Summary:
Immune checkpoint inhibitors (ICI) are now approved in numerous and diverse cancer types, in various combination regimens, and are now an established cornerstone of cancer therapeutics. Toxicities from ICI are autoimmune in nature and may affect any organ system in an unpredictable fashion, commonly including endocrine manifestations involving the thyroid, pituitary, adrenal, and pancreas. These events are a frequent source of acute and persistent morbidity and even mortality. Over the past few years, there has been a growing appreciation for the underlying pathogenesis of these processes, and in developing more effective management strategies. Herein, we review the current understanding of the pathobiology, clinical manifestations, and treatment approaches to endocrine toxicities arising from ICI.
Keywords: Nivolumab, pembrolizumab, ipilimumab, immune, toxicity, hypothyroidism, hypophysitis, hyperthyroidism, diabetes, adrenal
Introduction
Since their initial FDA approval in 2011, immune checkpoint inhibitors (ICI) have rapidly become an integral part of many cancer therapeutic regimens. These novel drugs can markedly improve survival rates in several forms of cancer, but they can also induce a wide array of immune-related adverse events (irAEs) that can range from mild to life-threatening. Among the most common of these irAEs are the ICI-induced endorinopathies. Due to the the relatively vague nature of symptoms associated with these adverse events, timely diagnosis requires a high index of suspicion. Prompt recognition of the conditions and initiation of treatment can have dramatic effects on the patients’ health and quality of life.
In this review, we discuss the general mechanisms and characteristics of ICI therapy and toxicities, followed by a specific review of the current state of the science regarding the epidemiology and pathophysiology of ICI-associated endocrinopathies. We also review the typical clinic presentation, recommended steps in screening, diagnosis, and management of these conditions.
ICI mechanism of action
ICI are monoclonal antibodies that are now widely used in cancer treatment.1 These agents are approved in 17 different cancer types and have emerged, alongside surgery, radiation, chemotherapy, and targeted therapy, as a fundamental pillar of cancer treatment. Approximately half of all metastatic cancer patients are now eligible for these treatments, with many more approvals and indications expected in the coming years.2 Effective combinations with dual ICI therapy, and regimens combined with targeted therapy and chemotherapy are now being widely used.3 Further, these agents are being used in the adjuvant or maintenance setting in melanoma, urothelial carcinoma, and non-small cell lung cancer, thus expanding their use to patients without active cancer.4–6
ICI function primarily in two key signaling pathways related to T cell activation and exhaustion. A full discussion of these pathways is beyond the scope of this review, and is shown schematically in Figure 1.1 The first ICI approved was ipilimumab, a monoclonal antibody targeting cytotoxic T lymphocyte antigen-4 (CTLA-4). This signaling node functions largely in the context of antigen-presenting cell (APC) engaging with T cells in the periphery (lymph nodes) although additional functionality in the tumor microenvironment may also be relevant. The second pathway involves engagement between programmed cell death-1 and its ligand (PD-1/PD-L1). This interaction is relevant in the context of an inflamed tumor microenvironment as well as other chronic inflammatory settings.
Figure 1:
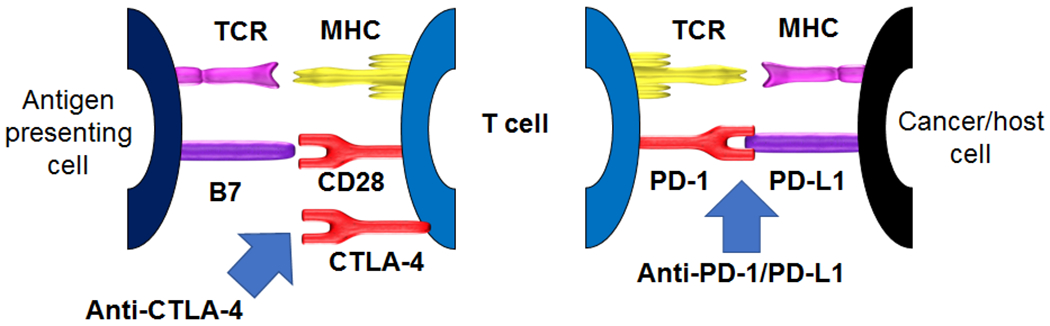
Schematic of immune checkpoint inhibitor activity; anti-CTLA-4 (left) and anti-PD-1/PD-L1 (right). T cell activation requires 1) engagement of a T cell receptor (TCR) with an antigen presented in the context of a major histocompatibility complex (MHC), and 2) a second signal consisting of engagement of CD28 with B7. CTLA-4 opposes this second signal, by binding to B7 at higher affinity than CD28, thus limiting T cell activation. Blocking CTLA-4 pharmacologically, therefore “removes the brakes” on T cell activation and allows for unopposed engagement of the second signal. PD-1, a receptor on T cells, binds PD-L1, expressed on a variety of cells including tumor cells and tumor-infiltrating macrophages, which triggers a cascade of T cell inhibitor processes known as T cell exhaustion. Blocking either side of this interaction (with PD-1 or PD-L1 targeting antibodies) prevents this engagement, precludes T cell exhaustion, and permits anti-tumor activity
ICI Clinical Activity
Ipilimumab (anti-CTLA-4) was the first approved ICI, receiving approval in 2011. Substantial clinical activity was observed primarily in melanoma, producing approximately 15-20% response rates, with 20% long-term survival, persisting even 10 years beyond treatment.7 This contrasted markedly with the efficacy of chemotherapy and even most targeted therapies in solid tumors (which are usually associated with acquired resistance and temporary responses), and provided an important proof of principle for immunotherapy activity. More importantly, agents targeting PD-1 (nivolumab, pembrolizumab, cemiplimab) and PD-L1 (atezolizumab, avelumab, durvalumab) have demonstrated more widespread efficacy, now receiving approvals in 17 different cancer types, including cancers of the skin, kidney, upper aeorodigestive tract, lung, head and neck, bladder, and many more. Response rates range from 15-25% (most solid tumors including lung cancer) to 40-60% (mismatch repair deficient cancers and skin cancers including melanoma) to 80-90% (Hodgkin Lymphoma).8 Many of these responses are durable, and may amount to a cure in some patients although longer term follow up is needed.
ICI-based combinations have also shown great promise. The combination of ipilimumab and nivolumab has demonstrated enhanced response rates in melanoma, lung cancer, renal cell carcinoma, and mismatch repair deficient colon cancer compared with single agent therapy, although at a cost of increased toxicities (see next section).9–11 Anti-PD-1/PD-L1 agents pared with chemotherapy have also improved response rates and survival in many diseases including lung cancer, triple negative breast cancer, head and neck squamous cell carcinoma, and others.12–14 Combinations of targeted agents and anti-PD-1/PD-L1 have also shown efficacy, most notably the combination of pembrolizumab and axitinib (an inhibitor of vascular growth factor inhibitor) in renal cell carcinoma.15 Literally hundreds of clinical trials involving combinations of ICI are now being conducted, suggesting that this field will continue to rapidly evolve.
