doi: 10.1080/15567030701457152. [DOI] [Google Scholar]
- Dogu, O., Pelucchi, M., Van De Vijver, R., Van Steenberge, P. H. M., D'hooge, D. R., Cuoci, A., Mehl, M., Frassoldati, A., Faravelli, T. & Van Geem, K. M. 2021. The chemistry of chemical recycling of solid plastic waste via pyrolysis and gasification: State-of-the-art, challenges, and future directions. Prog Energy Combust Sci, 84, 100901, https://doi.org/10.1016/j.pecs.2020.100901.
- Donaj P.J., Kaminsky W., Buzeto F., Yang W. Pyrolysis of polyolefins for increasing the yield of monomers' recovery. Waste Manag. 2012;32(5):840–846. doi: 10.1016/j.wasman.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Edwards J.C. Spectroscopic Analysis of Petroleum Products and Lubricants; ASTM International: 2011. A review of applications of NMR spectroscopy in the petroleum industry; p. 423. [Google Scholar]
- Eguchi A., Matsukami H., Takahashi A., Kajiwara N. Simultaneous determination of polybrominated diphenyl ethers and hexabromocyclododecane in plastic waste by short-column gas-chromatography-quadrupole mass spectrometry and electron capture detector. Chemosphere. 2021;277 doi: 10.1016/j.chemosphere.2021.130301. [DOI] [PubMed] [Google Scholar]
- Elliott P., Tyreman C.J., Prescott R. High Temperature Alloy Corrosion by Halogens. JOM. 1985;37(7):20–23. doi: 10.1007/BF03259691. [DOI] [Google Scholar]
- Epstein N. Thinking about Heat Transfer Fouling: A 5 × 5 Matrix. Heat Transf Eng. 1983;4(1):43–56. doi: 10.1080/01457638108939594. [DOI] [Google Scholar]
- Eschenbacher A., Varghese R.J., Abbas-Abadi M.S., Van Geem K.M. Maximizing light olefins and aromatics as high value base chemicals via single step catalytic conversion of plastic waste. Chem. Eng. J. 2022;428 doi: 10.1016/j.cej.2021.132087. [DOI] [Google Scholar]
- Fekhar B., Zsinka V., Miskolczi N. Value added hydrocarbons obtained by pyrolysis of contaminated waste plastics in horizontal tubular reactor: In situ upgrading of the products by chlorine capture. J Clean Prod. 2019;241 doi: 10.1016/j.jclepro.2019.118166. [DOI] [Google Scholar]
- Ferraz-Almeida R., Spokas K.A., De Oliveira R.C. Columns and Detectors Recommended in Gas Chromatography to Measure Greenhouse Emission and O2 Uptake in Soil: A Review. Commun. Soil Sci. Plant Anal. 2020;51(5):582–594. doi: 10.1080/00103624.2020.1729370. [DOI] [Google Scholar]
- Fitzsimons, S. 2020. Total And Plastic Energy Announce A Strategic Partnership And The Construction Of The First Chemical Recycling Plant In France [Online]. Available: https://plasticenergy.com/total-and-plastic-energy-announce-a-strategic-partnership-and-the-construction-of-the-first-chemical-recycling-plant-in-france/ [Accessed 04/01/2021 2020].
- Foppiano D., Tarik M., Schneebeli J., Calbry-Muzyka A., Biollaz S., Ludwig C. Siloxane compounds in biogas from manure and mixed organic waste: Method development and speciation analysis with GC-ICP-MS. Talanta. 2020;208 doi: 10.1016/j.talanta.2019.120398. [DOI] [PubMed] [Google Scholar]
- Frysinger G.S., Gaines R.B. Comprehensive Two-Dimensional Gas Chromatography with Mass Spectrometric Detection (GC × GC/MS) Applied to the Analysis of Petroleum. J. High Resolut. Chromatogr. 1999;22(5):251–255. doi: 10.1002/(sici)1521-4168(19990501)22:5<251::Aid-jhrc251>3.0.Co;2-v. [DOI] [Google Scholar]
- Gaile A.A., Zalishchevskii G.D., Gafur N.N., Semenov L.V., Varshavskii O.M., Fedyanin N.P., Koldobskaya L.L. Removal of Aromatic Hydrocarbons from Reforming Naphtha. Combined Extraction — Extractive-Azeotropic Distillation Process. Chem Technol Fuels Oils. 2004;40(4):215–221. doi: 10.1023/B:CAFO.0000041217.96743.22. [DOI] [Google Scholar]
- Gaines R.B., Frysinger G.S., Hendrick-Smith M.S., Stuart J.D. Oil Spill Source Identification by Comprehensive Two-Dimensional Gas Chromatography. Environ. Sci. Technol. 1999;33(12):2106–2112. doi: 10.1021/es9810484. [DOI] [Google Scholar]
- Garforth A.A., Ali S., Hernández-Martínez J., Akah A. Feedstock recycling of polymer wastes. Curr. Opin. Solid State Mater. Sci. 2004;8(6):419–425. doi: 10.1016/j.cossms.2005.04.003. [DOI] [Google Scholar]
- Gargiulo V., Apicella B., Alfè M., Russo C., Stanzione F., Tregrossi A., Amoresano A., Millan M., Ciajolo A. Structural Characterization of Large Polycyclic Aromatic Hydrocarbons. Part 1: The Case of Coal Tar Pitch and Naphthalene-Derived Pitch. Energy Fuels. 2015;29(9):5714–5722. doi: 10.1021/acs.energyfuels.5b01327. [DOI] [Google Scholar]
- Gary J.H., Handwerk G.E., Kaiser M.J. Fifth Edition. CRC Press; 2007. Petroleum Refining: Technology and Economics. [Google Scholar]
- Gazulla M.F., Rodrigo M., Orduña M., Ventura M.J., Andreu C. High precision measurement of silicon in naphthas by ICP-OES using isooctane as diluent. Talanta. 2017;164:563–569. doi: 10.1016/j.talanta.2016.12.023. [DOI] [PubMed] [Google Scholar]
- Geyer R., Jambeck J.R., Law K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017;3(7):1–5. doi: 10.1126/sciadv.1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia F., Murena F. Simultaneous catalytic hydroprocessing of chlorine-, nitrogen-, and sulphur-containing aromatic compounds. J Hazard Mater. 1998;57(1):177–192. doi: 10.1016/S0304-3894(97)00082-4. [DOI] [Google Scholar]
- Goto M. Chemical recycling of plastics using sub- and supercritical fluids. J. Supercrit. Fluids. 2009;47(3):500–507. doi: 10.1016/j.supflu.2008.10.011. [DOI] [Google Scholar]
- Grause G., Furusawa M., Okuwaki A., Yoshioka T. Pyrolysis of tetrabromobisphenol-A containing paper laminated printed circuit boards. Chemosphere. 2008;71(5):872–878. doi: 10.1016/j.chemosphere.2007.11.033. [DOI] [PubMed] [Google Scholar]
- Griffing M.E., Rozek A., Snyder L.J., Henderson S.R. Determination of Trace Amounts of Lead in Gasolines and Naphthas. Anal. Chem. 1957;29(2):190–195. doi: 10.1021/ac60122a005. [DOI] [Google Scholar]
- Groenenboom, C. J. 1989. Process for cracking metal-containing hydrocarbon feedstocks. US patent application.
- Guisnet M., Magnoux P. Organic chemistry of coke formation. Appl Catal A Gen. 2001;212(1):83–96. doi: 10.1016/S0926-860X(00)00845-0. [DOI] [Google Scholar]
- Gutzeit, J. 2000. Effect of Organic Chloride Contamination of Crude Oil on Refinery Corrosion. Corrosion 2000. Orlando, Florida: NACE International.
- Hall W.J., Williams P.T. Fast Pyrolysis of Halogenated Plastics Recovered from Waste Computers. Energy Fuels. 2006;20(4):1536–1549. doi: 10.1021/ef060088n. [DOI] [Google Scholar]
- Hall W.J., Williams P.T. Pyrolysis of brominated feedstock plastic in a fluidised bed reactor. J. Anal. Appl. Pyrol. 2006;77(1):75–82. doi: 10.1016/j.jaap.2006.01.006. [DOI] [Google Scholar]
- Hall W.J., Williams P.T. Removal of organobromine compounds from the pyrolysis oils of flame retarded plastics using zeolite catalysts. J. Anal. Appl. Pyrol. 2008;81(2):139–147. doi: 10.1016/j.jaap.2007.09.008. [DOI] [Google Scholar]
- Haraguchi H. Metallomics as integrated biometal science. J. Anal. At. Spectrom. 2004;19(1):5–14. doi: 10.1039/B308213J. [DOI] [Google Scholar]
- Herrera M., Matuschek G., Kettrup A. Main products and kinetics of the thermal degradation of polyamides. Chemosphere. 2001;42(5):601–607. doi: 10.1016/S0045-6535(00)00233-2. [DOI] [PubMed] [Google Scholar]
- Herrera M., Wilhelm M., Matuschek G., Kettrup A. Thermoanalytical and pyrolysis studies of nitrogen containing polymers. J. Anal. Appl. Pyrol. 2001;58–59:173–188. doi: 10.1016/S0165-2370(00)00193-5. [DOI] [Google Scholar]
- Hirschberg, E. H., Bertolacini, R. J. & Modica, F. S. 1982. Passivating metals on cracking catalysts with zinc. US patent application.