ICI toxicities: Pathobiology
The targets of ICI (CTLA-4, PD-1, and PD-L1) are key regulators of immune tolerance, and prevent autoimmunity in the physiologic state. These nodes of self-tolerance are upregulated by ongoing inflammation (particularly by interferon gamma) and serve to mitigate inflammation in a variety of contexts (autoimmunity, tumor inflammation, tissue injury). Immune checkpoints are hijacked by the cancer cell to achieve immune evasion and avoid T cell killing. Pharmacologic blockade, therefore, can result in not only anti-tumor immunity, but autoinflammation at other sites, which clinically manifest as immune related adverse events.16
Although the general mechanisms of toxicity are understood (removal of self-tolerance and activation of T cells), the specific mechanisms of individual toxicities in particular patients are only beginning to be unraveled. Several studies have developed early data to propose potential mechanisms of toxicity (note: mechanisms of endocrine toxicity specifically will be discussed in each toxicity subsection below). First, shared antigens between tumor and inflamed tissue could play a role. One study of ICI-dermatitis showed a high frequency of shared T cell clones, including those that targeted keratins, were present in the tumor and inflamed skin after anti-PD-1 treatment.17 Another study of two fatal cases of myocarditis showed overexpression of cardiac proteins in the tumors of the affected patients, also potentially suggesting an overlap.18 Second, an environmental trigger has been proposed. One study suggested that ICI-colitis correlated with particular strains of microbial flora colonization,19 and other suggested a potentially causative role of Epstein-Barr Virus infection or reactivation in a case of ICI-encephalitis.20 Third, pre-existing, smoldering inflammation that preceded ICI but unleashed by treatment could play a role.
ICI toxicities: Clinical Presentation
As autoimmune-like phenomenon, irAEs may affect any organ system with inflammatory manifestations.21 The diversity of presentations requires a high index of suspicion by treating physicians for early diagnosis and treatment. The most common organs affected are the skin, colon, lungs, liver, and thyroid. Any type of irAE occurs in 80-90% of patients, with higher grade events (as categorized by the Common Terminology Criteria for Adverse Events) occurring in 15-20% of patients treated with anti-PD-1/PD-L1 monotherapy, and up to 60% of patients treated with combination of ipilimumab and nivolumab.9 Of interest, CTLA-4 blockade (alone or in combination with anti-PD-1/PD-L1) produces toxicities in a dose-dependent fashion.22,23 Rarely, some events may be fatal, including ~0.35% of those treated with anti-PD-1/PD-L1, and 1.2% treated with combination therapy.24 In addition, a subset of events result in chronic symptoms, particularly including endocrine and rheumatologic toxicities.25,26
Regarding risk factors, CTLA-4 blockade (alone or in combination with anti-PD-1/PD-L1) produces toxicities in a dose-dependent fashion. By contrast, PD-1 or PD-L1 blockade does not appear to have a dose-toxicity relationship at clinically tested doses (including over a 100-fold range; 0.1mg/kg to 10mg/kg of nivolumab).27 There may be a slight distinction in toxicity profile by age, as several studies have suggested increased incidence of rheumatologic events in older patients, and gender, as one study suggested female patients may be at higher risk of myocarditis.28,29 Patients with pre-existing autoimmune disease may have somewhat higher risk of irAEs, although these usually relate to increased risks of low-grade flares of their pre-existing disease rather than de novo events.30–32 Finally, patients with pre-existing organ dysfunction (e.g. steatohepatitis, smoking history) are speculated to have somewhat higher risk.33,34
Treatment for these toxicities revolve around three distinct pillars (and of note, are somewhat different from endocrine events, discussed in detail in the following sections).35,36 First, severe events require holding or discontinuation of the ICI. Second, the long pharmacologic and pharmacodynamic effects of ICI (lasting several months) requires that simply holding the drug will not reverse the effects in a clinically relevant timeframe. Thus, steroids are needed to mitigate the ongoing inflammation. Mild events may be treated with low/moderate doses of steroids (e.g. 0.5mg/kg of prednisone or equivalent), whereas clinically severe irAEs require high-dose steroids (1-2mg/kg or equivalent) tapered over 4-6 weeks. Second-line immunosuppressant options are also available for patients who fail steroids or who experience flares during the steroid taper; for example, infliximab is often effective in resolving steroid-refractory ICI-colitis. It should be noted, however, that all treatment for irAEs are based on expert-opinion and clinical-judgment rather than large-scale clinical trials or high level evidence. Third, supportive management is often needed, for example anti-inflammatories for ICI-arthritis, oxygen for ICI-pneumonitis, cardiac monitoring and anti-arrhythmic therapy for ICI-myocarditis, and anti-diarrheal agents for ICI-colitis.
Endocrine manifestations of ICI therapy
Endocrine events are among the most common toxicities experienced from ICI, affecting up to 40% of treated patients37, depending on the drugs used. The most common endocrine organs affected by ICI (in descending order) are thyroid (usually hypothyroid which may be preceded by transient thyroiditis-induced thyrotoxicosis), pituitary (panhypopituitarism or hypophysitis), adrenal (primary adrenal insufficiency), and beta cells of the pancreatic islets (insulin-deficient diabetes, similar to type 1 diabetes). These are distinct events from those caused by traditional cytotoxic chemotherapy or even newer molecular targeted therapies, which rarely cause endocrine dysfunction.
Incidence of endocrine events varies based on type of ICI used (see Figure 2).38 Symptoms typically present within 6 months of ICI initiation, but the timing of onset is quite unpredictable, and may arise any time on therapy or even several months after therapy discontinuation (see Figure 3). The severity of these events is also widely divergent; whereas hypothyroidism is usually accompanied by mild symptoms, cases of diabetes mellitus have proven fatal when presenting in diabetic ketoacidosis (DKA). While ICI-induced endocrinopathies are rarely fatal, these conditions can have dramatic effects on patients’ quality of life, and initiation of hormone therapy often markedly improves their sense of well-being. Although typically manageable with prompt recognition and treatment, the subtle and non-specific manifestations and unpredictable timing of endocrinopathies requires a high index of suspicion by treating oncologists. As these symptoms often overlap with cancer-related or other therapy-related complications (e.g. fatigue, loss of libido, depression, nausea), prophylactic symptom and hormone level monitoring are critical. Importantly, oncologists should screen patients regularly, as detailed below, and refer to endocrinology following positive screening tests if the diagnosis is unclear and for ongoing management of hormone replacement.
Figure 2:
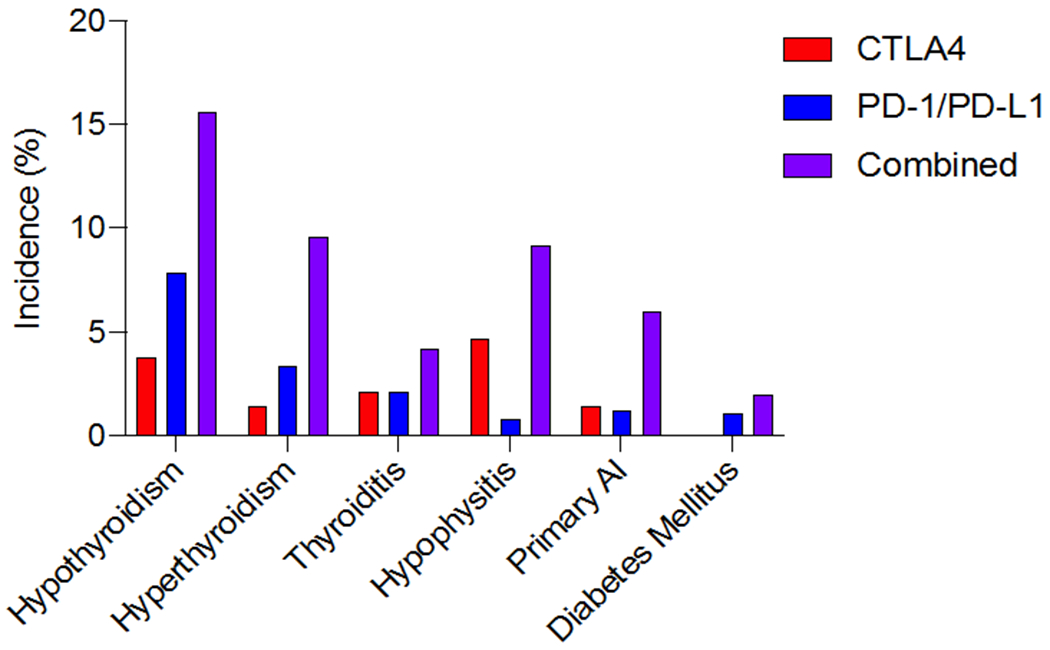
Incidence of endocrine adverse events varies by organ affected and by type of ICI therapy. Data presented as percent of patients treated with indicated ICI therapy that develop the indicated hormonal dysfunction. AI, adrenal insufficiency. Adapted from data reported in de Filette et al, 2019.
Figure 3:
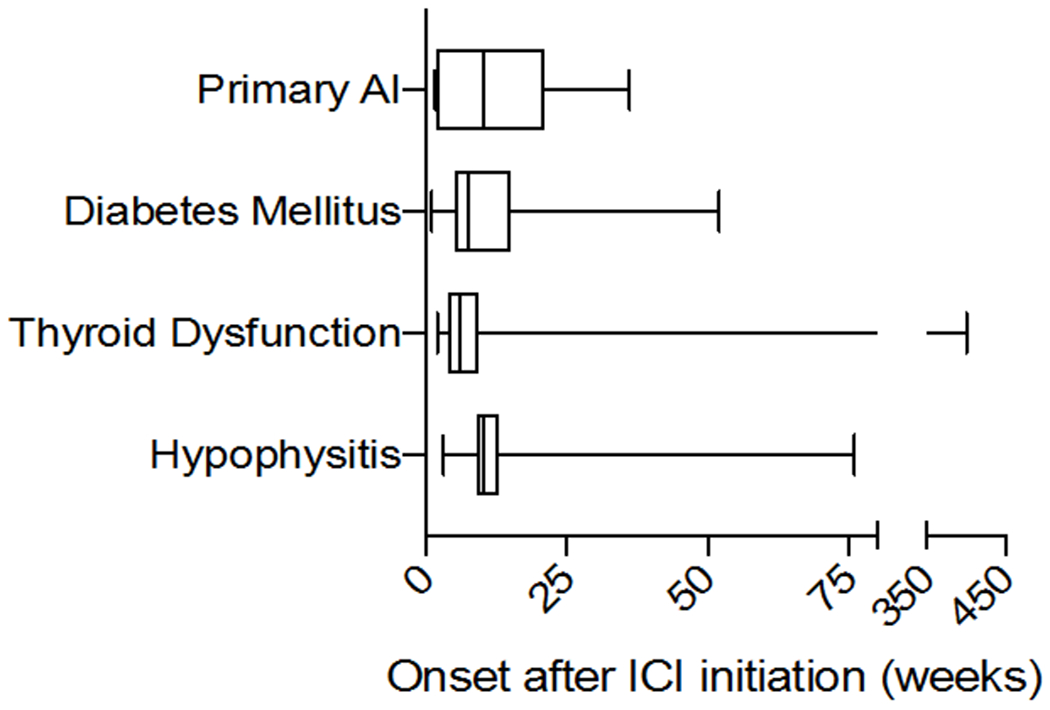
Onset of endocrinopathy can occur at any time after ICI initiation. Median time (in weeks) to onset of AI, diabetes, thyroid dysfunction and hypophysitis, with interquartile range and range indicated by boxes and whiskers, respectively. AI, adrenal insufficiency. Adapted from data reported in Tan et al, 2019.
Endocrine toxicities are distinct in several ways from other organ systems affected by ICI. First, based on comparison to spontaneous forms of autoimmune endocrinopathies, they are presumed to result in permanent, irreversible endocrine dysfunction. Most other systems are affected by transient inflammation that resolves with steroid therapy and restoration of normal organ function (although up to half of rheumatologic events and rarely other toxicities also become chronic in nature).25,26 A possible explanation for this difference is that the immune activation damages or destroys all (or nearly all) of the relatively small number of hormone producing cells, thus precluding the ability to resume hormone production, though histologic confirmation of this hypothesis is lacking. Furthermore, the inflammatory phase of the toxicity is often asymptomatic, and symptoms arise only in the terminal phase when most hormone-secreting cells have been destroyed and anti-inflammatory therapy is unlikely to be of benefit. Second, for management, endocrine events typically do not require the first two pillars of management (drug discontinuation or high-dose steroids) since the long-term hormone insufficiency usually occurs regardless of treatment. Third, supportive management (in the form of hormone replacement) is the foundation of treatment and is particularly critical to achieving optimal patient outcomes. Lifelong hormone supplementation of the deficient gland is generally required. This is a particularly relevant therapeutic complication for patients (especially young patients) who are treated in the adjuvant setting (e.g. for prevention of high-risk melanoma which may have already been cured by surgery).
Thyroid toxicities
Epidemiology/pathophysiology
Thyroid events occur in approximately 10% of patients treated with anti-PD-1/PD-L1 monotherapy, and up to 15-20% of those treated with combination PD-1/CTLA-4 blockade (as opposed to only 5% of those treated with ipilimumab monotherapy).9,38 Hypothyroidism comprises the overwhelming majority of cases, and is preceded by a destructive thyroiditis in 30-40% of anti-PD-1/PD-L1 induced cases, and up to two-thirds of combination-induced cases. It has not been well-described whether particular age, gender, or other demographics predispose to thyroid dysfunction. Several studies have suggested that thyroid dysfunction is associated with improved overall survival from a cancer standpoint, although the mechanism for this type of association is not well understood.39,40
Patients with pre-existing anti-thyroid antibodies are at higher risk compared with patients lacking these antibodies; for example three studies showed that patients with antithyroglobulin or anti-thyroid peroxidase antibodies had a much higher risk of thyroid dysfunction compared with patients negative for these antibodies (20-50% vs. 1-2.5%).41–43 This suggests that smoldering anti-thyroid autoimmunity was present prior to treatment and was unmasked by an additional removal of self-tolerance. Interestingly, thyroid transcription factor-1 (TTF-1) is expressed in the thyroid and about half of lung cancers; TTF-1 expression in the tumor did not correlate with the development of thyroid dysfunction following anti-PD-1.44 Evidence of early-on-treatment cytokine dysregulation also seems to correlate with thyroid dysfunction.45 Limited numbers of thyroid biopsies have been performed in this setting for detailed characterization of the causative immune cells, although one fine needle aspiration obtained during active ICI-induced thyroiditis showed a lymphocytic infiltrate along with CD163+ histiocytes.46 There is no evidence that patients with a history of autoimmune thyroid dysfunction experience more prominent toxicities when treated with ICI.