- Hoekstra E., Kersten S.R.A., Tudos A., Meier D., Hogendoorn K.J.A. Possibilities and pitfalls in analyzing (upgraded) pyrolysis oil by size exclusion chromatography (SEC) J. Anal. Appl. Pyrol. 2011;91(1):76–88. doi: 10.1016/j.jaap.2011.01.006. [DOI] [Google Scholar]
- Hua R., Li Y., Liu W., Zheng J., Wei H., Wang J., Lu X., Kong H., Xu G. Determination of sulfur-containing compounds in diesel oils by comprehensive two-dimensional gas chromatography with a sulfur chemiluminescence detector. J. Chromatogr. A. 2003;1019(1):101–109. doi: 10.1016/j.chroma.2003.08.048. [DOI] [PubMed] [Google Scholar]
- Ibrahim, H. a.-H. 2012. Fouling in Heat Exchangers. In: Katsikis, V. N. (ed.) MATLAB - A Fundamental Tool for Scientific Computing and Engineering Applications - Volume 3. BoD – Books on Demand.
- Ibrahim, H. a.-H., Safwat, A. & Hussamy, N. 2005. Investigation of the Fouling Mechanisms in the Heat Exchangers of a Hydrotreater. Engineering Journal of the University of Qatar, 18, 9-14, https://doi.org/10.13140/RG.2.1.2168.2648.
- Ioakeimidis C., Fotopoulou K.N., Karapanagioti H.K., Geraga M., Zeri C., Papathanassiou E., Galgani F., Papatheodorou G. The degradation potential of PET bottles in the marine environment: An ATR-FTIR based approach. Sci. Rep. 2016;6(1):23501. doi: 10.1038/srep23501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafarinejad, S. 2016. Control and treatment of sulfur compounds specially sulfur oxides (SOx) emissions from the petroleum industry: a review. Int J Chem, 2(4), 242-253, https://doi.org/10.31221/osf.io/bxmsr.
- Jai Kumar S., Gangadharan S. Determination of trace elements in naphtha by inductively coupled plasma mass spectrometry using water-in-oil emulsions. J. Anal. At. Spectrom. 1999;14(6):967–971. doi: 10.1039/A900894B. [DOI] [Google Scholar]
- Jakobi D., Karduck P. Corrosion; Phoenix, Arizona: 2018. Behavior of High-Temperature Tube Materials in Sulfur-Containing Steam-Cracking Conditions. [Google Scholar]
- Jin W., Shen D.K., Liu Q., Xiao R. Evaluation of the co-pyrolysis of lignin with plastic polymers by TG-FTIR and Py-GC/MS. Polym. Degrad. Stab. 2016;133:65–74. doi: 10.1016/j.polymdegradstab.2016.08.001. [DOI] [Google Scholar]
- Joo H.S., Guin J.A. Hydrocracking of a Plastics Pyrolysis Gas Oil to Naphtha. Energy Fuels. 1997;11(3):586–592. doi: 10.1021/ef960151g. [DOI] [Google Scholar]
- Juntarachat N., Bouvier N., Lepoutre J.-P., Roland A., Sainte-Beuve J., Granet F., Salmon J.-M., Rigou P., Chalier P. Identification by GC-O and GC-MS of new odorous compounds in natural rubber. J. Appl. Polym. Sci. 2013;130(3):1863–1872. doi: 10.1002/app.39356. [DOI] [Google Scholar]
- Kakuta Y., Hirano K., Sugano M., Mashimo K. Study on chlorine removal from mixture of waste plastics. Waste Manage. 2008;28(3):615–621. doi: 10.1016/j.wasman.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Kaminsky W. Chemical recycling of mixed plastics of pyrolysis. Adv. Polym. Tech. 1995;14(4):337–344. doi: 10.1002/adv.1995.060140407. [DOI] [Google Scholar]
- Kaminsky W., Kim J.-S. Pyrolysis of mixed plastics into aromatics. J. Anal. Appl. Pyrol. 1999;51(1):127–134. doi: 10.1016/S0165-2370(99)00012-1. [DOI] [Google Scholar]
- Kaminsky W., Predel M., Sadiki A. Feedstock recycling of polymers by pyrolysis in a fluidised bed. Polym. Degrad. Stab. 2004;85(3):1045–1050. doi: 10.1016/j.polymdegradstab.2003.05.002. [DOI] [Google Scholar]
- Kaminsky W., Schlesselmann B., Simon C.M. Thermal degradation of mixed plastic waste to aromatics and gas. Polym. Degrad. Stab. 1996;53(2):189–197. doi: 10.1016/0141-3910(96)00087-0. [DOI] [Google Scholar]
- Kaminsky W., Schmidt H., Simon C.M. Recycling of mixed plastics by pyrolysis in a fluidised bed. Macromol. Symp. 2000;152(1):191–199. doi: 10.1002/1521-3900(200003)152:1<191::AID-MASY191>3.0.CO;2-2. [DOI] [Google Scholar]
- Kannan P., Al Shoaibi A., Srinivasakannan C. Temperature Effects on the Yield of Gaseous Olefins from Waste Polyethylene via Flash Pyrolysis. Energy Fuels. 2014;28(5):3363–3366. doi: 10.1021/ef500516n. [DOI] [Google Scholar]
- Kawecki D., Scheeder P.R.W., Nowack B. Probabilistic Material Flow Analysis of Seven Commodity Plastics in Europe. Environ. Sci. Technol. 2018;52(17):9874–9888. doi: 10.1021/acs.est.8b01513. [DOI] [PubMed] [Google Scholar]
- Khan M.R., Al-Sayed E. Hydrocarbon Desulfurization to Clean Fuels by Selective Oxidation Versus Conventional Hydrotreating. Energ Source Part A. 2007;30(3):200–217. doi: 10.1080/02713680701638788. [DOI] [Google Scholar]
- Kicinski H.G., Adamek S., Kettrup A. Trace enrichment and HPLC analysis of polycyclic aromatic hydrocarbons in environmental samples, using solid phase extraction in connection with UV/VIS diode-array and fluorescence detection. Chromatographia. 1989;28(3):203–208. doi: 10.1007/BF02319648. [DOI] [Google Scholar]
- Kinghorn, R. M., Marriott, P. J. & Dawes, P. A. 1998. Longitudinal modulation studies for augmentation of injection and detection in capillary gas chromatography. J Microcolumn Sep, 10(7), 611-616, https://doi.org/10.1002/(sici)1520-667x(1998)10:7<611::Aid-mcs8>3.0.Co;2-j.
- Kiran N., Ekinci E., Snape C.E. Recyling of plastic wastes via pyrolysis. Resour. Conserv. Recycl. 2000;29(4):273–283. doi: 10.1016/S0921-3449(00)00052-5. [DOI] [Google Scholar]
- Kislov V.V., Sadovnikov A.I., Mebel A.M. Formation mechanism of polycyclic aromatic hydrocarbons beyond the second aromatic ring. J. Phys. Chem. A. 2013;117(23):4794–4816. doi: 10.1021/jp402481y. [DOI] [PubMed] [Google Scholar]
- Kleinhans K., Hallemans M., Huysveld S., Thomassen G., Ragaert K., Van Geem K.M., Roosen M., Mys N., Dewulf J., De Meester S. Development and application of a predictive modelling approach for household packaging waste flows in sorting facilities. Waste Manage. 2021;120:290–302. doi: 10.1016/j.wasman.2020.11.056. [DOI] [PubMed] [Google Scholar]
- Kopinke F.D., Zimmermann G., Reyniers G.C., Froment G.F. Relative rates of coke formation from hydrocarbons in steam cracking of naphtha. 2. Paraffins, naphthenes, mono-, di-, and cycloolefins, and acetylenes. Ind. Eng. Chem. Res. 1993;32(1):56–61. doi: 10.1021/ie00013a009. [DOI] [Google Scholar]
- Kopinke F.D., Zimmermann G., Reyniers G.C., Froment G.F. Relative rates of coke formation from hydrocarbons in steam cracking of naphtha. 3. Aromatic hydrocarbons. Ind. Eng. Chem. Res. 1993;32(11):2620–2625. doi: 10.1021/ie00023a027. [DOI] [Google Scholar]
- Krishna K., Kurakalva R.M., Satyanarayanan M. Non-destructive Sampling Method for Environmental and Geochemical Exploration Studies and XRF Determination of Cl, F, and Br. Atomic Spectroscopy -Norwalk Connecticut- 2012;33:108–116. [Google Scholar]
- Kukes S.G., Battiste D.R. Demetallization of heavy oils with phosphorous acid. US Patent. 1985;US 4522702 [Google Scholar]
- Kunzru D. Allied Publishers; 2000. Recent Advances In The Steam Cracking Of Hydrocarbons. Petroleum Refining and Petrochemical Based Industries in Eastern India. [Google Scholar]
- Kusch, P. 2017. Chapter 7 - Application of Pyrolysis-Gas Chromatography/Mass Spectrometry (Py-GC/MS). In: Rocha-Santos, T. a. P. & Duarte, A. C. (eds.) Comprehensive Analytical Chemistry. Elsevier, 169-207.