Clinical Presentation and Diagnosis
The median time to onset of thyroid dysfunction, most of which is hypothyroidism, is 6 weeks after ICI initiation47, although this may occur essentially any time on therapy. ICI-related hypothyroidism, which is related to thyroiditis, may present with fatigue, depressed mood, mild weight gain, constipation, and altered cognition when severe, though many patients are completely or relatively asymptomatic. Thyroiditis leading to thyrotoxicosis may be asymptomatic but symptoms typical for thyrotoxicosis (agitation, palpitations, etc.) may be present. Cases of thyroid storm are very rare48,49. Cases of ICI-related Graves’ disease have been rarely reported due to either CTLA-450–53 or PD-1 targeted therapies54,55. Toxic autonomous nodules or toxic multinodular goiter are common causes of thyrotoxicosis in the non-cancer setting and may be coincident with ICI therapy but are not likely affected by ICI therapy per se.
ICI-related hypothyroidism may have a decreased free thyroxine (FT4), but thyroid stimulating hormone (TSH) is the preferred and more sensitive test with an elevated TSH in primary hypothyroidism and low (or inappropriately normal) TSH in secondary hypothyroidism. The diagnosis of secondary hypothyroidism warrants further investigation into pituitary dysfunction (see hypophysitis, below). Anti-thyroid antibodies are not helpful in diagnosing hypothyroidism in non-pregnant adults, since primary hypothyroidism is overwhelmingly caused by autoimmune destruction of the gland. ICI-related thyrotoxicosis is characterized by an elevated FT4 or total T3 and a low or suppressed TSH. The vast majority of ICI-related thyrotoxicosis is caused by thyroiditis, resulting from destruction of thyroid follicular cells with spillage of preformed thyroid hormone. In ICI-related thyrotoxicosis, symptoms may be minimal or mild. If so, diagnostic testing beyond measurement of TSH, free T4, and total T3 may not be required and thyroid function tests should be reassessed 6 weeks later. However, if initial symptoms are prominent, the FT4 is markedly elevated, or signs of Graves’ eye disease (e.g. orbitopathy) are present, or if symptoms last more than 6 weeks, assessment should include measurement of thyroid-stimulating antibodies and/or thyroid scanning to investigate for Graves’ Disease (elevated thyroid uptake). One meta-analysis reported that ~68% of patients with thyroiditis progressed to hypothyroidism, with a median of 6 weeks from thyroiditis diagnosis to hypothyroid diagnosis47; thus patients should be closely monitored for evolution to hypothyroidism.
Monitoring and Treatment
Thyroid function should be screened by measuring TSH and FT4 every 8 weeks or sooner if clinically indicated, although many practitioners obtain these tests at every cycle of treatment.56 If the TSH is markedly elevated (> 10 uIU/ml), then further testing is not needed and thyroid hormone replacement should be started. A low TSH value can signal thyrotoxicosis (elevated FT4) or secondary hypothyroidism (FT4 low or in the normal range). Importantly, the TSH is not an accurate assessment of thyroid status in those with pituitary disease or hypophysitis. Hypothyroidism (whether or not preceded by thyroiditis) should be treated with thyroid hormone replacement when the TSH is > 10uIU/ml (on two occasions). The standard starting dose of levothyroxine is 1.6mcg/kg daily, with retesting every 6 weeks for dose adjustment. In elderly individuals, a lower initial dose should be used. The TSH level is helpful for adjusting doses in primary hypothyroidism, but should not be used to monitor the adequacy of replacement in secondary hypothyroidism with the FT4 being used instead. High-dose (or low-dose) steroids are not indicated, and patients may continue to receive their ICI treatment. One study of 151 patients with ICI-thyroid disorders, administration of high-dose glucocorticoids did not alter the time from thyrotoxicosis to hypothyroidism, or mean dose of levothyroxine needed.57 High-dose steroids may be indicated in rare cases of thyroid storm (in conjunction with standard management for this condition). Treatment for thyroiditis-associated thyrotoxicosis is generally supportive with beta blockers for tachycardia and symptomatic relief. Graves’ disease treatment may include anti-thyroid medications, radioactive iodine, or surgery depending on the clinical setting and patient preference.
Pituitary toxicities
Epidemiology/Pathophysiology
Hypophysitis, or inflammation of the pituitary gland, is a vanishingly rare condition outside the context of ICI. However, hypophysitis or hypopituitarism occurs in up to 10% of patients who receive anti-CTLA-4 based therapy (either with ipilimumab monotherapy, and slightly more commonly with combination CTLA-4/PD-1 blockade).38 The condition is less common with anti-PD-1/PD-L1 monotherapy, occurring in 0.5-1% of patients. Male gender and older age may be risk factors although this has only been observed in one small series.58
One proposed mechanism of hypophysitis relates to a type II hypersensitivity reaction, which is quite distinct from other proposed toxicity mechanisms. A mouse model of hypophysitis (induced by repeated injections of anti-CTLA-4 into C57BL/6 mice) resulted in lymphocytic infiltration into the pituitary gland and circulating anti-pituitary autoantibodies specific to thyrotropin-, gonadotropin-, and corticocotropin secreting cells.59 Interestingly, pituitary glands expressed CTLA-4 in a subset of these cells, which were targets for CTLA-4 antibody binding, and resulted in complement deposition. This potentially explains why anti-CTLA-4 therapy, but only rarely PD-1/PD-L1 blockade, results in hypophysitis. Another study using large scale autoantibody screening identified autoantibodies targeting guanine nucleotide-binding protien G(olf) subunit alpha (GNAL) and integral membrane protein 2B (ITM2B) in the serum of a patient who developed hypophysitis; further study revealed that these proteins were expressed in the pituitary gland.60
Clinical Presentation and Diagnosis
In contrast to thyroid disorders, most patients with pituitary dysfunction or hypophysitis present with clinical symptoms, rather than being detected by laboratory screening. Median time to presentation is 9-12 weeks into treatment for ipilimumab-treated patients (monotherapy or combination) and 26 weeks into treatment for anti-PD-1/PD-L1.61 These symptoms are commonly related to neuro-compression, including headache, nausea, emesis, diplopia, and visual field defects, or more often, to secondary adrenal insufficiency including fatigue and nausea. Rarely, severe cases can present with hypotension or adrenal crisis precipitated by a concurrent illness. Secondary hypothyroidism and secondary hypogonadism are also quite common (see Figure 4).
Figure 4:
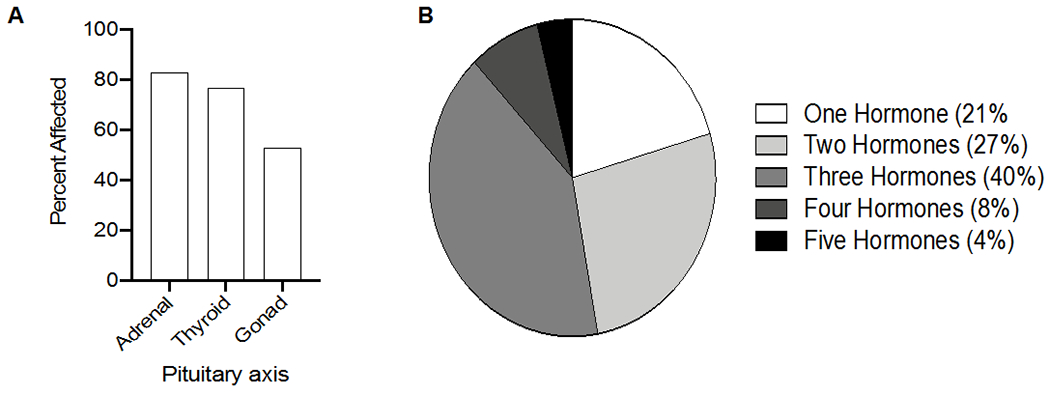
A) Percentage of patients with ICI-associated hypophysitis with adrenal, thyroid, or gonadal axes affected. B) Percentage of patients with the indicated number of hormonal axes affected. The majority of patients have 2-3 axes affected.
Adapted from data reported in Tan et al, 2019.
A low morning cortisol (< 5 mcg/dL) and low ACTH are indicative of secondary adrenal insufficiency, with low TSH and low free T4 indicative of secondary hypothyroidism, and low sex hormones, FSH, and LH indicative of secondary hypogonadism. Any recent acute steroid doses or chronic steroid therapy can confound the assessment for adrenal insufficiency and endocrinologic consultation should be considered. If the cortisol value is not in the normal range for the time of day, then cortrosyn stimulation testing should be considered as it can diagnose either primary or secondary (chronic) adrenal insufficiency. Patients with headaches should have brain imaging, preferably brain MRI with pituitary windows. This may reveal an enlarged pituitary which may even be called radiographically as a “pituitary adenoma” or an empty sella. Normal brain imaging does not rule out hypopituitarism/hypophysitis, however.
Monitoring and Treatment
Routine monitoring for pituitary (or adrenal) dysfunction is controversial. In our practice, we obtain morning cortisol +/− ACTH in patients receiving ipilimumab-based treatment at every cycle, although we do not perform routine testing in patients on anti-PD-1/PD-L1 in the absence of symptoms. Patients with adrenal insufficiency should be treated with replacement dose glucocorticoids (e.g., prednisone 5 mg daily or hydrocortisone 10-20mg in the morning and 5-10mg in the evening), with thyroid replacement if indicated.35,36 Testosterone and estrogen replacement should be started when indicated and not otherwise contraindicated. Hemodynamically stable patients with adrenal insufficiency, but who are ill, should be treated with 2-3x maintenance dose of glucocorticoids orally until the illness resolves and then resume daily maintenance glucocorticoid replacement. If the patient with adrenal insufficiency is unable to take oral glucocorticoid replacement or is hemodynamically unstable, hospitalization and treatment with increased doses of parenteral glucocorticoids is needed. Patients with severe compressive symptoms from the hypophysitis (e.g. severe headaches, diplopia, visual field defects) may receive prednisone 1-2mg/kg with rapid taper (over 1-2 weeks). One study suggested that patients who developed ipilimumab-induced hypophysitis treated with high-dose steroids have inferior anti-tumor outcomes compared to those treated with replacement-dose steroids.62 Thus, it is usually recommended to avoid high-dose steroids in the absence of severe symptoms. As with thyroid disorders, patients may continue their ICI therapy although it is reasonable to delay resuming therapy until severe symptoms resolve.
Primary Adrenal insufficiency
Primary adrenal insufficiency is a rare complication of ICI therapy.63 Clinical presentation is similar to secondary hypocortisolism, with malaise, fatigue, and nausea, but differs in that it is more frequently associated with hypotension and/or adrenal crisis, due to mineralocorticoid deficiency in combination with severe glucocorticoid deficiency. Laboratory testing reveals decreased morning cortisol and elevated ACTH levels. If mineralocorticoid-producing cells are affected, metabolic acidosis and hyperkalemia may also be present. Given the life-threatening nature of severe adrenal insufficiency, whether primary or secondary, it is appropriate to initiate treatment while awaiting confirmatory testing or subtype classification. Treatment consists of glucocorticoid replacement as for secondary adrenal insufficiency related to hypopituitarism, and may require mineralocorticoid supplementation in the outpatient setting. As with hypophysitis, patients may continue ICI following acute stabilization.
Diabetes Mellitus
Epidemiology/Pathophysiology
ICI-associated diabetes mellitus (ICI-DM), functionally defined as severe and persistent insulin deficiency following treatment with ICI 64, occurs in slightly less than 1% of patients treated with ICI 65 and has been increasing in recognition and diagnosis.66 Exacerbation of underlying type 2 diabetes may also occur, though frequency of this is unknown. Approximately 97% of all reported ICI-DM cases have arisen with anti-PD-1/PD-L1 or combination treated patients, whereas reports of cases on CTLA-4 monotherapy are very rare and may be confounded by prior immunotherapies66–68. Demographics of ICI-DM, including age, sex, and ethnicity, likely reflect the demographics of people treated with ICI. ICI-DM is frequently co-morbid with other irAEs, including pancreatitis, thyroidits, hypophysitis, or colitis, though the causative or prognostic relationship between irAEs is unknown.