- Lee K.-H. Pyrolysis of municipal plastic wastes separated by difference of specific gravity. J. Anal. Appl. Pyrol. 2007;79(1):362–367. doi: 10.1016/j.jaap.2006.12.020. [DOI] [Google Scholar]
- Lee K.-H. Thermal and catalytic degradation of pyrolytic oil from pyrolysis of municipal plastic wastes. J. Anal. Appl. Pyrol. 2009;85(1):372–379. doi: 10.1016/j.jaap.2008.11.032. [DOI] [Google Scholar]
- Lee K.-H., Jeon S.-G., Kim K.-H., Noh N.-S., Shin D.-H., Park J., Seo Y., Yee J.-J., Kim G.-T. Thermal and catalytic degradation of waste high-density polyethylene (HDPE) using spent FCC catalyst. Korean J. Chem. Eng. 2003;20(4):693–697. doi: 10.1007/BF02706909. [DOI] [Google Scholar]
- Letzsch W.S., Ashton A.G. In: Studies in Surface Science and Catalysis. Magee J.S., Mitchell M.M., editors. Elsevier; 1993. Chapter 12 The Effect of Feedstock on Yields and Product Quality; pp. 441–498. [Google Scholar]
- Li, H., Mahyoub, S. a. A., Liao, W., Xia, S., Zhao, H., Guo, M. & Ma, P. 2017. Effect of pyrolysis temperature on characteristics and aromatic contaminants adsorption behavior of magnetic biochar derived from pyrolysis oil distillation residue. Bioresour Technol, 223, 20-26, https://doi.org/10.1016/j.biortech.2016.10.033. [DOI] [PubMed]
- Li J., Xia H., Wu Q., Hu Z., Hao Z., Zhu Z. Hydrocracking of the crude oil from thermal pyrolysis of municipal wastes over bi-functional Mo–Ni catalyst. Catal. Today. 2016;271:172–178. doi: 10.1016/j.cattod.2015.08.034. [DOI] [Google Scholar]
- Li, X., Wu, B. & Zhu, J. 2018. Hazards of Organic Chloride to Petroleum Processing in Chinese Refineries and Industrial Countermeasures. PPS, 2(3), https://doi.org/10.31031/PPS.2018.02.000539.
- Li, Y., Watkinson, P., Herrera, P., Fahiminia, F. & James, M. Formation of Gum and Deposits in an Oxygenated Naphtha Stream. ECI Symposium Series, Volume RP5: Proceedings of 7th International Conference on Heat Exchanger Fouling and Cleaning - Challenges and Opportunities, 2007 Tomar, Portugal. Engineering Conferences International.
- Libardoni M., Fix C., Waite J.H., Sacks R. Design and performance evaluation of a two-stage resistively-heated thermal modulator for GC × GC. Anal. Methods. 2010;2(7):936–943. doi: 10.1039/C0AY00090F. [DOI] [Google Scholar]
- Lienemann C.P., Dreyfus S., Pecheyran C., Donard O.F.X.J.O., Science G., Ifp T.-R. Trace Metal Analysis in Petroleum Products: Sample Introduction Evaluation in ICP-OES and Comparison with an ICP-MS Approach. Oil Gas Sci Technol. 2007;62(1):69–77. doi: 10.2516/ogst:2007006. [DOI] [Google Scholar]
- Lingaiah N., Uddin M.A., Muto A., Imai T., Sakata Y. Removal of organic chlorine compounds by catalytic dehydrochlorination for the refinement of municipal waste plastic derived oil. Fuel. 2001;80(13):1901–1905. doi: 10.1016/S0016-2361(01)00046-1. [DOI] [Google Scholar]
- Linge K.L. Trace Element Determination by ICP-AES and ICP-MS: Developments and Applications Reported During 2006 and 2007. Geostand Geoanalytical Res. 2008;32(4):453–468. doi: 10.1111/j.1751-908X.2008.00917.x. [DOI] [Google Scholar]
- Lissitsyna K., Huertas S., Quintero L.C., Polo L.M. Novel simple method for quantitation of nitrogen compounds in middle distillates using solid phase extraction and comprehensive two-dimensional gas chromatography. Fuel. 2013;104:752–757. doi: 10.1016/j.fuel.2012.08.054. [DOI] [Google Scholar]
- Liu G., Liao Y., Ma X. Thermal behavior of vehicle plastic blends contained acrylonitrile-butadiene-styrene (ABS) in pyrolysis using TG-FTIR. Waste Manage. 2017;61:315–326. doi: 10.1016/j.wasman.2017.01.034. [DOI] [PubMed] [Google Scholar]
- López A., De Marco I., Caballero B.M., Laresgoiti M.F., Adrados A. Pyrolysis of municipal plastic wastes: Influence of raw material composition. Waste Manage. 2010;30(4):620–627. doi: 10.1016/j.wasman.2009.10.014. [DOI] [PubMed] [Google Scholar]
- López A., De Marco I., Caballero B.M., Laresgoiti M.F., Adrados A. Dechlorination of fuels in pyrolysis of PVC containing plastic wastes. Fuel Process. Technol. 2011;92(2):253–260. doi: 10.1016/j.fuproc.2010.05.008. [DOI] [Google Scholar]
- López A., De Marco I., Caballero B.M., Laresgoiti M.F., Adrados A. Influence of time and temperature on pyrolysis of plastic wastes in a semi-batch reactor. Chem. Eng. J. 2011;173(1):62–71. doi: 10.1016/j.cej.2011.07.037. [DOI] [Google Scholar]
- López A., De Marco I., Caballero B.M., Laresgoiti M.F., Adrados A., Aranzabal A. Catalytic pyrolysis of plastic wastes with two different types of catalysts: ZSM-5 zeolite and Red Mud. Appl. Catal. B. 2011;104(3):211–219. doi: 10.1016/j.apcatb.2011.03.030. [DOI] [Google Scholar]
- Lopez G., Artetxe M., Amutio M., Bilbao J., Olazar M. Thermochemical routes for the valorization of waste polyolefinic plastics to produce fuels and chemicals A review. Renew Sust Energ Rev. 2017;73:346–368. doi: 10.1016/j.rser.2017.01.142. [DOI] [Google Scholar]
- Lorentz C., Laurenti D., Zotin J.L., Geantet C. Comprehensive GC×GC chromatography for the characterization of sulfur compound in fuels: A review. Catal. Today. 2017;292:26–37. doi: 10.1016/j.cattod.2017.04.052. [DOI] [Google Scholar]
- Luong J., Hua Y., Gras R., Shellie R.A. Uniformity and Sensitivity Improvements in Comprehensive Two-Dimensional Gas Chromatography Using Flame Ionization Detection with Post-Column Reaction. Anal. Chem. 2019;91(17):11223–11230. doi: 10.1021/acs.analchem.9b02159. [DOI] [PubMed] [Google Scholar]
- Ma C., Yu J., Wang B., Song Z., Xiang J., Hu S., Su S., Sun L. Chemical recycling of brominated flame retarded plastics from e-waste for clean fuels production: A review. Renew. Sust. Energ Rev. 2016;61:433–450. doi: 10.1016/j.rser.2016.04.020. [DOI] [Google Scholar]
- Maier C., Calafut T. In: Polypropylene. Maier C., Calafut T., editors. William Andrew Publishing; Norwich, NY: 1998. 3 - Additives; pp. 27–47. [Google Scholar]
- Marsman J.H., Wildschut J., Mahfud F., Heeres H.J. Identification of components in fast pyrolysis oil and upgraded products by comprehensive two-dimensional gas chromatography and flame ionisation detection. J. Chromatogr. A. 2007;1150(1):21–27. doi: 10.1016/j.chroma.2006.11.047. [DOI] [PubMed] [Google Scholar]
- Mashyanov N. Mercury in gas and oil deposits: corrosion problem. E3S Web of Conf. 2021;225:4. doi: 10.1051/e3sconf/202122501009. [DOI] [Google Scholar]
- Matar S., Hatch L.F. Elsevier Science; 2001. Chemistry of Petrochemical Processes. [Google Scholar]
- Mattsson C., Andersson S.-I., Belkheiri T., Åmand L.-E., Olausson L., Vamling L., Theliander H. Using 2D NMR to characterize the structure of the low and high molecular weight fractions of bio-oil obtained from LignoBoost™ kraft lignin depolymerized in subcritical water. Biomass Bioenergy. 2016;95:364–377. doi: 10.1016/j.biombioe.2016.09.004. [DOI] [Google Scholar]
- Mello P.A., Pereira J.S.F., Mesko M.F., Barin J.S., Flores E.M.M. Sample preparation methods for subsequent determination of metals and non-metals in crude oil—A review. Anal. Chim. Acta. 2012;746:15–36. doi: 10.1016/j.aca.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Mercader F.D.M., Groeneveld M.J., Kersten S.R.A., Venderbosch R.H., Hogendoorn J.A. Pyrolysis oil upgrading by high pressure thermal treatment. Fuel. 2010;89(10):2829–2837. doi: 10.1016/j.fuel.2010.01.026. [DOI] [Google Scholar]
- Meys R., Kätelhön A., Bachmann M., Winter B., Zibunas C., Suh S., Bardow A. Achieving net-zero greenhouse gas emission plastics by a circular carbon economy. Science. 2021;374(6563):71–76. doi: 10.1126/science.abg9853. [DOI] [PubMed] [Google Scholar]
- Miandad R., Barakat M.A., Aburiazaiza A.S., Rehan M., Ismail I.M.I., Nizami A.S. Effect of plastic waste types on pyrolysis liquid oil. Int. Biodeterior. Biodegrad. 2017;119:239–252. doi: 10.1016/j.ibiod.2016.09.017. [DOI] [Google Scholar]
- Miller S.J., Shah N., Huffmann G.P. Feedstock Recycling and Pyrolysis of Waste Plastics. John Wiley & Sons, Ltd; 2006. Production of Premium Oil Products from Waste Plastic by Pyrolysis and Hydroprocessing; pp. 345–362. [Google Scholar]
- Miranda R., Pakdel H., Roy C., Vasile C. Vacuum pyrolysis of commingled plastics containing PVC II Product analysis. Polym. Degrad. Stab. 2001;73(1):47–67. doi: 10.1016/s0141-3910(01)00066-0. [DOI] [Google Scholar]
- Miranda R., Yang J., Roy C., Vasile C. Vacuum pyrolysis of commingled plastics containing PVC I. Kinetic study. Polym Degrad Stab. 2001;72(3):469–491. doi: 10.1016/s0141-3910(01)00048-9. [DOI] [Google Scholar]
- Miskolczi N. Co-pyrolysis of petroleum based waste HDPE, poly-lactic-acid biopolymer and organic waste. J. Ind. Eng. Chem. 2013;19(5):1549–1559. doi: 10.1016/j.jiec.2013.01.022. [DOI] [Google Scholar]
- Miskolczi N., Angyal A., Bartha L., Valkai I. Fuels by pyrolysis of waste plastics from agricultural and packaging sectors in a pilot scale reactor. Fuel Process. Technol. 2009;90(7):1032–1040. doi: 10.1016/j.fuproc.2009.04.019. [DOI] [Google Scholar]
- Miskolczi N., Ateş F. Thermo-catalytic co-pyrolysis of recovered heavy oil and municipal plastic wastes. J. Anal. Appl. Pyrol. 2016;117:273–281. doi: 10.1016/j.jaap.2015.11.005. [DOI] [Google Scholar]
- Miskolczi N., Ateş F., Borsodi N. Comparison of real waste (MSW and MPW) pyrolysis in batch reactor over different catalysts. Part II: Contaminants, char and pyrolysis oil properties. Bioresour. Technol. 2013;144:370–379. doi: 10.1016/j.biortech.2013.06.109. [DOI] [PubMed] [Google Scholar]
- Miskolczi N., Bartha L. Investigation of hydrocarbon fractions form waste plastic recycling by FTIR, GC, EDXRFS and SEC techniques. J. Biochem. Bioph. Methods. 2008;70(6):1247–1253. doi: 10.1016/j.jbbm.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Miskolczi N., Bartha L., Deák G., Jóver B. Thermal degradation of municipal plastic waste for production of fuel-like hydrocarbons. Polym. Degrad. Stab. 2004;86(2):357–366. doi: 10.1016/j.polymdegradstab.2004.04.025. [DOI] [Google Scholar]
- Miskolczi N., Hall W.J., Angyal A., Bartha L., Williams P.T. Production of oil with low organobromine content from the pyrolysis of flame retarded HIPS and ABS plastics. J. Anal. Appl. Pyrol. 2008;83(1):115–123. doi: 10.1016/j.jaap.2008.06.010. [DOI] [Google Scholar]
- Miskolczi N., Hall W.J., Borsodi N., Williams P.T., Angyal A. A comparison of different analytical techniques (energy dispersive X-ray fluorescent spectrometry, bomb calorimetry combined with ion chromatography and inductively coupled plasma–optical emission spectrometry) for the determination of the bromine and antimony content of samples. Microchem. J. 2011;99(1):60–66. doi: 10.1016/j.microc.2011.03.009. [DOI] [Google Scholar]
- Mondello L., Casilli A., Tranchida P.Q., Dugo P., Costa R., Festa S., Dugo G. Comprehensive multidimensional GC for the characterization of roasted coffee beans. J. Sep. Sci. 2004;27(5–6), 442–450:1615–9306. doi: 10.1002/jssc.200301662. [DOI] [PubMed] [Google Scholar]
- Mondello L., Tranchida P.Q., Dugo P., Dugo G. Comprehensive two-dimensional gas chromatography-mass spectrometry: A review. Mass Spectrom. Rev. 2008;27(2):101–124. doi: 10.1002/mas.20158. [DOI] [PubMed] [Google Scholar]
- Monteiro N.K.V., Firme C.L. Hydrogen-Hydrogen Bonds in Highly Branched Alkanes and in Alkane Complexes: A DFT, ab initio, QTAIM, and ELF Study. J. Phys. Chem. A. 2014;118(9):1730–1740. doi: 10.1021/jp500131z. [DOI] [PubMed] [Google Scholar]
- Montezin F., Cuesta J.-M.L., Crespy A., Georlette P. Flame retardant and mechanical properties of a copolymer PP/PE containing brominated compounds/antimony trioxide blends and magnesium hydroxide or talc. Fire Mater. 1997;21(6):245–252. doi: 10.1002/(SICI)1099-1018(199711/12)21:6<245::AID-FAM616>3.0.CO;2-F. [DOI] [Google Scholar]
- Müller-Steinhagen, H. 2010. C4 Fouling of Heat Exchanger Surfaces. In: Vdi (ed.) VDI Heat Atlas. SpringerMaterials.
- Müller-Steinhagen H., Malayeri M.R., Watkinson A.P. Heat Exchanger Fouling: Mitigation and Cleaning Strategies. Heat Transf Eng. 2011;32(3–4):189–196. doi: 10.1080/01457632.2010.503108. [DOI] [Google Scholar]
- Murata K., Brebu M., Sakata Y. The effect of PVC on thermal and catalytic degradation of polyethylene, polypropylene and polystyrene by a continuous flow reactor. J. Anal. Appl. Pyrol. 2009;86(1):33–38. doi: 10.1016/j.jaap.2009.04.003. [DOI] [Google Scholar]
- Murata K., Hirano Y., Sakata Y., Uddin M.A. Basic study on a continuous flow reactor for thermal degradation of polymers. J. Anal. Appl. Pyrol. 2002;65(1):71–90. doi: 10.1016/S0165-2370(01)00181-4. [DOI] [Google Scholar]
- Murata K., Sato K., Sakata Y. Effect of pressure on thermal degradation of polyethylene. J. Anal. Appl. Pyrol. 2004;71(2):569–589. doi: 10.1016/j.jaap.2003.08.010. [DOI] [Google Scholar]
- Murena F., Gioia F. Catalytic hydroprocessing of chlorobenzene–pyridine mixtures. J Hazard Mater. 1998;60(3):271–285. doi: 10.1016/S0304-3894(98)00151-4. [DOI] [Google Scholar]
- Muscalu A.M., Reiner E.J., Liss S.N., Chen T., Ladwig G., Morse D. A routine accredited method for the analysis of polychlorinated biphenyls, organochlorine pesticides, chlorobenzenes and screening of other halogenated organics in soil, sediment and sludge by GCxGC-μECD. Anal. Bioanal. Chem. 2011;401(8):2403–2413. doi: 10.1007/s00216-011-5114-0. [DOI] [PubMed] [Google Scholar]
- Nanda S., Berruti F. Thermochemical conversion of plastic waste to fuels: a review. Environ. Chem. Lett. 2021;19(1):123–148. doi: 10.1007/s10311-020-01094-7. [DOI] [Google Scholar]
- Navarro M.S., Ulbrich H.H.G.J., Andrade S., Janasi V.A. Adaptation of ICP–OES routine determination techniques for the analysis of rare earth elements by chromatographic separation in geologic materials: tests with reference materials and granitic rocks. J. Alloy. Compd. 2002;344(1):40–45. doi: 10.1016/S0925-8388(02)00302-X. [DOI] [Google Scholar]
- Nelms S. Blackwell; 2005. Inductively Coupled Plasma Mass Spectrometry Handbook. [Google Scholar]
- Nicolotti L., Cordero C., Bressanello D., Cagliero C., Liberto E., Magagna F., Rubiolo P., Sgorbini B., Bicchi C. Parallel dual secondary column-dual detection: A further way of enhancing the informative potential of two-dimensional comprehensive gas chromatography. J. Chromatogr. A. 2014;1360:264–274. doi: 10.1016/j.chroma.2014.07.081. [DOI] [PubMed] [Google Scholar]
- Okuwaki A. Feedstock recycling of plastics in Japan. Polym. Degrad. Stab. 2004;85(3):981–988. doi: 10.1016/j.polymdegradstab.2004.01.023. [DOI] [Google Scholar]
- Okuwaki, A., Yoshioka, T., Asai, M., Tachibana, H., Wakai, K. & Tada, K. 2006. The Liquefaction of Plastic Containers and Packaging in Japan. In: Scheirs, J. & Kaminsky, W. (eds.) Feedstock Recycling and Pyrolysis of Waste Plastics. 663–708.
- Oliveira, C. & Van Dril, T. 2021. Decarbonisation options for large volume organic chemicals production, Sabic geleen. Available: https://www.pbl.nl/sites/default/files/downloads/pbl-2021-decarbonisation-options-for-large-volume-organic-chemicals-production-sabic-geleen_3718.pdf.