Similar to classic type 1 diabetes (T1D), ICI-DM is presumably caused by immune destruction of pancreatic beta cells. PD-L1 expression is increased in pancreatic beta cells of older mice and in mice with immune infiltration of their islets69,70, and disruption of PD-1/PD-L1 signaling induces diabetes in the non-obese diabetic (NOD) mouse model 71, possibly mediated through increased proliferation of autoreactive T cells72,73. In humans, PD-L1 expression in beta cells is increased in donors with T1D compared to normal donors 69, but other mechanistic studies on human tissue has been very limited to this point. In the only published report of histologic examination of pancreatic tissue from a patient with ICI-DM, Yoneda et al noted increased peri-islet infiltration of CD8+ T lymphocytes74, as is often seen in early onset T1D. In contrast to classic T1D, they also noted an almost complete absence of insulin-positive cells, suggesting that ICI-DM may be characterized by a more severe and rapid destruction of pancreatic beta cells than T1D. Other notable differences between the two two types of diabetes suggest that their pathogenic mechanisms may be distinct. Approximately 40-50% of individuals with ICI-DM have detectable islet auto-antibodies at diagnosis, compared to >90% in T1D 64,65. Individuals with islet auto-antibodies developed diabetes earlier after ICI treatment than those without65. In one case series of 10 patients, auto-antibody negative patients did not subsequently develop auto-antibodies as far as 32 months after diagnosis67, though further studies are needed to understand the mechanistic and prognostic role that islet auto-antibodies may play in ICI-DM. Genetic factors also likely contribute to ICI-DM susceptibility. Human leukocyte antigen (HLA) haplotypes linked with classic T1D (DR3-DQ2 and DR4-DQ8) and fulminant diabetes in the Asian population (DR4-DQ4 and DR9-DQ9) are over-represented among ICI-DM patients68, with a particularly strong association with HLA-DR465. On the other hand, when multiple HLA and non-HLA T1D risk genes were evaluated in a patient with ICI-DM as part of a genetic risk score (GRS) analysis, the ICI-DM patient had a GRS below the 5th percentile for patients with T1D 75, suggesting that genetic risk factors for ICI-DM may differ from classic T1D. Further studies are needed to better understand the HLA and non-HLA genes that may contribute to ICI-DM predisposition. Finally, exocrine pancreatic inflammation may also be involved in ICI-DM pathogenesis, since elevated amylase and lipase were present in about one-third of patients presenting with ICI-DM65. Collectively, this suggests that ICI-DM has a distinct pathophysiology from classic T1D; further studies are needed to better understands its etiology and risk factors.
Clinical Presentation and Diagnosis
Time to onset from first ICI infusion is widely variable with a median of 7-17 weeks and a range of 1-228 weeks 64. Most patients develop ICI-DM while on therapy, though diabetes has developed several months after ICI cessation 66. A review of reported cases showed that a large subset of patients (38-71%) present in diabetic ketoacidosis (DKA) with relatively mild hemoglobin A1C (A1C) elevation, suggesting rapid onset and abrupt decline in insulin secretory function. It is possible, though, that less severe cases of diabetes have been under-recognized or under-reported in the literature. Given this relative acuity in onset of diabetes, A1c may not be helpful in establishing the diagnosis. Rather, random blood glucose and basic metabolic panel are more useful in screening. Patients should also be advised regarding symptoms, and treating physicians should have a high index of suspicion for this and other ICI-related toxicities. Since ICI-DM is generally insulin-dependent diabetes, it is also possible that exacerbation of underlying type 2 diabetes remains unrecognized, and standard treatment and screening for type 2 diabetes should continue. Presenting symptoms of ICI-DM include polyuria, polydipsia, and fatigue, as well as abdominal pain and nausea if presenting in DKA.
Monitoring and Treatment
Random glucose monitoring at every cycle of ICI is the primary monitoring strategy. In patients with new-onset hyperglycemia, basic metabolic panel, C-peptide, and A1C should be measured, and can help in distinguishing the etiology of hyperglycemia when other causes may be suspected (type 2 diabetes, stress hyperglycemia, steroid-induced, etc). Amylase and lipase elevations may be supportive of the diagnosis but may be normal. Treatment primarily consists of standard therapy for DKA, specifically with insulin therapy and fluid resuscitation. As with other endocrinopathies, high-dose steroids are not indicated, as no evidence suggests that steroids will assist with gland function. Patients almost certainly will require lifelong insulin replacement therapy, similar to management of patients with classic T1D, though glucose management may be more challenging in ICI-DM. There have been two reported cases of ICI-DM remission 76,77, though both cases lacked confirmation of insulinopenia64, so it is possible that they were cases of acute hyperglycemia related to severe illness rather than true ICI-DM.
Rare endocrinopathies
Primary hypoparathyroidism, diabetes insipidus, syndrome of inappropriate anti-diuretic hormone, and Cushing’s disease (ACTH-dependent cortisol excess) have been described in case reports. Details are limited due to the exceedingly rare nature of these conditions, and should be diagnosed and managed according to clinical symptoms and standard care.
Conclusions
ICI therapy has completely transformed cancer management during the past decade, and appears poised for continued growth. Thus, understanding organ-specific toxicities are critical goals for not only oncologists, but for many other specialists who encounter these patients throughout their treatment course. As among the most frequent side effects, management of endocrine toxicities remains a key objective for practicing endocrinologists. In addition, the somewhat predictable onset (at least in a defined proportion of patients) of ICI related endocrine toxicities represents a unique opportunity to explore the pathobiology of endocrine syndromes, including type 1 diabetes and hypothyroidism both within and outside the context of ICI, perhaps offering novel opportunities for developing therapeutics.
Table 1:
Summary of screening, testing, and treatment of ICI-associated endocrinopathies.
| Gland | ICI | Hormone | Disease | Testing | Results | Treatment |
|---|---|---|---|---|---|---|
| Pituitary (anterior) | C >> P | n/a | Hypophysitis | Pituitary MRI if compressive symptoms, hormonal tests as below | Sellar mass, hypophyseal enlargement | Prednisone/methylprednisolone 1-2mg/kg/day if severe symptoms |
| ACTH | Secondary AI | AM cortisol and ACTH (with symptoms or every CTLA-4 cycle) | Cortisol low ACTH low |
Glucocorticoid replacement (HC 20mg AM and 10mg PM, starting dose) | ||
| TSH | Secondary hypothyroidism | TSH, FT4 (every 4-6 weeks) | TSH low or low-normal with FT4 low |
Levothyroxine replacement (1.6mcg/kg starting dose) | ||
| LH/FSH | Secondary hypogonadism | AM testosterone or estrogen, LH, FSH (with symptoms) | AM testosterone low x2, Estrogen low, LH and FSH low | Testosterone/Estrogen replacement if not contraindicated | ||
| Thyroid | D > P > C | Thyroxine | Thyrotoxicosis | TSH, FT4 (every 4-6 weeks) | TSH low, FT4 high | Supportive, +/− beta blocker, evaluate for other causes if persistent > 6 weeks |
| Hypothyroidism | TSH high, FT4 low | Levothyroxine replacement if TSH>10 (1.6mcg/kg starting dose) | ||||
| Pancreas | P | Insulin | ICI-DM | Random or fasting BG (every 4-6 weeks) | BG high | Evaluate and treat DKA as appropriate, Insulin (basal-bolus regimen) |
| Adrenal | D > C = P | Cortisol +/− Aldosterone | Primary AI | AM cortisol and ACTH, BMP (with symptoms) | Cortisol low, ACTH high, Na low, K high, CO2 low | Glucocorticoid replacement (HC 20mg AM and 10mg PM, starting dose) +/− Mineralicorticoid replacement (fludricortisone 0.1mg daily, starting dose) |
For detailed testing and management recommendations, see clinical practice guidelines by the National Comprehensive Cancer Network56 and by the American Society of Clinical Oncology78.
ACTH, adrenocorticotropic hormone; TSH, thyroid stimulating hormone; LH, luteinizing hormone; FSH, follicle stimulating hormone; AI, adrenal insufficiency; ICI-DM, immune checkpoint inhibitor associated diabetes mellitus; BMP, basic metabolic panel; FT4, free thyroxine; DKA, diabetic ketoacidosis; HC, hydrocortisone; ; C, CTLA-4 inhibition; P, PD-1/PD-L1 inhibition; D, dual therapy.