- Oña-Ruales J.O., Ruiz-Morales Y., Wise S.A. Identification and quantification of seven fused aromatic rings C26H14 peri-condensed benzenoid polycyclic aromatic hydrocarbons in a complex mixture of polycyclic aromatic hydrocarbons from coal tar. J. Chromatogr. A. 2016;1442:83–93. doi: 10.1016/j.chroma.2016.02.082. [DOI] [PubMed] [Google Scholar]
- Onwudili J.A., Insura N., Williams P.T. Composition of products from the pyrolysis of polyethylene and polystyrene in a closed batch reactor: Effects of temperature and residence time. J. Anal. Appl. Pyrol. 2009;86(2):293–303. doi: 10.1016/j.jaap.2009.07.008. [DOI] [Google Scholar]
- Orriss, R. Effects of Contaminants in Ethylene Plants: Sodium and Iron. AIChE Spring National Meeting, Ethylene Producers Conference, 1996 New Orleans, Louisiana.
- Österlund H., Rodushkin I., Ylinenjärvi K., Baxter D.C. Determination of total chlorine and bromine in solid wastes by sintering and inductively coupled plasma-sector field mass spectrometry. Waste Manage. 2009;29(4):1258–1264. doi: 10.1016/j.wasman.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Palos R., Gutiérrez A., Vela F.J., Olazar M., Arandes J.M., Bilbao J. Waste Refinery: The Valorization of Waste Plastics and End-of-Life Tires in Refinery Units A Review. Energy Fuels. 2021;35(5):3529–3557. doi: 10.1021/acs.energyfuels.0c03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S.K., Alawani N.A., Lajami A.R., Al-Qunaysi T.A., Muller H. Characterization of aromatic hydrocarbons and sulfur heterocycles in Saudi Arabian heavy crude oil by gel permeation chromatography and ultrahigh resolution mass spectrometry. Fuel. 2019;235:1420–1426. doi: 10.1016/j.fuel.2018.07.118. [DOI] [Google Scholar]
- Pandey, S., Ralli, D., Saxena, A. & Alamkhan, W. 2004. Physicochemical characterization and applications of naphtha. J Sci Ind Res (India), 63, 276-282, https://doi.org/0975-1084..
- Papari S., Bamdad H., Berruti F. Pyrolytic Conversion of Plastic Waste to Value-Added Products and Fuels A Review. Materials. 2021;14(10) doi: 10.3390/ma14102586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.J., Park K., Kim J.-S., Maken S., Song H., Shin H., Park J.-W., Choi M.-J. Characterization of Styrene Recovery from the Pyrolysis of Waste Expandable Polystyrene. Energy Fuels. 2003;17(6):1576–1582. doi: 10.1021/ef030102l. [DOI] [Google Scholar]
- Park K.-B., Jeong Y.-S., Guzelciftci B., Kim J.-S. Two-stage pyrolysis of polystyrene: Pyrolysis oil as a source of fuels or benzene, toluene, ethylbenzene, and xylenes. Appl. Energy. 2020;259 doi: 10.1016/j.apenergy.2019.114240. [DOI] [Google Scholar]
- Patil, M., Djokic, M. R., Marin, G. B., Reyniers, M.-F. & Van Geem, K. M. Effect of Contaminants on Coke Formation during Steam Cracking of Propane. AIChE Spring National Meeting, Ethylene Producers Conference, 2019 New Orleans, Louisiana.
- Patil M., Sarris S.A., Verbeken K., Reyniers M.-F., Van Geem K.M. Catalytic Effect of Dimethyl Disulfide on Coke Formation on High-Temperature Alloys: Myth or Reality? Ind. Eng. Chem. Res. 2020;59(34):15165–15178. doi: 10.1021/acs.iecr.0c01693. [DOI] [Google Scholar]
- Pellizzari E.D. Electron capture detection in gas chromatography. J. Chromatogr. A. 1974;98(2):323–361. doi: 10.1016/S0021-9673(00)92077-6. [DOI] [Google Scholar]
- Pereira L.S.F., Frohlich A.C., Duarte F.A., Burrow R.A., Muller E.I., Flores E.M.M. Determination of halogens and sulfur in pitch from crude oil by plasma-based techniques after microwave-induced combustion. J. Anal. At. Spectrom. 2015;30(8):1822–1827. doi: 10.1039/C5JA00143A. [DOI] [Google Scholar]
- Phillips J.B., Beens J. Comprehensive two-dimensional gas chromatography: a hyphenated method with strong coupling between the two dimensions. J. Chromatogr. A. 1999;856(1):331–347. doi: 10.1016/S0021-9673(99)00815-8. [DOI] [PubMed] [Google Scholar]
- Phillips J.B., Gaines R.B., Blomberg J., Van Der Wielen F.W.M., Dimandja J.-M., Green V., Granger J., Patterson D., Racovalis L., De Geus H.-J., De Boer J., Haglund P., Lipsky J., Sinha V., Ledford E.B., Jr. A Robust Thermal Modulator for Comprehensive Two-Dimensional Gas Chromatography. J. High Resolut. Chromatogr. 1999;22(1):3–10. doi: 10.1002/(sici)1521-4168(19990101)22:1<3::Aid-jhrc3>3.0.Co;2-u. [DOI] [Google Scholar]
- Phillips J.B., Xu J. Comprehensive multi-dimensional gas chromatography. J. Chromatogr. A. 1995;703(1):327–334. doi: 10.1016/0021-9673(95)00297-Z. [DOI] [Google Scholar]
- Philp R.P., Bishop A.N., Del Rio J.C., Allen J. Characterization of high molecular weight hydrocarbons (>C<sub>40</sub>) in oils and reservoir rocks. Geological Society, London, Special Publications. 1995;86(1):71. doi: 10.1144/GSL.SP.1995.086.01.06. [DOI] [Google Scholar]
- Pilkington, B. 2020. The Future of Chemical Recycling to Turn Plastic into Virgin-Quality Materials [Online]. Available: https://www.azom.com/article.aspx?ArticleID=19986 [Accessed 04/01/2021].
- Pinto F., Costa P., Gulyurtlu I., Cabrita I. Pyrolysis of plastic wastes. 1. Effect of plastic waste composition on product yield. J. Anal. Appl. Pyrol. 1999;51(1):39–55. doi: 10.1016/S0165-2370(99)00007-8. [DOI] [Google Scholar]
- Pivnenko K., Granby K., Eriksson E., Astrup T.F. Recycling of plastic waste: Screening for brominated flame retardants (BFRs) Waste Manage. 2017;69:101–109. doi: 10.1016/j.wasman.2017.08.038. [DOI] [PubMed] [Google Scholar]
- Plastics Europe 2020. Plastics - The Facts 2020.
- Plasticseurope. 2021. Waste Collection, Pre-treatment and Sorting [Online]. Available: https://www.plasticseurope.org/en/focus-areas/circular-economy/zero-plastics-landfill/waste-collection-pre-treatment-and-sorting [Accessed 15/02/2021].
- Poole C.F. Ionization-based detectors for gas chromatography. J. Chromatogr. A. 2015;1421:137–153. doi: 10.1016/j.chroma.2015.02.061. [DOI] [PubMed] [Google Scholar]
- Powers G.W., Martin R.L., Piehl F.J., Griffin J.M. Arsenic in Naphthas. Anal. Chem. 1959;31(9):1589–1593. doi: 10.1021/ac60153a047. [DOI] [Google Scholar]
- Prado G.H.C., Rao Y., De Klerk A. Nitrogen Removal from Oil: A Review. Energy Fuels. 2017;31(1):14–36. doi: 10.1021/acs.energyfuels.6b02779. [DOI] [Google Scholar]
- Prahlada Rao S., Sunkada S. Making sense of boiling points and melting points. Resonance. 2007;12(6):43–57. doi: 10.1007/s12045-007-0059-5. [DOI] [Google Scholar]
- Predel M., Kaminsky W. Pyrolysis of mixed polyolefins in a fluidised-bed reactor and on a pyro-GC/MS to yield aliphatic waxes. Polym. Degrad. Stab. 2000;70(3):373–385. doi: 10.1016/S0141-3910(00)00131-2. [DOI] [Google Scholar]
- Pursch M., Eckerle P., Biel J., Streck R., Cortes H., Sun K., Winniford B. Comprehensive two-dimensional gas chromatography using liquid nitrogen modulation: set-up and applications. J. Chromatogr. A. 2003;1019(1):43–51. doi: 10.1016/j.chroma.2003.07.016. [DOI] [PubMed] [Google Scholar]
- Pyl S.P., Schietekat C.M., Reyniers M.-F., Abhari R., Marin G.B., Van Geem K.M. Biomass to olefins: Cracking of renewable naphtha. Chem. Eng. J. 2011;176–177:178–187. doi: 10.1016/j.cej.2011.04.062. [DOI] [Google Scholar]
- Qin L., Han J., Zhao B., Wang Y., Chen W., Xing F. Thermal degradation of medical plastic waste by in-situ FTIR, TG-MS and TG-GC/MS coupled analyses. J. Anal. Appl. Pyrol. 2018;136:132–145. doi: 10.1016/j.jaap.2018.10.012. [DOI] [Google Scholar]
- Ragaert K., Delva L., Van Geem K. Mechanical and chemical recycling of solid plastic waste. Waste Manage. 2017;69(1):24–58. doi: 10.1016/j.wasman.2017.07.044. [DOI] [PubMed] [Google Scholar]
- Rahmi D., Zhu Y., Fujimori E., Umemura T., Haraguchi H. Multielement determination of trace metals in seawater by ICP-MS with aid of down-sized chelating resin-packed minicolumn for preconcentration. Talanta. 2007;72(2):600–606. doi: 10.1016/j.talanta.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Rankin-Turner, S. & Heaney, L. M. 2021. Applications of ambient ionization mass spectrometry in 2020: An annual review. ASA, n/a(n/a), https://doi.org/10.1002/ansa.202000135. [DOI] [PMC free article] [PubMed]
- Reid, J. & Nowowiejski, G. Overview of oxygenates in olefin units in relation to corrosion, fouling, product specifications, and safety. AIChE Spring National Meeting, Ethylene Producers Conference, 2003 New Orleans, Louisiana. American Institute of Chemical Engineers New York.
- Reyniers M.-F.S.G., Froment G.F. Influence of Metal Surface and Sulfur Addition on Coke Deposition in the Thermal Cracking of Hydrocarbons. Ind. Eng. Chem. Res. 1995;34(3):773–785. doi: 10.1021/ie00042a009. [DOI] [Google Scholar]
- Richter H., Howard J.B. Formation of polycyclic aromatic hydrocarbons and their growth to soot—a review of chemical reaction pathways. Prog. Energy Combust. Sci. 2000;26(4):565–608. doi: 10.1016/S0360-1285(00)00009-5. [DOI] [Google Scholar]
- Ristic N., Djokic M., Van Geem K., Marin G. On-line analysis of nitrogen containing compounds in complex hydrocarbon matrixes. J Vis Exp. 2016;114(1):1–12. doi: 10.3791/54236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic N.D., Djokic M.R., Delbeke E., Gonzalez-Quiroga A., Stevens C.V., Van Geem K.M., Marin G.B. Compositional Characterization of Pyrolysis Fuel Oil from Naphtha and Vacuum Gas Oil. Energy Fuels. 2018;32(2):1276–1286. doi: 10.1021/acs.energyfuels.7b03242. [DOI] [Google Scholar]
- Ristic N.D., Djokic M.R., Konist A., Van Geem K.M., Marin G.B. Quantitative compositional analysis of Estonian shale oil using comprehensive two dimensional gas chromatography. Fuel Process. Technol. 2017;167:241–249. doi: 10.1016/j.fuproc.2017.07.008. [DOI] [Google Scholar]
- Robinson P.R. In: Advances in Clean Hydrocarbon Fuel Processing. Khan M.R., editor. Woodhead Publishing; 2011. 10 - Hydroconversion processes and technology for clean fuel and chemical production; pp. 287–325. [Google Scholar]
- Rodríguez E., Elordi G., Valecillos J., Izaddoust S., Bilbao J., Arandes J.M., Castaño P. Coke deposition and product distribution in the co-cracking of waste polyolefin derived streams and vacuum gas oil under FCC unit conditions. Fuel Process. Technol. 2019;192:130–139. doi: 10.1016/j.fuproc.2019.04.012. [DOI] [Google Scholar]
- Rodríguez E., Gutiérrez A., Palos R., Vela F.J., Arandes J.M., Bilbao J. Fuel production by cracking of polyolefins pyrolysis waxes under fluid catalytic cracking (FCC) operating conditions. Waste Manage. 2019;93:162–172. doi: 10.1016/j.wasman.2019.05.005. [DOI] [PubMed] [Google Scholar]
- Roosen M., Mys N., Kusenberg M., Billen P., Dumoulin A., Dewulf J., Van Geem K.M., Ragaert K., De Meester S. Detailed Analysis of the Composition of Selected Plastic Packaging Waste Products and Its Implications for Mechanical and Thermochemical Recycling. Environ. Sci. Technol. 2020;54(20):13282–13293. doi: 10.1021/acs.est.0c03371. [DOI] [PubMed] [Google Scholar]
- Ross A.B., Junyapoon S., Bartle K.D., Jones J.M., Williams A. Development of pyrolysis–GC with selective detection: coupling of pyrolysis–GC to atomic emission detection (py–GC–AED) J. Anal. Appl. Pyrol. 2001;58–59:371–385. doi: 10.1016/S0165-2370(00)00179-0. [DOI] [Google Scholar]
- Ruiz-Guerrero R., Vendeuvre C., Thiébaut D., Bertoncini F., Espinat D. Comparison of Comprehensive Two-Dimensional Gas Chromatography Coupled with Sulfur-Chemiluminescence Detector to Standard Methods for Speciation of Sulfur-Containing Compounds in Middle Distillates. J. Chromatogr. Sci. 2006;44(9):566–573. doi: 10.1093/chromsci/44.9.566. [DOI] [PubMed] [Google Scholar]
- Santak P., Conduit G. Predicting physical properties of alkanes with neural networks. Fluid Phase Equilib. 2019;501 doi: 10.1016/j.fluid.2019.112259. [DOI] [Google Scholar]
- Sarris S.A., Olahova N., Verbeken K., Reyniers M.-F., Marin G.B., Van Geem K.M. Optimization of the in Situ Pretreatment of High Temperature Ni–Cr Alloys for Ethane Steam Cracking. Ind. Eng. Chem. Res. 2017;56(6):1424–1438. doi: 10.1021/acs.iecr.6b04537. [DOI] [Google Scholar]
- Sasse, F. & Emig, G. 1998. Chemical Recycling of Polymer Materials. Chem Eng Technol, 21(10), 777-789, https://doi.org/10.1002/(SICI)1521-4125(199810)21:10<777::AID-CEAT777>3.0.CO;2-L.
- Scanlon J.T., Willis D.E. Calculation of Flame Ionization Detector Relative Response Factors Using the Effective Carbon Number Concept. J. Chromatogr. Sci. 1985;23(8):333–340. doi: 10.1093/chromsci/23.8.333. [DOI] [Google Scholar]
- Schaberg A., Wroblowski R., Goertz R. Comparative study of the thermal decomposition behaviour of different amino acids and peptides. J. Phys. Conf. Ser. 2018;1107 doi: 10.1088/1742-6596/1107/3/032013. [DOI] [Google Scholar]
- Schlummer M., Brandl F., Mäurer A., Van Eldik R. Analysis of flame retardant additives in polymer fractions of waste of electric and electronic equipment (WEEE) by means of HPLC–UV/MS and GPC–HPLC–UV. J. Chromatogr. A. 2005;1064(1):39–51. doi: 10.1016/j.chroma.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Schofield K. The enigmatic mechanism of the flame ionization detector: Its overlooked implications for fossil fuel combustion modeling. Prog. Energy Combust. Sci. 2008;34(3):330–350. doi: 10.1016/j.pecs.2007.08.001. [DOI] [Google Scholar]
- Schubert T., Lehner M., Karner T., Hofer W., Lechleitner A. Influence of reaction pressure on co-pyrolysis of LDPE and a heavy petroleum fraction. Fuel Process. Technol. 2019;193:204–211. doi: 10.1016/j.fuproc.2019.05.016. [DOI] [Google Scholar]
- Seo Y.-H., Lee K.-H., Shin D.-H. Investigation of catalytic degradation of high-density polyethylene by hydrocarbon group type analysis. J. Anal. Appl. Pyrol. 2003;70(2):383–398. doi: 10.1016/S0165-2370(02)00186-9. [DOI] [Google Scholar]
- Sevcik J.G.K. Elsevier Science; 2011. Detectors in Gas Chromatography. [Google Scholar]
- Sgorbini B., Cagliero C., Boggia L., Liberto E., Reichenbach S.E., Rubiolo P., Cordero C., Bicchi C. Parallel dual secondary-column-dual detection comprehensive two-dimensional gas chromatography: a flexible and reliable analytical tool for essential oils quantitative profiling. Flavour Fragr J. 2015;30(5):366–380. doi: 10.1002/ffj.3255. [DOI] [Google Scholar]
- Shen Y., Zhao R., Wang J., Chen X., Ge X., Chen M. Waste-to-energy: Dehalogenation of plastic-containing wastes. Waste Manage. 2016;49:287–303. doi: 10.1016/j.wasman.2015.12.024. [DOI] [PubMed] [Google Scholar]
- Siddiqui M.N., Redhwi H.H. Pyrolysis of mixed plastics for the recovery of useful products. Fuel Process. Technol. 2009;90(4):545–552. doi: 10.1016/j.fuproc.2009.01.003. [DOI] [Google Scholar]
- Simon C.M., Kaminsky W., Schlesselmann B. Pyrolysis of polyolefins with steam to yield olefins. J. Anal. Appl. Pyrol. 1996;38(1):75–87. doi: 10.1016/S0165-2370(96)00950-3. [DOI] [Google Scholar]
- Singh S., Wu C., Williams P.T. Pyrolysis of waste materials using TGA-MS and TGA-FTIR as complementary characterisation techniques. J. Anal. Appl. Pyrol. 2012;94:99–107. doi: 10.1016/j.jaap.2011.11.011. [DOI] [Google Scholar]
- Sinha A.E., Prazen B.J., Fraga C.G., Synovec R.E. Valve-based comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometric detection: instrumentation and figures-of-merit. J. Chromatogr. A. 2003;1019(1):79–87. doi: 10.1016/j.chroma.2003.08.047. [DOI] [PubMed] [Google Scholar]
- Sogancioglu M., Yel E., Ahmetli G. Pyrolysis of waste high density polyethylene (HDPE) and low density polyethylene (LDPE) plastics and production of epoxy composites with their pyrolysis chars. J. Clean Prod. 2017;165:369–381. doi: 10.1016/j.jclepro.2017.07.157. [DOI] [Google Scholar]
- Sojak L., Kubinec R., Jurdakova H., Hajekova E., Bajus M. GC-MS of polyethylene and polypropylene thermal cracking products. Pet. Coal. 2006;48(1), 1–14:1337–7027. [Google Scholar]
- Soják L., Kubinec R., Jurdáková H., Hájeková E., Bajus M. High resolution gas chromatographic–mass spectrometric analysis of polyethylene and polypropylene thermal cracking products. J. Anal. Appl. Pyrol. 2007;78(2):387–399. doi: 10.1016/j.jaap.2006.09.012. [DOI] [Google Scholar]
- Solis M., Silveira S. Technologies for chemical recycling of household plastics – A technical review and TRL assessment. Waste Manage. 2020;105:128–138. doi: 10.1016/j.wasman.2020.01.038. [DOI] [PubMed] [Google Scholar]
- Speight J.G. Wiley; 2015. Handbook of Petroleum Product Analysis. [Google Scholar]
- Srakeaw V., Yodjai S., Wetwatana U. Catalytic Pyrolysis of LDPE Plastic Wastes over Mortar Cement Catalyst. Adv Mat Res. 2014;931–932:47–51. doi: 10.4028/www.scientific.net/AMR.931-932.47. [DOI] [Google Scholar]
- Staš M., Auersvald M., Kejla L., Vrtiška D., Kroufek J., Kubička D. Quantitative analysis of pyrolysis bio-oils: A review. TrAC, Trends Anal. Chem. 2020;126 doi: 10.1016/j.trac.2020.115857. [DOI] [Google Scholar]
- Sundaram, K. M. & Stancato, B. How much is too much? Feed contaminants and their consequences. AIChE Spring National Meeting, Ethylene Producers Conference, 22-26 April 2018 Orlando, Florida. 24.