Acknowledgments and research support:
This study was supported by NIH/NCI K23 CA204726 (DBJ), NIH R01CA227481 (DBJ), DK20593 (ACP), NIDDK T32DK007061 (JJW), the James C. Bradford Jr. Melanoma Fund (DBJ), and the Melanoma Research Foundation (DBJ).
Conflicts of interest:
DBJ serves on advisory boards for Array Biopharma, Bristol Myers Squibb, Catalyst Biopharma, Iovance, Jansen, Merck, Novartis, and Oncosec, and receives research funding from Bristol Myers Squibb and Incyte.
References
- 1.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359:1350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haslam A, Prasad V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw Open 2019;2:e192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vilgelm AE, Johnson DB, Richmond A. Combinatorial approach to cancer immunotherapy: strength in numbers. Journal of leukocyte biology 2016;100:275–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. The New England journal of medicine 2018;379:2342–50. [DOI] [PubMed] [Google Scholar]
- 5.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. The New England journal of medicine 2017;377:1824–35. [DOI] [PubMed] [Google Scholar]
- 6.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. The New England journal of medicine 2018;378:1789–801. [DOI] [PubMed] [Google Scholar]
- 7.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine 2010;363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. The New England journal of medicine 2017;377:2500–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. The New England journal of medicine 2019;381:1535–46. [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. The New England journal of medicine 2018;378:1277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. The New England journal of medicine 2019;381:2020–31. [DOI] [PubMed] [Google Scholar]
- 12.Horn L, Mansfield AS, Szczesna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. The New England journal of medicine 2018;379:2220–9. [DOI] [PubMed] [Google Scholar]
- 13.Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. The lancet oncology 2020;21:44–59. [DOI] [PubMed] [Google Scholar]
- 14.Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019;394:1915–28. [DOI] [PubMed] [Google Scholar]
- 15.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. The New England journal of medicine 2019;380:1116–27. [DOI] [PubMed] [Google Scholar]
- 16.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. The New England journal of medicine 2018;378:158–68. [DOI] [PubMed] [Google Scholar]
- 17.Berner F, Bomze D, Diem S, et al. Association of Checkpoint Inhibitor-Induced Toxic Effects With Shared Cancer and Tissue Antigens in Non-Small Cell Lung Cancer. JAMA Oncol 2019;5:1043–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson DB, Balko JM, Compton ML, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. The New England journal of medicine 2016;375:1749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubin K, Callahan MK, Ren B, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nature communications 2016;7:10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson DB, McDonnell WJ, Gonzalez-Ericsson PI, et al. A case report of clonal EBV-like memory CD4(+) T cell activation in fatal checkpoint inhibitor-induced encephalitis. Nature medicine 2019;25:1243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson DB, Chandra S, Sosman JA. Immune Checkpoint Inhibitor Toxicity in 2018. JAMA : the journal of the American Medical Association 2018;320:1702–3. [DOI] [PubMed] [Google Scholar]
- 22.Lebbe C, Meyer N, Mortier L, et al. Evaluation of Two Dosing Regimens for Nivolumab in Combination With Ipilimumab in Patients With Advanced Melanoma: Results From the Phase IIIb/IV CheckMate 511 Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2019;37:867–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarhini AA, Lee SJ, Hodi FS, et al. Phase III Study of Adjuvant Ipilimumab (3 or 10 mg/kg) Versus High-Dose Interferon Alfa-2b for Resected High-Risk Melanoma: North American Intergroup E1609. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2020;38:567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang DY, Salem JE, Cohen JV, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4:1721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braaten TJ, Brahmer JR, Forde PM, et al. Immune checkpoint inhibitor-induced inflammatory arthritis persists after immunotherapy cessation. Ann Rheum Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson DB, Friedman DL, Berry E, et al. Survivorship in Immune Therapy: Assessing Chronic Immune Toxicities, Health Outcomes, and Functional Status among Long-term Ipilimumab Survivors at a Single Referral Center. Cancer immunology research 2015;3:464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine 2012;366:2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Betof AS, Nipp RD, Giobbie-Hurder A, et al. Impact of Age on Outcomes with Immunotherapy for Patients with Melanoma. The oncologist 2017;22:963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zamami Y, Niimura T, Okada N, et al. Factors Associated With Immune Checkpoint Inhibitor-Related Myocarditis. JAMA Oncol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menzies AM, Johnson DB, Ramanujam S, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2017;28:368–76. [DOI] [PubMed] [Google Scholar]
- 31.Johnson DB, Sullivan RJ, Ott PA, et al. Ipilimumab Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders. JAMA Oncol 2016;2:234–40. [DOI] [PubMed] [Google Scholar]
- 32.Leonardi GC, Gainor JF, Altan M, et al. Safety of Programmed Death-1 Pathway Inhibitors Among Patients With Non-Small-Cell Lung Cancer and Preexisting Autoimmune Disorders. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2018;36:1905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanz BA, Pollack MH, Johnpulle R, et al. Safety and efficacy of anti-PD-1 in patients with baseline cardiac, renal, or hepatic dysfunction. J Immunother Cancer 2016;4:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naidoo J, Wang X, Woo KM, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017;35:709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2018;36:1714–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 2017;5:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nature Reviews Clinical Oncology 2019;16:563–80. [DOI] [PubMed] [Google Scholar]
- 38.de Filette J, Andreescu CE, Cools F, Bravenboer B, Velkeniers B. A Systematic Review and Meta-Analysis of Endocrine-Related Adverse Events Associated with Immune Checkpoint Inhibitors. Horm Metab Res 2019;51:145–56. [DOI] [PubMed] [Google Scholar]