- Symoens S.H., Olahova N., Muñoz Gandarillas A.E., Karimi H., Djokic M.R., Reyniers M.-F., Marin G.B., Van Geem K.M. State-of-the-art of Coke Formation during Steam Cracking: Anti-Coking Surface Technologies. Ind. Eng. Chem. Res. 2018;57(48):16117–16136. doi: 10.1021/acs.iecr.8b03221. [DOI] [Google Scholar]
- Takamoto D.Y., Petrich M.A. Effect of Heterogeneous Secondary Pyrolysis Reactions on the Thermal Decomposition of Polyurethane Scrap. Ind. Eng. Chem. Res. 1994;33(12):3004–3009. doi: 10.1021/ie00036a015. [DOI] [Google Scholar]
- Takeshita Y., Kato K., Takahashi K., Sato Y., Nishi S. Basic study on treatment of waste polyvinyl chloride plastics by hydrothermal decomposition in subcritical and supercritical regions. J. Supercrit Fluid. 2004;31(2):185–193. doi: 10.1016/j.supflu.2003.10.006. [DOI] [Google Scholar]
- Tatterson D.F., Vasalos I.A. US patent application. 1981. Fluid catalytic cracking of heavy petroleum fractions. [Google Scholar]
- Thiäner J.B., Nett L., Zhou S., Preibisch Y., Hollert H., Achten C. Identification of 7–9 ring polycyclic aromatic hydrocarbons in coals and petrol coke using High performance liquid chromatography – Diode array detection coupled to Atmospheric pressure laser ionization – Mass spectrometry (HPLC-DAD-APLI-MS) Environ. Pollut. 2019;252:723–732. doi: 10.1016/j.envpol.2019.05.109. [DOI] [PubMed] [Google Scholar]
- Thunman H., Berdugo Vilches T., Seemann M., Maric J., Vela I.C., Pissot S., Nguyen H.N.T. Circular use of plastics-transformation of existing petrochemical clusters into thermochemical recycling plants with 100% plastics recovery. SM&T. 2019;22 doi: 10.1016/j.susmat.2019.e00124. [DOI] [Google Scholar]
- Thunman, H. & Seemann, M. C. First Experiences with the New Chalmers Gasifier. In: Yue, G., Zhang, H., Zhao, C. & Luo, Z., eds. Proceedings of the 20th International Conference on Fluidized Bed Combustion, 2010// 2010 Berlin, Heidelberg. Springer Berlin Heidelberg, 659-663.
- Tjong S.C., Meng Y.Z. Morphology and performance of potassium titanate whisker-reinforced polypropylene composites. J. Appl. Polym. Sci. 1998;70(3):431–439. doi: 10.1002/(SICI)1097-4628(19981017)70:3<431::AID-APP2>3.0.CO;2-O. [DOI] [Google Scholar]
- Tjong S.C., Meng Y.Z. Performance of potassium titanate whisker reinforced polyamide-6 composites. Polymer. 1998;39(22):5461–5466. doi: 10.1016/S0032-3861(97)10294-4. [DOI] [Google Scholar]
- Toraman H.E., Dijkmans T., Djokic M.R., Van Geem K.M., Marin G.B. Detailed compositional characterization of plastic waste pyrolysis oil by comprehensive two-dimensional gas-chromatography coupled to multiple detectors. J. Chromatogr. A. 2014;1359(1):237–246. doi: 10.1016/j.chroma.2014.07.017. [DOI] [PubMed] [Google Scholar]
- Toraman H.E., Franz K., Ronsse F., Van Geem K.M., Marin G.B. Quantitative analysis of nitrogen containing compounds in microalgae based bio-oils using comprehensive two-dimensional gas-chromatography coupled to nitrogen chemiluminescence detector and time of flight mass spectrometer. J. Chromatogr. A. 2016;1460:135–146. doi: 10.1016/j.chroma.2016.07.009. [DOI] [PubMed] [Google Scholar]
- Towfighi J., Sadrameli M., Niaei A. Coke Formation Mechanisms and Coke Inhibiting Methods in Pyrolysis Furnaces. J. Chem. Eng. Jpn. 2002;35(10):923–937. doi: 10.1252/jcej.35.923. [DOI] [Google Scholar]
- Tranchida P.Q., Mondello L. Elsevier Science; 2019. Hyphenations of Capillary Chromatography with Mass Spectrometry. [Google Scholar]
- Tranchida P.Q., Salivo S., Franchina F.A., Mondello L. Flow-Modulated Comprehensive Two-Dimensional Gas Chromatography Combined with a High-Resolution Time-of-Flight Mass Spectrometer: A Proof-of-Principle Study. Anal. Chem. 2015;87(5):2925–2930. doi: 10.1021/ac5044175. [DOI] [PubMed] [Google Scholar]
- Tsuge S., Ohtani H., Watanabe C. Elsevier B.V; 2011. Pyrolysis-gc/ms data book of synthetic polymers pyrograms, thermograms and ms of pyrolyzates. [Google Scholar]
- Turner A., Filella M. The influence of additives on the fate of plastics in the marine environment, exemplified with barium sulphate. Mar. Pollut. Bull. 2020;158 doi: 10.1016/j.marpolbul.2020.111352. [DOI] [PubMed] [Google Scholar]
- Ügdüler S., Van Geem K.M., Roosen M., Delbeke E.I.P., De Meester S. Challenges and opportunities of solvent-based additive extraction methods for plastic recycling. Waste Manage. 2020;104:148–182. doi: 10.1016/j.wasman.2020.01.003. [DOI] [PubMed] [Google Scholar]
- Uzumkesici E.S., Casal-Banciella M.D., Mcrae C., Snape C.E., Taylor D. Co-processing of single plastic waste streams in low temperature carbonisation. Fuel. 1999;78(14):1697–1702. doi: 10.1016/S0016-2361(99)00117-9. [DOI] [Google Scholar]
- Van Deursen M.M., Beens J., Janssen H.G., Leclercq P.A., Cramers C.A. Evaluation of time-of-flight mass spectrometric detection for fast gas chromatography. J. Chromatogr. A. 2000;878(2):205–213. doi: 10.1016/S0021-9673(00)00300-9. [DOI] [PubMed] [Google Scholar]
- Van Dongen R.H., Bode D., Van Der Eijk H., Van Klinken J. Hydrodemetallization of Heavy Residual Oils in Laboratory Trickle-Flow Liquid Recycle Reactors. Ind Eng Chem Process Des Dev. 1980;19(4):630–635. doi: 10.1021/i260076a021. [DOI] [Google Scholar]
- Van Geem K.M., Dhuyvetter I., Prokopiev S., Reyniers M.-F., Viennet D., Marin G.B. Coke Formation in the Transfer Line Exchanger during Steam Cracking of Hydrocarbons. Ind. Eng. Chem. Res. 2009;48(23):10343–10358. doi: 10.1021/ie900124z. [DOI] [Google Scholar]
- Van Geem K.M., Pyl S.P., Reyniers M.-F., Vercammen J., Beens J., Marin G.B. On-line analysis of complex hydrocarbon mixtures using comprehensive two-dimensional gas chromatography. J. Chromatogr. A. 2010;1217(43):6623–6633. doi: 10.1016/j.chroma.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Van Stee, L. L. P., Beens, J., Vreuls, R. J. J. & Brinkman, U. a. T. 2003. Comprehensive two-dimensional gas chromatography with atomic emission detection and correlation with mass spectrometric detection: principles and application in petrochemical analysis. J Chromatogr A, 1019(1), 89-99, https://doi.org/10.1016/S0021-9673(03)01301-3. [DOI] [PubMed]
- Van Stee, L. L. P., Brinkman, U. a. T. & Bagheri, H. 2002. Gas chromatography with atomic emission detection: a powerful technique. TrAC, Trends Anal Chem, 21(9), 618–626, https://doi.org/10.1016/S0165-9936(02)00810-5.