- 39.Kotwal A, Kottschade L, Ryder M. PD-L1 Inhibitor-Induced Thyroiditis Is Associated with Better Overall Survival in Cancer Patients. Thyroid 2020;30:177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakakida T, Ishikawa T, Uchino J, et al. Clinical features of immune-related thyroid dysfunction and its association with outcomes in patients with advanced malignancies treated by PD-1 blockade. Oncol Lett 2019;18:2140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okada N, Iwama S, Okuji T, et al. Anti-thyroid antibodies and thyroid echo pattern at baseline as risk factors for thyroid dysfunction induced by anti-programmed cell death-1 antibodies: a prospective study. British journal of cancer 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobayashi T, Iwama S, Yasuda Y, et al. Patients With Antithyroid Antibodies Are Prone To Develop Destructive Thyroiditis by Nivolumab: A Prospective Study. J Endocr Soc 2018;2:241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toi Y, Sugawara S, Sugisaka J, et al. Profiling Preexisting Antibodies in Patients Treated With Anti-PD-1 Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol 2019;5:376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koyama J, Horiike A, Yoshizawa T, et al. Correlation between thyroid transcription factor-1 expression, immune-related thyroid dysfunction, and efficacy of anti-programmed cell death protein-1 treatment in non-small cell lung cancer. J Thorac Dis 2019;11:1919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurimoto C, Inaba H, Ariyasu H, et al. Predictive and sensitive biomarkers for thyroid dysfunctions during treatment with immune-checkpoint inhibitors. Cancer Sci 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Angell TE, Min L, Wieczorek TJ, Hodi FS. Unique Cytologic Features of Thyroiditis Caused by Immune Checkpoint Inhibitor Therapy for Malignant Melanoma. Genes Dis 2018;5:46–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan MH, Iyengar R, Mizokami-Stout K, et al. Spectrum of immune checkpoint inhibitors-induced endocrinopathies in cancer patients: a scoping review of case reports. Clin Diabetes Endocrinol 2019;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu C, Chopra IJ, Ha E. A novel melanoma therapy stirs up a storm: ipilimumab-induced thyrotoxicosis. Endocrinol Diabetes Metab Case Rep 2015;2015:140092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yonezaki K, Kobayashi T, Imachi H, et al. Combination therapy of ipilimumab and nivolumab induced thyroid storm in a patient with Hashimoto’s disease and diabetes mellitus: a case report. J Med Case Rep 2018;12:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Min L, Vaidya A, Becker C. Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur J Endocrinol 2011;164:303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gan EH, Mitchell AL, Plummer R, Pearce S, Perros P. Tremelimumab-Induced Graves Hyperthyroidism. Eur Thyroid J 2017;6:167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borodic G, Hinkle DM, Cia Y. Drug-induced graves disease from CTLA-4 receptor suppression. Ophthalmic Plast Reconstr Surg 2011;27:e87–8. [DOI] [PubMed] [Google Scholar]
- 53.Azmat U, Liebner D, Joehlin-Price A, Agrawal A, Nabhan F. Treatment of Ipilimumab Induced Graves’ Disease in a Patient with Metastatic Melanoma. Case Rep Endocrinol 2016;2016:2087525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iadarola C, Croce L, Quaquarini E, et al. Nivolumab Induced Thyroid Dysfunction: Unusual Clinical Presentation and Challenging Diagnosis. Front Endocrinol (Lausanne) 2018;9:813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brancatella A, Viola N, Brogioni S, et al. Graves’ Disease Induced by Immune Checkpoint Inhibitors: A Case Report and Review of the Literature. Eur Thyroid J 2019;8:192–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thompson JA, Schneider BJ, Brahmer J, et al. Management of Immunotherapy-Related Toxicities, Version 1.2019. J Natl Compr Canc Netw 2019;17:255–89. [DOI] [PubMed] [Google Scholar]
- 57.Ma C, Hodi FS, Giobbie-Hurder A, et al. The Impact of High-Dose Glucocorticoids on the Outcome of Immune-Checkpoint Inhibitor-Related Thyroid Disorders. Cancer immunology research 2019;7:1214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Faje AT, Sullivan R, Lawrence D, et al. Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab 2014;99:4078–85. [DOI] [PubMed] [Google Scholar]
- 59.Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Science translational medicine 2014;6:230ra45. [DOI] [PubMed] [Google Scholar]
- 60.Tahir SA, Gao J, Miura Y, et al. Autoimmune antibodies correlate with immune checkpoint therapy-induced toxicities. Proceedings of the National Academy of Sciences of the United States of America 2019;116:22246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Faje A, Reynolds K, Zubiri L, et al. Hypophysitis secondary to nivolumab and pembrolizumab is a clinical entity distinct from ipilimumab-associated hypophysitis. Eur J Endocrinol 2019. [DOI] [PubMed] [Google Scholar]
- 62.Faje AT, Lawrence D, Flaherty K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 2018;124:3706–14. [DOI] [PubMed] [Google Scholar]
- 63.Grouthier V, Lebrun-Vignes B, Moey M, et al. Immune Checkpoint Inhibitor-Associated Primary Adrenal Insufficiency: WHO VigiBase Report Analysis. The oncologist 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Quandt Z, Young A, Anderson M. Immune checkpoint inhibitor diabetes mellitus: a novel form of autoimmune diabetes. Clin Exp Immunol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stamatouli AM, Quandt Z, Perdigoto AL, et al. Collateral Damage: Insulin-Dependent Diabetes Induced With Checkpoint Inhibitors. Diabetes 2018;67:1471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wright JJ, Salem JE, Johnson DB, et al. Increased Reporting of Immune Checkpoint Inhibitor-Associated Diabetes. Diabetes Care 2018;41:e150–e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsang VHM, McGrath RT, Clifton-Bligh RJ, et al. Checkpoint Inhibitor-Associated Autoimmune Diabetes Is Distinct From Type 1 Diabetes. J Clin Endocrinol Metab 2019;104:5499–506. [DOI] [PubMed] [Google Scholar]
- 68.de Filette JMK, Pen JJ, Decoster L, et al. Immune checkpoint inhibitors and type 1 diabetes mellitus: a case report and systematic review. Eur J Endocrinol 2019;181:363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Osum KC, Burrack AL, Martinov T, et al. Interferon-gamma drives programmed death-ligand 1 expression on islet beta cells to limit T cell function during autoimmune diabetes. Sci Rep 2018;8:8295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rui J, Deng S, Arazi A, Perdigoto AL, Liu Z, Herold KC. beta Cells that Resist Immunological Attack Develop during Progression of Autoimmune Diabetes in NOD Mice. Cell Metab 2017;25:727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ansari MJ, Salama AD, Chitnis T, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med 2003;198:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martinov T, Spanier JA, Pauken KE, Fife BT. PD-1 pathway-mediated regulation of islet-specific CD4(+) T cell subsets in autoimmune diabetes. Immunoendocrinology (Houst) 2016;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pauken KE, Jenkins MK, Azuma M, Fife BT. PD-1, but not PD-L1, expressed by islet-reactive CD4+ T cells suppresses infiltration of the pancreas during type 1 diabetes. Diabetes 2013;62:2859–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoneda S, Imagawa A, Hosokawa Y, et al. T-Lymphocyte Infiltration to Islets in the Pancreas of a Patient Who Developed Type 1 Diabetes After Administration of Immune Checkpoint Inhibitors. Diabetes care 2019;42:e116–e8. [DOI] [PubMed] [Google Scholar]
- 75.Lowe JR, Perry DJ, Salama AK, Mathews CE, Moss LG, Hanks BA. Genetic risk analysis of a patient with fulminant autoimmune type 1 diabetes mellitus secondary to combination ipilimumab and nivolumab immunotherapy. J Immunother Cancer 2016;4:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hansen E, Sahasrabudhe D, Sievert L. A case report of insulin-dependent diabetes as immune-related toxicity of pembrolizumab: presentation, management and outcome. Cancer Immunol Immunother 2016;65:765–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Trinh B, Donath MY, Laubli H. Successful Treatment of Immune Checkpoint Inhibitor-Induced Diabetes With Infliximab. Diabetes care 2019;42:e153–e4. [DOI] [PubMed] [Google Scholar]
- 78.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714–68. [DOI] [PMC free article] [PubMed] [Google Scholar]