- Vasile C., Brebu M.A., Karayildirim T., Yanik J., Darie H. Feedstock recycling from plastics and thermosets fractions of used computers II. Pyrolysis oil upgrading. Fuel. 2007;86(4):477–485. doi: 10.1016/j.fuel.2006.08.010. [DOI] [Google Scholar]
- Velghe I., Carleer R., Yperman J., Schreurs S. Study of the pyrolysis of municipal solid waste for the production of valuable products. J. Anal. Appl. Pyrol. 2011;92(2):366–375. doi: 10.1016/j.jaap.2011.07.011. [DOI] [Google Scholar]
- Vinu R., Broadbelt L.J. Unraveling Reaction Pathways and Specifying Reaction Kinetics for Complex Systems. Annu. Rev. Chem. Biomol. Eng. 2012;3(1):29–54. doi: 10.1146/annurev-chembioeng-062011-081108. [DOI] [PubMed] [Google Scholar]
- Wang F.C.-Y., Robbins W.K., Greaney M.A. Speciation of nitrogen-containing compounds in diesel fuel by comprehensive two-dimensional gas chromatography. J. Sep. Sci. 2004;27(5–6):468–472. doi: 10.1002/jssc.200301643. [DOI] [PubMed] [Google Scholar]
- Wauters S., Marin G.B. Kinetic Modeling of Coke Formation during Steam Cracking. Ind. Eng. Chem. Res. 2002;41(10):2379–2391. doi: 10.1021/ie010822k. [DOI] [Google Scholar]
- Weiss I.M., Muth C., Drumm R., Kirchner H.O.K. Thermal decomposition of the amino acids glycine, cysteine, aspartic acid, asparagine, glutamic acid, glutamine, arginine and histidine. BMC Biophys. 2018;11(1):2. doi: 10.1186/s13628-018-0042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel M.D., Jurs P.C. Prediction of Normal Boiling Points of Hydrocarbons from Molecular Structure. J Chem Inform Comput Sci. 1995;35(1):68–76. doi: 10.1021/ci00023a010. [DOI] [Google Scholar]
- Westmoreland P.R., Dean A.M., Howard J.B., Longwell J.P. Forming benzene in flames by chemically activated isomerization. J. Phys. Chem. 1989;93(25):8171–8180. doi: 10.1021/j100362a008. [DOI] [Google Scholar]
- Wilhelm S.M. Risk analysis for operation of aluminum heat exchangers contaminated by mercury. Process Saf. Prog. 2009;28(3):259–266. doi: 10.1002/prs.10322. [DOI] [Google Scholar]
- Wilhelm S.M., Bloom N. Mercury in petroleum. Fuel Process. Technol. 2000;63(1):1–27. doi: 10.1016/S0378-3820(99)00068-5. [DOI] [Google Scholar]
- Wilkie C.A. TGA/FTIR: an extremely useful technique for studying polymer degradation. Polym. Degrad. Stab. 1999;66(3):301–306. doi: 10.1016/S0141-3910(99)00054-3. [DOI] [Google Scholar]
- Williams E.A., Williams P.T. Analysis of products derived from the fast pyrolysis of plastic waste. J. Anal. Appl. Pyrol. 1997;40–41:347–363. doi: 10.1016/S0165-2370(97)00048-X. [DOI] [Google Scholar]
- Williams, E. A. & Williams, P. T. 1997b. The pyrolysis of individual plastics and a plastic mixture in a fixed bed reactor. J Chem Technol Biotechnol, 70(1), 9–20, https://doi.org/10.1002/(SICI)1097-4660(199709)70:1<9::AID-JCTB700>3.0.CO;2-E.
- Williams P.T., Williams E.A. Fluidised bed pyrolysis of low density polyethylene to produce petrochemical feedstock. J. Anal. Appl. Pyrol. 1999;51(1–2):107–126. doi: 10.1016/s0165-2370(99)00011-x. [DOI] [Google Scholar]
- Williams P.T., Williams E.A. Interaction of Plastics in Mixed-Plastics Pyrolysis. Energy Fuels. 1999;13(1):188–196. doi: 10.1021/ef980163x. [DOI] [Google Scholar]
- Wong H.-W., Broadbelt L.J. Tertiary Resource Recovery from Waste Polymers via Pyrolysis: Neat and Binary Mixture Reactions of Polypropylene and Polystyrene. Ind. Eng. Chem. Res. 2001;40(22):4716–4723. doi: 10.1021/ie010171s. [DOI] [Google Scholar]
- Wongkhorsub C., Chindaprasert N. A Comparison of the Use of Pyrolysis Oils in Diesel Engine. EPE. 2013;05:350–355. doi: 10.4236/epe.2013.54B068. [DOI] [Google Scholar]
- Wu J., Chen T., Luo X., Han D., Wang Z., Wu J. TG/FTIR analysis on co-pyrolysis behavior of PE PVC and PS. Waste Manage. 2014;34(3):676–682. doi: 10.1016/j.wasman.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Wysocka I. Determination of rare earth elements concentrations in natural waters – A review of ICP-MS measurement approaches. Talanta. 2021;221 doi: 10.1016/j.talanta.2020.121636. [DOI] [PubMed] [Google Scholar]
- Yan X. Detection by ozone-induced chemiluminescence in chromatography. J. Chromatogr. A. 1999;842(1):267–308. doi: 10.1016/S0021-9673(99)00177-6. [DOI] [Google Scholar]
- Yan X. Sulfur and nitrogen chemiluminescence detection in gas chromatographic analysis. J. Chromatogr. A. 2002;976(1):3–10. doi: 10.1016/S0021-9673(02)01231-1. [DOI] [PubMed] [Google Scholar]
- Yan X. Unique selective detectors for gas chromatography: Nitrogen and sulfur chemiluminescence detectors. J. Sep. Sci. 2006;29(12):1931–1945. doi: 10.1002/jssc.200500507. [DOI] [PubMed] [Google Scholar]
- Yang X., Sun L., Xiang J., Hu S., Su S. Pyrolysis and dehalogenation of plastics from waste electrical and electronic equipment (WEEE): A review. Waste Manage. 2013;33(2):462–473. doi: 10.1016/j.wasman.2012.07.025. [DOI] [PubMed] [Google Scholar]
- Ye Q., Ma X., Li B., Jin Z., Xu Y., Fang C., Zhou X., Ge Y., Ye F. Development and Investigation of Lanthanum Sulfadiazine with Calcium Stearate and Epoxidised Soyabean Oil as Complex Thermal Stabilizers for Stabilizing Poly(vinyl chloride) Polymers. 2019;11(3) doi: 10.3390/polym11030531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon, H. C. & Jung, S. J. Calcium Removal from Heavy Crude Oil Using Aqueous Solution Containing a Calcium Removal Agent. AIChE Spring National Meeting and Global Congress on Process Safety, 2016 Houston, Texas.
- Young, I. 2021. Sabic, Plastic Energy to start building plant in Netherlands for chemical recycling of plastics [Online]. Available: https://chemweek.com/CW/Document/116768/Sabic-Plastic-Energy-to-start-building-plant-in-Netherlands-for-chemical-recycling-of-plastics [Accessed 24/01/2021].
- Yu D., Wu J., Zhou L., Xie D., Wu S. The dielectric and mechanical properties of a potassium-titanate-whisker-reinforced PP/PA blend. Compos. Sci. Technol. 2000;60(4):499–508. doi: 10.1016/S0266-3538(99)00149-9. [DOI] [Google Scholar]
- Zacher A.H., Olarte M.V., Santosa D.M., Elliott D.C., Jones S.B. A review and perspective of recent bio-oil hydrotreating research. Green Chem.. 2014;16(2):491–515. doi: 10.1039/C3GC41382A. [DOI] [Google Scholar]
- Zanella D., Focant J.-F., Franchina F.A. 30th Anniversary of comprehensive two-dimensional gas chromatography: Latest advances. ASA. 2021;1–12 doi: 10.1002/ansa.202000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Horrocks A.R. A review of flame retardant polypropylene fibres. Prog. Polym. Sci. 2003;28(11):1517–1538. doi: 10.1016/j.progpolymsci.2003.09.001. [DOI] [Google Scholar]
- Ziegler M., Jilbert T., De Lange G.J., Lourens L.J., Reichart G.-J. Bromine counts from XRF scanning as an estimate of the marine organic carbon content of sediment cores. Geochem. Geophys. Geosyst. 2008;9(5) doi: 10.1029/2007gc001932. [DOI] [Google Scholar]
- Zushi Y., Hashimoto S., Tamada M., Masunaga S., Kanai Y., Tanabe K. Retrospective analysis by data processing tools for comprehensive two-dimensional gas chromatography coupled to high resolution time-of-flight mass spectrometry: A challenge for matrix-rich sediment core sample from Tokyo Bay. J. Chromatogr. A. 2014;1338:117–126. doi: 10.1016/j.chroma.2014.02.015. [DOI] [PubMed] [Google Scholar]